Abstract
Marine heatwaves can cause coral bleaching and reduce coral cover on reefs, yet few studies have identified “bright spots,” where corals have recently shown a capacity to survive such pressures. We analyzed 7714 worldwide surveys from 1997 to 2018 along with 14 environmental and temperature metrics in a hierarchical Bayesian model to identify conditions that contribute to present‐day coral cover. We also identified locations with significantly higher (i.e., “bright spots”) and lower coral cover (i.e., “dark spots”) than regionally expected. In addition, using 4‐km downscaled data of Representative Concentration Pathways (RCPs) 4.5 and 8.5, we projected coral cover on reefs for the years 2050 and 2100. Coral cover on modern reefs was positively associated with historically high maximum sea‐surface temperatures (SSTs), and negatively associated with high contemporary SSTs, tropical‐cyclone frequencies, and human‐population densities. By 2100, under RCP8.5, we projected relative decreases in coral cover of >40% on most reefs globally but projected less decline on reefs in Indonesia, Malaysia, the central Philippines, New Caledonia, Fiji, and French Polynesia, which should be focal localities for multinational networks of protected areas.
Keywords: bright spots, climate change, coral cover, coral cover projections, coral ecology, hierarchical Bayesian model
This study identified "bright spots," where corals have recently shown a capacity to survive marine heatwaves. The study also projected future coral cover, globally, based on temperature changes. By 2100, under RCP8.5, the study projected a relative decline in coral cover >40% on most reefs, globally, but less of a decline on reefs in Indonesia, eastern Malaysia, the central Philippines, New Caledonia, Fiji, and French Polynesia. These projections provide information for management decisions at a local level and predict localities that could be considered for multinational, marine‐protected‐area networks.
1. INTRODUCTION
Tropical coral reefs are the world's most diverse marine ecosystems that provide billions of dollars in economic value through coastal protection, food, tourism, and other goods and services (Costanza et al., 2014; Spalding et al., 2017). Yet most coral reefs are increasingly under threat from local and global disturbances (Anthony et al., 2011; Halpern et al., 2008; Hughes et al., 2017; Sheppard et al., 2020). A recent estimate indicates that the capacity of coral reefs to provide ecosystem services has declined by half since the 1950s (Eddy et al., 2021). For example, over the past three decades percent coral cover has declined at three quarters of the coral reefs monitored in the Caribbean (Jackson et al., 2014). Similarly, the Great Barrier Reef (GBR) recently lost 10% of its coral cover from 1985 to 2012 because of coral bleaching alone (De'ath et al., 2012). Other reefs globally have been affected, including reefs in the western Indian Ocean (Obura et al., 2017), and in the northern (Couch et al., 2017) and central Pacific Ocean (Barkley et al., 2018). Globally, coral cover has declined by half since the 1950s, and the associated biodiversity has similarly declined (Eddy et al., 2021). One of the major causes of the global decline in coral cover has been the increasing intensity and frequency of marine heatwaves.
The rate of ocean warming has more than doubled since 1993 and marine heatwaves have intensified and doubled in frequency since 1982 (IPCC, 2019). Marine heatwaves are not, however, uniform across the globe (IPCC, 2013, 2019). For example, a recent study found that the last two decades have been most severe for coral bleaching at mid‐tropical latitudes compared with other latitudes (Sully et al., 2019). Moreover, the ambient conditions and the frequency of sea‐surface temperature (SST) variability can influence the response of corals to marine heatwaves (Safaie et al., 2018; Thompson & van Woesik, 2009). For example, Thompson and van Woesik (2009) showed that during marine heatwaves, corals at sites with a high frequency of historical SST anomalies (SSTAs) experienced less bleaching than corals at sites with a low frequency. Similarly, Guest et al. (2012) and Safaie et al. (2018) found that thermal histories had a significant influence on bleaching responses, and Sully et al. (2019) showed that during marine heatwaves coral bleaching was less common at sites with a high variance in SSTAs. Yet even where corals are exposed to similar SST stresses the responses can vary locally and regionally (McClanahan et al., 2007; McClanahan & Maina, 2003; Safaie et al., 2018; van Woesik et al., 2012). Furthermore, factors other than thermal stress can be associated with coral cover. For example, cyclones can damage reefs, and human activity (Darling et al., 2019; Zinke et al., 2018) and reef management (Donovan et al., 2021) can affect coral cover.
Although marine heatwaves have caused extensive bleaching and coral mortality, some coral populations may be adjusting (Dixon et al., 2015; Kenkel & Matz, 2016; Maynard et al., 2008). For example, Maynard et al. (2008) found that corals on the GBR exhibited higher thermal tolerance in 2002 than in 1997, and Sully et al. (2019) showed that the onset of coral bleaching from 2007 to 2017 had occurred at 0.5°C higher than from 1997 to 2006. Processes that increase the thermal tolerance of corals may range from differential mortality of more susceptible genotypes (Sampayo et al., 2008) to pre‐adaptation (Kenkel & Matz, 2016) and epigenetics (van Oppen et al., 2015). Irrespective of the adjustment process, given the high biodiversity and socio‐economic value of coral reefs, it is critically important to accurately project how corals in different regions of the world will respond to future rates of ocean warming.
Previous studies (Cacciapaglia & van Woesik, 2015; McManus et al., 2020) have predicted considerable spatial variability in changes in coral cover from future ocean warming. For example, McManus et al. (2020) predicted that the Philippines will experience a more severe decline in coral cover in the future than Papua New Guinea and northern Australia. They suggested that these geographical differences will be largely dependent on increases in absolute and inter‐annual variation in SST. An Indo‐Pacific wide projection by Cacciapaglia and van Woesik (2015) predicted twelve potential climate‐change refuges and predicted that the GBR was unlikely to fare well under future ocean warming. Subsequent observations by Hughes et al. (2018) were consistent with this prediction. However, most predictive models have used geographically coarse‐grained outputs from global climate models (GCMs) typically on a scale of approximately 100 km2 (Balaji et al., 2018), thus potentially losing the details of where climate refuges might help protect some coral reefs.
Here, we project future percent coral cover globally for the years 2050 and 2100 by using 4‐km high‐resolution SST rasters downscaled from GCMs (van Hooidonk et al., 2016) for two different scenarios of future greenhouse gas emissions (IPCC, 2019)—Representative Concentration Pathway (RCP) scenarios 4.5 and 8.5. The objectives of this global study were to (1) determine which key environmental variables have been associated with coral cover from 1997 to 2018, (2) identify modern “bright” and “dark” spots of coral cover, and (3) project future “bright” and “dark” spots of coral cover for the years 2050 and 2100 under RCPs 4.5 and 8.5.
2. MATERIALS AND METHODS
2.1. Field data
The coral cover data were collected by scientists and trained and certified citizen‐scientists following a standardized transect protocol of Reef Check (Hodgson, 1999). The Reef Check tropical program was designed carefully and specifically for volunteers in 1995, approved by an expert group of 20 coral‐reef scientists, peer‐reviewed during a multi‐day workshop in 1998, and peer‐reviewed again in 2004. The Reef Check protocol was based on using easy‐to‐identify, proxy indicators of coral‐reef health. Reef Check teams collect multiple types of data including data derived from a quantitative point‐sample survey that estimates the percentage of the benthos covered by different substrate types on four 20‐m sections of a 100‐m transect. Each section is separated by a 5 m gap. Therefore, percent coral cover along at least 80 m of reef was measured for each survey, at each study site.
More than 60% of Reef Check's data were collected by research teams comprised entirely of Masters or Ph.D. level professional marine biologists who are affiliated with research institutes, national survey teams, or marine‐protected‐area teams. Most of the data collectors who are not professional scientists are experienced scuba diving professionals, such as dive masters and dive instructors. Depending on their initial knowledge level, the volunteers undergo a 4‐ to 5‐day training course in both the classroom and field and use a 100‐page instruction manual. All surveyors must pass classroom‐based and field‐based tests including tests of substrate analyses of corals, fishes, invertebrates, and algae. All teams are led by at least one professional marine biologist with a Masters or Ph.D. degree who has taken a Training of Trainers course and who checks the data as they are being collected and reviewed (Hodgson et al., 2006). The Reef Check data have been used in global and regional analyses (Bruno et al., 2009; Done et al., 2017; Donovan et al., 2021; Hodgson, 1999; Sully et al., 2019). The data, collected from 1997 to 2018, comprised more than 5000 sites. The number of sites included in the present study was filtered down to 2949, based on the availability of environmental data from other sources (i.e., turbidity and modern and historical SSTs; Figures S1–S17). Sites were surveyed at irregular time intervals. The numbers of surveys per site ranged from 1 to 37 over the entire 1997 to 2018 timeframe. Mean number of surveys per site was 2.6, and the standard deviation of number of surveys per site was 2.9. It should be noted that the mean percent coral cover in each oceanic region (Table 1) may be somewhat high because divers were instructed to select reefs that appeared to have relatively high coral cover; no surveys were conducted on reefs that had been completely decimated and report zero coral cover. These instructions were uniform for all divers across all regions. Such consistency allows for useful comparisons among regions.
