Abstract
The ability to experience, use and eventually control anger is crucial to maintain well‐being and build healthy relationships. Despite its relevance, the neural mechanisms behind individual differences in experiencing and controlling anger are poorly understood. To elucidate these points, we employed an unsupervised machine learning approach based on independent component analysis to test the hypothesis that specific functional and structural networks are associated with individual differences in trait anger and anger control. Structural and functional resting state images of 71 subjects as well as their scores from the State–Trait Anger Expression Inventory entered the analyses. At a structural level, the concentration of grey matter in a network including ventromedial temporal areas, posterior cingulate, fusiform gyrus and cerebellum was associated with trait anger. The higher the concentration, the higher the proneness to experience anger in daily life due to the greater tendency to orient attention towards aversive events and interpret them with higher hostility. At a functional level, the activity of the default mode network (DMN) was associated with anger control. The higher the DMN temporal frequency, the stronger the exerted control over anger, thus extending previous evidence on the role of the DMN in regulating cognitive and emotional functions in the domain of anger. Taken together, these results show, for the first time, two specialized brain networks for encoding individual differences in trait anger and anger control.
Keywords: anger control, brain networks, machine learning, resting state, source‐based morphometry, trait anger
Structural and functional resting state images of 71 subjects as well as their scores from the State–Trait Anger Expression Inventory were analysed through the unsupervised machine learning approach based on the independent component analysis. At a structural level, the concentration of grey matter in a network was associated with trait anger. At a functional level, the activity of the default mode network was associated with anger control.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- BOLD
blood‐oxygen‐level‐dependent
- CAT12
computational anatomy toolbox
- CN
cerebellar network
- DAN
dorsal attention network
- DMN
default mode network
- FC
functional connectivity
- fMRI
functional magnetic resonance imaging
- FPN
fronto‐parietal network
- IC(s)
independent component(s)
- ICA
independent component analysis
- LN
language network
- MDL
minimum description length
- MNI
Montreal Neurological Institute
- mPFC
medial prefrontal cortex
- MRI
magnetic resonance imaging
- ROI
region of interest
- SBM
source‐based morphometry
- SCID‐I
Structured Clinical Interview for DSM‐IV
- SMN
sensorimotor network
- SN
salience network
- SPM12
statistical parametric mapping
- STAXI
State–Trait Anger Expression Inventory
- TIV
total intracranial volume
- VBM
voxel‐based morphometry
- VN
visual network
1. INTRODUCTION
Anger has been defined as an intense emotional state involving a strong uncomfortable and hostile response to a perceived provocation, hurt or threat (Sorella et al., 2021; Videbeck, 2006). Anger has a survival value, given its fundamental role in alerting us and in fight‐or‐flight reactions. It also helps us to pose limits to unfair behaviours of others and to show assertiveness (Grecucci, Giorgetta, Bonini, & Sanfey, 2013; Grecucci, Giorgetta, van Wout, et al., 2013; Sorella et al., 2021). When this transitory emotional state (e.g. state anger) is experienced with a certain frequency and intensity, it becomes trait anger: a long‐lasting individual difference in frequency and duration of anger episodes (Spielberger, 1988, p. 1). Although anger leads to instrumental actions, it can become detrimental if excessively experienced or poorly controlled. Indeed, high levels of trait anger can lead not only to psychological and interpersonal consequences (Baron et al., 2006) but also to physiological drawbacks such as higher incidence of cardiovascular diseases (Smith et al., 2004), tobacco use (Spielberger et al., 1995) and excessive alcohol intake (Litt et al., 2000).
Besides some recent attempts to study specific aspects of anger, such as anger experience and anger perception (Sorella et al., 2021), there is poor evidence on the neural bases of individual differences in trait anger. The vast majority of studies focused on specific sub‐aspects of trait anger instead of looking at the complete picture. For example, the tendency to generate hostile attributions, one main feature of trait anger according to the integrative cognitive model (Wilkowski & Robinson, 2008), has been associated with different brain areas such as the temporo‐parietal junction (Carlson et al., 2012; Giardina et al., 2011; Quan et al., 2019) and medial brain areas linked to emotional conceptualization (Lindquist et al., 2012). Also, the posterior cingulate cortex has been associated with the feeling of certainty (Luttrell et al., 2016), which is known to characterize trait anger (Lerner & Keltner, 2000).
Another line of evidence comes from studies on attentional biases. Increased tendency to attend aversive events characterizes trait anger (Wilkowski & Robinson, 2008). Indeed, its scores have been associated with both attentional biases while inspecting angry facial expressions (Honk et al., 2001; Veenstra et al., 2016) and bilateral dorsal amygdala activity (Carré et al., 2010). In fact, amygdala lesions impair the automatic allocation of attention to aversive stimuli (Anderson & Phelps, 2001) and socially relevant stimuli (Kennedy & Adolphs, 2010; Piretti et al., 2020).
Besides individual differences in trait anger, another important aspect not yet studied in the neuroimaging literature concerns anger control. Controlling anger may be vital in certain interpersonal situations, and lack of anger control is usually punished by society and law. The integrative cognitive model considers anger control as an effortful capacity to control anger expression by calming down and monitoring its outcomes (Wilkowski & Robinson, 2010). This is exerted by frontal brain regions. These regions have been hypothesized to control anger through their role in top‐down modulation of subcortical brain regions. In particular, an effort to control anger after an insult (recreated in experimental settings with anger provocation paradigms) increases the connectivity between the amygdala and prefrontal cortices, which are responsible for top‐down control (Denson et al., 2013). Accordingly, another study (Fulwiler et al., 2012) showed a positive correlation between anger control and the functional connectivity (FC) of the amygdala with the contralateral orbitofrontal cortex.
Moreover, a poor anger control has been associated with a greater tendency to react with angry expression and aggressive behaviours towards hostile stimuli (Bettencourt et al., 2006; Dodge & Coie, 1987; Mattevi et al., 2019). Further, aggressive outbreaks and maladaptive interpersonal behaviours are particularly common in different disorders such as borderline personality disorder (Dadomo et al., 2016, 2018; de Panfilis et al., 2019; Kernberg, 2012), antisocial personality disorder (Kolla et al., 2016) and the intermittent explosive disorder (Coccaro et al., 2014). On the other hand, excessive inhibition (excessive control) of anger characterizes, among others, anxiety disorders (Grecucci et al., 2020; Grecucci, Giorgetta, Brambilla, et al., 2013) and dependent personality disorder (Kernberg, 2012). From a neural point of view, it has been reported that resting state activity of violent offenders after anger provocation showed increased amygdala–paralimbic connectivity and decreased amygdala–medial prefrontal cortex (mPFC) connectivity (Siep et al., 2018), suggesting that a lack of mPFC regulation can lead to reactive aggression and anger expression. Authors concluded that this area may be particularly involved in anger control (Gilam et al., 2015, 2018; Jacob et al., 2018).
However, one limitation of the previously cited studies is that they measured anger by using anger provocation paradigms or the perception of angry facial expressions (Sorella et al., 2021), but there is poor evidence on how individual differences mediate trait anger and anger control. Moreover, previous paradigms usually focused only on one specific aspect of anger. For example, because high trait anger individuals are characterized by the perception of high levels of hostility in environmental cues, scholars have started to investigate the relationship between the perception of angry faces and aggressive tendencies (Beyer et al., 2015; Buades‐Rotger et al., 2016). However, trait anger also refers to other features, such as the frequency in which anger is elicited in life or a self‐centred point of view characterized by high certainty. Therefore, we decided to investigate the individual differences of anger, taking into account more comprehensive measures of anger.
In addition, previous studies relied on a priori defined brain regions (e.g. region of interest [ROI] studies) and massive univariate approaches, thus ignoring the possibility that different regions together may play a role in anger. Furthermore, previous studies have focused on functional magnetic resonance imaging (fMRI), neglecting possible structural markers of anger.
To overcome all these limitations, we decided to apply an unsupervised machine learning approach based on the independent component analysis (ICA) to structural and functional data (Lapomarda, Grecucci, et al., 2021; Lapomarda, Pappaianni, et al., 2021; Pappaianni et al., 2019; Saviola et al., 2020). In particular, the unsupervised machine learning (Vu et al., 2018) ICA (Brown et al., 2001), part of the so‐called blind source separation methods (Müller et al., 2004), was applied to both structural and functional (resting state) images of 71 healthy individuals. The goal was to understand whether specific structural features (i.e. grey matter concentration) and patterns of connectivity (i.e. the temporal variability and frequency of functional networks) were associated with individual differences in trait anger, anger control, anger‐out and anger‐in.
Specifically, these anger facets were measured with the State–Trait Anger Expression Inventory (STAXI; Spielberger, 1988), a self‐report measure. Trait anger refers to the frequency and duration of anger episodes, whereas anger control refers to the ability to control and regulate this emotion. Finally, anger‐out represents the tendency to externalize anger, and anger‐in the tendency to internalize it (redirect it towards the self).
Because trait anger is considered a stable individual tendency, we expect to find evidence at a structural level, that is, grey matter concentration changes. In particular, given that trait anger is characterized by the perception of environmental cues as hostile, we expect to find grey matter changes in a network involving perceptual brain regions linked to emotional processing, such as paralimbic and temporal areas. However, we expect that trait anger can influence not only the perception but also the conceptualization of life events, thus involving the posterior cingulate cortex and temporal areas that are also associated with certainty (Luttrell et al., 2016) that characterizes anger (Lerner & Keltner, 2000).
On the other hand, we expect that anger control, anger‐out and anger‐in would be more related to functional activity, being transient brain states. On the basis of a recent review on anger (Alia‐Klein et al., 2020), we expect that anger‐out could be positively related to the habit network and negatively related to the self‐regulation network, also known as the dorso‐parietal network. On the other hand, anger‐in and anger control could be related to the self‐regulation network or to the default mode network (DMN). Indeed, different researchers have shown that DMN has a net role in regulating cognitive and emotional functions (Pan et al., 2018; Sripada et al., 2014), but there is a lack of evidence that this extends to anger. Prior studies have shown that the DMN could suppress limbic and paralimbic areas responsible for emotional processes and reactivity (Buckner et al., 2008; Harris & Friston, 2010). Moreover, alterations of the DMN have also been associated with anger expression in individuals with attention deficit hyperactivity disorder (ADHD) (Hasler et al., 2017) and to criminal psychopathic tendencies (Pujol et al., 2012). This evidence suggests that this network could be particularly involved in the regulation of violent acts. Furthermore, the DMN could be associated not only with anger control but also with anger‐out or anger‐in scores, in particular when considering the modulation of the DMN exerted on other brain networks (see, e.g. Weathersby et al., 2019).
