Abstract
Objective
To compare the efficacy and safety of avanafil as compared with sildenafil in the management of patients with erectile dysfunction.
Methods
It was a prospective, randomized, double‐blind, two‐arm, active‐controlled, parallel, multicenter, non‐inferiority clinical study carried out in patients with erectile dysfunction for at least 3 months and International Index of Erectile Function – Erectile Function domain score of <26 at enrolment.
Results
A total of 220 patients were randomized to receive either avanafil tablets 100 mg or sildenafil tablets 50 mg in 1:1 ratio. After 4 weeks of treatment, 40.0% of patients in the avanafil group and 45.6% of patients in the sildenafil group required dose escalation to a high dose (avanafil 200 mg/sildenafil 100 mg). The difference in the mean change of International Index of Erectile Function – Erectile Function score from baseline in the two groups increased from week 4 (1.1, 95% confidence interval −0.2 to 2.5) to week 8 (1.4, 95% confidence interval 0.1–2.7) and week 12 (2.1, 95% confidence interval 0.8–3.5), showing non‐inferiority at week 4, and superiority at week 8 and week 12. Avanafil showed a faster onset of action as shown by a significantly better response to modified Sexual Encounter Profile 1 in the avanafil group (84.8%) as compared with that in the sildenafil group (28.2%; P < 0.001). Both avanafil and sildenafil were well tolerated by all the patients in the study; the most common adverse event reported during the study was headache in both the groups.
Conclusion
Avanafil is superior to sildenafil in improving the International Index of Erectile Function – Erectile Function domain score at the end of 12 weeks of treatment with the added advantage of faster onset of action.
Keywords: avanafil, erectile dysfunction, phosphodiesterase 5 inhibitors, sildenafil
Abbreviations & Acronyms
- CI
confidence interval
- ED
erectile dysfunction
- IIEF‐EF
International Index of Erectile Function – Erectile Function
- i.p.
investigational product
- LOCF
last observation carried forward
- PDE5
phosphodiesterase 5
- SEP
Sexual Encounter Profile
- Tmax
time taken to reach the maximum concentration
Introduction
ED is defined as the inability to attain or maintain an erection sufficient for satisfactory sexual performance. Worldwide, more than 150 million men are reported to be affected by ED, with an estimated incidence rate of 26 new cases per 1000 men annually. With the expected rise in the aging population, the demand for therapies for the treatment of ED will continue to increase. 1 The causes of ED are often multifactorial, and consist of a mix of organic and psychogenic factors. 2 Various medical and interventional therapies are available for management of ED, such as PDE5 inhibitors, vacuum erection devices, penile self‐injection regimens (with vasoactive drugs such as alprostadil) and penile prostheses; however, because of the ease of use, good efficacy and favorable adverse effect profiles, PDE5 inhibitors have been recognized as the first‐line of therapy. 3
Various PDE5 inhibitors, such as sildenafil, tadalafil, udenafil and vardenafil, have shown efficacy rates as high as 80% in ED patients, associated with comorbidities, such as diabetes, hypertension, cardiovascular disease, neurological disorders (such as spinal cord injury, multiple sclerosis), renal insufficiency and a history of urological pelvic surgery. The efficacy of these available PDE5 inhibitors has been acceptable with both on‐demand and chronic use; however, they are often associated with adverse events, such as headache, flushing, dyspepsia, visual disturbances, back pain, tachycardia and nasal congestion, attributed to non‐selective inhibition of other PDE isoenzymes in the body. 1 , 3 , 4 , 5 Also, there are still a number of patients who fail to respond clinically to PDE5 treatment due to lack of efficacy or intolerable adverse effects or a combination of the two. Up to 70% of men who seek care for their ED discontinue treatment within 2–3 years of follow up. 1 , 6 Hence, physicians continue to seek out novel and alternative formulations to maximize efficacy and minimize adverse effects.
Avanafil (4‐[(3‐chloro‐4‐methoxybenzyl)amino]‐2‐[2‐(hydroxymethyl)‐1‐pyrrolidinyl]‐N‐(2‐pyrimidinylmethyl)‐5‐pyrimidinecarboxamide), a second‐generation selective PDE5 inhibitor, has a rapid onset of action (as early as 15 min), a Tmax of 30–45 min and a terminal half‐life of 3–5 h. The recommended starting dose of avanafil is 100 mg taken as early as approximately 15 min before sexual activity, on an as needed basis, and based on efficacy and/or tolerability, the dose can be increased to 200 mg taken as early as approximately 15 min before sexual activity, or decreased to 50 mg taken approximately 30 min before sexual activity. 7 Avanafil has already been approved in the USA since 2012, in the European Union since 2013 and various other countries, such as South Korea, Australia, New Zealand, Russia, Jordan, UAE, Turkey and Saudi Arabia, based on the favorable efficacy and safety profile of the product as confirmed in a number of double‐blind, placebo‐controlled, randomized clinical trials, carried out both in the general population and in “difficult” to treat patient subgroups, such as those with diabetes mellitus or those who have undergone nerve‐sparing radical prostatectomy. 8 , 9 , 10 , 11 , 12
Even after many years of approval and successful use in the management of ED, only limited data are available on comparative evaluation of the efficacy and safety of avanafil with other PDE5 inhibitors. To date, only a single study has been published comparing the efficacy and safety of avanafil to sildenafil, that too in difficult to treat subset of patients; that is, patients undergoing rehabilitation therapy after robot‐assisted unilateral nerve‐sparing prostatectomy. 13 The current study was planned to compare the efficacy and safety of avanafil to sildenafil in the management of Indian patients with ED.
