The terms ‘generalist’ and ‘specialist’ are commonly used to describe a species' ecology but their precise meaning is rarely considered. In particular, are specialists specialised in all aspects of their lives, or in only some or only one? In technical terms, is niche breadth correlated among niche dimensions? Recent research on three ecological guilds of fungi – arbuscular mycorrhizas, plant pathogens and saprotrophs – allows us to investigate the degree of co‐specialisation among niche dimensions. These studies suggest that fungi can specialise independently on climatic, edaphic and biotic conditions and resources, although evidence from saprotrophs suggests that specialisation on different climatic conditions is correlated. Quantifying niche shape has important implications for understanding how species will respond to global change.
The idea of the niche has been among the most important, yet controversial, in ecology for over a century (Chase & Leibold, 2003). The theory of evolution by natural selection considers a species' fitness in a particular environment, but it was Grinnell's (1917) paper on ‘The niche‐relationships of the California thrasher’ that explicitly defined the ecological niche as a set of environmental conditions occupied by a species. These conditions included dietary requirements, climatic tolerances and interactions with other species, such as predators. Some years later, Elton (1927) defined the niche from the perspective of a species' role in food webs and its impact on consumable resources such as prey populations. However, it was Hutchinson (1957) who introduced the revolutionary concept of the n‐dimensional hypervolume to define the niche and to allow, at least in theory, the niche to be empirically quantified. In Hutchinson's conceptual model, any number of limiting factors to species population growth, be they conditions such as temperature or resources such as prey, form dimensions of the hyperspace within which the species' fundamental niche sits (Blonder et al., 2014). The fundamental niche is defined as the niche in the absence of biotic interactions such as competition or facilitation (Carscadden et al., 2020). Ecological theories such as the Volterra–Gause competitive exclusion principle are conceptualised in Hutchinson's model, by stating that where the fundamental niche volumes of two species overlap, only the more competitive species will survive. The realised niche of a species is then any element of the fundamental niche that does not overlap with another, plus any parts of the intersection in which that species is the superior competitor (Hutchinson, 1957). Realised niches should therefore always be smaller than fundamental niches (Soberón & Arroyo‐Peña, 2017), except when positive interactions such as mutualism are considered (Carscadden et al., 2020). The concept of ecological specialisation, and comparison between specialists and generalists, can be visualised as variation in the extent of the niche along different axes or the volume of the n‐dimensional niche (Fig. 1). The magnitude of the niche on a particular axis is often termed the niche width or breadth (Sexton et al., 2017).
Fig. 1.
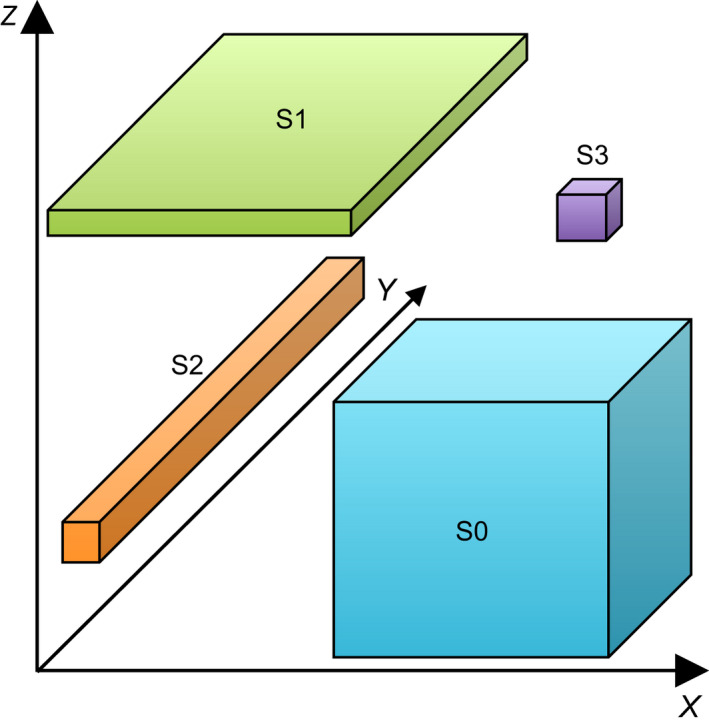
Ecological niches can be visualised as volumes in n‐dimensional niche space. This hypothetical example shows the niches of four species on three niche axes (x, y and z). Labels indicate the number of axes on which a species is specialised (i.e. has narrow niche breadth). S0 is a generalist on all axes, S3 is specialised on all axes. S1 and S2 specialise on one or two axes. Recent research on fungi allows us to consider whether specialisation on different niche axes is correlated (S0, S3) or uncorrelated (S1, S2). In some illustrations (e.g. Chaloner et al., 2020) the niche is depicted as a spheroid rather than a cuboid, implying interactions among niche axes.
