Abstract
Background and objective
The availability of chest computed tomography (CT) imaging can help diagnose comorbidities associated with chronic obstructive pulmonary disease (COPD). Their systematic identification and relationship with all‐cause mortality have not been explored. Furthermore, whether their CT‐detected prevalence differs from clinical diagnosis is unknown.
Methods
The prevalence of 10 CT‐assessed comorbidities was retrospectively determined at baseline in 379 patients (71% men) with mild to severe COPD attending pulmonary clinics. Anthropometrics, smoking history, dyspnoea, lung function, exercise capacity, BODE (BMI, Obstruction, Dyspnoea and Exercise capacity) index and exacerbations rate were recorded. The prevalence of CT‐determined comorbidities was compared with that recorded clinically. Over a median of 78 months of observation, the independent association with all‐cause mortality was analysed. A ‘CT‐comorbidome’ graphically expressed the strength of their association with mortality risk.
Results
Coronary artery calcification, emphysema and bronchiectasis were the most prevalent comorbidities (79.8%, 62.7% and 33.9%, respectively). All were underdiagnosed before CT. Coronary artery calcium (hazard ratio [HR] 2.09; 95% CI 1.03–4.26, p = 0.042), bronchiectasis (HR 2.12; 95% CI 1.05–4.26, p = 0.036) and low psoas muscle density (HR 2.61; 95% CI 1.23–5.57, p = 0.010) were independently associated with all‐cause mortality and helped define the ‘CT‐comorbidome’.
Conclusion
This study of COPD patients shows that systematic detection of 10 CT‐diagnosed comorbidities, most of which were not detected clinically, provides information of potential use to patients and clinicians caring for them.
Keywords: all‐cause mortality, chest CT, comorbidity, COPD, tomography
Short abstract
This multicentric study shows that chest computed tomography (CT) to evaluate the presence of 10 comorbidities detects important pathologies not diagnosed in the clinical management of those patients. While emphysema, coronary artery calcification (CAC) and bronchiectasis were the most prevalent CT‐detected comorbidities, CAC, bronchiectasis and low Psoas muscle density were independently associated with all‐cause mortality.
See related Editorial
INTRODUCTION
Chronic obstructive pulmonary disease (COPD), the third cause of death worldwide, 1 is frequently associated with a wide group of comorbidities that contribute substantially to its poor outcome. 2 , 3
Our group has identified the most prevalent clinically detected comorbidities in COPD and defined the ones with an independent impact on long‐term mortality. 3 Some of these are identifiable using validated chest computed tomography techniques (CT chest). These include coronary artery disease (CAD), 4 pulmonary hypertension (PH), 5 lung cancer, 6 interstitial lung disease, 7 osteoporosis 8 and liver disease. 9 They may impair functional capacity, reduce quality of life and increase hospitalizations and importantly mortality risk. 10
In patients with COPD, chest CT is frequently used to evaluate lung parenchyma and airways, presence of pulmonary infections and/or embolism. 11 In addition, multiple associations support the use of CT scan as a screening tool for early lung cancer detection. 6 , 12 Several experts have expressed the convenience of doing a chest CT in COPD patients to make use of the information available in those studies. 13 , 14
We conducted this study in a cohort of patients with COPD attending pulmonary clinics to (1) define the prevalence of CT‐assessed comorbidities, (2) compare the CT‐determined prevalence with their clinical recognition and (3) determine the association of these comorbidities with all‐cause mortality.
METHODS
Participants
This was a retrospective analysis of a multicentre, observational study, involving COPD outpatients prospectively enrolled between June 2012 and 2015 and followed up until January 2021. All patients had mild to severe airway limitation followed by pulmonologists from the BODE (BMI, Obstruction, Dyspnoea and Exercise capacity) collaborative group (Pamplona, Las Palmas, Tenerife and Zaragoza in Spain).
COPD was defined according to the GOLD criteria 10 and had to be stable (without exacerbations) for at least 8 weeks while receiving therapy according to the same guidelines. 10 Exclusion criteria were the presence of uncontrolled comorbidities such as malignancy including the working diagnosis of lung cancer. At baseline, age, gender, height, weight, BMI, body surface area, smoking status and pack‐year history and previous year exacerbations were recorded. Lung functions were measured following the ATS/ERS standards. 15 Patients' dyspnoea was evaluated with the modified Medical Research Council (mMRC) scale, 16 and 6‐min walking distance (6MWD) was performed following the ATS recommendations. 17 The forced expiratory volume in 1 s (FEV1%), BMI, 6MWD and mMRC scale values were integrated into the BODE index. 18 CT‐diagnosed comorbidities were compared with those previously recorded in patient's medical records.
