Abstract
Background
To date, no validated assessment of motor imagery (MI) ability with temporomandibular disorders (TMD) exists preventing identification of good imagers and appropriate MI use during TMD rehabilitation.
Objective
To assess the reliability and construct validity of the previously developed Tongue and Mouth Imagery Questionnaire (TMIQ) compared with the gold‐standard Kinaesthetic and Visual Imagery Questionnaire (KVIQ‐10).
Methods
Both KVIQ‐10 and TMIQ assess MI ability using vividness (i.e. clarity/brightness for visual MI, VMI; or intensity for kinesthetic MI, KMI) of MI using a 5‐point Likert scale (1: no image/sensation, 5: clear/intense image/sensation). The KVIQ‐10 was administered once (test) and the TMIQ twice (test–retest) to heathy participants and patients with TMD. Questionnaire validity was investigated using concurrent validity (Pearson correlation and paired t test); TMIQ‐test–retest reliability (intraclass correlation coefficients, ICCs); internal consistency (Cronbach ⍺) and the factorial structure (principal factor extraction).
Results
A total of 94 participants were included (n = 47 per group). The mean vividness scores of the KVIQ‐10 and the TMIQ were significantly correlated, and not significantly different for both groups indicating concurrent validity. ICCs in the control group (range: 0.82‐0.90), and in the TMD group (range: 0.75‐0.82) indicated good reproducibility. The Cronbach ⍺ values were all above 0.94, indicating excellent reliability. Two factors were extracted corresponding to VMI and KMI, and explained 66% of total variance.
Conclusion
The TMIQ is a valid and reproducible MI questionnaire showing excellent internal consistency and, therefore, can be used to assess imagined movements of the TM region in healthy individuals and patients with TMD.
Keywords: cronbach ⍺, factorial analysis, intraclass correlation coefficient, Physiotherapy, temporomandibular disorders
1. BACKGROUND
Temporomandibular disorders (TMD) are characterised by a triad of clinical features including muscle and/or joint pain, TM joint sounds and impaired mobility of the mandible during opening and closing movements. 1 , 2 Pain in the TM region appears relatively common, occurring in approximately 10% of the population over the age of 18 years. 3 It has been established that pain produces changes in motor behaviour due to peripheral and central mechanisms related to the central nervous system, 4 including a distortion of somatorepresentation, 5 with evidence of structural and functional changes in the prefrontal cortex and basal ganglia. 6 TMD management is patient‐centred, multidisciplinary 7 and based on a biopsychosocial matrix model. 8 Physiotherapy also plays a part in the treatment of such patients using a combination of brain training techniques and biobehavioural interventions, as appropriate, which elicits cortical reorganisation, 5 and has been found to be effective in improving mobility and reducing pain due to TMD. 9
Among the possible brain training techniques, explicit motor imagery (MI)—the conscious construction of a mental representation from an internal perspective based on either visual or kinaesthetic information without simultaneous physical execution 10 —has been shown to be effective to reduce pain and improve range of motion in musculoskeletal disorders, 11 increase strength 12 and limit the loss of strength due to immobilisation. 13 As such, MI has been extensively used to improve motor performance in athletes, 14 , 15 and individuals with neurological issues, including stroke 16 and spinal cord injury, 17 , 18 The effectiveness of MI (in adjunction to actual practice) is related to functional equivalence that consists of similar sensorimotor brain recruitment between actual and imagined practice. 19 Therefore, MI has been very recently recommended to be included in TMD rehabilitation to promote both clinical improvement and cortical reorganisation. 5
Notably, improvements observed in response to MI training are directly related to the MI ability of the individual. 20 , 21 The latter is usually assessed by measuring the vividness that refers to the clarity/brightness of the image (visual MI, vMI) and intensity of the sensation (kinaesthetic MI, KMI) of the mental reconstruction of the movement 22 during the administration of a specific questionnaire. Because all validated questionnaires exclusively assess the MI ability for the movements of limbs and trunk, we developed The Tongue and Mouth Imagery Questionnaire (TMIQ), a new questionnaire designed for the assessment of MI ability of movements of the TM region, 23 , 24 that is yet to be validated. The present study, therefore, aimed to assess the reliability and construct validity of the TMIQ with reference to the gold‐standard Kinaesthetic and Visual Imagery Questionnaire (KVIQ‐10).
2. METHODS
2.1. Trial design and ethics
This case‐control study was observational and prospective, and the present report conforms to STROBE guidelines. 25 Approval was obtained from the regional ethics committee (Comité de Protection des Personnes Sud‐Ouest et Outre‐Mer III 2018‐A02195‐50).
2.2. Role of the funding source
The study received no financial support. The sponsors only participated in the study design.
2.3. Eligibility criteria
French speaking volunteers aged between 18 and 75 years were considered for inclusion. Prior to the study inclusion, a physician independent of the study diagnosed TMD and delivered a written prescription for TMD rehabilitation specifying the diagnosis of TMD, and the physiotherapist classified the type of pain and intra‐articular temporomandibular disorders according to the diagnostic criteria for the temporomandibular disorders (DC/TMD); imaging was not used. 2 Healthy subjects included in the control group were age‐ and sex‐matched to TMD participants. Eligibility to participate in this study was then screened for each identified individual by an experienced physiotherapist (i.e. >5 years of TMD rehabilitation). The physiotherapist verified the absence of exclusion criteria defined as the presence of a short lingual frenulum, 26 lingual immaturity 27 and/or peripheral facial palsy; a history of orthognathic surgery or facial fracture during the 6 previous months; the current participation in another study to prevent any experimental bias; and TMD for inclusion in the control group. Eligible individuals received an informed consent document about the study mentioning that the participation was voluntary. In case, eligible individual gave written informed consent to participate in the study.
2.4. Settings
All patients with TMD were recruited in the physiotherapy facility (private practice) Cabinet Saint Alexandre (Lyon, France), that exclusively receives patients for TMD rehabilitation. Control participants were recruited within the family environment of either the patients with TMD or the authors, or in the authors’ professional entourage among the staff of the University of Lyon or the hospital.
