Abstract
This study evaluated venetoclax population pharmacokinetics (popPK) in patients with treatment‐naïve acute myeloid leukemia and assessed the relationship between venetoclax exposure and clinical response for venetoclax in combination with either a hypomethylating agent (HMA) or low‐dose cytarabine (LDAC). A total of 771 patients who received venetoclax from 5 Phase 1–3 studies were included in the popPK model. Exposure‐response analyses included data from 575 patients for venetoclax/placebo plus HMA and 279 patients for venetoclax/placebo plus LDAC. The popPK model successfully characterized venetoclax plasma concentrations over time and confirmed venetoclax exposure did not vary significantly with age, weight, sex, mild to moderate hepatic impairment, or mild to severe renal impairment. Asian patients had 67% higher mean relative bioavailability than non‐Asian patients, however the range of exposures in Asian patients was similar to non‐Asian patients. For all efficacy endpoints with both treatment combinations, efficacy was higher in the venetoclax treatment groups compared with the respective control arm of placebo plus azacitidine or LDAC. Within patients who received venetoclax, no significant exposure‐efficacy relationships were identified for either treatment combination, indicating that the beneficial effects of venetoclax were already maximized in the dose ranges studied. There was no apparent effect of venetoclax exposure on treatment‐emergent Grade ≥3 thrombocytopenia or infections for either combination. Rates of treatment‐emergent Grade ≥3 neutropenia were higher in the venetoclax treatment arms compared with the respective control arms; however, within patients who received venetoclax, there was only a shallow relationship or no apparent relationship with venetoclax exposure for venetoclax plus HMA or LDAC, respectively. Along with the efficacy and safety data previously published, the exposure‐response analyses support the venetoclax dose regimens of 400 mg once daily (QD) plus HMA and 600 mg QD plus LDAC in treatment‐naïve AML patients who are ineligible for intensive chemotherapy.
Keywords: acute myeloid leukemia, dose selection, exposure‐response, hypomethylating agents, low‐dose cytarabine, venetoclax
1. INTRODUCTION
Acute myeloid leukemia (AML) is one of the most aggressive forms of leukemia, and elderly patients who are ineligible for intensive chemotherapy due to co‐morbidities have an especially low 5‐year survival rate of less than 30%. 1 Venetoclax, an orally bioavailable BCL‐2 inhibitor, has been shown in preclinical studies to induce apoptosis in malignant cells that are dependent on BCL‐2 for survival. 2 Venetoclax demonstrated moderate activity as a single agent in patients with relapsed or refractory (R/R) AML, 3 but preclinical models suggested synergistic activity of a hypomethylating agent (HMA) or low‐dose cytarabine (LDAC) in combination with venetoclax. 4
In two Phase 1/2 non‐randomized trials in patients with newly diagnosed AML National Clinical Trial (NCT02203772, NCT02287233), higher overall response rates (ORRs) were observed for the combinations with venetoclax than with historical ORRs for HMA or LDAC alone. 5 , 6 Corresponding exposure‐response analyses showed that venetoclax 400 mg once daily (QD) plus HMA and venetoclax 600 mg QD plus LDAC maximized remission rates and maintained an acceptable safety profile. 7 Subsequently, two placebo‐controlled, randomized, Phase 3 trials were conducted to further establish the efficacy and safety of these venetoclax dose regimens (NCT02993523, VIALE‐A; NCT03069352, VIALE‐C) in newly diagnosed AML patients who were ineligible for intensive chemotherapy. 2 , 5 , 8
The pharmacokinetics (PK) of venetoclax have been well characterized and are described in the literature. 9 , 10 , 11 , 12 , 13 , 14 The objective of the current analyses was to characterize the PK characteristics of venetoclax in AML patients, and evaluate the exposure‐efficacy and exposure‐safety relationships of venetoclax plus HMA or venetoclax plus LDAC.
2. METHODS
2.1. Study designs and assessments
Patients included in the analyses participated in one of five Phase 1–3 trials of venetoclax monotherapy 3 (for PK analysis only), venetoclax plus HMA (azacitidine or decitabine), 2 , 5 or venetoclax plus LDAC 6 , 8 and are described in Table S1 with additional details on trial status, NCT number, doses studied, and efficacy assessments. In all studies, venetoclax was dosed with a daily ramp‐up to reach the target dose of 400–1200 mg QD.
2.2. Population PK analyses
2.2.1. Model parameterization
Population PK models were built using nonlinear mixed effects modeling based on NONMEM 7.4.2 compiled with a GNU Fortran compiler (Version 4.8.3). The first‐order conditional estimation method with n‐ε interaction (FOCE‐INT) was employed for model runs within NONMEM.
The population PK model of venetoclax in chronic lymphocytic leukemia (CLL)/small lymphocytic leukemia (SLL)/non‐Hodgkin lymphoma (NHL) patients and healthy volunteers was used to inform this model. 15 In particular, the structural, statistical (inter‐ and intra‐individual variability) and covariate components of the model were maintained. Population parameter estimates and the variance‐covariance matrix of the fixed effects and estimates for the random effects (inter‐ and intra‐individual variability) from the legacy model were used as informative priors via the $PRIOR TNPRI option in NONMEM and the data from the five studies in AML patients were used to re‐estimate all model parameters.
2.2.2. Covariate selection
Covariate selection was performed by the stepwise forward addition and backward elimination procedure with significance levels of α = 0.01 and α = 0.001, respectively. The influence of race (Asian vs. non‐Asian), azacitidine, decitabine, and LDAC on venetoclax apparent clearance (CL/F), apparent volume of distribution (Vd/F) of the central compartment, and relative bioavailability were tested by incorporating each as a multiplicative factor. Covariates previously included in the CLL/NHL/SLL model were not re‐evaluated for exclusion.
