Abstract
Aims
Anaemia is frequent among patients with heart failure (HF) and reduced ejection fraction (HFrEF) and is associated with poor outcomes. Sodium–glucose co‐transporter 2 inhibitors (SGLT2i) increase haematocrit and may correct anaemia. This study aims to investigate the impact of empagliflozin on haematocrit and anaemia, and whether anaemia influenced the effect of empagliflozin in EMPEROR‐Reduced.
Methods and results
Mixed‐effects models and survival analysis. A total of 3726 patients (out of 3730) had baseline haematocrit values, 3013 (81%) had no anaemia and 713 (19%) had anaemia. Patients with anaemia were older (70.4 vs. 66.0 years), had lower body mass index (26.6 vs. 28.2 kg/m2), lower estimated glomerular filtration rate (54.2 vs. 63.9 ml/min/1.73 m2), and higher N‐terminal pro‐B‐type natriuretic peptide (2362 vs. 1800 pg/ml). Compared to patients without anaemia, those with anaemia had 1.5 to 2.5‐fold higher rates of cardiovascular and all‐cause mortality, total HF hospitalizations, and kidney composite outcomes. The effect of empagliflozin to reduce the primary composite outcome of cardiovascular death or HF hospitalizations, total HF hospitalizations, and kidney composite outcome was not modified by baseline anaemia status (interaction p > 0.1 for all). Compared to placebo, empagliflozin rapidly (as early as week 4) increased haematocrit and haemoglobin and reduced the rates of new‐onset anaemia throughout the follow‐up (22.6% in placebo vs. 12.3% in empagliflozin; hazard ratio 0.49, 95% confidence interval 0.41–0.59; p < 0.001).
Conclusions
Anaemia was associated with poor outcomes. Empagliflozin reduced new‐onset anaemia throughout the follow‐up and improved HF and kidney outcomes irrespective of anaemia status at baseline.
Keywords: Empagliflozin, Anaemia, Haematocrit, Treatment effect
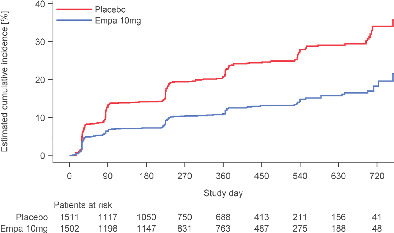
Effect of empagliflozin on new‐onset anaemia. Among patients without anaemia at baseline (1511 in the placebo group and 1502 in the empagliflozin group), the number patients with new‐onset anaemia was 341 (22.6%) in the placebo group and 184 (12.3%) in the empagliflozin group, corresponding to a hazard ratio of 0.49 (95% confidence interval 0.41–0.59; p < 0.001).
Introduction
Anaemia is a frequent co‐morbid condition among patients with heart failure (HF) and reduced ejection fraction (HFrEF). 1 Anaemia may have multiple aetiologies; however, in patients with HFrEF, anaemia is usually hypoproliferative caused by deficient iron utilization or low iron stores, vitamin B12 or folate deficiency, chronic inflammation, kidney disease, or conditions affecting the bone marrow. 2 Regardless of the underlying aetiology, anaemia is associated with low exercise capacity, impaired quality of life and a poor prognosis, including a higher risk of HF, kidney and fatal events. 3
Among HFrEF patients with iron deficiency or iron‐deficient anaemia, intravenous ferric carboxymaltose (compared to placebo) improved symptoms 4 , 5 , 6 and reduced HF rehospitalizations in patients with acute HF. 7 However, darbepoetin alfa, an erythropoiesis‐stimulating agent, increased haematocrit/haemoglobin and reduced the proportion of patients with anaemia compared to placebo, but did not improve outcomes in patients with HFrEF and anaemia, and increased the risk of thromboembolic adverse events. 8 These findings suggest that correcting anaemia is not sufficient by itself and the means by which anaemia is corrected may be more important.
Sodium–glucose co‐transporter 2 inhibitors (SGLT2i) increase haematocrit, 9 , 10 , 11 , 12 and ‘statistical mediation’ studies suggest that the rise in haematocrit may be relevant for the effect of SGLT2i. 13 However, the mechanisms by which SGLT2i increase haematocrit are not fully established.