2.5. Intervention
The study consisted of the administration of 2 questionnaires: the gold‐standard KVIQ‐10 and the TMIQ. The KVIQ‐10 was administered once (test) and the TMIQ twice (test–retest). The interval between test and retest was ≥7 days. For TMD participants the test and retest were integrated into the course of their TMD rehabilitation; the usual interval between 2 rehabilitation sessions being between 7 and 42 days. For controls, the test and retest were scheduled according to the availability of participants.
2.6. The KVIQ‐10
The KVIQ‐10 is a validated questionnaire used to assess MI ability through vividness, distinguishing VMI (i.e. clarity of the image) and KMI (i.e. intensity of the sensation). The KVIQ‐10 consists of a total of 5 different movements, of upper limb (n = 2), trunk (n = 1) and lower limb (n = 2). The examiner reads the questionnaire instructions and the participant performs successively an actual movement and an imagined movement using a first‐person perspective and VMI from the first to the fifth movement. This set of movements is then repeated but imagined movements are performed using KMI. After each imagined movement, the subject rates the vividness using the operational definition corresponding to a 5‐point Likert scale (i.e. ‘no image/ no sensation’ = 1 up to ‘image/sensation as clear/intense as during actual movement’ = 5). The examiner records a total of 10 vividness scores (i.e. VMI, n = 5 and KMI, n = 5). 24 This questionnaire was chosen to be the gold‐standard because movements instructed are restricted to an anatomical region (e.g. flexing the shoulder while maintaining the elbow extended) that is more close to the movements performed with the TM region contrary to other questionnaires assessing vividness of more complex and goal‐directed imagined movement such as walking, running or grasping. 23 , 28
2.7. The TMIQ
The structure of the TMIQ was similar to that of the KVIQ‐10. The TMIQ included 5 items representing gestures of the tongue (i. pointing to mouth commissure, ii. licking lips and teeth, iii. drawing an ‘m’ on the palate) and of the mandible (iv. laterally shift, v. maximal opening; see Appendix and Figure 1 for details). These were performed in the order presented above, and as for the KVIQ‐10, for each of these the actual movements were performed and followed by the VMI movements (item 1 to 5); this was repeated for actual and KMI movements. All movements were performed at a comfortable speed in a sitting position, with no rest between movements unless needed by the participant. After each imagined movement, the participant rated the vividness using the same 5‐point Likert scale used for the KVIQ‐10. 24 Like the KVIQ‐10, the TMIQ was administered by an examiner who read the instructions and recorded the vividness score. In case, an actual movement was inappropriately executed, the examiner asked the participant to repeat it immediately. In addition, manifestation of pain within the TM region occurring during questionnaire administration was systematically recorded after each item and rated using a numerical scale (0 = no pain, 10 = maximal pain). Hence, for each participant 10 vividness scores and 10 pain scores were recorded.
FIGURE 1.
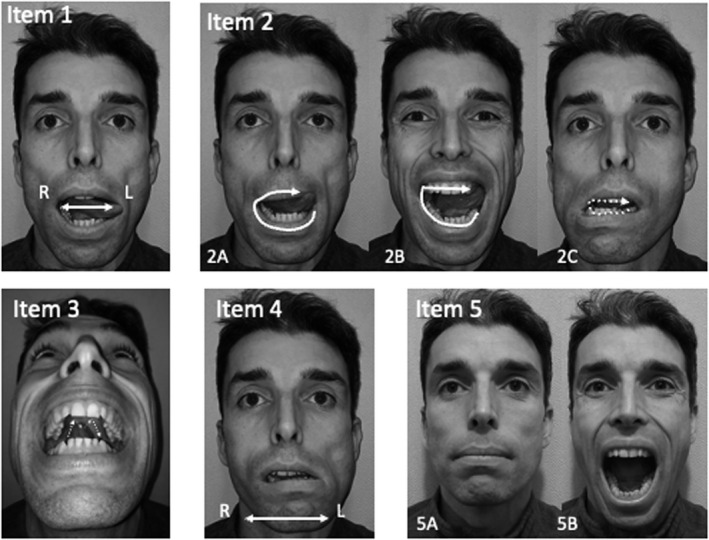
Illustration of the Tongue and Mouth Imagery Questionnaire (TMIQ). R: right, L: Left. Item 1. Pointing to mouth commissure, Participant was instructed to point with the tip of the tongue to the mouth commissures a total of 6 pointing (e.g. left, right, left, right, left, right); Item 2: Licking lip and teeth, Participant was instructed to lick (2A) the lower lip, then the upper lip, (2B) the anterior part of the mandible teeth then the anterior part of the maxilla teeth, (2C) the posterior part of the mandible teeth then the posterior part of the maxilla teeth (hence a total of 6 licking); Item 3: Drawing an ‘m’ on the palate with the tongue without touching the teeth; Item 4: Lateral shift of the mandible, Participant, maintaining the mouth slightly open, was instructed to laterally shift the mandible a total of 6 time (e.g. left, right, left, right, left, right); Item 5: Maximal opening of the tongue then closing 3 consecutive times
2.8. Outcomes
The vividness scores of both the KVIQ‐10 and TMIQ (i.e. Likert scale 1‐5) along with the pain intensity (Numerical scale 0‐10) were recorded using OpenSesame (version 3.2.4) software (https://osdoc.cogsci.nl/), 29 and stored as a csv file. This procedure was chosen to both automate and secure the data collection thus preventing the risk of data loss. In addition, maximal mouth opening was measured once using a calliper as recommended for both control participants and patients with TMD; 30 this measure was subsequently used for DC/TMD classification of intra‐articular temporomandibular disorders for patients.
2.9. Sample size
A moderate effect size was at least expected (i.e. Pearson correlation coefficient r = 0.4) 31 to evidence the concurrent validity between the gold‐standard KVIQ‐10 and the TMIQ. Using this a priori hypothesis, statistical significance threshold (5%) and power (80%), a total of 94 participants was needed to be included in the study (i.e. 47 participants per group).
2.10. Blinding
Because the questionnaire administration required active (either actual or imagined) movements of the participant and physiotherapist examiner supervision, neither the participant nor the assessor were blinded to the evaluation; therefore, physiotherapists assessed exclusively participants of one group.