Patients were followed up for a median of 78 (50–116) months. During this time, the investigators at each site determined the patient's survival status by reviewing medical records or contacting patient's family members as previously reported. 18
Chest CT protocol and CT‐assessed comorbidities
At baseline, while supine, subjects underwent a low‐dose chest CT examination acquired at end‐inspiration, using multidetector‐row (16‐detector or 64‐detector) CT scans, ordered following the discretion of the attending physician. The examination extended from the thoracic inlet to the upper abdomen. The following parameters were employed: 120 kV, 40 mAs, 32 × 0.6 mm detector collimation, pitch 1. Images were reconstructed with 5 mm and 1 mm slice thickness using soft tissue (B31f) and high‐resolution (B60f) reconstruction algorithms to evaluate the mediastinum and lung parenchyma, respectively. CT scans were retrospectively evaluated by two chest radiologists (AE and GB), blinded to clinical data. The 10 radiological variables with validated methodology and that are potentially useful for COPD prognosis were established by the same radiologists (AE and GB) and by COPD expert pulmonologists (BRC and JPdT; see Appendix S1 in the Supporting Information). They included lung abnormalities (emphysema, interstitial lung abnormalities [ILA] and bronchiectasis) 19 , 20 , 21 , 22 ; cardiovascular abnormalities (ascending aorta and pulmonary artery enlargement [PAE] and coronary artery calcification [CAC]) 4 , 23 , 24 , 25 , 26 ; low liver density 9 , 27 ; musculoskeletal abnormalities (osteoporosis and low psoas muscle density) 8 , 13 , 28 , 29 , 30 , 31 ; and hiatus hernia. 32
Statistical analysis
To explore the normality of the data distribution, we used the Kolmogorov–Smirnov test. Quantitative data with a normal distribution were described as mean and SD and, with non‐normal distribution, as median and interquartile range (IQR). Categorical data were described using relative frequencies (%).
The prevalence of each morbidity (as categorical variable) in survivors versus non‐survivors and between clinically diagnosed versus CT‐detected comorbidities was compared using chi‐square test. To evaluate the independent association of the detected comorbidities with all‐cause mortality, a Cox proportional regression analysis was performed. The relationship was adjusted by age, BMI, FEV1 and sex. Statistical analysis was performed with IBM SPSS Statistics for Macintosh, version 25.0 (IBM Corp., Armonk, NY, USA). A p‐value of <0.05 was considered statistically significant.
Development of the ‘CT‐comorbidome’
To evaluate the strength of the association of the comorbidities with the risk of death, we performed multivariate analyses using Cox proportional hazards regression including all 10 CT‐diagnosed comorbidities. A second multivariate analysis was performed including smoking status and FEV1 (%, already adjusted by sex, age and BMI). We integrated this information with the prevalence of the disease to construct the ‘CT‐comorbidome’, a graphical expression (orbital bubble chart) of the CT‐diagnosed comorbidity prevalence and risk of death.
RESULTS
Cohort characteristics
From a total of 406 patients, we obtained appropriate radiological information in 379 participants (Figure S1 in the Supporting Information). The patients' clinical and functional data are provided in Table 1. The sample included 297 men and 82 women, with a median age of 66 years (IQR, 60–73) with a good exercise capacity at the time of enrolment. One third of the subjects were current smokers with a median history of 50 pack‐years (IQR, 36–75). The mean FEV1% predicted value was 64.4 (22.1)%, with a low BODE score and less than one exacerbation in the year prior to enrolment. During the follow‐up time (78; 50–116 months), 32.7% (n = 124) of the participants died.
TABLE 1.
Demographic characteristics and functional data of participants at baseline (n = 379)
| Variables | |
|---|---|
| Age in years, median (IQR) | 66 (59–72) |
| Male sex, n (%) | 297 (78.4%) |
| Pack‐years, median (IQR) | 50 (36–75) |
| Current smoker, n (%) | 136 (37.3) |
| BMI kg/m2, median (IQR) | 27 (23.7–30.1) |
| BSA (kg/m2), mean (SD) | 1.9 (0.3) |
| FEV1/FVC (%), median (IQR) | 55 (44–63) |
| FEV1 %, mean (SD) | 64.4 (21.9) |
| FVC %, mean (SD) | 93.6 (22.4) |
| TLC %, median (IQR) | 107 (96–116) |
| DLCO %, mean (SD) | 62.1 (46.8) |
| 6MWD (m), median (IQR) | 480 (403–545) |
| MMRC, median (IQR) | 1 (0–2) |
| BODE, median (IQR) | 1 (0–2) |
| Spirometric GOLD stages (%) |
I (36.2) II (47.9) III (13.3) IV (2.7) |
| Exacerbations in the previous year, median (IQR) | 0 (0–1) |
| Exacerbations in the previous year, yes (%) | 97 (37.9) |
| Charlson index, median (IQR) | 1 (0–2) |
Abbreviations: 6MWD, 6‐min walking distance; BODE, BMI, Obstruction, Dyspnoea and Exercise capacity, GOLD Global Initiative for Obstructive Lung Disease; BSA, body surface area; DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IQR, interquartile range; mMRC, modified Medical Research Council; TLC total lung capacity.