TABLE 1.
Modern percent coral cover metrics for global coral reefs and for each of five oceanic regions from 1997 to 2018
| Oceanic region | Mean coral cover (%) | SD coral cover (%) | n | Reef area (km2) |
|---|---|---|---|---|
| Global | 31.7 | 19.4 | 2949 | 14,3075 |
| Red Sea | 37.6 | 15.0 | 37 | 8,886 |
| Arabian Gulf | 39.6 | 22.9 | 47 | 892 |
| Indian Ocean | 32.6 | 18.0 | 321 | 22,632 |
| Pacific Ocean | 35.6 | 19.6 | 1944 | 97,556 |
| Atlantic Ocean | 17.7 | 11.6 | 600 | 13,109 |
Abbreviations: n, number of sites per oceanic region; sd, standard deviation.
Bold indicates important values.
For each coral cover survey, we used the survey date and the latitude/longitude coordinates to obtain corresponding environmental metrics from other datasets. We used the global Coral Reef Temperature Anomaly Database (CoRTAD Version 6) from the National Oceanic and Atmospheric Administration (NOAA) (https://data.nodc.noaa.gov/cortad/Version6/) to derive SST data for each survey period at each site. All CoRTAD variables were weekly data provided on a grid‐cell basis of approximately 4‐km resolution, which extended from 1982 to 2020 (Table S1), except for mean SST and maximum SST, which we converted to monthly data, so that it was compatible with the temperature projection datasets that had a monthly resolution. We used NASA's (National Aeronautics and Space Administration's) Earth Observing System Data and Information System (EOSDIS) Modis‐Aqua satellite database to derive turbidity data, which has a 4‐km resolution beginning in mid‐2002 through to March 2020 (https://oceandata.sci.gsfc.nasa.gov/MODIS‐Aqua/Mapped/Monthly/4km/Kd_490/) (Figure S16). Turbidity was measured as Kd490 values, which is the diffuse attenuation coefficient of light at the 490 nm wavelength (NASA Goddard Space Flight Center 2010).
Historical SST data were derived from the World Climate Research Program Coupled Model Intercomparison Project Phase 6 (CMIP6) (https://esgf‐node.llnl.gov/projects/cmip6/). We used data with a 1° × 1° monthly resolution, averaged from 20 model outputs that were made available from the Community Earth System Model (CESM; Table S2). From these data, we calculated historical SST mean, historical SST maximum, and historical SST standard deviation from the years 1870 to 1980, which we used as the pre‐global coral‐bleaching years. Henceforth, we refer to both Pacific cyclones and typhoons and Caribbean hurricanes as tropical cyclones. Tropical‐cyclone frequency data were calculated from International Best Track Archive for Climate Stewardship (IBTrACS) (http://www.ncdc.noaa.gov/ibtracs/index.php?name=ibtracs‐data), available at a 9‐km resolution from 1964 to 2014 (Figure S17). The future SST data were from van Hooidonk et al. (2016). RCP4.5 projects emissions to have leveled off by mid‐century, whereas RCP8.5 projects a high‐emission scenario and continued increases in greenhouse gas concentrations. RCPs 4.5 and 8.5 were used because these data have been statistically downscaled to a 4‐km resolution (see van Hooidonk et al., 2016 for methods and models), which is the same resolution as the CoRTAD and turbidity data. Current greenhouse gas emissions continue to grow at a rate consistent with a high emission future (RCP8.5) without effective policies of climate change mitigation (IPCC, 2019). Notably, coral bleaching can vary at a much smaller scale than 4‐km. Nevertheless, for a global analysis, a 4‐km resolution is a considerable improvement over coarse‐grained GCMs.
Human activity such as fishing pressure or pollution can damage reefs, and reef management approaches can benefit reefs. We chose to consider human population as a proxy for human activity in our model. Modern human population data were derived from the Socioeconomic Data and Applications Center (SEDAC; https://sedac.ciesin.columbia.edu/) at a 1‐km resolution on a decadal scale (Center for International Earth Science Information Network (CIESIN), Columbia University, 2017). Human population was converted to an annual resolution by interpolating between decades. The human population corresponding to each Reef Check survey site was calculated as the human population within a 10‐km radius of the reef for the year of reef survey. From the SEDAC human population data, we used the “middle of the road” scenario of human population growth for the years 2050 and 2100 (Gao, 2017, 2020).
Coral diversity data were obtained from Veron et al. (2015) (Figure 1; Table S1; Figure S15). These coral species diversity data are the most complete global coral species data available, but are only available at the ecoregion scale, not at an individual reef scale. Therefore, coral species diversity was incorporated hierarchically in the model at the ecoregion level.
FIGURE 1.
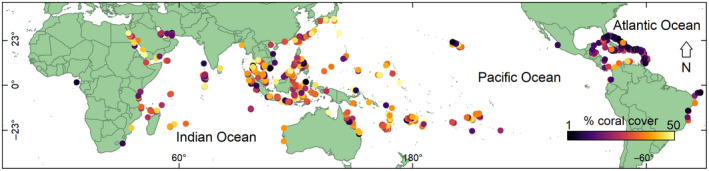
Modern global percent coral cover. The locations of 2949 survey sites and their percent coral cover measured during the years 1997–2018
2.2. Data analyses
To obtain summaries of coral cover across the oceans, we calculated the mean and standard deviation of percent coral cover for modern coral reefs, globally, and for each of five oceanic regions (i.e., the Red Sea, Arabian Gulf, Indian Ocean, Pacific Ocean, and Atlantic Ocean; Table 1). The most recent survey at each site was used in the calculations, so that some sites which were surveyed more frequently than others were not over‐represented in these ocean‐summary statistics. If multiple surveys at different depths were performed on the date of the most recent site survey, the mean percent coral cover over all depths was used for that site. We used only one survey per site to obtain coral‐cover summaries. Each site was assigned to one of five oceanic regions, and the mean and standard deviation of percent coral cover were calculated for the sites in each of the oceanic regions. To obtain estimates of the total reef area in each oceanic region, we overlaid reef shapefiles from ReefBase (ReefBase, 2021) with ecoregion shapefiles (Veron et al., 2015). For each oceanic region, all ecoregions within that ocean were included in the calculations, and the areas (km2) of each reef shapefile within each ecoregion were summed (Table 1). These summaries of oceanic regions were calculated to demonstrate that there are large‐scale spatial differences in coral cover and reef area, globally.
In a separate analysis to describe coral cover at a finer scale, we constructed a hierarchical Bayesian Beta model, which is a common model for proportional estimates (Lunn et al., 2013). Initially we used 35 covariates in the analysis (Table S1). A pair‐wise Pearson's correlation and a Brownian distance correlation (Székely et al., 2007) were used to determine which covariates were highly correlated (Figures S18 and S19). We discarded 21 predictor variables whose correlation coefficients were >0.7 with co‐occurring predictors (Darling et al., 2019). For highly correlated predictors, and to avoid overparameterization and multicollinearity, one of the paired variables was excluded based on ecological relevance, resulting in 14 predictor variables. We used a generalized linear mixed model within a Bayesian framework to examine the influence of the covariates on percent coral cover (Lunn et al., 2013). We standardized each covariate to improve the stability of our model. Each survey was included as a datapoint. Some sites were surveyed multiple times over different years, so the coral cover and corresponding environmental variables for each time a site was surveyed were incorporated as different data points. Additionally, to account for this repeated sampling, and avoid temporal pseudo‐replication, site was incorporated as a random effect in the model. Coral cover for a given observation (oi ) was assumed to follow a Beta distribution (pi ) using a log‐link function as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
where γ 0 is the intercept, γ1 : n are coefficients, x are environmental covariates, a are random effects of site (s), which hierarchically follow a normal distribution (norm) from the random effect (R) of ecoregion (r), γ div is the coefficient for diversity (d) introduced at the ecoregion level, μ is the overall mean, and τ and Τ are variance across site and ecoregion, respectively. Covariates were modeled with flat normal priors. The Bayesian model was implemented in R (R Core Team, 2007) and runs through the rjags package that calls JAGS (Plummer, 2016), with three chains, a burn‐in of 4000, and 15,000 iterations. The trace plots were examined for convergence, and posterior predictions were compared with simulated values from the same model (Hobbs & Hooten, 2015).
The model has a hierarchical structure. The model includes a ‘random effect of site,’ and sites are nested within ecoregions. Sites within the same ecoregion are treated as more similar to each other than they are to sites outside of that ecoregion. The hierarchical structure is also beneficial because it allows us to introduce variables at the scale at which they are available. Temperature variables are available at the site level, for example, but diversity is only available at the ecoregion level, globally. Therefore, using the hierarchical structure, we introduce temperature variables into the model at the site level and introduce diversity into the model at the ecoregion level. The random effect of site and the hierarchical structure help account for some regions having more surveys than other regions. In this way, we can examine coral cover globally, even though our survey densities are heterogeneous.
