Abstract
Most aerobic biodegradation pathways for hydrocarbons involve iron-containing oxygenases. In iron-limited environments, such as the rhizosphere, this may influence the rate of degradation of hydrocarbon pollutants. We investigated the effects of iron limitation on the degradation of toluene by Pseudomonas putida mt2 and the transconjugant rhizosphere bacterium P. putida WCS358(pWWO), both of which contain the pWWO (TOL) plasmid that harbors the genes for toluene degradation. The results of continuous-culture experiments showed that the activity of the upper-pathway toluene monooxygenase decreased but that the activity of benzyl alcohol dehydrogenase was not affected under iron-limited conditions. In contrast, the activities of three meta-pathway (lower-pathway) enzymes were all found to be reduced when iron concentrations were decreased. Additional experiments in which citrate was used as a growth substrate and the pathways were induced with the gratuitous inducer o-xylene showed that expression of the TOL genes increased the iron requirement in both strains. Growth yields were reduced and substrate affinities decreased under iron-limited conditions, suggesting that iron availability can be an important parameter in the oxidative breakdown of hydrocarbons.
Aerobic degradation of aromatic compounds by microorganisms proceeds via several oxidation steps that are catalyzed by oxygenases. Most of these oxygenases contain iron as a cofactor. Iron is also an important element because of its occurrence as a cofactor in various other proteins, including Krebs cycle enzymes, proteins of the respiratory pathway (6, 11), and enzymes involved in the virulence of pathogens (28). Thus, aerobic metabolism of hydrocarbons is expected to impose a specific iron requirement on cells (29).
The availability of iron in natural environments is usually very low. In the rhizosphere, for instance, the total concentration of iron is estimated to be 0.1 μM, and the concentration of dissolved iron, depending on the pH, can be as low as 10−18 M (4). Under these conditions, where competition is strong, iron availability and the efficiency of iron uptake may influence microbial activity. To compete for iron, microorganisms have developed specialized uptake systems for which they produce siderophores that bind extracellular iron; after binding the iron-siderophore complex can be taken up by the organism via high-affinity receptors (for an overview see references 8, 33, and 34). A well-known example is the production and uptake of siderophores by the root-colonizing organism Pseudomonas putida WCS358 (9, 15); these siderophores can increase the levels of iron available to this strain in the rhizosphere (16). The efficiency of the uptake systems is crucially important in the strong competition among microorganisms that colonize plant roots (7).
It has been shown that iron-limited conditions can lead to altered utilization patterns for various compounds (30) and that iron availability can alter the composition of plant root exudates (36). Very little is known about the effects of trace element limitation on the biodegradation of xenobiotic compounds. It has been shown that expression of the alkane hydroxylase (AlkB) in Pseudomonas oleovorans increases the iron requirement of this organism, but the effects of iron limitation on the capacity to degrade alkanes were not established (29). Expression of iron-containing oxygenases may increase the iron requirement of bacterial strains during growth on aromatic compounds and thereby jeopardize the competitive capabilities of the organisms.
One system that provides an excellent tool for studying the effects of iron limitation on expression of iron-containing oxygenases is the pathway for degradation of toluene and xylenes encoded by the pWWO (TOL) plasmid found in P. putida mt2 (18, 24). This well-studied pathway is encoded by two catabolic operons, the upper operon and the meta operon, both of which contain iron-binding and iron-free enzymes. By comparing iron-sufficient conditions and iron-limited conditions in a chemostat culture, it is possible to distinguish between loss of activity due to lower levels of expression and loss of activity due to formation of inactive apoenzyme. The former results in lower activities of the iron-containing enzymes as well as the iron-free enzymes, whereas the latter results in lower activities of the iron-containing enzymes while the levels of expression are not affected. In this paper we describe the effect of iron limitation on degradation of toluene by P. putida mt2 and P. putida WCS358(TOL).
MATERIALS AND METHODS
Bacterial strains.
P. putida mt2 (= ATCC 33015) harbors the TOL plasmid pWWO (3). P. putida WCS358(TOL) is a transconjugant of P. putida WCS358 in which TOL plasmid pWWO was introduced via triparental mating by A. Ooyevaar (University of Utrecht, Utrecht, The Netherlands).
Media.
The growth medium was a mineral salts medium (MM medium) described previously (20), except that no iron was added. This medium was supplemented with 20 mg of yeast extract per liter. Toluene or citrate (Na3C6H5O7 · 2H2O) was added to the medium as a sole carbon and energy source. Iron was added to media as FeCl3. In order to avoid contamination of media by residual iron, all glassware was soaked in a 0.5 M EDTA solution and rinsed extensively with double-distilled water. Luria-Bertani broth (26) was used to grow cells in rich medium. All media and carbon sources were analytical grade.
Continuous culture.
Fermentors with a working volume of 2.5 liters were used to grow the microorganisms in MM medium supplemented with 20 mg of yeast extract per liter. The pH of each culture was adjusted to 7 with autoclaved 1 M NaOH or 0.5 M H2SO4. The temperature was set at 28°C, and the impeller speed was 900 rpm. For growth on toluene, an air flow was passed through two bottles containing cooled (11°C) toluene via glass filters (P3; Elgebe, Leek, The Netherlands) prior to addition to the culture.