Methods
The present prospective, randomized, double‐blind, two‐arm, active‐controlled, parallel, multicenter, non‐inferiority phase III clinical trial was carried out at six tertiary care centers in India from January 2020 to October 2020. The study was carried out by consultant urologists and psychiatrists in compliance with the Indian Good Clinical Practice Guidelines and Ethical Principles of the Declaration of Helsinki. The study was approved by the Office of the Drug Controller General of India and was registered with the Clinical Trials Registry of India (www.ctri.nic.in; CTRI/2020/01/022798). The study was initiated after review and approval by the Institutional Ethics Committees at each of the six participating study centers. Written informed consent was obtained from all patients before initiation of any study related activity.
Patients
Patients aged ≥21 years were enrolled in the study. Patients were required to have a history of ED for at least 3 months; score of <26 on the IIEF‐EF domain; heterosexual, monogamous steady relationship for the past 6 months; and adequate literacy to complete the diary card and evaluation questionnaires.
Key exclusion criteria were: patients engaged in polygamy and homosexual relationship; had used PDE5 inhibitors or any other drug for ED in the past 4 weeks; tried PDE5 inhibitors in the past and had discontinued either due to lack of efficacy or significant side‐effects, or had known hypersensitivity to sildenafil, avanafil or other PDE5 inhibitors; patients with history of primary hypoactive sexual desire, hypogonadism, spinal cord injury, pelvic surgery and radical prostatectomy; history of or predisposition to priapism; presence of penile anatomical abnormalities; and use of penile implants. Patients were not allowed to use the following medications during the study period: nitrates/sodium nitroprusside/nitric oxide donors; alpha‐blockers; anticoagulants. except antiplatelet agents; androgens/anti‐androgens; cytochrome P450 3A4, inhibitors, such as HIV protease inhibiters (ritonavir, indinavir); anti‐mycotic agents (itraconazole, ketoconazole); erythromycin; any other medication for ED, including over‐the‐counter, herbal or Ayurveda products; antipsychotics/antidepressants; and amlodipine.
Study procedures and drugs
Patients satisfying the eligibility criteria were randomized in a 1:1 ratio to receive either the test (avanafil) or reference (sildenafil) drugs, in a double blind fashion. Avanafil 100 mg tablets were similar to sildenafil 50 mg tablets, whereas avanafil tablets 200 mg were similar to sildenafil 100 mg tablets. Patients were followed up for a total duration of 12 weeks, with scheduled visits at 4‐week intervals during the present study. Patients were asked to take the study drugs (test – avanafil tablets 100 mg/200 mg or reference – sildenafil tablets 50 mg/100 mg) before the initiation of sexual activity. All patients were given a low dose of the medication; that is, avanafil tablets 100 mg/sildenafil tablets 50 mg to begin with. After 4 weeks of treatment, if the dose was required to be increased, the patients were given avanafil tablets 200 mg/sildenafil tablets 100 mg. Patients were allowed to take only one dose of study medication in a day (calendar day), and were asked to take at least four doses of the medication (and attempt intercourse) on four separate occasions during each 4‐week follow‐up period.
Efficacy and safety assessments
The efficacy of the study drugs was evaluated by completing the 15‐item validated IIEF questionnaire based on the sexual experience of patients during the past 4 weeks at each visit.
In addition to the IIEF questionnaire, patients were also asked to answer the following three questions from SEP after taking each dose and each attempt at sexual intercourse in diary cards: were you able to achieve at least some erection (some enlargement of the penis) in 15 min after drug intake (modified SEP 1); were you able to insert your penis into your partner’s vagina (SEP 2); and did your erection last long enough for you to have successful intercourse (SEP 3).
The percentage of successful vaginal penetrations (based on SEP 2), percentage of successful intercourse (based on SEP 3) and percentage of doses with some erection in 15 min were calculated based on the number of doses taken and sexual attempts made by the patient between each 4‐week visit duration.
The safety of the study medication was assessed by recording the adverse events occurring during the course of the study. Routine hematological and biochemical laboratory investigations and electrocardiogram were carried out at screening and end of the study. All abnormalities found in the physical examination (including vitals) and laboratory investigations were dealt with as adverse events.
Statistical analysis
The primary end‐point of the study was the change in the IIEF‐EF domain score in the two groups. The secondary efficacy end‐points included the percentage of patients reaching a normal IIEF‐EF score; the IIEF score in domains other than EF in the two groups; percentage of successful vaginal penetration and successful intercourse in the two groups; and percentage of doses with some erection in 15 min in the two groups. All the end‐points were evaluated at the end of 4, 8 and 12 weeks of treatment.
