Abstract
Skin conditions associated with Tenacibaculum spp. constitute a significant threat to the health and welfare of sea‐farmed Atlantic salmon (Salmo salar L.) in Norway. Fifteen presumptive tenacibaculosis outbreaks distributed along the Norwegian coast during the late winter and spring of 2018 were investigated. Bacteriological culture confirmed the presence of Tenacibaculum spp. Seventy‐six isolates cultured from individual fish were selected and subjected to whole‐genome sequencing and MALDI‐TOF MS analysis. Average nucleotide identity and MALDI‐TOF analyses confirmed the presence of T. finnmarkense and T. dicentrarchi, with further division of T. finnmarkense into genomovars (gv.) finnmarkense and ulcerans. Core genome multilocus sequence typing (cgMLST) and single‐nucleotide polymorphism (SNP) analyses identified the presence of a genetically conserved cluster of gv. finnmarkense isolates against a background of relatively genetically diverse gv. finnmarkense and gv. ulcerans isolates in 13 of the 15 studied cases. This clustering strongly suggests a link between T. finnmarkense gv. finnmarkense and development of clinical tenacibaculosis in sea‐farmed Norwegian salmon in the late winter and spring. Analysis of 25 Tenacibaculum isolates collected during the spring of 2019 from similar cases identified a similar distribution of genotypes. Low water temperatures were common to all cases, and most incidences involved relatively small fish shortly after sea transfer, suggesting that these fish are particularly predisposed to Tenacibaculum infection.
Keywords: disease, Salmo salar, salmon, skin pathology, tenacibaculosis, Tenacibaculum
1. INTRODUCTION
Ulcerous skin conditions are among the most important causes of economic loss and poor welfare in sea‐farmed Atlantic salmon (Salmo salar) in Norway (Takle et al., 2015). Such cases appear to be more common from late autumn until spring when water temperatures are low, and are a greater problem in northern latitudes (Sommerset et al., 2020; Takle et al., 2015). While bacteriological investigation of lesions and internal organs may result in culture of a variety of different bacterial species, Moritella viscosa, Aliivibrio wodanis and various Tenacibaculum spp. have been most commonly associated with these conditions in Norwegian salmon farming (Grove et al. 2010; Lunder et al., 1995; Olsen et al., 2011; Olsen et al., 2020). The aetiological role of M. viscosa in the development of ‘winter ulcer’ is generally accepted (Lunder et al., 1995) and all Atlantic salmon transferred to sea in Norway are now vaccinated against this bacterium (Karlsen et al., 2017). Despite being regularly isolated from salmon displaying winter ulcer, Aliivibrio wodanis has not been related to primary pathogenesis of this condition (Karlsen et al., 2014). A number of different psychrophilic Tenacibaculum taxa have been identified from Atlantic salmon displaying skin lesions including both genomovars of T. finnmarkense (Bridel et al., 2018; Olsen et al., 2020; Småge, Brevik, et al., 2016), T. dicentrarchi (Avandeño‐Herrera et al., 2016; Klakegg et al., 2019; Olsen et al., 2017) and the recently described T. piscium (Olsen et al., 2020). All three species appear to have a wide geographical distribution and all have been associated with skin lesions in farmed Atlantic salmon in both Norway and Chile. T. maritimum, although present in Norwegian waters (Småge et al., 2016), has not been associated with skin disease in Atlantic salmon in Norway (Olsen et al., 2017; Norwegian Veterinary Institute statistics), despite its established aetiological role in ‘mouth rot’ (bacterial stomatitis) in Atlantic salmon farmed in British Columbia (Frisch et al., 2018).
Although there is no absolute demarcation, case histories of outbreaks of skin disease involving Tenacibaculum spp. in farmed salmon diagnosed by the Norwegian Veterinary Institute, indicate that many late winter/spring outbreaks differ in clinical presentation to most of those experienced in late autumn. While flank lesions are observed during both seasons, lesions involving the head appear more common in late winter and spring. The latter manifestation in particular may be associated with acute high mortality.
Given the heavy losses associated with tenacibaculosis in Norwegian salmon farming (Småge et al., 2017), the documented diversity of involved Tenacibaculum taxa and the possible presence of two distinct clinical manifestations, we decided to investigate a number of geographically disparate outbreaks (>2000 km) during the late winter and spring of 2018. To verify the findings based on the 2018 dataset, an additional 25 isolates sampled in 2019 were sequenced and analysed.