Modern‐day “bright spots” were defined as locations in which survey observations showed percent coral cover at levels of at least 1.5 standard deviations (30% total coral cover) greater than expected by the fitted beta model (Equation 9a). Conversely, “dark spots” were defined as locations in which survey observations showed coral cover at levels of at least 1.5 standard deviations less than expected by the fitted beta model (Equation 9b). The following equations were used to calculate “bright” and “dark” spots:
| (9a) |
| (9b) |
In an additional analysis, future percent coral cover at the Reef Check sites was projected by using the beta coefficients derived from the beta model that was fit to data on percent coral cover from 1997 to 2018. Mean SST values for the years 2050 and 2100, obtained from the RCP4.5 and RCP8.5 future SST datasets, were substituted for modern (from 1997 to 2018) mean SST values. Future human population data, obtained from SEDAC, were substituted for data on modern human populations from 1997 to 2018. The model was run to project percent coral cover for the years 2050 and 2100. The underlying assumption of these projections is that the response of coral cover to temperature going forward will be the same as responses to current temperatures. The R scripts used in the analyses are available at: https://github.com/InstituteForGlobalEcology/Present‐and‐future‐bright‐and‐dark‐spots‐for‐coral‐reefs‐through‐climate‐change.
3. RESULTS
Globally, modern percentage coral cover averaged ~32% between 1997 and 2018, with nearly a 20% standard deviation (Table 1). Overall, the Arabian Gulf and the Red Sea had the highest average coral cover, although the Arabian Gulf was considerably more variable than the Red Sea. Both regions had relatively fewer samples than other oceanic regions (Table 1). Coral cover in the Pacific and Indian Oceans were comparable both in terms of average percentage coral cover and in terms of variability (Table 1). The Atlantic Ocean had on average 20% less coral cover than the other oceanic regions, and variability was considerably lower than in other regions (Table 1).
Maximum historical SST and the standard deviation of the frequency of thermal‐stress anomalies (TSAs) were the only variables in the Bayesian model positively associated with modern (1997–2018) coral cover (Figure 2). The variables that were negatively associated with modern coral cover were as follows: depth (m), standard deviation of the frequency of SSTAs (SSTA_freq_sd), turbidity (Kd490), human population density (within a 10‐km radius of the reef site for the year in which the survey occurred), cyclone frequency (the average annual number of tropical cyclones at a given site from 1964 to 2014), latitude (°N or °S), and mean SST (°C; Figure 2).
FIGURE 2.
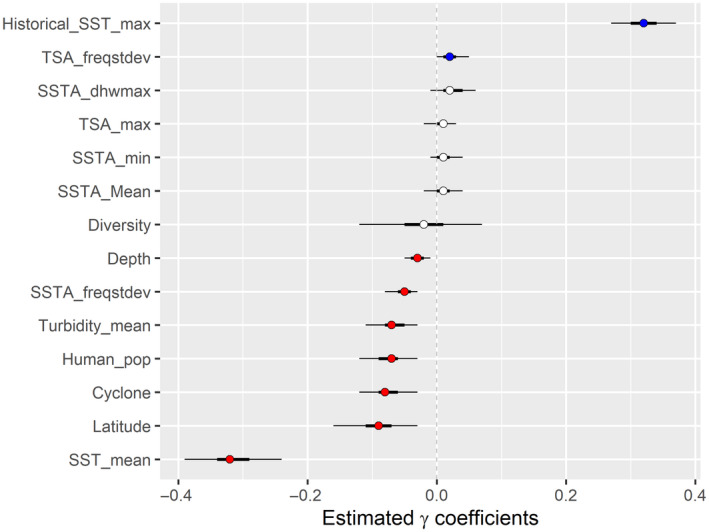
Model parameter coefficients of modern coral reefs globally. The relationships between the percentage of coral cover in each survey and the environmental variables are displayed within a Bayesian framework with mean values (circles) and 95% credible intervals (the thin black horizontal lines) as well as 50% credible intervals (the thick black horizontal lines) from 7714 Reef Check surveys at 2949 sites in 76 countries from 1997 to 2018. Blue dots show a positive association with percent coral cover, red dots show a negative association with percent coral cover, and white dots show no significant association with percent coral cover (95% credible interval crosses zero dashed line)
The beta model accurately estimated modern (from 1997 to 2018) global coral cover (Figure S20). However, some survey sites had a significantly higher percent coral cover (i.e., “bright spots”) or a significantly lower percent coral cover (i.e., “dark spots”) than expected by the model. Modern ‘bright spots’ were most apparent in Indonesia, Malaysia, the central Philippines, New Caledonia, Fiji, and French Polynesia (Figure 3; Table 2). Although less dense, there were also modern “bright spots” in Musandam Governorate (Oman), the Dog Islands (British Virgin Islands), Cocos (Keeling) Islands (Australia), Reunion (Mascarene Islands, France), Ari Atoll (the Maldives), Cauca (Columbia), Okinawa and Wakayama (southern islands of Japan), Tanintharyi region (in the eastern Andaman Sea in southern Myanmar), Maui in Hawai'i (USA), and Kien Giang (western Vietnam; Figure 3; Table 2). Modern “dark spots” were most apparent in northwestern Madagascar, eastern Africa, the northern GBR (Australia), and northern Java in Indonesia (Figure 3).
FIGURE 3.
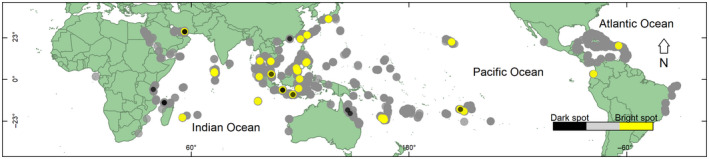
The sites of modern global coral reef “bright spots” and “dark spots” from 1997 to 2018. Gray dots are the survey sites where the measured percent coral cover was within 1.5 standard deviations (30% total coral cover) of expected coral cover. Yellow dots are the survey sites where the measured percent coral cover was at least 1.5 standard deviations greater than the expected percent coral cover. Dark circles are the survey sites where the observed percent coral cover was less than 1.5 standard deviations less than the expected percent coral cover
TABLE 2.
Modern global coral reef “bright spots.” The locations of “bright spots” globally from 1997 to 2018, including the latitude and longitude, the location, the country, and the oceanic region. Note, this list of modern “bright spots” is derived from 7714 Reef Check surveys at 2949 sites in 76 countries
| Latitude | Longitude | Location | Country | Oceanic region |
|---|---|---|---|---|
| 26.38 | 56.42 | Musandam Governorate | Oman | Arabian Gulf |
| 18.48 | −64.46 | Dog Islands | British Virgin Islands | Atlantic |
| −12.09 | 96.85 | Cocos (Keeling) Islands | Australia | Indian |
| −21.18 | 55.28 | Reunion | France, Mascarene Islands | Indian |
| 1.41 | 97.61 | North Sumatra | Indonesia | Indian |
| 3.63 | 72.95 | Ari Atoll | Maldives | Indian |
| 2.94 | −78.19 | Cauca | Colombia | Pacific |
| −17.62 | −149.62 | Society Islands | French Polynesia | Pacific |
| −16.50 | −151.78 | Society Islands | French Polynesia | Pacific |
| −5.90 | 110.43 | Central Java | Indonesia | Pacific |
| 0.14 | 119.81 | Central Sulawesi | Indonesia | Pacific |
| −5.05 | 119.33 | South Sulawesi | Indonesia | Pacific |
| −8.36 | 116.03 | West Nusa Tenggara | Indonesia | Pacific |
| 24.37 | 123.97 | Okinawa | Japan | Pacific |
| 33.34 | 135.70 | Wakayama | Japan | Pacific |
| 2.78 | 104.21 | Pahang | Malaysia | Pacific |
| 4.79 | 118.42 | Sabah | Malaysia | Pacific |
| 4.57 | 118.76 | Sabah | Malaysia | Pacific |
| 6.17 | 118.11 | Sabah | Malaysia | Pacific |
| 10.15 | 97.97 | Tanintharyi Region | Myanmar | Pacific |
| −21.38 | 164.97 | North Province | New Caledonia | Pacific |
| −22.34 | 166.24 | South Province | New Caledonia | Pacific |
| −22.32 | 166.46 | South Province | New Caledonia | Pacific |
| −21.59 | 166.26 | South Province | New Caledonia | Pacific |
| 9.07 | 123.27 | Central Visayas | Philippines | Pacific |
| 9.64 | 123.82 | Central Visayas | Philippines | Pacific |
| 20.67 | −156.44 | Maui, Hawai'i | United States | Pacific |
| 9.96 | 104.01 | Kien Giang | Vietnam | Pacific |
The major difference in coral cover projected under the RCP4.5 and RCP8.5 climate‐change scenarios (Figures S21–S24) was the amount of coral cover that was lost, not the geographical location where coral cover was lost. Therefore, we focused on absolute and relative coral losses in the worst‐case scenario—business‐as‐usual RCP8.5 (Figures 4 and 5). By 2050, under RCP8.5, we projected spatially variable changes in coral cover, with up to 5% decrease in absolute coral cover in the southern Caribbean, eastern Malaysia, southeastern Sulawesi (Indonesia), the central Philippines, eastern reefs in the Maldives, New Caledonia, Fiji, and French Polynesia. We also projected absolute changes in percent coral cover of 5%–14% (i.e., a 6%–100% decrease in relative coral cover; Figure 5) by 2050, under RCP8.5 at the GBR, southern New Caledonia, eastern Fiji, Java, the Andaman Sea, western Madagascar, eastern Africa, the Marshall Islands, western Sulawesi, the Red Sea, and the Gulf of Oman (Figure 4; Figure S25).