Prevalence of chest CT‐assessed comorbidities
The prevalence of the 10 CT‐assessed comorbidities is summarized in Table 2 and its specific prevalence in survivors versus non‐survivors is shown in Figure 1. A statistically significant higher prevalence was observed in non‐survivors for bronchiectasis, ILA, CAC, PAE and osteoporosis. Table S1 in the Supporting Information shows the prevalence of CT‐assessed comorbidities by sex. A higher prevalence of bronchiectasis and borderline higher prevalence of low Psoas density (PsD) was found in men while a higher prevalence of osteoporosis was found in women.
TABLE 2.
Prevalence of the different CT‐assessed comorbidities
| Morbidity detected | % of patients |
|---|---|
| Emphysema | 62.7 |
| Bronchiectasis | 33.9 |
| ILA | 9.2 |
| CAC | 79.8 |
| PAE (≥30 mm) | 15.6 |
| Ascending aorta enlargement | 16 |
| Hiatal hernia | 24.2 |
| Liver steatosis | 23.4 |
| Osteoporosis | 25.7 |
| Low PsD | 15.8 |
Abbreviations: CAC, coronary artery calcification; CT, computed tomography; ILA, interstitial lung abnormalities; PAE, pulmonary artery enlargement; PsD, Psoas density.
FIGURE 1.
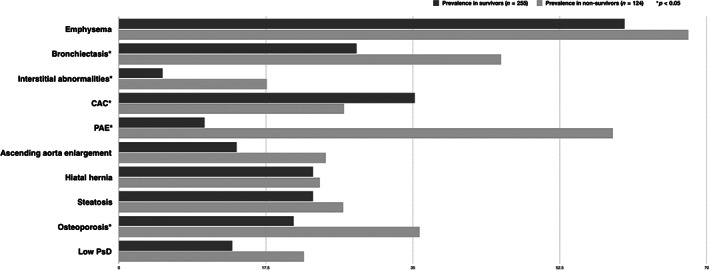
Prevalence of chest computed tomography‐assessed comorbidities in survivors and non‐survivors
Table S2 in the Supporting Information shows no significant differences in the treatment of comorbidities between survivors and non‐survivors, except for emphysema and CAD. Surprisingly, a significantly higher proportion of non‐survivors was receiving treatment for CAD perhaps reflecting more severe CAD and, thus, a higher mortality risk.
As shown in Table 3, chest CT increased the diagnostic prevalence for all the comorbidities compared to the clinically recognized diseases.
TABLE 3.
Contribution of chest CT to the diagnosis of comorbidities
| Morbidity | Clinically diagnosed | Radiologically detected | p‐value |
|---|---|---|---|
| Emphysema, % | 34.4 | 62.7 | 0.011 |
| Bronchiectasis, % | 25.9 | 33.9 | <0.001 |
| ILA, % | 4.2 | 9.2 | <0.001 |
| CAC, % | 15.6 | 79.8 | <0.001 |
| PAE (≥30 mm), % | 9 | 15.6 | <0.001 |
| Ascending aorta enlargement, % | 7.1 | 16 | <0.001 |
| Hiatal hernia, % | 21.6 | 24.2 | <0.001 |
| Liver steatosis, % | 15 | 23.4 | 0.018 |
| Osteoporosis, % | 12.9 | 25.7 | 0.039 |
| Muscle weakness versus low PsD, % | 0.3 | 15.8 | 0.021 |
Abbreviations: CAC, coronary artery calcification; CT, computed tomography; ILA, interstitial lung abnormalities; PAE, pulmonary artery enlargement; PsD, Psoas density.