To note, the majority of previous connectivity studies usually took into account the strength of connections between different brain regions and networks. However, although connectivity features were previously considered as static (such as the frequency, amplitude and phase), recent evidence proposed and started to show that connectivity patterns change over time (Calhoun et al., 2014; Chang & Glover, 2010). Therefore, we decided to take into consideration two available measures linked to the temporal dynamics of the ICA based networks: the temporal variability and frequency. Although few studies considered these measures, there is increasing evidence linking these features with different variables such as age, gender (Allen et al., 2011), cognitive states (Garrett et al., 2013), empathy and awareness (Stoica & Depue, 2020). Furthermore, some studies also showed alterations of temporal features of some networks in clinical population, such as higher frequency fluctuations in the DMN of schizophrenia patients (Garrity et al., 2007). Exploring these features of the brain circuits involved in individual anger‐related differences may shed new light on the neurocognitive mechanisms underlying angry outbursts and anger regulation and possibly pave the way to new forms of neuroscientific evidence‐based psychological treatments for individual who suffer from anger dyscontrol at a clinical and subclinical level (Frederickson et al., 2018; Grecucci et al., 2017, 2020).
2. MATERIALS AND METHODS
2.1. Participants
For this study, we took advantage of data from the Max Planck Institute sample (MPI‐S) dataset (OpenNeuro database, accession number ds000221), which contains behavioural, physiological and neuroimaging data from healthy subjects (Babayan et al., 2018). Absence of psychiatric conditions was controlled with the SCID‐I (Wittchen et al., 1995). For the purpose of this study, we selected participants according to age (20–40), availability of structural T1‐weighted images and 15‐min eyes‐open resting state data and availability of specific questionnaires' scales. In this study, we focused on the 44‐item STAXI (Spielberger, 1988), selecting self‐report measures to assess trait anger, anger control, anger‐out and anger‐in. The final sample included 71 subjects (M = 42, F = 29) ranging from 20 to 40 years, mean age 26.02 ± 3.53 (age was specified in a 5‐year bin, where the middle value was used for calculations). See Table 1 for more details.
TABLE 1.
Participants
| N | Age | Trait anger | Anger‐in | Anger‐out | Anger control | |
|---|---|---|---|---|---|---|
| Participants | 71 | 26.02 ± 3.53 | 18.38 ± 3.93 | 15.87 ± 4.32 | 12.03 ± 3.1 | 22.38 ± 3.82 |
| Males | 42 | 26.43 ± 3.41 | 17.95 ± 4.02 | 16.21 ± 3.78 | 11.52 ± 2.82 | 23.21 ± 3.52 |
| Females | 29 | 25.43 ± 3.66 | 19 ± 3.76 | 15.38 ± 5.04 | 12.76 ± 3.38 | 21.17 ± 3.97 |
| Difference M versus F (p‐value) | 0.244 | 0.272 | 0.428 | 0.099 | 0.026 |
2.2. Data acquisition
Neuroimaging data were acquired on a 3T Siemens Magnetom Verio Scanner. For our analyses, we considered T1‐weighted images, acquired using a MP2RAGE sequence (TR = 5000 ms, TE = 2.92 ms, TI1 = 700 ms, TI2 = 2500 ms, flip angle 1 = 4°, flip angle 2 = 5°, voxel size = 1.0 mm isotropic, duration = 8.22 min), and the 15‐min resting state data (voxel size = 2.3 mm isotropic, FOV = 202 × 202 mm2, imaging matrix = 88 × 88, 64 slices with 2.3 mm thickness, TR = 1,400 ms, TE = 39.4 ms, flip angle = 69°, echo spacing = 0.67 ms, bandwidth = 1776 Hz/Px, partial Fourier 7/8, no pre‐scan normalization, multiband acceleration factor = 4, 657 volumes, duration = 15 min 30 s).
2.3. Structural analyses
Structural images were analysed by using an unsupervised machine learning approach based on the ICA. ICA applied to structural images is also known as source‐based morphometry (SBM) (Grecucci et al., 2016; Gupta et al., 2019; Pappaianni et al., 2017, 2019; Sorella et al., 2019; Xu, Groth, et al., 2009; Xu, Pearlson, & Calhoun, 2009). This is a data‐driven multivariate alternative to voxel‐based morphometry (VBM), used to identify ‘source networks’ or groups of spatially distinct regions in the brain with common covariation among participants (Xu, Groth, et al., 2009). The main difference between VBM and SBM is the application of ICA to identify patterns of covariation of grey matter in different independent areas, detecting and decomposing the mixed signals coming from whole brain structural images. In this way, SBM identifies brain networks, taking into account the interrelationship among different voxels, rather than a voxel‐by‐voxel comparison (as in VBM). The multivariate nature of this approach makes it more efficient than other structural analyses because it reduces the noise while acting as a spatial filter (Grecucci et al., 2016; Pappaianni et al., 2017, 2019; Sorella et al., 2019). Before applying ICA, images were preprocessed with SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software) using the toolbox CAT12 (http://www.neuro.uni-jena.de/cat/) for image segmentation.
A visual check of data quality was performed in order to identify any distortion, such as head motion or stripes. Then, images were reoriented according to the origin and segmented in grey matter, white matter and cerebrospinal fluid through CAT12. For the purposes of the study, we only took into account the grey matter images. The registration was computed through Diffeomorphic Anatomical Registration using Exponential Lie algebra (DARTEL; https://github.com/scanUCLA/spm12-dartel) tools for SPM12, rather than using traditional approaches. The last step of the preprocessing included the normalization to MNI space with spatial smoothing (full width at half maximum of Gaussian smoothing kernel [8, 8, 8]).
After the preprocessing steps, ICA was applied to the data, allowing us to identify independent grey matter sources in the brain. ICA was applied to structural images through the sMRI modality of the Group ICA fMRI Toolbox (GIFT, http://mialab.mrn.org/software/gift).
The Infomax algorithm was used to maximize the recognition of IC from images' signal information (Bell & Sejnowski, 1995; Lee et al., 1999), whereas the ICASSO toolbox (http://research.ics.aalto.fi/ica/icasso/) was used to investigate the reliability of the ICA algorithm (RandInit mode, with 100 repetitions).
Finally, the ICA converted grey matter volumes of each component into numerical vectors; it returned an n × m matrix (n subjects in rows and m component in columns), which represents how a specific component is expressed in each participant (Grecucci et al., 2016; Pappaianni et al., 2017, 2019; Sorella et al., 2019). After obtaining the components, we performed statistical analyses to determine which networks are linked to anger. In particular, we performed the Pearson correlation between each component and the scores of the STAXI. Significant results were then corrected relying on partial correlation, considering gender, age, total intracranial volume (TIV) and a social desirability scale (Crowne & Marlowe, 1960). In addition, the same method was used to partial out the scores on the other subscales when finding a significant result with a particular scale.
Mango (http://ric.uthscsa.edu/mango/), Surf Ice (https://github.com/neurolabusc/surf-ice) and Matlab (https://it.mathworks.com/products/matlab.html) were used to visualize data.
2.4. Functional analyses
Similar to structural analyses, the same unsupervised machine learning approach based on the ICA was then applied to functional images. The usage of ICA also in resting state analyses shows many advantages. Besides its abilities to detect and remove noise, ICA maximizes the statistical independence in order to extract different networks with high consistency (Rajamanickam, 2020). Indeed, the main reason why we relied on this approach is to extract resting state networks without overlaying a predefined mask, but relying on the individual features of the participants included in the analysis. Thus, one of the main advantages that led us to rely on ICA‐based resting state analyses is to identify naturally grouping functional connections patterns of brain regions not restricted to predefined boundaries of network nodes (Kornelsen et al., 2020; Motoyama et al., 2019). This is especially important when considering that resting state networks are susceptible to individual differences.
The preprocessing steps were performed through the default processing pipeline of CONN software (https://web.conn-toolbox.org) for volume‐based analysis of resting states data. It includes the following steps: functional realignment and unwarping, translation and centring, functional outlier detection (conservative settings), functional direct segmentation and normalization (2 mm resolution), structural translation and centring, structural segmentation and normalization (2 mm resolution) and functional and structural smoothing (spatial convolution with Gaussian kernel 8 mm). Then, CONN includes a component‐based noise correction method (CompCor) for the physiological and other noise source reduction. This step applies linear regression and bandpass filtering in order to remove unwanted motion, physiological and other artefactual effects from the BOLD signal before computing connectivity measures. Data were checked through quality assurance plots. ICA was then applied, given that we did not want to restrict our analyses to a priori seeds/ROIs and rather investigate possible connectivity differences related to trait anger across the entire brain. Therefore, we selected voxel‐to‐voxel analysis, specifically the group‐ICA one. Through the ICA, we identified 20 networks (default settings) of highly functionally‐connected areas. This analysis is based on Calhoun's group‐level ICA approach (Calhoun et al., 2001) and includes the following steps: variance normalization preconditioning, subject concatenation of BOLD signal data along temporal dimension, group‐level dimensionality reduction, fastICA for estimation of independent spatial components and GICA1 back projection for individual subject‐level spatial map estimation (Nieto‐Castanon, 2020; Whitfield‐Gabrieli & Nieto‐Castanon, 2012).
Among the 20 networks, we used the CONN's preselected eight networks correspondent to well‐known resting state networks in literature (Soman et al., 2020) through the spatial match to template function. Through ICA.Temporal.Components, we considered the temporal variability, defined as a fluctuation of neural activity over time, and the temporal frequency, defined as distribution of neural activity fluctuations over various frequencies, of the BOLD signal time series associated with each network. Finally, we checked whether the temporal variability and the temporal frequency of the identified networks were related to STAXI anger scores of the 71 subjects through correlation coefficients. Significant results were then corrected relying on partial correlations, considering gender, age, TIV and a social desirability scale (Crowne & Marlowe, 1960). In addition, the same method was used to partial out the scores on the other subscales when finding a significant result with a particular scale.
3. RESULTS
3.1. Structural results
ICA applied to structural images automatically identified eight independent components (ICs) on the basis of grey matter changes through the subsampling scheme proposed by Li et al. (2007). This method is used to obtain a set of effectively independent and identically distributed samples on which the minimum description length (MDL) information‐theoretic criteria are then applied. See Figures 1 and 2. The consistency and reliability of the results were quantified with a quality index (Iq) ranging from 0 to 1, which reflects the difference between intracluster and extracluster similarity of the identified networks (Canessa et al., 2013; Himberg et al., 2004). All of them showed an Iq > 0.9, which indicates a highly stable ICA decomposition (Allen et al., 2011). ICs were then correlated with trait anger, anger control, anger‐out and anger‐in scores.
FIGURE 1.
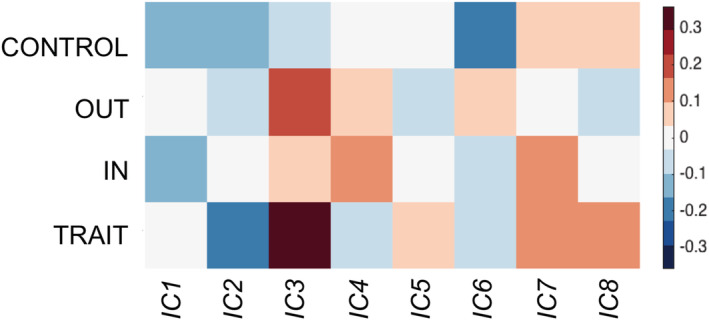
Heatmap of the structural results. Heatmap plot of the four analyses showing the correlations between the four anger scales (trait anger, anger‐in, anger‐out and anger control) with the structural networks
FIGURE 2.
