Abstract
Aim
The metabolite 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionic acid (CMPF) is a fatty fish–intake biomarker. We investigated the association between plasma levels of CMPF in relation to gingival inflammation and periodontitis case definition, as well as the extent and severity variables.
Materials and Methods
The Malmö Offspring Study is a population‐based study, and the Malmö Offspring Dental Study (MODS) is its dental arm, including periodontal charting. Plasma CMPF was measured using liquid chromatography–mass spectrometry and studied in relation to periodontal diagnosis and parameters using multivariable linear or logistic regression modelling adjusting for age, sex, education, body mass index, fasting glucose, and smoking.
Results
Metabolite data were available for 922 MODS participants. Higher CMPF levels were associated with less gingival inflammation (β = −2.12, p = .002) and lower odds of severe periodontitis (odds ratio [OR] = 0.74, 95% confidence interval [CI]: 0.56 to 0.98). Higher CMPF levels were also associated with more teeth (β = 0.19, p = .001), lower number of periodontal pockets (≥4 mm) (β = −1.07, p = .007), and lower odds of having two or more periodontal pockets of ≥6 mm (OR = 0.80, 95% CI: 0.65 to 0.98) in fully adjusted models.
Conclusions
CMPF, a validated biomarker of fatty fish consumption, is associated with less periodontal inflammation and periodontitis. Residual confounding cannot be ruled out, and future studies are warranted.
Keywords: CMPF, fish diet, gingival inflammation, metabolomics, periodontitis
Clinical Relevance.
Scientific rationale for study: The impact of nutrition on periodontitis is of emerging interest. We aimed to investigate the association between fish consumption via the biomarker 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionic acid (CMPF) and periodontal parameters.
Principal findings: A person with a 1 SD higher level of CMPF (a fish consumption biomarker) is less likely to have severe periodontitis and gingival inflammation after adjusting for age, sex, education, fasting plasma glucose, and smoking habits.
Practical implications: This study suggests that fish consumption is associated with less gingival inflammation and periodontal pockets.
1. INTRODUCTION
Periodontitis is an inflammatory disease with infectious aetiology causing loss of periodontal attachment and, if left untreated, tooth loss. Periodontitis is initiated through a dysbiosis of the commensal oral microbiota and its interaction with the host immune response (Kinane et al., 2017). About 30% of the population is affected by moderate periodontitis and about 8% by severe periodontitis according to the US National Health and Nutrition Examination Survey study (Eke et al., 2012).
Risk factors and risk indicators are, when considered together with clinical variables, important tools in preventing and treating periodontitis. Several risk factors of periodontitis have been established, including smoking, type 2 diabetes mellitus, and genetic predisposition (Genco & Borgnakke, 2013; Kinane et al., 2017). Micronutrients, including the metabolite vitamin D, have been associated with both periodontitis and gingival inflammation (Dietrich et al., 2004, 2005; Jönsson et al., 2013; Dommisch et al., 2018). The omega‐3 fatty acid docosahexaenoic acid was reported to be associated with lower incidence of periodontitis (Iwasaki et al., 2010) as well as more successful periodontal treatment outcome (Deore et al., 2014). Omega‐3 fatty acids in combination with aspirin have also been associated with improved periodontal healing when added to scaling and root planing compared to placebo (El‐Sharkawy et al., 2010). There are no reports on the relationship between fish intake and periodontitis in the general population.
3‐Carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionic acid (CMPF) is a metabolite that exists mainly in marine bacteria, plankton, and algae, and therefore, it accumulates in fish, particularly in oil‐rich fish. In humans, CMPF can be measured in blood plasma and urine. The levels of CMPF will increase with a diet including fish and fish oil (Hanhineva et al., 2015; Pallister et al., 2016; Wang et al., 2018; Shi et al., 2019). The current study hypothesizes that, given the periodontal health‐promoting effects of omega‐3 fatty acids, CMPF may be associated with periodontitis and gingival inflammation. The aim of the current study was therefore to investigate whether CMPF, as a biomarker of fatty fish intake, is associated with gingival inflammation and periodontitis.
2. MATERIALS AND METHODS
2.1. Study population
The Malmö Offspring Study (MOS) (Brunkwall et al., 2021) is a population‐based cohort study that recruited between 2013 and 2021, inviting adult children and grandchildren (age > 18 years) of index subjects (participants) from the Malmö Diet Cancer Study‐Cardiovascular Cohort (Berglund et al., 1993). Participants were recruited through letters inviting them for a visit at the Clinical Research Unit, Skåne University Hospital, Malmö, Sweden. At the visit, venous blood was drawn after overnight fasting and anthropometrics were measured during physical examination. Details of the study design and methods can be found in Brunkwall et al. (2021) and Ottosson et al. (2018).
The MOS participants were also informed about the dental arm of MOS, the Malmö Offspring Dental Study (MODS), and the participants were subsequently recruited to the dental arm via phone calls. In MODS, saliva as well as dental, tongue, and mucosal biofilms were sampled. In addition, the participants underwent a thorough dental examination including full‐mouth clinical examination by a dentist and panoramic and bitewing radiography. MOS and MODS were both approved by the ethical review board of Lund University (MOS: Dnr. 2012/594 and MODS: Dnr. 2013/761).