2.2.3. Model evaluation
The final population PK model was evaluated using goodness‐of‐fit plots and prediction‐corrected visual predicted checks (pcVPCs). For pcVPCs, 500 simulated replicates of the dataset were generated using NONMEM. To account for the different doses, covariates, dose modifications and interruptions, the simulated and observed concentrations were prediction corrected and plotted on selected percentile intervals of the simulated data to create pcVPC with time since last dose on the x‐axis.
The final population PK model was used to estimate the venetoclax exposure metric, area under the plasma concentration‐time curve at steady‐state (AUCss) given the planned dose of venetoclax, used in the exposure‐response analyses.
2.3. Exposure‐response analyses
2.3.1. Graphical analyses
The relationships between venetoclax exposure and overall survival (OS) and event‐free survival (EFS) were explored graphically by Kaplan–Meier curves implemented using the survfit function (survival package) with R version 3.5.2.
The relationships between venetoclax exposure and other efficacy endpoints (complete remission [CR], CR + CRi [CR with incomplete hematologic recovery], CR + CRh [CR with partial hematologic recovery], conversion to post‐baseline platelet or red blood cell [RBC] transfusion independence) and safety events were explored graphically using quartile plots.
2.3.2. Model parameterization
Cox proportional hazards models were utilized to describe exposure‐response relationships for survival endpoints and were implemented using the coxph function (survival package) with R version 3.5.2.
Logistic regression models, using the glm function in R version 3.5.2, were developed for all other efficacy and safety variables. Linear and E max models were explored for each endpoint and the better model was selected based on Akaike information criterion (AIC) values. Models were rejected if parameter estimates were not sufficiently precise or did not provide adequate representation of the observed data. Statistical significance of the linear slope or E max relationship was declared at p < 0.01.
2.3.3. Covariate selection
Covariate selection was performed by the stepwise forward addition and backward elimination procedure with significance levels of α = 0.01 and α = 0.001, respectively. Covariates tested in the exposure‐response models included sex, age, race (Asian vs. non‐Asian), concomitant use of cytochrome P450 3A(CYP3A)/P‐glycoprotein (P‐gp) inhibitors, baseline Eastern Cooperative Oncology Group (ECOG) performance status, prior HMA, cytogenetic risk (per National Comprehensive Cancer Network guidelines), AML type (de novo vs. secondary), AML with myelodysplasia‐related changes, HMA type, baseline platelets (exposure‐safety only), baseline neutrophils (exposure‐safety only), and molecular markers, such as FLT3, TP53, IDH1/2, and NPM1.
Covariates were evaluated on the slope (or E max) only if there was a significant exposure‐response relationship. Concomitant medications, such as CYP3A/P‐gp inhibitors or HMA type, were not tested on the intercept.
3. RESULTS
3.1. Population PK
A total of 771 patients with R/R or treatment‐naïve AML receiving at least 1 dose of venetoclax were included in the population PK analysis. The model described venetoclax PK in AML patients with minimal bias (Figure S1). The PK parameters estimated by the model are similar to those of the legacy model (Table S2). Race (Asian vs. non‐Asian) on relative bioavailability and azacitidine on venetoclax Vd/F were identified as significant covariates.
Asian patients had 67% higher relative bioavailability than non‐Asian patients (Figure 1A). Azacitidine was a significant covariate on venetoclax Vd/F (24% higher), but with no meaningful impact on total venetoclax exposure.
FIGURE 1.
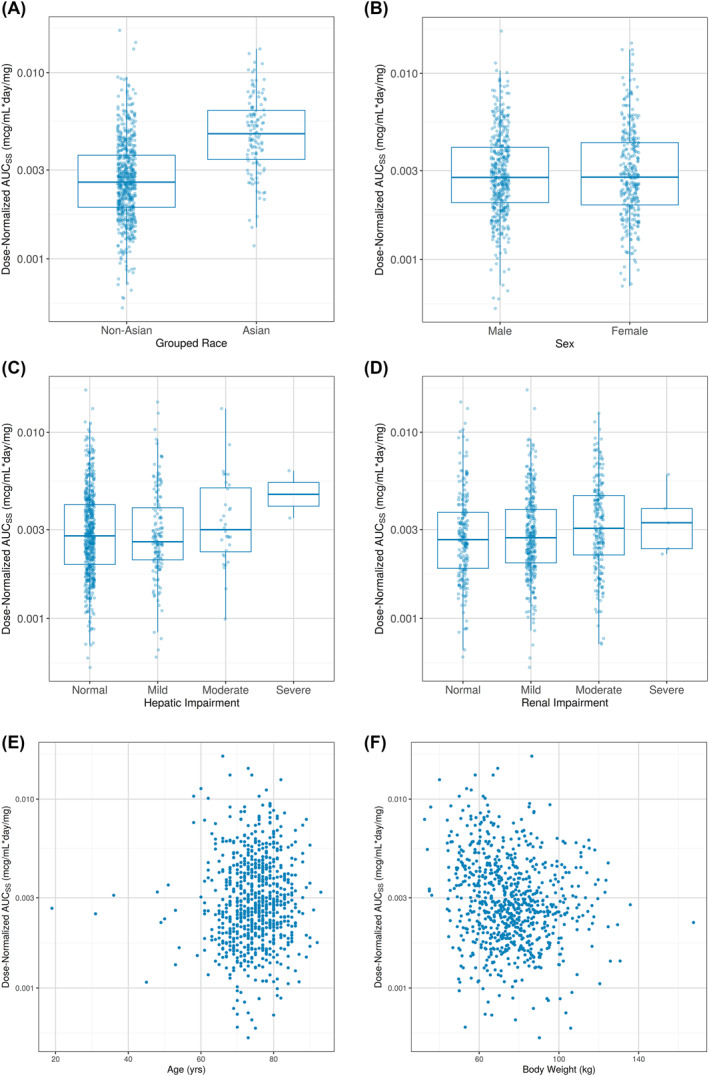
Boxplots and Scatterplots of Post Hoc Dose‐Normalized area under the plasma concentration‐time curve at steady‐state (AUCss) by Categorical and Continuous Covariates, respectively. Venetoclax AUCs s values, normalized for designated cohort dose, are plotted versus categorical covariates (A–D) or continuous covariates (E, F). (A) Asian versus Non‐Asian; (B) Sex; (C) Hepatic Impairment; (D) Renal Impairment; (E) Age; and (F) Body Weight
Sex, mild to moderate hepatic impairment, mild to severe renal impairment, age, and body weight did not appear to have any effect on venetoclax exposure (Figure 1B–F).
