Abstract
Background:
In patients with sarcoidosis, right ventricular (RV) abnormalities have been described on many imaging modalities. On cardiovascular magnetic resonance (CMR), RV abnormalities include RV systolic dysfunction quantified as an abnormal RV ejection fraction (RVEF), and RV late gadolinium enhancement (LGE).
Objectives:
We aimed to determine the prevalence on CMR of RV systolic dysfunction and RV LGE, their determinants, and their impact on long-term adverse outcomes in patients with sarcoidosis.
Methods:
We studied consecutive patients with biopsy-proven sarcoidosis who underwent CMR for suspected cardiac involvement. They were followed for two endpoints: all-cause death, and a composite arrhythmic endpoint of sudden cardiac death or significant ventricular arrhythmia.
Results:
Among 290 patients, RV systolic dysfunction (RVEF <40% in men and <45% in women) and RV LGE were present in 35 (12.1%) and 16 (5.5%) respectively. The median follow-up time was 3.2 years (interquartile range 1.6 to 5.7 years) for all-cause death and 3.0 years (interquartile range 1.4 to 5.5 years) for the arrhythmic endpoint. On Cox proportional hazards regression multivariable analyses, only RVEF was independently associated with all-cause death (HR 1.05 for every 1% decrease; 95% CI 1.01–1.09; p=0.022) after adjustment for LVEF, LV LGE extent, and the presence of RV LGE. RVEF was not associated with the arrhythmic endpoint (HR 1.01; 95% CI 0.96–1.06; p=0.67). Conversely, RV LGE was not associated with all-cause death (HR 2.78; 95% CI 0.36–21.66; p=0.33), while it was independently associated with the arrhythmic endpoint (HR 5.43; 95% CI 1.25–23.47; p=0.024).
Conclusions:
In our study of patients with sarcoidosis, RV systolic dysfunction and RV LGE had distinct prognostic associations; RV systolic dysfunction but not RV LGE was independently associated with all-cause death, while RV LGE but not RV systolic dysfunction was independently associated with sudden cardiac death or significant ventricular arrhythmia. Our findings may indicate distinct implications for the management of RV abnormalities in sarcoidosis.
Keywords: Outcomes, sarcoidosis, cardiovascular magnetic resonance, right ventricle, systolic dysfunction, late gadolinium enhancement
Graphical Abstract
INTRODUCTION
Sarcoidosis is a multisystem, granulomatous disorder of unknown etiology (1). It has heterogeneous manifestations and clinical outcomes, varying partly based on which organs are involved (2). Right ventricular (RV) involvement has been described in sarcoidosis since the late 1970s on thallium scintigraphy (3–6), echocardiography (4,7), invasive hemodynamic testing (7), and more recently on echocardiographic strain imaging (8,9), cardiovascular magnetic resonance imaging (CMR) (10–13), and 18F-fluorodeoxyglucose positron emission tomography (14–17).
RV abnormalities that have been described on CMR in patients with sarcoidosis include RV systolic dysfunction on cine CMR (11,12), and RV late gadolinium enhancement (LGE) on LGE CMR (10,13). The prevalence, correlates, and prognostic significance of these two abnormalities in relation to each other have not been studied. This knowledge has the potential to guide clinical decision-making and to contribute to improved outcomes. We hypothesized that RV systolic dysfunction and RV LGE are both independently associated with long-term adverse outcomes in patients with sarcoidosis.
We aimed to determine the prevalence of RV systolic dysfunction and RV LGE on CMR, their determinants, and their impact on long-term adverse outcomes, using a large cohort of patients with sarcoidosis.
METHODS
Patients and data collection
We included consecutive patients with biopsy-proven (extra-cardiac and/or cardiac) sarcoidosis who had CMR with late gadolinium enhancement (LGE) imaging for the evaluation of suspected cardiac sarcoidosis at the University of Minnesota. Study patients were identified from the University of Minnesota’s Cardiovascular Magnetic Resonance Registry (18–23).
Demographic data, medical history, co-morbidities, medications, and outcome data were collected blinded to CMR data. This retrospective cohort study was approved by the University of Minnesota’s Institutional Review Board with a waiver of informed consent.
CMR protocol
CMR was performed on clinical 1.5T scanners (Siemens Avanto or Siemens Aera, Malvern, Pennsylvania) using phased-array receiver coils according to standard recommendations (24). All CMRs were done using a standard CMR protocol consisting of: 1) localizers to identify the cardiac position, 2) cine CMR for anatomical and functional assessment using a steady-state free precession sequence (typical repetition time of 3.0 to 3.5 ms; echo time of 1.2 to 1.5 ms; in-plane spatial resolution of 1.8 mm x 1.4 mm; temporal resolution of 35 to 40 ms) in short-axis (every 10 mm to cover the entire LV from the mitral valve plane through the apex), and three (2-, 3-, and 4-chamber) long-axis views, and 3) LGE CMR for tissue characterization performed 10 to 15 min after gadolinium contrast administration, using a two-dimensional segmented inversion-recovery sequence (set to null viable myocardium using an inversion time of 250 to 350 ms; in-plane spatial resolution of 1.8 mm x 1.5 mm; temporal resolution of 180 to 200 ms; slice thickness of 6 mm), in identical views as the cine CMR images.
CMR analyses
CMR analyses were performed by the consensus of two investigators with expertise in CMR, blinded to clinical information. LV and RV ejection fractions (LVEFs and RVEFs) were determined by quantitative analysis according to standard recommendations (25). LV and RV volumes were quantified by planimetry of the end-diastolic and -systolic endocardial borders on short-axis cine CMR images acquired from base to apex, which were used to calculate the respective end-diastolic and -systolic volumes. EFs were obtained by subtracting the end-systolic volumes from the end-diastolic volumes and dividing the result by the end-diastolic volumes. Normal ranges from the UK Biobank population cohort were used to classify LVEFs and RVEFs as normal or abnormal (26). LGE was identified visually in both ventricles. In patients with LV LGE, the extent was quantified using the signal threshold versus reference myocardium approach (27). In the first step, the endocardial and epicardial borders were traced and a reference region of interest was placed over the largest contiguous portion of homogeneously nulled (i.e., normal) myocardium. Next, a signal threshold of >5 standard deviations (SD) above the mean signal of the reference myocardium was applied to derive the total LGE mass, which was then divided by total LV mass to obtain LV LGE extent as a percentage. The >5SD threshold was chosen because it is the best predictor of cardiovascular events in non-ischemic cardiomyopathy when compared to expert visual scoring and the >2SD and >3SD thresholds (28). RV LGE was defined as RV free wall LGE. The extent of RV LGE was not quantified since quantification of the RV mass has been noted to have high variability (29).
Clinical follow-up and endpoints
Follow-up data were collected through a review of electronic medical records from all hospitals and clinics within the University of Minnesota Health system. Two endpoints were studied. The first endpoint was all-cause death. Mortality status and death dates were obtained from the electronic medical records and the Minnesota State Department of Health’s Office of Vital Records. For patients who died outside the hospital, death certificates were reviewed to determine the cause of death.