2.2. Periodontal examination
Periodontitis was assessed using a periodontal probe with 1‐mm grading (Hu‐Friedy PCPUNC157), and periodontal pockets >2 mm at six surfaces per tooth were registered, as 2‐mm sulcus can be considered to constitute a healthy periodontium. In addition, the distances from the cementum–enamel junction to the depth of the pocket were registered using the same probe, that is, clinical attachment level. Periodontitis was classified according to the American Academy of Periodontology and Centers for Disease Control's classification at the time of the study (Page & Eke, 2007). Five trained and experienced dentists examined the participants between 2014 and 2018. Inter‐examiner agreement was set at ≥90% within ±1‐mm probing depth. The two‐way mixed inter‐class correlation coefficient with absolute agreement for inter‐examiner probing depth was >0.75 (0.753–0.791).
2.3. Questionnaire and biochemical measurements
In MODS, all participants were asked to fill out a comprehensive questionnaire, including questions on smoking habits (never, former, or current smoker) and education [(i) up to 9 years or completed 9 years of education, (ii) completed 12 years education, or (iii) completed university education]. In MOS, 84% filled out a questionnaire including questions on whether they had a job and how often they drank alcohol. Drinking habits were sorted into the following categories: (i) never, (ii) up to four times per month, or (iii) more often than twice per week.
Concentrations of fasting plasma glucose were measured using routine standard methods at the Department of Clinical Chemistry, Malmö University Hospital.
2.4. Metabolite profiling
Plasma metabolites were examined in 3430 MOS participants. Out of those, 927 participants had also undergone a full dental examination in MODS. The study size was not based on power analysis for CMPF and periodontitis, as the current study is the first study investigating the potential association.
Measurements of CMPF were performed using a liquid chromatography–quadrupole time‐of‐flight mass spectrometry system (Agilent 1290 LC, 6550 MS; Agilent Technologies, Santa Clara, CA). Plasma samples stored at −80°C were thawed and extracted by the addition of six volumes of the extraction solution consisting of 80:20 methanol/water. The samples were separated on an ACE C18 column (1.7 μm; 2.1 × 100 mm; Advanced Chromatography Technologies Ltd., Aberdeen, UK) using gradient elution (mobile phase A, water with 0.1% formic acid; mobile phase B, acetonitrile with 0.1% formic acid). Mass spectra were acquired in the negative ion mode. The flow rate, gradient elution schedule, column temperature, and ion‐source settings were set as previously described (Ottosson et al., 2018). Samples were analysed in batches of 180 samples, where the quality control samples were run at the start of each batch and after every 10 analytical samples. Fragmentation mass spectra for CMPF were collected by isolating the ion of m/z 239.092 and subjecting it to 20 eV of collision energy. The isolation width was 1.3 m/z.
2.5. Metabolite identification and data processing
CMPF was identified using a synthetically produced CMPF standard (Toronto Research Chemicals, Toronto, Canada). The identity of CMPF was confirmed by matching the plasma measurements of the m/z ratio, retention time, and fragmentation spectra (Figure S1) with data acquired from the synthetic standard. A correct match corresponds to the highest level of annotation confidence according to the Metabolite Standards Initiative (Sumner et al., 2007). CMPF peak areas were integrated using the Agilent Profinder B.06.00 (Agilent Technologies) and were normalized to measurements in the quality control samples (Supporting Material S1). CMPF values were ln‐transformed to better represent a normal distribution and were subsequently mean‐centred and univariance‐scaled to get interpretable coefficients from the regression models. The normalization was performed in R software (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria). The variability of measured CMPF in the quality control samples is presented in Figure S2.
2.6. Statistical analysis
Descriptive data are expressed as means and SD, or number (proportions [%]). A series of multivariable regressions were used to assess the associations between periodontal parameters and CMPF. Multinominal regression, with the effect estimate odds ratio (OR), 95% confidence intervals (CI), and p values, was used to regress no or mild (reference), moderate, or severe periodontitis. Logistic regression was performed for having more than two sites with pockets of ≥6 mm with the effect estimates OR with 95% CIs and p values. Linear regression with the effect estimates β, 95% CIs, and p values was used to regress the number of periodontal pockets of ≥4 mm and the percentage of sites bleeding on probing (%BoP). In all models with continuous CMPF, coefficients were expressed per SD increment of CMPF. All regression models were adjusted for confounders as follows: Model 1 was adjusted for age and sex; Model 2 was additionally adjusted for education, fasting plasma glucose, and body mass index (BMI); and in Model 3, smoking status was added. Quartile 1 refers to subjects from lowest level of CMPF to the first quartile, quartile 2 from first to the median, quartile 3 from median to third quartile, and quartile 4 from the third quartile to the highest value of CMPF. Model assumptions for linear and logistic regressions were met.
Sensitivity analysis was conducted in non‐smokers, including subjects that never smoked and previous smokers.
The statistics were computed in IBM SPSS version 27. p‐Values <.05 were regarded as statistically significant.
3. RESULTS
3.1. Cohort characteristics
The attendance rate in MOS was 47%, and 3428 of the participants had their plasma metabolites analysed. In total, 922 participants with analysed plasma metabolites had undergone a full periodontal examination in MODS. In one study participant, the periodontal charting was interrupted because of discomfort; however, the diagnosis of severe periodontitis could be made based on clinical appearance and radiographs. Therefore, the analyses of pockets and BoP and periodontal pockets are based on 921 participants. Table 1 presents details of the cohort that underwent periodontal examination.
TABLE 1.