In patients with chronic HFrEF enrolled in the Dapagliflozin and Prevention of Adverse outcomes in Heart Failure (DAPA‐HF) trial, 12 dapagliflozin corrected anaemia more frequently than placebo among patients with aanemia at baseline, and improved outcomes regardless of the anaemia status at baseline. 14
In patients with chronic HFrEF enrolled in the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR‐Reduced), 11 this study aims to compare the characteristics of the patients with and without anaemia at baseline, to investigate the impact of empagliflozin on haematocrit/haemoglobin and new‐onset anaemia throughout the follow‐up, and to assess whether the effect of empagliflozin on a broad range of outcomes was consistent irrespective of the anaemia status at baseline.
Methods
The EMPEROR‐Reduced trial was a randomized, double‐blind, parallel‐group, placebo‐controlled and event‐driven study, whose design has been described previously. 11 Participants were men or women with chronic HF (New York Heart Association functional class II, III or IV) with a left ventricular ejection fraction ≤40%, who were receiving appropriate background treatment for HF. We preferentially enrolled patients with an ejection fraction ≤30% by requiring those with a higher ejection fraction to have been hospitalized for HF within 12 months or to have markedly increased levels of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), i.e. ≥1000 pg/ml or ≥2500 pg/ml in those with an ejection fraction of 31%–35% or 36%–40%, respectively; these thresholds were doubled in patients with atrial fibrillation. Patients with an haemoglobin <9 g/dl at screening were excluded from the trial. The Ethics Committee of each of the 520 sites in 20 countries approved the protocol and all patients gave written informed consent.
Randomization
Patients were randomized double‐blind (in a 1:1 ratio) to receive placebo or empagliflozin 10 mg daily in addition to their usual therapy. Patients were periodically assessed at study visits for major outcomes, symptoms and functional capacity, vital signs, laboratory analysis (including haematocrit/haemoglobin) and adverse events. All randomized patients were followed for the occurrence of pre‐specified outcomes for the entire duration of the trial, regardless of whether the study participants were taking their study medications or adhered to the schedule of study visits. The median follow‐up time was 16 months.
Trial endpoints and definition of anaemia and erythrocytosis
The primary endpoint was the composite of adjudicated cardiovascular death or hospitalization for HF, analysed as time to first event. The occurrence of all adjudicated hospitalizations for HF (including first and recurrent events), and a composite of serious adverse renal events, defined by the need for chronic dialysis or renal transplant or a sustained ≥40% drop in estimated glomerular filtration rate (eGFR) or a sustained eGFR <15 ml/min/1.73 m2 (if baseline eGFR was ≥30 ml/min/1.73 m2) or < 10 ml/min/1.73 m2 (if baseline eGFR was <30 ml/min/1.73 m2) were also analysed. Additional analyses included cardiovascular and all‐cause mortality, and health status as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ).
Haematocrit and haemoglobin were measured at baseline, week 4, 12, 32, 52, 76, 100, and 124, or study end. Anaemia was defined by the presence of an haematocrit <39% in men or <36% in women. In addition, anaemia was also defined using sex‐specific baseline haemoglobin thresholds (men <13 g/dl and women <12 g/dl). Erythrocytosis was defined by an haematocrit >52% in men and >48% in women. 15
Statistical analysis
Baseline characteristics were compared between patients with and without anaemia at baseline using Chi‐squared test for categorical variables and t‐test for continuous variables. For time‐to‐first‐event analyses, differences between the placebo and empagliflozin groups were assessed using a Cox proportional hazards model, with pre‐specified baseline covariates of age, gender, geographical region, diabetes, left ventricular ejection fraction, and eGFR. For the analysis of total (first and recurrent) events, the differences between the placebo and empagliflozin groups were assessed using a joint frailty model, with cardiovascular death as competing risk. For the analysis of changes in KCCQ, haematocrit and haemoglobin over time, treatment effects were assessed based on changes from baseline using a mixed model for repeated measures. For the analysis of change from baseline to last value on treatment and 1‐month off treatment phase were assessed using an analysis of covariance model. The association of anaemia with subsequent outcomes was assessed in the placebo group using both the baseline and time‐updated anaemia status. The association of haematocrit as a continuous covariate and subsequent outcomes was also tested in the placebo group. The new initiation of iron supplements, erythropoietic therapy, and blood transfusions was retrieved from concomitant therapies. All analyses used the same covariates as in the Cox model and included the baseline variable as an additional covariate, where applicable. For all efficacy measures, separate analyses were performed according to the presence or absence of anaemia at baseline, and differences in the effect of empagliflozin were assessed by interaction terms. P‐values and 95% confidence intervals (CI) presented in this report have not been adjusted for multiplicity. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of patients with and without anaemia
A total of 3726 patients (out of 3730) had baseline haematocrit values, 3013 (81%) had no anaemia and 713 (19%) had anaemia. Compared to patients without anaemia, those with anaemia were older (70.4 vs. 66.0 years), had lower body mass index (26.6 vs. 28.2 kg/m2), lower eGFR (54.2 vs. 63.9 ml/min/1.73 m2), and higher NT‐proBNP (2362 vs. 1800 pg/ml) and left ventricular ejection fraction (28.5% vs. 27.2%). History of diabetes, ischaemic heart disease, coronary interventions and use of antiplatelet agents was more frequent among patients with anaemia (Table 1 ).