Use of strong and moderate CYP3A inhibitors decreased venetoclax CL/F by 83% and 19%, respectively (Figure 2A). Posaconazole, a strong CYP3A inhibitor commonly used for anti‐fungal prophylaxis in AML, had a similar effect on venetoclax CL/F as other strong CYP3A inhibitors (Figure 2B).
FIGURE 2.
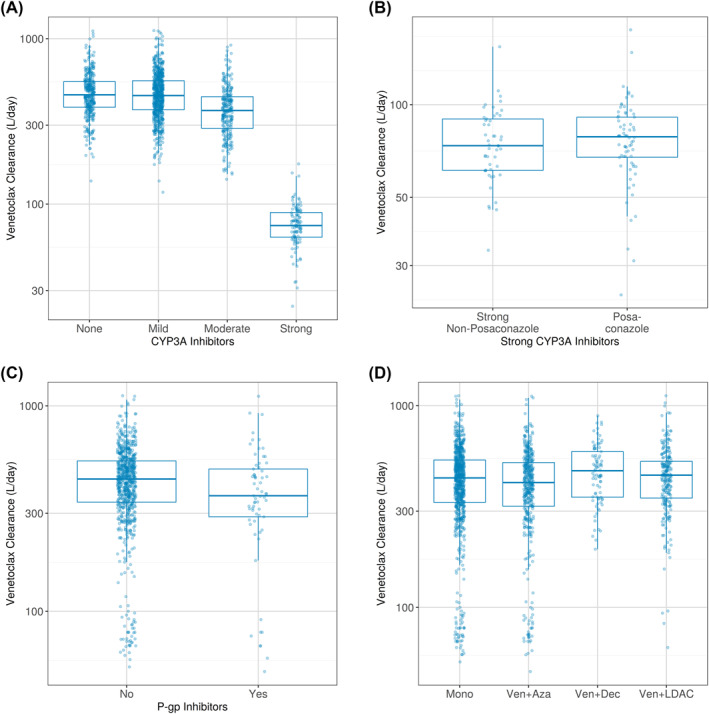
Boxplot of the Post Hoc CL/F by Co‐administration of (A) CYP3A Inhibitors, (B) Posaconazole, (C) P‐gp Inhibitors, (D) AZA, DEC, and LDAC. Patients may appear in more than one category if co‐administration of CYP3A/P‐gp inhibitors changed during treatment. Patients on venetoclax plus AZA, DEC, or LDAC may also appear in venetoclax alone (Mono) if pharmacokinetic data were collected while on venetoclax alone. AZA, azacitidine; CYP3A, cytochrome P450 3A; DEC, decitabine; CL/F, apparent clearance; LDAC, low‐dose cytarabine
Co‐administration of venetoclax with P‐gp inhibitors, mild CYP3A inhibitors, azacitidine, decitabine, or LDAC had no effect on venetoclax CL/F (Figure 2C,D).
3.2. Exposure‐efficacy analyses
3.2.1. Venetoclax plus HMA
Exposure‐response analyses for venetoclax plus HMA (azacitidine or decitabine) were performed using data from patients with treatment‐naïve AML from the Phase 1b non‐randomized study (venetoclax 400, 800, or 1200 mg QD plus azacitidine or decitabine) and all patients from the Phase 3 randomized, double‐blind, placebo‐controlled study (venetoclax 400 mg QD plus azacitidine vs. placebo plus azacitidine). Data from 431 patients taking venetoclax plus HMA and 144 patients taking placebo plus azacitidine were available for analysis.
For all efficacy variables evaluated, venetoclax AUCss quartile plots showed higher efficacy with venetoclax plus HMA than placebo plus azacitidine (Figure 3), but there were no significant exposure‐efficacy relationships identified without the inclusion of the patients in the control arm.
FIGURE 3.
