Abstract
Background
The occurrence of overt hepatic encephalopathy (OHE) is associated with increased mortality. HE is commonly precipitated by infection, but whether HE predisposes to new infection is unclear. This study aimed to test if OHE predisposes to de novo infection during hospitalisation and its association with short‐term mortality.
Aims and Methods
Seven hundred and fifty‐nine consecutive patients were identified at two institutions from prospectively maintained clinical databases of cirrhotic patients admitted with acute decompensation (AD). Infection and HE data were collected on the day of admission, and the occurrence of de novo infections was assessed for 28 days after admission. EASL‐CLIF organ failure criteria were used to determine the presence of organ failures. Multivariable analysis using the logistic regression model was used to assess predictors of 28‐day mortality and de novo infection.
Results
Patients were divided into four groups; no baseline OHE or infection (n = 352); OHE with no baseline Infection (n = 221); no OHE but baseline infection (n = 100) and OHE with baseline infection (n = 86). On multivariate analyses, OHE (OR, 1.532 [95% CI, 1.061–2.300, P = 0.024]), and admission to ITU (OR, 2.303 [95% CI, 1.508–3.517, P < 0.001]) were independent risk factors for de novo infection. 28‐day mortality was 25.3%, 60.2%, 55.0% and 72.1% in the 4‐groups respectively. Age, INR and creatinine were independently predictive of mortality. The presence of overt HE, infection, coagulation, kidney, circulatory, respiratory and liver failures were significantly associated with higher mortality.
Conclusion
OHE is an independent risk factor for de novo infection in cirrhotic patients with AD.

1. INTRODUCTION
Overt hepatic encephalopathy (OHE) complicates the course of cirrhosis occurring in up to one‐third of patients at some point during their clinical course. 1 HE is also a major contributor to repeated hospital admissions in this cohort and has a massive impact on health‐related quality of life for both the patients and their caregivers 2 and is associated with high mortality (36% survival rate at 1 year and 15% at 5 years). 3 , 4 , 5 Even higher mortality is observed in patients with acute‐on‐chronic liver failure (ACLF). 6 Overt HE may occur spontaneously or because of other precipitating events such as infection, gastrointestinal bleeding, dehydration, constipation, hypovolemia, shock, high dietary protein intake, hypokalaemia, alkalosis or medications such as opiates and benzodiazepines. 7 , 8
Community‐acquired and healthcare‐related infections occur in more than 50% of hospitalised patients with cirrhosis 9 with an admission incidence of infection of 25%‐35%, which is four‐ to fivefold greater than that for the general population. 10 The most common infections in the setting of cirrhosis are spontaneous bacterial peritonitis (SBP), urinary tract infections (UTI), pneumonia and cellulitis. 9 , 11 Some of these infections might be caused by multidrug‐resistant organisms (MDROs), bearing in mind that antibiotic resistance is a growing complex issue among patients with advanced cirrhosis and can negatively affect their prognosis. 12 Infection is a common cause for hospital admission and is associated with progression to HE, other organ failures and mortality in patients with cirrhosis. 13 , 14 , 15 , 16 Those who develop one to three episodes of infection have an almost threefold risk of developing HE compared to patients without any infection. 17
The high risk of infection in cirrhotic patients is partly explained by the impaired immunity, bacterial translocation from the intestinal lumen because of intestinal bacterial overgrowth, increased permeability and decreased motility. 18 , 19 , 20 However, despite the best available treatment for managing HE and intensive care support, the risk of mortality in patients with HE remains high. 21 Several studies in the neurology literature provide compelling data showing a strong interaction between acute disorders of the nervous system and immune dysfunction as exemplified by an increased risk of infection even in patients with silent stroke. 22 Ammonia, which is thought to be central in the pathophysiology of HE is also known to induce impairment in neutrophil phagocytosis. 23 , 24 , 25 In addition, elevated ammonia levels are associated with other organ failure and mortality in patients with cirrhosis and acute decompensation (AD). 26 These data support the hypothesis that the occurrence of HE may predispose to the development of de novo infection. Therefore, in this study, we aimed to determine whether the occurrence of overt HE defines the risk of developing “de novo infection” in patients with cirrhosis and AD.
2. PATIENTS AND METHODS
2.1. Patients
The study included a total of 759 cirrhosis patients with AD from two different institutions; Royal Free Hospital (RFH), London, UK, and All India Institute of Medical Sciences (AIIMS), New Delhi, India. For the patients recruited to the study in New Delhi, India, the institute’s ethics committee approved the study. All patients provided informed consent and were recruited prospectively. For patients lacking the capacity to consent, assent from the next of kin was obtained with retrospective consent from the patient, following the 1975 Declaration of Helsinki. At the RFH, data were retrieved from a prospectively maintained registry of all patients with cirrhosis and AD admitted to the intensive care unit (ICU) of the RFH. Parts of this registry has previously been used to address other questions. 27 , 28
Patients were included if they were hospitalised with liver‐related complications of cirrhosis (AD) and met the diagnosis of cirrhosis either clinically, histologically or radiologically. Model for End‐Stage Liver Disease (MELD) 29 was calculated based on laboratory tests and clinical evaluation performed at admission, and EASL‐CLIF criteria were used to determine the presence of organ failures and grade of ACLF. 30 The West Haven (WH) classification was used to assess the severity of HE. 31 , 32 Patients admitted for reasons other than AD or with severe comorbidity, pregnancy, malignancy, infection with HIV and history of surgery were excluded.
2.1.1. Cohort 1
This comprised a population of 294 patients with cirrhosis and AD admitted to the intensive care unit in RFH, London, between January 2005 and April 2012.
2.1.2. Cohort 2
Consecutive AD cirrhotic patients (n = 465) admitted to the Department of Gastroenterology, AIIMS, New Delhi, between January 2012 and December 2018, were prospectively recruited.
2.2. Diagnostic criteria of bacterial infections
Infection was diagnosed based on the following criteria:
SBP: Polymorphonuclear (PMN) count in ascitic fluid ≥250 mm3
Urinary tract infection (UTI): Urinary sediment (>10 leukocytes/high power field) and positive urinary culture or culture‐negative but with uncountable leukocytes per high power field.
Bacteraemia: positive blood cultures.
Pneumonia: clinical signs of infection and infiltrates on chest X‐ray.
Bronchitis: clinical features of infection, no radiographic infiltrates and positive sputum culture.
Skin and soft tissue infections (SSTI): clinical signs of infection (swelling, erythema, heat and tenderness in the skin) and/or positive swab.
Spontaneous bacterial empyema (SBE): PMN count in pleural fluid ≥500 mm3 (250 mm3 if positive culture).