Table 1.
Baseline characteristics of the patients by anaemia status (n = 3726)
| Characteristics | No anaemia (n = 3013) | Anaemia a (n = 713) | p‐value |
|---|---|---|---|
| Age, years | 66.0 ± 11.0 | 70.4 ± 10.5 | <0.001 |
| Male sex, n (%) | 2271 (75.4) | 562 (78.8) | 0.052 |
| Region | <0.001 | ||
| North America | 316 (10.5) | 109 (15.3) | |
| Latin America | 1097 (36.4) | 187 (26.2) | |
| Europe | 1100 (36.5) | 251 (35.2) | |
| Asia | 381 (12.6) | 112 (15.7) | |
| Other | 119 (3.9) | 54 (7.6) | |
| BMI, kg/m2 | 28.2 ± 5.4 | 26.6 ± 5.2 | <0.001 |
| LVEF, % | 27.2 ± 6.0 | 28.5 ± 6.0 | <0.001 |
| NT‐proBNP, pg/ml | 1800 [1070–3233] | 2362 [1438–4482] | <0.001 |
| Heart rate, bpm | 71.3 ± 11.7 | 71.1 ± 11.8 | 0.69 |
| SBP, mmHg | 122.2 ± 15.6 | 121.3 ± 15.7 | 0.18 |
| eGFR, ml/min/1.73 m2 | 63.9 ± 21.3 | 54.2 ± 21.2 | <0.001 |
| Haemoglobin, g/dl | 14.2 ± 1.3 | 11.5 ± 0.9 | <0.001 |
| Haematocrit, % | 43.8 ± 3.9 | 35.4 ± 2.6 | <0.001 |
| NYHA class III/IV, n (%) | 743 (24.7) | 185 (25.9) | 0.65 |
| KCCQ‐TSS, points | 74.6 ± 22.4 | 73.6 ± 23.6 | 0.27 |
| HF duration, years | 6.1 ± 6.2 | 6.3 ± 6.7 | 0.57 |
| Ischaemic HF, n (%) | 1509 (50.1) | 419 (58.8) | <0.001 |
| History of AF/flutter, n (%) | 1152 (38.2) | 288 (40.4) | 0.32 |
| History of diabetes, n (%) | 1436 (47.7) | 417 (58.5) | <0.001 |
| History of hypertension, n (%) | 2163 (71.8) | 532 (74.6) | 0.13 |
| PCI/CABG, n (%) | 1191 (39.5) | 331 (46.4) | 0.001 |
| HHF within 12 months, n (%) | 903 (30.0) | 247 (34.6) | 0.015 |
| ACEi/ARB/ARNi, n (%) | 2685 (89.1) | 605 (84.9) | 0.002 |
| Beta‐blocker, n (%) | 2866 (95.1) | 664 (93.1) | 0.032 |
| MRA, n (%) | 2185 (72.5) | 472 (66.2) | 0.001 |
| Loop diuretic, n. (%) | 2518 (83.6) | 629 (88.2) | 0.002 |
| ICD/CRT, n (%) | 943 (31.3) | 227 (31.8) | 0.78 |
| Antiplatelet agents, n (%) | 1553 (51.5) | 438 (61.4) | <0.001 |
| Anticoagulants, n (%) | 1185 (39.3) | 273 (38.3) | 0.61 |
Values are given as mean ± standard deviation or median [25th–75th percentile] unless otherwise indicated.
ACEi, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; CABG, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; HHF, hospitalization for heart failure; ICD, implantable cardioverter‐defibrillator; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
Anaemia defined by haematocrit <39% in men or <39% in women.
Association of anaemia with subsequent outcomes
Compared to patients without anaemia, the presence of anaemia (either defined by haematocrit or haemoglobin) was associated with a higher rate of cardiovascular and all‐cause mortality, total HF hospitalizations, and kidney composite outcomes (online supplementary Tables S1 and S2 ).