The sample size of the study was based on the primary efficacy end‐point (change in the IIEF‐ED domain score at the end of the study). A total of 220 patients were required to be enrolled in the study to achieve 90% power with 2.5% one‐sided level of significance, considering a non‐inferiority margin of 3 (less than the minimum score defining clinically significant change i.e. 4), 14 and assuming no difference between the test and the reference groups in the change in the IIEF‐ED domain score at the end of the study (week 12), common standard deviation of 6.5 and a dropout rate of 10%.
The LOCF method has been used to impute missing data in patients who were lost to follow up or dropouts.
Results
A total of 220 patients with ED were enrolled in the present randomized, double‐blind, active‐controlled, multicenter, phase III clinical trial. A total of 111 patients were assigned to the avanafil group, and 109 were assigned to the sildenafil group. The flow of patients in the study is shown in Figure 1. A total of 108 patients in the avanafil group, and 103 patients in the sildenafil group were considered for intention‐to‐treat efficacy analysis. The demographic profile and baseline characteristics of patients enrolled in the study were similar in the two groups (Table 1). Most of the patients enrolled in the study had moderate‐to‐severe ED.
Fig. 1.
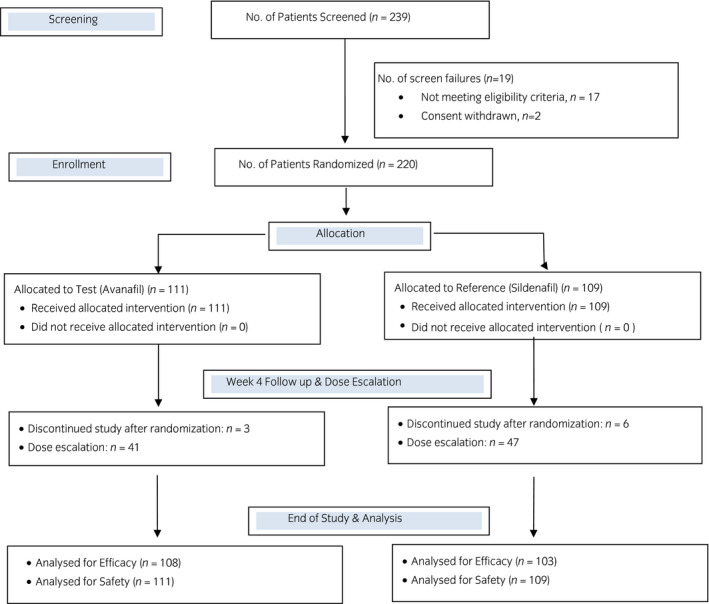
Flow of patients in the study.
Table 1.
Demographic and baseline characteristics of all enrolled patients
| Avanafil (n = 111) | Sildenafil (n = 109) | P‐value | |
|---|---|---|---|
| Age (years) | 36.4 ± 9.0 (34.7–38.1) | 37.1 ± 8.9 (35.4–38.7) | 0.60 |
| Height (cm) | 169.6 ± 3.8 (168.9–170.3) | 169.4 ± 4.5 (168.6–170.3) | 0.74 |
| Weight (kg) | 71.0 ± 6.8 (69.7–72.3) | 70.7 ± 7.6 (69.3–72.2) | 0.79 |
| BMI (kg/m2) | 24.7 ± 2.0 (24.3–25.0) | 24.6 ± 2.3 (24.2–25.1) | 0.91 |
| History of ED (months) | 8.6 ± 5.6 (7.6–9.7) | 7.9 ± 4.4 (7.1–8.7) | 0.27 |
| Mean erectile function (IIEF‐EF) score | 12.5 ± 3.3 (11.8–13.1) | 12.2 ± 3.4 (11.6–12.9) | 0.61 |
| ED severity at baseline† | |||
| Mild (22–25) | 1 (0.9%) | 2 (1.8%) | 0.37‡ |
| Mild to moderate (17–21) | 16 (14.4%) | 8 (7.3%) | |
| Moderate (11–16) | 64 (57.7%) | 68 (62.3%) | |
| Severe (1–10) | 30 (27.0%) | 31 (28.4%) | |
Data expressed as mean ± SD (95% CI). P‐values based on unpaired t‐test.
Data expressed as n (%).
P‐value based on the χ2‐test.
Efficacy
All the patients enrolled in the study consumed a minimum of four doses of study medication and made attempts at sexual intercourse thereafter between any two visits, as per the protocol. There was no difference in the mean consumption of medication and attempts made (in 4‐week intervals) in the two groups (avanafil group: 7.2 ± 2.9; sildenafil group: 7.1 ± 3.4; P > 0.05). The number of doses consumed and attempts at sexual intercourse ranged from four to 20 in the avanafil group, and four to 16 in the sildenafil group during any 4‐week interval between the visits. A total of 41 of the 108 patients (40.0%) in the avanafil group, and 47 of the 103 patients (45.6%) in the sildenafil group required dose escalation after 4 weeks of study treatment.