Secondary peritonitis: PMN count in ascitic fluid ≥250 mm3 and evidence of an intraabdominal source of infection.
Clostridium difficile infection (CDI): positive stool toxin in a patient with diarrhoea.
Unproven bacterial infection: the presence of fever (≥38°C) and leucocytosis (white blood cell count ≥12 000 mm3) requiring antibiotic therapy without any identifiable source.
2.3. Criteria for diagnosing de novo infection
Infections were qualified as de novo when they were detected between day 2 and day 28 after admission in patients that had no infection prior to admission. In the patients with infection at study enrolment, de novo infection was diagnosed when the patient developed a new infection, at least 48 hours after they were free of clinical and microbiological evidence of infection. Infection with multi‐drug resistant organisms (MDR) was defined as acquired non‐susceptibility to at least one agent in 3 or more antimicrobial categories. 12 , 13
2.4. Data collection
Baseline demographic, clinical and biochemical data were recorded prospectively at the time of enrolment. Prognostic scores (CPT, MELD, CLIF‐SOFA and CLIF‐C ACLF) were subsequently calculated. HE grades were recorded at admission using the West Haven criteria. Infection data were collected from the results of protocol screening tests done to detect possible sepsis, including blood, urine, stool and ascitic fluid cultures. Follow‐up was for 28 days from inclusion or until death or liver transplantation, if before.
2.5. Statistical analysis
Patients were subdivided into four groups for analyses 1 : no OHE and no baseline infection (n = 352), 2 OHE with no baseline infection (n = 221), 3 no OHE with baseline infection (n = 100), and 4 OHE with baseline infection (n = 86).
Continuous variables were expressed as median (interquartile range). Categorical data were presented as proportions. Comparison of demographics and clinical features in the four groups mentioned above was performed using chi‐squared or Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. Predictors of infection and mortality at 28‐days were assessed by logistic regression model. All variables significant with P < 0.10 on univariate analysis, were entered in the multivariable model for proper adjustment. Multiple models were generated using individual laboratory parameters and OFs independently. The Kaplan–Meier method was used to generate survival curves. The data were analysed using SPSS statistics software (version 20.0) and Medcalc software (version 15.11.4, MedCalc Software).
3. RESULTS
A total of 759 cirrhotic patients with AD were divided into four groups. (1) No OHE and no baseline infection (n = 359), (2) OHE with no baseline infection (n = 222), (3) no OHE with baseline infection (n = 93), and (4) OHE with baseline infection (n = 85). Most patients (74%) were male, with a median age of 43.9, 45.6, 44.8 and 48.7 in the four groups respectively. The most common aetiology of cirrhosis was alcohol (55.1%), followed by hepatitis B (11.7%). Four hundred and fifty‐two patients (59.6%) had no or mild HE (grade 0/1). Of the 307 patients with overt HE, 142 (46.2%) patients had grade 2 HE, whereas 165 (53.7%) had advanced HE (grade 3 or 4). ACLF was more common in those with OHE, with 85.9% of OHE patients having ACLF compared with 55.9% who were in the non‐OHE groups. The median MELD scores were 24.3, 26.4, 25.3 and 27.1 in the four groups respectively. Comparison of baseline demographic profile, clinical presentations and other characteristics in four groups are outlined in Table 1.
TABLE 1.
Patient characteristics stratified by different grades of HE and baseline infection
| Baseline characteristic | No baseline OHE, no baseline infection (n = 352) Group 1 | OHE, no baseline infection (n = 221) Group 2 | No OHE, baseline infection (n = 100) Group 3 | OHE, baseline infection (n = 86) Group 4 | P** |
|---|---|---|---|---|---|
| Predisposition | |||||
| Age (years) | 43.9 ± 13.6 | 45.6 ± 12.4 | 44.8 ± 11.7 | 48.7 ± 13.3 | 0.007* |
| Males:Females | 260 (73.9%): 92 (26.1%) | 162 (73.3%): 59 (26.7%) | 75 (75.0%): 25 (25.0%) | 69 (80.2%): 17 (19.8%) | 0.627 |
| Aetiology (CLD) | |||||
| HBV | 48 (13.6%) | 29 (13.1%) | 6 (6.0%) | 6 (7.0%) | 0.001 |
| Alcohol | 171 (48.6%) | 118 (63.4%) | 68 (68.0%) | 61 (59.3%) | |
| AIH | 40 (11.4%) | 14 (6.3%) | 3 (3.0%) | 6 (7.0%) | |
| Other | 78 (22.2%) | 46 (20.8%) | 20 (20.0%) | 13 (15.1) | |
| HCV | 11 (3.1%) | 4 (1.8%) | 1 (1.0%) | 3 (3.5%) | |
| Viral + alcohol | 4 (1.1%) | 10 (4.5%) | 2 (2.0%) | 7 (8.1%) | |
| Organ failures | |||||
| Liver | 142 (40.3%) | 69 (31.2%) | 30 (30.0%) | 26 (30.2%) | 0.052 |
| Kidney | 72 (20.5%) | 83 (37.6%) | 41 (41.0%) | 41 (47.7%) | <0.001 |
| Brain | 0 | 119 (53.8%) | 0 | 50 (58.1%) | <0.001 |
| Coagulation | 97 (27.6%) | 97 (43.9%) | 35 (35.0%) | 36 (41.9%) | <0.001 |
| Circulation | 65 (18.5%) | 54 (24.4%) | 25 (25.0%) | 30 (34.9%) | 0.010 |
| Respiratory | 85 (24.1%) | 119 (53.8%) | 35 (35.0%) | 54 (62.8%) | <0.001 |
| Laboratory values | |||||
| Haemoglobin | 9.6 ± 2.4 | 9.4 ± 2.6 | 9.2 ± 2.3 | 9.2 ± 2.3 | 0.356 |
| TLC (×109) | 10.3 ± 6.5 | 10.4 ± 5.9 | 14.1 ± 8.6 | 16.0 ± 11.9 | <0.001(1 & 3,1 & 4, 2 & 3, 2 & 4) |
| Platelets (×109) | 98 (62–150) | 91 (62–134) | 83 (58–137) | 90 (62–145) | 0.522 |
| Bilirubin (mg/dl) | 12.9 ± 11.6 | 10.7 ± 9.7 | 10.5 ± 9.7 | 10.8 ± 9.3 | 0.188 |
| INR | 2.2 ± 1.0 | 2.7 ± 1.4 | 2.3 ± 0.9 | 2.6 ± 1.3 | <0.001 (1 & 2, 1 & 4) |
| Albumin (g/dl) | 2.7 ± 0.7 | 2.6 ± 0.6 | 2.4 ± 0.6 | 2.4 ± 0.7 | 0.003 (1 & 3) |
| Creatinine (mg/dl) | 0.9 (0.6–1.6) | 1.3 (0.7–2.2) | 1.4 (0.8–2.5) | 1.7 (0.9–2.7) | <0.001 (1 & 3, 1 & 4, 2 & 4) |
| Mean arterial pressure (mm Hg) | 82 ± 13 | 83 ± 35 | 81 ± 12 | 81 ± 14 | 0.801 |
| Scores | |||||
| ACLF grades | |||||
| No ACLF | 174 (49.4%) | 38 (17.2%) | 25 (25.0%) | 5 (5.8%) | <0.001 |
| ACLF 1 | 45 (12.8%) | 21 (9.5%) | 24 (24.0%) | 9 (10.5%) | |
| ACLF 2 | 88 (25.0%) | 66 (29.9%) | 28 (28.0%) | 24 (27.9%) | |
| ACLF 3 | 45 (12.8%) | 96 (43.4%) | 23 (23.0%) | 48 (55.8%) | |
| MELD | 24.3 ± 8.6 | 26.4 ± 9.1 | 25.3 ± 9.3 | 27.1 ± 9.9 | 0.014 (1 &2) |
| CLIF‐C ACLF (those with ACLF) | 46.6 ± 8.6 | 51.7 ± 8.5 | 47.7 ± 8.3 | 53.4 ± 10.1 | <0.001 (1 & 2, 1 & 4, 2 & 3, 3 & 4) |
| De novo infection | 67 (19.0%) | 63 (28.5%) | 14 (14.0%) | 18 (20.9%) | 0.011 |
| 28‐day mortality (%) | 89 (25.3%) | 133 (60.2%) | 55 (55.0%) | 62 (72.1%) | <0.001 |
Note: All data are expressed as n (%) or median (interquartile range) unless otherwise specified.