Effect of empagliflozin versus placebo on outcomes by baseline anaemia status
The effect of empagliflozin to reduce the primary composite outcome of cardiovascular death or HF hospitalizations, total HF hospitalizations, and the kidney composite outcome, as well as health‐related quality of life improvement, was not modified by baseline anaemia status (interaction p > 0.1 for all). The effect of fatal events was not modified by anaemia status either (interaction p > 0.1 for both cardiovascular and all‐cause mortality) (Table 2 for anaemia using haematocrit definition, and online supplementary Table S3 for anaemia using haemoglobin definition).
Table 2.
Effect of empagliflozin versus placebo on outcomes by baseline anaemia status (using haematocrit a )
| Outcome | Placebo | Empagliflozin | HR (95% CI) | Interaction p‐value | ||
|---|---|---|---|---|---|---|
| Events, n/N (%) | Event rate, per 100 py | Events, n/N (%) | Event rate, per 100 py | |||
| CV death or HFH | 0.59 | |||||
| No anaemia | 355/1511 (23.5) | 19.8 | 273/1502 (18.2) | 14.7 | 0.74 (0.63–0.86) | |
| Anaemia | 106/355 (29.9) | 26.3 | 88/358 (24.6) | 20.5 | 0.81 (0.61–1.07) | |
| Total HFH b | 0.93 | |||||
| No anaemia | 418 | – | 295 | – | 0.71 (0.57–0.88) | |
| Anaemia | 135 | – | 93 | – | 0.69 (0.45–1.05) | |
| Kidney composite c | 0.18 | |||||
| No anaemia | 36/1511 (2.4) | 2.4 | 23/1502 (1.5) | 1.5 | 0.62 (0.36–1.04) | |
| Anaemia | 22/355 (6.2) | 6.2 | 7/358 (2.0) | 1.9 | 0.31 (0.13–0.73) | |
| KCCQ‐TSS change week 52 d | 0.94 | |||||
| No anaemia | 5.2 (0.5) | – | 6.9 (0.5) | – | 1.7 (0.7) | |
| Anaemia | 5.0 (1.1) | – | 6.6 (1.1) | – | 1.6 (1.5) | |
| CV death | 0.96 | |||||
| No anaemia | 154/1511 (10.2) | 7.7 | 143/1502 (9.5) | 7.2 | 0.93 (0.74–1.17) | |
| Anaemia | 47/355 (13.2) | 10.0 | 44/358 (12.3) | 9.3 | 0.92 (0.61–1.39) | |
| All‐cause death | 0.48 | |||||
| No anaemia | 196/1511 (13.0) | 9.7 | 190/1502 (12.6) | 9.5 | 0.96 (0.79–1.17) | |
| Anaemia | 69/355 (19.4) | 14.7 | 59/358 (16.5) | 12.4 | 0.83 (0.59–1.18) | |
CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HFH, hospitalization for heart failure; HR, hazard ratio; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; py, patient‐years.
Anaemia defined by haematocrit <39% in men or <36% in women.
Total number of events, i.e. first and recurrent heart failure hospitalizations analysed by a joint frailty model.
Composite of sustained worsening kidney function ≥40% from baseline (sustained means in two consecutive visits separated by a minimum of 30 days), or end‐stage kidney disease (renal transplant, sustained eGFR <15 ml/min/1.73 m2 for patients with baseline eGFR ≥30 ml/min/1.73 m2 or eGFR <10 ml/min/1.73 m2 for patients with baseline eGFR <30 ml/min/1.73 m2 or chronic dialysis).
Mixed model for repeated measures with results presented as adjusted mean (standard error) for the change in KCCQ‐TSS points from baseline to week 52.
Effect of empagliflozin on haematocrit and haemoglobin over time
Compared to placebo, empagliflozin rapidly (detected at the first visit, i.e. week 4, but could be present earlier) increased haematocrit and haemoglobin. In patients with anaemia at baseline, the differences between empagliflozin and placebo in haematocrit and hemoglobin were 1.12% and 0.35 g/dl at week 4, and 1.64% and 0.69 g/dl at week 12, respectively, a difference that persisted throughout the trial (e.g. week 52 difference = 1.94% and 0.74 g/dl) (p < 0.05 at all time‐points). The effect size was similar in patients without anaemia (Figure 1 ).
Figure 1.