For growth on citrate, MM medium containing 20 mg of yeast extract per liter was supplemented with 15 mM citrate. The xyl genes were induced by adding o-xylene at a concentration of 420 μmol per liter of fresh medium in the same way that toluene was added. Extra water-saturated air was added to the cultures in order to supply sufficient oxygen. The flow rates of air, toluene, and o-xylene were controlled with mass flow controllers (type F201C-FA-11-V; Bronkhorst High-Tec BV, Veenendaal, The Netherlands). All gasses were filter sterilized before addition to the cultures. Each outgoing gas stream was passed through a water column with slight overpressure, which facilitated detection of possible leakage.
The chemostats were inoculated with toluene-pregrown batch cultures so that the densities were approximately 5 mg (dry weight) of cells liter−1. The organisms were grown on toluene in fed-batch mode to densities of around 250 mg (dry weight) of cells liter−1 before a dilution rate of 0.1 h−1 was applied. The concentrations of toluene and o-xylene in the in- and outgoing gas streams were determined by gas chromatography. In order to check the purity of the cultures during operation of the chemostats, culture samples were regularly plated onto Luria-Bertani agar plates, which were incubated at 30°C.
Iron assay.
To determine the iron concentrations in culture supernatants and medium samples, a colorimetric bathophenanthroline-based method was used (37). Three equivalents of 4,7-diphenyl-1,10-phenanthroline disulfonic acid and 1 equivalent of Fe2+ stoichiometrically react to form a red complex, which strongly absorbs at 537 nm. The detection limit of this method was an iron concentration of 0.3 μM.
Culture density.
The culture density was estimated by measuring the optical density at 450 nm with a Pharmacia Novaspec II Rapid spectrophotometer and correlating the values to dry weights of cells. The latter values were determined by centrifuging duplicate 100-ml samples of a culture (15 min, 6,000 × g, 4°C), washing the pellets with the same volume of cold demineralized water, and drying the pellets to a constant weight in a preweighed aluminum cup for 3 days at 80°C. Steady-state biomass concentrations were measured after five volume changes and stayed constant for at least 24 h.
Preparation of crude cell extracts.
Cells from 150-ml culture samples were harvested by centrifugation (15 min, 6,000 × g, 4°C) and washed twice with ice-cold 0.1 M Tris-HCl (pH 7) containing 0.1 mM 1,4-dithiothreitol (TD buffer). After resuspension in a small volume of TD buffer, the cells were disrupted by sonication and centrifuged in an ultracentrifuge for 1 h at 150,000 × g and 4°C in order to remove cell debris. The protein concentrations of the crude cell extracts were determined by the Bradford method using bovine serum albumin as the standard.
Enzyme assays.
Benzyl alcohol dehydrogenase activities were measured by determining NAD reduction at 340 nm (ɛNADH = 6,300 liters mol−1 cm−1). The reaction mixtures contained 20 μmol of Tris-HCl (pH 7), 2 μmol of NAD, 0.4 μmol of benzyl alcohol, and 1 to 2 mg of protein in a total volume of 1 ml. Catechol-2,3-dioxygenase activities were measured by determining formation of 2-hydroxy-6-oxohepta-2,4-dienoate (HMS) from catechol at 375 nm (ɛHMS = 36,000 liters mol−1 cm−1) (22). The reaction mixtures contained 30 μmol of Tris-HCl (pH 7.0), 0.5 μmol of catechol, and 0.1 to 1 mg of protein in a total volume of 1 ml. Hydroxymuconic semialdehyde hydrolase (HMSH) activities were measured by determining the breakdown of HMS at 375 nm. The reaction mixtures contained 30 μmol of Tris-HCl (pH 7.0), about 40 nmol of freshly prepared HMS, and 1 to 2 mg of protein in a total volume of 1 ml. HMS was prepared as described previously (19).
Oxygen uptake measurements.
Oxygen uptake experiments were carried out in order to estimate the activities of the membrane-bound toluene monooxygenase and the three-component benzoate-1,2-dioxygenase. The cells in 150-ml culture samples were harvested by centrifugation (15 min, 6,000 × g, 4°C), washed twice with ice-cold iron-free MM medium, and resuspended in 2 ml of MM medium. Cells were added to a 10-ml stirred incubation vessel filled with oxygen-saturated iron-free MM medium to a concentration of 0.3 to 0.75 mg (dry weight) of cells per ml. The vessel was sealed with a lid to which an oxygen electrode was connected. After the endogenous oxygen consumption rate was determined, toluene or benzoate was added to the cell suspension to a final concentration of about 2 mM. The difference between the oxygen consumption rates before and after addition of the substrate was used to calculate the specific oxidation rate of the substrate in micromoles per minute per gram (dry weight) of cells.
Estimation of kinetic parameters.
Kinetic parameters (Km and Vmax) for toluene degradation were obtained from toluene depletion curves that were determined with cells taken from chemostat cultures (25). At different steady states, a 25-ml sample of a chemostat culture was added to a magnetically stirred (700 rpm) 120-ml stainless steel incubation vessel that was sealed and temperature controlled at 30°C. After a pulse of toluene was added to the reaction vessel, depletion of toluene was measured by on-line analysis of the toluene concentration in the headspace by gas chromatography. Gas was continuously withdrawn from the headspace with a micro membrane pump (model NMP 02LU; KNF Neuberger GmbH, Freiburg-Munzingen, Germany). After passing a Valco 6 port sampling injector (Vici AG, Schenkon, Switzerland) to which a 35-μl sample loop was connected, the gas was injected back into the liquid phase of the incubation vessel. The contents of the sample loop were injected every minute into a gas chromatograph (model CP 9001; Chrompack, Middelburg, The Netherlands) equipped with a CPsil 5 CB column (Chrompack). Stainless steel tubing and a glass-embedded magnetic stirrer were used in order to minimize adsorption of toluene.