The independent association between CT‐detected morbidity with all‐cause mortality is displayed in Figure 2 as an orbital bubble chart (‘CT‐comorbidome’). The bigger the size of the bubble, the higher the prevalence. The closer to the centre, the higher the hazard ratio (HR) for mortality risk, with those included within the dotted orbit reaching statistical significance. CAC, emphysema and bronchiectasis were present in 79.8%, 62.7% and 33.9% of the patients, respectively. The distribution of the visually assessed emphysema severity was mild (51.5%), moderate (31.2%) and severe (17.3%) disease. Adjusting for other important cofounders (age, sex, BMI, pack‐years history and FEV1), bronchiectasis, CAC and low PsD were independently associated with all‐cause mortality (Table 4 and Figure 2). CAC had added prognostic value to patients with low BODE index (BODE ≤ 4) (Figures S2–S4 and Tables S3–S5 in the Supporting Information).
FIGURE 2.
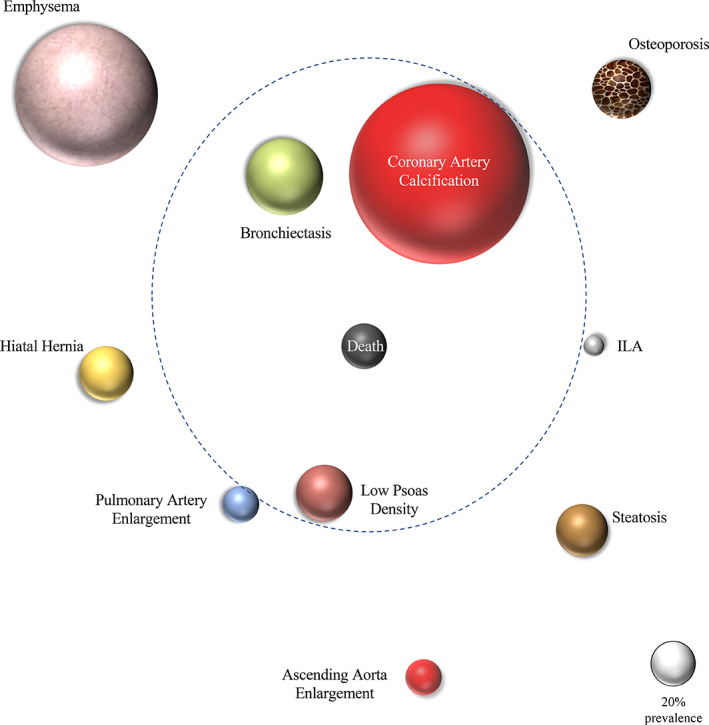
‘Computed tomography (CT)‐comorbidome’: an orbital bubble chart showing the prevalence of the 10 CT‐assessed comorbidities and the strength of their association with all‐cause mortality
TABLE 4.
Multivariate analysis exploring the CT‐determined comorbidities and risk of all‐cause mortality
| Variables | HR (95% CI) | p‐value |
|---|---|---|
| Emphysema (yes vs. no) | 1.06 (0.53–2.14) | 0.89 |
| Bronchiectasis (yes vs. no) | 2.12 (1.05–4.26) | 0.036 |
| ILA (yes vs. no) | 1.93 (0.79–4.74) | 0.151 |
| CAC (low risk vs. high risk) | 2.09 (1.03–4.26) | 0.042 |
| PAE (≥30 mm) | 1.98 (0.69–5.73) | 0.21 |
| Ascending aorta enlargement (yes vs. no) | 1.18 (0.48–2.90) | 0.724 |
| Hiatus hernia (yes vs. no) | 1.53 (0.69–3.36) | 0.269 |
| Liver steatosis (yes vs. no) | 1.39 (0.66–2.94) | 0.392 |
| Osteoporosis by CT (yes vs. no) | 1.09 (0.44–2.68) | 0.864 |
| Low PsD (yes vs. no) | 2.61 (1.23–5.57) | 0.013 |
Note: Adjusted for age, sex, BMI, pack‐year history and FEV1.
Abbreviations: CAC, coronary artery calcification; CT, computed tomography; FEV1, forced expiratory volume in 1 s; HR, hazard ratio; ILA, interstitial lung abnormalities; PAE, pulmonary artery enlargement; PsD, Psoas density.
DISCUSSION
This study shows that, in COPD patients, the systematic evaluation of data obtained from a chest CT significantly increases the prevalence of clinically unrecognized comorbidities. Importantly, we observed a significant association between these comorbidities and the risk of death after a median follow‐up of 6 years. The graphical representation of their prevalence and the strength of association with all‐cause mortality (‘CT‐comorbidome’) provides a novel visualization of the relevance of these comorbidities in COPD patients.