IIEF domain scores
The IIEF‐EF domain score increased in both the groups with duration of treatment, the details are shown in Figure 2. The difference in the mean change of IIEF‐EF score from baseline in the two groups increased from week 4 to week 8 and week 12, thereby achieving non‐inferiority as per the defined criteria at week 4 (lower limit of 95% more than −3), and superiority at week 8 and week 12 (lower limit of 95% CI >0; P < 0.05).
Fig. 2.
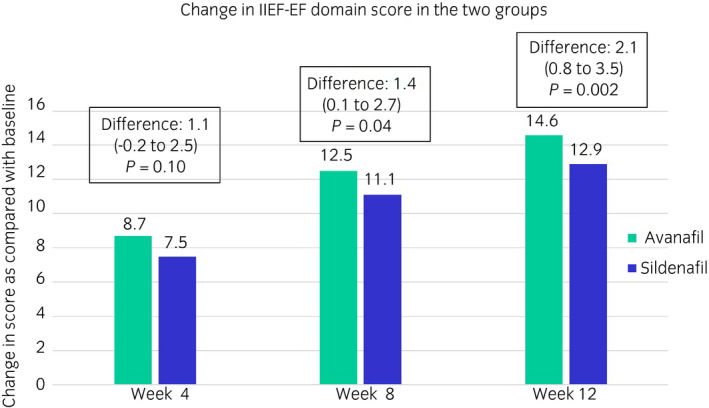
IIEF‐EF domain scores in the two groups during the study.
The percentage of patients reaching a normal IIEF‐EF score (≥26) steadily increased in both the groups with time. The distribution of patients in various categories based on the IIEF‐EF domain score are shown in Table 1 (baseline) and Figure 3 (week 4–12 after initiation of treatment). The distribution of patients in the various categories was significantly better in the avanafil group as compared with the sildenafil group.
Fig. 3.
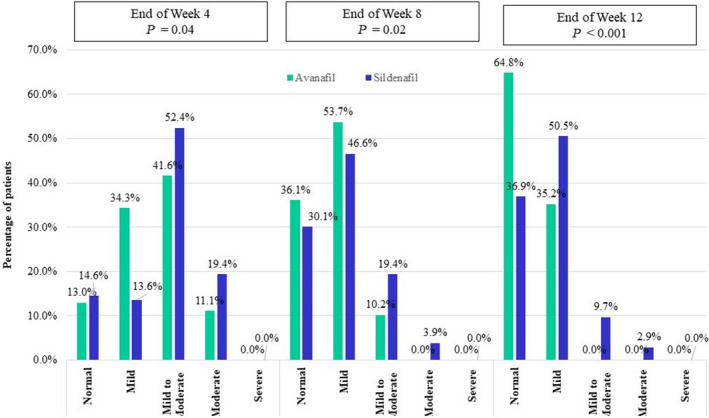
Distribution of patients in various categories based on the IIEF‐EF domain score at week 4, 8 and 12 after initiation of treatment (P‐value as compared with baseline).
The change in IIEF score in the other four domains; that is, orgasmic function, sexual desire, intercourse satisfaction and overall satisfaction, also increased with time, as compared with baseline in both the groups. The change was significantly better in the avanafil group in some domains at few time points, as compared with the sildenafil group. The details are shown in Table 2.
Table 2.
IIEF domain score other than EF domain in the two groups
| Avanafil (n = 108) | Sildenafil (n = 103) | P‐value | |
|---|---|---|---|
| End of week 4 | |||
| Orgasmic function | 3.1 ± 2.0 (2.7–3.5) | 2.3 ± 2.0 (1.9–2.7) | <0.01 |
| Sexual desire | 2.0 ± 1.8 (1.6–2.3) | 1.5 ± 1.9 (1.2–1.9) | 0.08 |
| Intercourse satisfaction | 4.0 ± 2.8 (3.5–4.6) | 3.6 ± 2.9 (3.1–4.2) | 0.28 |
| Overall satisfaction | 2.7 ± 2.0 (2.3–3.1) | 2.3 ± 2.0 (1.9–2.7) | 0.11 |
| End of week 8 | |||
| Orgasmic function | 4.1 ± 1.8 (3.8–4.5) | 3.5 ± 1.9 (3.2–3.9) | 0.02 |
| Sexual desire | 3.1 ± 1.8 (2.8–3.5) | 2.6 ± 1.9 (2.2–3.0) | 0.03 |
| Intercourse satisfaction | 5.4 ± 2.7 (4.9–6.0) | 4.8 ± 2.9 (4.3–5.4) | 0.11 |
| Overall satisfaction | 4.2 ± 1.9 (3.8–4.5) | 3.5 ± 2.1 (3.1–3.9) | 0.02 |
| End of week 12 | |||
| Orgasmic function | 4.7 ± 1.8 (4.3–5.0) | 4.1 ± 2.1 (3.7–4.5) | 0.02 |
| Sexual desire | 3.6 ± 1.9 (3.3–4.0) | 3.1 ± 2.1 (2.7–3.5) | 0.04 |
| Intercourse satisfaction | 6.2 ± 2.6 (5.7–6.6) | 5.5 ± 3.1 (4.9–6.1) | 0.07 |
| Overall satisfaction | 4.8 ± 1.8 (4.5–5.2) | 4.1 ± 2.3 (3.7–4.6) | 0.01 |
Data expressed as mean ± SD (95% CI). All scores are change as compared with baseline. P‐value based on unpaired t‐test.