A fundamental question in evolutionary ecology is: what determines niche breadth and how does it evolve (Sexton et al., 2017; Carscadden et al., 2020)? In the absence of competition, we might expect a species to evolve the capacity to survive under all conditions and exploit all resources. However, there must be competitive advantages to specialising, notwithstanding any biological constraints such as the implausibility of the capacity to consume prey of any size. Many models of niche evolution expect a trade‐off between performance and breadth, summed up by the phrase ‘Jack of all trades, master of none’. However, such trade‐offs remain only weakly supported by empirical evidence, and trade‐off mechanisms such as antagonistic pleiotropy operate mainly under specific conditions (Bono et al., 2017). Other niche evolution models indicate that specialisation can evolve without the need for trade‐offs, for example when specialists spend more time in particular environments and so beneficial alleles are fixed more rapidly than in generalist subpopulations (Whitlock, 1996).
Whereas niche breadth evolution over one niche axis has been investigated by numerous models and empirical studies (reviewed in Sexton et al., 2017), the relationship between niche breadths on different axes remains largely unexplored (Carscadden et al., 2020). Is specialisation on niche axes correlated, or uncorrelated? Is a specialist a specialist in all niche dimensions? Grinnell (1917) describes the California thrasher as preferring warm, moist environments (i.e. narrow abiotic niche axes) but having a very diverse omnivorous diet (i.e. wide resource niche axis). No correlations among niche axes were detected within the genus Lasthenia, an annual plant of ephemeral pools in California (Emery et al., 2012), whereas in global analyses of amphibians and reptiles, temperature and precipitation niche breadths were positively correlated (Bonetti & Wiens, 2014; Lin & Wiens, 2017; Liu et al., 2020). Whether niche breadths are correlated or not has important implications for issues such as climate change impacts on populations. For species with narrow thermal tolerances, migration to track optimal climates will be facilitated if other niche axes are broad (Carscadden et al., 2020).
Recent research on different guilds of fungi (Table 1) allows the question of niche breadth correlations to be addressed (Maynard et al., 2019; Chaloner et al., 2020; Davison et al., 2021). Writing in the New Phytologist, Davidson et al used 327 soil samples from around the world to estimate mean and standard deviation (SD) of some soil chemical properties and two climatic parameters, mean annual temperature and mean annual precipitation, for each of 230 arbuscular mycorrhizal fungal (AMF) taxa (Davison et al., 2021). They defined niche breath as the standard deviation of the values across sampled sites of each parameter per taxon. Strictly, geographical distributions can give an incomplete estimate of the realised niche because dispersal limitation can prevent a species from filling all of its potential range (Soberón & Nakamura, 2009; Soberón & Peterson, 2020). However, AMF are geographically widespread with little dispersal limitation (Davison et al., 2015; Kivlin, 2020), so this bias may be small. Davison et al tested for significant specialisation or generalisation by bootstrap resampling of their soil and climate data. They found that approximately half of the taxa exhibited significantly narrower niches than expected from random resampling, particularly for temperature and pH. They did not estimate correlations in niche breadths among axes, which we now present (Fig. 1a,b). We found a range of correlations among niche axes (Fig. 1a, upper triangle), from moderately negative (e.g. phosphorus and mean annual precipitation) to strongly positive (e.g. organic carbon and nitrogen). However, these correlations are themselves strongly correlated to matching correlations among the soil and climatic variables (Fig. 1b, lower triangle; Fig. 2). In other words, when abiotic conditions are correlated among sites, we see stronger correlations among niche breadths inferred from the species presences at those sites. For negatively correlated soil variables, niche breadths were ranged between −0.3 and 0.3, while for positively correlated soil variables, niche breadth correlations were generally positive but weaker than the soil correlations. The results suggest that apparent positive or negative correlations among niche axis widths could well be due to correlations among climatic and edaphic variables in this observational dataset, and that specialisation is largely independent among abiotic niche axes.