2.11. Statistical methods
2.11.1. Preparation
The total vividness scores of VMI, KMI and MI were computed for each participant and each questionnaire by summing the vividness scores of respectively 10 items and 5 items (VMI and KMI range: 5‐25; MI range: 10‐50 respectively). The mean of the 10 pain scores with 95% confidence intervals (95%CI) were calculated for each participant for the TMIQ‐test and for ‐retest. Shapiro test analyses showed that vividness scores of each group was normally distributed; parametric tests were, therefore, subsequently used for the analyses.
2.11.2. Concurrent validity
The concurrent validity of the TMIQ against the KVIQ‐10 was investigated using the Pearson’s correlation test, separating groups and MI modality (i.e. VMI and KMI). In addition, differences in the total vividness scores between the TMIQ‐test and the KVIQ‐10 were evaluated for each group using the paired Student’s t test and reported as mean difference and 95%CI
2.11.3. Test–retest reliability of the TMIQ
Difference in the vividness scores of VMI, KMI and MI of the TMIQ between test and retest was investigated using the paired Student’s t test. Then the test–retest reproducibility of the TMIQ was estimated using intraclass correlation coefficients (ICCs) in a one‐way random effect model, 32 with 95%CI. 33 ICC values <0.50 are indicative of poor reliability, values between 0.50 and 0.75 indicate moderate reliability, values between 0.75 and 0.90 indicate good reliability, and values >0.90 indicate excellent reliability. 34 The test–retest standard error of measurement (SEM) was calculated by multiplying the standard deviation of the test results by the square root of one minus the ICC (). 34 These analyses were conducted for each group on the total vividness scores of VMI, KMI and MI; 24 these analyses were repeated considering separately tongue and mandible (i.e. respectively items 1‐3 and 4&5). In addition, the estimated components of variance related to participants, time and random error were computed for each group from the analysis of variance analyses testing the total vividness scores (for VMI, KMI and MI) using time and within‐participants as factors.
2.11.4. Internal consistency and factor analysis
The homogeneity of items composing the TMIQ was evaluated using Cronbach’s ⍺ coefficient and 95%CI; ⍺ > 0.75 were considered as indicative of good reliability, and ⍺ > 0.90 as indicative of excellent reliability. 34 The corrected item‐total correlations were computed between the score of each item and the total score from the questionnaire in which that item has been replaced by a rationally equivalent item; 35 correlation >0.30 evidence that all items correlate with the total score, indicating a reliable questionnaire. 36 The latent structure of the TMIQ was assessed with the principal factor extraction technique using an oblique ‘oblimin’ rotation since the visual and kinaesthetic factors were expected to correlate. 36 Communalities were computed, and these represent the proportion of variance explained by the extracted factors; item communality >0.40 is considered as acceptable. 37 The χ2 statistic was used to verify whether the number of factors extracted was sufficient. 34
2.11.5. Participants’ MI ability
To investigate an effect of TMD on MI ability, the mean VMI vividness score of the TMD group was compared to that of the control group using the paired Student’s t test for each questionnaire (i.e. KVIQ‐10, TMIQ‐test and TMIQ‐retest); the analyses were also performed for the KMI vividness score. To investigate MI dominance, the mean VMI vividness score were compared to the mean KMI vividness score using the paired Student’s t test and Pearson correlation for each group and each questionnaire (i.e. KVIQ‐10, TMIQ‐test and TMIQ‐retest). Furthermore, exploratory analyses were conducted computing for each category of the DC/TMD the mean and 95%CI (calculated from the mean) of the vividness scores; overlapping vividness score 95%CI indicated no significant difference in MI ability between categories of the DC/TMD, therefore, suggesting no influence of the type of pain or the type of intra‐articular disorder on MI ability. We also investigated whether the ability to open the mouth was related to MI ability by computing separately for the control and TMD groups Pearson’s’ correlation between maximal mouth opening and vividness scores for VMI, KMI of the TMIQ at test and retest.
2.11.6. Pain while imagining
The median and 95%CI (calculated from the Wilcoxon test) of the occurrence of pain during the administration of the TMIQ was reported for each group and the TMIQ‐test and ‐retest. Pain scores was compared between TMIQ‐test and ‐retest for each group using Wilcoxon test
All analyses were performed using R 3.5.3. 32 Statistical significance was set at 5% (p < 0.05).
3. RESULTS
All 94 screened individuals gave the written informed consent attesting that they agreed to participate in the study and were subsequently included, and all participants completed the study. Inclusions started September 26, 2018, and the study was completed on December 31, 2019 (Figure 2). The mean, standard deviation (SD) age of the TMD group (38 (16) years) was not significantly different to that of the control group (38 (15) years, p = 0.99). The mean (SD) interval between test and retest was significantly longer for patients with TMD (28 (13) days) than for control participants (12 (11) days, p < 0.001). Accordingly to the DC/TMD, all patients reported myalgia – local myalgia (n = 6, 13%), myofascial pain (n = 21, 45%), myofascial pain with referral (n = 20, 23%); among the 47 patients with TMD, 17 (36%) had no intra‐articular disorder, 18 (38%) had a disc displacement with reduction, 10 (21%) had a disc displacement without reduction and with limited opening, and 2 (4%) and a degenerative joint disease (Supplementary Table S1).
FIGURE 2.