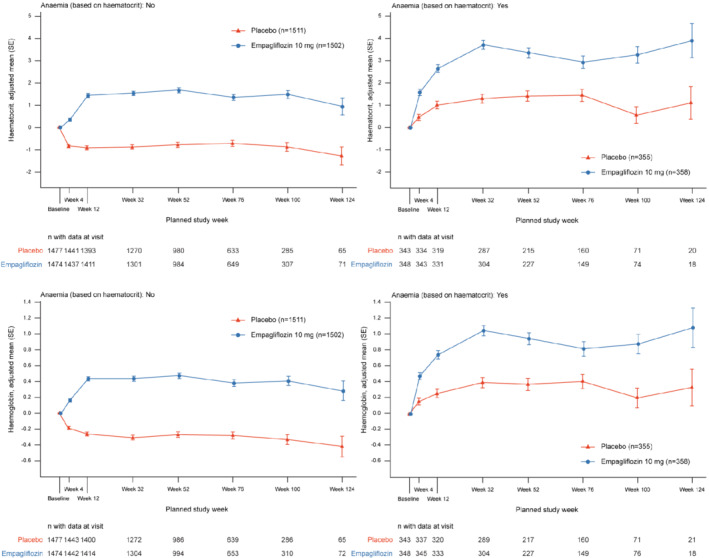
Change in haematocrit over time by anaemia status. Compared to placebo, empagliflozin increased haematocrit and haemoglobin at all time‐points in patients with and without anaemia at baseline. SE, standard error.
Effect of empagliflozin on anaemia and erythrocytosis over time
Compared to placebo, empagliflozin reduced the proportion of patients with anaemia throughout the follow‐up, with a difference of 4.9% at week 4, 7.0% at week 12, and 8.5% at week 52 (p < 0.001 at all time‐points) and of similar magnitude irrespective of anaemia definition, i.e. by haematocrit or haemoglobin (Figure 2 ). Among patients without anaemia at baseline (1511 in the placebo group and 1502 in the empagliflozin group), the number of patients with new‐onset anaemia was 341 (22.6%) in the placebo group and 184 (12.3%) in the empagliflozin group, corresponding to a hazard ratio of 0.49 (95% CI 0.41–0.59; p < 0.001) (Graphical Abstract).
Figure 2.
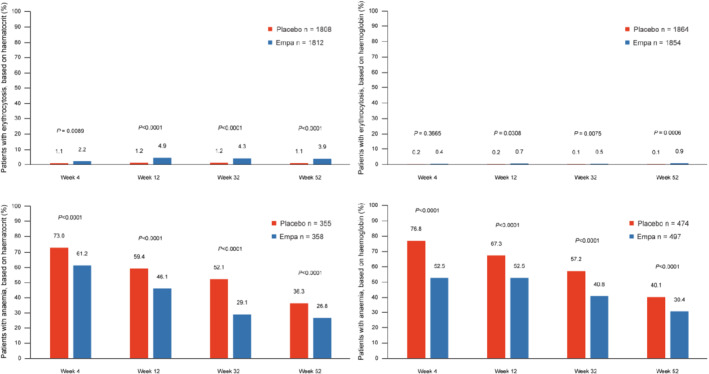
Frequency of patients with anaemia over time by treatment group. Empagliflozin significantly reduced the proportion of patients with anaemia at all time‐points, regardless of anaemia definition.
Compared to placebo, empagliflozin increased the proportion of patients with erythrocytosis throughout the follow‐up, with a difference of 1.2% at week 4, 4.0% at week 12, and 3.2% at week 52 (online supplementary Figure S1 ).
Among the subset of patients in whom haematocrit data at baseline, last value on treatment, and 1‐month after treatment discontinuation were available (n = 948), the effect of empagliflozin to increase haematocrit did not persist 1 month after treatment discontinuation, returning to levels similar to those observed at baseline [empagliflozin group change (standard error) from baseline to last value on‐treatment = +1.9% (0.2), change from last value on treatment to 1 month after treatment discontinuation = −1.5% (0.1), and change from baseline to 1 month after treatment discontinuation = +0.2% (0.2)]. Still, compared with the baseline haematocrit value, at 1 month after treatment discontinuation, haematocrit decreased in the placebo [−0.5% (0.2)] but not in the empagliflozin group [+0.2% (0.2)], with an empagliflozin versus placebo difference at 1 month after treatment discontinuation of +0.8% (0.3) (p < 0.003) (online supplementary Table S4 ).