The second endpoint was a composite arrhythmic endpoint consisting of sudden cardiac death, resuscitated cardiac arrest with documented ventricular tachycardia, or significant ventricular arrhythmia, including sustained ventricular tachycardia (duration >30 seconds) and appropriate implantable cardioverter-defibrillator (ICD) therapy (shock or anti-tachycardia pacing). Sudden cardiac death was defined according to the 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials (30). The appropriateness of ICD therapies was adjudicated by board-certified cardiac electrophysiologists as part of the patients’ clinical care using intracardiac electrograms recorded by the ICD, and based on tachycardia rate, onset, stability, atrioventricular association, and the QRS morphology.
Statistical analyses
Statistical analyses were performed using the statistical environment R (RStudio version 1.2, Boston, Massachusetts). Normally distributed continuous variables were expressed as mean ± SD, and non-normally distributed continuous variables were presented as medians with interquartile range (IQR). Categorical variables were expressed as counts with percentages. Comparisons between groups were performed with a 2-sample Student t test for continuous, normally distributed variables, and Wilcoxon rank-sum test for continuous, non-normally distributed data. Chi-square tests were used to compare discrete data between groups; in those cases where the expected cell count was <5, Fisher exact test was used. The cumulative incidence of the study endpoints was estimated using the Kaplan-Meier method, and hazard ratios (HR) were calculated using Cox proportional hazards regression and presented with their associated 95% confidence intervals (CIs). After fitting the Cox regression models, the proportional hazards assumption was tested by analysis of Schoenfeld residuals for the global test and scaled Schoenfeld residuals for the individual covariates. The incremental prognostic value of the variable of interest (RVEF or RV LGE) was evaluated by comparing the final models with those in which the variable of interest was not included, using the likelihood ratio chi-square test. All statistical comparisons were two-tailed, and a p value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics
Two hundred and ninety consecutive patients with biopsy-proven sarcoidosis were included in the study. Demographic, clinical and CMR characteristics are provided in Tables 1 and 2. Of the 290 patients, 284 (98%) had biopsy-proven extra-cardiac sarcoidosis including 2 with concomitant biopsy-proven cardiac sarcoidosis, while 6 had biopsy-proven cardiac sarcoidosis without clinical findings characteristic of sarcoidosis in any other organ. Forty-nine percent were women and 80% were White. Ninety percent had pulmonary sarcoidosis and 17% had pulmonary hypertension (PH). On CMR, the median LVEF was 57% and the median RVEF was 53%. Thirty percent had LGE with a mean LGE extent of 2.1% of the LV myocardial mass.
Table 1.
Patient characteristics for all patients, patients with and without an abnormal RVEF, and patients with and without LGE
| All patients (n = 290) | Abnormal RVEF (n = 35) | Normal RVEF (n = 255) | p value | RV LGE (n = 16) | No RV LGE (n = 274) | p value | |
|---|---|---|---|---|---|---|---|
| Age, years (SD) | 53.1 (12.2) | 54.3 (12.9) | 53.0 (12.2) | 0.56 | 55.9 (11.5) | 52.9 (12.3) | 0.36 |
| Women, n (%) | 141 (48.6) | 12 (34.3) | 129 (50.6) | 0.07 | 6 (37.5) | 135 (49.3) | 0.36 |
| Race | |||||||
| White, n (%) | 233 (80.3) | 24 (68.6) | 209 (82.0) | 0.06 | 14 (87.5) | 219 (79.9) | 0.46 |
| Black, n (%) | 50 (17.2) | 9 (25.7) | 41 (16.1) | 0.16 | 1 (6.3) | 49 (17.9) | 0.23 |
| Extra-cardiac sarcoidosis involvement (may be more than one) | |||||||
| Lung, n (%) | 260 (89.7) | 27 (77.1) | 233 (91.4) | 0.009 | 9 (56.3) | 251 (91.6) | <0.001 |
| Skin, n (%) | 27 (9.3) | 3 (8.6) < | 24 (9.4) | 0.87 | 3 (18.8) | 24 (8.8) | 0.18 |
| Liver, n (%) | 23 (7.9) | 4 (11.4) | 19 (7.5) | 0.42 | 2 (12.5) | 21 (7.7) | 0.49 |
| Eye, n (%) | 19 (6.6) | 1 (2.9) | 18 (7.1) | 0.35 | 1 (6.3) | 18 (6.6) | 0.96 |
| Nervous system, n (%) | 11 (3.8) | 1 (2.9) | 10 (3.9) | 0.76 | 0 (0.0) | 11 (4.0) | 0.41 |
| Other, n (%) | 98 (33.8) | 12 (34.3) | 86 (33.7) | 0.95 | 4 (25.0) | 94 (34.3) | 0.45 |
| Comorbidities | |||||||
| Hypertension, n (%) | 153 (52.8) | 21 (60.0) | 132 (51.7) | 0.36 | 7 (43.8) | 146 (53.3) | 0.46 |
| Dyslipidemia, n (%) | 136 (46.9) | 15 (42.9) | 121 (47.5) | 0.61 | 8 (50.0) | 128 (46.7) | 0.80 |
| Diabetes mellitus, n (%) | 69 (23.8) | 11 (31.4) | 58 (22.7) | 0.26 | 4 (25.0) | 65 (23.7) | 0.91 |
| Former tobacco use, n (%) | 120 (41.4) | 15 (42.9) | 105 (41.2) | 0.85 | 4 (25.0) | 116 (42.3) | 0.17 |
| Current tobacco use, n (%) | 28 (9.7) | 1 (2.9) | 27 (10.6) | 0.15 | 2 (12.5) | 26 (9.5) | 0.69 |
| Coronary artery disease, n (%) | 31 (10.7) | 9 (25.7) | 22 (8.6) | 0.002 | 3 (18.8) | 28 (10.2) | 0.28 |
| Pulmonary hypertension, n (%) | 48 (16.6) | 14 (40.0) | 34 (13.3) | <0.001 | 1 (6.3) | 47 (17.2) | 0.26 |
| Cardiac symptoms | |||||||
| Palpitations, n (%) | 97 (33.4) | 6 (17.1) | 91 (35.7) | 0.029 | 7 (43.8) | 90 (32.8) | 0.37 |