FIGURE 4.
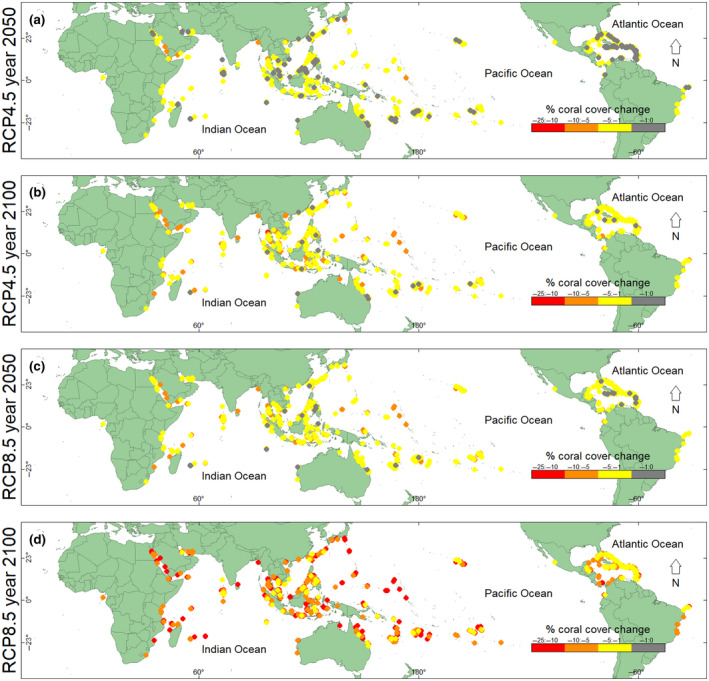
Projected absolute change in percent coral cover at Reef Check sites globally. The difference between modern (1997–2018) expected percent coral cover and future expected percent coral cover at Reef Check sites globally. Change in percent coral cover: (a) by the year 2050 for RCP4.5, (b) by the year 2100 for RCP4.5, (c) by the year 2050 for RCP8.5, and (d) by the year 2100 for RCP8.5
FIGURE 5.
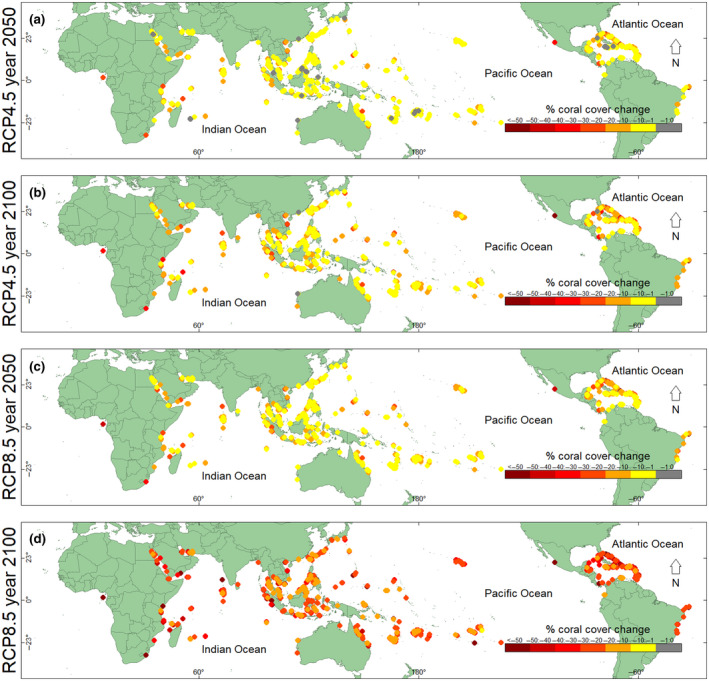
Projected relative change in percent coral cover at Reef Check sites globally. The relative difference between modern (1997–2018) expected percent coral cover and future expected percent coral cover at Reef Check sites globally. Relative change in percent coral cover: (a) by the year 2050 for RCP4.5, (b) by the year 2100 for RCP4.5, (c) by the year 2050 for RCP8.5, and (d) by the year 2100 for RCP8.5
By 2100, under RCP8.5, we projected absolute decreases in coral cover between 5% and 15% globally, which equates to relative decreases in coral cover of more than 40% globally (Figures 4, 5, 6). We also projected the smallest decreases in coral cover for some reefs in Malaysia, some western reefs in the Maldives, the central Philippines, New Caledonia, eastern Fiji, and French Polynesia by 2100, under RCP8.5. The largest decreases in absolute coral cover of 10%–21% (or a 12%–100% decrease in relative coral cover) were projected for reefs in Taiwan, the northern and central GBR, the southern Red Sea, the Gulf of Oman, eastern reefs in the Maldives, Java, southwestern Sulawesi, Madagascar, and eastern Africa (Figure 4; Figure S25). The northern Caribbean was also projected to lose considerable relative coral cover; however, the losses in absolute coral cover were projected to be small because Caribbean coral cover was already low at the start of this study period (i.e., in 1997; Figures 4 and 5).
FIGURE 6.
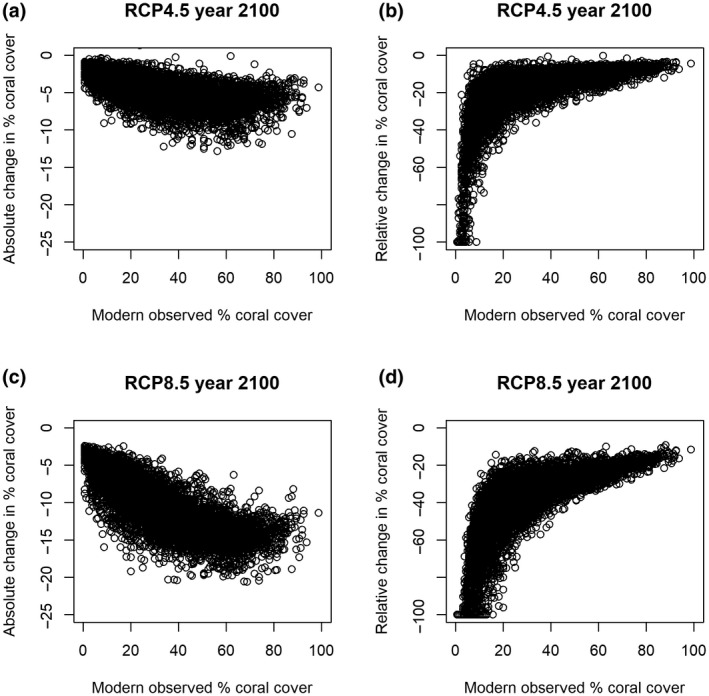
Absolute and relative change in future global percent coral cover under Representative Concentration Pathways (RCPs) 4.5 and 8.5 in 2100. (a) Absolute change under climate change scenario RCP4.5, (b) relative change under RCP4.5, (c) absolute change under RCP8.5, and (d) relative change under RCP8.5
Overall, 26% of the 2949 sites (25% of the 76 countries) were projected to lose at least 50% of their relative coral cover by 2100 under RCP8.5. Only 10% of sites, globally, were projected to lose less than 25% of their relative coral cover (Figure 6). Countries that have reefs that are projected to lose less than 25% of their relative coral cover also have other reefs that were projected to lose significantly more coral cover. Considered together, no country is projected to maintain more than 75% of its total coral cover in the next 80 years. Note however, that as the initial value of absolute coral cover decreases, the percentage of relative loss in coral cover increases disproportionately (Figure 6).
4. DISCUSSION
The results of this study showed that worldwide the percent coral cover on modern (from 1997 to 2018) coral reefs was negatively associated with mean SST and was positively associated with maximum historical SST. The strong negative association of coral cover with mean SST in the present study was expected. We know that marine heatwaves cause coral bleaching (Loya et al., 2001) and that the recent high frequency and intensity of thermal‐stress events are associated with climate change (Hoegh‐Guldberg et al., 2014; IPCC, 2013). SST changes are spatially variable however, as are coral responses to SST. For example, Sheppard (Sheppard, 2003) projected that corals at the Lakshadweep Islands, in the northern Indian Ocean would experience significantly lower probabilities of repeatedly crossing bleaching thresholds than elsewhere in the Indian Ocean. Our results similarly projected the western Indian Ocean faring worse in the near future than the central and eastern Indian Ocean. Our model also projected greater stability and less change in percent coral cover in southeastern Asia, particularly on the reefs of Malaysia, Indonesia, and the central Philippines by the year 2100. These geographic projections of changes in coral cover aligned with results from coral bleaching studies, showing significantly lower bleaching in the western tropical Pacific Ocean, and the highest probability of coral bleaching between the latitudes 15° and 20° north and south of the Equator (McClanahan et al., 2020; Sully et al., 2019).