Abbreviations: ACLF, acute on chronic liver failure; AIH, autoimmune hepatitis; CLD, chronic liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalised ratio; MELD, The Model for End‐Stage Liver Disease; TLC, total leukocyte count.
**P‐values describe comparison between respective groups.
The median age was higher, with more female patients among the RFH cohort (32.7% vs 20.9%). The commonest aetiology of cirrhosis was alcohol in both cohorts, but HBV‐related cirrhosis was higher among AIIMS group (15.9% vs 5.1%), and HCV‐related cirrhosis was more frequent among the RFH group (6.1% vs 0.2%). The differences in clinical characteristics of the two cohorts are outlined in Table 2.
TABLE 2.
Comparison of AIIMS and RFH cohort
| Baseline characteristic | AIIMS (n = 465) | UCL (n = 294) | P |
|---|---|---|---|
| Predisposition | |||
| Age (years) | 40.9 ± 12.3 | 51.5 ± 11.4 | <0.001 |
| Males: Females | 368 (79.1%): 97(20.9%) | 198 (67.3%): 96(32.7%) | <0.001 |
| Aetiology (CLD) | |||
| HBV | 74 (15.9%) | 15 (5.1%) | <0.001 |
| Alcohol | 235 (50.5%) | 173 (58.8%) | |
| AIH | 41 (8.8%) | 22 (7.5%) | |
| Other | 110 (23.7%) | 47 (16.0%) | |
| HCV | 1 (0.2%) | 18 (6.1%) | |
| Viral + alcohol | 4 (0.9%) | 19 (6.5%) | |
| Organ failures | |||
| Liver | 222 (47.7%) | 45 (15.3%) | <0.001 |
| Kidney | 150 (32.3%) | 87 (29.6%) | 0.470 |
| Brain | 123 (26.5%) | 46 (15.6%) | <0.001 |
| Coagulation | 197 (42.4%) | 68 (23.1%) | <0.001 |
| Circulation | 58 (12.5%) | 116 (39.5%) | <0.001 |
| Respiratory | 108 (23.2%) | 185 (62.9%) | <0.001 |
| Laboratory values | |||
| Haemoglobin | 9.2 ± 2.5 | 9.8 ± 2.2 | 0.003 |
| TLC (×109) | 11.5 ± 7.8 | 11.3 ± 7.6 | 0.645 |
| Platelets (×109) | 100 (65–153) | 86 (58–127) | 0.006 |
| Bilirubin (mg/dl) | 14.2 ± 10.3 | 7.8 ± 9.9 | <0.001 |
| INR | 2.6 ± 1.2 | 2.1 ± 1.1 | <0.001 |
| Albumin (g/dl) | 2.6 ± 0.7 | 2.5 ± 0.7 | 0.013 |
| Creatinine (mg/dl) | 1.2 (0.8–2.4) | 0.9 (0.7–1.6) | 0.001 |
| Mean arterial pressure (mm Hg) | 83 (73–88) | 80 (70–90) | 0.225 |
| Scores | |||
| ACLF grades | |||
| No ACLF | 186 (40.0%) | 56 (19.0%) | <0.001 |
| ACLF 1 | 37 (8.0%) | 62 (21.1%) | |
| ACLF 2 | 110 (23.7%) | 96 (32.7%) | |
| ACLF 3 | 132 (28.4%) | 80 (27.2%) | |
| MELD | 28.7 ± 7.7 | 20.2 ± 8.6 | <0.001 |
| CLIF‐C ACLF (those with ACLF) | 49.8 ± 9.8 | 49.5 ± 8.4 | 0.749 |
| ITU admission | 71 (15.3%) | 294 (100%) | <0.001 |
| De novo infection | 74 (15.9%) | 88 (29.9%) | <0.001 |
| 28‐day mortality (%) | 209 (44.9) | 130 (44.2) | 0.881 |
Note: All data are expressed as n (%) or median (interquartile range) unless otherwise specified.
Abbreviations: CLD, chronic liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; AIH, autoimmune hepatitis; TLC, total leukocyte count; INR, international normalised ratio; ACLF, acute on chronic liver failure; MELD, The Model for End‐Stage Liver Disease.
3.1. Factors associated with infection
At baseline, 186 patients had infection; 177 were culture‐positive and 9 culture‐negative. A total of 215 organisms were isolated. Escherichia coli was the most common organism isolated in the whole cohort. Infection with Gram‐negative E. coli was the most common isolated organism in the AIIMS cohort, whereas Gram‐positive bacteria (Staphylococcus followed by MRSA) were the most commonly isolated organisms in the RFH cohort (Table S1). The commonest sites of infection were urine followed by blood in AIIMS group, and blood followed by respiratory in RFH group (Table S2). 37.6% of culture‐positive baseline infections (out of 133, which the MDR data are available) were caused by MDR organisms (Table 3).