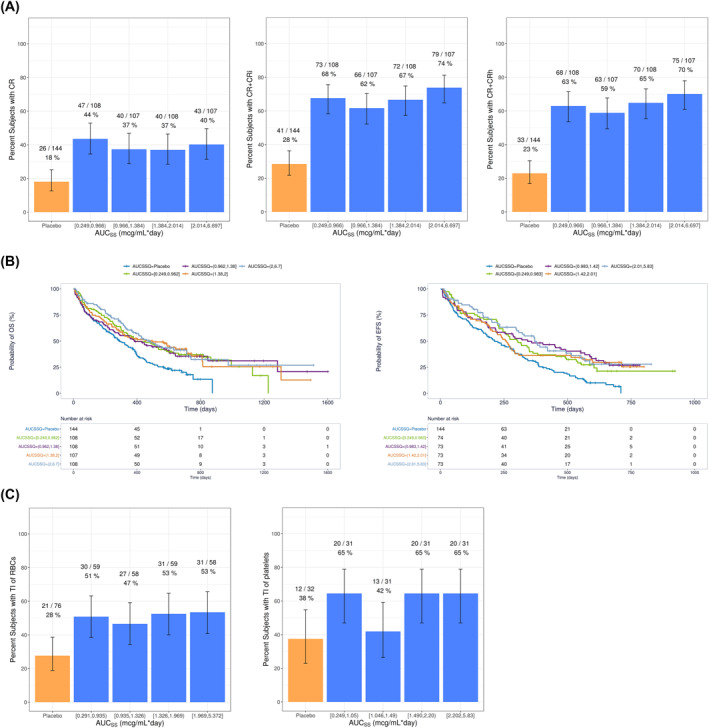
(A) Observed Response Rates and 95% Binomial CIs for Venetoclax Plus HMA by AUCss Quartiles, (B) Kaplan–Meier Curves of OS and EFS by Venetoclax AUCss Quartiles for Venetoclax Plus HMA, and (C) Observed Conversion to Post Baseline Transfusion Independence and 95% Binomial CIs for Venetoclax Plus HMA by Venetoclax AUCss Quartiles. AUCss, Area under the plasma concentration‐time curve at steady state; CR, complete remission; CRi, CR with incomplete hematologic recovery; CRh, CR with partial hematologic recovery; CI, confidence intervals; EFS, event‐free survival; HMA, hypomethylating agent; OS, Overall survival; RBC, red blood cell; TI, transfusion independence
With the inclusion of data from placebo plus azacitidine, there was a statistically significant exposure‐response relationship between venetoclax exposure and probability of response of CR + CRi and CR + CRh (Figure 3A). A maximal effect (E max) model best described the data, with response rates plateauing in the dose range studied (400–1200 mg QD; Figure S2). Similarly, significant (p < 0.01) exposure‐response relationships were observed for OS and EFS, but no significant exposure‐response relationships were identified without the inclusion of patients in the control arm (placebo plus azacitidine; Figure 3B). No significant exposure‐response relationships were identified for conversion to post‐baseline transfusion independence for either RBCs or platelets after accounting for treatment effect (Figure 3C). TP53 mutations were found to be a negative predictor of OS. Intermediate cytogenetic risk was identified as a predictor for better OS and EFS compared to poor cytogenetic risk (Table S3).
3.2.2. Venetoclax plus LDAC
Exposure‐response analyses for venetoclax plus LDAC were performed using data from patients with treatment‐naïve AML from the Phase 1/2 non‐randomized study (venetoclax 600 or 800 mg QD plus LDAC) and all patients from the Phase 3 randomized, double‐blind, placebo‐controlled study (venetoclax 600 mg QD plus LDAC vs. placebo plus LDAC). Data from 211 patients taking venetoclax plus LDAC and 68 patients taking placebo plus LDAC were available for analysis.
For all efficacy variables evaluated, venetoclax AUCss quartile plots showed higher efficacy with venetoclax plus LDAC than placebo plus LDAC (Figure 4), but there were no significant exposure‐efficacy relationships identified without the inclusion of the patients in the control arm.
FIGURE 4.
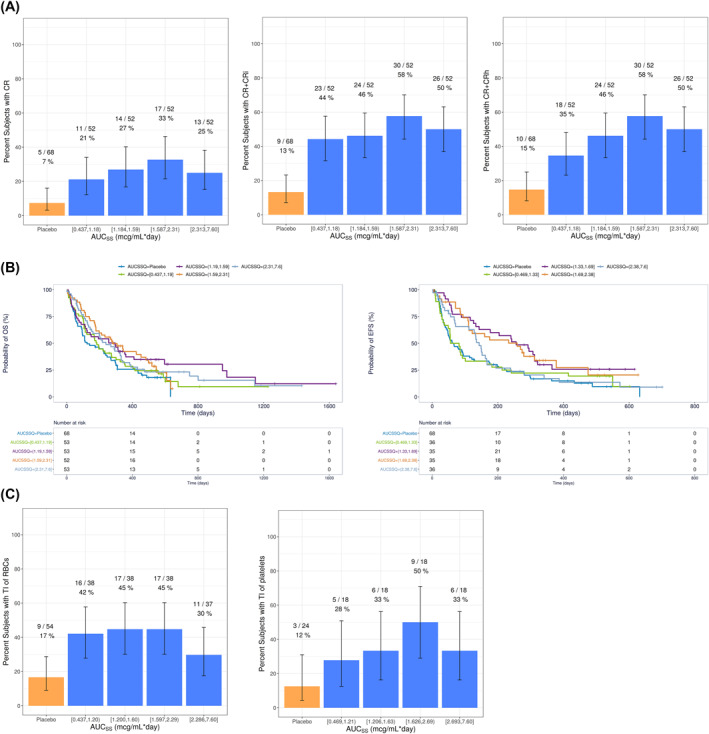
(A) Observed Response Rates and 95% Binomial CIs for Venetoclax Plus LDAC by AUCss Quartiles, (B) Kaplan–Meier Curves of OS and EFS by Venetoclax AUCss Quartiles for Venetoclax Plus LDAC, and (C) Observed Conversion to Post Baseline Transfusion Independence and 95% Binomial CIs for Venetoclax Plus LDAC by Venetoclax AUCss Quartiles. AUCss, Area under the plasma concentration‐time curve at steady state; CR, complete remission; CRi, CR with incomplete hematologic recovery; CRh, CR with partial hematologic recovery; CI, confidence intervals; EFS, event‐free survival; LDAC, low‐dose cytarabine; OS, Overall survival; RBC, red blood cell; TI, transfusion independence
With the inclusion of data from placebo plus LDAC, there was a statistically significant exposure‐response relationship between venetoclax exposure and probability of response of CR, CR + CRi, and CR + CRh (Figure 4A). An E max model best described the data (Figure S3).
For OS, EFS, and conversion from baseline transfusion dependence to post‐baseline transfusion independence (RBC or platelets), there was a trend for better response with venetoclax plus LDAC than placebo plus LDAC, but no significant (p > 0.01) exposure‐response relationships (Figure 4B,C). Covariate analysis identified TP53 mutations as a negative predictor of both OS and EFS and that patients with ECOG scores ≥2 had worse OS (Table S4).