The substrate depletion curves were fitted with a model in which a Monod type of equation and the gas-liquid mass transfer of substrate are used with the gas and liquid phase concentrations (Cg and Cl, respectively) as variables. The dimensionless Henry's coefficient (H) for toluene at 30°C is 0.27 (27). The mass transfer coefficient (kLa) for toluene was determined to be 0.57 min−1 by a previously described procedure (32). The volumes of the gas and liquid phases (Vg and Vl, respectively) were 0.025 and 0.095 liter, respectively. The concentration of biomass (X) was determined in separate experiments. Since the measurements were obtained over short time periods (less than 30 min), bacterial growth could be neglected, which led to a model consisting of two equations:
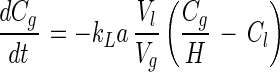 |
1 |
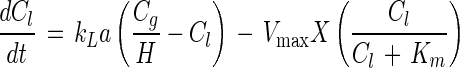 |
2 |
The parameters Km and Vmax and the initial concentrations of toluene in the gas and liquid phases (Cg,0 and Cl,0, respectively) were fitted to numerically integrated equations 1 and 2 by using the Episode routine in Scientist for Windows 2.0 (Micromath Scientific Software, Salt Lake City, Utah).
RESULTS
Iron-limited growth on toluene.
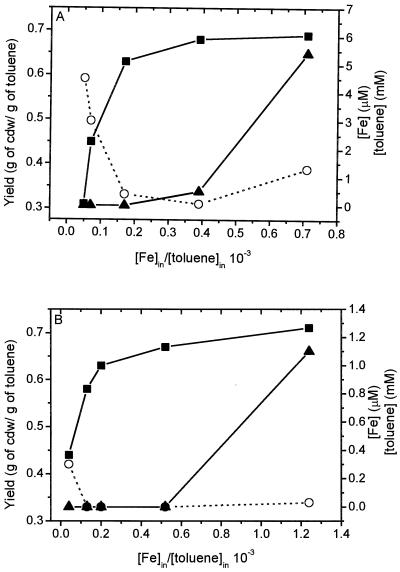
To investigate the effects of iron limitation on degradation of toluene, residual toluene and iron levels were determined in steady-state chemostat cultures of P. putida mt2 and WCS358(TOL) operated at iron/toluene ratios varying from 0.04 × 10−3 to 1.24 × 10−3 moles of iron per mole of toluene. At iron/toluene ratios less than 0.16 × 10−3, no residual iron could be detected in the culture fluids, while only 60 to 70% of the toluene was removed by both P. putida mt2 (Fig. 1A) and P. putida WCS358(pWWO) (Fig. 1B), showing that growth was iron limited. When the iron/toluene ratio was increased, complete removal of both toluene and iron was observed, indicating dual-nutrient-limited growth. At iron/toluene ratios greater than 0.5 × 10−3, toluene-limited growth occurred since more than 98.5% of the toluene was removed while only 64% of the total iron was taken up by the cells.
FIG. 1.
Effects of iron/toluene ratio on substrate removal and yield for P. putida mt2 (A) and P. putida WCS358(pWWO) (B). Cell cultures were grown on mineral medium in a chemostat at a fixed dilution rate of 0.1 h−1. The toluene concentration was kept constant, while the iron concentration was varied. The yield (expressed in grams [dry weight] of cells [cdw] per gram of toluene consumed) (■), residual iron concentration (▴), and residual toluene concentration (○) were determined.
Determination of the growth yields (expressed in grams [dry weight] of cells per gram of toluene removed) showed that for both strains the yield on toluene under iron-limited conditions was significantly lower than the yield under iron-excess conditions (Fig. 1). Increasing the iron/toluene ratio in the iron-limited range resulted in an increase in the growth yield. A further increase in the iron/toluene ratio resulted in a smaller increase in the growth yield, showing that the increase in yield was not dependent only on the iron concentration, which is consistent with dual-nutrient-limited growth. No significant increase in yield was observed under toluene-limited conditions if the iron concentration was elevated further. The lower growth yields on toluene observed under iron-limited conditions indicated that there were significant changes in toluene metabolism. At iron/toluene ratios lower than 0.04 × 10−3 the P. putida mt2 culture washed out at a dilution rate of 0.1 h−1.
Effects of iron limitation on the activity of the TOL enzymes.
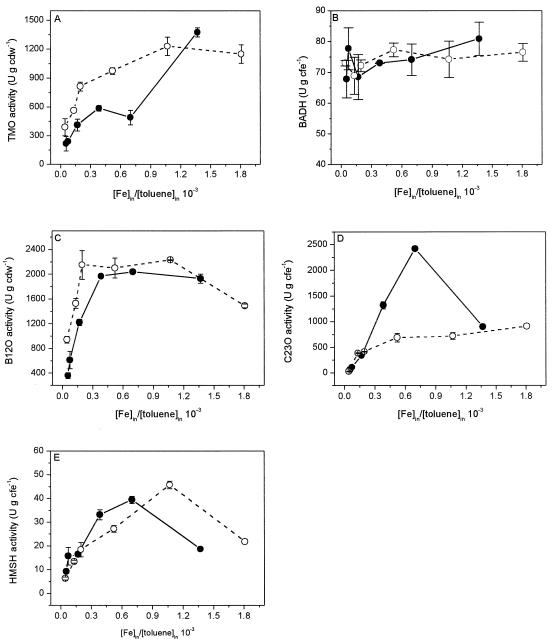
To obtain more insight in the effects of iron limitation on the activity of a catabolic pathway that requires iron-containing enzymes, we determined the activities of two upper-pathway enzymes and three meta-pathway enzymes encoded by the TOL plasmid. The activities were determined at steady states in which the iron/toluene ratio varied from an iron-limited value of 0.04 × 10−3 to an iron-excess value of 1.24 × 10−3. The specific activity of the iron-containing toluene monooxygenase (XylMA) encoded by the upper pathway in P. putida WCS358(pWWO) and mt2 clearly decreased three- to sevenfold when the iron/toluene ratio was decreased (Fig. 2A). The activity was generally 30 to 50% higher for the transconjugant P. putida WCS358(pWWO) than for P. putida mt2. In contrast to the toluene monooxygenase activities, the activities of the upper-pathway iron-free benzyl alcohol dehydrogenase (XylB) at different iron/toluene ratios were not significantly changed (Fig. 2B).