| Chest pain, n (%) | 100 (34.5) | 13 (37.1) | 87 (34.1) | 0.72 | 5 (31.3) | 95 (34.7) | 0.78 |
| Pre-syncope, n (%) | 67 (23.1) | 6 (17.1) | 61 (23.9) | 0.37 | 7 (43.8) | 60 (21.9) | 0.044 |
| Syncope, n (%) | 14 (4.8) | 3 (8.6) | 11 (4.3) | 0.27 | 3 (18.8) | 11 (4.0) | 0.008 |
| NYHA functional class (I/II/III/IV), n (%) | 92/43/22/13 (31.7/14.8/7.6/4.5) | 10/5/7/5 (28.6/14.3/20.0/14.3) | 82/38/15/8 (32.2/14.9/5.9/3.1) | <0.001 | 4/3/1/2 (25.0/18.8/6.3/12.5) | 88/40/21/11 (32.1/14.6/7.7/4.0) | 0.57 |
| Arrhythmia prior to CMR | |||||||
| PVCs, n (%) | 70 (24.1) | 12 (34.3) | 58 (22.7) | 0.14 | 7 (43.8) | 63 (23.0) | 0.06 |
| Ventricular arrhythmia, n (%) | 20 (6.9) | 4 (11.4) | 16 (6.3) | 0.26 | 7 (43.8) | 13 (4.7) | <0.001 |
| Supraventricular tachycardia, n (%) | 28 (9.7) | 4 (11.4) | 24 (9.4) | 0.71 | 3 (18.8) | 25 (9.1) | 0.21 |
| Atrial fibrillation/Flutter, n (%) | 34 (11.7) | 5 (14.3) | 29 (11.4) | 0.62 | 4 (25.0) | 30 (10.9) | 0.09 |
| AVB (≥2nd degree), n (%) | 16 (5.5) | 5 (14.2) | 11 (4.3) | 0.016 | 5 (31.3) | 11 (4.0) | <0.001 |
| Pulmonary function testing (n = 217) | |||||||
| FVC %predicted | 85 (66 – 96) | 76 (60 – 87) | 86(68 – 96) | 0.025 | 87(83 – 97) | 85 (66 – 96) | 0.45 |
| FEV1 %predicted | 79 (62 – 92) | 67 (54 – 83) | 80 (63 – 93) | 0.012 | 90 (77 –96) | 79(61 – 92) | 0.10 |
| DLCO %predicted | 83 (56 – 100) | 64 (30 – 86) | 86(59 – 103) | 0.004 | 81 (66 – 91) | 83 (55 – 101) | 0.95 |
| Medications | |||||||
| Aspirin, n (%) | 90 (31.0) | 11 (31.4) | 79 (31.0) | 0.96 | 8 (50.0) | 82 (29.9) | 0.09 |
| Beta-blockers, n (%) | 87 (30.0) | 13 (37.1) | 74 (29.0) | 0.33 | 6 (37.5) | 81 (29.6) | 0.50 |
| ACE-inhibitor/Angiotensin receptor blocker, n (%) | 87 (30.0) | 18 (51.4) | 69 (27.1) | 0.003 | 7 (43.8) | 80 (29.2) | 0.22 |
| Statin, n (%) | 91 (31.4) | 11 (31.4) | 80 (31.4) | 0.99 | 7 (43.8) | 84 (30.7) | 0.27 |
| Steroids, n (%) | 98 (33.8) | 14 (40.0) | 84 (32.9) | 0.41 | 2 (12.5) | 96 (35.0) | 0.06 |
| Non-steroidal immune modulatory agents, n (%) | 56 (19.3) | 9 (25.7) | 47 (18.4) | 0.31 | 2 (12.5) | 54 (19.7) | 0.48 |
Values are n (%) or mean (SD). ACE = angiotensin converting enzyme, AVB = atrioventricular block; CMR = cardiovascular magnetic resonance; DLCO = diffusion capacity of lungs for carbon monoxide; EF = ejection fraction; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; LGE = late gadolinium enhancement; LV = left ventricle; NYHA = New York Heart Association; PVC = premature ventricular complexes; RV = right ventricle; SD = standard deviation
Table 2.
CMR characteristics for all patients, patients with and without an abnormal RVEF, and patients with and without LGE
| All patients (n = 290) | Abnormal RVEF (n = 35) | Normal RVEF (n = 255) | p value | RV LGE (n = 16) | No RV LGE (n = 274) | p value | |
|---|---|---|---|---|---|---|---|
| LV EDVI, ml/m2 (IQR) | 59.9 (50.3 – 72.0) | 63.9 (54.4 – 92.7) | 59.9 (49.8 – 70.6) | 0.07 | 72.2 (63.3 – 96.5) | 59.0 (50.0 – 70.8) | 0.002 |
| LV ESVI, ml/m2 (IQR) | 26.3 (20.7 – 32.5) | 33.0 (27.0 – 71.4) | 25.7 (20.5 – 31.3) | <0.001 | 45.6 (30.7 – 70.3) | 26.0 (20.5 – 31.4) | <0.001 |
| LV EF, % (IQR) | 56.6 (53.2 – 60.4) | 41.9 (29.1 – 54.1) | 57.0 (54.6 – 60.7) | <0.001 | 39.7 (26.9 – 53.6) | 56.9 (53.9 – 60.5) | <0.001 |
| RV EDVI, ml/m2 (IQR) |
62.7 (53.3 – 72.8) | 85.1 (69.3 – 96.1) | 61.1 (52.0 – 69.9) | <0.001 | 74.9 (53.5 – 93.3) | 62.4 (53.3 – 71.8) | 0.043 |
| RV ESVI, ml/m2 (IQR) |
28.9 (23.6 – 36.8) | 56.6 (46.9 – 64.7) | 27.4 (23.2 – 33.7) | <0.001 | 48.3 (26.2 – 58.5) | 28.6 (23.4 – 35.8) | 0.004 |
| RV EF, % (IQR) | 52.5 (49.0 – 56.9) | 34.8 (30.2 – 38.9) | 53.0 (50.5 – 57.7) | <0.001 | 40.2 (32.7 – 50.7) | 52.7 (49.7 – 57.2) | <0.001 |
| LV LGE presence, n (%) | 87 (30.0) | 26 (74.3) | 61 (23.9) | <0.001 | 16 (100.0) | 71 (25.9) | <0.001 |
| LV LGE extent, % (SD) | 2.1 (5.4) | 6.6 (9.2) | 1.5 (4.3) | <0.001 | 17.3 (10.4) | 1.2 (3.3) | <0.001 |
| RV LGE presence, n (%) | 16 (5.5) | 9 (25.7) | 7.0 (2.7) | <0.001 | 16 (100.0) | 0 (0.0) | N/A |
Values are n (%), mean (SD), or median (interquartile range). CMR = cardiovascular magnetic resonance; EDVI = end-diastolic volume index; EF = ejection fraction; ESVI = end-systolic volume index; IQR = interquartile range; LGE = late gadolinium enhancement; LV = left ventricle; RV = right ventricle; SD = standard deviation
Prevalence and correlates of RV systolic dysfunction
RV systolic dysfunction defined as an abnormal RVEF of <40% in men and <45% in women (26) was present in 35 (12.1%) patients. Compared with patients with normal RVEF, those with abnormal RVEF had a lower prevalence of pulmonary sarcoidosis but had worse pulmonary function on all tests (forced vital capacity, forced expiratory volume in 1s, and diffusing capacity of lungs for carbon monoxide), suggestive of more extensive pulmonary parenchymal disease. They had a higher rate of PH, with a prevalence of 40%, and higher rates of coronary artery disease and heart failure symptoms. Arrhythmic manifestations such as pre-syncope, syncope, ventricular arrhythmias, and atrioventricular block were not significantly different between patients with normal RVEFs and those with abnormal RVEFs.