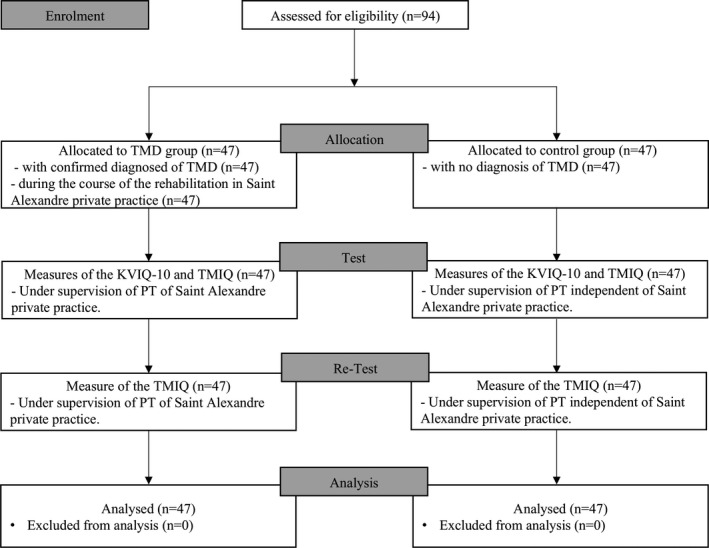
Flow chart detailing the enrolment, allocation, test, retest and analysis of the TMIQ study. n: number of participants
3.1. Concurrent validity
All tested correlations between TMIQ‐test and KVIQ‐10 were significant for both groups and modalities (all p < 0.0001). For the TMD group, the correlation coefficient (r) were 0.72 for VMI and 0.80 for KMI; for the control group this was 0.81 for both VMI and KMI (Figure 3).
FIGURE 3.
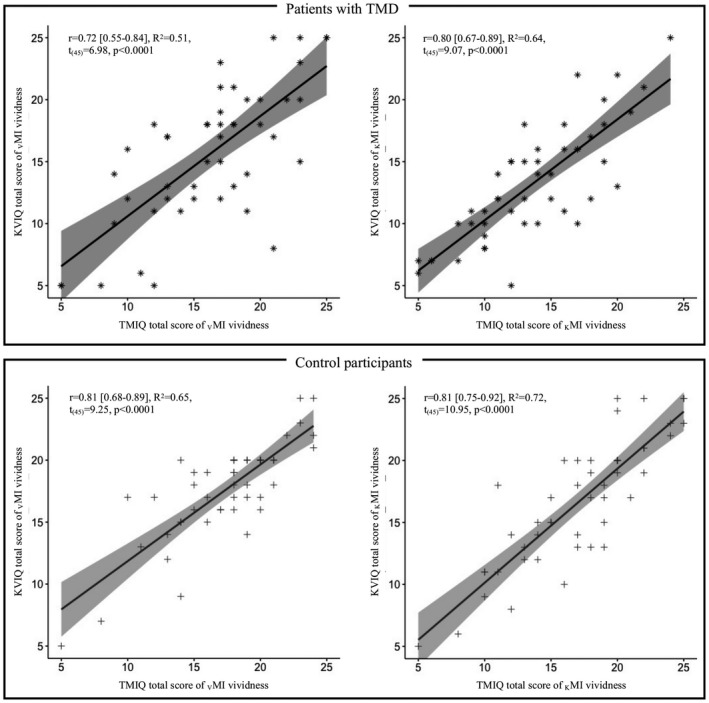
Concurrent validity of the TMIQ against the KVIQ‐10 for participants with TMD (upper panel) and healthy participants (lower panel) separating visual and kinaesthetic motor imagery (left and right panels respectively)
3.2. Test–retest reliability of the TMIQ
There was no significant difference in the mean value of the TMIQ‐test and ‐retest vividness scores (all p > 0.05). The ICCs ranged from 0.82 to 0.90 in the control group and from 0.75 to 0.82 in the TMD group, indicating good reproducibility. ICCs of the items referring to the tongue were >0.75 for KMI and MI for both control and TMD groups, indicating good reproducibility; for VMI this was also the case in the control group and was 0.72 in the TMD group, indicating moderate reproducibility. For the control group the ICCs of the items referring to the mandible were >0.75 for KMI and MI, indicating good reproducibility, and 0.74 for VMI, indicating moderate reproducibility; for the TMD group the ICCs were ≥0.67 for VMI and MI, indicating moderate reproducibility and 0.45 for KMI, indicating poor reproducibility. The mean SEM of total vividness scores was higher for the TMD than the control group for VMI, KMI and MI either considering all items of the TMIQ or items referring to the tongue or the mandible (Table 1). The estimated components of variances due to participants, time and random error were higher for the TMD group as compared to the control group (Table 2).
TABLE 1.
Test–retest reliability of the TMIQ and of its parts in the control and temporomandibular disorder groups
| Control group (n = 47) | TMD group (n = 47) | |||||
|---|---|---|---|---|---|---|
| VMI 1 | KMI 1 | MI 2 | VMI 1 | KMI 1 | MI 2 | |
| TMIQ‐test 3 | 17.3 ± 4.1 | 17.1 ± 4.8 | 34.4 ± 7.6 | 16.2 ± 5.0 | 13.6 ± 4.6 | 29.9 ± 8.3 |
| TMIQ‐retest 3 | 17.4 ± 4.5 | 16.9 ± 4.5 | 34.3 ± 7.8 | 16.8 ± 4.7 | 14.1 ± 5.0 | 30.9 ± 8.7 |
| p 4 | 0.78 | 0.60 | 0.90 | 0.21 | 0.40 | 0.19 |
| TMIQall items ICC 5 | 0.82 [0.72; 0.89] | 0.89 [0.83; 0.93] | 0.90 [0.84; 0.94] | 0.79 [0.68; 0.87] | 0.75 [0.62; 0.84] | 0.82 [0.72; 0.88] |
| TMIQall items SEM 6 | 1.75 [1.39. 2.17] | 1.57 [1.24; 1.97] | 2.39 [1.89; 3.00] | 2.27 [1.81; 2.80] | 2.31 [1.85; 2.83] | 3.59 [2.86; 4.45] |
| TMIQitems 1‐3 ICC 5 | 0.77 [0.65; 0.85] | 0.83 [0.74; 0.89] | 0.87 [0.80; 0.92] | 0.72 [0.58; 0.82] | 0.76 [0.64; 0.85] | 0.80 [0.69; 0.87] |
| TMIQitems 1‐3 SEM 6 | 1.27 [1.02; 1.56] | 1.24 [0.98; 1.54] | 2.4 [1.92. 2.97] | 1.62 [1.30; 1.98] | 1.55 [1.25; 1.91] | 2.40 [1.92; 2.97] |
| TMIQitems 4‐5 ICC 5 | 0.74 [0.60; 0.83] | 0.79 [0.69; 0.87] | 0.84 [0.75; 0.90] | 0.67 [0.52; 0.79] | 0.45 [0.23; 0.62] | 0.68 [0.52; 0.79] |
| TMIQitems 4‐5 SEM 6 | 0.91 [0.73; 1.11] | 0.87 [0.69; 1.07] | 1.96 [1.59; 2.38] | 1.30 [1.05; 1.58] | 1.38 [1.15; 1.63] | 1.96 [1.59; 2.38] |
TMIQ—Tongue and Mouth Imagery Questionnaire; n—number of participants.
total vividness score is 25.
total vividness score is 25.
values are expressed in mean ± SD.
p of the paired Student’s t test.