FIG. 2.
Activities of the TOL pathway enzymes in continuous cultures of P. putida mt2 (●) and P. putida WCS358(pWWO) (○) grown on toluene. The toluene monooxygenase (TMO) (A) and benzoate-1,2-dioxygenase (B12O) (C) activities are described by the rate of oxygen consumption (micromoles per minute per gram [dry weight] of cells, equivalent to units per gram [dry weight] of cells). The benzyl alcohol dehydrogenase (BADH) (B), catechol-2,3-dioxygenase (C23O) (D), and HMSH (E) activities are described by the rate of substrate conversion (micromoles per minute per gram of protein in cell extract, equivalent to units per gram of protein in cell extract). cdw, dry weight of cells.
The activity of the meta-pathway iron-containing benzoate-1,2-dioxygenase (XylXYZ) clearly increased when the iron/toluene ratio was increased from 0.04 × 10−3 to 0.39 × 10−3 (Fig. 2C). The increase in activity leveled off at higher iron/toluene ratios, at which dual-nutrient-limited growth or toluene-limited growth occurred. The activities increased two- and sixfold for P. putida WCS358(pWWO) and mt2, respectively, when the lowest and highest iron concentrations used were compared. An increase in activity with an increase in the iron supply was also observed for the second iron-containing meta-pathway dioxygenase, catechol-2,3-dioxygenase (XylE) (Fig. 2D). From the lowest iron/toluene ratio to the highest iron/toluene ratio the activity increased by a factor 28 for P. putida WCS358(pWWO) and by a factor 40 for P. putida mt2.
In contrast to the lack of dependence of the iron-free benzyl alcohol dehydrogenase activity on the iron concentration, the iron-free hydroxy muconic semialdehyde hydrolase (HMSH) (XylF) exhibited the same iron dependence that was observed for the two iron-containing oxygenases of the meta pathway (Fig. 2E). From the lowest iron concentration to the highest iron concentration the activity increased by a factor 7 for P. putida WCS358(pWWO) and by a factor 4 for P. putida mt2. The activities under toluene-limited conditions were similar for the two strains.
Effects of iron limitation on the kinetic parameters for toluene degradation.
Since enzyme levels may influence the kinetics of substrate removal by a culture (31), the kinetic parameters for toluene degradation were determined at different iron concentrations in order to investigate the effect of iron limitation on the overall kinetics of substrate removal by the strains. Cells were obtained from steady states with iron/toluene ratios ranging from 0.04 × 10−3 to 1.24 × 10−3. Values for the maximal specific substrate conversion rate (Vmax) and the substrate affinity constant (Km) were obtained by least-squares fits from toluene depletion measurements that were obtained with resting cell suspensions. For P. putida mt2, a clear decrease in Vmax from 0.102 to 0.03 μmol mg (dry weight) of cells−1 min−1 was observed when cells were cultivated at iron/toluene ratios that decreased from 0.71 × 10−3 to 0.05 × 10−3 (Table 1). The values obtained for Km fluctuated some but were similar to the Km values obtained for the transconjugant P. putida WCS358(pWWO) (data not shown). A clear decrease in degradation performance, as defined by Vmax/Km (5), was observed as the iron/toluene ratio decreased, suggesting that the incomplete removal of toluene observed in the chemostat at low iron/toluene ratios resulted from reduced performance of the cells in terms of degradation kinetics.
TABLE 1.
Kinetic parameters for toluene degradation by P. putida mt2 cells grown in continuous culture with different iron/toluene ratiosa
| Iron/toluene ratio | Km (μM)b | Vmax (μmol mg [dry wt] of cells−1 min−1)b | Vmax/Km (liter mg [dry wt] of cells−1 min−1) |
|---|---|---|---|
| 0.71 × 10−3 | 2.4 | 0.102 | 0.043 |
| 0.39 × 10−3 | 5.2 | 0.085 | 0.017 |
| 0.17 × 10−3 | 14.5 | 0.076 | 0.006 |
| 0.07 × 10−3 | 4.2 | 0.026 | 0.007 |
| 0.05 × 10−3 | 4.9 | 0.030 | 0.006 |
Values were obtained by numerically fitting data from toluene depletion experiments in batch incubations of freshly harvested cells.
Data are averages from two curves; the standard error was approximately 10%.
Effects of iron limitation during growth on citrate.