Prevalence and correlates of RV LGE
RV LGE was present in 16 (5.5%) patients. Compared with patients without RV LGE, those with RV LGE had a lower prevalence of pulmonary sarcoidosis but not significantly different pulmonary function. The rates of PH, coronary artery disease, and heart failure symptoms were also not significantly different. However, patients with RV LGE had significantly higher rates of arrhythmic manifestations such as pre-syncope, syncope, ventricular arrhythmias, and atrioventricular block. All patients with RV LGE also had LV LGE.
Clinical management and outcomes
During follow up, over half the patients received steroids, of which 18% received them for suspected cardiac sarcoidosis. Thirty-nine percent received non-steroidal immune-modulatory agents, of which, 13% received them for suspected cardiac sarcoidosis. Thirteen percent had ICDs implanted. Two had left ventricular assist devices implanted while three underwent cardiac transplantation (Table 3).
Table 3.
Management of all patients, patients with and without an abnormal RVEF, and patients with and without LGE
| All patients (n = 290) | Abnorma l RVEF (n = 35) | Normal RVEF (n = 255) | p value | RV LGE (n = 16) | No RV LGE (n = 274) | p value | |
|---|---|---|---|---|---|---|---|
| Endomyocardial biopsy performed, n (%) | 22 (7.6) | 10 (28.6) | 12 (4.7) | <0.001 | 9 (56.3) | 13 (4.7) | <0.001 |
| Steroids for any sarcoidosis indication, n (%) | 153 (52.8) | 26 (74.3) | 127 (49.8) | <0.001 | 10 (62.5) | 143 (52.2) | 0.43 |
| Non-steroidal immune modulatory agents for any sarcoidosis indication, n (%) | 114 (39.3) | 18 (51.4) | 96 (37.6) | 0.12 | 9 (56.3) | 105 (38.3) | 0.15 |
| Steroids for suspected cardiac sarcoidosis, n (%) | 53 (18.3) | 15 (42.9) | 38 (14.9) | <0.001 | 10 (62.5) | 43 (15.7) | <0.001 |
| Non-steroidal immune modulatory agents for suspected cardiac sarcoidosis, n (%) | 39 (13.4) | 10 (28.6) | 29 (11.4) | 0.005 | 9 (56.3) | 30 (10.9) | <0.001 |
| Pacemaker-only implantation, n (%) | 9 (3.1) | 2 (5.7) | 7 (2.7) | 0.34 | 3 (18.8) | 6 (2.2) | <0.001 |
| ICD implantation, n (%) | 37 (12.8) | 12 (34.3) | 25 (9.8) | <0.001 | 14 (87.5) | 23 (8.4) | <0.001 |
| Left ventricular assist device placement, n (%) | 2 (0.7) | 1 (2.9) | 1 (0.4) | 0.10 | 1 (6.3) | 1 (0.4) | 0.006 |
| Orthotopic heart transplantation, n (%) | 3 (1.0) | 2 (5.7) | 1 (0.4) | 0.004 | 2 (12.5) | 1 (0.4) | <0.001 |
Values are n (%). EF = ejection fraction; ICD = implantable cardioverter-defibrillator; LGE = late gadolinium enhancement; LV = left ventricle; RV = right ventricle
The median follow-up time was 3.2 years (IQR 1.6 to 5.7 years) for all-cause death and 3.0 years (IQR 1.4 to 5.5 years) for the arrhythmic endpoint. There was a total of 1,063 patient-years of follow-up for all-cause death and 1,019 patient-years for the arrhythmic endpoint. No patients were lost to follow-up. Forty-two (14.5%) patients died. Of these deaths, 15 were primarily attributed to respiratory failure or sepsis of pulmonary origin, one to severe PH, three to cardiac sarcoidosis (two heart failure deaths and one sudden cardiac death), four to coronary artery disease, and 12 to non-cardiac, non-pulmonary causes; the causes of death for seven could not be ascertained. Eighteen (6.2%) patients reached the arrhythmic endpoint (one sudden cardiac death and 17 significant ventricular arrhythmias). Three patients underwent orthotopic heart transplantation and were censored at the time of their transplantation.
Associations of RV systolic dysfunction with clinical outcomes
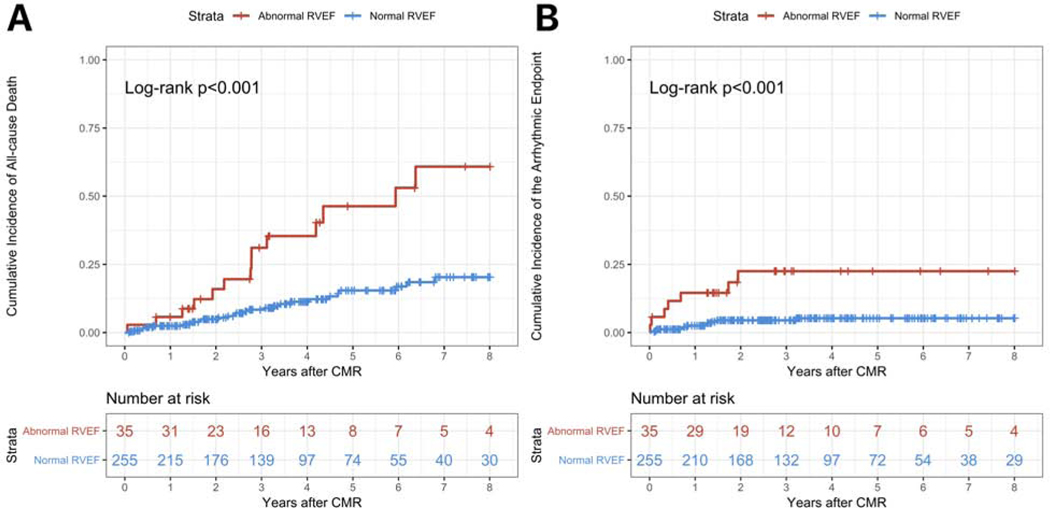
On Kaplan-Meier analyses, the cumulative incidence of all-cause death was significantly higher in patients with abnormal RVEF compared with those with normal RVEF (p<0.001) (Figure 1 Panel A). The cumulative incidence of the arrhythmic endpoint was also significantly higher (p<0.001) (Figure 1 Panel B).
Figure 1. Kaplan-Meier analyses for the study endpoints for RV systolic dysfunction on CMR.
Panel A shows the cumulative incidence of all-cause death for patients with abnormal RVEF (red line) versus those with normal RVEF (blue line). Panel B shows the cumulative incidence of the arrhythmic endpoint for patients with abnormal RVEF (red line) versus those with normal RVEF (blue line). Each vertical tick on the curves displays a censored patient. See the text for further details.
On Cox proportional hazards regression analyses, RVEF was associated on univariable analyses with both all-cause death and the arrhythmic endpoint (Table 4). On multivariable analysis, RVEF was the only variable independently associated with all-cause death after adjustment for LVEF, LV LGE extent, and RV LGE. For every 1% decrease in RVEF, the risk of all-cause death increased by 5% (HR 1.05; 95% CI 1.01–1.09; p = 0.022]. However, RVEF was not independently associated with the arrhythmic endpoint (HR 1.01; 95% CI 0.96–1.06; p = 0.67) (Table 5).