Effect of empagliflozin on iron supplements, erythropoietic therapy and blood transfusions
Throughout the follow‐up, 106 out of 1794 (5.9%) patients required iron supplementation, erythropoietic therapy or blood transfusions in the placebo group compared with 89 out of 1798 (4.9%) patients in the empagliflozin group, corresponding to a HR of 0.83 (95% CI 0.62–1.10; p = 0.19), without effect differences between patients with and without anaemia at baseline (interaction p = 0.38).
Correlation of changes in haematocrit with other clinical variables
Changes in haematocrit from baseline to week 4 and week 52 among patients taking empagliflozin were not correlated with changes in body weight, NT‐proBNP (log‐transformed), systolic blood pressure or eGFR in patients with empagliflozin (week 4 Rho = −0.17, −0.11, 0.03, and −0.12, respectively; and week 52 Rho = 0.03, −0.06, 0.11, and −0.06, respectively).
Discussion
This study confirms that anaemia is frequent and associated with poor outcomes in patients with HFrEF. The effect of empagliflozin to reduce the composite of HF hospitalizations or cardiovascular death and kidney composite events was not affected by the presence or absence of anaemia. Consistently with the findings of DAPA‐HF, empagliflozin increased haematocrit/haemoglobin and reduced the proportion of patients with anaemia throughout the follow‐up in patients with HFrEF.
In EMPEROR‐Reduced, anaemia was present in almost 1 of 5 patients at baseline. Compared to patients without anaemia, those with anaemia were older, had poorer renal function, lower body mass index, more frequent coronary interventions with use of antiplatelet agents, and higher NT‐proBNP levels. Not surprisingly and as noted by others, 1 , 3 patients with anaemia had a worse prognosis. 16 Compared to placebo, empagliflozin reduced the proportion of patients who had anaemia at baseline by 8.5% at 1 year, decreasing the proportion of patients with anaemia at baseline from nearly 1 in 5 to 1 in 12 at 1 year. These findings were accompanied by a 51% reduction of new‐onset anaemia among patients without anaemia at baseline and a non‐significant 17% reduction in the need for iron supplements, erythropoietic agents or blood transfusions. Together, these findings support a clinically meaningful effect of empagliflozin to correct anaemia.
Empagliflozin, and other SGLT2i, increase erythropoietin levels and promote erythrocytosis through effects that are not fully established and that are likely multifactorial. 17 SGLT2i may cause a rise in haematocrit by reducing oxidative stress in the kidney which may restore erythropoietin production, through activation of nutrient deprivation pathways which may stimulate the production of hypoxia‐inducible factors and erythropoietin gene transcription, or by an enhanced natriuresis which may reduce afferent renal blood flow and stimulate erythropoietin production. 17 , 18 , 19 , 20 The effect of SGLT2i on red blood cell morphology, reduction of ferritin and iron stores suggest that haemoconcentration is not a major driver of haematocrit rise. 17 Such hypothesis is supported by the lack of correlation between the changes in haematocrit and changes in body weight, NT‐proBNP, blood pressure, and eGFR, suggesting that ‘volume contraction’ is not associated with the rise in haematocrit.
When performing ‘statistical mediation analysis’ on the effect of SGLT2i, haematocrit appears as the most important ‘statistical mediator’, 13 which does not mean that SGLT2i act solely via a rise in haematocrit. 20 In fact, in the Reduction of Events by Darbepoetin Alfa in Heart Failure (RED‐HF) trial, 8 darbepoetin alfa, an erythropoiesis‐stimulating agent, increased haemoglobin levels by 1.5 g/dl on average versus placebo, which is a similar difference to that observed with empagliflozin versus placebo in EMPEROR‐Reduced of 0.74 g/dl at 1 year. However, while in EMPEROR‐Reduced empagliflozin significantly improved cardiovascular and renal outcomes, 11 in RED‐HF darbepoetin alfa did not improve cardiovascular outcomes and increased thromboembolic adverse events. 8 Empagliflozin slightly increased the proportion of patients with polycythaemia, but it did not increase thromboembolic events (including stroke). A meta‐analysis of SGLT2i trials involving 75 540 participants did not find differences in the risk of stroke with SGLT2i compared to placebo. 21
The most frequently found form of anaemia in patients with chronic HFrEF is ‘chronic disease anaemia’, which is often concomitant with chronic kidney disease (present in almost 50% of EMPEROR‐Reduced patients) characterized by low‐grade inflammation and an incapacity to adequately use iron stores, i.e. relative iron deficiency. 22 , 23 Beyond the stimulation of erythropoiesis, it is possible that the reduction of inflammation with SGLT2i may help improving the utilization of iron stores. 24 , 25 , 26 Nonetheless, studies on the effect of SGLT2i on iron metabolism are warranted.