TABLE 3.
Comparison of AIIMS and RFH cohorts according to baseline infection with/without MDR and survival
| N = 177 | AIIMS | RFH | Alive | Died | |
|---|---|---|---|---|---|
| MDR+ | 50 | 40 (40.8%) | 10 (12.7%) | 17 (34.0%) | 33 (66.0%) |
| MDR− | 127 | 58 (59.2%) | 69 (87.3%) | 48 (37.8%) | 79 (62.2%) |
| Total | 98 (100%) | 79 (100%) | 65 (100%) | 112 (100%) |
Note: P = 0.730.
Multi‐drug resistant organisms (MDR) are defined as acquired non‐susceptibility to at least one agent in 3 or more antimicrobial categories.
Of the 186 patients with infection‐ 177 are culture‐positive and 9 culture negative.
One hundred and sixty‐two patients developed de novo infections (n = 162). One hundred and forty‐three (n = 143) were culture‐positive (total of 171 organisms isolated) and 19 were culture‐negative, with most de novo infections occurring in the first and second weeks of admission (58.6%, and 25.9% respectively). The highest de novo infection rates were observed in those with OHE (no baseline infection: 28.5%, with baseline infection: 20.9%). Of the 162 de novo infections, a higher proportion of infection occurred among patients admitted to ITU (n = 108/365, accounting for 29.6%) than those in the ward (n = 54/394, accounting for 13.7%), with P < 0.001.
Overall, E. coli and Klebsiella pneumoniae were the most isolated organisms (Table S3). The commonest sites of infection were urine followed by blood in the AIIMS cohort, and blood followed by respiratory in the RFH patients (Table S4). 30.8% of de novo infections were caused by MDR organisms, with 52.3% mortality compared with 45.5% if no MDR was isolated (P = 0.47) (Table 4).
TABLE 4.
Comparison of AIIMS and RFH according to de novo infection with/without MDR, and survival
| N = 143 | AIIMS | RFH | Alive | Died | |
|---|---|---|---|---|---|
| MDR + | 44 (30.8%) | 26 (44.1%) | 18 (21.4%) | 21 (47.7%) | 23 (52.3%) |
| MDR − | 99 (69.2%) | 33 (55.9%) | 66 (78.6%) | 54(54.5%) | 45 (45.5%) |
| Total | 59 (100%) | 84 (100%) | 75 (100%) | 68 (100%) |
Note: P = 0.473.
Multi‐drug resistant organisms (MDR)are defined as acquired non‐susceptibility to at least one agent in three or more antimicrobial categories.
On univariate analysis, OHE, age, admission to ITU, respiratory and circulatory failures were predictive of the development of de novo infections, with odds ratio (OR) 1.642, 1.020, 2.646, 1.499 and 1.512 respectively. On multivariate analysis, OHE (OR, 1.532 [1.061–2.300], 95% CI, P = 0.024), and admission to ITU (OR, 2.303 [1.508–3.517], 95% CI, P < 0.001) independently predicted de novo infections (Table 5).
TABLE 5.
Univariate and multivariate analysis of predictors of de novo infections
| OR | P | Multivariate −1 | P | Multivariate model‐2 | P | |
|---|---|---|---|---|---|---|
| Age (years) | 1.020 (1.007–1.034) | 0.003 | 1.009 (0.994–1.024) | 0.250 | 1.009 (0.994–1.024) | 0.258 |
| Sex (Female) | 0.992 (0.666–1.479) | 0.969 | ||||
| TLC (×109) | 0.988 (0.964–1.012) | 0.320 | ||||
| INR | 0.853 (0.719–1.011) | 0.067 | 0876(0.3737–1.041) | 0.876 | 0.876 (0.738–1.040) | 0.130 |
| Creatinine (mg/dl) | 1.037 (0.957–1.124) | 0.376 | ||||
| Total bilirubin (mg/dl) | 0.998 (0.982–1.015) | 0.830 | ||||
| Albumin (g/dl) | 0.928 (0.717–1.201) | 0.570 | ||||
| Mean arterial pressure (mm Hg) | 1.006 (0.998–1.013) | 0.134 | ||||
| Overt‐HE | 1.642 (1.157–2.329) | 0.005 | 1.532 (1.061–2.300) | 0.024 | ||
| Organ failures | ||||||
| HE | 1.467 (0.987–2.181) | 0.058 | ||||
| Liver | 0.870 (0.602–1.258) | 0.460 | ||||
| Kidney | 1.302 (0.903–1.878) | 0.157 | ||||
| Coagulation | 1.203 (0.840–1.723) | 0.313 | ||||
| Circulatory | 1.512 (1.022–2.238) | 0.039 | 1.175 (0.757–1.824) | 0.472 | 1.213 (0.777–1.894) | 0.395 |
| Respiratory | 1.499 (1.055–2.129) | 0.024 | 0.830 (0.545–1.264) | 0.385 | 0.837 (0.547–1.281) | 0.412 |
| Baseline HE and infection groups | ||||||
| No baseline HE no infection | 1 | 1 | ||||
| Overt HE no baseline infection | 1.696 (1.143–2.518) | 0.009 | 1.547 (1.001–2.391) | 0.049 | ||
| No baseline HE baseline infection yes | 0.692 (0.371–1.293) | 0.249 | 0.524 (0.267–1.030) | 0.061 | ||
| Overt HE baseline infection yes | 1.126 (0.628–2.019) | 0.690 | 0.954 (0.509–1.787) | 0.883 | ||
| ITU admission | 2.646 (1.837–3.810) | <0.001 | 2.303 (1.508–3.517) | <0.001 | 2.403 (1.566–3.688) | <0.001 |
Abbreviations: HE, hepatic encephalopathy; INR, international normalised ratio; TLC, total leucocyte count.
3.2. Factors associated with survival
The 28‐day mortality in our cohort was 44.7%, with the highest mortality rates among the OHE groups (72.1% and 60.2% in those with and without infection at baseline respectively). The Kaplan–Meier survival curves of the four groups are shown in Figure 1 (P < 0.001).
FIGURE 1.