Table 4.
Univariate CMR predictors of the study endpoints
| Variable | All-cause death | Arrhythmic endpoint | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value | |
| LV EDVI, per 1 ml/m2 increase | 1.01 | 1.00 – 1.03 | 0.049 | 1.05 | 1.03 – 1.06 | <0.001 |
| LV ESVI, per 1 ml/m2 increase | 1.02 | 1.01 – 1.03 | 0.006 | 1.05 | 1.03 – 1.06 | <0.001 |
| LV EF, per 1% decrease | 1.04 | 1.01 – 1.06 | 0.002 | 1.08 | 1.05 – 1.12 | <0.001 |
| RV EDVI, per 1 ml/m2 increase | 1.03 | 1.01 – 1.04 | 0.001 | 1.04 | 1.02 – 1.06 | <0.001 |
| RV ESVI, per 1 ml/m2 increase | 1.03 | 1.02 – 1.05 | <0.001 | 1.04 | 1.02 – 1.05 | <0.001 |
| RV EF, per 1% decrease | 1.06 | 1.03 – 1.09 | <0.001 | 1.08 | 1.04 – 1.12 | <0.001 |
| LV LGE presence | 4.13 | 2.21 – 7.70 | <0.001 | 13.63 | 3.94 – 47.12 | <0.001 |
| LV LGE extent, per 1% increase | 1.02 | 0.97 – 1.07 | 0.39 | 1.20 | 1.15 – 1.25 | <0.001 |
| RV LGE presence | 1.04 | 0.25 – 4.32 | 0.96 | 0.02 | 0.01 – 0.07 | <0.001 |
CI = confidence interval; CMR = cardiovascular magnetic resonance; EDVI = end-diastolic volume index; EF = ejection fraction; ESVI = end-systolic volume index; LGE = late gadolinium enhancement; LV = left ventricle; RV = right ventricle
Table 5.
Multivariable CMR predictors of the study endpoints
| Variable | All-cause death | Arrhythmic endpoint | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value | |
| LV EF, per 1% decrease | 1.02 | 0.99 – 1.05 | 0.28 | 1.00 | 0.94 – 1.05 | 0.87 |
| RV EF, per 1% decrease | 1.05 | 1.01 – 1.09 | 0.022 | 1.01 | 0.96 – 1.06 | 0.67 |
| LV LGE extent, per 1% increase | 1.01 | 0.94 – 1.09 | 0.73 | 1.15 | 1.08 – 1.23 | <0.001 |
| RV LGE presence | 2.78 | 0.36 – 21.66 | 0.33 | 5.43 | 1.25 – 23.47 | 0.024 |
CI = confidence interval; CMR = cardiovascular magnetic resonance; EF = ejection fraction; LGE = late gadolinium enhancement; LV = left ventricle; RV = right ventricle
The proportional hazards assumption was valid for all covariates individually and the model overall for both multivariable models; the p values for the global test were 0.96 and 0.67 for the all-cause death and arrhythmic endpoint models respectively.
The addition of RVEF to a Cox model that included LVEF, LV LGE extent, and RV LGE resulted in a significantly improved model fit as assessed by the likelihood ratio test (p = 0.023), suggesting an incremental prognostic value of RVEF for all-cause death.
Associations of RV LGE with clinical outcomes
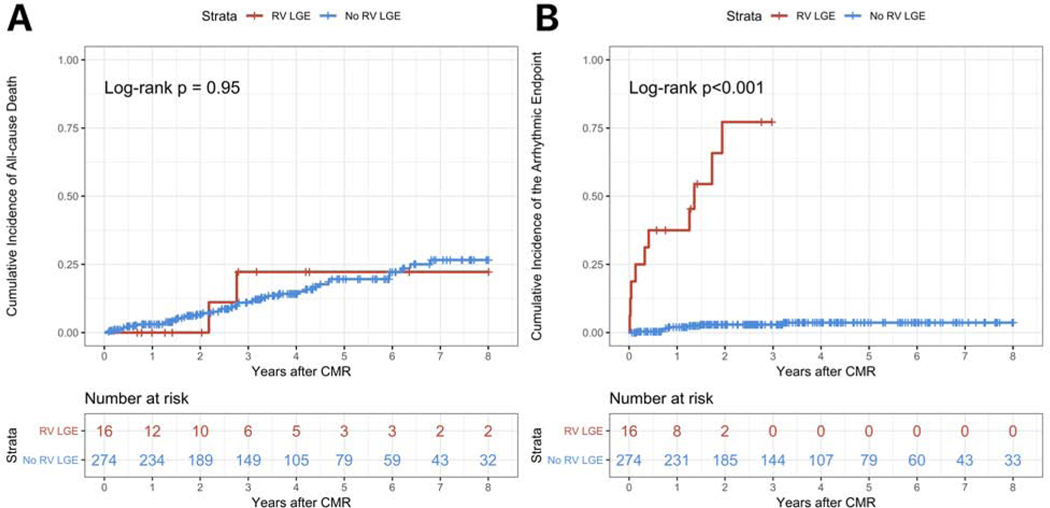
On Kaplan-Meier analyses, the cumulative incidence of all-cause death was not different in patients with RV LGE compared with those without RV LGE (p = 0.95) (Figure 2 Panel A). Conversely, the cumulative incidence of the arrhythmic endpoint was significantly higher (p<0.001) (Figure 2 Panel B).
Figure 2. Kaplan-Meier analyses for the study endpoints for RV LGE on CMR.
Panel A shows the cumulative incidence of all-cause death for patients with RV LGE (red line) versus those with no RV LGE (blue line). Panel B shows the cumulative incidence of the arrhythmic endpoint for patients with RV LGE (red line) versus those with no RV LGE (blue line). Each vertical tick on the curves displays a censored patient. See the text for further details.
On Cox proportional hazards regression analyses, RV LGE was not associated on univariable analyses with all-cause death, but it was associated with the arrhythmic endpoint (Table 4). Similarly, on multivariable analysis, RV LGE was not associated with all-cause death (HR 2.78; 95% CI 0.36–21.66; p = 0.33), while it was independently associated with the arrhythmic endpoint (HR 5.43; 95% CI 1.25–23.47; p = 0.024) after adjustment for LVEF, RVEF, and LV LGE extent (Table 5).
The addition of RV LGE to a Cox model that included LVEF, RVEF, and LV LGE extent, resulted in a significantly improved model fit as assessed by the likelihood ratio test (p = 0.019), suggesting an incremental prognostic value of RV LGE for the arrhythmic endpoint.
DISCUSSION
In a large cohort of patients with sarcoidosis who underwent CMR, we found that RV systolic dysfunction and RV LGE were present in 12.1% and 5.5% respectively, and they may have distinct prognostic implications with respect to all-cause death and ventricular arrhythmias.