Characteristics of participants in the Malmö Offspring Dental Study in all subjects
| Variables | All |
|---|---|
| n | 922 |
| Age (years) | 44.8 ± 14.2 |
| Sex (women) | 460 (49.9) |
| Fasting plasma glucose (mmol/L) | 5.5 ± 1.0 |
| BMI (kg/m2) | 26.3 ± 4.7 |
| Education | |
| Education ≤12 years | 50 (5.4) |
| Education 12 years | 513 (55.6) |
| Education at university level | 359 (38.9) |
| Smoking habit | |
| Never smoked | 550 (59.7) |
| Current smoker | 99 (10.7) |
| Former smoker | 273 (29.6) |
| Mean years since stopped smoking | 15.7 ± 10.8 |
| CDC/AAP case definition | |
| No or mild periodontitis | 592 (64.2) |
| Moderate periodontitis | 266 (28.9) |
| Severe periodontitis | 64 (6.9) |
| Bleeding on probing | 27.1 ± 18.4 |
| Sites with PPD ≥4 mm | 8.2 ± 11.5 |
| Percentage of teeth with PPD ≥4 mm | 18.6 ± 17.3 |
| Prevalence of ≥2 sites with PPD ≥6 mm | 97 (10.5) |
| Prevalence of ≥2 sites with CAL ≥6 mm | 65 (7) |
Note: Continuous variables are expressed as mean ± SD and categorical variables as number (percentage).
Abbreviations: AAP, American Academy of Periodontology; BMI, body mass index; CAL, clinical attachment loss; CDC, Centers for Disease Control; PPD, probing pocket depth.
3.2. Periodontitis
Higher CMPF levels were associated with a statistically significant lower risk of severe periodontitis (no or mild periodontitis as reference group) when adjusting for age, sex, education, plasma glucose levels, BMI, and smoking status (OR = 0.74, 95% CI: 0.56 to 0.98). Being in the highest versus lowest quartile of CMPF levels (quartile 4 vs. 1) was significantly associated with lower odds of having severe periodontitis, when adjusting for age, sex, fasting blood glucose, education, and BMI (OR = 0.40, 95% CI: 0.17 to 0.93). However, this association was weak and not significant when performing additional adjustments for smoking status (OR = 0.55, 95% CI: 0.23to 1.32) (Table 2).
TABLE 2.
Multi‐variable multi‐nominal regression with no or mild periodontitis/moderate periodontitis/severe periodontitis as dependent variables
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| CMPF | ||||||
| Moderate | 0.93 (0.79 to 1.09) | .38 | .94 (0.80 to 1.11) | .48 | 0.95 (0.80 to 1.12) | .51 |
| Severe | 0.64 (0.49 to 0.83) | .001 | .68 (0.52to 0.89) | .005 | 0.74 (0.56 to 0.98) | .03 |
| CMPF Q4 versus Q1 | ||||||
| Moderate | 0.96 (0.61 to 1.54) | .86 | 1.05 (0.64 to 1.75) | .84 | 1.11 (0.66 to 1.85) | .70 |
| Severe | 0.31 (0.14 to 0.67) | .003 | 0.40 (0.17 to 0.93) | .03 | 0.55 (0.23 to 1.32) | .18 |
| CMPF Q3 versus Q1 | ||||||
| Moderate | 1.32 (0.84 to 2.07) | .23 | 1.34 (0.85 to 2.11) | .22 | 1.33 (0.84 to 2.10) | .23 |
| Severe | 0.41 (0.19 to 0.88) | .02 | 0.48 (0.22 to 1.07) | .07 | 0.55 (0.24 to 1.25) | .15 |
| CMPF Q2 versus Q1 | ||||||
| Moderate | 1.15 (0.74 to 1.79) | .54 | 1.19 (0.76 to 1.86) | .45 | 1.23 (0.79 to 1.92) | .35 |
| Severe | 0.38 (0.17 to 0.87) | .02 | 0.52 (0.22 to 1.25) | .14 | 0.57 (0.24 to 1.37) | .22 |
| N | 922 | 922 | 922 | |||
Note: No or mild periodontitis is the reference category. Z‐ and ln‐transformed CMPF as well as comparing the fourth, third, and second quartile of CMPF levels with the first quartile. Model 1 (M1) adjusted for age and sex; Model 2 (M2) adjusted for M1 + level of education, fasting plasma glucose level, and body mass index; and Model 3 (M3) adjusted for M2 + smoking status. CMPF 4 versus 1 is the fourth versus first quartile of CMPF; CMPF 3 versus 1 is the third versus first quartile of CMPF; and CMPF 2 versus 1 is the second versus first quartile of CMPF. Significant values are indicated with bold font.
Abbreviations: CI, confidence interval; CMPF, 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionic acid; OR, odds ratio.
There was no significant association between CMPF levels and moderate periodontitis, with no or mild periodontitis as reference group, as shown in Table 2.
To further elucidate the impact of smoking on the association between periodontitis and CMPF, a separate analysis on non‐smokers (never smokers and previous smokers) was conducted (Table S1). Here, in a reduced cohort, CMPF levels had a slightly weakened association with severe periodontitis (OR = 0.74, 95% CI: 0.54 to 1.01). Because previous smokers had not smoked for a mean of 15.7 years, they were here merged with non‐smokers rather than with current smokers.