Intraclass Correlation Coefficients (ICCs) are computed using a one‐way random effect model (with the R software) 32 and expressed in coefficient and 95% confidence interval.
Standardised error of the Measure (SEMs) are expressed in mean and 95% confidence interval; i items 1 to 3 of the TMIQ refers to the tongue; ii items 4 and 5 of the TMIQ refers to the mandible.
TABLE 2.
Estimated components of variance for the analysis of variance for the TMIQ for the VMI, KMI and MI vividness scores in the control and temporomandibular disorder groups
| Control group (n = 47) | TMD group (n = 47) | |||||
|---|---|---|---|---|---|---|
| VMI | KMI | MI | VMI | KMI | MI2 | |
| σ2 participants | 33.37 | 40.87 | 112.4 | 42.3 | 39.95 | 131.7 |
| σ2 time | 0.27 | 0.68 | 0.10 | 7.76 | 4.26 | 23.5 |
| σ2 random error | 3.38 | 2.38 | 5.99 | 4.82 | 5.78 | 13.2 |
TMD: Temporomandibular Disorder; n: number of participants; σ2: variance (mean square error) due to participants, time and random error.
3.3. Internal consistency and factor analysis
The Cronbach ⍺ values were 0.94 95%CI [0.91; 0.97] for VMI, and 0.96 95%CI [0.94; 0.98] for KMI of the control group; these were 0.94 95%CI [0.91; 0.96] for VMI and 0.94 95%CI [0.91; 0.96] for KMI of the TMD group indicating excellent reliability. All corrected item‐total correlation were >0.3 for the control group (range: 0.60 to 0.80) and for the TMD group (range: 0.50 to 0.83), indicating a reliable questionnaire. Two factors were extracted and explained 65% of the total variance. These 2 factors were sufficient, as confirmed by the χ2 statistic (χ2 (26) = 611.1, p < 0.001). All the kinaesthetic items were explained by the first factor, and all the remaining visual items were explained by the second factor. Communalities ranged between 0.46 and 0.78 indicating that the proportion of variance explained by the extracted factors was acceptable. The correlation coefficient between the two factors was 0.51 (Table 3).
TABLE 3.
Principal factors solution with oblique (‘oblimin’) rotation of the TMIQ
| Pattern matrix | Structure matrix | ||||
|---|---|---|---|---|---|
| TMIQ‐10 items | Factor 1 | Factor 2 | Factor 1 | Factor 2 | Communality |
| Item1 V | 0.67 | 0.35 | 0.68 | 0.46 | |
| Item2 V | 0.85 | 0.33 | 0.80 | 0.65 | |
| Item3 V | 0.69 | 0.47 | 0.75 | 0.58 | |
| Item4 V | 0.81 | 0.52 | 0.87 | 0.76 | |
| Item5 V | 0.75 | 0.33 | 0.72 | 0.53 | |
| Item1 K | 0.80 | 0.85 | 0.50 | 0.73 | |
| Item2 K | 0.91 | 0.88 | 0.40 | 0.78 | |
| Item3 K | 0.84 | 0.84 | 0.43 | 0.71 | |
| Item4 K | 0.85 | 0.82 | 0.37 | 0.68 | |
| Item5 K | 0.70 | 0.78 | 0.51 | 0.63 | |
Data of 94 participants was used for the principal factor extraction technique; Proportion of variance explained was 35% and 30% for Factor 1 and 2, respectively. The correlation coefficient between Factors 1 and 2 was 0.51.
3.4. Participants MI ability
Participants of the TMD group had significantly lower mean (SD) KMI vividness scores (13.2 (4.7)) compared to control group for the KVIQ‐10 (16.6 (5.2); p = 0.001); for the TMIQ‐test (13.6 (4.6) vs. 17.1(4.8); p < 0.001); and TMIQ‐retest (14.1 (5.0) vs. 16.9 (4.5); p = 0.003); there was no significant difference between the mean VMI vividness scores of the control and TMD groups (all p > 0.05). For the TMD group, the mean (SD) VMI vividness score (15.6 (5.6)) was significantly higher than the KMI vividness score for KVIQ‐10 (13.2 (4.7); p < 0.01); for the TMIQ‐test (16.2 (5.0) vs. 13.6 (4.6); p = 0.001); and TMIQ‐retest (16.8 (4.7) vs. 14.1 (5.0); p < 0.0001); for the control group there was no significant difference in these comparisons (all p > 0.05). There was a significant correlation of VMI and KMI vividness scores for both groups and all questionnaires (all p ≤ 0.002; Figure 4). Furthermore, all 95%CI of the vividness scores overlapped indicating no significant difference, thus suggesting no effect of the DC/TMD category on MI ability (Table S1). There was also no significant correlation between maximal mouth opening and vividness scores at both TMIQ‐test and ‐retest suggesting that MI ability was independent of the capacity of maximal mouth opening for either control participants and patients with TMD (see Figure S1 and Figure S2).
FIGURE 4.