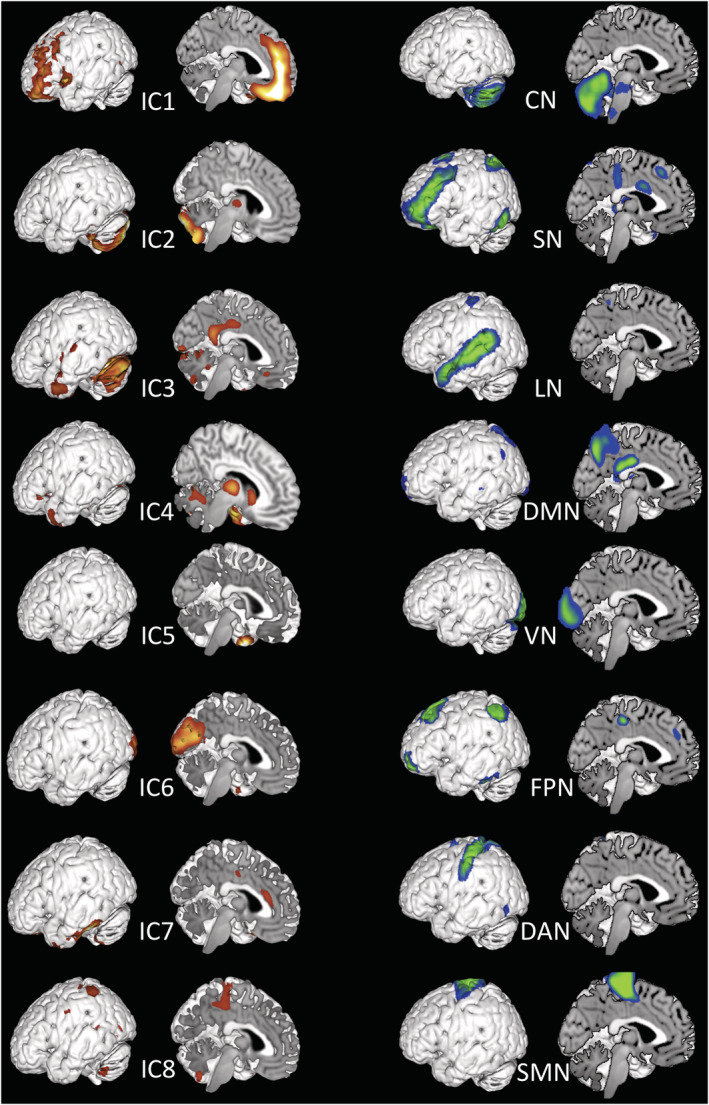
Structural and functional independent networks. A machine learning method known as independent component analysis was applied to both structural and functional neuroimaging data. The eight structural ICs are displayed on the left (first column in hot colours); the eight functional ICs are displayed on the right (second column in cold colours). The functional networks included the cerebellar network (CN), the salience network (SN), the language network (LN), the default mode network (DMN), the visual network (VN), the fronto‐parietal network (FPN), the dorsal attention network (DAN) and the sensorimotor network (SMN)
3.1.1. Trait anger
The analysis showed a positive significant correlation between Independent Component 3 (IC 3, Iq = 0.97) and trait anger (r = 0.34, p = 0.004), even after correcting for gender (r = 0.327, p = 0.0058), age (r = 0.339, p = 0.0041), TIV (r = 0.338, p = 0.0042), social desirability (r = 0.376, p = 0.0013) and the other three anger's subscales of the STAXI (r = 0.2694; p = 0.0263). Results remained significant after correcting for the number of components (n = 8, Bonferroni corrected p‐critic = 0.006). The IC 3 included portions of the fusiform gyrus, different parts of the cerebellum, the temporal pole, the lingual gyrus, the visual association area, the posterior cingulate cortex and the inferior parietal lobule. See Table 2 and Figure 3. The other seven components were not significantly correlated with trait anger (r = −0.007, p = 0.954; r = −0.178, p = 0.137; r = −0.040; p = 0.740; r = 0.070, p = 0.563; r = −0.079, p = 0.511; r = 0.147, p = 0.220; r = 0.112, p = 0.352).
TABLE 2.
Morphometric results associated with trait anger
| ICA network 3 | Volume (cc) | Random effects: max value (x, y, z) | Brodmann | Label (nearest grey matter within 5 mm) |
|---|---|---|---|---|
| 4.8/6.7 | 5.6 (−33, −55, −16)/6.3 (36, −56, −16) | 19,20,37 | Fusiform gyrus | |
| 2.4/5.9 | 5.3 (−30, −52, −16)/6.2 (34, −52, −16) | 19,20,37 | Fusiform gyrus | |
| 0.8/3.0 | 4.7 (−36, −55, −14)/6.0 (39, −53, −16) | 19,20,37 | Fusiform gyrus | |
| 0.3/1.0 | 4.1 (−43, −68, −23)/5.1 (48, −52, −25) | No Brodmann area | Cerebellum‐tuber | |
| 0.1/0.2 | 4.4 (−39, −55, −16)/5.8 (34, −66, −13) | 19,20,37 | Fusiform gyrus | |
| 0.1/0.0 | 4.0 (−22, −56, 8)/− | 23 | Posterior cingulate cortex | |
| 0.1/0.0 | 3.9 (−37, −70, −23)/− | No Brodmann area | Cerebellum‐uvula | |
| 0.0/2.7 | −/5.7 (43, −50, −38) | No Brodmann area | Cerebellar tonsil | |
| 0.0/0.9 | −/4.7 (36, −69, −39) | No Brodmann area | Cerebellum‐inferior semi‐lunar lobule | |
| 0.0/0.8 | −/5.0 (4, −84, −10) | 18 | Lingual gyrus | |
| 0.0/0.3 | −/5.0 (21, 3, −37) | 38 | Parahippocampal gyrus | |
| 0.0/0.2 | −/4.0 (36, −75, −34) | No Brodmann area | Cerebellum‐pyramis | |
| 0.0/0.1 | −/4.4 (1, −83, −7) | 18 | Visual association area | |
| 0.0/0.1 | −/3.8 (53, −16, 27) | 40 | Inferior parietal lobule | |
| 0.0/0.1 | −/3.7 (36, −39, 39) | 40 | Inferior parietal lobule | |
| 0.0/0.1 | −/3.6 (46, 10, −26) | 38 | Temporal pole |
Note: ICA 3 significantly correlates with trait anger.
FIGURE 3.
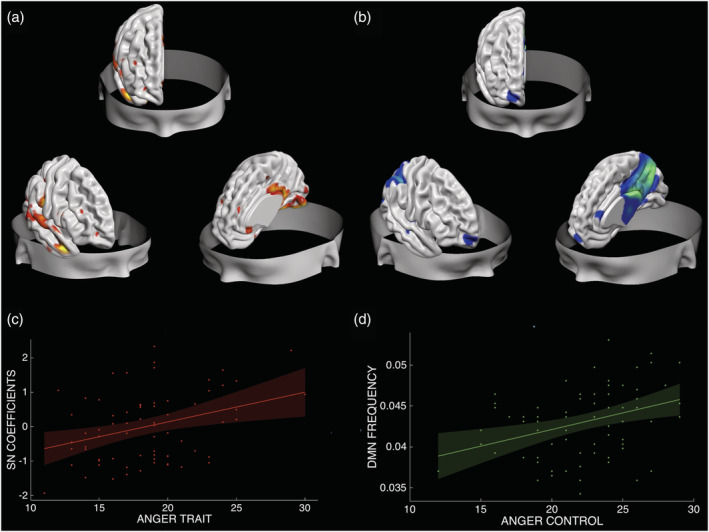
Structural and functional networks associated with trait anger and anger control. The structural network (a) includes portions of the temporal pole, the posterior cingulate cortex and the inferior parietal lobule, the lingual gyrus, the visual association area, fusiform gyrus and parts of the cerebellum, which positively correlates with trait anger (r = 0.3386, p = 0.004) (c). The functional network, that is, the default mode network, IC14 (b), whose frequency positively correlates with anger control (R 2 = 0.13, p = 0.002) (d)
3.1.2. Anger control
The analysis showed no significant correlations with any of the eight components (r = −0.103, p = 0.394; r = −0.126, p = 0.295; r = −0.092, p = 0.445; r = 0.021, p = 0.862; r = 0.020, p = 0.867; r = −0.206, p = 0.085; r = 0.072, p = 0.550; r = 0.035, p = 0.772).
3.1.3. Anger‐out
The analysis showed no significant correlations with any of the eight components (r = −0.006, p = 0.961; r = −0.048, p = 0.691; r = 0.212, p = 0.076; r = 0.053, p = 0.660; r = −0.078, p = 0.518; r = 0.080, p = 0.507; r = 0.013, p = 0.912; r = −0.073, p = 0.546).
3.1.4. Anger‐in
The analysis showed no significant correlations with any of the eight components (r = −0.107, p = 0.373; r = −0.023, p = 0.851; r = −0.072, p = 0.554; r = 0.118, p = 0.326; r = 0.010, p = 0.933; r = −0.086, p = 0.474; r = 0.121, p = 0.314; r = −0.016, p = 0.897).
3.2. Functional results
The resting state ICA identified 20 functional networks. Among them, the temporal variability and frequency of the eight standard resting state networks have been regressed with anger control scores. These networks are the following: the cerebellar network (ICA1), the salience network (ICA3), the language network (ICA12), DMN (ICA14), the visual network (ICA15), the fronto‐parietal network (ICA17), the dorsal attention network (ICA18) and the sensorimotor network (ICA19). See Figures 2 and 4.
FIGURE 4.
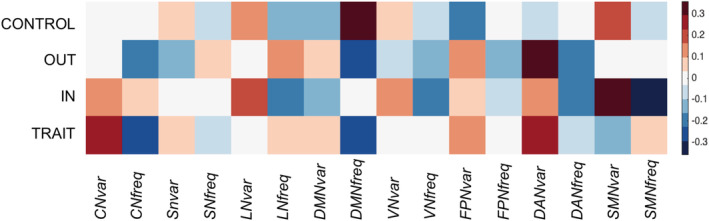
Heatmap of the functional results. Heatmap plot of the four analyses showing the correlations between the four anger scales (trait anger, anger‐in, anger‐out and anger control) with the functional networks (CN, cerebellar network; DAN, dorsal attention network; DMN, default mode network; FPN, fronto‐parietal network; LN, language network; SMN, sensorimotor network; SN, salience network; VN, visual network)
3.2.1. Trait anger
The analysis showed no significant correlations with trait anger when considering the variability (ICA1: r = 0.25, p = 0.033; ICA3: r < 0.01, p = 0.730; ICA12: r < 0.01, p = 0.970; ICA14: r = 0.1, p = 0.544; ICA15: r < 0.01, p = 0.811; ICA17: r = 0.1, p = 0.359; ICA18: r = −0.25, p = 0.039; ICA19: r = −0.141, p = 0.242) or the frequency (ICA1: r = −0.25, p = 0.042; ICA3: r < 0.01, p = 0.719; ICA12: r < 0.01, p = 0.693; ICA14: r = −0.283, p = 0.021; ICA15: r < 0.01, p = 0.973; ICA17: r < 0.01, p = 0.982; ICA18: r = 0.1, p = 0.433; ICA19: r < 0.01, p = 0.723) of each network, after correcting for the number of components (n = 8 considering both the variability and the frequency of each; Bonferroni corrected p critic = 0.003).