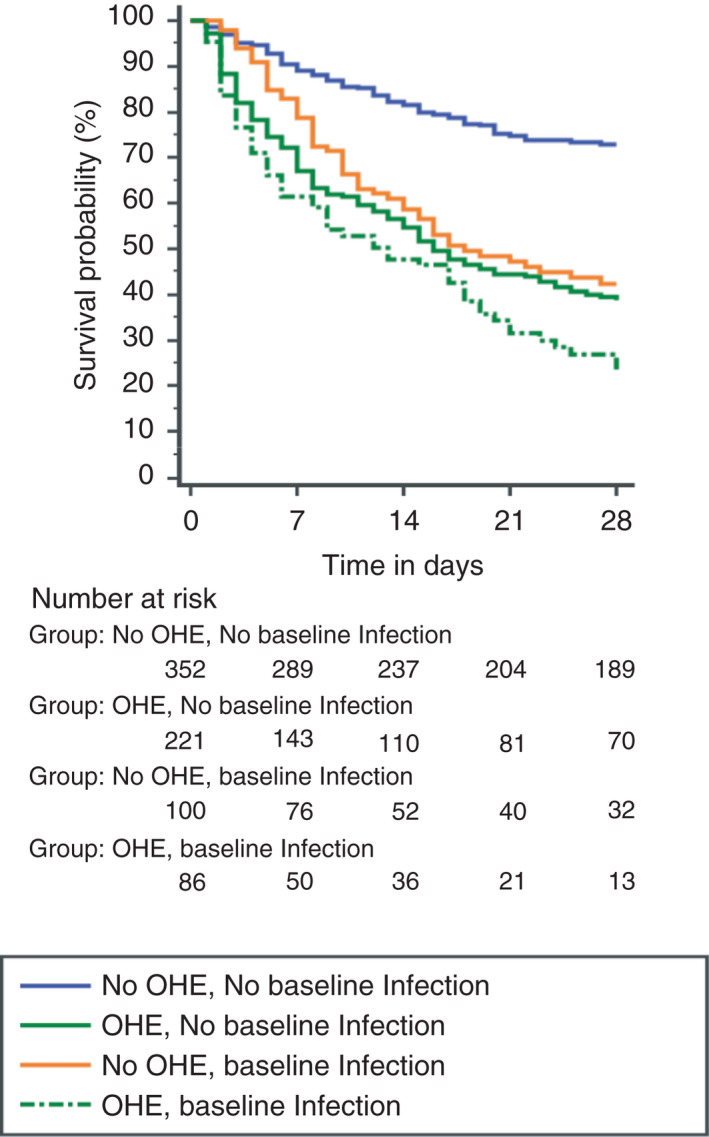
Kaplan–Meier graph of 28‐day survival in the four groups. Probability of survival at day 28 in patients, based on infection/OHE at baseline. The highest mortality rates are amongst the OHE groups (72.1% and 60.2% in those with and without infection at baseline, respectively). (p < 0.001)
On univariate analysis, OHE, age, total leukocyte count (TLC), INR, creatinine, albumin and mean arterial pressure were predictive of higher mortality. Different multivariate models were used to analyse which factors remained independently associated with 28‐day mortality. Model one included age, sex, TLC, creatinine, INR, total bilirubin, albumin, MAP and the OHE and infection groups. Presence of OHE, no infection (OR, 3.711; P < 0.001), OHE and infection (OR, 7.634; P < 0.001), no baseline HE and infection (OR, 3.612; P < 0.001), age (OR, 1.022; P = 0.003), INR (OR, 1.579; P < 0.001), and creatinine (OR, 1.160; P = 0.013) remained independently predictive of 28‐day mortality. On the other hand, in model two including organ failures (OF), presence of OHE, no infection (OR, 3.312; P < 0.001), OHE, infection (OR, 4.586; P < 0.001), no baseline HE and infection (OR, 3.018; P < 0.001), coagulation (OR, 2.781; P < 0.001), kidney (OR, 2.442; P < 0.001), respiratory (OR, 2.057; P < 0.001), and liver failure (OR, 1.814; P = 0.004) remained significantly associated with higher mortality (Table 6).
TABLE 6.
Univariate and multivariate analysis of factors defining the risk of death at 28 days
| OR (95% CI) | P | Multivariate model 1 (OR) | Multivariate model 1 (P) | Multivariate model 2 (OFs) (OR) | Multivariate model 2 (P) | |
|---|---|---|---|---|---|---|
| Age (years) | 1.013 (1.002–1.025) | 0.019 | 1.022 (1.008–1.036) | 0.003 | 1.014 (1.000–1.029) | 0.052 |
| Sex (Female) | 0.727 (0.521–1.015) | 0.061 | 0.850 (0.575–1.256) | 0.414 | 0.799 (0.539–1.185) | 0.265 |
| TLC (×109) | 1.043 (1.022–1.064) | <0.001 | 1.012 (0.989–1.037) | 0.307 | 1.006 (0.983–1.030) | 0.623 |
| INR | 1.742 (1.493–2.033 | <0.001 | 1.579 (1.320–1.889) | <0.001 | ||
| Creatinine (mg/dl) | 1.391 (1.241–1.559) | <0.001 | 1.160 (1.032–1.304) | 0.013 | ||
| Total bilirubin (mg/dl) | 1.013 (1.000–1.027) | 0.058 | 1.013 (0.995–1.031) | 0.151 | ||
| Albumin (g/dl) | 0.745 (0.600–0.926) | 0.008 | 0.862 (0.667–1.114) | 0.257 | 0.878 (0.679–1.135) | 0.320 |
| Mean arterial pressure (mm Hg) | 0.988 (0.978–0.998) | 0.022 | 0.995 (0.986–1.003) | 0.240 | ||
| Overt‐HE | 3.724 (2.744–5.053) | <0.001 | ||||
| Organ failures | ||||||
| HE | 4.462 (3.064–6.497) | <0.001 | ||||
| Liver | 1.378 (1.021–1.859) | 0.036 | 1.814 (1.215–2.708) | 0.004 | ||
| Kidney | 3.982 (2.873–5.519) | <0.001 | 2.442 (1.669–3.572) | <0.001 | ||
| Coagulation | 3.355 (2.456–4.583) | <0.001 | 2.781 (1.930–4.007) | <0.001 | ||
| Circulatory | 2.213 (1.567–3.125) | <0.001 | 1.534 (1.007–2.337) | 0.046 | ||
| Respiratory | 2.605 (1.930–3.518) | <0.001 | 2.057 (1.403–3.017) | <0.001 | ||
| Baseline HE and infection groups | ||||||
| No baseline HE no infection | 1 | 1 | 1 | |||
| Overt HE no baseline infection | 4.466 (3.113–6.408) | <0.001 | 3.711 (2.488–5.536) | <0.001 | 3.312 (2.193–5.002) | <0.001 |
| No baseline HE baseline infection | 3.612 (2.277–5.730) | <0.001 | 3.181 (1.918–5.275) | <0.001 | 3.018 (1.802–5.053) | <0.001 |
| Overt HE baseline infection | 7.634 (4.498–12.956) | <0.001 | 5.388 (3.021–9.611) | <0.001 | 4.586 (2.540–8.277) | <0.001 |
Abbreviations: HE, hepatic encephalopathy; INR, international normalised ratio; TLC, total leucocyte count.