Sexual encounter profile
The mean percentage of successful vaginal penetrations (i.e. response to SEP 2) was similar in the two groups at week 4, but significantly increased in the avanafil group as compared with the sildenafil group at the end of week 8 and 12 (P < 0.05). Similarly the mean percentage of successful intercourse (i.e. response to SEP 3) was also similar in the two groups at the end of week 4, but the difference steadily increased between the avanafil and sildenafil groups at week 8 and 12 to reach significant levels. However, contrary to the results of SEP 2 and 3, the mean percentage of doses taken by trial patients having some erection in 15 min (i.e. response to modified SEP 1) was significantly more in the avanafil group at all the three time points, as compared with that in the sildenafil group (P < 0.001). The detailed results of the SEP questionnaire are shown in Figure 4.
Fig. 4.
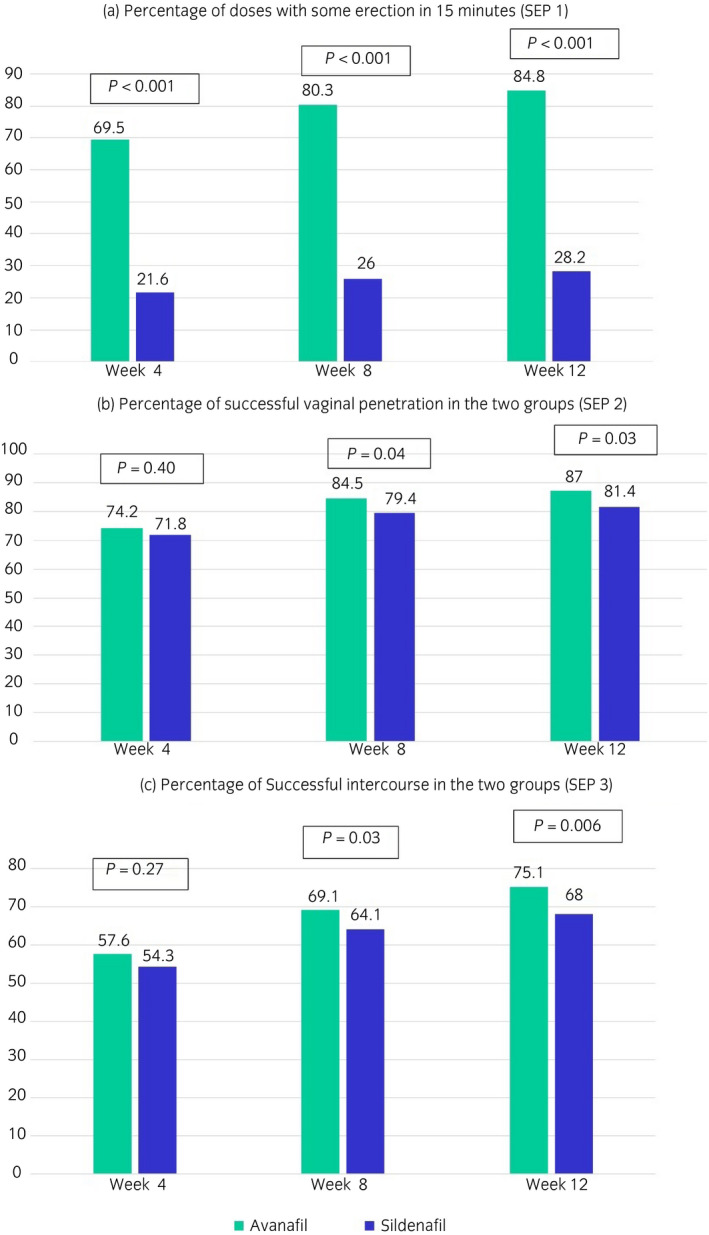
Results of SEP questionnaire in the two groups during the study.
Safety
Both the drugs were well tolerated by all the patients in the study. A total of 13 adverse events were reported in 11 patients in the avanafil group (adverse event rate 9.9%), and 13 adverse events were reported in 12 patients in the sildenafil group (adverse event rate 11.0%) during the 12‐week treatment period. Most of the adverse events; that is, 12 (92.3%) adverse events reported in the avanafil group and 11 (84.6%) adverse events in the sildenafil group, were ‘mild’ in severity. No severe or serious adverse event was reported by any of the patients enrolled in the study. All the reported adverse events resolved completely with/without symptomatic treatment during the study period. None of the adverse events required any change in i.p. dosing/discontinuation of i.p. during the entire course of the study. The most common adverse event reported during the study was headache in both the groups. The list of adverse events reported during the study is given in Table 3.