Table 1.
Summary of niche breadth comparisons for three fungal guilds.
| Guild | Plant pathogens | Arbuscular mycorrhizal fungi | Saprotrophs | |||
| Source | Chaloner et al. (2020) | Davison et al. (2021) | Maynard et al. (2019) | |||
| Taxa | 209 a | 268 | 23 | |||
| Scale | Global b | Global | Regional | |||
| Axis | Temperature | Host range | Climate c | Soil chemistry d | Temperature | Moisture |
| Method | Experiment | Observation | Observation | Observation | Experiment | Experiment |
| Niche | Fundamental | Realised | Realised | Realised | Fundamental | Fundamental |
Temperature responses for growth in culture, 187 fungi and 27 oomycetes.
Probable sampling bias toward temperate climate species (Chaloner et al., 2021).
Mean annual temperature and mean annual precipitation.
Includes pH, organic C, N, P, K, Mg, Ca.
Fig. 2.
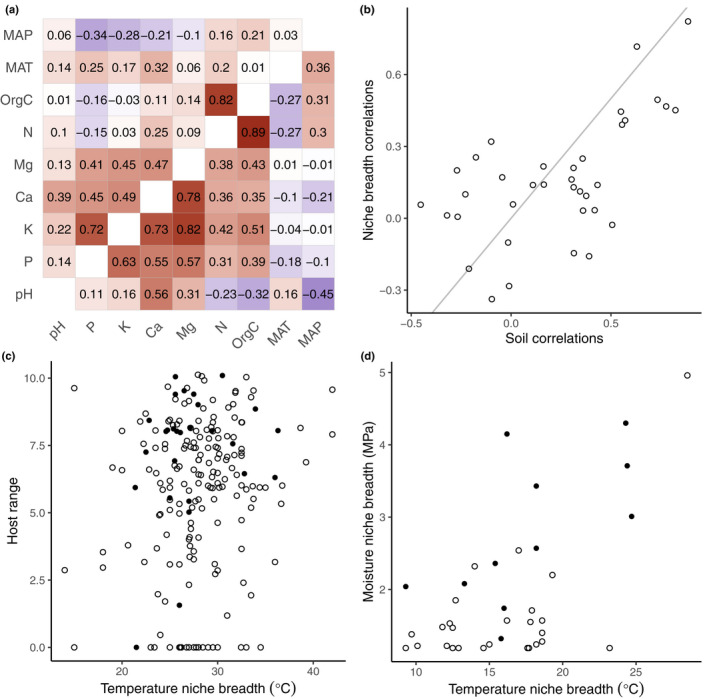
Niche breadth correlations. (a) Heat map with niche breadth correlations in upper triangle and soil property correlations in lower triangle for arbuscular mycorrhizal fungi (AMF) (data from Davison et al., 2021). For example, soil organic carbon (OrgC) niche breadth is strongly correlated with soil nitrogen (N) niche breadth (r = 0.82, upper triangle). However, soil OrgC is also strongly correlated with soil N content (r = 0.89, lower triangle). Red indicates a positive correlation, blue a negative correlation. MAP, mean annual precipitation; MAT, mean annual temperature. (b) Niche breadth correlations vs soil property correlations for all pairs of variables in (a). Pearson correlation 0.59 (bootstrap percentile 95% confidence interval 0.35–0.77). Line of equality in grey. (c) Host range vs temperature niche breadth (growth in culture) for fungal (open circles) and oomycete (closed circles) pathogens of plants (data from Chaloner et al., 2020). Host range is the log‐transformed phylogenetic diversity of known host plants. Pearson correlation 0.14 (bootstrap 95% CI 0.00–0.26). (d) Moisture niche breadth vs temperature niche breadth for AMF (data from Maynard et al., 2019). Pearson correlation 0.60 (bootstrap 95% CI 0.26–0.80).