3.3. Exposure‐safety analyses
3.3.1. Venetoclax plus HMA
Of the safety variables evaluated, only treatment‐emergent Grade ≥3 neutropenia showed any relationship to venetoclax (Figure 5A). An E max model best described the statistically significant (p < 0.01) relationship between treatment‐emergent Grade ≥3 neutropenia and venetoclax exposure (Table S5). Covariate analysis identified that patients with an Asian race were more likely to have treatment‐emergent Grade ≥3 neutropenia, regardless of whether they received venetoclax plus HMA or placebo plus azacitidine, and that patients with higher baseline neutrophils had a slightly increased E max effect of venetoclax (Figure S4; Table S5).
FIGURE 5.
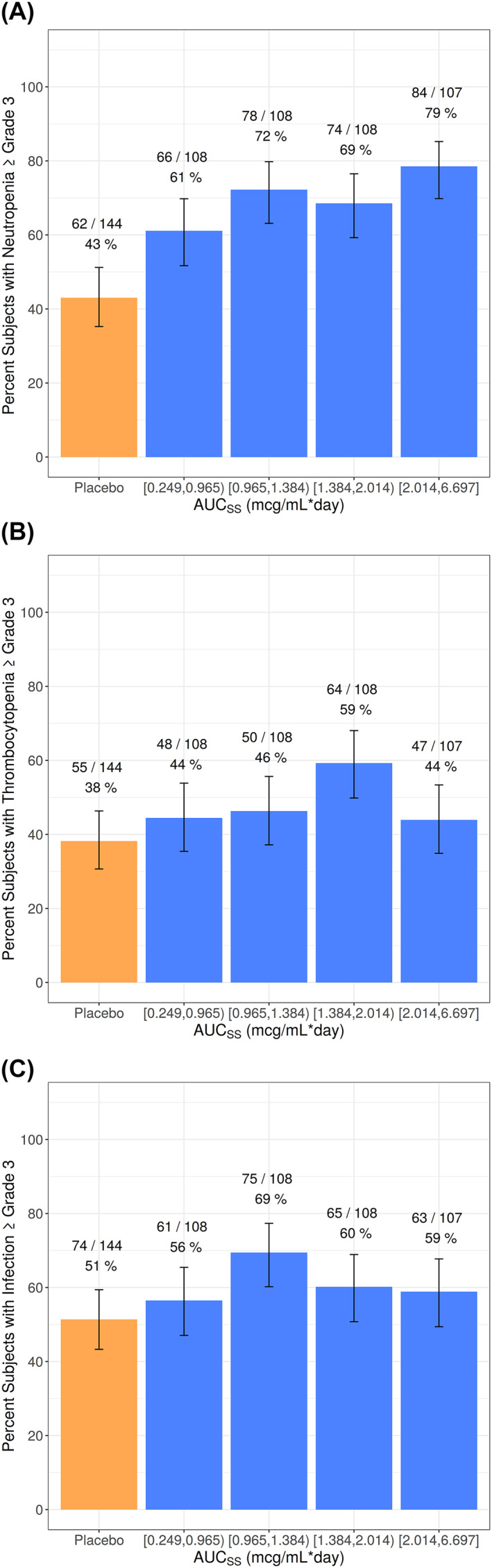
Occurrence of Observed Treatment‐Emergent Grade 3 or Worse Adverse Events and 95% Binomial CIs for Venetoclax Plus HMA by Venetoclax AUCss Quartiles (A) Neutropenia, (B) Thrombocytopenia, (C) Infections. AUCSS, area under the plasma concentration‐time curve at steady state; CI, confidence interval; HMA, hypomethylating agent
Model predictions show that, when compared with placebo plus azacitidine, venetoclax plus HMA had an approximately 20%–25% higher probability of treatment‐emergent Grade ≥3 neutropenia. This magnitude of difference between venetoclax plus HMA and placebo plus azacitidine was similar for patients with an Asian race and for patients with a non‐Asian race (Table 1). When data from placebo plus azacitidine were excluded from the analysis, there was only a shallow but not statistically significant (p > 0.01) exposure‐response relationship between treatment‐emergent Grade ≥3 neutropenia and higher venetoclax exposures. Irrespective of whether data from placebo plus azacitidine was included, there were no apparent relationships between venetoclax exposure and treatment‐emergent Grade ≥3 thrombocytopenia or treatment‐emergent Grade ≥3 infections (Figure 5B,C).
TABLE 1.
Median model‐predicted probabilities of treatment‐emergent Grade ≥3 neutropenia for venetoclax plus HMA or placebo plus AZA
| Race | N | Median exposure (AUCss) at 400 mg venetoclax (µg*day/ml) | Predicted probability (%) for PBO + AZA | Predicted probability (%) for VEN 400 mg + HMA | Difference (%) between VEN + HMA and PBO + AZA | P‐value a |
|---|---|---|---|---|---|---|
| Non‐Asian | 464 b | 1.11 | 36.4 | 61.0 | 24.6 | ‐‐ |
| Asian | 111 | 1.85 | 64.4 | 84.9 | 20.5 | 0.98 |
Abbreviations: AZA, azacitidine; HMA, hypomethylating agent; PBO, placebo; VEN, venetoclax.
Effect of Asian race on E max parameter (venetoclax effect) in final model.
Includes 5 subjects with missing race information.
3.3.2. Venetoclax plus LDAC
Venetoclax plus LDAC had a higher incidence of treatment‐emergent Grade ≥3 neutropenia than placebo plus LDAC; however, within patients who received venetoclax plus LDAC, there was no statistically significant relationship between venetoclax exposure and treatment‐emergent Grade ≥3 neutropenia (Figure 6A). There were no apparent exposure‐response relationships between venetoclax exposure and incidence of treatment‐emergent Grade ≥3 thrombocytopenia or treatment‐emergent Grade ≥3 infections (Figure 6B,C).