In the present study, the strong positive association of percent coral cover with historical maximum SST may indicate that corals living on reefs historically exposed to relatively high maximum temperatures have suffered less from recent marine heatwaves than corals living elsewhere (McClanahan et al., 2020; Sully et al., 2019). This association may be a consequence of higher survival of corals pre‐conditioned and locally adapted to high historical SSTs. Hughes et al. (2003) suggested that bleaching susceptibilities may change over time through genotypic adjustments to SST, and Thompson and van Woesik (2009) found that reefs which historically experienced frequent thermal anomalies were less likely to bleach during recent thermal‐stress events. In addition, prior heat stress has been found to reduce the impact of subsequent heat stress on the GBR (Maynard et al., 2008; Middlebrook et al., 2008) and at Palmyra Atoll (Williams et al., 2010). Although coral cover was used as a universal integrator of change on reefs in the present study, future models could consider the moderating influence of differential adaptation (McManus et al., 2020), which may vary geographically (Selmoni et al., 2020).
By the end of the century, SSTs may rise by more than 3°C under climate change scenario RCP8.5 (IPCC, 2013). This increase in ocean temperature will also likely open higher latitudes for coral colonization, which presently do not have reefs (Greenstein & Pandolfi, 2008; Precht & Aronson, 2004; Yamano et al., 2011). However, any potential benefits that reefs may have expanding to high latitudes may be offset by ocean acidification (van Hooidonk et al., 2014). Many other factors can also influence the capacity of corals to expand their geographic distributions, such as the need for suitable substrate (Lauria et al., 2021), connectivity to other reefs (Veron, 1995; Wood et al., 2014), and light (Muir et al., 2015). Therefore, latitudinal expansion is not simply related to optimal temperature. In addition, corals may be forced deeper with an increasing frequency in marine heatwaves, even though this study found less coral cover on average with increasing depth, and other studies have found that deeper corals may be less fecund than shallow corals, independent of species (Shlesinger et al., 2018).
Our model also showed a negative relationship with tropical cyclones, corroborating previous work by Darling et al. (2019). The strong negative relationship between coral cover with tropical‐cyclone frequency was expected, because tropical cyclones physically damage reefs, resulting in coral loss (Gouezo et al., 2015; Heron et al., 2007). A decrease in annual cyclone frequency would therefore be beneficial for reefs and correspond to an increase in coral cover. Tropical cyclones are however expected to increase in intensity and to shift their trajectories toward lower latitudes because of climate change (IPCC, 2013; Wu & Wang, 2004). Therefore, we anticipate that coral cover will decrease in localities where tropical cyclones were historically infrequent, such as in the lower latitudes in the western Pacific (Gouezo et al., 2015), especially under RCP8.5. Future models could consider inputting both projected changes in tropical‐cyclone intensity and changes in their geographical trajectories (IPCC, 2013; Wu & Wang, 2004).
The present study projected that under future climate‐change scenarios RCP4.5 and RCP8.5, relative percent coral cover will decrease by more than 40% globally by the year 2100, which is an absolute decline of more than 10%. The models showed that such declines will vary geographically. For example, corals on reefs in some parts of southeastern Asia may fare better than in other regions, and therefore these reefs should receive high‐priority conservation attention. These same reefs are also however associated with large human populations, which have detrimental effects (Figure 2) through pollution, land‐use change, and river discharge. The modern ‘bright spot’ analysis, as well as the future projections (see Google Earth overlays) could help guide local managers and policymakers to develop solutions for individual reefs, particularly in the Coral Triangle, which includes reefs of Indonesia, Malaysia, the Philippines, Papua New Guinea, the Democratic Republic of Timor‐Leste, and the Solomon Islands. Even though we identify some “bright spots” and identify a few reefs that may lose relatively little coral cover, the extreme global loss of coral cover under RCP8.5 is serious. A few bright spots may not be sufficient for many coral species to survive, and for humans to experience their benefits through storm‐surge and wave barriers. But sites where corals have the potential to survive climate change should be focal sites for conservation. Modern “dark spots” and reefs that are projected to lose the most coral cover by 2050 and 2100 should, however, not be ignored nor abandoned by management. Many of these reefs are crucial to biodiversity, and effective local management can reduce local pressures on “dark spots” and ameliorate local disturbances (Donovan et al., 2021).
We note that our study provides KML maps, readable in Google Earth, as a decision‐support tool for geographical conservation efforts. Yet, the percentage coral cover displayed on the maps may be marginally higher than neighboring reefs because Reef Check divers were instructed to select sites with relatively high coral cover. The consistency of the instructions across all regions, however, allows for useful comparisons, including the associations of environmental variables with coral cover (Figure 2). Other variables, including the variance and frequency of SSTAs, the frequency of TSAs, and cyclone activity, were also found to be significantly correlated with contemporary coral cover. Yet the projections made in this study were only based on future mean SST and future human populations. Therefore, future studies could include projections of these additional variables to improve the accuracy of future projections.
Identifying climate refuges is imperative and coordinating conservation efforts in potential refuges can reduce the risk of widespread failure at a global scale (Beyer et al., 2018). Beyer et al. (2018), identified potential climate‐change refuges using modern portfolio theory (Markowitz, 1952) and recommended high priority reefs for conservation near Kalimantan (Indonesia), the Philippines, Malaysia, French Polynesia, and the southern Red Sea. Similarly, Beger et al. (2015) identified many reefs in the Coral Triangle with high conservation value. Although the objectives of Beger et al. (2015) differed from the objectives of the present study, we nonetheless identified overlapping localities in the Coral Triangle region that may continue to support relatively high percent coral cover with ocean warming. These areas included eastern Malaysia, the central Philippines, and southeastern Sulawesi in Indonesia. Similarly, McClanahan et al. (2020) identified Sulawesi, Fiji, and the Solomon Islands as having high resistance to thermal stress, and they identified Madagascar, eastern Africa, and ecoregions near Japan as having lower resistance to thermal stress. Our global study also projected that the Coral Triangle region, in general, will have higher percent coral cover under future climate change scenarios than other reefs (Figures 4 and 5; Figures S25–S108), making the Coral Triangle a region of particular interest for coral conservation. Our results contrast with McManus et al. (2021) who, by simulating the cover of two coral types in the Coral Triangle under a networked eco‐evolutionary framework, suggested that coral cover will decline throughout the Coral Triangle region. Our results however corroborate McClanahan et al.'s (2020) work which demonstrated that reefs in the Coral Triangle have a higher resistance to thermal stress than reefs outside the Coral Triangle.
In conclusion, the model of the present study is unique in that it used a fine resolution (~4 km) of modern and projected SSTs to identify modern global climate change refuges or “bright spots” and predicts where corals have the highest likelihood of survival in the future. Knowing where corals may survive is of utmost societal importance, especially for island nations that are immediately affected by sea‐level rise and where millions of people depend on the goods and services from coral reefs for sustenance and protection from storm waves. Local and regional conservation efforts should not be a substitute for global reductions in carbon emissions but should instead be additional strategies used to conserve coral reefs into the future.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The Kd490 data were provided by NASA (National Aeronautics and Space Administration), Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group, and the SeaWiFS (Sea‐viewing Wide Field‐of‐view Sensor) Ocean Color Data. We thank the Reef Check coordinators and the thousands of volunteer scientists and citizen scientists who have collected Reef Check data since 1997 (Table S3). The CoRTAD data were provided by GHRSST and the US National Centers for Environmental Information, which was supported in part by a grant from the NOAA Climate Data Record (CDR) Program for satellites. We acknowledge the World Climate Research Programme, which, through its Working Group on Coupled Modelling, coordinated and promoted CMIP6. We thank the climate modeling groups for producing and making available their model output, the Earth System Grid Federation (ESGF) for archiving the data and providing access, and the multiple funding agencies who support CMIP6 and ESGF. We thank NASA EOSDIS SEDAC for making human population data available, and we thank NOAA's International Best Track Archive for Climate Stewardship (IBTrACS) for making their tropical‐cyclone data accessible. We would like to thank NOAA's Coral Reef Conservation Program for providing downscaled SST projections, and particularly Ruben van Hooidonk who provided the downscaled temperature data for RCP4.5 and RCP8.5. Many thanks go to Chelsey Kratochwill for her untiring assistance with the database. We would like to thank Zack H. for sharing his expertise in Linux and converting netcdf data. We would also like to thank Sandra van Woesik for her valuable editorial comments on the manuscript. Funding was provided by the National Science Foundation, OCE 1829393. This is contribution number 239 from the Institute for Global Ecology at the Florida Institute of Technology.