Chaloner et al. (2020) compiled a database of niche breadths along two axes, one an abiotic condition (temperature), the other an abiotic resource (host range), for a large number of fungal and oomycete plant pathogens. They defined abiotic niche breadth as the temperature range between the minimum and maximum temperatures of some biological processes, including growth in culture and spore germination, and biotic niche breadth as the phylogenetic distances among all known plant hosts of each pathogen. They found no statistically significant positive or negative correlations among these two measures of niche breadth (once significance levels had been corrected for multiple comparisons), nor even any hints of correlations that we may not have confirmed for lack of statistical power (Fig. 1c). Acknowledging certain weaknesses in the pathogen dataset, which was compiled from older, mostly experimental results and observed rather than complete host ranges, the data indicated that specialisation on these niche axes is uncorrelated. In other words, rather than a generalist being ‘Jack of all trades, master of none’, a species can be ‘Jack of some trades, master of others’. Plant pathogens may therefore be host generalists, able to infect a diverse range of plants while also being climate specialists with narrow temperature performance curves or any other combination of niche shape.
A study on North American basidiomycetes allows us to investigate niche breadth correlations in a third guild of fungi, the saprotrophs (Maynard et al., 2019). Here, temperature and moisture performance curves of 37 fungal isolates in 16 genera and 23 species were experimentally determined, with temperature and moisture niche breadths estimated from those curves. The correlation between temperature and moisture niche breadths is strongly positive when all isolates are included (Fig. 1d; fig. 2 in Maynard et al., 2019). Armillaria is represented by four species, and Armillaria gallica by eight isolates. When A. gallica is considered alone, niche breadths remained strongly correlated (r = 0.67). If Armillaria is removed from the dataset, the correlation remains strongly positive (r = 0.58), largely because Xylobolus subpileatus is an outlier with very wide niche axes (r = 0.14 if X. subpileatus removed). This analysis of wood decay fungi suggests significant co‐specialisation on climate niche axes, even within species.
These three studies in different fungal (and oomycete) ecological guilds allow us to consider co‐specialisation on different niche axes and therefore the shape of the ecological niche. The AMF and saprotroph studies only considered abiotic niche axes (soil chemistry and climate), whereas the plant pathogen analysis used one biotic axis (host range) and one abiotic condition (temperature). For plant pathogens and saprotrophs, temperature and moisture niches for growth in culture were quantified experimentally and can be considered estimates of the fundamental niche. Niche breadths of the AMF were derived from geographical distributions and therefore estimate the realised niche. Given the correlations among soil properties and climate variables used to estimate niche breadth, specialisation on these factors is probably independent in AMF. For plant pathogens, the known host range estimates a subset of the fundamental niche restricted by dispersal limitation, that is plant pathogens have not reached all potential hosts (Bebber et al., 2014). In this fungal guild, there is no indication of co‐specialisation on climatic and biotic niche axes. In saprotrophs, there is evidence for co‐specialisation on two climatic niche axes. Therefore, taken together, we can tentatively conclude that co‐specialisation may be more likely to occur within a particular class of niche axis (e.g. climatic) than across classes (e.g. climatic and biotic).
The quantity and quality of data varied considerably among the three studies. Arbuscular mycorrhizal fungal and plant pathogens are represented by hundreds of species, but the saprotrophs by only a few. However, niche data on the saprotrophs were obtained by controlled experiment and so may be more reliable than data on the other guilds, which were obtained either by inference from geographic occurrence, or by collation of observations and historical experimental data. Further experimental studies, as well as inference from distributions, will be required to better understand niche breadth correlations (Carscadden et al., 2020). Temperature niche breadths are the most comparable, indicating similar variability in pathogens and saprotrophs (Fig. 1c,d). Arbuscular mycorrhizal fungal temperature niche breadths are reported as the SD of mean annual temperature at the sampled sites of each taxon (Davison et al., 2021). Most values lie between 1 and 10°C, and so are broadly comparable with the ranges estimated for growth in culture in the other guilds, if we assume that the majority of values lies within 2 SD of the mean. Host ranges, or biotic niche breadths, were available for pathogens but not for AMF or saprotrophs. Arbuscular mycorrhizal fungal are thought to have very low host specificity, although host preferences have been reported (van der Heijden et al., 2015). Host ranges of saprotrophs appear to be evolutionarily labile (Krah et al., 2018). White rot fungi switch frequently between generalism and angiosperm specialism, while most brown rot fungi are generalists but often switch to and from gymnosperm specialism. Phylogenetic diversities of known host plants could, in principle, be calculated for AMF using information in the MaarjAM database (Öpik et al., 2010) and for saprotrophs using the USDA Fungus–Host Distribution Database (Farr & Rossman, 2022) to allow biotic and abiotic niche breadths to be compared.