Table 3.
Adverse events reported during the study
| Adverse events | Avanafil (n = 111) | Sildenafil (n = 109) | P‐value |
|---|---|---|---|
| Headache | 9 (8.1%) | 10 (9.2%) | 0.81 |
| Flushing | 0 (0.0%) | 1 (0.9%) | 0.50 |
| Myalgia | 0 (0.0%) | 1 (0.9%) | 0.50 |
| Nasal congestion | 0 (0.0%) | 1 (0.9%) | 0.50 |
| Nausea | 1 (0.9%) | 0 (0.0%) | 0.99 |
| Fever | 1 (0.9%) | 0 (0.0%) | 0.99 |
| Back pain | 2 (1.8%) | 0 (0.0%) | 0.50 |
Data presented as n (%). P‐value based on χ2‐test/Fisher’s exact test.
Discussion
This study presents the results of the randomized, double‐blind study comparing the efficacy and safety of avanafil with sildenafil in the treatment of ED in Indian patients. Although the study was designed to show non‐inferiority of avanafil as compared with sildenafil, the study showed statistical superiority of avanafil over sildenafil in almost all the efficacy variables, including the IIEF‐EF domain scores and modified SEP 1, SEP 2 and SEP 3 patient diary questions at the end of 12 weeks of treatment.
The results of the present study are comparable to the results of the other internationally published studies with avanafil. A randomized, double‐blind, placebo‐controlled phase III clinical trial evaluating the safety and efficacy of avanafil in 646 patients with ED showed that the IIEF‐EF domain score increased from 12.6 to 20.9 and 12.8 to 22.2 at the end of 12 weeks of treatment with avanafil 100 and 200 mg tablets, respectively. The percentage of successful vaginal penetrations (SEP 2) was 74/77%, whereas the percentage of successful intercourses (SEP 3) was 57% at end of 12 weeks of treatment with 100 and 200 mg avanafil tablets. 9 Another randomized, double‐blind, placebo‐controlled phase III clinical trial evaluating different doses of avanafil in 200 patients with ED showed that the IIEF‐EF domain score increased by 8.5 and 8.8 with 100 and 200 mg avanafil tablets, respectively, at the end of 12 weeks of treatment. The study also showed that avanafil increased all the IIEF domain scores at the end of 12 weeks of treatment, as compared with the baseline. 15 Overall, the change in IIEF‐EF domain scores observed in the current study was numerically higher in both the study groups, as compared with that in the published literature. This could be ascribed to the subjective nature of the scoring scale.
Since their introduction, PDE5 inhibitors have been considered as the first‐line of treatment for ED irrespective of the cause of the disease. Although effective, the first‐generation PDE5 inhibitors are associated with few concerns; for example, reduced efficacy due to improper time of dosing (related to delayed onset of action) and side‐effects due to off‐target action of the drugs (inhibition of other PDE isoenzymes). 16 Avanafil addresses both these problems with its favorable pharmacokinetic and pharmacodynamic profile.
PDE5 inhibitors have often been used as an on‐demand therapy, and patients tend to use the drug just before sexual intercourse; however, the currently available PDE5 inhibitors have a late onset of action (30–60 min for sildenafil and 15–45 min for tadalafil), often leading to reduced therapeutic efficacy. Avanafil has a distinct advantage in terms of its onset of action, which starts well within 15 min of drug intake. 17 This effect was also seen in the present study, as the patients reported start of drug activity (i.e. some erection) within 15 min of drug intake in >84% doses of avanafil, as compared with just 28% doses of sildenafil consumed during the study. This earlier onset of action with avanafil is linked to the better pharmacokinetic profile of the drug; the Tmax of avanafil is 30–45 min, as compared with 60 min for sildenafil and vardenafil, and 120 min for tadalafil. 17 A separate randomized, double‐blind placebo controlled study has been carried out to specifically evaluate the therapeutic effect of avanafil 15 min after dosing in men with mild‐to‐severe ED. The study showed significantly greater percentages of successful intercourse attempts (SEP 3) within approximately 15 min after dosing with avanafil 100 mg (mean 25.9%) and 200 mg (mean 29.1%), as compared with a placebo (mean 14.9%). This significant difference in the proportion of successful intercourse attempts (SEP 3) was noted as early as 10 min in the 200 mg group and 12 min in the 100 mg group, as compared with a placebo. 18 In another randomized double‐blind placebo‐controlled study, the proportions of successful intercourse by time interval from dose to attempt was evaluated, and it was found that avanafil has a similar success rate irrespective of the time interval from dose to attempt; that is, whether the attempt is made within 15 min of drug intake or even after 6 h of drug intake. 9 Based on the rapid onset of action, avanafil is recommended to be taken 15 min before the desired sexual activity on an as needed basis, 7 whereas sildenafil is recommended to be taken approximately 1 h before the desired sexual activity. 19