FIGURE 6.
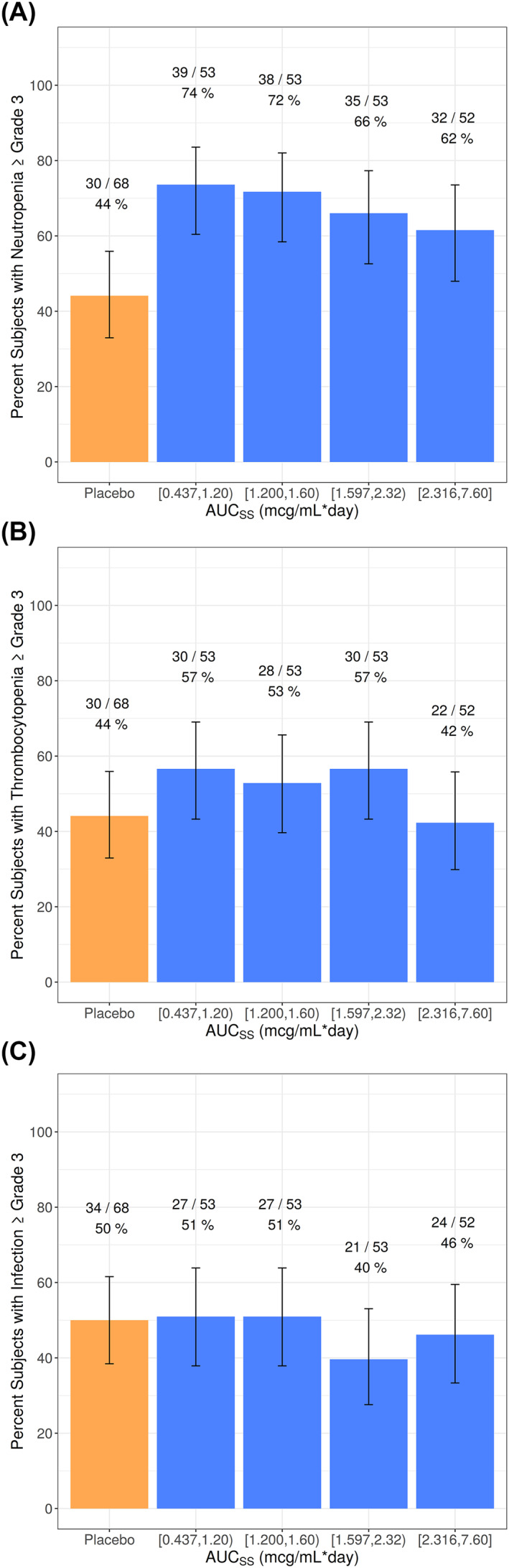
Occurrence of Observed Treatment‐Emergent Grade 3 or Worse Adverse Events and 95% Binomial CIs for Venetoclax Plus LDAC by Venetoclax AUCss Quartiles (A) Neutropenia, (B) Thrombocytopenia, (C) Infections. AUCSS, area under the plasma concentration‐time curve at steady state; CI, confidence interval; LDAC, low‐dose cytarabine
4. DISCUSSION
The analyses presented here were based on data from venetoclax plus HMA or venetoclax plus LDAC in patients with treatment‐naïve AML who were ineligible for intensive chemotherapy. These analyses characterized the exposure‐response relationships for these venetoclax combination regimens, including population PK analysis to inform dosing in special populations (i.e., renal impairment). The exposure‐response relationships of venetoclax plus HMA and venetoclax plus LDAC for ORRs and key safety endpoints using data from non‐randomized Phase 1/2 studies were previously published 7 ; the current analyses, which have included data from two randomized placebo‐controlled Phase 3 studies, are consistent with those findings. Additionally, the current analyses have evaluated the exposure‐response relationships for efficacy endpoints of survival and conversion to post‐baseline transfusion independence for RBCs or platelets.
The updated venetoclax population PK model is generally consistent with the legacy model developed by Jones et al. 15 The venetoclax PK in patients with AML was similar to that in patients with other hematological malignancies. Additionally, this is the first population PK analysis of venetoclax that included an adequate number of Asian patients to characterize the effect of Asian versus non‐Asian on the PK of venetoclax, and Asian patients had 67% higher relative bioavailability than non‐Asian patients. While the mean change was higher, the range of individual exposures in Asian patients was generally comparable to the range of exposures in non‐Asian patients (Figure 1A). When considering this in combination with the lack of meaningful exposure‐safety relationships observed with venetoclax, no dose adjustments are recommended for Asian patients treated with venetoclax.
Strong and moderate CYP3A inhibitors decreased venetoclax CL/F by 83% and 19%, respectively, consistent with results from previous drug‐drug interaction studies. 16 , 17 The present analysis showed that posaconazole had a similar magnitude of effect on venetoclax CL/F as other strong CYP3A inhibitors (Figure 2B), suggesting that a uniform dose adjustment for all strong CYP3A inhibitors may be adequate. Although the population PK analysis identified azacitidine as increasing venetoclax Vd/F by 24%, co‐administration does not affect total venetoclax exposure and is not likely to be clinically relevant. Azacitidine is not a known inhibitor or inducer of metabolizing enzymes or transporters, and therefore there is little biological rationale for an interaction between azacitidine and venetoclax.
The population PK model included concentration data from five patients with severe renal impairment (creatinine clearance of ≥15 ml/min and <30 ml/min), which has not been previously studied. The model showed comparable venetoclax exposure in these patients as patients with normal renal function or mild to moderate renal impairment. This supports that no dose adjustment is necessary in patients with severe renal impairment based on PK.