The decrease in growth yield and worse degradation kinetics under iron-limited conditions could have been caused by a general effect on cell metabolism or by a specific increase in demand for iron due to expression of the TOL pathway enzymes. Therefore, the effects of induction of the TOL enzymes on the iron requirement and growth yield were investigated during growth on citrate. Both strains were grown on 15 mM citrate in continuous cultures in which the TOL pathway enzymes were either not induced or induced by the gratuitous inducer o-xylene (17). The iron levels were varied between 10 and 1 μM FeCl3.
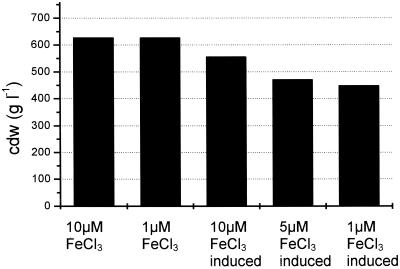
The cell densities during growth on citrate when the TOL pathway enzymes were induced by o-xylene were found to be significantly lower than the cell densities in uninduced cultures (Fig. 3). A 10% decrease in cell density was observed when there was excess iron (10 μM FeCl3), and this decrease may have been caused by toxic effects of the o-xylene or by increased energy consumption related to expression of the TOL proteins. At a low FeCl3 concentration of 1 μM, at which no iron limitation effects were observed for the uninduced cultures, there was an additional 20% decrease in cell density in the induced cultures compared to the uninduced cultures, suggesting that expression of the TOL genes increased the iron requirement of the strains and that the efficiency of biomass production was reduced.
FIG. 3.
Total biomass (expressed in grams [dry weight] of cells [cdw] per liter) formed during growth of P. putida mt2 in continuous culture on medium containing 15 mM citrate amended with 10, 5, or 1 μM FeCl3. The TOL pathway enzymes were either not induced or induced by the gratuitous inducer o-xylene.
The data obtained for iron removal by cultures of P. putida WCS358(pWWO) and mt2 during growth on citrate supported these findings. Incomplete removal of iron was observed in all uninduced cultures, irrespective of the iron concentration available, suggesting that iron was not growth limiting even at an FeCl3 concentration of 1 μM (Table 2). In contrast, the data show that there was a clear increase in iron uptake during growth on citrate when the TOL pathway enzymes were induced by o-xylene. Thus, expression of the TOL pathway enzymes required additional iron. Additionally, complete iron removal was observed in induced cultures grown with 1 μM FeCl3, suggesting that expression of the TOL enzymes induced iron limitation at a low iron concentration (1 μM FeCl3). Iron consumption values (expressed in micromoles of FeCl3 per gram [dry weight] of cells) were significantly higher during growth under iron-excess conditions, while induction of the TOL genes increased the uptake even more.
TABLE 2.
Effects of carbon source on iron utilization by P. putida mt2 and P. putida WCS358(pWWO) under iron-excess (10 μM FeCl3) and iron-limited (1 μM FeCl3) conditions
| Growth substrate | FeCl3 concn (μM) |
P. putida mt2
|
P. putida WCS358(pWWO)
|
||
|---|---|---|---|---|---|
| % of iron removeda | Iron consumption (μmol of Fe/g [dry wt] of cells)b | % of iron removeda | Iron consumption (μmol of Fe/g [dry wt] of cells)b | ||
| Toluene (10 mM) | 10 | 46 | 4.6 | 89 | 14.69 |
| 1 | 100 | 1.81 | 100 | 1.08 | |
| Citrate (15 mM) | 10 | 58 | 8.9 | 74 | 10.7 |
| 1 | 20 | 1.0 | 20 | 0.4 | |
| Citrate (15 mM) + o-xylene | 10 | 64 | 11.1 | 92 | 14.2 |
| 1 | 100 | 1.84 | 100 | 1.9 | |
The percentage of iron removed was determined by measuring the iron concentrations in the in- and outgoing growth media.
Iron consumption was defined as the amount of iron taken up per gram (dry weight) of cells.
TOL pathway enzyme activities during iron-limited growth on citrate.
The effects of iron limitation on the induction and activities of the TOL pathway enzymes were determined during growth on 15 mM citrate. The FeCl3 levels were varied between 10 and 1 μM. As the activity of the toluene monooxygenase decreases during iron-limited growth, the amount of upper-pathway metabolite formed may be reduced. Thus, to avoid regulation of meta-pathway expression by iron-dependent levels of an upper-pathway product (24), the pathways were induced with the gratuitous inducer o-xylene. As this compound is not converted, no intermediate-dependent expression of the meta pathway can occur (1). In this case expression of the meta pathway is affected only by high levels of XylS (24).
Both P. putida mt2 (Table 3) and P. putida WCS358(pWWO) (data not shown) exhibited low levels of activity in uninduced cultures, irrespective of the amount of iron present. The levels of activity of the iron-containing toluene monooxygenase (XylMA), benzoate-1,2-dioxygenase (XylXYZ), and catechol-2,3-dioxygenase (XylE) all were highest in induced cultures with an FeCl3 concentration of 10 μM and decreased significantly as the FeCl3 concentration was decreased to 5 or 1 μM. As observed previously during growth on toluene, the benzyl alcohol dehydrogenase activities stayed constant for both strains irrespective of the amount of iron present, indicating that transcription was not influenced by the availability of iron. The activity of the iron-free HMSH depended on the iron concentration in both strains. In induced cultures of P. putida mt2, the HMSH activity decreased by 59% when the FeCl3 concentration was decreased from 10 to 1 μM. As this effect cannot be accounted for by a lower level of intermediate, the data suggest that the meta-pathway enzymes are expressed in an iron-dependent fashion.