3.2.2. Anger control
The analysis showed a positive significant correlation between temporal frequency in the DMN and anger control (r = 0.36, p = 0.002), even when correcting for gender (r = 0.33, p = 0,006), age (r = 0.35, p = 0.004), TIV (r = 0.3665, p = 0.0018), social desirability (r = 0.3499, p = 0.0030) and the other three anger's subscales of the STAXI (r = 0.3075, p = 0.0108). Results remained significant after correcting for the number of components (n = 8, considering both the variability and the frequency of each; Bonferroni corrected p critic = 0.003). See Table 3 and Figure 3. None of the other networks showed significant correlation with anger control when considering the variability (ICA1: r < 0.01, p = 0.95; ICA3: r = 0.10, p = 0.48; ICA12: r = 0.17, p = 0.19; ICA14: r = 0.14, p = 0.27; ICA15: r < 0.01, p = 0.61; ICA17: r = 0.22, p = 0.07; ICA18: r < 0.01, p = 0.64; ICA19: r = 0.20, p = 0.11) or the frequency (ICA1: r < 0.01, p = 0.80; ICA3: r < 0.01, p = 0.55; ICA12: r < 0.01, p = 0.20; ICA15: r < 0.01, p = 0.67; ICA17: r < 0.01, p = 0.94; ICA18: r < 0.01, p = 0.93; ICA19: r < 0.01, p = 0.56) of each network.
TABLE 3.
Resting state results associated with anger control
| Default mode network | Max value (x, y, z) | Brodmann | Label |
|---|---|---|---|
| +10–66 + 38 | 7 | Posterior cingulate | |
| +38 + 38–6 | 47 | Ventral PFC | |
| +42–80–34 | No Brodmann area | Cerebellum | |
| –40–78–32 | No Brodmann area | Cerebellum‐ | |
| −22 + 58 + 2 | 10, 32 | Anterior cingulate | |
| +28–90–10 | 18 | Visual association cortex | |
| −28–94–10 | 18 | Visual association cortex | |
| +6 + 34 + 16 | 32 | Anterior cingulate | |
| +0–90 + 32 | 32 | Cerebellum‐inferior semi‐lunar lobule | |
| +42–60–52 | 19 | Visual extrastriate cortex | |
| +56–20–10 | 22 | Superior temporal gyrus | |
| −44–52–44 | No Brodmann area | Cerebellum | |
| −32 + 8 + 58 | 6 | Premotor cortex | |
| −14 + 12–10 | No Brodmann area | Putamen | |
| −60–40 + 50 | 40 | Parietal lobule | |
| +62–40 + 50 | 40 | Parietal lobule | |
| −30 + 44 + 44 | 9 | Medial prefrontal cortex | |
| +14 + 12–10 | No Brodmann area | Nucleus accumbens | |
| +6–84–42 | No Brodmann area | Cerebellum |
Note: DMN's frequency significantly correlates with anger control.
3.2.3. Anger‐out
The analysis showed no significant correlations with trait anger when considering the variability (ICA1: r = 0.1, p = 0.416; ICA3: r < 0.01, p = 0.976; ICA12: r = 0.2, p = 0.091; ICA14: r = −0.10, p = 0.382; ICA15: r = 0.141, p = 0.306; ICA17: r = 0.1, p = 0.495; ICA18: r = 0.173, p = 0.181; ICA19: r = 0.332, p = 0.005) or the frequency (ICA1: r < 0.01, p = 0.583; ICA3: r < 0.01, p = 0.991; ICA12: r = −0.2, p = 0.090; ICA14: r < 0.01, p = 0.946; ICA15: r = −0.173, p = 0.149; ICA17: r < 0.01, p = 0.678; ICA18: r = 0.224, p = 0.073; ICA19: r = 0.316, p = 0.009) of each network, after correcting for the number of components (n = 8 considering both the variability and the frequency of each; Bonferroni corrected p critic = 0.003).
3.2.4. Anger‐in
The analysis showed no significant correlations with anger‐in when considering the variability (ICA1: r < 0.01, p = 0.978; ICA3: r = 0.10, p = 0.329; ICA12: r < 0.01, p = 0.972; ICA14: r < 0.01, p = 0.785; ICA15: r = −0.1, p = 0.521; ICA17: r = 0.141, p = 0.247; ICA18: r = 0.316, p = 0.007; ICA19: r = <0.10, p = 0.888) or the frequency (ICA1: r = −0.224, p = 0.059; ICA3: r = 0.10, p = 0.549; ICA12: r = 0.141, p = 0.254; ICA14: r = −0.245, p = 0.035; ICA15: r = −0.10, p = 0.376; ICA17: r = −0.141, p = 0.215; ICA18: r = −0.2, p = 0.086; ICA19: r < 0.01, p = 0.986) of each network, after correcting for the number of components (n = 8 considering both the variability and the frequency of each; Bonferroni corrected p critic = 0.003).
4. DISCUSSION
Anger is a fundamental emotion that helps us to respond to perceived provocations, hurts or threats (Sorella et al., 2021). In this paper, we explored important aspects of anger by relying on self‐report measures of trait anger, anger control, anger‐out and anger‐in of the STAXI questionnaire. As predicted, we found neural correlates of trait anger, defined as a stable personality tendency to experience anger, and anger control or the ability individuals have to regulate this emotion. In particular, we hypothesized that trait anger may be more related to structural properties of the brain and that anger control, anger‐out and anger‐in may be more expressed at a functional level. In line with our predictions, we found evidence of a structural network associated with trait anger and separate functional evidence for anger control abilities in the DMN. No significant result was associated with anger‐out and anger‐in scores. We discussed these results in more details in the next sections.
4.1. The structural side of trait anger
ICA applied to structural images revealed that trait anger is associated with a network including the right temporal pole, the right ventromedial temporal area, the posterior cingulate, the bilateral fusiform gyrus (anterior and posterior regions) and the cerebellum. Scholars suggested that during anger‐eliciting situations, a mutual interaction between posterior and anterior temporal areas can be responsible for retrieving semantic memories of past experiences associated with anger, enhancing the elicitation of this emotion (Potegal & Stemmler, 2009; Sorella et al., 2021). Medial and anterior temporal areas are involved in mnemonic and emotional processing (Grecucci et al., 2010; Grecucci, Giorgetta, Bonini, & Sanfey, 2013; Grecucci, Giorgetta, van Wout, et al., 2013; Harris & Friston, 2010; Roshtstein et al., 2011), and they can have a key role in anger‐related experiences. Indeed, temporal lobectomy has been shown to cause social withdrawal in monkeys and to reduce aggression and rage in both monkeys and humans (Fenwick, 1989; Olson et al., 2007; Potegal & Stemmler, 2009). In particular, the temporal pole found in our analysis is consistent with previous research showing its involvement in both anger‐inducing paradigms (Damasio et al., 2000; Dougherty et al., 1999; Foster & Harrison, 2002; Grecucci, Giorgetta, Bonini, & Sanfey, 2013; Grecucci, Giorgetta, van Wout, et al., 2013; Kimbrell et al., 1999; Klimecki et al., 2018; Olson et al., 2007) and aggressive and/or violent habits (Bufkin & Luttrell, 2005; Potegal & Stemmler, 2009). This area seems to bind highly processed perceptual inputs to visceral emotional responses, allowing the formation of a personal semantic memory through perception–emotion linkages (Olson et al., 2007), especially when considering anger‐related social cues used to interpret the behaviours of others (Sorella et al., 2021).
The fusiform gyrus area has previously been associated with emotional valence (Mattek et al., 2020), especially when considering anger during mental imagery (Drexler et al., 2000). In addition, the right anterior fusiform gyrus is crucial for associative semantic knowledge (Mion et al., 2010) and could be responsible for the initial elicitation of anger‐associated beliefs (stored in our semantic system), such as the unfairness of the situation (Fernandez & Wasan, 2009; Smedslund, 1993).
Therefore, these temporal regions can underlie a link between perceptions, memories and emotion that is characterized by higher grey matter concentration in individuals with high trait anger. In addition, this higher concentration of grey matter could be responsible for hostile interpretations of environmental cues related to trait anger (Wilkowski & Robinson, 2007, 2008, 2010), especially when a more explicit conceptualization of events is considered. This process in particular may rely on the posterior cingulate cortex, whose activity is related to the conceptualization of self‐relevant cues (Ochsner et al., 2005; Rameson et al., 2010), especially when they are socially significant (Johnson et al., 2006). In addition, these conceptualizations are characterized by high certainty in high trait anger individuals (Lerner & Keltner, 2000). Accordingly, the posterior cingulate has been previously associated with high certainty (Luttrell et al., 2016), and different meta‐analyses revealed that this area is also implicated during both anger‐inducing life experiences and anger perception (Murphy et al., 2003; Phan et al., 2002; Sorella et al., 2021).
Lastly, the cerebellum has been found to play a role in negative emotions such as anger and disgust, and it seems particularly relevant for emotional control when goal‐directed behaviour is needed in social contexts (Schraa‐Tam et al., 2012). In addition, a recent meta‐analysis found involvement of the cerebellum in both perceptual and aggressive mechanisms associated with anger (Klaus & Schutter, 2021). Furthermore, the authors found that cerebellar activity was functionally connected with several networks, such as the somatomotor and the DMN. Therefore, the role of the cerebellum in anger could be modulated by these connectivity patterns, where the DMN could play a regulatory role, as explained in the following paragraph.
4.2. The functional side of anger control
Functional analyses revealed that individual differences in anger control correlate with the temporal frequency of the DMN, whereas the temporal variability of the networks showed no significant correlations. Even if the interpretation of the temporal frequency of the DMN is still under debate, there is some evidence that shows its relevance when considering different variables, such as physiological activity (Yuen et al., 2019), age, gender (Allen et al., 2011) and mental illness (Garrity et al., 2007). Our results extend this evidence to the study of emotional control. Indeed, they confirm and extend previous findings on the topic, showing for the first time an association between anger control and the DMN temporal frequency, rather than other widely used connectivity measures, such as the role of different areas in the network or the strength of their connections. Nevertheless, our results are in line with previous studies suggesting that some areas of the DMN are implicated in the regulation of emotions (Grecucci et al., 2019). It has been shown that DMN alterations are associated with anger in both clinical and non‐clinical populations. For example, a study by Hasler et al. (2017) suggested that inter‐hemispheric DMN asymmetry (e.g. in the inferior parietal lobule and in the medial frontal gyrus) is related to anger expression in individuals with ADHD, which are characterized by impaired anger regulation. Furthermore, criminal psychopathic individuals show altered FC in the DMN (Pujol et al., 2012), suggesting that this network is also involved in the regulation of violent acts.
Other studies with non‐clinical populations suggest that negative mood states, such as anger, might result from impaired connectivity between DMN regions. In particular, anger is negatively associated with connectivity between dorsal and ventral regions in the DMN (Dong et al., 2017). In addition, the modulation of the DMN activity following acute tryptophan depletion (which reduces serotonin levels) is associated with mood change: Specifically, there is a link between low‐frequency spontaneous BOLD activity in the superior parietal lobule and self‐reported scores of anger and hostility (Kunisato et al., 2011). Another study showed associations between the activation of the anterior cingulate cortex and anger control capacity; males with high trait aggression show a decreased activation in this area during a frustration task, suggesting that this region could play a role in the control of anger (Pawliczek et al., 2013).