Three comorbidities deserve special attention, because they were independently associated with increased risk of death, but also because they were significantly under‐recognized clinically and all of which can be treated, thus able to modify their outcome. As expected, the added prognostic value of these comorbidities (especially CAC) was found in patients with low BODE index (BODE ≤ 4). 3
The strongest association to risk of death was observed for bronchiectasis, a finding that has been previously described. 22 However, our study highlights how frequently it is under‐recognized clinically (known vs. unknown: 25.6% vs. 33.9%, p < 0.001). In this cohort, the systematic CT detection of bronchiectasis increased up to 34% the percentage of patients who could benefit from its recognition as there currently are available and effective treatments for its occurrence. 33
The prevalence of chest CT‐defined low PsD was significantly higher in non‐survivors compared with survivors. Muscle weakness, which seems to occur in 22% of COPD patients, has been related to exercise intolerance and increased mortality in COPD. 30 , 34 Chest CT‐assessed PsD, which can be a marker of muscle weakness, is independently associated with long‐term mortality in COPD patients. 29 Because a regimen of rehabilitation and nutritional support may help revert muscle dysfunction and atrophy, 35 identification of PsD is clinically meaningful.
In our study, CAC reached a statistically significant independent association with mortality (p = 0.042). Importantly, CT increased the diagnostic prevalence by five times compared with the known clinical diagnosis (15.6% in clinical compared with 79.8% by CT). CAC is a marker of coronary atherosclerosis associated with both all‐cause and cardiovascular mortalities. 4 , 36 , 37 , 38 Although CAC is highly prevalent in COPD patients, it remains largely underdiagnosed clinically. CAC identification should lead to the implementation of different interventions for secondary prevention of myocardial injury. 35 , 38
PH was underdiagnosed in our cohort (known vs. unknown: 9% vs. 15.6%, p < 0.001), with a higher percentage of non‐survivors having PAE (Figure 2). PH is associated with reduced exercise capacity, increased number of exacerbations and mortality. 24 , 25 , 39 In this study, we did not find an increased risk of death for this finding likely because previous studies 24 , 25 did not compare this parameter with other important comorbidities. Although there have been no studies evaluating specific therapies for this image finding, potentially reversible causes should be explored and treated, including hypoxaemia and obstructive sleep apnoea. 40
The finding of other comorbidities that were not associated with mortality over 6 years cannot be minimized, because several of them impact health status and functional capacity.
We found that 25.7% of the patients had CT‐defined osteoporosis, a prevalence similar to other studies 31 and double the number of clinically diagnosed cases in our cohort (Table 4). Because osteoporosis increases the risk of bone fractures, we believe the systematic chest CT detection of this morbidity should prompt the implementation of guideline‐directed treatment for osteopenia and osteoporosis. 41
We also found a high prevalence of emphysema in this study population (60%), mainly of mild to moderate degree. Although we defined emphysema as an associated comorbidity in the present work, we acknowledge that it is also a disease characteristic that defines a precise phenotype of the disease. 42 We observed no association with all‐cause mortality as has been reported for more severe patients. 43 , 44 Zulueta et al. explored a large lung cancer screening cohort, finding that visually detected emphysema was associated with mortality. 45 Similar findings have been reported from several large population‐based cohorts using software‐based emphysema detection (−950 HU as the cut‐off value). 46 , 47 , 48 Those findings are in conflict with the results of our study, perhaps due to differences in population type (population based or lung cancer screening cohorts vs. COPD cohort followed at university hospitals), differences in the prevalence of emphysema (Zulueta 30%, Han 27%, Oelsner 5%, Johannessen 40% vs. 62% of the present study) or method of emphysema detection (software based vs. visual based). However, an emphysema diagnosis is associated with subsequent risk of lung cancer and as such it could help clinicians reaffirm the strategy for secondary prevention over time. 49
The prevalence of participants with ILA in the present investigation (9.2%) was similar to that reported in other COPD cohorts. 21 Currently, there is no defined strategy to implement the correct way to follow these patients, but identification of ILA should prompt a call to evaluate potential causes and imaging follow‐up.
Liver density has emerged as a relevant CT‐assessed comorbidity associated with metabolic disorders. In our study, the prevalence of CT‐assessed steatosis (23.4%) was slightly lower than previously reported in COPD patients (ranging from 30% to 41%), but higher than the one reported in the general population (5%–20% prevalence). 27 Interestingly, non‐alcoholic fatty liver disease was found to be independently related to the risk of developing ischaemic heart disease in COPD patients, regardless of classical risk factors. 50
Finally, the presence of hiatus hernia is related to gastroesophageal reflux and this has been associated with exacerbations in the ECLIPSE study. 51 Its finding should help clinicians to look for gastroesophageal reflux disease symptoms and, if required, provide its appropriate treatment.