Sully, S. , Hodgson, G. , & van Woesik, R. (2022). Present and future bright and dark spots for coral reefs through climate change. Global Change Biology, 28, 4509–4522. 10.1111/gcb.16083
DATA AVAILABILITY STATEMENT
All the R code, Reef Check data, and diversity data for the analysis are available at the GitHub repository for the Institute for Global Ecology (https://github.com/InstituteForGlobalEcology/Present‐and‐future‐bright‐and‐dark‐spots‐for‐coral‐reefs‐through‐climate‐change). All Coral Reef Temperature Anomaly Database (CoRTAD) data used in this analysis are publicly available at NOAA's National Centers for Environmental Information (NCEI) webpage (https://data.nodc.noaa.gov/cortad/Version6/). Kd490 data are publicly available at the NASA, Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group, SeaWiFS (Sea‐viewing Wide Field‐of‐view Sensor) Ocean Color Data webpage (https://oceandata.sci.gsfc.nasa.gov/MODIS‐Aqua/Mapped/Monthly/4km/Kd_490/). Human population data are available at https://sedac.ciesin.columbia.edu/data/set/popdynamics‐global‐pop‐count‐time‐series‐estimates/data‐download#close and https://sedac.ciesin.columbia.edu/data/set/popdynamics‐1‐km‐downscaled‐pop‐base‐year‐projection‐ssp‐2000‐2100‐rev01. Tropical‐cyclone data are available from International Best Track Archive for Climate Stewardship (IBTrACS; www.ncdc.noaa.gov/ibtracs/index.php?name=ibtracsdata). Reef shapefiles were available from ReefBase (http://www.reefbase.org). The methods for determining annual tropical‐cyclone frequency are available in a study by Cacciapaglia and van Woesik (2020). The future RCP4.5 and RCP8.5 SST data from NOAA's Coral Reef Conservation Program are available in van Hooidonk et al. (2016).
REFERENCES
- Anthony, K. R. N. , Maynard, J. A. , Diaz‐pulido, G. , Mumby, P. J. , Marshall, P. A. , Cao, L. , & Hoegh‐guldberg, O. (2011). Ocean acidification and warming will lower coral reef resilience. Global Change Biology, 17, 1798–1808. 10.1111/j.1365-2486.2010.02364.x [DOI] [Google Scholar]
- Balaji, V. , Taylor, K. E. , Juckes, M. , Lawrence, B. N. , Durack, P. J. , Lautenschlager, M. , Blanton, C. , Cinquini, L. , Denvil, S. , Elkington, M. , Guglielmo, F. , Guilyardi, E. , Hassell, D. , Kharin, S. , Kindermann, S. , Nikonov, S. , Radhakrishnan, A. , Stockhause, M. , Weigel, T. , & Williams, D. (2018). Requirements for a global data infrastructure in support of CMIP6. Geoscientific Model Development, 11, 3659–3680. 10.5194/gmd-11-3659-2018 [DOI] [Google Scholar]
- Barkley, H. C. , Cohen, A. L. , Mollica, N. R. , Brainard, R. E. , Rivera, H. E. , DeCarlo, T. M. , Lohmann, G. P. , Drenkard, E. J. , Aplert, A. E. , Young, C. W. , & Vargas‐Angel, B. (2018). Repeat bleaching of a central Pacific coral reef over the past six decades (1960–2016). Communication Biology, 1, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger, M. , McGowan, J. , Treml, E. A. , Green, A. L. , White, A. T. , Wolff, N. H. , Klein, C. J. , Mumby, P. J. , & Possingham, H. P. (2015). Integrating regional conservation priorities for multiple objectives into national policy. Nature Communications, 6, 8208. 10.1038/ncomms9208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer, H. L. , Kennedy, E. V. , Beger, M. , Chen, C. A. , Cinner, J. E. , Darling, E. S. , Eakin, C. M. , Gates, R. D. , Heron, S. F. , Knowlton, N. , Obura, D. O. , Palumbi, S. R. , Possingham, H. P. , Puotinen, M. , Runting, R. K. , Skirving, W. J. , Spalding, M. , Wilson, K. A. , Wood, S. , … Hoegh‐Guldberg, O. (2018). Risk‐sensitive planning for conserving coral reefs under rapid climate change. Conservation Letters, 11, e12587. 10.1111/conl.12587 [DOI] [Google Scholar]
- Bruno, J. F. , Sweatman, H. , Precht, W. F. , Selig, E. R. , & Schutte, V. G. W. (2009). Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology, 90(6), 1478–1484. 10.1890/08-1781.1 [DOI] [PubMed] [Google Scholar]
- Cacciapaglia, C. W. , & van Woesik, R. (2015). Reef‐coral refugia in a rapidly changing ocean. Global Change Biology, 21, 2272–2283. 10.1111/gcb.12851 [DOI] [PubMed] [Google Scholar]
- Cacciapaglia, C. W. , & van Woesik, R. (2020). Reduced carbon emissions and fishing pressure are both necessary for equatorial coral reefs to keep up with rising seas. Ecography, 43, 1–12. 10.1111/geb.13191 [DOI] [Google Scholar]
- Center for International Earth Science Information Network (CIESIN) , & Columbia University . (2017). Global population count grid time series estimates. NASA Socioeconomic Data and Applications Center (SEDAC). https://sedac.ciesin.columbia.edu/data/set/popdynamics‐global‐pop‐count‐time‐series‐estimates/data‐download#close [Google Scholar]
- Costanza, R. , de Groot, R. , Sutton, P. , van der Ploeg, S. , Anderson, S. J. , Kubiszewski, I. , Farber, S. , & Turner, R. K. (2014). Changes in the global value of ecosystem services. Global Environmental Change, 26, 152–158. 10.1016/j.gloenvcha.2014.04.002 [DOI] [Google Scholar]
- Couch, C. S. , Burns, J. H. R. , Liu, G. , Steward, K. , Gutlay, T. N. , Kenyon, J. , Eakin, C. M. , & Kosaki, R. K. (2017). Mass coral bleaching due to unprecedented marine heatwave in Papahānaumokuākea Marine National Monument (Northwestern Hawaiian Islands). PLoS One, 12, e0185121. 10.1371/journal.pone.0185121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling, E. S. , McClanahan, T. R. , Maina, J. , Gurney, G. G. , Graham, N. A. J. , Januchowski‐Hartley, F. , Cinner, J. E. , Mora, C. , Hicks, C. C. , Maire, E. , Puotinen, M. , Skirving, W. J. , Adjeroud, M. , Ahmadia, G. , Arthur, R. , Bauman, A. G. , Beger, M. , Berumen, M. L. , Bigot, L. , … Mouillot, D. (2019). Social–environmental drivers inform strategic management of coral reefs in the Anthropocene. Nature Ecology & Evolution, 3(9), 1341–1350. 10.1038/s41559-019-0953-8 [DOI] [PubMed] [Google Scholar]
- De'ath, G. , Fabricius, K. E. , Sweatman, H. , & Puotinen, M. (2012). The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proceedings of the National Academy of Sciences of the United States of America, 109(44), 17995–17999. 10.1073/pnas.1208909109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, G. B. , Davies, S. W. , Aglyamova, G. V. , Meyer, E. , Bay, L. K. , & Matz, M. V. (2015). Genomic determinants of coral heat tolerance across latitudes. Science, 348, 1460–1462. 10.1126/science.1261224 [DOI] [PubMed] [Google Scholar]
- Done, T. , Roelfsema, C. , Harvey, A. , Schuller, L. , Hill, J. , Schläppy, M.‐L. , Lea, A. , Bauer‐Civiello, A. , & Loder, J. (2017). Reliability and utility of citizen science reef monitoring data collected by Reef Check Australia, 2002–2015. Marine Pollution Bulletin, 117, 148–155. 10.1016/j.marpolbul.2017.01.054 [DOI] [PubMed] [Google Scholar]
- Donovan, M. K. , Burkepile, D. E. , Kratochwill, C. , Shlesinger, T. , Sully, S. , Oliver, T. A. , Hodgson, G. , Freiwald, J. , & van Woesik, R. (2021). Local conditions magnify coral loss after marine heatwaves. Science, 372(6545), 977–980. 10.1126/science.abd9464 [DOI] [PubMed] [Google Scholar]
- Eddy, T. D. , Lam, V. W. Y. , Reygondeau, G. , Cisneros‐Montemayor, A. M. , Greer, K. , Palomares, M. L. D. , Bruno, J. F. , Ota, Y. , & Cheung, W. W. L. (2021). Global decline in capacity of coral reefs to provide ecosystem services. One Earth, 4, 1278–1285. 10.1016/j.oneear.2021.08.016 [DOI] [Google Scholar]
- Gao, J. (2017). Downscaling Global Spatial Population Projections from 1/8‐degree to 1‐km Grid Cells. NCAR Technical Note, NCAR/TN‐537+STR. 10.5065/D60Z721H [DOI] [Google Scholar]
- Gao, J. (2020). Global 1‐km downscaled population base year and projection grids based on the shared socioeconomic pathways, revision 01. NASA Socioeconomic Data and Applications Center (SEDAC). https://sedac.ciesin.columbia.edu/data/set/popdynamics‐1‐km‐downscaled‐pop‐base‐year‐projection‐ssp‐2000‐2100‐rev01 [Google Scholar]