Causes of RV systolic dysfunction in patients with sarcoidosis
Broadly, RV systolic dysfunction could be a consequence of an RV cardiomyopathy (primary) or RV pressure overload (secondary) (31). In patients with sarcoidosis, primary RV involvement can occur due to cardiac sarcoidosis and can manifest on CMR as both RV systolic dysfunction and RV LGE. Thus, the presence of RV LGE identifies a primary RV cardiomyopathy due to cardiac sarcoidosis. Secondary RV involvement can occur due to sarcoidosis-associated PH, which is thought to be multifactorial and potentially fit all five classes of the accepted World Health Organization classification of PH (32), despite its official classification as Group 5 (33).
The most common cause of sarcoidosis-associated PH is likely advanced pulmonary parenchymal fibrosis from pulmonary sarcoidosis, resulting in hypoxia (34) (Group 3). Sarcoidosis-associated PH could occur due to cardiac sarcoidosis involving the LV resulting in increased LV end-diastolic, pulmonary venous, and eventually, pulmonary arterial pressures (Group 2). Pulmonary veno-occlusive disease could occur in sarcoidosis and lead to PH (35–37) (Group 4). Pulmonary artery vasculitis is possible in sarcoidosis due to granulomatous involvement of the pulmonary vasculature (38) (Group 1). Another possible Group 1 cause of sarcoidosis-related PH is liver failure due to granulomatous involvement of the liver, resulting in portal and subsequently, portopulmonary hypertension (39). Lastly, sarcoidosis-related PH could also occur from extrinsic pulmonary arterial compression by lymphadenopathy (37). Independent of PH, cardiac sarcoidosis involving the LV, particularly the septum, could result in systolic ventricular interdependence and up to 40% reduction in RV systolic pressure and volume outflow (40). Similarly, LV pressure or volume overload from cardiac sarcoidosis could result in diastolic ventricular interdependence, and consequently, RV systolic dysfunction (41).
We found a higher prevalence of pulmonary parenchymal dysfunction, PH, coronary artery disease, and concomitant RV LGE in patients with RV systolic dysfunction. Among the various causes for RV systolic dysfunction, secondary involvement from sarcoidosis-associated PH appears to be more frequent than primary RV involvement from cardiac sarcoidosis since RV LGE was present in only a quarter of patients with RV systolic dysfunction.
The greater contribution of sarcoidosis-associated PH to RV systolic dysfunction may explain the prognostic associations; sarcoidosis-associated PH is independently associated with increased mortality (42–44). In a study of 130 sarcoidosis patients with persistent dyspnea despite immunosuppressive therapy, those with PH and LV dysfunction – defined as a pulmonary arterial wedge pressure of ≥15 mm Hg – had an increased mortality compared with those without PH, but those with PH in the absence of LV dysfunction had an even higher mortality (43). This suggests a greater contribution of non-LV causes of sarcoidosis-associated PH to mortality compared with the LV-related causes described above.
In contrast, RV LGE has exclusively been described as a manifestation of cardiac sarcoidosis directly involving the RV free wall (10,13), always accompanying LV LGE related to cardiac sarcoidosis (10). We found a higher prevalence of arrhythmic manifestations of cardiac sarcoidosis, but not pulmonary parenchymal dysfunction, PH, or coronary artery disease in patients with RV LGE compared with those without RV LGE.
In a recent study of gross pathological findings in patients with histologically diagnosed cardiac sarcoidosis that had died from cardiac sarcoidosis or undergone cardiac transplantation, RV involvement was seen in 90.7% of cases (45). Supporting these data, patients with RV LGE in our study had a higher risk for events associated with cardiac sarcoidosis: sudden cardiac death or significant ventricular arrhythmia.
Clinical implications
In patients with sarcoidosis and RV systolic dysfunction on CMR, hemodynamic evaluation by right heart catheterization may be considered if not previously done. This can not only identify sarcoidosis-associated PH but also provide insights into the contribution of the LV-related causes (43). After a diagnosis of sarcoidosis-associated PH is made, treatment options include immunosuppressive medications for the underlying sarcoidosis and PH-directed therapies such as endothelial receptor antagonists or phosphodiesterase-5 inhibitors (46). Studies of PH-directed therapies in sarcoidosis have shown improvements in pulmonary hemodynamics (46–50). Identification of sarcoidosis-associated PH due to non-LV causes could trigger consideration of lung transplantation before deterioration beyond the eligibility criteria for transplantation. Similarly, identification of LV causes of PH with LV systolic dysfunction, such as cardiac sarcoidosis and ischemic cardiomyopathy, could prompt consideration of combined heart-lung transplantation.
In patients with RV LGE, an implantable cardioverter-defibrillator may be considered if not already implanted. RV LGE is always accompanied by LV LGE as noted in all 13 patients with RV LGE in the study by Crawford et al. (10) and all 16 patients in our study. Additionally, RV LGE was also associated with a significantly larger LV LGE extent compared with those without RV LGE in the study by Crawford et al. (10) and the current study. In the 2017 American Heart Association/American College of Cardiology/Heart Rhythm Society Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death, extensive LGE is a criterion for a Class IIa recommendation for ICD implantation to prevent sudden cardiac death in patients with LVEF >35% (21,51). While extensive LGE is not defined in the Guideline, our findings suggest that the presence of RV LGE could be used as a marker of extensive LGE to meet the recommendation for ICD implantation.
Limitations
Our study was limited by its retrospective cohort study design and modest number of outcomes. Our cohort consists of sarcoidosis patients seen at a tertiary care academic medical center who underwent CMR for evaluation of known or suspected cardiac sarcoidosis. Thus, our findings may not be generalizable to all-comers with sarcoidosis. Eighty percent of our cohort is White. Because of the modest number of outcomes, we could not adjust for possible clinical predictors of the outcomes in the multivariable models. Our follow up duration is limited. Thus, our findings need replication in a larger, multicenter, more racially diverse cohort with longer follow up.