TABLE 3.
Effects of iron concentration on the activities of the TOL pathway enzymes in continuous cultures of P. putida mt2 growing on 15 mM citrate
| Culturea | Fe concn (μM) | Upper-pathway enzyme activities
|
meta-Pathway enzyme activities
|
|||
|---|---|---|---|---|---|---|
| TMO (U/g [dry wt] of cells)b | BADH (U/g of cfe)c | B12O (U/g [dry wt] of cells)b | C23O (U/g of cfe)c | HMSH (U/g of cfe)c | ||
| Uninduced | 10 | 5.8 | 2.1 | 2.6 | 1.2 | 1.3 |
| 1 | 0.0 | 1.0 | 20 | 1.0 | 0.9 | |
| 10 | 200 | 35 | 151 | 66 | 18 | |
| Induced | 5 | 25 | 39 | 24 | 41 | 14 |
| 1 | 4.8 | 35 | 11 | 3.1 | 12 | |
Cultures were either not induced or induced by addition of o-xylene.
Toluene monooxygenase (TMO) and benzoate-1,2-dioxygenase (B12O) activities are described by the rate of oxygen consumption (micromoles per minute per gram [dry weight] of cells, equivalent to units per gram [dry weight] of cells).
Benzyl alcohol dehydrogenase (BADH), catechol-2,3-dioxygenase (C23O), and HMSH activities are described by the rate of substrate conversion (micromoles per minute per gram of protein in cell extract, equivalent to units per gram of protein in cell extract).
The effects of iron limitation were found to be most pronounced for P. putida mt2. For this strain, 85% reductions in toluene monooxygenase activity and benzoate-1,2-dioxygenase activity were observed when the FeCl3 concentration was reduced from 10 to 5 μM. For the transconjugant P. putida WCS358(TOL) the decreases were only 50 to 60%. At the lowest iron concentrations the two strains suffered equally from iron limitation.
DISCUSSION
As aerobic degradation of many hydrocarbons is performed by iron-containing oxygenases, the kinetics of degradation of these compounds may be influenced by iron limitation. It was found that degradation of toluene by P. putida mt2 and WCS358(TOL) was clearly affected by the iron-limited conditions used in our experiments. The substrate depletion experiments showed that the efficiency of toluene degradation by P. putida mt2 and WCS358(TOL) was reduced when the iron concentration was low. The maximal specific substrate conversion rate (Vmax) and the affinity (Vmax/Km) decreased as the iron/toluene ratio decreased. Additionally, the growth yield on toluene was clearly reduced under iron-limited conditions. This may have resulted from inefficient conversion of the substrate due to rearrangements of metabolic flux (2) or from production of secondary metabolites, such as siderophores.
A more extensive investigation of the effects of iron limitation on the TOL pathway enzymes revealed that the TOL upper and meta pathways were affected differently by a decrease in iron availability. The data for the upper-pathway enzymes show that the activity of the iron-containing toluene monooxygenase is significantly lower under iron-limited conditions, whereas the activity of the iron-free benzyl alcohol dehydrogenase was found to be the same irrespective of the iron concentration used. The decrease in activity of the iron-containing toluene monooxygenase may be the result of insufficient iron to bind to the enzyme, which could result in an unstable or inactive apoenzyme.
In contrast, all of the meta-pathway enzymes tested exhibited iron-dependent activity, which may have been due to iron-dependent regulation of expression of the genes of the meta operon. Additional evidence that supports this hypothesis was obtained during growth on citrate in the presence of o-xylene. The level of meta-pathway enzyme activities depended on the amount of iron present, a result similar to the result obtained during growth on toluene. This rules out the possibility that the influence of iron on the activities of these enzymes was indirectly due to an effect of iron on the level of a meta-pathway-inducing intermediate formed by the upper pathway.
The effects of iron limitation on the activity of catechol-2,3-dioxygenase were found to be much more pronounced than the effects on the activities of the other meta-pathway enzymes. The activity of this enzyme decreased 28- and 40-fold in P. putida WCS358(TOL) and mt2, respectively, compared to decreases of only 2- to 7-fold for benzoate-1,2-dioxygenase and HMSH. It has been shown that catechol-2,3-dioxygenase is inactivated by oxidation of the ferrous ion present in the active enzyme (14, 23). The ferric ion is then released, and only binding of a new ferrous ion can reactivate the enzyme, a process that requires a 2Fe-2S-ferredoxin encoded by xylT (12). Therefore, it may be more likely that cells suffer from loss of activity in iron-limited environments, which could explain the pronounced effect of iron limitation on the activity of catechol-2,3-dioxygenase.
During iron-limited growth, iron consumption was found to be much greater on the growth substrate toluene than on citrate. Increases in iron consumption in citrate-grown cultures during induction of the TOL pathway by o-xylene can be explained by iron binding by enzymes of the TOL pathway, which reduces the amount of iron available for iron-dependent enzymes involved in citrate degradation. An estimate of the amount of iron bound to the enzymes involved in toluene degradation based on the assumption that 10% of the cell biomass consists of TOL pathway enzymes is consistent with an increase in iron consumption of 0.8 to 1.5 μmol of FeCl3 per g (dry weight) of cells in induced cultures during growth on citrate. Yields on iron of 1 g (dry weight) of cells per μmol of FeCl3 for P. putida mt2 during growth on citrate correspond well to values obtained for Escherichia coli W3110(pGEc47) growing on glucose (29). Additionally, induction of the alkane monooxygenase AlkB in the latter organism resulted in a reduced yield, 0.35 g (dry weight) of cells per μmol of FeCl3, which is comparable to a yield of 0.55 g (dry weight) of cells per μmol of FeCl3 determined during induction of the TOL pathway enzymes. The large amounts of iron taken up under iron-excess conditions exceed the cell requirements for iron and may be stored in the cells in ferritin-like structures (10, 13).