Iron‐deficient anaemia can also be present in some HFrEF patients, particularly those using antiplatelet agents, who are more prone to asymptomatic blood loss. In such cases of absolute iron deficiency, SGLT2i may be less effective in rising haematocrit and correcting anaemia, which may explain why some patients did not have their anaemia corrected with SGLT2i treatment. In any case, a thorough investigation of anaemia and adequate iron supplementation are essential for the optimal treatment of HF patients, 2 and empagliflozin improved clinical outcomes irrespective of anaemia at baseline.
Limitations
This is a secondary analysis of a randomized controlled trial without control for multiplicity of tests given the exploratory nature of this work; thus, some of the findings might have occurred by chance. However, the consistency of the effect of empagliflozin on haematocrit, haemoglobin and anaemia, and the extensive replication of similar findings with other SGLT2i in multiple populations, provide robustness to our data. We do not have data on parameters of iron metabolism nor erythropoietin levels; therefore, we cannot display in more detail the mechanisms by which empagliflozin increased haematocrit.
Conclusions
In EMPEROR‐Reduced, anaemia was associated with poor outcomes. Empagliflozin reduced the proportion of patients with anaemia throughout the follow‐up and improved HF and kidney outcomes irrespective of anaemia status at baseline.
Funding
The EMPEROR‐Reduced trial was funded by Boehringer Ingelheim and Eli Lilly (EMPEROR‐Reduced ClinicalTrials.gov Identifier: NCT03057977).
Conflict of interest: J.P.F. reports consulting fees from Boehringer Ingelheim during the conduct of the study. S.D.A. reports grants from Vifor; personal fees from Vifor, Bayer, Boehringer Ingelheim, Novartis, Servier, Impulse Dynamics, Cardiac Dimensions, and Thermo Fisher Scientific; and grants and personal fees from Abbott Vascular, outside the submitted work. J.B. reports consultancy fees from Boehringer Ingelheim during the conduct of the study; and consultancy fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, BerlinCures, Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V‐Wave, and Vifor, outside the submitted work. G.F. reports receiving payment from Boehringer Ingelheim for being a trial committee member during the conduct of the study and from Medtronic, Vifor, Servier, and Novartis for being a trial committee member outside the submitted work. T.I., A.S., and C.Z. are employees of Boehringer Ingelheim. S.J.P. reports personal fees from Boehringer Ingelheim during the conduct of the study. F.Z. reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from Janssen, Novartis, Boston Scientific, Amgen, CVRx, AstraZeneca, Vifor Fresenius, Cardior, Cereno pharmaceutical, Applied Therapeutics, Merck, Bayer, and Cellprothera outside the submitted work; and other support from CVCT and Cardiorenal, outside the submitted work. M.P. reports personal fees from Boehringer Ingelheim, during the conduct of the study; personal fees from AbbVie, Akcea, Amarin, AstraZeneca, Amgen, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, Lilly, Novartis, Pfizer, Relypsa, Sanofi, Synthetic Biologics, Theravance, and NovoNordisk, outside the submitted work.
Supporting information
Table S1. Event rates per 100 patient‐years by anaemia status (using haematocrit) at baseline and time‐updated in the placebo group.
Table S2. Event rates per 100 patient‐years by anaemia status (using haemoglobin) at baseline and time‐updated in the placebo group.
Table S3. Effect of empagliflozin vs. placebo on outcomes according to baseline anaemia status (using haemoglobin).
Table S4. Comparison of haematocrit last value on treatment with 1‐month after treatment discontinuation.
Figure S1. Frequency of patients with erythrocytosis over time by treatment group.
Data availability statement
Data will be made available upon request in adherence with transparency conventions in medical research and through requests to the corresponding author. The executive committee of EMPEROR has developed a comprehensive analysis plan and numerous pre‐specified analyses, which will be presented in future scientific meetings and publications. At a later time‐point, the full database will be made available in adherence with the transparency policy of the sponsor (available at https://trials.boehringer‐ingelheim.com/transparency_policy.html).