4. DISCUSSION
The most important observation of this study among cirrhotic patients with AD and ACLF was that OHE was an independent risk factor for the development of de novo infections. In patients with cirrhosis and AD, particularly in those with ACLF, the mortality of patients with OHE significantly exceeds that observed in those without. 4 The mechanisms underlying this increased risk of death are not explained by the severity of the underlying liver disease. However, the observed association between OHE and de novo infection may be an operative mechanism for the higher 28‐day mortality. Other data in the literature support this observation. 33 , 34 , 35 In patients with non‐paracetamol induced acute liver failure, similar observations were made by Vaquero et al. 33 The authors investigated the link between infection and HE in acute liver failure patients and showed that although the occurrence of infection preceded the development of HE in patients with paracetamol‐induced ALF, the reverse was observed in patients with non‐paracetamol induced acute liver failure. In a sub‐analysis of the CANONIC study in patients with AD of cirrhosis, a similar association between HE and the occurrence of de novo infection was observed. 34 It was intriguing to note that this association is also observed in patients with milder forms of HE. In a prospective study, Thomsen et al. followed a group of patients with Grade 1 HE and those with no or mHE and showed that the patients with Grade 1 HE had more marked evidence of systemic inflammation, higher spontaneous neutrophil respiratory burst, bacterial translocation and subsequent infection, with infection being the most common complication necessitating hospital admission in those patients. 35 Taken together, the data provide evidence that the occurrence of HE predisposes to the risk of infection.
Similar findings of a link between acute CNS disorders such as traumatic brain injury (TBI), stroke and spinal cord injury (SCI), and high susceptibility to infection were observed and attributed to what was described as CNS injury‐induced immunodepression (CIDS). In animal models, middle cerebral artery occlusion resulted in a state of immune dysfunction with impaired ability to clear the iatrogenically inoculated infection into the lungs. 36 The underlying mechanisms that are thought to be involved include the effect of alterations in the sympathetic nervous system (SNS), the hypothalamic–pituitary–adrenal (HPA) axis and the parasympathetic nervous system that are known to regulate immune function. 23 , 24 Pneumonia is the most encountered serious complication in patients with stroke, occurring in about 22% of these patients. 37 This is the most commonest cause of death and increases stroke‐associated acute and long‐term mortality by 2.5‐fold. 38 , 39 , 40 , 41 This risk of infection and mortality has also been observed in patients with silent infarcts. 42 , 43 In contrast, our data in cirrhosis patients show that UTI and septicaemia account for most of the infections.
MDR infections are a growing healthcare problem, particularly in the setting of decompensated cirrhosis and ACLF, and carry a poor prognosis. 12 In this study, 28.2% of culture‐positive baseline infections were caused by MDR organisms (Table 3), while 36% of culture‐positive de novo infections were attributed to MDR organisms. (Table 4). Most MDR de novo infections occurred in the first 2 weeks of admission (61.4%, and 27.3% in the first and second weeks respectively).
Univariate analysis showed that OHE, age, admission to ITU, respiratory and circulatory failures were predictors of de novo infections. OHE remained as an independent predictor of de novo infection on multivariate analysis, (OR, 1.532; P = 0.024), emphasising the strong association between OHE and the increased risk of infection. We also found that ITU admission was associated with a higher risk of de novo infection. The higher rate of mechanical ventilation, instrumentation and the fact that patients admitted to ITU have more organ dysfunction may account for that association. Other factors have been linked with increased risk of infections with decompensated cirrhosis in different studies. 10 , 12 , 34 , 44 Fernandez et al. showed that ITU admission, recent hospitalisation and nosocomial origin of infection were independent risk factors for MDR infection. 12 B‐lactam use within the previous 3 months, long‐term norfloxacin prophylaxis and MDR infection in the last 6 months were found to increase the risk of MDR infection 10 and CLIF‐C ACLF score at diagnosis is an independent risk factor of bacterial infections. 34 Recent study by Martinez et al. in patients with acute variceal bleeding, showed that Child‐Pugh B and C, and Grade III/IV hepatic encephalopathy were independently associated with bacterial infection. 44
The mechanisms underlying the interaction between the immune and nervous systems are not entirely clear but are possibly mediated by the neural pathways that are known to regulate the immune system. 45 , 46 The central nervous system can affect the immune function through the HPA directly through the innervation of the immune organs/cells. Nerve fibres of the SNS innervate the mucosa and gut‐associated lymphoid tissue, 45 and cytokines released by immune cells can influence the nervous system. 45 , 47 SNS is known to be activated in advanced cirrhosis 48 , 49 , 50 exerting strong immunosuppressive actions. Worlicek et al. showed that splanchnic sympathectomy prevents spontaneous bacterial translocation from the gut to mesenteric lymph nodes and decreases the incidence and severity of the systemic spread of E. coli after its intraperitoneal application in ascitic cirrhotic rats. 51 The CNS/immune interaction in patients with stroke results in a state known as CIDS predisposing to infection through different mechanisms, including impaired natural killer (NK) and T‐ cell activity, reduced peripheral blood lymphocytes with reduced proliferation and cytokine production. 36 It was also found that pro‐inflammatory cytokines produced by damaged brain tissue can directly activate HPA and increase the risk of developing an infection. 36 , 52 It is of note that similar changes are seen in patients with HE 53 and might play a role in the interaction between HE and infection.
Additionally, ammonia, a key molecule known to be clinically and pathophysiologically involved in the pathogenesis of hepatic encephalopathy is elevated in patients with HE. 22 , 23 Besides being toxic to astrocytes, ammonia impairs neutrophil function which is mediated by activation of the p38 mitogen‐activated protein kinase (p38 MAPK) pathway, with excess reactive oxygen species release, systemic inflammation, oxidative stress, high spontaneous oxidative burst (OB), and decreased phagocytosis, which is associated with a significantly greater risk of infection, organ failure and mortality. 23 , 24 Although we did not measure ammonia levels in this study, the existing literature suggests that a diagnosis of HE is incompatible with normal ammonia levels. 54
OHE was independently associated with death. The risk of mortality was higher when OHE and infection both were present together than either alone. In addition, the factors independently associated with 28‐day mortality were age, INR and creatinine. Of organ failures; liver, kidney, coagulation, circulation and respiratory independently predicted mortality. These data are in keeping with the observations made in the CANONIC study, where cerebral failure did not independently define the occurrence of ACLF and required dysfunction of the kidneys. 6
The results of this study should be interpreted considering the following limitations. First, merging the data from two separate institutions can be difficult due to demographic differences and the prevalence of infection. However, the strength of our approach was the prospective collection of the data in a relatively large number of patients and a degree of internal validation of the observations. Second, we may have underestimated the presence of infection as isolated shadowing on the chest X‐ray was excluded from the diagnosis of infection. The data presented here assessed the impact of HE on confirmed bacterial infections. Third, given the retrospective nature of the study, the classification of the severity of HE may be inaccurate. However, the data for this study were collected prospectively and as we have analysed the groups according to the presence or the absence of OHE, misclassification is less likely as the clinical diagnosis is usually clear. Despite these potential limitations, we believe that the data are robust as most of the data were complete.