As a consequence of expression of the TOL pathway enzymes during growth on citrate, iron consumption increased and the growth yield decreased under iron-limited conditions. The affinity of the cells for iron and the mechanism of iron uptake may be complicated by the chelating character of citrate. However, a different mechanism of uptake would not explain the reduced yield, as this is a matter of mass balance. Thus, the 10% lower yield on citrate under iron-excess conditions can be explained by production of useless TOL enzymes, the effects on a possible citrate-iron uptake system, or toxic effects of o-xylene. The 30% lower yields at low iron concentrations were caused by the increased iron requirement of the cells.
A comparison of the two strains showed that the effects of iron limitation are not significantly altered in the transconjugant strain. This shows that the TOL plasmid can be successfully introduced into an excellent root colonizer without significantly affecting the way that the strain responds to iron limitation compared to the natural host strain, derivatives of which have been shown to be rhizosphere colonizers (21). As a result, these strains may be used in rhizoremediation, a strategy proposed for fast and effective removal of aromatic hydrocarbons from soil (35).
ACKNOWLEDGMENTS
This work was financed by STW/ALW under project 790-43-868.
We thank Pieter Wietzes for technical support.
REFERENCES
- 1.Abril M A, Michan C, Timmis K N, Ramos J L. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J Bacteriol. 1989;171:6782–6790. doi: 10.1128/jb.171.12.6782-6790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansell R, Adler L. The effect of iron limitation on glycerol production and expression of the isogenes for NAD(+)-dependent glycerol 3-phosphate dehydrogenase in Saccharomyces cerevisiae. FEBS Lett. 1999;461:173–177. doi: 10.1016/s0014-5793(99)01456-8. [DOI] [PubMed] [Google Scholar]
- 3.Assinder S J, Williams P A. The TOL plasmids: determinants of the catabolism of toluene and the xylenes. Adv Microb Physiol. 1990;31:1–69. doi: 10.1016/s0065-2911(08)60119-8. [DOI] [PubMed] [Google Scholar]
- 4.Braun V, Killmann H. Bacterial solutions to the iron-supply problem. Trends Biochem Sci. 1999;24:104–109. doi: 10.1016/s0968-0004(99)01359-6. [DOI] [PubMed] [Google Scholar]
- 5.Button D K. Kinetics of nutrient-limited transport and microbial growth. Microbiol Rev. 1985;49:270–297. doi: 10.1128/mr.49.3.270-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter P A, Darie S, Gunsalus R P. The effect of iron limitation on expression of the aerobic and anaerobic electron transport pathway genes in Escherichia coli. FEMS Microbiol Lett. 1992;79:227–232. doi: 10.1111/j.1574-6968.1992.tb14045.x. [DOI] [PubMed] [Google Scholar]
- 7.de Weger L A, van Arendonk J J, Recourt K, van der Hofstad G A, Weisbeek P J, Lugtenberg B. Siderophore-mediated uptake of Fe3+ by the plant growth-stimulating Pseudomonas putida strain WCS358 and by other rhizosphere microorganisms. J Bacteriol. 1988;170:4693–4698. doi: 10.1128/jb.170.10.4693-4698.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escolar L, Perez-Martin J, de Lorenzo V. Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol. 1999;181:6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geels F P, Schippers B. Reduction in yield depression in high frequency potato cropping soil after seed tuber treatments with antagonistic fluorescent Pseudomonas spp. Phytopathol Z. 1983;108:207–221. [Google Scholar]
- 10.Harrison P M, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;3:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard J A, Lewandowska K B, Hughes M N, Poole R K. Effects of iron-limitation of Escherichia coli on growth, the respiratory chains and gallium uptake. Arch Microbiol. 1986;146:80–86. doi: 10.1007/BF00690163. [DOI] [PubMed] [Google Scholar]
- 12.Hugo N, Armengaud J, Gaillard J, Timmis K N, Jouanneau Y. A novel -2Fe-2S- ferredoxin from Pseudomonas putida mt2 promotes the reductive reactivation of catechol 2,3-dioxygenase. J Biol Chem. 1998;273:9622–9629. doi: 10.1074/jbc.273.16.9622. [DOI] [PubMed] [Google Scholar]
- 13.Ilari A, Stefanini S, Chiancone E, Tsernoglou D. The dodecameric ferritin from Listeria innocua contains a novel intersubunit iron-binding site. Nat Struct Biol. 2000;7:38–43. doi: 10.1038/71236. [DOI] [PubMed] [Google Scholar]
- 14.Kita A, Kita S, Fujisawa I, Inaka K, Ishida T, Horiike K, Nozaki M, Miki K. An archetypical extradiol-cleaving catecholic dioxygenase: the crystal structure of catechol 2,3-dioxygenase (metapyrocatechase) from Pseudomonas putida mt-2. Struct Fold Des. 1999;7:25–34. doi: 10.1016/s0969-2126(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 15.Koster M, Ovaa W, Bitter W, Weisbeek P. Multiple outer membrane receptors for uptake of ferric pseudobactins in Pseudomonas putida WCS358. Mol Gen Genet. 1995;248:735–743. doi: 10.1007/BF02191714. [DOI] [PubMed] [Google Scholar]