References
- 1. O'Meara E, Clayton T, McEntegart MB, McMurray JJ, Lang CC, Roger SD, et al.; CHARM Committees and Investigators . Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Circulation. 2006;113:986–94. [DOI] [PubMed] [Google Scholar]
- 2. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138:80–98. [DOI] [PubMed] [Google Scholar]
- 3. Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new‐onset heart failure. Circulation. 2003;107:223–5. [DOI] [PubMed] [Google Scholar]
- 4. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al.; FAIR‐HF Trial Investigators . Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–48. [DOI] [PubMed] [Google Scholar]
- 5. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, et al.; EFFECT‐HF Investigators . Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, et al.; CONFIRM‐HF Investigators . Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, et al.; AFFIRM‐AHF Investigators . Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet. 2020;396:1895–904. [DOI] [PubMed] [Google Scholar]
- 8. Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, et al.; RED‐HF Committees ; RED‐HF Investigators . Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–9. [DOI] [PubMed] [Google Scholar]
- 9. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306. [DOI] [PubMed] [Google Scholar]
- 10. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al.; EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 11. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al.; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. [DOI] [PubMed] [Google Scholar]
- 12. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al.; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 13. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care. 2018;41:356–63. [DOI] [PubMed] [Google Scholar]
- 14. Docherty KF, Curtain JP, Anand IS, Bengtsson O, Inzucchi SE, Køber L, et al.; DAPA‐HF Investigators and Committees . Effect of dapagliflozin on anaemia in DAPA‐HF. Eur J Heart Fail. 2021;23:617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McMullin MF. Diagnosis and management of congenital and idiopathic erythrocytosis. Ther Adv Hematol. 2012;3:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anand IS, Kuskowski MA, Rector TS, Florea VG, Glazer RD, Hester A, et al. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val‐HeFT. Circulation. 2005;112:1121–7. [DOI] [PubMed] [Google Scholar]
- 17. Mazer CD, Hare GM, Connelly PW, Gilbert RE, Shehata N, Quan A, et al. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. 2020;141:704–7. [DOI] [PubMed] [Google Scholar]
- 18. Chen R, Xu M, Hogg RT, Li J, Little B, Gerard RD, et al. The acetylase/deacetylase couple CREB‐binding protein/Sirtuin 1 controls hypoxia‐inducible factor 2 signaling. J Biol Chem. 2012;287:30800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eckardt KU, Kurtz A. Regulation of erythropoietin production. Eur J Clin Invest. 2005;35:13–9. [DOI] [PubMed] [Google Scholar]
- 20. Packer M. Cardioprotective effects of Sirtuin‐1 and its downstream effectors: potential role in mediating the heart failure benefits of SGLT2 (sodium‐glucose cotransporter 2) inhibitors. Circ Heart Fail. 2020;13:e007197. [DOI] [PubMed] [Google Scholar]
- 21. Guo M, Ding J, Li J, Wang J, Zhang T, Liu C, et al. SGLT2 inhibitors and risk of stroke in patients with type 2 diabetes: a systematic review and meta‐analysis. Diabetes Obes Metab. 2018;20:1977–82. [DOI] [PubMed] [Google Scholar]
- 22. Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. 2006;113:2454–61. [DOI] [PubMed] [Google Scholar]
- 23. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103:2055–9. [DOI] [PubMed] [Google Scholar]
- 24. Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62:1154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11:2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sukhanov S, Higashi Y, Yoshida T, Mummidi S, Aroor AR, Jeffrey Russell J, et al. The SGLT2 inhibitor empagliflozin attenuates interleukin‐17A‐induced human aortic smooth muscle cell proliferation and migration by targeting TRAF3IP2/ROS/NLRP3/Caspase‐1‐dependent IL‐1β and IL‐18 secretion. Cell Signal. 2021;77:109825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Event rates per 100 patient‐years by anaemia status (using haematocrit) at baseline and time‐updated in the placebo group.
Table S2. Event rates per 100 patient‐years by anaemia status (using haemoglobin) at baseline and time‐updated in the placebo group.
Table S3. Effect of empagliflozin vs. placebo on outcomes according to baseline anaemia status (using haemoglobin).
Table S4. Comparison of haematocrit last value on treatment with 1‐month after treatment discontinuation.
Figure S1. Frequency of patients with erythrocytosis over time by treatment group.
Data Availability Statement
Data will be made available upon request in adherence with transparency conventions in medical research and through requests to the corresponding author. The executive committee of EMPEROR has developed a comprehensive analysis plan and numerous pre‐specified analyses, which will be presented in future scientific meetings and publications. At a later time‐point, the full database will be made available in adherence with the transparency policy of the sponsor (available at https://trials.boehringer‐ingelheim.com/transparency_policy.html).