Out of the subjects included, 84.7% (781 of 922) answered questions on socio‐economic status (SES) and alcohol consumption in the MOS questionnaire. A question on whether the participants have a job and another on how often they drink alcohol were included in Model 2 in Tables S3 and S4. A total of 65.9% had a job. In relation to alcohol consumption, 6.1% never drank, 52.6% drank up to four times per month, and 29.7% drank alcohol on several occasions per week. Adding these confounders resulted in a slightly weaker association with no significant association between CMPF levels and severe periodontitis in the fully adjusted model (OR = 0.74, 95% CI: 0.54 to 1.01) (Table S3).
3.3. Gingival inflammation
Higher CMPF levels were associated with less gingival inflammation, measured as %BoP and adjusting for all confounders, as shown in Table 3. The fully adjusted β values were −2.12 (95% CI: −3.38 to –0.86) for CMPF levels, −5.73 (95% CI: −9.60 to –1.86) comparing CMPF quartile 4 to 1, −4.32 (95% CI: −8.00 to –0.64) quartile 3 versus 1, and −0.96 (95% CI: −4.53 to 2.61) quartile 2 versus 1 (Table 3a).
TABLE 3.
(a) Multivariable linear regression with the clinical parameters number of sites with probing pocket depth (PPD) ≥ 4 mm, % bleeding on probing (BoP), and number of teeth as dependent variables; (b) Multivariable logistic regression for number of sites with PPD ≥ 6 mm (0 or 1 versus 2 or more)
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dependent variable | Independent variable | Model 1 | Model 2 | Model 3 | N | |||
| β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |||
| Pockets ≥ 4 mm | CMPF | −1.42 (−2.20 to 0.64) | <.001 | −1.21 (−1.99 to –0.43) | .002 | −1.07 (−1.85 to 0.29) | .007 | 921 |
| CMPF 4 versus 1 | −3.34 (−5.76 to 0.91) | .007 | −2.41 (−4.85 to 0.04) | .05 | −1.77 (−4.21 to 0.68) | .16 | 461 | |
| CMPF 3 versus 1 | −1.94 (−4.19 to 0.31) | .09 | −1.40 (−3.61 to 0.80) | .21 | −1.22 (−3.43 to 0.98) | .25 | 459 | |
| CMPF 2 versus 1 | −1.40 (−3.56 to 0.76) | .20 | −0.57 (−2.66 to 1.53) | .60 | −0.46 (−2.56 to 1.64) | .67 | 459 | |
| %BoP | CMPF | −2.43 (−3.68 to –1.19) | <.001 | −2.16 (−3.41 to −0.91) | .001 | −2.12 (−3.38 to –0.86) | .002 | 921 |
| CMPF 4 versus 1 | −6.68 (−10.43 to 2.92) | .001 | −5.83 (−9.66 to –2.00) | .003 | −5.73 (−9.60 to –1.86) | .004 | 461 | |
| CMPF 3 versus 1 | −4.73 (−8.13 to –1.10) | .01 | −4.45 (−8.12 to –0.79) | .02 | −4.32 (−8.00 to –0.64) | .02 | 459 | |
| CMPF 2 versus 1 | −1.98 (−5.52 to 1.57) | .27 | −1.03 (−4.58 to 2.53) | .57 | −0.96 (−4.53 to 2.61) | .60 | 459 | |
| Number of teeth | CMPF | 0.22 (0.12 to 0.33) | <.001 | 0.20 (0.09 to 0.30) | .001 | 0.19 (0.08 to 0.29) | .001 | 922 |
| CMPF 4 versus 1 | 0.73 (0.39 to 1.08) | <.001 | 0.62 (0.27 to 0.97) | .001 | 0.58 (0.22 to 0.93) | .001 | 462 | |
| CMPF 3 versus 1 | 0.50 (0.17 to 0.83) | .003 | 0.44 (0.11 to 0.77) | .009 | 0.40 (0.07 to 0.73) | .02 | 460 | |
| CMPF 2 versus 1 | 0.45 (0.15 to 0.75) | .004 | 0.36 (0.06 to 0.65) | .02 | 0.33 (0.03 to 0.63) | .03 | 460 | |
| (b) | |||||||
|---|---|---|---|---|---|---|---|
| No or 1 versus ≥2 deep pockets | Model 1 | Model 2 | Model 3 | N | |||
| Independent variable | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| CMPF | 0.74 (0.61 to 0.91) | .005 | 0.78 (0.63 to 0.96) | .02 | 0.80 (0.65 to 0.98) | .035 | 921 |
| CMPF 4 versus 1 | 0.49 (0.26 to 0.92) | .03 | 0.64 (0.32 to 1.25) | .19 | 0.76 (0.38 to 1.53) | .44 | 461 |
| CMPF 3 versus 1 | 0.71 (0.40 to 1.29) | .26 | 0.79 (0.44 to 1.45) | .45 | 0.82 (0.44 to 1.55) | .55 | 459 |
| CMPF 2 versus 1 | 0.67 (0.37 to 1.23) | .20 | 0.82 (0.44 to 1.55) | .55 | 0.83 (0.44 to 1.58) | .57 | 459 |
Note: (a) Independent variables are CMPF levels as well as CMPF quartile 1–4 and comparing the fourth, third, and second quartile of CMPF levels with the first quartile. Effect sizes are reported with 95% CI. (b). Z‐ and ln‐transformed CMPF as well as comparing quartiles of CMPF as the independent variable. Model 1 (M1) adjusted for age and sex; Model 2 (M2) adjusted for M1 + level of education, fasting plasma glucose level, and BMI; Model 3 (M3) adjusted for M2 + smoking status. CMPF 4 versus 1 is the fourth versus first quartile of CMPF; CMPF 3 versus 1 is the third versus first quartile of CMPF; CMPF 2 versus 1 is the second versus first quartile of CMPF. Statistically significant effect estimates are indicated with bold font. N is the number of subjects in the model.