In conclusion, the results of our study show a significant relationship between OHE and the risk of de novo infection in the setting of cirrhosis with AD. Therefore, patients with OHE should be considered at high risk of a new infection suggesting the need for regular surveillance with a low threshold to start antibiotics early. Further studies should address the role of prophylactic antibiotics in HE patients and assess the underlying mechanisms of this risk.
AUTHORSHIP
Guarantor of the article: Prof Rajiv Jalan and Dr Shalimar
Author contributions: EA, S and RJ participated in the study design. EA, MFS, BA, S and SA participated in data collection. EA, S, SA, MP and RJ participated in data analysis and interpretation. EA, S and RJ participated in the writing up. All the authors approve the publication of this article and this version of the manuscript in Alimenary Pharmacology and Therapeutics.
Supporting information
Table S1–S4
ACKNOWLEDGEMENT
Declaration of personal interests: Eman Alabsawy, Shalimar, Mohammed Faisal Sheikh, Subrat Kumar Acharya, Banwari Agarwal: none to declare. Rajiv Jalan: Rajiv Jalan has research collaborations with Yaqrit and Takeda. Rajiv Jalan is the inventor of OPA which has been patented by University College London and licensed to Mallinckrodt Pharma. He is also a founder of Yaqrit limited, a spin‐out company from University College London. He has also co‐founded Hepyx Ltd. and Cyberliver Ltd.
FUNDING
No funding has been received from any source for this work.
Alabsawy EP, Shalimar, Sheikh MF, et al. Overt hepatic encephalopathy is an independent risk factor for de novo infection in cirrhotic patients with acute decompensation. Aliment Pharmacol Ther.;55:722–732. doi: 10.1111/apt.16790
Eman Alabsawy and Shalimar joint 1st authors.
Subrat Kumar Acharya, Banwari Agarwal and Rajiv Jalan are senior authors.
The Handling Editor for this article was Professor Dr Stephen Ryder, and it was accepted for publication after full peer‐review.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Blei AT, Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K. Hepatic encephalopathy – definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th world congresses of gastroenterology, Vienna, 1998. Hepatology. 2002;35:716‐721. [DOI] [PubMed] [Google Scholar]
- 2. Nabi E, Thacker LR, Wade JB, et al. Diagnosis of covert hepatic encephalopathy without specialized tests. Clin Gastroenterol Hepatol. 2014;12:1384‐1389.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Orr JG, Morgan CL, Jenkins‐Jones S, et al. P478 resource use associated with hepatic encephalopathy in patients with liver disease. J Hepatol. 2014;60:S228‐S229. [Google Scholar]
- 4. Romero‐Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute‐on‐chronic liver failure. J Hepatol. 2015;62:437‐447. [DOI] [PubMed] [Google Scholar]
- 5. Jalan R, Stadlbauer V, Sen S, Cheshire L, Chang YM, Mookerjee RP. Role of predisposition, injury, response and organ failure in the prognosis of patients with acute‐on‐chronic liver failure: a prospective cohort study. Crit Care. 2012;16:R227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moreau R, Jalan R, Gines P, et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426‐1437. [DOI] [PubMed] [Google Scholar]
- 7. Association A, Diseases L, Association E . Clinical Practice guidelines hepatic encephalopathy in chronic liver disease : 2014 Practice Guideline by the European Association for the Study of the liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61:642‐659. [DOI] [PubMed] [Google Scholar]
- 8. Vilstrup H, Amodio P, Bajaj J, et al. AASLD PRACTICE GUIDELINE hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. 2014;2014:715‐735. [DOI] [PubMed] [Google Scholar]
- 9. Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care‐associated bacterial infections. YJCGH. 2010;8:979‐985. [DOI] [PubMed] [Google Scholar]
- 10. Fernández J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551‐1561. [DOI] [PubMed] [Google Scholar]
- 11. Fern J. Microbiology and management of pediatric liver abscesses: two cases caused by Streptococcus anginosus Group. Manage Liver Dis. 2012;2012:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández J, Prado V, Trebicka J, et al. Multidrug‐resistant bacterial infections in patients with decompensated cirrhosis and with acute‐on‐chronic liver failure in Europe. J Hepatol. 2019;70:398‐411. [DOI] [PubMed] [Google Scholar]
- 13. Shalimar RG, Jadaun SS, Ranjan G, Kedia S, Gunjan D, et al. Prevalence, predictors and impact of bacterial infection in acute on chronic liver failure patients. Dig Liver Dis. 2018;50:1225‐1231. [DOI] [PubMed] [Google Scholar]
- 14. Dionigi E, Garcovich M, Borzio M, et al. Bacterial infections change natural history of cirrhosis irrespective of liver disease severity. Am J Gastroenterol. 2017;112:588‐596. [DOI] [PubMed] [Google Scholar]
- 15. Fernández J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute‐on‐chronic liver failure: prevalence, characteristics, and impact on prognosis. Gut. 2018;67:1870‐1880. [DOI] [PubMed] [Google Scholar]
- 16. Piano S, Singh V, Caraceni P, et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology. 2019;156:1368‐1380.e10. [DOI] [PubMed] [Google Scholar]
- 17. Yuan LT, Chuah SK, Yang SC, et al. Multiple bacterial infections increase the risk of hepatic encephalopathy in patients with cirrhosis. PLoS ONE. 2018;13:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piotrowski D, Boroń‐Kaczmarska A. Bacterial infections and hepatic encephalopathy in liver cirrhosis–prophylaxis and treatment. Adv Med Sci. 2017;62:345‐356. [DOI] [PubMed] [Google Scholar]
- 19. Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cell Mol Life Sci. 2004;61:2322‐2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575‐581. [DOI] [PubMed] [Google Scholar]
- 21. Cordoba J, Ventura‐Cots M, Simón‐Talero M, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute‐on‐chronic liver failure (ACLF). J Hepatol. 2014;60:275‐281. [DOI] [PubMed] [Google Scholar]