With regard to study limitations, the most important problem in our results is that of diagnosing an incidental disease to a person who may be undergoing the test for a different reason. This can lead to significant anxiety that may not affect individual's outcome and may lead to physicians ordering additional tests. However, most of the comorbidities described here directly impact the outcomes and many can be modified with early secondary prevention and specific treatment. A second limitation is that most of the patients studied were men and the findings need to be confirmed in larger groups of women with COPD. Third, only COPD patients with a baseline chest CT scan were included in our study as the CT had to be ordered for suspicion of some underlying problem. However, all the comorbidities were underdiagnosed clinically, suggesting that the diseases detected were not the product of that bias. We also acknowledge that the CT‐investigated comorbidities were not systematically assessed at recruitment, but we believe this is ‘real’ under diagnosis in general practice. Fourth, the all‐cause mortality was identified from medical records and family contacts, so the specific cause of death could not be clearly determined. Specific causes of death would have helped define which outcomes could be most helped with CT findings. Finally, only those patients without clinical or radiological signs of lung cancer were included in the study introducing an important selection bias.
In summary, using a systematic method to determine the presence of 10 comorbidities in clinically obtained chest CT increases their prevalence above that already clinically diagnosed. Visualization of the prevalence of these chest CT‐assessed comorbidities and the strength of their association to risk of death can be expressed as a ‘CT‐comorbidome’. As many of the comorbidities are treatable, their systematic evaluation is of practical use for clinicians and patients alike. Whether this approach can result in better outcomes needs to be tested.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Ana Ezponda: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Ciro Casanova: Conceptualization (equal); project administration (equal); resources (equal); writing – review and editing (equal). Miguel Divo: Conceptualization (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal). Marta Marín‐Oto: Data curation (equal); writing – review and editing (equal). Carlos Cabrera: Conceptualization (equal); resources (equal); writing – review and editing (equal). Jose M. Marín: Resources (equal); writing – review and editing. Gorka Bastarrika: Conceptualization (equal); methodology (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal). Víctor Pinto‐Plata: Resources (equal); writing – review and editing. Ángela Martin‐Palmero: Visualization (equal); writing – review and editing (equal). Francesca Polverino: Validation (equal); writing – original draft (supporting); writing – review and editing (equal). Bartolome R. Celli: Conceptualization; investigation (equal); methodology (equal); supervision (equal); validation (equal); visualization (equal); writing – review and editing. Juan P. de Torres: Conceptualization; investigation (equal); methodology; supervision (equal); validation (equal); visualization (equal); writing‐ review and editing.
HUMAN ETHICS APPROVAL DECLARATION
The study protocol was approved by the Institution's ethics committee (IRB approval no. 28/2012) and the patients signed the informed consent to participate in this study.
Supporting information
Supporting Information.
Ezponda A, Casanova C, Divo M, Marín‐Oto M, Cabrera C, Marín JM, et al. Chest CT‐assessed comorbidities and all‐cause mortality risk in COPD patients in the BODE cohort. Respirology. 2022;27:286–293. 10.1111/resp.14223
Associate Editor: Sanjay Haresh Chotirmall; Senior Editor: Paul King
See related Editorial
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. World Health Organization . Global initiative for chronic obstructive lung disease. Geneva: World Health Organization; 2002.