The ecological literature has had little to say on the shape of the n‐dimensional hypervolume, or more directly the degree of specialisation on different environmental conditions and resources within a species (Blonder et al., 2014; Soberón & Peterson, 2020). Major reviews of the niche concept commonly illustrate the niche as a sphere or cube rather than ovoid or oblong, implying an assumption of correlated specialisation (e.g. Chase & Leibold, 2003). Carscadden et al. (2020) proposed that the degree of niche breadth correlation can be determined by environmental drivers or functional constraints. Correlations in the variability of environmental conditions, rather than their levels, should determine niche breadth correlations. For example, due to opposing latitudinal gradients in plant species richness (which can be thought of as biotic variability) and annual temperature variation, we might expect temperature niche breadth to be negatively correlated with host niche breadth for plant‐associated species. Similarly, tropical plants and animals tend to have narrower thermal and wider precipitation niche breadths than temperate species (Liu et al., 2020). Functional constraints could inhibit the degree to which species can evolve niches to best fit environmental conditions and resources. Positive correlations in niche breadths would occur if sets of genes convey tolerances to a range of stressors, for example. This is largely speculation in the absence of data and we have little theoretical framework to consider, although new global datasets on fungal distributions derived from sequencing data may help us to answer this question (Větrovský et al., 2020).
Despite the apparent conceptual clarity that Hutchinson brought to the niche concept, the utility of the niche in understanding species ecology faced criticism during the 1970s and 1980s, leading ultimately to proposals that patterns of diversity and population dynamics in nature could be explained without recourse to any differences among species (Chase & Leibold, 2003). However, the close relationship between niche space and geographic distributions (Soberón & Nakamura, 2009) means that predicting the impact of global change on the biosphere requires an understanding of species' resource requirements and environmental tolerances, that is the shape of the ecological niche.
Acknowledgements
TMC was funded by a BBSRC SWBio Doctoral Training Award.
Data availability
Data used in the publication are openly available from the publications cited as sources in the manuscript.
References
- Bebber DP, Holmes T, Gurr SJ. 2014. The global spread of crop pests and pathogens. Global Ecology and Biogeography 23: 1398–1407. [Google Scholar]
- Blonder B, Lamanna C, Violle C, Enquist BJ. 2014. The n‐dimensional hypervolume. Global Ecology and Biogeography 23: 595–609. [Google Scholar]
- Bonetti MF, Wiens JJ. 2014. Evolution of climatic niche specialization: a phylogenetic analysis in amphibians. Proceedings of the Royal Society of London B: Biological Sciences 281: 20133229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono LM, Smith LB Jr, Pfennig DW, Burch CL. 2017. The emergence of performance trade‐offs during local adaptation: insights from experimental evolution. Molecular Ecology 26: 1720–1733. [DOI] [PubMed] [Google Scholar]
- Carscadden KA, Emery NC, Arnillas CA, Cadotte MW, Afkhami ME, Gravel D, Livingstone SW, Wiens JJ. 2020. Niche breadth: causes and consequences for ecology, evolution, and conservation. The Quarterly Review of Biology 95: 179–214. [Google Scholar]
- Chaloner TM, Gurr SJ, Bebber DP. 2020. Geometry and evolution of the ecological niche in plant‐associated microbes. Nature Communications 11: 2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloner TM, Gurr SJ, Bebber DP. 2021. Plant pathogen infection risk tracks global crop yields under climate change. Nature Climate Change 11: 710–715. [Google Scholar]
- Chase JM, Leibold MA. 2003. Ecological niches: linking classical and contemporary approaches. Chicago, IL, USA: University of Chicago Press. [Google Scholar]
- Davison J, Moora M, Öpik M, Adholeya A, Ainsaar L, Bâ A, Burla S, Diedhiou AG, Hiiesalu I, Jairus T et al. 2015. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 349: 970–973. [DOI] [PubMed] [Google Scholar]
- Davison J, Moora M, Semchenko M, Adenan SB, Ahmed T, Akhmetzhanova AA, Alatalo JM, Al‐Quraishy S, Andriyanova E, Anslan S et al. 2021. Temperature and pH define the realised niche space of arbuscular mycorrhizal fungi. New Phytologist 231: 763–776. [DOI] [PubMed] [Google Scholar]
- Elton CS. 1927. Animal ecology. London, UK: Sidgwick & Jackson. [Google Scholar]
- Emery NC, Forrestel EJ, Jui G, Park MS, Baldwin BG, Ackerly DD. 2012. Niche evolution across spatial scales: climate and habitat specialization in California Lasthenia (Asteraceae). Ecology 93: S151–S166. [Google Scholar]
- Farr DF, Rossman AY. (2022). Fungal databases, U.S. National fungus collections, ARS, USDA. [WWW document] URL https://nt.ars‐grin.gov/fungaldatabases/ [accessed 11 November 2021].