- Gouezo, M. , Golbuu, Y. , van Woesik, R. , Rehm, L. , Koshiba, S. , & Doropoulos, C. (2015). The impacts of two sequential super typhoons on coral‐reef communities in Palau. Marine Ecology Progress Series, 540, 73–85. [Google Scholar]
- Greenstein, B. J. , & Pandolfi, J. M. (2008). Escaping the heat: Range shifts of reef coral taxa in 599 coastal Western Australia. Global Change Biology, 14, 513–528. 10.1111/j.1365-6002486.2007.01506.x [DOI] [Google Scholar]
- Guest, J. R. , Baird, A. H. , Maynard, J. A. , Muttaqin, E. , Edwards, A. J. , Campbell, S. J. , Yewdall, K. , Affendi, Y. A. , & Chou, L. M. (2012). Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS One, 7(3), e33353. 10.1371/journal.pone.0033353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern, B. S. , Walbridge, S. , Selkoe, K. A. , Kappel, C. V. , Micheli, F. , D'Agrosa, C. , Bruno, J. F. , Casey, K. S. , Ebert, C. , Fox, H. E. , Fujita, R. , Heinemann, D. , Lenihan, H. S. , Madin, E. M. P. , Perry, M. T. , Selig, E. R. , Spalding, M. , Steneck, R. , & Watson, R. (2008). A global map of human impact on marine ecosystems. Science, 310(5865), 948–952. 10.1126/science.1149345 [DOI] [PubMed] [Google Scholar]
- Heron, S. F. , Morgan, J. A. , Eakin, C. M. , & Skirving, W. J. (2007). Hurricanes and their effects on coral reefs. In Wilkinson C. & Souter W. D. (Eds.), Status of Caribbean coral reefs after bleaching and hurricanes in 2005 (pp. 31–36). Global Coral Reef Monitoring Network, and Reef and Rainforest Research Centre. [Google Scholar]
- Hobbs, N. T. , & Hooten, M. B. (2015). Bayesian models: A statistical primer for ecologists. Princeton University Press, 320 pp. [Google Scholar]
- Hodgson, G. (1999). A global assessment of human effects on coral reefs. Marine Pollution Bulletin, 38, 345–355. 10.1016/S0025-326X(99)00002-8 [DOI] [Google Scholar]
- Hodgson, G. , Hill, J. , Kiene, W. , Maun, L. , Mihaly, J. , Liebeler, J. , Shuman, C. , & Torres, R. (2006). Reef check instruction manual: A guide to reef check coral reef monitoring. Reef Check Foundation, 97 pp. [Google Scholar]
- Hoegh‐Guldberg, O. , Cai, R. , Poloczanska, E. S. , Brewer, P. G. , Sundby, S. , Hilmi, K. , Fabry, V. J. , & Jung, S. (2014). Climate change 2014: Impacts, adaptation, and vulnerability. Part B: Regional aspects. Contribution of working group II to the fifth assessment report of the Intergovernmental Panel on Climate Change (Ch. 30, pp. 1655–1731). Cambridge University Press. [Google Scholar]
- Hughes, T. P. , Baird, A. H. , Bellwood, D. R. , Card, M. , Connolly, S. R. , Folke, C. , Grosberg, R. , Hoegh‐Guldberg, O. , Jackson, J. B. C. , Kleypas, J. , Lough, J. M. , Marshall, P. , Nyström, M. , Palumbi, S. R. , Pandolfi, J. M. , Rosen, B. , & Roughgarden, J. (2003). climate change, human impacts, and the resilience of coral reefs. Science, 301, 929–933. 10.1126/science.1085046 [DOI] [PubMed] [Google Scholar]
- Hughes, T. P. , Barnes, M. L. , Bellwood, D. R. , Cinner, J. E. , Cumming, G. S. , Jackson, J. B. C. , Kleypas, J. , van de Leemput, I. A. , Lough, J. M. , Morrison, T. H. , Palumbi, S. R. , van Nes, E. H. , & Scheffer, M. (2017). Coral reefs in the Anthropocene. Nature, 546, 82–90. 10.1038/nature22901 [DOI] [PubMed] [Google Scholar]
- Hughes, T. P. , Kerry, J. T. , Baird, A. H. , Connolly, S. R. , Dietzel, A. , Eakin, C. M. , Heron, S. F. , Hoey, A. S. , Hoogenboom, M. O. , Liu, G. , McWilliam, M. J. , Pears, R. J. , Pratchett, M. S. , Skirving, W. J. , Stella, J. S. , & Torda, G. (2018). Global warming transforms coral reef assemblages. Nature, 556, 492–496. 10.1038/s41586-018-0041-2 [DOI] [PubMed] [Google Scholar]
- IPCC . (2013). Climate change: The physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press. [Google Scholar]
- IPCC . (2019). Summary for policymakers. In Pörtner H.‐O., Roberts D. C., Masson‐Delmotte V., Zhai P., Tignor M., Poloczanska E., Mintenbeck K., Alegría A., Nicolai M., Okem A., Petzold J., Rama B., & Weyer N. M. (Eds.), IPCC special report on the ocean and cryosphere in a changing climate. IPCC. [Google Scholar]
- Jackson, J. , Donovan, M. , Cramer, K. , & Lam, V. (2014). Status and trends of Caribbean coral reefs: 1970–2012. IUCN, Global Coral Reef Monitoring Network. [Google Scholar]
- Kenkel, C. D. , & Matz, M. V. (2016). Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nature Ecology & Evolution, 1. 10.1038/s41559-016-0014 [DOI] [PubMed] [Google Scholar]
- Lauria, V. , Massi, D. , Fiorentino, F. , Milisenda, G. , & Cillari, T. (2021). Habitat suitability mapping of the black coral Leiopathes glaberrima to support conservation of vulnerable marine ecosystems. Scientific Reports, 11, 15661. 10.1038/s41598-021-95256-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loya, Y. , Sakai, K. , Yamazato, K. , Nakano, Y. , Sambali, H. , & van Woesik, R. (2001). Coral bleaching: The winners and the losers. Ecology Letters, 4(2), 122–131. 10.1046/j.1461-0248.2001.00203.x [DOI] [Google Scholar]
- Lunn, D. , Jackson, C. , Best, N. , Thomas, A. , & Spiegelhalter, D. (2013). The BUGS book: A practical introduction to Bayesian analysis. CRC Press. [Google Scholar]
- Markowitz, H. (1952). Portfolio selection. The Journal of Finance, 7(1), 77–91. [Google Scholar]
- Maynard, J. A. , Anthony, K. R. N. , Marshall, P. A. , & Masiri, I. (2008). Major bleaching events can lead to increased thermal tolerance in corals. Marine Biology, 155, 173–182. 10.1007/s00227-008-1015-y [DOI] [Google Scholar]
- McClanahan, T. R. , Ateweberhan, M. , Muhando, C. A. , Maina, J. , & Mohammed, M. S. (2007). Effects of climate and seawater temperature variation on coral bleaching and mortality. Ecological Monographs, 77, 503–525. 10.1890/06-1182.1 [DOI] [Google Scholar]
- McClanahan, T. R. , & Maina, J. (2003). Response of coral assemblages to the interaction between natural temperature variation and rare warm‐water events. Ecosystems, 6, 551–563. 10.1007/s10021-002-0104-x [DOI] [Google Scholar]
- McClanahan, T. R. , Maina, J. M. , Darling, E. S. , Guillaume, M. M. M. , Muthiga, N. A. , D’agata, S. , Leblond, J. , Arthur, R. , Jupiter, S. D. , Wilson, S. K. , Mangubhai, S. , Ussi, A. M. , Humphries, A. T. , Patankar, V. , Shedrawi, G. , Julius, P. , Ndagala, J. , & Grimsditch, G. (2020). Large geographic variability in the resistance of corals to thermal stress. Global Ecology and Biogeography, 29(12), 2229–2247. 10.1111/geb.13191 [DOI] [Google Scholar]
- McManus, L. C. , Forrest, D. L. , Tekwa, E. W. , Schindler, D. E. , Colton, M. A. , Webster, M. M. , Essington, T. E. , Palumbi, S. R. , Mumby, P. J. , & Pinsky, M. L. (2021). Evolution and connectivity influence the persistence and recovery of coral reefs under climate change in the Caribbean, Southwest Pacific, and Coral Triangle. Global Change Biology, 27, 4307–4321. 10.1111/gcb.15725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus, L. C. , Vasconcelos, V. V. , Levin, S. A. , Thompson, D. M. , Kleypas, J. A. , Castruccio, F. S. , Curchitser, E. N. , & Watson, J. R. (2020). Extreme temperature events will drive coral decline in the Coral Triangle. Global Change Biology, 26, 2120–2133. 10.1111/gcb.14972 [DOI] [PubMed] [Google Scholar]
- Middlebrook, R. , Hoegh‐Guldberg, O. , & Leggat, W. (2008). The effect of thermal history on the susceptibility of reef‐building corals to thermal stress. Journal of Experimental Biology, 211, 1050–1056. 10.1242/jeb.013284 [DOI] [PubMed] [Google Scholar]
- Muir, P. R. , Wallace, C. C. , Done, T. , & Aguirre, J. D. (2015). Limited scope for latitudinal extension of reef corals. Science, 348(6239), 1135–1138. 10.1126/science.1259911 [DOI] [PubMed] [Google Scholar]
- Obura, D. , Gudka, M. , Abdou Rabi, F. , Bacha Gian, S. , Bijoux, J. , Freed, S. , Maharavo, J. , Mwaura, J. , Porter, S. , Sola, E. , & Wickel, J. (2017). Coral reef status report for the western Indian Ocean. Global Coral Reef Monitoring Network (GCRMN)/International Coral Reef Initiative (ICRI) CORDIO East Africa. [Google Scholar]