In addition, many studies show that the mPFC (another hub of the DMN) can suppress limbic activation of subcortical, paralimbic and temporal areas (for a review, see Harris & Friston, 2010). A possible explanation could be that the DMN, through the FC of frontal regions with limbic and perceptual brain areas, can reduce the effect of hostile interpretations and angry reactions through an effortful control (Wilkowski & Robinson, 2010). Consistently, the DMN has been associated with monitoring potential alternative courses of action (Allegra et al., 2018), possibly modulating inferences about the beliefs and intentions of others (Laird et al., 2011; Li et al., 2014; Schilbach et al., 2008). Although anger is characterized by high external causality attributed to others and high goal‐directed motivation, the DMN is known to reduce this motivation and to improve social understanding and representation of others' mental states (Greicius et al., 2003; Gusnard & Raichle, 2001; Li et al., 2014; Samson et al., 2004). Therefore, the DMN can induce a self‐referential process that can regulate and control anger while increasing social and moral emotions, such as guilt (Colasante et al., 2015; Stuewig et al., 2010).
5. CONCLUSIONS AND LIMITATIONS
Separating different aspects of anger is of fundamental importance to fully understand this complex emotion (Sorella et al., 2021). The study of how trait anger and anger control capacity are implemented in our brain has been poorly addressed by neuroscience. In this paper, for the first time, we found evidence of a structural network associated with trait anger and a functional network associated with anger control capacity. Because trait anger is a stable personality trait, this becomes sedimented in specific parts of the brain (posterior perceptual, amnestic and paralimbic brain regions), where the grey matter concentration correlates with trait anger scores. On the other hand, functional results show a positive correlation between anger control capacity and the frequency of the DMN. In addition to the theoretical relevance of such results, clinicians can benefit from understanding the functional and structural bases of anger to develop psychological treatments based on neuroscientific evidence. For example, by targeting the areas found in the present study relying on pharmacology or neurostimulation methods, new treatments could be used to improve anger regulation in clinical and non‐clinical populations suffering from anger dysregulation.
However, a limitation of the study is that it only concerns healthy subjects. Therefore, further evidence is needed to generalize our results to clinical populations characterized by anger dysregulation.
Another limitation, in contrast with previous studies on anger (Alia‐Klein et al., 2020; Gilam & Hendler, 2015; Sorella et al., 2021), concerns the absence of the amygdala in our results. This is probably due to the difficulty to identify subcortical regions in whole brain multivariate analyses, thereby not allowing the identification of cortico–subcortical networks (Malherbe et al., 2014). However, future studies should take into account the possible relationship between the DMN and the amygdala in anger and its control. Indeed, although it is known that the mPFC plays a regulatory role on the amygdala during affective processes, such as during the regulation of anger (Alia‐Klein et al., 2020), some studies also suggest that the entire DMN could be involved in emotion regulation, given its suppression of limbic and paralimbic areas (Buckner et al., 2008; Harris & Friston, 2010) and its alterations associated with anger expression and violence (Hasler et al., 2017; Pujol et al., 2012). For example, it has been observed that individuals with a genotype associated with aggression are characterized by DMN deactivations during inhibitory control (Ma et al., 2018), although posterior cingulate deactivations were observed in psychopathic individuals during moral dilemmas (Pujol et al., 2012). Our results are in line with this evidence and suggest that the DMN can be involved in the regulation of emotions such as anger and in moral judgements. In particular, the mentalization process associated with the DMN could play a key role in the regulation of anger, for example, when considering the possible consequences of anger expression. However, future studies are needed to clarify and sustain these hypotheses.
In addition, we analysed structural and functional data separately. Future studies can further investigate the relation between structural and functional brain networks involved in emotional experiences, especially when considering emotional control and the common brain areas involved in these analyses. For example, the posterior cingulate emerged both in the structural and in the functional networks. On a speculative account, its role could be modulated according to its connectivity with other brain areas. For example, it has been previously linked to both certainty (Luttrell et al., 2016), characteristic of high trait anger and moral judgement (Pujol et al., 2012), which could be involved in the control of anger when other areas of the DMN are also activated.
Finally, it should be noted that the DMN identified by the ICA mainly involved posterior brain regions. Other studies relying on the same analysis found similar effects (see, e.g. Motoyama et al., 2019). In multivariate analyses, this could be explained by the fact that posterior regions of the DMN seem to be more robust (Kim & Lee, 2011). Future studies are needed to better understand this result, in particular considering the anterior and posterior regions of the DMN and their link with affective processes.
CONFLICT OF INTEREST
The authors have no competing interests to declare that are relevant to the content of this article.
AUTHOR CONTRIBUTIONS
Sara Sorella: Conceptualization, methodology, formal analysis, writing–original draft preparation. Valentina Vellani: Methodology, formal analysis, writing–original draft preparation. Roma Siugzdaite: Methodology, formal analysis, writing–reviewing and editing. Paola Feraco: Writing–reviewing and editing. Alessandro Grecucci: Formal analysis, supervision, conceptualization, writing–original draft preparation, writing–reviewing and editing. All listed authors should have contributed to the manuscript substantially and have agreed to the final submitted version.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15537.
ACKNOWLEDGEMENT
We thank Stepheni Uh (MRC Cognition and Brain Science Unit, Cambridge University, UK) for her assistance with language revision of our manuscript. Open Access Funding provided by Universita degli Studi di Trento within the CRUI‐CARE Agreement. [Correction added on 20 May 2022, after first online publication: CRUI funding statement has been added.]
Sorella, S. , Vellani, V. , Siugzdaite, R. , Feraco, P. , & Grecucci, A. (2022). Structural and functional brain networks of individual differences in trait anger and anger control: An unsupervised machine learning study. European Journal of Neuroscience, 55(2), 510–527. 10.1111/ejn.15537
Edited by: John Foxe
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in OpenNeuro at [https://openneuro.org/datasets/ds000221/versions/00002], reference number [ds000221].
REFERENCES
- Alia‐Klein, N. , Gan, G. , Gilam, G. , Bezek, J. , Bruno, A. , Denson, T. F. , Hendler, T. , Lowe, L. , Mariotti, V. , Muscatello, M. R. , Palumbo, S. , Pellegrini, S. , Pietrini, P. , Rizzo, A. , & Verona, E. (2020). The feeling of anger: From brain networks to linguistic expressions. Neuroscience & Biobehavioral Reviews, 108, 480–497. 10.1016/j.neubiorev.2019.12.002 [DOI] [PubMed] [Google Scholar]
- Allegra, M. , Seyed‐Allaei, S. , Schuck, N. W. , Amati, D. , Laio, A. , & Reverberi, C. (2018). Brain network dynamics during spontaneous strategy shifts and incremental task optimization. 10.1101/481838 [DOI] [PubMed]
- Allen, E. A. , Erhardt, E. B. , Damaraju, E. , Gruner, W. , Segall, J. M. , Silva, R. F. , Havlicek, M. , Rachakonda, S. , Fries, J. , Kalyanam, R. , Michael, A. M. , Caprihan, A. , Turner, J. A. , Eichele, T. , Adelsheim, S. , Bryan, A. D. , Bustillo, J. , Clark, V. P. , Feldstein Ewing, S. W. , … Calhoun, V. D. (2011). A baseline for the multivariate comparison of resting‐state networks. Frontiers in Systems Neuroscience, 5, 1–23. 10.3389/fnsys.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, A. K. , & Phelps, E. A. (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature, 411, 305–309. 10.1038/35077083 [DOI] [PubMed] [Google Scholar]
- Babayan, A. , Baczkowski, B. , Cozatl, R. , Dreyer, M. , Engen, H. , Erbey, M. , Falkiewicz, M. , Farrugia, N. , Gaebler, M. , Golchert, J. , Golz, L. , Gorgolewski, K. , Haueis, P. , Huntenburg, J. , Jost, R. , Kramarenko, Y. , Krause, S. , Kumral, D. , Lauckner, M. , … Villringer, A. (2018). MPI‐Leipzig_Mind‐Brain‐Body. OpenNeuro, ds000221, version 00002. 10.18112/openneuro.ds000221.v1.0.0 [dataset] [DOI]
- Baron, K. G. , Smith, T. W. , Butner, J. , Nealey‐Moore, J. , Hawkins, M. W. , & Uchino, B. N. (2006). Hostility, anger, and marital adjustment: Concurrent and prospective associations with psychosocial vulnerability. Journal of Behavioral Medicine, 30(1), 1–10. 10.1007/s10865-006-9086-z [DOI] [PubMed] [Google Scholar]
- Bell, A. J. , & Sejnowski, T. J. (1995). An information‐maximization approach to blind separation and blind deconvolution. Neural Computation, 7, 1129–1159. 10.1162/neco.1995.7.6.1129 [DOI] [PubMed] [Google Scholar]
- Bettencourt, B. A. , Talley, A. , Benjamin, A. J. , & Valentine, J. (2006). Personality and aggressive behavior under provoking and neutral conditions: A meta‐analytic review. Psychological Bulletin, 132(5), 751–777. 10.1037/0033-2909.132.5.751 [DOI] [PubMed] [Google Scholar]
- Beyer, F. , Münte, T. F. , Göttlich, M. , & Krämer, U. M. (2015). Orbitofrontal cortex reactivity to angry facial expression in a social interaction correlates with aggressive behavior. Cerebral Cortex, 25(9), 3057–3063. 10.1093/cercor/bhu101 [DOI] [PubMed] [Google Scholar]
- Brown, G. D. , Yamada, S. , & Sejnowski, T. J. (2001). Independent component analysis at the neural cocktail party. Trends in Neuroscience, 24(1), 54–63. 10.1016/S0166-2236(00)01683-0 [DOI] [PubMed] [Google Scholar]