- Grinnell J. 1917. The niche‐relationships of the California thrasher. The Auk 34: 427–433. [Google Scholar]
- van der Heijden MGA, Martin FM, Selosse M‐A, Sanders IR. 2015. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist 205: 1406–1423. [DOI] [PubMed] [Google Scholar]
- Hutchinson GE. 1957. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22: 415–427. [Google Scholar]
- Kivlin SN. 2020. Global mycorrhizal fungal range sizes vary within and among mycorrhizal guilds but are not correlated with dispersal traits. Journal of Biogeography 47: 1994–2001. [Google Scholar]
- Krah F‐S, Bässler C, Heibl C, Soghigian J, Schaefer H, Hibbett DS. 2018. Evolutionary dynamics of host specialization in wood‐decay fungi. BMC Evolutionary Biology 18: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L‐H, Wiens JJ. 2017. Comparing macroecological patterns across continents: evolution of climatic niche breadth in varanid lizards. Ecography 40: 960–970. [Google Scholar]
- Liu H, Ye Q, Wiens JJ. 2020. Climatic‐niche evolution follows similar rules in plants and animals. Nature Ecology & Evolution 4: 753–763. [DOI] [PubMed] [Google Scholar]
- Maynard DS, Bradford MA, Covey KR, Lindner D, Glaeser J, Talbert DA, Tinker PJ, Walker DM, Crowther TW. 2019. Consistent trade‐offs in fungal trait expression across broad spatial scales. Nature Microbiology 4: 846–853. [DOI] [PubMed] [Google Scholar]
- Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij JM, Reier Ü, Zobel M. 2010. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytologist 188: 223–241. [DOI] [PubMed] [Google Scholar]
- Sexton JP, Montiel J, Shay JE, Stephens MR, Slatyer RA. 2017. Evolution of ecological niche breadth. Annual Review of Ecology, Evolution, and Systematics 48: 183–206. [Google Scholar]
- Soberón J, Arroyo‐Peña B. 2017. Are fundamental niches larger than the realized? Testing a 50‐year‐old prediction by Hutchinson. PLoS ONE 12: e0175138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberón J, Nakamura M. 2009. Niches and distributional areas: concepts, methods, and assumptions. Proceedings of the National Academy of Sciences, USA 106: 19644–19650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberón J, Peterson AT. 2020. What is the shape of the fundamental Grinnellian niche? Theoretical Ecology 13: 105–115. [Google Scholar]
- Větrovský T, Morais D, Kohout P, Lepinay C, Algora C, Awokunle Hollá S, Bahnmann BD, Bílohnědá K, Brabcová V, D’Alò F et al. 2020. GlobalFungi, a global database of fungal occurrences from high‐throughput‐sequencing metabarcoding studies. Scientific Data 7: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock MC. 1996. The red queen beats the Jack‐of‐all‐trades: the limitations on the evolution of phenotypic plasticity and niche breadth. The American Naturalist 148: S65–S77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in the publication are openly available from the publications cited as sources in the manuscript.