- 2. Agusti A, Caverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Characterization of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto‐Plata V, et al. Cocomorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–61. [DOI] [PubMed] [Google Scholar]
- 4. Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr. 2017;11:74–84. [DOI] [PubMed] [Google Scholar]
- 5. Ascha M, Renapurkar RD, Tonelli AR. A review of imaging modalities in pulmonary hypertension. Ann Thorac Med. 2017;12:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wender R, Fontham E, Barrera E, Colditz GA, Church TR, Ettinger DS, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sverzellati N, Guerci L, Randi G, Calabrò E, La Vecchia C, Marchianò A, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38:392–400. [DOI] [PubMed] [Google Scholar]
- 8. Romme EA, Murchison JT, Phang KF, Jansen FH, Rutten EP, Wouters EF, et al. Bone attenuation on routine chest CT correlates with bone mineral density on DXA in patients with COPD. J Bone Miner Res. 2012;27(11):2338–43. [DOI] [PubMed] [Google Scholar]
- 9. Hamer OW, Aguirre DA, Casola G, Jansen FH, Rutten EP, Wouters EF, et al. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26(6):1637–53. [DOI] [PubMed] [Google Scholar]
- 10. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management and Prevention of ChronicObstructive Lung Disease 2017 Report: GOLD Executive Summary. Respirology. 2017;22(3):575–601. doi: 10.1111/resp.13012 [DOI] [PubMed] [Google Scholar]
- 11. Hersh CP, Washko GR, Estépar RS, Lutz S, Friedman PJ, Han MK, et al. Paired inspiratory‐expiratory chest CT scans to assess for small airways disease in COPD. Respir Res. 2013;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moyer VA, U.S. Preventive Services Task Force . Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–8. [DOI] [PubMed] [Google Scholar]
- 13. Singhvi D, Bon J. CT imaging and cocomorbidities in COPD: beyond lung cancer screening. Chest. 2021. Jan;159(1):147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhatt SP, Washko GR, Hoffman EA, Newell JD Jr, Bodduluri S, Diaz AA, et al. Imaging advances in chronic obstructive pulmonary disease. Insights from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) study. Am J Respir Crit Care Med. 2019. Feb 1;199(3):286–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Celli BR, MacNee W, ATS/ERS Task Force . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. [DOI] [PubMed] [Google Scholar]
- 16. Mahler D, Weels C. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–6. [DOI] [PubMed] [Google Scholar]
- 17. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 18. Celli BR, Cote C, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body mass index, airflow obstruction, dyspnea, exercise performance (BODE) index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12. [DOI] [PubMed] [Google Scholar]
- 19. National Emphysema Treatment Trial Research Group , Fishman A, Fessler H, Martinez F, McKenna RJ Jr, Naunheim K, et al. Patients at high risk of death after lung‐volume ± reduction surgery. N Engl J Med. 2001;345:1075–83. [DOI] [PubMed] [Google Scholar]
- 20. Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy‐Jardin M, et al. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med. 2020. Jul;8(7):726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Association between interstitial lung abnormalities and all‐cause mortality. JAMA. 2016;315(7):672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martínez‐García MA, de la Rosa Carrillo D, Soler‐Cataluña JJ, Donat‐Sanz Y, Serra PC, Lerma MA, et al. Prognostic value of bronchiectasis in patients with moderate‐to‐severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:823–31. [DOI] [PubMed] [Google Scholar]
- 23. Turkbey EB, Jain A, Johnson C, Redheuil A, Arai AE, Gomes AS, et al. Determinants and normal values of ascending aortic diameter by age, gender, and race/ethnicity in the Multi‐Ethnic Study of Atherosclerosis (MESA). J Magn Reson Imaging. 2014;39(2):360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Torres JP, Ezponda A, Alcaide AB, Campo A, Berto J, Gonzalez J, et al. Pulmonary arterial enlargement predicts long‐term survival in COPD patients. PLoS One. 2018. Apr 25;13(4):e0195640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LaFon DC, Bhatt SP, Labaki WW, Rahaghi FN, Moll M, Bowler RP, et al. Pulmonary artery enlargement and mortality risk in moderate to severe COPD: results from COPDGene. Eur Respir J. 2020;55(2):1901812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chiles C, Duan F, Gladish G, Ravenel JG, Baginski SG, Snyder BS, et al. Association of coronary artery calcification and mortality in the National Lung Screening Trial: a comparison of three scoring methods. Radiology. 2015;276(1):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Viglino D, Martin M, Almeras N, Ravenel JG, Baginski SG, Snyder BS, et al. Low liver density is linked to cardiovascular comorbidity in COPD: an ECLIPSE cohort analysis. Int J Chron Obstruct Pulmon Dis. 2019;14:3053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoo T, Lo WD, Evans DC. Computed tomography measured psoas density predicts outcomes in trauma. Surgery. 2017;162(2):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ezponda A, Casanova C, Cabrera C, Martin‐Palmero Á, Marin‐Oto M, Marín JM, et al. Psoas muscle density evaluated by chest CT and long‐term mortality in COPD patients. Arch Bronconeumol (Engl Ed). 2021; doi: 10.1016/j.arbres.2021.04.012 [DOI] [PubMed] [Google Scholar]