Abbreviations: CI, confidence interval; CMPF, 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionic acid; OR, odds ratio.
Separate analysis in non‐smokers had no major impact on the models (CMPF levels β = −2.28, 95% CI: −3.62 to –0.94), nor had the addition of SES and alcohol consumption covariates (CMPF levels β = −2.22, 95% CI: −3.62 to –0.82), as shown in Tables S2 and S4, respectively.
3.4. Periodontal pockets
Higher CMPF levels were significantly associated with lower numbers of periodontal pockets (≥4 mm) when adjusting for age, sex, education, BMI, plasma glucose levels, and smoking (β = −1.07, 95% CI: −1.85 to –0.29). Comparing the quartiles of CMPF levels was not significantly associated with periodontal pockets >4 mm, as shown in Table 3.
Because of the skewed distribution, deep periodontal pockets (≥6 mm) were analysed using logistic regression (0 or 1 deep pocket vs. ≥2 pockets). Each SD increase in the CMPF level was associated with an OR of 0.80 (95% CI: 0.65 to 0.98) for having ≥2 deep periodontal pockets, adjusting for age, sex, fasting blood glucose, education, BMI, and smoking status. Comparing quartiles of CMPF was not significantly associated with deep periodontal pockets, as shown in Table 3b.
Separate analysis of non‐smokers had no major impact on the association between CMPF levels and ≥4 mm pockets (β = −1.08, 95% CI: −1.84 to –0.31). Odds of having ≥2 pockets of ≥6 mm based on higher CMPF levels was weakened in non‐smokers (OR = 0.81, 95% CI: 0.64 to 1.02), as shown in Figure S2A,B.
Adding alcohol consumption and having a job to the covariates had no major impact on the association between CMPF levels and ≥4 mm pockets (β = −1.07, 95% CI: −1.84 to –0.30) (Table S4A). The impact of the additional covariates on OR of having ≥2 pockets of ≥6 mm based on higher CMPF levels was more substantial (OR = 0.86, 95% CI: 0.67 to 1.10), as shown in Table S4B.
3.5. Number of teeth
A 1 SD higher CMPF level was associated with 0.19 (95% CI: 0.08 to 0.29) more teeth. Comparing the fourth to the first quartile of CMPF levels was associated with 0.58 (95% CI: 0.22 to 0.93) more teeth, third to first quartile with 0.40 (95% CI: 0.07 to 0.73), and second to first quartile with 0.33 (95% CI: 0.03 to 0.63) in fully adjusted models (Table 3a).
Separate analysis of non‐smokers had no major impact on the models (CMPF levels β = 0.13, 95% CI: 0.03 to 0.23), nor had the addition of SES and alcohol consumption covariates (CMPF levels β = 0.22, 95% CI: 0.10 to 0.34).
4. DISCUSSION
To the best of our knowledge, this is the first study to report an association between CMPF levels and severe periodontitis and gingival inflammation. This is particularly interesting because CMPF is a validated marker for oil‐rich fish and fish oil consumption (Hanhineva et al., 2015; Pallister et al., 2016; Wang et al., 2018; Shi et al., 2019). Considering all confounding factors (age, sex, education level, plasma glucose levels, BMI, and smoking status), a 1 SD higher plasma CMPF level was associated with a 26% reduction in the odds of severe periodontitis, 2.2% less BoP, one fewer periodontal pocket of ≥4 mm, 0.2 more teeth, and a 20% reduction in the odds of having ≥2 pockets with ≥6 mm. It should, however, be noted that the association between CMPF levels and severe periodontitis as well as ≥6 mm pockets was less robust than the association between CMPF levels and BoP, ≥4 mm pockets, and number of teeth. This was illustrated by the fully adjusted models of CMPF levels and severe periodontitis as well as ≥6 mm pockets losing statistical significance only when analysed in non‐smoking subjects or when adding the covariates of having a job and alcohol consumption in a reduced subset of the cohort.
The clinical relevance of 0.2 teeth is unclear. However, one more periodontal pocket ≥4 mm and a 2.2% reduction in BoP are clinically relevant. The current findings can help to better understand the potential benefits of fish‐rich food on gingival inflammation and periodontitis, laying the foundation for future intervention trials designed to test causality.
High fish consumption is connected to high intake of omega‐3 fatty acids. Omega‐3 fatty acids can have a protective effect against periodontitis and inhibit the progression of the disease (El‐Sharkawy et al., 2010; Iwasaki et al., 2010; Deore et al., 2014), plausibly via the antioxidant effect of omega‐3 fatty acids (Heshmati et al., 2019) and resolvins (Calder, 2015). Neutrophils are considered important contributors to oxidative stress and inflammation in periodontitis. Subjects with periodontitis have higher levels of peroxidases in their saliva (Grant et al., 2018) due to the oxidative stress in the periodontal lesions, which is considered a major contributor to tissue destruction in periodontitis (Chapple & Matthews, 2007). Although no studies have shown increased action of resolvins in association with increased CMPF levels, the omega‐3 fatty acid derivatives resolvins constitute one of the anti‐oxidative pathways. Indeed, resolvin E1 has been shown to stop the progression of periodontitis in animal models (Hasturk et al., 2007). This could constitute a possible mechanism responsible for the periodontal‐protective effect of fish consumption.