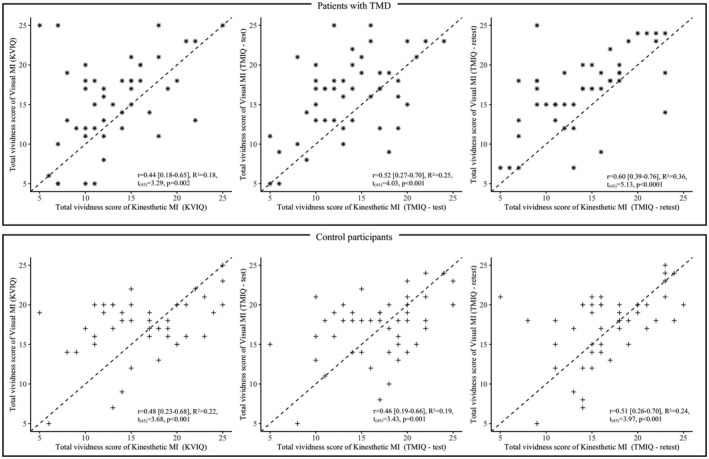
Motor imagery (MI) dominance evidenced by correlation between total vividness score of visual and Kinaesthetic MI for participants with TMD (upper panel) and healthy control participants (lower panel) for respectively KVIQ‐10 (left), TMIQ‐test (centre) and TMIQ‐retest (right)
3.5. Pain while imagining
No participant of the control group reported pain while imagining movements of the temporomandibular region (for both TMIQ‐test and ‐retest median = 0.0, 95%CI [0.0; 0.0]). Patients with TMD reported a significantly higher mean pain in the TM region as compared to control at both TMIQ‐test and ‐retest (respectively, median = 0.9 95%CI [0.7; 1.2] and median = 0.8 95%CI [0.6; 0.9]) but comparison between TMIQ‐test and ‐retest was non‐significant (W = 2448, p = 0.05).
4. DISCUSSION
The present study found that the TMIQ is a valid MI questionnaire; the absence of significant difference in vividness scores between the TMIQ and the KVIQ‐10 along with the significant correlation between the vividness score of these 2 questionnaires for both groups confirmed the concurrent validity of the TMIQ. Furthermore, the test–retest analyses indicated both that there was no significant difference in vividness scores between TMIQ‐test –retest, and ICC scores were all >0.75, which indicated good reliability. The TMIQ showed a better reliability than the Movement Imagery Question (second revised version; MIQ‐RS), which had only moderate reliability; 28 but comparable reliability to the KVIQ‐10 that also had good reliability. 24 Similarly, the correlation between TMIQ and KVIQ‐10 (r > 0.72 for VMI and 0.80 for KMI) was greater than that between Vividness of Movement Imagery Questionnaire (VMIQ‐2) and VMIQ (r = 0.65 for VMI and r = 0.73 for KMI). 23 Taken together, this suggests that questionnaires investigating MI vividness using simpler single‐joint movements (i.e. KVIQ‐10 for the head, trunk and limbs, and TMIQ for the TM region) are more reliable as compared to questionnaires using more complex and goal‐directed movements (i.e. the MIQ and VMIQ‐2). The high Cronbach ⍺ values of the TMIQ confirmed the internal consistency of this questionnaire. Again, the TMIQ had better internal consistency than both the MIQ‐RS (⍺ = 0.87 for VMI and 0.90 for KMI) 28 and the KVIQ‐10 (⍺ = 0.89 for VMI and 0.87 for KMI); 24 only the KVIQ‐20 (⍺ = 0.94 for VMI and 0.92 for KMI) 24 and the VMIQ‐2 (⍺ = 0.95 for VMI and 0.93 for KMI) 23 had internal consistency comparable to the TMIQ. Taken together, the results support the use of the TMIQ to reliably assess MI vividness of imagined movements of the TM region in both healthy persons and patients with TMD.
The factor analysis confirmed the bifactorial structure of the TMIQ, which indicates that the TMIQ assesses separately 2 dimensions of MI, namely the VMI and KMI. This is consistent with previous reports evidencing the bifactorial structure of other questionnaires (MIQ‐RS, 28 KVIQ‐10, 24 and VMIQ‐2 23 ), and hence, imagining movements of the TM region is similar in this regard to imagining movements of the rest of the body. This suggests a ‘general’ MI ability that is not specifically related to certain body parts.
The present study has several clinical implications. (i) Imagining movement of the TM region did not increase pain for patients with TMD, conversely to a previous report that found a transient increase in pain (lasting 1 h) after MI practice; 38 this suggests the safety of using explicit MI in these individuals. (ii) Patients with TMD showed similar VMI ability but significantly lower KMI ability as compared to healthy controls, and only patients with TMD exhibited a VMI dominance with significantly lower KMI ability. This is consistent with a previous report of individual differences in MI experience, 39 and suggests that one can use at least VMI of the TM region during the rehabilitation of patients with TMD. (iii) Although TMD could be responsible for a decrease in KMI ability, there is evidence indicating that KMI vividness could be improved both during and after MI practice for individuals with tetraplegia and stroke. 40 , 41 Therefore, one can also use KMI in patients with TMD expecting KMI improvement in response to its practice. (iv) Regarding the administration of the TMIQ, one should start with VMI and continue with KMI as it has been described in this study, and as it has been previously recommended for the KVIQ‐10. 24 (v) In absence of causal relationship between the ability to open the mouth and MI ability evidenced in the present study and in line with recent recommendations of using MI as a brain training technique during TMD rehabilitation, 5 one would expect an improvement TM joint active range of motion (i.e. therefore restauration of normal mouth opening) and decrease in pain that is consistent with previous evidence in chronic musculoskeletal pain conditions. 11 , 42
Among the limitations of the present study, there is a possible risk of evaluation bias that can be considered as low since the examiners independently assessed the groups and used a software for recording the vividness scores, thus preventing any further manipulation of the data. In addition, a significantly greater test–retest interval in the TMD group along with measuring MI ability during rehabilitation (in the TMD group) could be sources of greater variation as compared to the control group. However, the risk of possible confounding effect of concomitant rehabilitation on the motor ability of patients with TMD appears limited since practice is more likely to be insufficient to elicit change. For example, a total of 15 motor imagery sessions scheduled over 5 weeks and representing 675 minutes of practice has been documented to improve MI ability in patient with tetraplegia. 40 Finally, exploratory analyses evidenced no effect of the DC/TMD classification (for pain and intra‐articular joint disorders), which suggests that the results may be generalisable to the population of patients with TMD. Nevertheless, future studies should confirm these results and investigate the effect of higher level of pain (>1/10) on explicit MI ability in patients with TMD since a previous study that investigated a laterality judgement task of rotated image of the hand, foot or head found that patients with TMD and pain (mean 3.9/10) had a decreased implicit MI ability as compared to healthy participants. 43
5. CONCLUSIONS
TMIQ is a valid and reproducible motor imagery questionnaire showing excellent internal consistency. Given its good psychometric properties, the TMIQ can be used to specifically assess imagined movements of the TM region in healthy individuals and patients with TMD.