Exposure‐efficacy and exposure‐safety analyses were conducted for patients with treatment‐naïve AML receiving venetoclax or placebo plus an HMA or LDAC, consistent with previous analyses of venetoclax given as monotherapy or combination therapy across several malignancies. 18 , 19 , 20 , 21 , 22 , 23 For patients receiving venetoclax plus HMA, increased efficacy was seen for all venetoclax treatment groups compared with the control arm. However, when patients receiving placebo were excluded from the analysis, there was no relationship between venetoclax exposure and any efficacy endpoint, suggesting that the beneficial effect of venetoclax is already maximized in the dose range studied (400–1200 mg QD venetoclax) and that 400 mg QD venetoclax was sufficient to obtain maximum efficacy in combination with an HMA. There were no apparent relationships between venetoclax exposure and treatment‐emergent Grade ≥3 infections or thrombocytopenia. Neutropenia is a common manifestation of AML and an identified risk of venetoclax administration. An E max model best described the statistically significant relationship between venetoclax exposure and treatment‐emergent Grade ≥3 neutropenia for patients taking venetoclax plus HMA. Within patients who received venetoclax plus HMA, there was a shallow but not statistically significant relationship between treatment‐emergent Grade ≥3 neutropenia and higher venetoclax exposure, further supporting the venetoclax 400 mg QD regimen plus HMA.
Covariate analysis identified Asian patients as having a higher likelihood of treatment‐emergent Grade ≥3 neutropenia regardless of whether they received venetoclax plus HMA or placebo plus azacitidine. Table 1 shows that the net effect of venetoclax on the risk of neutropenia is similar between Asian patients and non‐Asian patients, even after accounting for the difference in expected exposures between the two groups. Therefore, this finding seems to be independent of venetoclax exposure. It is unknown what other intrinsic or extrinsic factors could have contributed to this.
For patients receiving venetoclax plus LDAC, a similar exposure‐efficacy relationship was observed, with increased efficacy observed for all venetoclax treatment groups compared with the control arm. Again, without the inclusion of patients in the control arm, there was no relationship between venetoclax exposure and any efficacy endpoint, suggesting that the beneficial effect of venetoclax is already maximized in the dose range studied (600–800 mg QD venetoclax) and that 600 mg venetoclax was sufficient to obtain maximum efficacy in combination with LDAC. Although 400 mg QD venetoclax plus LDAC was not evaluated, the analyses supporting the recommended Phase 3 dose of 600 mg QD identified that approximately 10% higher response rates were expected with 600 mg QD as compared to 400 mg QD. 7 For venetoclax plus LDAC, there were no relationships between venetoclax exposure and treatment‐emergent Grade ≥3 infections or thrombocytopenia. Patients taking venetoclax plus LDAC had higher rates of treatment‐emergent Grade ≥3 neutropenia than those taking placebo plus LDAC. However, within patients who received venetoclax plus LDAC, there was no apparent relationship between venetoclax exposure and treatment‐emergent Grade ≥3 neutropenia.
5. CONCLUSIONS
The population PK model successfully characterized venetoclax plasma concentrations over time and was able to identify the key intrinsic and extrinsic factors affecting venetoclax PK in AML patients. Together with the efficacy and safety data previously published, 2 , 8 the venetoclax exposure‐efficacy and exposure‐safety analyses support the venetoclax dose regimens of 400 mg QD plus HMA and 600 mg QD plus LDAC in treatment‐naïve AML patients who are ineligible for intensive chemotherapy.
CONFLICT OF INTEREST
Deanna Brackman, Doerthe Eckert, Rajeev Menon, Ahmed Hamed Salem, Jalaja Potluri, John Hayslip, Sven Mensing, and Jiuhong Zha are employees of AbbVie and may hold AbbVie stock. Sathej Gopalakrishnan is a former employee of AbbVie and may hold AbbVie stock. Douglas Smith served as a consultant for Novartis, Jubilant, Pfizer, Takeda, BMS, Jazz and on the Data and Safety Monitoring Board for BMS/Celgene, Cellularity. Andrew H. Wei served as a consultant for Celgene, AbbVie, Roche, Janssen, Astellas, Novartis, Amgen, MacroGenics, Servier, received research funding from Novartis, Celgene, AbbVie, and Servier, and is a former employee of the Walter and Eliza Hall Institute and receives a fraction of its royalty stream related to venetoclax. Dale Miles is an employee of Genentech Inc. and may own Roche stock.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/hon.2964.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
AbbVie, Genentech/Roche, and the authors thank the patients and investigators who participated in the trials. Medical writing support was provided by Mia DeFino, MS, ELS, a freelance medical writer under contract with AbbVie. The studies were supported by AbbVie in collaboration with Genentech/Roche. Venetoclax (ABT‐199/GDC‐0199) is being developed in collaboration between AbbVie and Genentech. AbbVie and Genentech provided financial support for the studies and participated in the study design, study conduct, and analysis and interpretation of data and the writing, review, and approval of the manuscript.
Brackman D, Eckert D, Menon R, et al. Venetoclax exposure‐efficacy and exposure‐safety relationships in patients with treatment‐naïve acute myeloid leukemia who are ineligible for intensive chemotherapy. Hematol Oncol. 2022;40(2):269‐279. 10.1002/hon.2964
Deanna Brackman and Doerthe Eckert have contributed equally.
Affiliation of John Hayslip and Sathej Gopalakrishnan was at the time of conducting this work.
Contributor Information
Deanna Brackman, Email: deanna.brackman@abbvie.com.
Jiuhong Zha, Email: jiuhong.zha@abbvie.com.
DATA AVAILABILITY STATEMENT
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our‐science/clinical‐trials/clinical‐trials‐data‐and‐information‐sharing/data‐and‐information‐sharing‐with‐qualified‐researchers.html.