The aim of the study was to characterize the disease and the Tenacibaculum spp. involved.
2. MATERIALS AND METHODS
2.1. Outbreak identification
A number of fish health practitioners in Norway submitted samples from disease outbreaks in sea‐farmed Atlantic salmon based on gross findings (primarily head injury and mortality) consistent with tenacibaculosis.
2.2. Production data
Production data (site level data) recorded on a monthly basis for the 15 affected sites was retrieved from a statutory, central database (https://havbruksdata.no; not publicly available). Data retrieved included water temperature, total number of fish held on site, total number of dead fish and average fish weight (g). Site‐level mortality was estimated by dividing the total number of dead fish by the total number of fish held on the site for the month of sampling.
2.3. Bacteriology
Fish health practitioners were encouraged to submit the following materials from suspected tenacibaculosis outbreaks: (1) Bacterial swabs from representative lesions (one head and one flank lesion, if both present) and head‐kidney of five affected fish, plated on blood agar with 2% NaCl (BAS) and marine agar (MA, Difco 2216), sent chilled overnight to the laboratory. (2) Bacterial swabs from the skin of five clinically healthy fish, sampled from an unaffected neighbouring cage. Bacteriological skin swabs were also collected from fish in five control sites where the fish did not display observable skin lesions.
Upon arrival in the laboratory, primary cultures were evaluated and colonies enumerated (sparse, moderate or rich growth) following 2, 5 and 7 days incubation at 15°C. Round to ovoid, yellow‐pigmented colonies consisting of filamentous, Gram‐negative, non‐motile rods (by phase contrast microscopy), morphologically consistent with Tenacibaculum spp., were sub‐cultured on marine agar. Colonies consistent with other recognized fish‐pathogenic bacteria were also recovered, registered and archived. Bacterial isolates were archived at −80°C.
2.4. Histopathology
To establish the general health of the studied fish and to exclude other significant infections, tissue samples from pseudobranch, gill, heart, kidney, pyloric caeca and pancreas, liver, spleen, and affected and non‐affected skin musculature, from the same five fish investigated bacteriologically from all farms, were fixed in 10% phosphate‐buffered formalin (pH 7.2–7.4). The tissue samples were then processed and embedded separately in paraffin, and sections (3 µm) stained with haematoxylin and eosin (H&E).
2.5. Immunohistochemistry
Selected ulcer sections were tested for the presence of T. finnmarkense, M. viscosa and A. wodanis by immunohistochemical methods, using polyclonal rabbit antisera raised against clinical isolates. In short, an enzyme‐linked streptavidin procedure with fast red as the chromogen was used. After blocking with bovine serum albumin, the sections were incubated with diluted primary antiserum, followed by incubation with biotin‐labelled secondary goat anti‐rabbit antibody and streptavidin alkaline phosphatase complex. Substrate‐chromogen solution was applied for desired colour development and haematoxylin was used for counterstaining.
2.6. Matrix‐assisted laser desorption ionization time of flight mass spectrometry (MALDI‐TOF MS) analysis
MALDI‐TOF MS analysis was performed on a Biotyper Microflex LT (Bruker Daltonics). As the Bruker reference library does not include reference spectra for psychrophilic Tenacibaculum spp., in‐house main spectral profiles (MSPs) were generated for T. finnmarkense gv. finnmarkense (CCUG 73831T), T. finnmarkense gv. ulcerans (CCUG 73832T), T. piscium (CCUG 7383T), T. maritimum (NCIMB 2154T) and T. dicentrarchi (NCIMB 14598T).
For in‐house MSP generation, proteins from each reference strain were extracted according to the manufacturer's protocol and processed using various software and reagents specified below (all Bruker Daltonics). In short, 1 µl of bacterial cells was suspended in 300 µl SDW and 900 µl 96% ethanol. After centrifugation for 2 min at 11,357 g (Eppendorf Minispin®) and removal of the supernatant, proteins were extracted from the bacterial pellet with formic acid and acetonitrile (ACN). Eight individual 1‐µl aliquots of each ACN extraction were then spotted onto a polished steel MALDI target plate, dried at room temperature, overlaid with 1 µl HCCA matrix solution (saturated α‐cyano‐4‐hydroxycinnamic acid in 50% ACN 2.5% trifluoroacetic acid), and allowed to air dry. Three spectra were generated for each spot (N = 24) using the MBT‐AutoX method in FlexControl 3.4 software. Peak lists were created using the MBT‐Standard FAMS Method followed by baseline subtraction and smoothing in FlexAnalysis software. Each spectrum was quality controlled for flatline spectrum, outlier peaks and peak shifts >500 ppm. Spectra passing the quality check were included in the strain‐specific MSP generated in MBT Compass Explorer software. MSPs were constructed based on 18–23 individual spectra per isolate. Prior to, and concurrently during each MSP creation, the instrument was calibrated with E. coli bacterial test standard (BTS, Bruker Daltonics).