6. LIST OF ABBREVIATION
TMIQ—Tongue and Mouth Imagery Questionnaire; TMD—temporomandibular disorders; KVIQ‐10—Kinaesthetic and Visual Imagery Questionnaire, 10 items; MI—motor imagery; VMI—visual MI; KMI—kinesthetic MI; ICC—intraclass correlation coefficient; DC/TMD—diagnostic criteria for the temporomandibular disorders.
7. REGISTRATION
The study was registered on ClinicalTrial.gov with identification number NCT04102306.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
All authors listed in the manuscript meet all the criteria stated by the International Committee of Medical Journal Editors Recommendations for the conduct, reporting, editing and the publication of Scholarly Work in Medical Journals (ICMJE Recommendations 2018).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/joor.13309.
Supporting information
Fig S1
Fig S2
Table S1
Alvarado C, Arminjon A, Damieux‐Verdeaux C, Lhotte C, Condemine C, Mateo S. Tongue and mouth imagery questionnaire (TMIQ) for assessing motor imagery vividness of the temporomandibular region: A reliability and validity case‐control study. J Oral Rehabil. 2022;49:381–390. doi: 10.1111/joor.13309
Funding information
This study did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article and its supplementary information files.
REFERENCES
- 1. Poveda Roda R, Díaz Fernández JM, Hernández Bazán S, Jiménez Soriano Y, Margaix M, Sarrión G. A review of temporomandibular joint disease (TMJD). Part II: Clinical and radiological semiology. Morbidity processes. Med Oral Patol Oral Cir Bucal. 13(2):E102‐E109. [PubMed] [Google Scholar]
- 2. Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache. 2014;28(1):6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. LeResche L. Epidemiology of Temporomandibular Disorders: Implications for the Investigation of Etiologic Factors. Crit Rev Oral Biol Med. 1997;8(3):291–305. doi: 10.1177/10454411970080030401 [DOI] [PubMed] [Google Scholar]
- 4. Gil‐Martinez A, Paris‐Alemany A, López‐de‐Uralde‐Villanueva I, La Touche R. Management of pain in patients with temporomandibular disorder (TMD): challenges and solutions. J Pain Res. 2018;11:571–587. doi: 10.2147/JPR.S127950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Piekartz H, Paris‐Alemany A. Assessment and Brain Training of Patients Experiencing Head and Facial Pain with a Distortion of Orofacial Somatorepresentation: A Narrative Review. Appl Sci. 2021;11(15):6857. doi: 10.3390/app11156857 [DOI] [Google Scholar]
- 6. Lin CS. Brain signature of chronic orofacial pain: a systematic review and meta‐analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PLoS One. 2014;9(4):e94300. doi: 10.1371/journal.pone.0094300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez‐de‐las‐Penas C, Svensson P. Myofascial Temporomandibular Disorder. Curr Rheumatol Rev. 2016;12(1):40–54. doi: 10.2174/1573397112666151231110947 [DOI] [PubMed] [Google Scholar]
- 8. Ettlin DA, Napimoga MH, Meira e Cruz M, Clemente‐Napimoga JT. Orofacial musculoskeletal pain: An evidence‐based bio‐psycho‐social matrix model. Neurosci Biobehav Rev. 2021;8:12–20. doi: 10.1016/j.neubiorev.2021.06.008 [DOI] [PubMed] [Google Scholar]
- 9. Dickerson SM, Weaver JM, Boyson AN, et al. The effectiveness of exercise therapy for temporomandibular dysfunction: a systematic review and meta‐analysis. Clin Rehabil. 2017;31(8):1039–1048. doi: 10.1177/0269215516672275 [DOI] [PubMed] [Google Scholar]
- 10. Decety J, Grèzes J. Neural mechanisms subserving the perception of human actions. Trends Cogn Sci. 1999;3(5):172–178. doi: 10.1016/S1364-6613(99)01312-1 [DOI] [PubMed] [Google Scholar]
- 11. Yap BWD, Lim ECW. The Effects of Motor Imagery on Pain and Range of Motion in Musculoskeletal Disorders: A Systematic Review Using Meta‐Analysis. Clin J Pain. 2019;35(1):87–99. doi: 10.1097/AJP.0000000000000648 [DOI] [PubMed] [Google Scholar]
- 12. Yue G, Cole KJ. Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol. 1992;67(5):1114–1123. [DOI] [PubMed] [Google Scholar]
- 13. Slimani M, Tod D, Chaabene H, Miarka B, Chamari K. Effects of Mental Imagery on Muscular Strength in Healthy and Patient Participants: A Systematic Review. J Sci Med Sport. 2016;15(3):434–450. [PMC free article] [PubMed] [Google Scholar]
- 14. Driskell JE, Copper C, Moran A. Does mental practice enhance performance? J Appl Psychol. 1994;79(4):481–492. doi: 10.1037/0021-9010.79.4.481 [DOI] [Google Scholar]
- 15. Feltz DL, Landers DM. The effects of mental practice on motor skill learning and performance: A meta‐analysis. J Sport Psychol. 1983;5(1):25–57. [Google Scholar]
- 16. Guerra ZF, Lucchetti ALG, Lucchetti G. Motor Imagery Training After Stroke: A Systematic Review and Meta‐analysis of Randomized Controlled Trials. J Neurol Phys Ther. 2017;41(4):205–214. doi: 10.1097/NPT.0000000000000200 [DOI] [PubMed] [Google Scholar]
- 17. Mateo S, Di Rienzo F, Bergeron V, Guillot A, Collet C. Rode G. neurobiofeedback. Front Behav Neurosci. 2015;9:1–12. doi: 10.3389/fnbeh.2015.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mateo S, Di Rienzo F, Reilly KT, et al. Improvement of grasping after motor imagery in C6–C7 tetraplegia: a kinematic and MEG pilot study. Restor Neurol Neurosci. 2015;33(4):543–555. doi: 10.3233/RNN-140466 [DOI] [PubMed] [Google Scholar]