Avanafil is also advantageous in terms of side‐effects due to its high selectivity for PDE5. Preclinical studies have reported that avanafil strongly inhibited PDE5 (half maximal inhibitory concentration, 5.2 nmol/L) in a competitive manner. In an in vitro receptor‐binding study comparing the inhibitory effects of avanafil on 11 PDE isozymes with those of sildenafil, vardenafil and tadalafil, avanafil potently inhibited PDE5 activity without significant inhibition of other PDE isozymes. In contrast, sildenafil, vardenafil and tadalafil produced inhibitory activity for other PDE isozymes (PDE1, PDE6 and PDE11). Avanafil has higher selectivity against PDE6 (120‐fold) than sildenafil (16‐fold) and vardenafil (21‐fold); against PDE1 (>10 000‐fold), as compared with sildenafil (380‐fold) and vardenafil (1000‐fold); and against PDE11 (>19 000), as compared with tadalafil (25 fold). As avanafil has relatively little cross‐reactivity with other PDE isoenzymes, especially PDE1, PDE6 and PDE11, which are causes of various side‐effects (PDE1 isozyme affects vascular smooth muscle contraction and its inhibition leads to vasodilation and symptomatic hypotension, headache and flushing; PDE6 is involved in phototransduction in the retina, and its inhibition leads to inability to discriminate between blue and green [blue/green] and transient cyanopsia; whereas inhibition of PDE11 leads to increased incidence of back pain and myalgia), it is expected to confer improved tolerability in long‐term clinical use, as compared with the other PDE5 inhibitors. 20 In fact, on comparing the safety profile of various PDE5 inhibitors from published literature, it was found that avanafil 200 mg has the lowest rate of common adverse events in terms of headache (9.3% vs sildenafil 100 mg 12.8% vs tadalafil 20 mg 14.5% and vs vardenafil 20 mg 16%), flushing (3.7% vs sildenafil 100 mg 10.4% vs tadalafil 20 mg 4.1% and vardenafil 20 mg 12%), abnormal vision (none vs sildenafil 100 mg 1.9% and vardenafil 20 mg <2%) and back pain/myalgia (<2% vs tadalafil 6.5%/5.7%). 17
One of the limitations of the present study was the small sample size of the study, which was powered to show the difference in terms of the efficacy parameters between the two drugs, but was not powered enough to detect any significant difference in the safety profile of the two drugs. However, showing such differences in the safety profile is as such difficult in randomized controlled studies, and is usually carried out in long‐term large post‐marketing studies. Another limitation of the study was that we used only subjective parameters to compare the efficacy of the two drugs; however, the scoring scales used in the study are validated scales, and have been used in various randomized studies to establish the efficacy of PDE5 inhibitors in patients of ED. We recommend carrying out further studies to compare the efficacy of avanafil with other PDE inhibitors in patients with ED and also compare its effect with other PDE5 inhibitors in patients associated with comorbidities, such as diabetes, hypertension, cardiovascular disease, neurological disorders (such as spinal cord injury, multiple sclerosis), renal insufficiency and a history of urological pelvic surgery.
The results of the present prospective, randomized, double‐blind, two‐arm, active‐controlled, parallel, multicenter, non‐inferiority clinical trial show that avanafil is superior to sildenafil in improving the IIEF‐EF domain score at the end of 12 weeks of treatment. Avanafil was also found to be non‐inferior/superior to sildenafil in other efficacy parameters, including percentage of attempts with successful vaginal penetration and successful intercourse, and improvement in the IIEF scores in the other domains. Also, avanafil was found to be superior to sildenafil in the percentage of doses that led to an erection in <15 min, further establishing its faster onset of action, as compared with sildenafil.
Acknowledgment
This study was sponsored and funded by Zydus Healthcare Limited, India.
Author contributions
Manish Kumar: Data curation, Investigation, Supervision, Writing – review & editing. Amey D Pathade: Data curation, Investigation, Supervision, Writing – review & editing. S VijayaBhaskara Gupta: Data curation, Investigation, Supervision, Writing – review & editing. Sanjay Goyal: Data curation, Investigation, Supervision, Writing – review & editing, Debadarshi Rath: Data curation, Investigation, Supervision, Writing – review & editing. Manish Thakre: Data curation, Investigation, Supervision, Writing – review & editing. Jayesh Sanmukhani: Conceptualization, Formal analysis, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. Ravindra Mittal: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing.
Conflict of interest
Manish Kumar, Amey D Pathade, S VijayaBhaskara Gupta, Sanjay Goyal, Debadarshi Rath and Manish Thakre have received honoraria for conducting the study. Jayesh Sanmukhani and Ravindra Mittal are employees of Zydus Healthcare Limited.
Approval of the research protocol by an Institutional Reviewer Board
The study was initiated after review and approval by the Institutional Ethics Committees at each of the six participating study centers.
Informed consent
Written informed consent was obtained from all patients before initiation of any study‐related activity.
Registry and the Registration No. of the study/trial
The study was registered with the Clinical Trials Registry of India (www.ctri.nic.in; CTRI/2020/01/022798) before enrolment of the first participant in the study.
Animal studies
Not applicable.