- Plummer, M. (2016). Package rjags: Bayesian graphical models using MCMC. Version 4–6. Retrieved from https://CRAN.R‐project.org/package=rjags
- Precht, W. F. , & Aronson, R. B. (2004). Climate flickers and range shifts of reef corals. Frontiers in Ecology and the Environment, 2, 307–314. 10.1890/1540-9295(2004)002%5B0307:CFARSO%5D2.0.CO;2 [DOI] [Google Scholar]
- R Core Team (2007). R: A language and environment for statistical computing. http://www.R‐project.org [Google Scholar]
- ReefBase . (2021). A global information system for coral reefs. http://www.reefbase.org [Google Scholar]
- Safaie, A. , Silbiger, N. J. , McClanahan, T. R. , Pawlak, G. , Barshis, D. J. , Hench, J. L. , Rogers, J. S. , Williams, G. J. , & Davis, K. A. (2018). High frequency temperature variability reduces the risk of coral bleaching. Nature Communications, 9(16), 1671. 10.1038/s41467-018-04074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampayo, E. M. , Ridgway, T. , Bongaerts, P. , & Hoegh‐Guldberg, O. (2008). Bleaching susceptibility and mortality of corals are determined by fine‐scale differences in symbiont type. Proceedings of the National Academy of Sciences of the United States of America, 105(30), 10444–10449. 10.1073/pnas.0708049105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmoni, O. , Rochat, E. , Lecellier, G. , Berteaux‐Lecellier, V. , & Joost, S. (2020). Seascape genomics as a new tool to empower coral reef conservation strategies: An example on north‐western Pacific Acropora digitifera. Evolutionary Applications, 13(8), 1923–1938. 10.1111/eva.12944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard, C. (2003). Predicted recurrences of mass coral mortality in the Indian Ocean. Nature, 425, 294–297. 10.1038/nature01987 [DOI] [PubMed] [Google Scholar]
- Sheppard, C. , Sheppard, A. , & Fenner, D. (2020). Coral mass mortalities in the Chagos Archipelago over 40 years: Regional species and assemblage extinctions and indications of positive feedbacks. Marine Pollution Bulletin, 154, 111075. 10.1016/j.marpolbul.2020.111075 [DOI] [PubMed] [Google Scholar]
- Shlesinger, T. , Grinblat, M. , Rapuano, H. , Amit, T. , & Loya, Y. (2018). Can mesophotic reefs replenish shallow reefs? Reduced coral reproductive performance casts a doubt. Ecology, 99(2), 421–437. 10.1002/ecy.2098 [DOI] [PubMed] [Google Scholar]
- Spalding, M. , Burke, L. , Wood, S. A. , Ashpole, J. , Hutchison, J. , & zu Ermgassen, P. (2017). Mapping the global value and distribution of coral reef tourism. Marine Policy, 82, 104–113. 10.1016/j.marpol.2017.05.014 [DOI] [Google Scholar]
- Sully, S. , Burkepile, D. E. , Donovan, M. K. , Hodgson, G. , & van Woesik, R. (2019). A global analysis of coral bleaching over the past two decades. Nature Communications, 10, 1264. 10.1038/s41467-019-09238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Székely, G. J. , Rizzo, M. L. , & Bakirov, N. K. (2007). Measuring and testing dependence by correlation of distances. The Annals of Statistics, 35(6), 2769–2794. MR2382665; Zentralblatt MATH identifier: 1129.62059. [Google Scholar]
- Thompson, D. , & van Woesik, R. (2009). Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proceedings of the Royal Society B: Biological Sciences, 276, 2893–2901. 10.1098/rspb.2009.0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooidonk, R. , Maynard, J. A. , Manzello, D. , & Planes, S. (2014). Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Global Change Biology, 20(1), 103–112. 10.1111/gcb.12394 [DOI] [PubMed] [Google Scholar]
- van Hooidonk, R. , Maynard, J. , Tamelander, J. , Gove, J. , Ahmadia, G. , Raymundo, L. , Williams, G. , Heron, S. F. , & Planes, S. (2016). Local‐scale projections of coral reef futures and implications of the Paris Agreement. Scientific Reports, 6, 39666. 10.1038/srep39666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen, M. J. H. , Oliver, J. K. , Putnam, H. M. , & Gates, R. D. (2015). Building coral reef resilience through assisted evolution. Proceedings of the National Academy of Sciences of the United States of America, 112(8), 2307–2313. 10.1073/pnas.1422301112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woesik, R. , Houk, P. , Isechal, A. L. , Idechong, J. W. , Victor, S. , & Golbuu, Y. (2012). Climate‐change refugia in the sheltered bays of Palau: Analogs of future reefs. Ecology and Evolution, 2, 2474–2484. 10.1002/ece3.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron, J. E. N. (1995). Corals in space and time: the biogeography and evolution of the Scleractinia. Comstock/Cornell. [Google Scholar]
- Veron, J. , Stafford‐Smith, M. , DeVantier, L. , & Turak, E. (2015). Overview of distribution patterns of zooxanthellate Scleractinia. Frontiers in Marine Science, 1, 81. 10.3389/fmars.2014.00081 [DOI] [Google Scholar]
- Williams, G. J. , Knapp, I. S. , Margos, J. E. , & Davy, S. K. (2010). Modeling patterns of coral bleaching at a remote Central Pacific atoll. Marine Pollution Bulletin, 60(9), 1467–1476. 10.1016/j.marpolbul.2010.05.009 [DOI] [PubMed] [Google Scholar]
- Wood, S. , Paris, C. B. , Ridgwell, A. , & Hendy, E. J. (2014). Modelling dispersal and connectivity of broadcast spawning corals at the global scale. Global Ecology and Biogeography, 23, 1–11. 10.1111/geb.12101 [DOI] [Google Scholar]
- Wu, L. , & Wang, B. (2004). Assessing impacts of global warming on tropical cyclone tracks. Journal of Climate, 17, 1686–1698. [DOI] [Google Scholar]
- Yamano, H. , Sugihara, K. , & Nomura, K. (2011). Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophysical Research Letters, 38, L04601. 10.1029/2010GL046474 [DOI] [Google Scholar]
- Zinke, J. , Gilmour, J. P. , Fisher, R. , Puotinen, M. , Maina, J. , Darling, E. , Stat, M. , Richards, Z. T. , McClanahan, T. R. , Beger, M. , Moore, C. , Graham, N. A. J. , Feng, M. , Hobbs, J.‐P. , Evans, S. N. , Field, S. , Shedrawi, G. , Babcock, R. C. , & Wilson, S. K. (2018). Gradients of disturbance and environmental conditions shape coral community structure for south‐eastern Indian Ocean reefs. Diversity and Distributions, 24, 605–620. 10.1111/ddi.12714 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All the R code, Reef Check data, and diversity data for the analysis are available at the GitHub repository for the Institute for Global Ecology (https://github.com/InstituteForGlobalEcology/Present‐and‐future‐bright‐and‐dark‐spots‐for‐coral‐reefs‐through‐climate‐change). All Coral Reef Temperature Anomaly Database (CoRTAD) data used in this analysis are publicly available at NOAA's National Centers for Environmental Information (NCEI) webpage (https://data.nodc.noaa.gov/cortad/Version6/). Kd490 data are publicly available at the NASA, Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group, SeaWiFS (Sea‐viewing Wide Field‐of‐view Sensor) Ocean Color Data webpage (https://oceandata.sci.gsfc.nasa.gov/MODIS‐Aqua/Mapped/Monthly/4km/Kd_490/). Human population data are available at https://sedac.ciesin.columbia.edu/data/set/popdynamics‐global‐pop‐count‐time‐series‐estimates/data‐download#close and https://sedac.ciesin.columbia.edu/data/set/popdynamics‐1‐km‐downscaled‐pop‐base‐year‐projection‐ssp‐2000‐2100‐rev01. Tropical‐cyclone data are available from International Best Track Archive for Climate Stewardship (IBTrACS; www.ncdc.noaa.gov/ibtracs/index.php?name=ibtracsdata). Reef shapefiles were available from ReefBase (http://www.reefbase.org). The methods for determining annual tropical‐cyclone frequency are available in a study by Cacciapaglia and van Woesik (2020). The future RCP4.5 and RCP8.5 SST data from NOAA's Coral Reef Conservation Program are available in van Hooidonk et al. (2016).