CONCLUSIONS
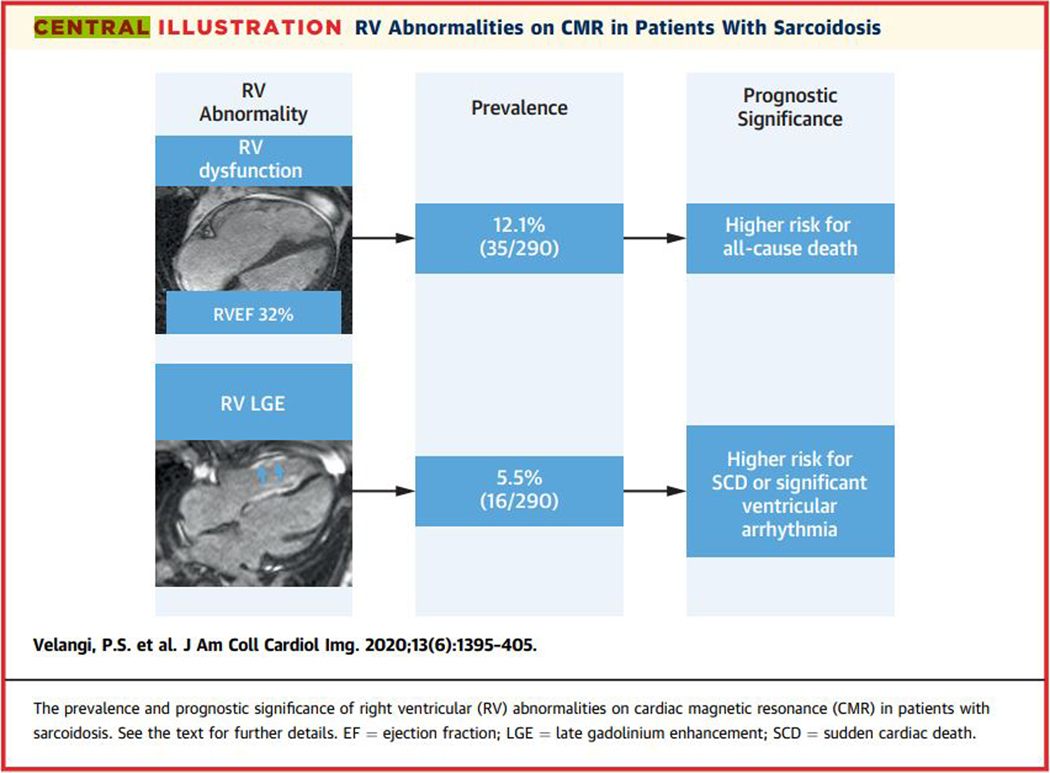
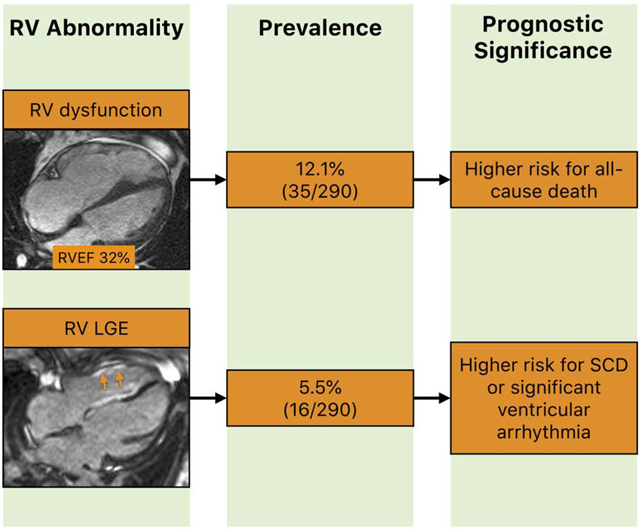
In a large cohort of sarcoidosis patients that had CMR for known or suspected cardiac sarcoidosis, RV systolic dysfunction and RV LGE were present in 12.1% and 5.5% respectively. At a median follow-up of 3.2 years for all-cause death and 3.0 years for the arrhythmic endpoint, RV systolic dysfunction but not RV LGE was independently associated with all-cause death, while RV LGE but not RV systolic dysfunction was independently associated with sudden cardiac death or significant ventricular arrhythmia (Central figure). These findings may indicate distinct implications for the management of patients with sarcoidosis and RV abnormalities.
Central figure. The prevalence and prognostic significance of RV abnormalities on CMR in patients with sarcoidosis.
See the text for further details.
COMPETENCY IN PATIENT CARE:
RV systolic dysfunction on CMR may prompt evaluation for and management of sarcoidosis-associated PH, while RV LGE may prompt consideration of ICD implantation for prevention of sudden cardiac death.
TRANSLATIONAL OUTLOOK:
Future studies are needed to replicate our findings in a larger, multicenter, more racially diverse cohort with longer follow-up, and to investigate whether interventions guided by CMR findings of RV systolic dysfunction and RV LGE improve patient outcomes.
Funding:
Pratik Velangi was supported by a Travel Grant from the Foundation for Sarcoidosis Research (FSR), Chicago, IL to present these data at the 2019 Americas Association of Sarcoidosis and Other Granulomatous Disorders (AASOG) Annual Conference. Mehmet Akçakaya was supported by NIH grant R00HL111410. Chetan Shenoy was supported by NIH grant K23HL132011, a University of Minnesota Clinical and Translational Science Institute KL2 Scholars Career Development Program Award (NIH grant KL2TR000113-05), and NIH grant UL1TR000114.
Abbreviations:
- CI
confidence interval
- CMR
cardiovascular magnetic resonance
- EF
ejection fraction
- HR
hazard ratio
- ICD
implantable cardioverter defibrillator
- IQR
interquartile range
- LGE
late gadolinium enhancement
- LV
left ventricle
- LVAD
left ventricular assist device
- OHT
orthotopic heart transplantation
- PH
pulmonary hypertension
- RV
right ventricle
- SCD
sudden cardiac death
- SD
standard deviation
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maier LA, Crouser ED, Martin WJ 2nd, Eu J Executive Summary of the NHLBI Workshop Report: Leveraging Current Scientific Advancements to Understand Sarcoidosis Variability and Improve Outcomes. Ann Am Thorac Soc 2017;14:S415–S420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerke AK, Judson MA, Cozier YC, Culver DA, Koth LL. Disease Burden and Variability in Sarcoidosis. Ann Am Thorac Soc 2017;14:S421–S428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulkley BH, Rouleau JR, Whitaker JQ, Strauss HW, Pitt B. The use of 201thallium for myocardial perfusion imaging in sarcoid heart disease. Chest 1977;72:27–32. [DOI] [PubMed] [Google Scholar]
- 4.KinneL, JacksoL, ReeveC, ZeliR. Thallium-scan myocardial defects and echocardiographic abnormalities in patients with sarcoidosis without clinical cardiac dysfunction. An analysis of 44 patients. Am J Med 1980;68:497–503. [DOI] [PubMed] [Google Scholar]
- 5.Haywood LJ, Sharma OP, Siegel ME et al. Detection of myocardial sarcoidosis by thallium 201 imaging. J Natl Med Assoc 1982;74:959–64. [PMC free article] [PubMed] [Google Scholar]
- 6.Baughman RP, Gerson M, Bosken CH. Right and left ventricular function at rest and with exercise in patients with sarcoidosis. Chest 1984;85:301–6. [DOI] [PubMed] [Google Scholar]
- 7.Rizzato G, Pezzano A, Sala G et al. Right heart impairment in sarcoidosis: haemodynamic and echocardiographic study. Eur J Respir Dis 1983;64:121–8. [PubMed] [Google Scholar]
- 8.Joyce E, Kamperidis V, Ninaber MK et al. Prevalence and Correlates of Early Right Ventricular Dysfunction in Sarcoidosis and Its Association with Outcome. J Am Soc Echocardiogr 2016;29:871–8. [DOI] [PubMed] [Google Scholar]
- 9.Patel MB, Mor-Avi V, Murtagh G et al. Right Heart Involvement in Patients with Sarcoidosis. Echocardiography 2016;33:734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford T, Mueller G, Sarsam S et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol 2014;7:1109–15. [DOI] [PubMed] [Google Scholar]
- 11.Murtagh G, Laffin LJ, Beshai JF et al. Prognosis of Myocardial Damage in Sarcoidosis Patients With Preserved Left Ventricular Ejection Fraction: Risk Stratification Using Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging 2016;9:e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekstrom K, Lehtonen J, Hanninen H, Kandolin R, Kivisto S, Kupari M. Magnetic Resonance Imaging as a Predictor of Survival Free of Life-Threatening Arrhythmias and Transplantation in Cardiac Sarcoidosis. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smedema JP, van Geuns RJ, Ainslie G, Ector J, Heidbuchel H, Crijns H. Right ventricular involvement in cardiac sarcoidosis demonstrated with cardiac magnetic resonance. ESC Heart Fail 2017;4:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blankstein R, Osborne M, Naya M et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manabe O, Yoshinaga K, Ohira H et al. Right ventricular (18)F-FDG uptake is an important indicator for cardiac involvement in patients with suspected cardiac sarcoidosis. Ann Nucl Med 2014;28:656–63. [DOI] [PubMed] [Google Scholar]
- 16.Omote K, Naya M, Koyanagawa K et al. (18)F-FDG uptake of the right ventricle is an important predictor of histopathologic diagnosis by endomyocardial biopsy in patients with cardiac sarcoidosis. J Nucl Cardiol 2019. [DOI] [PubMed] [Google Scholar]