- Buades‐Rotger, M. , Engelke, C. , Beyer, F. , Keevil, B. G. , Brabant, G. , & Krämer, U. M. (2016). Endogenous testosterone is associated with lower amygdala reactivity to angry faces and reduced aggressive behavior in healthy young women. Scientific Reports, 6(1), 1–14. 10.1038/srep38538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , Andrews‐Hanna, J. R. , & Schacter, D. L. (2008). The brain's default net‐ work: Anatomy, function, and relevance to disease. Ann NY Acad Sci, 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Bufkin, J. L. , & Luttrell, V. E. (2005). Neuroimaging studies of aggressive and violent behavior. Trauma, Violence and Abuse, 6, 176–191. 10.1177/1524838005275089 [DOI] [PubMed] [Google Scholar]
- Calhoun, V. , Adali, T. , Pearlson, G. , & Pekar, J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14(3), 140–151. 10.1002/hbm.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun, V. D. , Miller, R. , Pearlson, G. , & Adalı, T. (2014). The chronnectome: Time‐varying connectivity networks as the next frontier in fmri data discovery. Neuron, 84(2), 262–274. 10.1016/j.neuron.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa, N. , Crespi, C. , Motterlini, M. , Baud‐Bovy, G. , Chierchia, G. , Pantaleo, G. , Tettamanti, M. , & Cappa, S. F. (2013). The functional and structural neural basis of individual differences in loss aversion. The Journal of Neuroscience, 33(36), 14307–14317. 10.1523/JNEUROSCI.0497-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, J. M. , Beacher, F. , Reinke, K. S. , Habib, R. , Harmon‐Jones, E. , Mujica‐Parodi, L. R. , & Hajcak, G. (2012). Nonconscious attention bias to threat is correlated with anterior cingulate cortex gray matter volume: A voxel‐based morphometry result and replication. NeuroImage, 59(2), 1713–1718. 10.1016/j.neuroimage.2011.09.040 [DOI] [PubMed] [Google Scholar]
- Carré, J. M. , Fisher, P. M. , Manuck, S. B. , & Hariri, A. R. (2010). Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Social Cognitive and Affective Neuroscience, 7(2), 213–221. 10.1093/scan/nsq101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C. , & Glover, G. H. (2010). Time‐frequency dynamics of resting‐state brain connectivity measured with fMRI. NeuroImage, 50(1), 81–98. 10.1016/j.neuroimage.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro, E. F. , Lee, R. , & Mccloskey, M. S. (2014). Relationship between psychopathy, aggression, anger, impulsivity, and intermittent explosive disorder. Aggressive Behavior, 40(6), 526–536. 10.1002/ab.21536 [DOI] [PubMed] [Google Scholar]
- Colasante, T. , Zuffianò, A. , & Malti, T. (2015). Do moral emotions buffer the anger‐aggression link in children and adolescents? Journal of Applied Developmental Psychology, 41, 1–7. 10.1016/j.appdev.2015.06.001 [DOI] [Google Scholar]
- Crowne, D. P. , & Marlowe, D. (1960). A new scale of social desirability independent of psychopathology. Journal of Consulting Psychology, 24, 349–354. 10.1037/h0047358 [DOI] [PubMed] [Google Scholar]
- Dadomo, H. , Grecucci, A. , Giardini, I. , Ugolini, E. , Carmelita, A. , & Panzeri, M. (2016). Schema therapy for emotional dysregulation: Theoretical implication and clinical application. Frontiers in Psychology, 7, 1987. 10.3389/fpsyg.2016.01987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadomo, H. , Panzeri, M. , Caponcello, D. , Carmelita, A. , & Grecucci, A. (2018). Schema therapy for emotional dysregulation in personality disorders: A review. Current Opinion in Psychiatry, 31(1), 43–49. 10.1097/YCO.0000000000000380 [DOI] [PubMed] [Google Scholar]
- Damasio, A. R. , Grabowski, T. J. , Bechara, A. , Damasio, H. , Ponto, L. L. , Parvizi, J. , & Hichwa, R. D. (2000). Subcortical and cortical brain activity during the feeling of self‐ generated emotions. Nature Neuroscience, 3, 1049–1056. 10.1038/79871 [DOI] [PubMed] [Google Scholar]
- de Panfilis, C. , Schito, G. , Generali, I. , Gozzi, L. , Ossola, P. , Marchesi, C. , & Grecucci, A. (2019). Emotions at the border: Increased punishment behavior during fair interpersonal exchanges in borderline personality disorder. Journal of Abnormal Psychology, 128(2), 162–172. 10.1037/abn0000404 [DOI] [PubMed] [Google Scholar]
- Denson, T. F. , Ronay, R. , Hippel, W. V. , & Schira, M. M. (2013). Endogenous testosterone and cortisol modulate neural responses during induced anger control. Social Neuroscience, 8(2), 165–177. 10.1080/17470919.2012.655425 [DOI] [PubMed] [Google Scholar]
- Dodge, K. A. , & Coie, J. D. (1987). Social‐information‐processing factors in reactive and proactive aggression in children's peer groups. Journal of Personality and Social Psychology, 53, 1146–1158. 10.1037/0022-3514.53.6.1146 [DOI] [PubMed] [Google Scholar]
- Dong, G. , Li, H. , Wang, L. , & Potenza, M. N. (2017). The correlation between mood states and functional connectivity within the default mode network can differentiate internet gaming disorder from healthy controls. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 77, 185–193. 10.1016/j.pnpbp.2017.04.016 [DOI] [PubMed] [Google Scholar]
- Dougherty, D. D. , Shin, L. M. , Alpert, N. M. , Pitmann, R. K. , Orr, S. P. , Lasko, M. , Macklin, M. L. , Fischman, A. J. , & Rauch, S. L. (1999). Anger in healthy men: A PET study using script‐driven imagery. Biological Psychiatry, 46, 466–472. 10.1016/S0006-3223(99)00063-3 [DOI] [PubMed] [Google Scholar]
- Drexler, K. , Schweitzer, J. B. , Quinn, C. K. , Gross, R. , Ely, T. D. , Muhammad, F. , & Kilts, C. D. (2000). Neural activity related to anger in cocaine‐dependent men: A possible link to violence and relapse. American Journal on Addictions, 9(4), 331–339. 10.1080/105504900750047382 [DOI] [PubMed] [Google Scholar]
- Fenwick, P. (1989). The nature and management of aggression in epilepsy. Journal of Neuropsychiatry & Clinical Neurosciences, 1, 418–425. [DOI] [PubMed] [Google Scholar]
- Fernandez, E. , & Wasan, A. (2009). The anger of pain sufferers: Attributions to agents and appraisals of wrongdoings. In Potegal M., Stemmler G., & Spielberger C. (Eds.), International handbook of anger (pp. 449–464). Springer. [Google Scholar]
- Foster, P. S. , & Harrison, D. W. (2002). The relationship between magnitude of cerebral activation and intensity of emotional arousal. International Journal of Neuroscience, 112(12), 1463–1477. 10.1080/00207450290158359 [DOI] [PubMed] [Google Scholar]
- Frederickson, J. , Messina, I. , & Grecucci, A. (2018). Dysregulated affects and dysregulating defenses: Toward an emotion regulation informed dynamic psychotherapy. Frontiers in Psychology, 9, 2054. 10.3389/fpsyg.2018.02054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler, C. E. , King, J. A. , & Zhang, N. (2012). Amygdala–orbitofrontal resting‐state functional connectivity is associated with trait anger. Neuroreport, 23(10), 606–610. 10.1097/wnr.0b013e3283551cfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, D. D. , Kovacevic, N. , McIntosh, A. R. , & Grady, C. L. (2013). The modulation of BOLD variability between cognitive states varies by age and processing speed. Cerebral Cortex, 23(3), 684–693. 10.1093/cercor/bhs055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity, A. G. , Pearlson, G. D. , McKiernan, K. , Lloyd, D. , Kiehl, K. A. , & Calhoun, V. D. (2007). Aberrant “default mode” functional connectivity in schizophrenia. The American Journal of Psychiatry, 164, 450–457. 10.1176/ajp.2007.164.3.450 [DOI] [PubMed] [Google Scholar]
- Giardina, A. , Caltagirone, C. , & Oliveri, M. (2011). Temporo‐parietal junction is involved in attribution of hostile intentionality in social interactions: An rTMS study. Neuroscience Letters, 495(2), 150–154. 10.1016/j.neulet.2011.03.059 [DOI] [PubMed] [Google Scholar]
- Gilam, G. , Abend, R. , Gurevitch, G. , Erdman, A. , Baker, H. , Ben‐Zion, Z. , & Hendler, T. (2018). Attenuating anger and aggression with neuromodulation of the vmPFC: A simultaneous tDCS‐fMRI study. Cortex, 109, 156–170. 10.1016/j.cortex.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Gilam, G. , & Hendler, T. (2015). Deconstructing anger in the human brain. In Wöhr M. & Krach S. (Eds.), Social behavior from rodents to humans (Vol. 30). Current Topics in Behavioral Neurosciences. (pp. 257–273). Springer. [DOI] [PubMed] [Google Scholar]
- Gilam, G. , Lin, T. , Raz, G. , Azrielant, S. , Fruchter, E. , Ariely, D. , & Hendler, T. (2015). Neural substrates underlying the tendency to accept anger‐infused ultimatum offers during dynamic social interactions. NeuroImage, 120, 400–411. 10.1016/j.neuroimage.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Grecucci, A. , Frederickson, J. , & Job, R. (2017). How dare you not recognize the role of my contempt: Insights from experimental psychopathology. Behavioral and Brain Sciences, 40, e238. 10.1017/S0140525X16000777 [DOI] [PubMed] [Google Scholar]
- Grecucci, A. , Giorgetta, C. , Bonini, N. , & Sanfey, A. (2013). Reappraising social emotions: The role of inferior frontal gyrus, temporo‐parietal junction and insula in interpersonal regulation. Frontiers in Human Neuroscience, 7, 523. 10.3389/fnhum.2013.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci, A. , Giorgetta, C. , Brambilla, P. , Zanon, S. , Perini, L. , Balestrieri, M. , Bonini, N. , & Sanfey, A. (2013). Anxious ultimatums. How anxiety affects socio‐economic decisions. Cognition & Emotion., 27(2), 230–244. 10.1080/02699931.2012.698982 [DOI] [PubMed] [Google Scholar]
- Grecucci, A. , Giorgetta, C. , van Wout, M. , Bonini, N. , & Sanfey, A. (2013). Reappraising the ultimatum: An fMRI study of emotion regulation and decision‐making. Cerebral Cortex, 23(2), 399–410. 10.1093/cercor/bhs028 [DOI] [PubMed] [Google Scholar]
- Grecucci, A. , Messina, I. , Amodeo, L. , Lapomarda, G. , Crescentini, C. , Dadomo, H. , Panzeri, M. , Theuninck, A. , & Frederickson, J. (2020). A dual route model for regulating emotions: Comparing models, techniques and biological mechanisms. Frontiers in Psychology, 11, 930. 10.3389/fpsyg.2020.00930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci, A. , Rubicondo, D. , Siugzdaite, R. , Surian, L. , & Job, R. (2016). Uncovering social deficits in autistic individuals: A source‐based morphometry study. Frontiers in Neuroscience, 31(10), 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci, A. , Soto, D. , Rumiati, R. , Humphreys, G. W. , & Roshtstein, P. (2010). The interrelations between verbal working memory and visual selection of emotional faces. Journal of Cognitive Neuroscience, 22(6), 1189–1200. 10.1162/jocn.2009.21276 [DOI] [PubMed] [Google Scholar]