- 16.Loper J E, Henkels M D. Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl Environ Microbiol. 1999;65:5357–5363. doi: 10.1128/aem.65.12.5357-5363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques S, Holtel A, Timmis K N, Ramos J L. Transcriptional induction kinetics from the promoters of the catabolic pathways of TOL plasmid pWW0 of Pseudomonas putida for metabolism of aromatics. J Bacteriol. 1994;176:2517–2524. doi: 10.1128/jb.176.9.2517-2524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marques S, Ramos J L. Transcriptional control of the Pseudomonas putida TOL plasmid catabolic pathways. Mol Microbiol. 1993;9:923–929. doi: 10.1111/j.1365-2958.1993.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 19.Mars A E, Kingma J, Kaschabek S R, Reineke W, Janssen D B. Conversion of 3-chlorocatechol by various catechol 2,3-dioxygenases and sequence analysis of the chlorocatechol dioxygenase region of Pseudomonas putida GJ31. J Bacteriol. 1999;181:1309–1318. doi: 10.1128/jb.181.4.1309-1318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mars A E, Prins G T, Wietzes P, de Koning W, Janssen D B. Effect of trichloroethylene on the competitive behavior of toluene-degrading bacteria. Appl Environ Microbiol. 1998;64:208–215. doi: 10.1128/aem.64.1.208-215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina L, Ramos C, Duque E, Ronchel M C, Garcia J M, Wyke L, Ramos J L. Survival of Pseudomonas putida KT2440 in soil and in the rhizosphere of plants under greenhouse and environmental conditions. Soil Biol Biochem. 2000;32:315–321. [Google Scholar]
- 22.Nozaki M, Kotani S, Ono K, Seno S. Metapyrocatechase. 3. Substrate specificity and mode of ring fission. Biochim Biophys Acta. 1970;220:213–223. doi: 10.1016/0005-2744(70)90007-0. [DOI] [PubMed] [Google Scholar]
- 23.Nozaki M, Ono K, Nakazawa T, Kotani S, Hayaishi O. Metapyrocatechase. II. The role of iron and sulfhydryl groups. J Biol Chem. 1968;243:2682–2690. [PubMed] [Google Scholar]
- 24.Ramos J L, Mermod N, Timmis K N. Regulatory circuits controlling transcription of TOL plasmid operon encoding meta-cleavage pathway for degradation of alkylbenzoates by Pseudomonas. Mol Microbiol. 1987;1:293–300. doi: 10.1111/j.1365-2958.1987.tb01935.x. [DOI] [PubMed] [Google Scholar]
- 25.Robinson J A, Tiedje J M. Nonlinear estimation of Monod growth kinetic parameters from a single substrate depletion curve. Appl Environ Microbiol. 1983;45:1453–1458. doi: 10.1128/aem.45.5.1453-1458.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Schwarzenbach R P, Gschwend P M, Imboden D M. Environmental organic chemistry. 1st ed. New York, N.Y: J. Wiley and Sons, Inc.; 1995. [Google Scholar]
- 28.Somerville G, Mikoryak C A, Reitzer L. Physiological characterization of Pseudomonas aeruginosa during exotoxin A synthesis: glutamate, iron limitation, and aconitase activity. J Bacteriol. 1999;181:1072–1078. doi: 10.1128/jb.181.4.1072-1078.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staijen I E, Witholt B. Synthesis of alkane hydroxylase of Pseudomonas oleovorans increases the iron requirement of alk+ bacterial strains. Biotechnol Bioeng. 1998;57:228–237. [PubMed] [Google Scholar]
- 30.Takeda S. Influence of iron availability on nutrient consumption ratio of diatoms in oceanic waters. Nature. 1998;393:774–777. [Google Scholar]
- 31.Van Den Wijngaard A J, Wind R D, Janssen D B. Kinetics of bacterial growth on chlorinated aliphatic compounds. Appl Environ Microbiol. 1993;59:2041–2048. doi: 10.1128/aem.59.7.2041-2048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hylckama Vlieg J E T, de Koning W, Janssen D B. Transformation kinetics of chlorinated ethenes by Methylosinus trichosporium OB3b and detection of unstable epoxides by on-line gas chromatography. Appl Environ Microbiol. 1996;62:3304–3312. doi: 10.1128/aem.62.9.3304-3312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasil M L, Ochsner U A. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Mol Microbiol. 1999;34:399–413. doi: 10.1046/j.1365-2958.1999.01586.x. [DOI] [PubMed] [Google Scholar]
- 34.Venturi V, Weisbeek P, Koster M. Gene regulation of siderophore-mediated iron acquisition in Pseudomonas: not only the Fur repressor. Mol Microbiol. 1995;17:603–610. doi: 10.1111/j.1365-2958.1995.mmi_17040603.x. [DOI] [PubMed] [Google Scholar]
- 35.Walton B T, Anderson T A. Microbial degradation of trichloroethylene in the rhizosphere: potential application to biological remediation of waste sites. Appl Environ Microbiol. 1990;56:1012–1016. doi: 10.1128/aem.56.4.1012-1016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang C H, Crowley D E. Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl Environ Microbiol. 2000;66:345–351. doi: 10.1128/aem.66.1.345-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang D, Okada S, Kawabata T, Yasuda T. An improved simple colorimetric method for quantitation of non-transferrin-bound iron in serum. Biochem Mol Biol Int. 1995;35:635–641. [PubMed] [Google Scholar]