REFERENCES
- 1. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291‐299. 10.1182/blood-2015-01-621664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617‐629. [DOI] [PubMed] [Google Scholar]
- 3. Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bogenberger JM, Delman D, Hansen N, et al. Ex vivo activity of BCL‐2 family inhibitors ABT‐199 and ABT‐737 combined with 5‐azacytidine in myeloid malignancies. Leuk Lymphoma. 2015;56(1):226‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non‐randomised, open‐label, phase 1b study. Lancet Oncol. 2018;19(2):216‐228. [DOI] [PubMed] [Google Scholar]
- 6. Wei AH, Strickland SA, Jr. , Hou JZ, et al. Venetoclax combined with low‐dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agarwal S, Gopalakrishnan S, Mensing S, et al. Optimizing venetoclax dose in combination with low intensive therapies in elderly patients with newly diagnosed acute myeloid leukemia: an exposure‐response analysis. Hematol Oncol. 2019;37(4):464‐473. [DOI] [PubMed] [Google Scholar]
- 8. Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo‐controlled trial. Blood. 2020;135(24):2137‐2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheung TT, Salem AH, Menon RM, Munasinghe WP, Bueno OF, Agarwal SK. Pharmacokinetics of the BCL‐2 inhibitor venetoclax in healthy Chinese subjects. Clin Pharmacol Drug Dev. 2018;7(4):435‐440. [DOI] [PubMed] [Google Scholar]
- 10. Liu H, Michmerhuizen MJ, Lao Y, et al. Metabolism and disposition of a novel B‐cell lymphoma‐2 inhibitor venetoclax in humans and characterization of its unusual metabolites. Drug Metab Dispos. 2017;45(3):294‐305. [DOI] [PubMed] [Google Scholar]
- 11. Agarwal SK, Tong B, Bueno OF, Menon RM, Salem AH. Effect of azithromycin on venetoclax pharmacokinetics in healthy volunteers: implications for dosing venetoclax with P‐gp inhibitors. Adv Ther. 2018;35(11):2015‐2023. [DOI] [PubMed] [Google Scholar]
- 12. Salem AH, Dave N, Marbury T, et al. Pharmacokinetics of the BCL‐2 inhibitor venetoclax in subjects with hepatic impairment. Clin Pharmacokinet. 2019;58(8):1091‐1100. [DOI] [PubMed] [Google Scholar]
- 13. Salem AH, Dunbar M, Agarwal SK. Pharmacokinetics of venetoclax in patients with 17p deletion chronic lymphocytic leukemia. Anti Cancer Drugs. 2017;28(8):911‐914. [DOI] [PubMed] [Google Scholar]
- 14. Chiney MS, Menon RM, Bueno OF, Tong B, Salem AH. Clinical evaluation of P‐glycoprotein inhibition by venetoclax: a drug interaction study with digoxin. Xenobiotica. 2018;48(9):904‐910. 10.1080/00498254.2017.1381779 [DOI] [PubMed] [Google Scholar]
- 15. Jones AK, Freise KJ, Agarwal SK, Humerickhouse RA, Wong SL, Salem AH. Clinical predictors of venetoclax pharmacokinetics in chronic lymphocytic leukemia and non‐Hodgkin's lymphoma patients: a pooled population pharmacokinetic analysis. AAPS J. 2016;18(5):1192‐1202. [DOI] [PubMed] [Google Scholar]
- 16. Agarwal SK, Salem AH, Danilov AV, et al. Effect of ketoconazole, a strong CYP3A inhibitor, on the pharmacokinetics of venetoclax, a BCL‐2 inhibitor, in patients with non‐Hodgkin lymphoma. Br J Clin Pharmacol. 2017;83(4):846‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agarwal SK, DiNardo CD, Potluri J, et al. Management of venetoclax‐posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther. 2017;39(2):359‐367. [DOI] [PubMed] [Google Scholar]
- 18. Freise KJ, Jones AK, Eckert D, et al. Impact of venetoclax exposure on clinical efficacy and safety in patients with relapsed or refractory chronic lymphocytic leukemia. Clin Pharmacokinet. 2017;56(5):515‐523. [DOI] [PubMed] [Google Scholar]
- 19. Freise KJ, Jones AK, Menon RM, et al. Relationship between venetoclax exposure, rituximab coadministration, and progression‐free survival in patients with relapsed or refractory chronic lymphocytic leukemia: demonstration of synergy. Hematol Oncol. 2017;35(4):679‐684. [DOI] [PubMed] [Google Scholar]
- 20. Parikh A, Gopalakrishnan S, Freise KJ, et al. Exposure‐response evaluations of venetoclax efficacy and safety in patients with non‐Hodgkin lymphoma. Leuk Lymphoma. 2018;59(4):871‐879. [DOI] [PubMed] [Google Scholar]
- 21. Deng R, Gibiansky L, Lu T, et al. Exposure‐response analysis of venetoclax in combination with rituximab in patients with relapsed or refractory chronic lymphocytic leukemia: pooled results from a phase 1b study and the phase 3 MURANO study. Leuk Lymphoma. 2020;61(1):56‐65. [DOI] [PubMed] [Google Scholar]
- 22. Freise KJ, Dunbar M, Jones AK, et al. Venetoclax does not prolong the QT interval in patients with hematological malignancies: an exposure‐response analysis. Cancer Chemother Pharmacol. 2016;78(4):847‐853. [DOI] [PubMed] [Google Scholar]
- 23. Freise KJ, Jones AK, Verdugo ME, Menon RM, Maciag PC, Salem AH. Moving beyond maximum tolerated dose for targeted Oncology drugs: use of clinical utility index to optimize venetoclax dosage in multiple myeloma patients. Clin Pharmacol Ther. 2017;102(6):970‐976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our‐science/clinical‐trials/clinical‐trials‐data‐and‐information‐sharing/data‐and‐information‐sharing‐with‐qualified‐researchers.html.