The current study has some limitations that need to be discussed. Because of the cross‐sectional nature of this study, we are unable to provide any evidence for a causal relationship between CMPF and improved periodontal health. An alternative study design would be a longitudinal follow‐up comparing development or incidence of periodontitis in subjects with low and high fish consumption and CMPF measurements at baseline. Moreover, the quantification of CMPF levels was based on the relative abundance, making the data less generic than absolute levels. Confounding bias can never be excluded, even though we used regression models adjusting for known confounders. However, there is a risk for residual confounders, as fish consumption may be a part of a healthy lifestyle, which is difficult to recognize as a confounding variable. Although adding several more SES covariates may have improved that aspect, it would also have caused an overfitting of the models. Therefore, information on whether the individual had a job was added in the models presented in Tables S3 and S4 besides education, which is included in the original models. The reason for adding only smoking in Model 3 is to illustrate the substantial impact of smoking in all the regression models. To further elucidate the impact of smoking on the models, all regression models were re‐run on non‐smokers only, with similar results as in Model 2 in the entire population, minimizing the risk of residual confounding (Tables S1 and S2). As several years had passed since the majority of former smokers quit the habit (mean 15.7 years), former smokers were included as non‐smokers. Unfortunately, pack‐years were not available in this dataset, adding a further limitation to the study.
Adding the covariates of having a job and alcohol consumption had only a minor weakening effect on the linear regression models, indicating that the models are robust. The fully adjusted models for CMPF levels and severe periodontitis and CMPF levels and having ≥2 pockets of ≥6 mm were less robust, causing the models to lose statistical significance when adding the covariates and also reducing the size of the cohort.
An additional weakness was that only periodontal pockets of >2 mm were recorded in this study, thus limiting the derivation of certain periodontitis variables, such as the use of mean pocket depth as a dependent variable.
Fasting plasma glucose was included in the analysis rather than HbA1c, as HbA1c was measured only in a subset of the MOS cohort. However, fasting plasma glucose was assessed in all participants.
MOS recruited 47% of the eligible offspring from the Malmö Diet Cancer Study (Figure 1), and only about 30% of the MOS participants were also included in MODS. The most common reason for not participating in MODS was having recently visited the dentist. When comparing the descriptive from MOS (Brunkwall et al., 2021) and MODS, the data are similar. While the relatively low recruitment rate may cause a healthy selection bias, that may have a limited impact on the association between CMPF and gingival inflammation/periodontitis.
FIGURE 1.
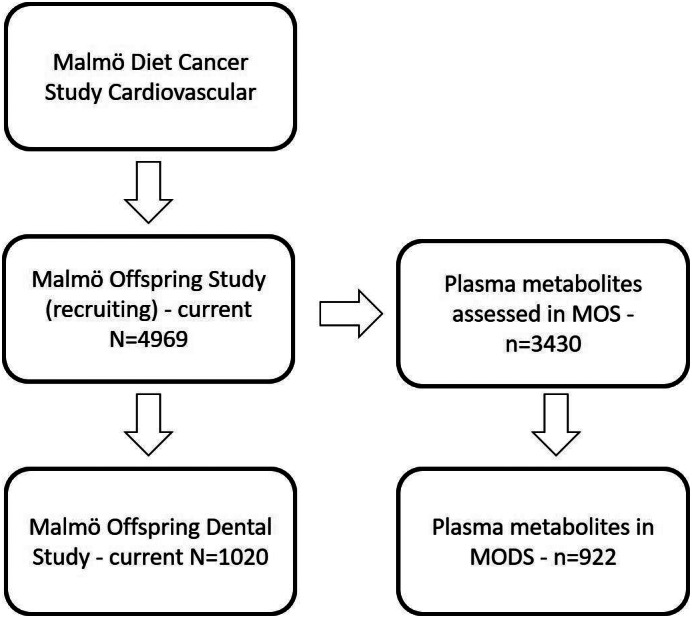
Malmö Offspring Study (MOS) invites the children and grandchildren of the participants from the Malmö Diet Cancer Study. Malmö Offspring Dental Study (MODS) is a sub‐study of MOS. Plasma metabolites were assessed in 3430 MOS participants, and out of these, data were available for 922 on 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionic acid and clinical dental data in MODS
Finally, our finding needs to be confirmed in other cohorts before any general conclusions about fish consumption, CMPF, and periodontal status can be drawn.
5. CONCLUSION
In the MODS cohort, high levels of a validated biomarker for oil‐rich fish intake, namely CMPF, were found to be associated with lower odds of severe periodontitis and having ≥2 pockets of ≥6 mm, as well as with less gingival inflammation, higher number of teeth, and fewer periodontal pockets in regression models adjusting for age, sex, education level, plasma glucose levels, BMI, and smoking status. The models on gingival inflammation, >4 mm pockets, and the number of teeth were more robust than severe periodontitis and having >2 pockets of >6 mm. Although fish consumption may be a part of a healthy lifestyle and is difficult to fully adjust for, the results suggest that fish consumption may constitute an overlooked protective factor for gingival inflammation and periodontitis.
CONFLICT OF INTEREST
The authors report no conflict of interest.
ETHICS STATEMENT
MOS and MODS were both approved by the Swedish Ethical Review Authority (MOS: Dnr. 2012/594, and MODS: Dnr. 2013/761).