- 22. Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51:1062‐1069. [DOI] [PubMed] [Google Scholar]
- 23. Shawcross DL, Wright GAK, Stadlbauer V, et al. Ammonia impairs neutrophil phagocytic function in liver disease. Hepatology. 2008;48:1202‐1212. [DOI] [PubMed] [Google Scholar]
- 24. Mookerjee RP, Stadlbauer V, Lidder S, et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831‐840. [DOI] [PubMed] [Google Scholar]
- 25. Jalan R, Ph D, Perricone G. Acute‐on‐chronic liver failure : a new disease or an old one hiding in plain sight? Clin Liver Dis (Hoboken). 2020;2:S45‐S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shalimar SMF, Mookerjee RP, Agarwal B, Acharya SK, Jalan R. Prognostic role of ammonia in patients with cirrhosis. Hepatology. 2019;70:982‐994. [DOI] [PubMed] [Google Scholar]
- 27. Engelmann C, Thomsen KL, Zakeri N, et al. Validation of CLIF‐C ACLF score to define a threshold for futility of intensive care support for patients with acute‐on‐chronic liver failure. Crit Care. 2018;22:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weiss E, de la Grange P, Defaye M, et al. Characterization of blood immune cells in patients with decompensated cirrhosis including ACLF. Front Immunol. 2021;5:619039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001;33:464‐470. [DOI] [PubMed] [Google Scholar]
- 30. Jalan R, Pavesi M, Saliba F, et al. Development and validation of the CLIF‐C AD score for the prognosis of patients with acute decompensation (AD) of cirrhosis not fulfilling diagnostic criteria for acute‐on‐chronic liver failure (ACLF). Hepatology. 2014;61:1038‐1047. [Google Scholar]
- 31. Parsons‐Smith BG, Summerskill WHJ, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet. 1957;270:867‐871. [DOI] [PubMed] [Google Scholar]
- 32. Maggi DC, Borgonovo A, Bansho ET, et al. Serial assessment of hepatic encephalopathy in patients hospitalised for acute decompensation of cirrhosis. Ann Hepatol. 2019;18:331‐337. [DOI] [PubMed] [Google Scholar]
- 33. Vaquero J, Polson J, Chung C, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125:755‐764. [DOI] [PubMed] [Google Scholar]
- 34. Fernández J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute‐on‐chronic liver failure: prevalence, characteristics and impact on prognosis. 2017;66:1‐11. [DOI] [PubMed] [Google Scholar]
- 35. Thomsen KL, Macnaughtan J, Tritto G, Mookerjee RP. Clinical and pathophysiological characteristics of cirrhotic patients with grade 1 and minimal hepatic encephalopathy. 2016;1–14. [DOI] [PMC free article] [PubMed]
- 36. Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury‐induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775‐786. [DOI] [PubMed] [Google Scholar]
- 37. Johnston KC, Li JY, Lyden PD, et al. Medical and neurological complications of ischemic stroke experience from the RANTTAS trial. RANTTAS Investigators. Stroke. 1998;29:447‐453. [DOI] [PubMed] [Google Scholar]
- 38. Vernino S, Brown RD, Sejvar JJ, Sicks JD, Petty GW, WMO F. Cause‐specific mortality after first cerebral infarction. 2003;34:1832. [DOI] [PubMed] [Google Scholar]
- 39. German T, Registers S, Group S . Predictors of in‐hospital mortality and attributable risks of death after ischemic stroke. 2020;164. [DOI] [PubMed] [Google Scholar]
- 40. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665‐671. [DOI] [PubMed] [Google Scholar]
- 41. Perry L, Love CP. Screening for dysphagia and aspiration in acute stroke: a systematic review. 2001;18:7‐18. [DOI] [PubMed] [Google Scholar]
- 42. Nakagawa T, Sekizawa K, Nakajoh K, Tanji H, Arai H, Sasaki H. Silent cerebral infarction: a potential risk for pneumonia in the elderly. 2000. Feb;247:255‐259. [DOI] [PubMed] [Google Scholar]
- 43. Nakagawa T, Sekizawa K, Arai H, Kikuchi R, Manabe K, Sasaki H. High incidence of pneumonia in elderly patients with basal ganglia infarction. Arch Intern Med 1997; 157: 321±4. [PubMed] [Google Scholar]
- 44. Martínez J, Hernández‐Gea V, Rodríguez‐de‐Santiago E, et al. International variceal bleeding observational study group and Baveno cooperation. Bacterial infections in patients with acute variceal bleeding in the era of antibiotic prophylaxis. J Hepatol. 2021;75:342‐350. [DOI] [PubMed] [Google Scholar]
- 45. Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98:477‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dunn AJ. Nervous system‐immune system interactions: an overview. J Recept Res. 1988;8:589‐607. [DOI] [PubMed] [Google Scholar]
- 47. Kenney MJ, Ganta CK. Autonomic nervous system and immune system interactions. Compr Physiol. 2014;4:1177‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stadlbauer V, Wright GA, Banaji M, et al. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology. 2008;134:111‐119. [DOI] [PubMed] [Google Scholar]
- 49. Estrela HF, Damásio ES, Fonseca EK, Bergamaschi CT, Campos RR. Differential sympathetic vasomotor activation induced by liver cirrhosis in rats. PLoS ONE. 2016;11:e0152512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abrahamovych M, Abrahamovych O, Fayura O, Fayura L, Tolopko S. The effect of oxidative stress on the AUTONOMIC nervous system in patients with liver cirrhosis. Georgian Med News. 2020;298:94‐99. [PubMed] [Google Scholar]
- 51. Worlicek M, Knebel K, Linde HJ, et al. Splanchnic sympathectomy prevents translocation and spreading of E. coli but not S aureus in liver cirrhosis. Gut. 2010;59:1127‐1134. [DOI] [PubMed] [Google Scholar]
- 52. Dirnagl U, Klehmet J, Braun JS, et al. Stroke‐induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770‐773. [DOI] [PubMed] [Google Scholar]
- 53. Genesca J, Gonzalez A, Segura R, et al. Interleukin‐6, nitric oxide, and the clinical and hemodynamic alterations of patients with liver cirrhosis. Am J Gastroenterol. 1999;94:169‐177. [DOI] [PubMed] [Google Scholar]
- 54. Rose CF, Amodio P, Bajaj JS, et al. Seminar hepatic encephalopathy : novel insights into classification, pathophysiology, and therapy. J Hepatol. 2020;73:1526‐1547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S4
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