- Grecucci, A. , Sulpizio, S. , Vespignani, F. , & Job, R. (2019). Seeing emotions, reading emotions: Behavioral and ERPs evidence of the regulation of visual and linguistic stimuli. PLoS ONE, 14(5), 0209461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius, M. D. , Krasnow, B. , Reiss, A. L. , & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings. National Academy of Sciences. United States of America, 100, 253–258. 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, C. N. , Turner, J. A. , & Calhoun, V. D. (2019). Source‐based morphometry: A decade of covarying structural brain patterns. Brain Structure & Function, 224, 3031–3044. 10.1007/s00429-019-01969-8 [DOI] [PubMed] [Google Scholar]
- Gusnard, D. A. , & Raichle, M. E. (2001). Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews. Neuroscience, 2, 685–694. 10.1038/35094500 [DOI] [PubMed] [Google Scholar]
- Harris, C. R. L. , & Friston, K. J. (2010). The default‐mode, ego‐functions and free‐energy: A neurobiological account of Freudian ideas. Brain, 133(4), 1265–1283. 10.1093/brain/awq010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler, R. , Preti, M. G. , Meskaldji, D. E. , Prados, J. , Adouan, W. , Rodriguez, C. , & Sinanaj, I. (2017). Inter‐hemispherical asymmetry in default‐mode functional connectivity and BAIAP2 gene are associated with anger expression in ADHD adults. Psychiatry Research: Neuroimaging, 269, 54–61. 10.1016/j.pscychresns.2017.09.004 [DOI] [PubMed] [Google Scholar]
- Himberg, J. , Hyvärinen, A. , & Esposito, F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage, 22, 1214–1222. CrossRef Medline [DOI] [PubMed] [Google Scholar]
- Honk, J. V. , Tuiten, A. , Haan, E. D. , Hout, M. V. , & Stam, H. (2001). Attentional biases for angry faces: Relationships to trait anger and anxiety. Cognition & Emotion, 15(3), 279–297. 10.1080/02699930126112 [DOI] [Google Scholar]
- Jacob, Y. , Gilam, G. , Lin, T. , Raz, G. , & Hendler, T. (2018). Anger modulates influence hierarchies within and between emotional reactivity and regulation networks. Frontiers in Behavioral Neuroscience, 12, 60. 10.3389/fnbeh.2018.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. K. , Raye, C. L. , Mitchell, K. J. , Touryan, S. R. , Greene, E. J. , & Nolen‐Hoeksema, S. (2006). Dissociating medial frontal and posterior cingulate activity during self‐reflection. Social Cognitive and Affective Neuroscience, 1(1), 56–64. 10.1093/scan/nsl004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, D. P. , & Adolphs, R. (2010). Impaired fixation to eyes following amygdala damage arises from abnormal bottom‐up attention. Neuropsychologia, 48, 3392–3398. 10.1016/j.neuropsychologia.2010.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernberg, O. F. (2012). The inseparable nature of love and aggression: Clinical and theoretical perspectives. American Psychiatric Pub. [Google Scholar]
- Kim, D.‐Y. , & Lee, J.‐H. (2011). Are posterior default‐mode networks more robust than anterior default‐mode networks? Evidence from resting‐state fmri data analysis. Neuroscience Letters, 498(1), 57–62. 10.1016/j.neulet.2011.04.062 [DOI] [PubMed] [Google Scholar]
- Kimbrell, T. A. , George, M. S. , Parakh, P. I. , Ketter, T. A. , Podell, D. M. , Danielson, A. L. , Repella, J. D. , Benson, B. E. , Willis, M. W. , Herscovitch, P. , & Post, R. M. (1999). Regional brain activity during transient self‐induced anxiety and anger in healthy adults. Biological Psychiatry, 46, 454–465. 10.1016/S0006-3223(99)00103-1 [DOI] [PubMed] [Google Scholar]
- Klaus, J. , & Schutter, D. (2021). Functional topography of anger and aggression in the human cerebellum. NeuroImage, 226, 117582. 10.1016/j.neuroimage.2020.117582 [DOI] [PubMed] [Google Scholar]
- Klimecki, O. M. , Sander, D. , & Vuilleumier, P. (2018). Distinct brain areas involved in anger versus punishment during social interactions. Scientific Reports, 8(1), 10556. 10.1038/s41598-018-28863-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla, N. J. , Meyer, J. H. , Bagby, R. M. , & Brijmohan, A. (2016). Trait anger, physical aggression, and violent offending in antisocial and borderline personality disorders. Journal of Forensic Sciences, 62(1), 137–141. 10.1111/1556-4029.13234 [DOI] [PubMed] [Google Scholar]
- Kornelsen, J. , Wilson, A. , Labus, J. S. , Witges, K. , Mayer, E. A. , & Bernstein, C. N. (2020). Brain resting‐state network alterations associated with Crohn's disease. Frontiers in Neurology, 11, 48. 10.3389/fneur.2020.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisato, Y. , Okamoto, Y. , Okada, G. , Aoyama, S. , Demoto, Y. , Munakata, A. , & Yamawaki, S. (2011). Modulation of default‐mode network activity by acute tryptophan depletion is associated with mood change: A resting state functional magnetic resonance imaging study. Neuroscience Research, 69(2), 129–134. 10.1016/j.neures.2010.11.005 [DOI] [PubMed] [Google Scholar]
- Laird, A. R. , Fox, P. M. , Eickhoff, S. B. , Turner, J. A. , Ray, K. L. , McKay, D. R. , Glahn, D. C. , Beckmann, C. F. , Smith, S. M. , & Fox, P. T. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognition, 23, 4022–4037. 10.1162/jocn_a_00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapomarda, G. , Grecucci, A. , Messina, I. , Pappaianni, E. , & Dadomo, H. (2021). Common and different gray and white matter alterations in bipolar and borderline personality disorder. Brain Research, 1762, 147401. 10.1016/j.brainres.2021.147401 [DOI] [PubMed] [Google Scholar]
- Lapomarda, G. , Pappaianni, E. , Siugzdaite, R. , Sanfey, A. G. , Rumiati, R. I. , & Grecucci, A. (2021). Out of control: An altered parieto‐occipital‐cerebellar network for impulsivity in bipolar disorder. Behavioural Brain Research, 406, 113228. [DOI] [PubMed] [Google Scholar]
- Lee, T. W. , Girolami, M. , & Sejnowski, T. J. (1999). Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Computation, 11, 417–441. 10.1162/089976699300016719 [DOI] [PubMed] [Google Scholar]
- Lerner, J. S. , & Keltner, D. (2000). Beyond valence: Toward a model of emotion‐specific influences on judgment and choice. Cognition and Emotion, 14, 473–493. 10.1080/026999300402763 [DOI] [Google Scholar]
- Li, W. , Mai, X. , & Liu, C. (2014). The default mode network and social understanding of others: What do brain connectivity studies tell us. Frontiers in Human Neuroscience, 8, 74. 10.3389/fnhum.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. O. , Adali, T. , & Calhoun, V. D. (2007). Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping, 28(11), 1251–1266. 10.1002/hbm.20359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, K. A. , Wager, T. D. , Kober, H. , Bliss‐Moreau, E. , & Barrett, L. F. (2012). The brain basis of emotion: A meta‐analytic review. The Behavioral and Brain Sciences, 35(3), 121–143. 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt, M. D. , Cooney, N. L. , & Morse, P. (2000). Reactivity to alcohol‐related stimuli in the laboratory and in the field: Predictors of craving in treated alcoholics. Addiction, 95(6), 889–900. 10.1046/j.1360-0443.2000.9568896.x [DOI] [PubMed] [Google Scholar]
- Luttrell, A. , Stillman, P. E. , Hasinski, A. E. , & Cunningham, W. A. (2016). Neural dissociations in attitude strength: Distinct regions of cingulate cortex track ambivalence and certainty. Journal of Experimental Psychology: General, 145(4), 419–433. 10.1037/xge0000141 [DOI] [PubMed] [Google Scholar]
- Ma, R. , Gan, G. , Zhang, J. , Ming, Q. , Jiang, Y. , Gao, Y. , Wang, X. , & Yao, S. (2018). MAOA genotype modulates default mode network deactivation during inhibitory control. Biological Psychology, 138, 27–34. 10.1016/j.biopsycho.2018.08.006 [DOI] [PubMed] [Google Scholar]
- Malherbe, C. , Messé, A. , Bardinet, E. , Pélégrini‐Issac, M. , Perlbarg, V. , Marrelec, G. , & Benali, H. (2014). Combining spatial independent component analysis with regression to identify the subcortical components of resting‐state fMRI functional networks. Brain Connectivity, 4(3), 181–192. 10.1089/brain.2013.0160 [DOI] [PubMed] [Google Scholar]
- Mattek, A. M. , Burr, D. A. , Shin, J. , Whicker, C. L. , & Kim, M. J. (2020). Identifying the representational structure of affect using fMRI. Affective Science, 1, 42–56. 10.1007/s42761-020-00007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattevi, A. , Sorella, S. , Vellani, V. , Job, R. , & Grecucci, A. (2019). Regolare la rabbia: Quale strategia? Uno studio preliminare. Giornale italiano di Psicologia. [Google Scholar]
- Mion, M. , Patterson, K. , Acosta‐Cabronero, J. , Pengas, G. , Izquierdo‐Garcia, D. , Hong, Y. T. , Fryer, T. D. , Williams, G. B. , Hodges, J. R. , & Nestor, P. J. (2010). What the left and right anterior fusiform gyri tell us about semantic memory. Brain, 133(11), 3256–3268. 10.1093/brain/awq272 [DOI] [PubMed] [Google Scholar]
- Motoyama, Y. , Oshiro, Y. , Takao, Y. , Sato, H. , Obata, N. , Izuta, S. , Mizobuchi, S. , & Kan, S. (2019). Resting‐state brain functional connectivity in patients with chronic pain who responded to subanesthetic‐dose ketamine. Scientific Reports, 9, 12912. 10.1038/s41598-019-49360-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, K.‐R. , Vigário, R. , Meinecke, F. , & Ziehe, A. (2004). Blind source separation techniques for decomposing event related brain signals. International Journal of Bifurcation and Chaos, 14(2), 773–792. 10.1142/S0218127404009466 [DOI] [Google Scholar]
- Murphy, F. C. , Nimmo‐Smith, I. , & Lawrence, A. D. (2003). Functional neuroanatomy of emotions: A meta‐analysis. Cognitive, Affective, & Behavioral Neuroscience, 3, 207–233. 10.3758/CABN.3.3.207 [DOI] [PubMed] [Google Scholar]
- Nieto‐Castanon, A. (2020). Handbook of fcMRI methods in CONN. Hilbert Press. [Google Scholar]
- Ochsner, K. N. , Beer, J. S. , Robertson, E. R. , Cooper, J. C. , Gabrieli, J. D. , Kihsltrom, J. F. , & Desposito, M. (2005). The neural correlates of direct and reflected self‐knowledge. NeuroImage, 28(4), 797–814. 10.1016/j.neuroimage.2005.06.069 [DOI] [PubMed] [Google Scholar]
- Olson, I. R. , Plotzker, A. , & Ezzyat, Y. (2007). The enigmatic temporal pole: A review of findings on social and emotional processing. Brain, 130(7), 1718–1731. 10.1093/brain/awm052 [DOI] [PubMed] [Google Scholar]