AUTHOR CONTRIBUTIONS
Filip Ottosson and Celine Fernandez analysed the metabolites in plasma. Cecilia Kennbäck and Margaretha Persson recruited Malmö Offspring Study (MOS) participants and were responsible for blood sampling and anthropometric measurements. Peter M. Nilsson, Gunnar Engström, Olle Melander, and Marju Orho‐Melander conceived and planned MOS. Olle Melander, Celine Fernandez, and Filip Ottosson conceived and planned metabolite analysis in MOS. Daniel Jönsson, Björn Klinge, and Ryan T. Demmer conceived and planned MODS. Daniel Jönsson and Filip Ottosson analysed CMPF in relation to periodontal disease. Daniel Jönsson, Filip Ottosson, and Lina Hultgren drafted the manuscript. All authors discussed the results and commented on the manuscript.
Supporting information
Data S1. Supporting information.
ACKNOWLEDGEMENTS
Malmö Offspring Dental Study (MODS) was supported by grants from Oral Health Related Research by Region Skåne, the Crafoord Foundation, the Albert Påhlsson Foundation, and the Public Dental Service of Skåne. Malmö Offspring Study (MOS) was supported by the Research Council of Sweden, the Heart and Lung Foundation, and the Region Skåne County Council (ALF) grant. The authors would like to extend their gratitude to the following people working within the MODS study: the dentists, who examined the participants, (Liselott Bennvid, Elisabeth Hansson, Demir Cirgic, and Elisabeth Larsson) and biomedical scientists and nurses (Johanna Karlsson, Anna Hallberg, Christina Neroth, Anders Holm, and Ingegärd Lundgren Svensson). We are also profoundly grateful to the MOS/MODS participants for their participation in this research.
Ottosson, F. , Hultgren, L. , Fernandez, C. , Engström, G. , Orho‐Melander, M. , Kennbäck, C. , Persson, M. , Demmer, R. T. , Melander, O. , Klinge, B. , Nilsson, P. M. , & Jönsson, D. (2022). The inverse association between a fish consumption biomarker and gingival inflammation and periodontitis: A population‐based study. Journal of Clinical Periodontology, 49(4), 353–361. 10.1111/jcpe.13602
Funding information Crafoordska Stiftelsen; Direktör Albert Påhlssons Stiftelse; Hjärt‐Lungfonden; Oral Health Related Research by Region Skåne; Public Dental Service of Skåne; Region Skane County Council (ALF) Grant; Vetenskapsrådet
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Berglund, G. , Elmstahl, S. , Janzon, L. , & Larsson, S. A. (1993). The Malmo Diet and Cancer Study. Design and feasibility. Journal of Internal Medicine, 233(1), 45–51. [DOI] [PubMed] [Google Scholar]
- Brunkwall, L. , Jönsson, D. , Ericson, U. , Hellstrand, S. , Kennback, C. , Ostling, G. , Jujic, A. , Melander, O. , Engström, G. , Nilsson, J. , Ohlsson, B. , Klinge, B. , Orho‐Melander, M. , Persson, M. , & Nilsson, P. M. (2021). The Malmo Offspring Study (MOS): Design, methods and first results. European Journal of Epidemiology, 36(1), 103–116. 10.1007/s10654-020-00695-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder, P. C. (2015). Marine omega‐3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et Biophysica Acta, 1851(4), 469–484. 10.1016/j.bbalip.2014.08.010 [DOI] [PubMed] [Google Scholar]
- Chapple, I. L. , & Matthews, J. B. (2007). The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology, 2000(43), 160–232. 10.1111/j.1600-0757.2006.00178.x [DOI] [PubMed] [Google Scholar]
- Deore, G. D. , Gurav, A. N. , Patil, R. , Shete, A. R. , Naiktari, R. S. , & Inamdar, S. P. (2014). Omega 3 fatty acids as a host modulator in chronic periodontitis patients: A randomised, double‐blind, palcebo‐controlled, clinical trial. Journal of Periodontal and Implant Science, 44(1), 25–32. 10.5051/jpis.2014.44.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, T. , Joshipura, K. J. , Dawson‐Hughes, B. , & Bischoff‐Ferrari, H. A. (2004). Association between serum concentrations of 25‐hydroxyvitamin D3 and periodontal disease in the US population. American Journal of Clinical Nutrition, 80(1), 108–113. [DOI] [PubMed] [Google Scholar]
- Dietrich, T. , Nunn, M. , Dawson‐Hughes, B. , & Bischoff‐Ferrari, H. A. (2005). Association between serum concentrations of 25‐hydroxyvitamin D and gingival inflammation. American Journal of Clinical Nutrition, 82(3), 575–580. [DOI] [PubMed] [Google Scholar]
- Dommisch, H. , Kuzmanova, D. , Jönsson, D. , Grant, M. , & Chapple, I. (2018). Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontology 2000, 78(1), 129–153. 10.1111/prd.12233 [DOI] [PubMed] [Google Scholar]