- 30. Bui KL, Nyberg A, Rabinovich R, Saey D, Maltais F. The relevance of limb muscle dysfunction in chronic obstructive pulmonary disease: a review for clinicians. Clin Chest Med. 2019. Jun;40(2):367–83. [DOI] [PubMed] [Google Scholar]
- 31. Lehouck A, Boonen S, Decramer M, Janssens W. COPD, bone metabolism, and osteoporosis. Chest. 2011;139(3):648–57. [DOI] [PubMed] [Google Scholar]
- 32. Kim C, Ouyang W, Dass C, Zhao H, Criner GJ. Hiatal hernia on chest high‐resolution computed tomography and exacerbation rates in COPD individuals. Chronic Obstr Pulm Dis. 2016;3(2):570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de la Rosa Carrillo D, López‐Campos JL, Alcázar Navarrete B, Calle Rubio M, Cantón Moreno R, García‐Rivero JL, et al. Consensus document on the diagnosis and treatment of chronic bronchial infection in chronic obstructive pulmonary disease. Arch Bronconeumol. 2020. Oct;56(10):651–64. [DOI] [PubMed] [Google Scholar]
- 34. Benz E, Trajanoska K, Lahousse L, Calle Rubio M, Cantón Moreno R, García‐Rivero JL, et al. Sarcopenia in COPD: a systematic review and meta‐analysis. Eur Respir Rev. 2019;28(154):190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cote CG, Celli BR. New treatment strategies for COPD. Pairing the new with the tried and true. Postgrad Med. 2005. Mar;117(3):27–34. [DOI] [PubMed] [Google Scholar]
- 36. Onishi K. Total management of chronic obstructive pulmonary disease (COPD) as an independent risk factor for cardiovascular disease. J Cardiol. 2017;70(2):128–34. [DOI] [PubMed] [Google Scholar]
- 37. Shemesh J, Henschke CI, Shaham D, Yip R, Farooqi AO, Cham MD, et al. Ordinal scoring of coronary artery calcifications on low‐dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010. Nov;257(2):541–8. [DOI] [PubMed] [Google Scholar]
- 38. Atar D, Jukema JW, Molemans B, Taub PR, Goto S, Mach F, et al. New cardiovascular prevention guidelines: how to optimally manage dyslipidaemia and cardiovascular risk in 2021 in patients needing secondary prevention? Atherosclerosis. 2021;319:51–61. [DOI] [PubMed] [Google Scholar]
- 39. Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012. Sep 6;367(10):913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–31. [DOI] [PubMed] [Google Scholar]
- 41. Romme EA, Geusens P, Lems WF, Rutten EP, Smeenk FW, van den Bergh JP, et al. Fracture prevention in COPD patients; a clinical 5‐step approach. Respir Res. 2015. Mar 7;16(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Celli BR, Locantore N, Tal‐Singer R, Rutten EP, Smeenk FW, van den Bergh JP, et al. Emphysema and extrapulmonary tissue loss in COPD: a multi‐organ loss of tissue phenotype. Eur Respir J. 2018. Feb 7;51(2):1702146. [DOI] [PubMed] [Google Scholar]
- 43. Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138(3):635–64. [DOI] [PubMed] [Google Scholar]
- 44. Kurashima K, Fukuda C, Nakamoto K, Takaku Y, Hijikata N, Hoshi T, et al. CT‐diagnosed emphysema and prognosis of chronic airflow obstruction: a retrospective study. BMJ Open. 2013;3(11):e003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zulueta JJ, Wisnivesky JP, Henschke CI, Yip R, Farooqi AO, McCauley DI, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141(5):1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Han MK, Tayob N, Murray S, Woodruff PG, Curtis JL, Kim V, et al. Association between emphysema and chronic obstructive pulmonary disease outcomes in the COPDGene and SPIROMICS cohorts: a post hoc analysis of two clinical trials. Am J Respir Crit Care Med. 2018;198(2):265–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oelsner EC, Hoffman EA, Folsom AR, Carr JJ, Enright PL, Kawut SM, et al. Association between emphysema‐like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161(12):863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602–8. [DOI] [PubMed] [Google Scholar]
- 49. de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, et al. Assessing the relationship between lung cancer risk and emphysema detected on low‐dose CT of the chest. Chest. 2007;132(6):1932–8. [DOI] [PubMed] [Google Scholar]
- 50. Moon SW, Kim SY, Jung JY, Kang YA, Park MS, Kim YS, et al. Relationship between obstructive lung disease and non‐alcoholic fatty liver disease in the Korean population: Korea National Health and Nutrition Examination Survey, 2007–2010. Int J Chron Obstruct Pulmon Dis. 2018;13:2603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Benson VS, Müllerová H, Vestbo J, Wedzicha JA, Patel A, Hurst JR, et al. Associations between gastro‐oesophageal reflux, its management and exacerbations of chronic obstructive pulmonary disease. Respir Med. 2015. Sep;109(9):1147–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