References
- 1. Zurawin JL, Stewart CA, Anaissie JE, Yafi FA, Hellstrom WJ. Avanafil for the treatment of erectile dysfunction. Expert Rev. Clin. Pharmacol. 2016; 9: 1163–70. [DOI] [PubMed] [Google Scholar]
- 2. Ralph D, McNicholas T. UK management guidelines for erectile dysfunction. BMJ 2000; 321: 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doggrell SA. Comparison of clinical trials with sildenafil, vardenafil and tadalafil in erectile dysfunction. Expert Opin. Pharmacother. 2005; 6: 75–84. [DOI] [PubMed] [Google Scholar]
- 4. JQ Yuan, RJ Zhang, ZY Yang et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta‐analysis. Eur. Urol. 2013; 63: 902–12. [DOI] [PubMed] [Google Scholar]
- 5. Hatzimouratidis K, Hatzichristou DG. A comparative review of the options for treatment of erectile dysfunction: which treatment for which patient? Drugs 2005; 65: 1621–50. [DOI] [PubMed] [Google Scholar]
- 6. Corona G, Rastrelli G, Burri A, Jannini EA, Maggi M. The safety and efficacy of Avanafil, a new 2(nd) generation PDE5i: comprehensive review and metaanalysis. Expert Opin. Drug Saf. 2016; 15: 237–47. [DOI] [PubMed] [Google Scholar]
- 7. Information P. Stendra (Avanafil Tablets). Mist Pharmaceuticals. Cranford, NJ, 2018. [Google Scholar]
- 8. Goldstein I, Jones LA, Belkoff LH et al. Avanafil for the treatment of erectile dysfunction: a multicenter, randomized, double‐blind study in men with diabetes mellitus. Mayo Clin. Proc. 2012; 87: 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldstein I, McCullough AR, Jones LA et al. A randomized, double‐blind, placebo‐controlled evaluation of the safety and efficacy of avanafil in subjects with erectile dysfunction. J. Sex. Med. 2012; 9: 1122. [DOI] [PubMed] [Google Scholar]
- 10. Mulhall JP, Burnett AL, Wang R et al. A phase 3, placebo controlled study of the safety and efficacy of avanafil for the treatment of erectile dysfunction after nerve sparing radical prostatectomy. J. Urol. 2012; 189: 2229. [DOI] [PubMed] [Google Scholar]
- 11. Jung J, Choi S, Cho SH et al. Tolerability and pharmacokinetics of avanafil, a phosphodiesterase type 5 inhibitor: a single‐ and multiple‐dose, double‐blind, randomized, placebo‐controlled, dose‐escalation study in healthy Korean male volunteers. Clin. Ther. 2010; 32: 1178–87. [DOI] [PubMed] [Google Scholar]
- 12. Belkoff LH, McCullough A, Goldstein I et al. An open‐label, long term evaluation of the safety, efficacy and tolerability of avanafil in male patients with mild to severe erectile dysfunction. Int. J. Clin. Pract. 2013; 67: 333. [DOI] [PubMed] [Google Scholar]
- 13. Della Camera PA, Tellini R, Cito G et al. Efficacy and safety of avanafil 200 mg versus sildenafil 100 mg in the treatment of erectile dysfunction after robot‐assisted unilateral nerve‐sparing prostatectomy: a prospective multicentre study. Urologia 2020; 87: 23–8. [DOI] [PubMed] [Google Scholar]
- 14. Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur. Urol. 2011; 60: 1010–6. [DOI] [PubMed] [Google Scholar]
- 15. Zhao C, Kim SW, Yang DY et al. Efficacy and safety of avanafil for treating erectile dysfunction: results of a multicentre, randomized, double‐blind, placebo‐controlled trial. BJU Int. 2012; 110: 1801–6. [DOI] [PubMed] [Google Scholar]
- 16. Park HJ, Kim SW, Kim JJ et al. A randomized, placebo‐controlled, double‐blind, multi‐center therapeutic confirmatory study to evaluate the safety and efficacy of avanafil in korean patients with erectile dysfunction. J. Korean Med. Sci. 2017; 32: 1016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boeri L, Capogrosso P, Ventimiglia E et al. Avanafil – a further step to tailoring patient needs and expectations. Expert Rev. Clin. Pharmacol. 2016; 9: 1171–81. [DOI] [PubMed] [Google Scholar]
- 18. Hellstrom WJ, Kaminetsky J, Belkoff LH et al. Efficacy of Avanafil 15 minutes after dosing in men with erectile dysfunction: a randomized, double‐blind, placebo controlled study. J. Urol. 2015; 194: 485–92. [DOI] [PubMed] [Google Scholar]
- 19. Information P. Viagra (Sildenafil Tablets). Pfizer Labs, NY, New York, 2017. [Google Scholar]
- 20. Wang R, Burnett AL, Heller WH et al. Selectivity of avanafil, a PDE5 inhibitor for the treatment of erectile dysfunction: Implications for clinical safety and improved tolerability. J. Sex. Med. 2012; 9: 2122–9. [DOI] [PubMed] [Google Scholar]