- 17.Tuominen H, Haarala A, Tikkakoski A, Kahonen M, Nikus K, Sipila K. FDG-PET in possible cardiac sarcoidosis: Right ventricular uptake and high total cardiac metabolic activity predict cardiovascular events. J Nucl Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H, Nijjar PS, Misialek JR et al. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: comparison with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2017;19:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin LQ, Kazmirczak F, Chen KA et al. Impact of Cardiovascular Magnetic Resonance Imaging on Identifying the Etiology of Cardiomyopathy in Patients Undergoing Cardiac Transplantation. Sci Rep 2018;8:16212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.KazmirczaF, NijjaS, ZhanL et al. Safety and prognostic value of regadenoson stress cardiovascular magnetic resonance imaging in heart transplant recipients. J Cardiovasc Magn Reson 2019;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazmirczak F, Chen KA, Adabag S et al. Assessment of the 2017 AHA/ACC/HRS Guideline Recommendations for Implantable Cardioverter-Defibrillator Implantation in Cardiac Sarcoidosis. Circ Arrhythm Electrophysiol 2019;12:e007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes A, Okasha O, Farzaneh-Far A et al. Myocardial Fibrosis and Prognosis in Heart Transplant Recipients. Circ Cardiovasc Imaging 2019;12:e009060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velangi PS, Choo C, Chen KA et al. Long-Term Embolic Outcomes After Detection of Left Ventricular Thrombus by Late Gadolinium Enhancement Cardiovascular Magnetic Resonance Imaging: A Matched Cohort Study. Circ Cardiovasc Imaging 2019;12:e009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E, Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized P. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 2013;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz-Menger J, Bluemke DA, Bremerich J et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen SE, Aung N, Sanghvi MM et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson 2017;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bondarenko O, Beek AM, Hofman MB et al. Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson 2005;7:481–5. [DOI] [PubMed] [Google Scholar]
- 28.Mikami Y, Cornhill A, Heydari B et al. Objective criteria for septal fibrosis in non-ischemic dilated cardiomyopathy: validation for the prediction of future cardiovascular events. J Cardiovasc Magn Reson 2016;18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mooij CF, de Wit CJ, Graham DA, Powell AJ, Geva T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J Magn Reson Imaging 2008;28:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicks KA, Mahaffey KW, Mehran R et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. Circulation 2018;137:961–972. [DOI] [PubMed] [Google Scholar]
- 31.Sanz J, Sanchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;73:1463–1482. [DOI] [PubMed] [Google Scholar]
- 32.Baughman RP. Pulmonary hypertension associated with sarcoidosis. Arthritis Res Ther 2007;9 Suppl 2:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simonneau G, Robbins IM, Beghetti M et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54:S43–54. [DOI] [PubMed] [Google Scholar]
- 34.Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest 2005;128:1483–9. [DOI] [PubMed] [Google Scholar]
- 35.Hoffstein V, Ranganatha N, Mullen JB. Sarcoidosis simulating pulmonary veno-occlusive disease. Am Rev Respir Dis 1986;134:809–11. [DOI] [PubMed] [Google Scholar]
- 36.Portier F, Lerebours-Pigeonniere G, Thiberville L et al. [Sarcoidosis simulating a pulmonary veno-occlusive disease]. Rev Mal Respir 1991;8:101–2. [PubMed] [Google Scholar]
- 37.Nunes H, Humbert M, Capron F et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax 2006;61:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemura T, Matsui Y, Saiki S, Mikami R. Pulmonary vascular involvement in sarcoidosis: a report of 40 autopsy cases. Hum Pathol 1992;23:1216–23. [DOI] [PubMed] [Google Scholar]
- 39.Salazar A, Mana J, Sala J, Landoni BR, Manresa F. Combined portal and pulmonary hypertension in sarcoidosis. Respiration 1994;61:117–9. [DOI] [PubMed] [Google Scholar]
- 40.Santamore WP, Dell’Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis 1998;40:289–308. [DOI] [PubMed] [Google Scholar]
- 41.Taylor RR, Covell JW, Sonnenblick EH, Ross J Jr, . Dependence of ventricular distensibility on filling of the opposite ventricle. Am J Physiol 1967;213:711–8. [DOI] [PubMed] [Google Scholar]
- 42.Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest 2003;124:922–8. [PubMed] [Google Scholar]
- 43.Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest 2010;138:1078–85. [DOI] [PubMed] [Google Scholar]
- 44.Kirkil G, Lower EE, Baughman RP. Predictors of Mortality in Pulmonary Sarcoidosis. Chest 2018;153:105–113. [DOI] [PubMed] [Google Scholar]
- 45.Okasha O, Kazmirczak F, Chen KA, Farzaneh-Far A, Shenoy C. Myocardial Involvement in Patients With Histologically Diagnosed Cardiac Sarcoidosis: A Systematic Review and Meta-Analysis of Gross Pathological Images From Autopsy or Cardiac Transplantation Cases. J Am Heart Assoc 2019;8:e011253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boucly A, Cottin V, Nunes H et al. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J 2017;50. [DOI] [PubMed] [Google Scholar]
- 47.Baughman RP, Judson MA, Lower EE et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2009;26:110–20. [PubMed] [Google Scholar]
- 48.Barnett CF, Bonura EJ, Nathan SD et al. Treatment of sarcoidosis-associated pulmonary hypertension. A two-center experience. Chest 2009;135:1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baughman RP, Culver DA, Cordova FC et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest 2014;145:810–817. [DOI] [PubMed] [Google Scholar]
- 50.Bonham CA, Oldham JM, Gomberg-Maitland M, Vij R. Prostacyclin and oral vasodilator therapy in sarcoidosis-associated pulmonary hypertension: a retrospective case series. Chest 2015;148:1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Khatib SM, Stevenson WG, Ackerman MJ et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017. [DOI] [PubMed] [Google Scholar]