- Eke, P. I. , Dye, B. A. , Wei, L. , Thornton‐Evans, G. O. , Genco, R. J. , & CDC Periodontal Disease Surveillance Workgroup: Beck, J., Douglass, G., Page, R . (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of Dental Research, 91(10), 914–920. 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- El‐Sharkawy, H. , Aboelsaad, N. , Eliwa, M. , Darweesh, M. , Alshahat, M. , Kantarci, A. , Hasturk, H. , & Van Dyke, T. E. (2010). Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega‐3 fatty acids and low‐dose aspirin. Journal of Periodontology, 81(11), 1635–1643. 10.1902/jop.2010.090628 [DOI] [PubMed] [Google Scholar]
- Genco, R. J. , & Borgnakke, W. S. (2013). Risk factors for periodontal disease. Periodontology 2000, 62(1), 59–94. 10.1111/j.1600-0757.2012.00457.x [DOI] [PubMed] [Google Scholar]
- Grant, M. , Kilsgård, O. , Åkerman, S. , Klinge, B. , Demmer, R. T. , Malmstrom, J. , & Jönsson, D. (2018). The human salivary antimicrobial peptide profile according to the oral microbiota in health, periodontitis and smoking. Journal of Innate Immunity, 1‐12, 432–444. 10.1159/000494146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanhineva, K. , Lankinen, M. A. , Pedret, A. , Schwab, U. , Kolehmainen, M. , Paananen, J. , de Mello, V. , Sola, R. , Lehtonen, M. , Poutanen, K. , Uusitupa, M. , & Mykkanen, H. (2015). Nontargeted metabolite profiling discriminates diet‐specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. Journal of Nutrition, 145(1), 7–17. 10.3945/jn.114.196840 [DOI] [PubMed] [Google Scholar]
- Hasturk, H. , Kantarci, A. , Goguet‐Surmenian, E. , Blackwood, A. , Andry, C. , Serhan, C. N. , & Van Dyke, T. E. (2007). Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. Journal of Immunology, 179(10), 7021–7029. 10.4049/jimmunol.179.10.7021 [DOI] [PubMed] [Google Scholar]
- Heshmati, J. , Morvaridzadeh, M. , Maroufizadeh, S. , Akbari, A. , Yavari, M. , Amirinejad, A. , Maleki‐Hajiagha, A. , & Sepidarkish, M. (2019). Omega‐3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta‐analysis of clinical trials. Pharmacological Research, 149, 104462. 10.1016/j.phrs.2019.104462 [DOI] [PubMed] [Google Scholar]
- Iwasaki, M. , Yoshihara, A. , Moynihan, P. , Watanabe, R. , Taylor, G. W. , & Miyazaki, H. (2010). Longitudinal relationship between dietary omega‐3 fatty acids and periodontal disease. Nutrition, 26(11–12), 1105–1109. 10.1016/j.nut.2009.09.010 [DOI] [PubMed] [Google Scholar]
- Jönsson, D. , Aggarwal, P. , Nilsson, B. O. , & Demmer, R. T. (2013). Beneficial effects of hormone replacement therapy on periodontitis are vitamin D associated. Journal of Periodontology, 84(8), 1048–1057. 10.1902/jop.2012.120434 [DOI] [PubMed] [Google Scholar]
- Kinane, D. F. , Stathopoulou, P. G. , & Papapanou, P. N. (2017). Periodontal diseases. Nature Reviews. Disease Primers, 3, 17038. 10.1038/nrdp.2017.38 [DOI] [PubMed] [Google Scholar]
- Ottosson, F. , Brunkwall, L. , Ericson, U. , Nilsson, P. M. , Almgren, P. , Fernandez, C. , Melander, O. , & Orho‐Melander, M. (2018). Connection between BMI related plasma metabolite profile and gut microbiota. Journal of Clinical Endocrinology and Metabolism, 103, 1491–1501. 10.1210/jc.2017-02114 [DOI] [PubMed] [Google Scholar]
- Page, R. C. , & Eke, P. I. (2007). Case definitions for use in population‐based surveillance of periodontitis. Journal of Periodontology, 78(7 Suppl), 1387–1399. 10.1902/jop.2007.060264 [DOI] [PubMed] [Google Scholar]
- Pallister, T. , Jennings, A. , Mohney, R. P. , Yarand, D. , Mangino, M. , Cassidy, A. , MacGregor, A. , Spector, T. D. , & Menni, C. (2016). Characterizing blood metabolomics profiles associated with self‐reported food intakes in female twins. PLoS One, 11(6), e0158568. 10.1371/journal.pone.0158568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L. , Brunius, C. , Bergdahl, I. A. , Johansson, I. , Rolandsson, O. , Donat Vargas, C. , Kiviranta, H. , Hanhineva, K. , Åkesson, A. , & Landberg, R. (2019). Joint analysis of metabolite markers of fish intake and persistent organic pollutants in relation to type 2 diabetes risk in Swedish adults. Journal of Nutrition, 149(8), 1413–1423. 10.1093/jn/nxz068 [DOI] [PubMed] [Google Scholar]
- Sumner, L. W. , Amberg, A. , Barrett, D. , Beale, M. H. , Beger, R. , Daykin, C. A. , Fan, T. W.‐M. , Fiehn, O. , Goodacre, R. , Griffin, J. L. , Hankemeier, T. , Hardy, N. , Harnly, J. , Higashi, R. , Kopka, J. , Lane, A. N. , Lindon, J. C. , Marriott, P. , Nicholls, A. W. , … Viant, M. R. (2007). Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics, 3(3), 211–221. 10.1007/s11306-007-0082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Gapstur, S. M. , Carter, B. D. , Hartman, T. J. , Stevens, V. L. , Gaudet, M. M. , & McCullough, M. L. (2018). Untargeted metabolomics identifies novel potential biomarkers of habitual food intake in a cross‐sectional study of postmenopausal women. Journal of Nutrition, 148(6), 932–943. 10.1093/jn/nxy027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