- 19. Hanakawa T, Dimyan MA, Hallett M. Motor Planning, Imagery, and Execution in the Distributed Motor Network: A Time‐Course Study with Functional MRI. Cereb Cortex. 2008;18(12):2775–2788. doi: 10.1093/cercor/bhn036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin KA, Moritz SE, Hall CR. Imagery use in sport: A literature review and applied model. Sport Psychol. 1999;13(3):245–268. [Google Scholar]
- 21. Roure R, Collet C, Deschaumes‐Molinaro C, Delhomme G, Dittmar A, Vernet‐Maury E. Imagery quality estimated by autonomic response is correlated to sporting performance enhancement. Physiol Behav. 1999;66(1):63–72. [DOI] [PubMed] [Google Scholar]
- 22. Lequerica A, Rapport L, Axelrod BN, Telmet K, Whitman RD. Subjective and Objective Assessment Methods of Mental Imagery Control: Construct Validations of Self‐Report Measures. J Clin Exp Neuropsyc. 2002;24(8):1103–1116. doi: 10.1076/jcen.24.8.1103.8370 [DOI] [PubMed] [Google Scholar]
- 23. Roberts R, Callow N, Hardy L, Markland D, Bringer J. Movement imagery ability: development and assessment of a revised version of the vividness of movement imagery questionnaire. J Sport Exerc Psychol. 2008;30(2):200–221. [DOI] [PubMed] [Google Scholar]
- 24. Malouin F, Richards CL, Jackson PL, Lafleur MF, Durand A, Doyon J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for Assessing Motor Imagery in Persons with Physical Disabilities: A Reliability and Construct Validity Study. J Neurol Phys Ther. 2007;31(1):20–29. doi: 10.1097/01.NPT.0000260567.24122.64 [DOI] [PubMed] [Google Scholar]
- 25. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marchesan IQ. Lingual frenulum: quantitative evaluation proposal. Int J Orofacial Myology. 2005;31(1):39–48. [PubMed] [Google Scholar]
- 27. Jouannaud B, Bregeon F, Tardieu C, Tardieu G. Development of imitative lingual praxis in normal children. Application to their evaluation in language disorders of cerebral palsy. Rev Neuropsychiatr Infant. 1972;20(8):673–680. [PubMed] [Google Scholar]
- 28. Gregg M, Hall C, Butler A. The MIQ‐RS: A Suitable Option for Examining Movement Imagery Ability. Evid Based Complement Alternat Med. 2010;7(2):249–257. doi: 10.1093/ecam/nem170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathôt S, Schreij D, Theeuwes J. OpenSesame: An open‐source, graphical experiment builder for the social sciences. Behav Res. 2012;44(2):314–324. doi: 10.3758/s13428-011-0168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Best N, Best S, Loudovici‐Krug D, Smolenski UC. Measurement of Mandible Movements Using a Vernier Caliper – An Evaluation of the Intrasession‐, Intersession‐ and Interobserver Reliability. CRANIO®. 2013;31(3):176–180. doi: 10.1179/crn.2013.028 [DOI] [PubMed] [Google Scholar]
- 31. Cohen J. Statistical Power Analysis for the Behavioral Sciences . 2nd ed. L. Erlbaum Associates; 1988. [Google Scholar]
- 32. R Core Team. R . A Language and Environment for Statistical Computing. R Foundation for Statistical. Computing. 2013; http://www.R‐project.org/ [Google Scholar]
- 33. Shrout PE, Fleiss JL. Intraclass Correlations : Uses in Assessing Rater Reliability. Psychol Bull. 1979;86(2):420–427. [DOI] [PubMed] [Google Scholar]
- 34. Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Pearson/Prentice Hall. 2009. . 892. [Google Scholar]
- 35. Cureton EE. Corrected item‐test correlations. Psychometrika. 1966;31(1):93–96. doi: 10.1007/BF02289461 [DOI] [Google Scholar]
- 36. Field AP, Miles J, Field Z. Discovering Statistics Using R. Sage. 2012. [Google Scholar]
- 37. Costello AB, Osborne J. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Exploratory Factor Analysis. 2005;10(7):10. [Google Scholar]
- 38. Moseley GL, Zalucki N, Birklein F, Marinus J, van Hilten JJ, Luomajoki H. Thinking about movement hurts: The effect of motor imagery on pain and swelling in people with chronic arm pain. Arthritis Rheum. 2008;59(5):623–631. doi: 10.1002/art.23580 [DOI] [PubMed] [Google Scholar]
- 39. Isaac AR, Marks DF. Individual differences in mental imagery experience: developmental changes and specialization. Br J Psychol. 1994;85(4):479–500. [DOI] [PubMed] [Google Scholar]
- 40. Mateo S, Reilly KT, Collet C, Rode G. Descriptive pilot study of vividness and temporal equivalence during motor imagery training after quadriplegia. Ann Phys Rehabil Med. 2018;61(5):300–308. [DOI] [PubMed] [Google Scholar]
- 41. Leifert‐Fiebach G, Welfringer A, Babinsky R, Brandt T. Motor imagery training in patients with chronic neglect: a pilot study. NeuroRehabilitation. 2013;32(1):43–58. doi: 10.3233/NRE-130822 [DOI] [PubMed] [Google Scholar]
- 42. Suso‐Martí L, La Touche R, Angulo‐Díaz‐Parreño S, Cuenca‐Martínez F. Effectiveness of motor imagery and action observation training on musculoskeletal pain intensity: A systematic review and meta‐analysis. Eur J Pain. 2020;24(5):886–901. doi: 10.1002/ejp.1540 [DOI] [PubMed] [Google Scholar]
- 43. Uritani D, Nishida T, Sakaguchi N, Kawakami T, Jones LE, Kirita T. Difference in Response to a Motor Imagery Task: A Comparison between Individuals with and without Painful Temporomandibular Disorders. Pain Res Manag. 2018;2018:1–8. doi: 10.1155/2018/6810412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.