- Pan, J. , Zhan, L. , Hu, C. , Yang, J. , Wang, C. , Gu, L. , Zhong, S. , Huang, Y. , Wu, Q. , Xie, X. , Chen, Q. , Zhou, H. , Huang, M. , & Wu, X. (2018). Emotion regulation and complex brain networks: Association between expressive suppression and efficiency in the fronto‐parietal network and default‐mode network. Frontiers in Human Neuroscience, 12, 70. 10.3389/fnhum.2018.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappaianni, E. , de Pisapia, N. , Siugzdaite, R. , Crescentini, C. , Calcagnì, A. , Job, R. , & Grecucci, A. (2019). Less is more: Psychological and morphometric differences between low vs high reappraisers. Cognitive, Affective, & Behavioral Neuroscience, 20, 128–140. 10.3758/s13415-019-00757-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappaianni, E. , Siugzdaite, R. , Vettori, S. , Venuti, P. , Job, R. , & Grecucci, A. (2017). Three shades of gray: Detecting brain abnormalities in children with autism using source‐, voxel‐ and surface‐ based morphometry. European Journal of Neuroscience, 47, 690–700. 10.1111/ejn.13704 [DOI] [PubMed] [Google Scholar]
- Pawliczek, C. M. , Derntl, B. , Kellermann, T. , Gur, R. C. , Schneider, F. , & Habel, U. (2013). Anger under control: Neural correlates of frustration as a function of trait aggression. PLoS ONE, 8(10), e78503. 10.1371/journal.pone.0078503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan, K. L. , Wager, T. , Taylor, S. F. , & Liberzon, I. (2002). Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. NeuroImage, 16, 331–348. 10.1006/nimg.2002.1087 [DOI] [PubMed] [Google Scholar]
- Piretti, L. , Pappaianni, E. , Lunardelli, A. , Zorzenon, I. , Ukmar, M. , Pesavento, V. , & Grecucci, A. (2020). The role of amygdala in self‐conscious emotions in a patient with acquired bilateral damage. Frontiers in Neuroscience, 14, 677. 10.3389/fnins.2020.00677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potegal, M. , & Stemmler, G. (2009). Constructing a neurology of anger. In Potegal M., Stemmler G., & Spielberger C. (Eds.), International handbook of anger (pp. 39–59). Springer. [Google Scholar]
- Pujol, J. , Batalla, I. , Contreras‐Rodriguez, O. , Harrison, B. J. , Pera, V. , Hernandez‐ Ribas, R. , Real, E. , Bosa, L. , Soriano‐Mas, C. , Deus, J. , López‐Solà, M. , Pifarré, J. , Menchón, J. M. , & Cardoner, N. (2012). Breakdown in the brain network subserving moral judgment in criminal psychopathy. Social Cognitive and Affective Neuroscience, 7, 917–923. 10.1093/scan/nsr075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, F. , Zhu, W. , Dong, Y. , Qiu, J. , Gong, X. , Xiao, M. , Zheng, Y. , Zhao, Y. , Chen, X. , & Xia, L.‐X. (2019). Brain structure links trait hostile attribution bias and attitudes toward violence. Neuropsychologia, 125, 42–50. 10.1016/j.neuropsychologia.2019.01.015 [DOI] [PubMed] [Google Scholar]
- Rajamanickam, K. (2020). A mini review on different methods of functional‐MRI data analysis. Archives of Internal Medicine Research, 03(01), 044–060. 10.26502/aimr.0022 [DOI] [Google Scholar]
- Rameson, L. T. , Satpute, A. B. , & Lieberman, M. D. (2010). The neural correlates of implicit and explicit self‐relevant processing. NeuroImage, 50(2), 701–708. 10.1016/j.neuroimage.2009.12.098 [DOI] [PubMed] [Google Scholar]
- Roshtstein, P. , Soto, D. , Grecucci, A. , Geng, J. J. , & Humphreys, G. W. (2011). The role of the pulvinar in resolving competition between memory and visual selection: A functional connectivity study. Neuropsychologia, 49(6), 1544–1552. 10.1016/j.neuropsychologia.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Samson, D. , Apperly, I. A. , Chiavarino, C. , & Humphreys, G. W. (2004). Left temporoparietal junction is necessary for representing someone else's belief. Nature Neuroscience, 7, 499–500. 10.1038/nn1223 [DOI] [PubMed] [Google Scholar]
- Saviola, F. , Pappaianni, E. , Monti, A. , Grecucci, A. , Jovicich, J. , & de Pisapia, N. (2020). Separating trait and state anxiety in the brain: Evidence from source‐based morphometry and functional connectivity study. Scientific Reports, 10, 11112. 10.1038/s41598-020-68008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach, L. , Eickhoff, S. B. , Rska‐Jagiela, A. R. , Fink, G. R. , & Vogeley, K. (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and Cognition, 17, 457–467. 10.1016/j.concog.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Schraa‐Tam, C. K. , Rietdijk, W. J. , Verbeke, W. J. , Dietvorst, R. C. , Van Den Berg, W. E. , Bagozzi, R. P. , & de Zeeuw, C. I. (2012). fMRI activities in the emotional cerebellum: A preference for negative stimuli and goal‐directed behavior. The Cerebellum, 11(1), 233–245. 10.1007/s12311-011-0301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep, N. , Tonnaer, F. , Ven, V. V. D. , Arntz, A. , Raine, A. , & Cima, M. (2018). Anger provocation increases limbic and decreases medial prefrontal cortex connectivity with the left amygdala in reactive aggressive violent offenders. Brain Imaging and Behavior, 13(5), 1311–1323. 10.1007/s11682-018-9945-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedslund, J. (1993). How shall the concept of anger be defined? Theory & Psychology, 3(1), 5–33. 10.1177/0959354393031001 [DOI] [Google Scholar]
- Smith, T. W. , Glazer, K. , Ruiz, J. M. , & Gallo, L. C. (2004). Hostility, anger, aggressiveness, and coronary heart disease: An interpersonal perspective on personality, emotion, and health. Journal of Personality, 72, 1217–1270. 10.1111/j.1467-6494.2004.00296.x [DOI] [PubMed] [Google Scholar]
- Soman, S. M. , Raghavan, S. , Rajesh, P. G. , Mohanan, N. , Thomas, B. , Kesavadas, C. , & Menon, R. N. (2020). Does resting state functional connectivity differ between mild cognitive impairment and early Alzheimer's dementia? Journal of the Neurological Sciences, 418, 117093. 10.1016/j.jns.2020.117093 [DOI] [PubMed] [Google Scholar]
- Sorella, S. , Grecucci, A. , Piretti, L. , & Job, R. (2021). Do anger perception and the experience of anger share common neural mechanisms? Coordinate‐based meta‐analytic evidence of similar and different mechanisms from functional neuroimaging studies. NeuroImage, 230, 117777. [DOI] [PubMed] [Google Scholar]
- Sorella, S. , Lapomarda, G. , Messina, I. , Frederickson, J. J. , Siugzdaite, R. , Job, R. , & Grecucci, A. (2019). Testing the expanded continuum hypothesis of schizophrenia and bipolar disorder. Neural and psychological evidence for shared and distinct mechanisms. NeuroImage: Clinical, 23, 101854. 10.1016/j.nicl.2019.101854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. D. (1988). Professional manual for the state‐trait anger expression inventory (STAXI) (Research ed.). Odessa, FL: Psychological Assessment Resources.
- Spielberger, C. D. , Foreyt, J. P. , Goodrick, G. , & Reheiser, E. C. (1995). Personality characteristics of users of smokeless tobacco compared with cigarette smokers and non‐users of tobacco products. Personality and Individual Differences, 19(4), 439–448. 10.1016/0191-8869(95)00084-j [DOI] [Google Scholar]
- Sripada, C. , Angstadt, M. , Kessler, D. , Phan, K. L. , Liberzon, I. , Evans, G. W. , Welsh, R. C. , Kim, P. , & Swain, J. E. (2014). Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. NeuroImage, 89, 110–121. 10.1016/j.neuroimage.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica, T. , & Depue, B. E. (2020). Shared characteristics of intrinsic connectivity networks underlying interoceptive awareness and empathy. 10.1101/2020.04.30.070490 [DOI] [PMC free article] [PubMed]
- Stuewig, J. , Tangney, J. P. , Heigel, C. , Harty, L. , & McCloskey, L. (2010). Shaming, blaming, and maiming: Functional links among the moral emotions, externalization of blame, and aggression. Journal of Research in Personality, 44(1), 91–102. 10.1016/j.jrp.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra, L. , Schneider, I. K. , Bushman, B. J. , & Koole, S. L. (2016). Drawn to danger: Trait anger predicts automatic approach behaviour to angry faces. Cognition and Emotion, 31(4), 765–771. 10.1080/02699931.2016.1150256 [DOI] [PubMed] [Google Scholar]
- Videbeck, S. L. (2006). Psychiatric mental health nursing (3rd ed.). Lippincott Williams & Wilkins. [Google Scholar]
- Vu, M. T. , Adali, T. , Ba, D. , Buzsáki, G. , Carlson, D. , Heller, K. , Liston, C. , Rudin, C. , Sohal, V. S. , Widge, A. S. , Mayberg, H. S. , Sapiro, G. , & Dzirasa, K. (2018). A shared vision for machine learning in neuroscience. The Journal of Neuroscience, 38(7), 1601–1607. 10.1523/JNEUROSCI.0508-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathersby, F. L. , King, J. B. , Fox, J. C. , Loret, A. , & Anderson, J. S. (2019). Functional connectivity of emotional well‐being: Overconnectivity between default and attentional networks is associated with attitudes of anger and aggression. Psychiatry Research. Neuroimaging, 291, 52–62. 10.1016/j.pscychresns.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wilkowski, B. M. , & Robinson, M. D. (2007). Keeping your cool: Trait anger, hostile thoughts, and the recruitment of limited capacity control. Personality and Social Psychology Bulletin, 33, 1201–1213. 10.1177/0146167207301031 [DOI] [PubMed] [Google Scholar]
- Wilkowski, B. M. , & Robinson, M. D. (2008). The cognitive basis of trait anger and reactive aggression: An integrative analysis. Personality and Social Psychology Review, 12(1), 3–21. 10.1177/1088868307309874 [DOI] [PubMed] [Google Scholar]
- Wilkowski, B. M. , & Robinson, M. D. (2010). The anatomy of anger: An integrative cognitive model of trait anger and reactive aggression. Journal of Personality, 78(1), 9–38. 10.1111/j.1467-6494.2009.00607.x [DOI] [PubMed] [Google Scholar]
- Wittchen, H.‐U. , Kessler, R. C. , Zhao, S. , & Abelson, J. (1995). Reliability and clinical validity of UM‐CIDI DSM‐III‐R generalized anxiety disorder. Journal of Psychiatric Research, 29, 95–110. 10.1016/0022-3956(94)00044-R [DOI] [PubMed] [Google Scholar]
- Xu, L. , Groth, K. M. , Pearlson, G. , Schretlen, D. J. , & Calhoun, V. D. (2009). Source‐based morphometry: The use of independent component analysis to identify gray matter differences with application to schizophrenia. Human Brain Mapping, 30(3), 711–724. 10.1002/hbm.20540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L. , Pearlson, G. , & Calhoun, V. D. (2009). Joint source based morphometry identifies linked gray and white matter group differences. NeuroImage, 44(3), 777–789. 10.1016/j.neuroimage.2008.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen, N. H. , Osachoff, N. , & Chen, J. J. (2019). Intrinsic frequencies of the resting‐state fMRI signal: The frequency dependence of functional connectivity and the effect of mode mixing. Frontiers in Neuroscience, 13, 900. 10.3389/fnins.2019.00900 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available in OpenNeuro at [https://openneuro.org/datasets/ds000221/versions/00002], reference number [ds000221].