Following incorporation of constructed in‐house MSPs into the reference library, single colonies of putative Tenacibaculum spp. were subjected to MALDI‐TOF analysis by the Direct Transfer Method according to the manufacturer's instructions. Single colonies were smeared with a toothpick as a thin film onto two successive spots on a MALDI‐TOF target plate. After drying at room temperature, the spots were overlaid with HCCA matrix and air‐dried. Identification was then performed using the standard Biotyper Database supplemented with the five new Tenacibaculum MSPs. Similarity scores >2.0 in relation to any particular MSP were considered reliable for identification at the species/gv. level.
2.7. Whole‐genome sequencing and analysis
Three to seven Tenacibaculum isolates from each of the 15 affected farms were selected for whole‐genome sequencing, resulting in 76 assemblies. Stock cultures (−80°C) were plated onto marine agar and incubated at 15°C for 48 h. Cells were harvested and DNA extracted on a QIAcube (Qiagen) with a QIAamp DNA Mini QIAcube kit (Qiagen). After quality checks by UV spectroscopy, the samples were submitted to the Norwegian Sequencing Centre (www.sequencing.uio.no) for library preparation with the Nextera DNA Flex kit (Illumina) and sequenced on a MiSeq (Illumina) platform providing 300 bp paired‐end reads. Read quality was assessed with fastQC v0.11.7. Adaptor removal and trimming was performed with Trimmomatic v. 0.39 (Bolger et al., 2014) with adaptor sequences provided by Illumina and using the MAXINFO:300:0.995 option and a minimum read length of 36 bp. PhiX174 was removed with BBduk from the BBmap (38.08) package using NC001422. Unpaired reads were removed prior to assembly with SPAdes v 3.12.0 (Bankevich et al., 2012) using the careful option. The reads were mapped back on the assemblies with BWA (0.7.12‐r1039), before the assemblies were polished with Pilon v 1.23 (Walker et al., 2014). The assemblies were submitted to NCBI (https://www.ncbi.nlm.nih.gov/genbank/, see Table S2 for details).
Average nucleotide identity (ANI) values between the polished assemblies and references were calculated using fastANI v 1.1 (Jain et al., 2018). A cgMLST scheme consisting of 1802 genes was generated with chewBBACA v 2.0.16 (Silva et al., 2018). cgMLST cladograms were generated by hierarchal UPGMA clustering in R using ape v 5.3 and subsequently plotted with ggtree v 1.17 (Yu et al., 2017) in combination with ggplot2 v 3.2 (Wickham et al., 2016). A multiple sequence alignment was generated with Snippy v 4.6.0 using GCF_900239185.1 (T. finnmarkense gv. finnmarkense TNO006T) as reference. Maximum likelihood (ML) trees based on single‐nucleotide polymorphisms (SNPs), with and without removal of putative recombination events (Gubbins v 2.3.4), were generated for T. finnmarkense gv. finnmarkense in Iqtree v 2.0.3 using a generalized time reversible (GTR) model with ascertainment bias correction and empirical base frequencies. Bootstrapping was performed with 1000 replicates of the ultrafast bootstrap option. The tree was subsequently plotted in R v 3.5.0. with ggtree v 1.17 (Yu et al., 2017) in combination with ggplot2 v 3.2 (Wickham et al., 2016). To study whether the genetic diversity observed during 2018 was representative of the epidemiological situation the following year, 25 Tenacibaculum isolates recovered from 8 clinical cases during the spring of 2019 were sequenced (see Table S2 for details). Four cases involved larger fish between 1 and 4 kg in weight.
3. RESULTS
During 2018, samples were submitted from 15 suspected outbreaks of tenacibaculosis covering an area in excess of 2000 km of the Norwegian coastline (Figure 1).
FIGURE 1.
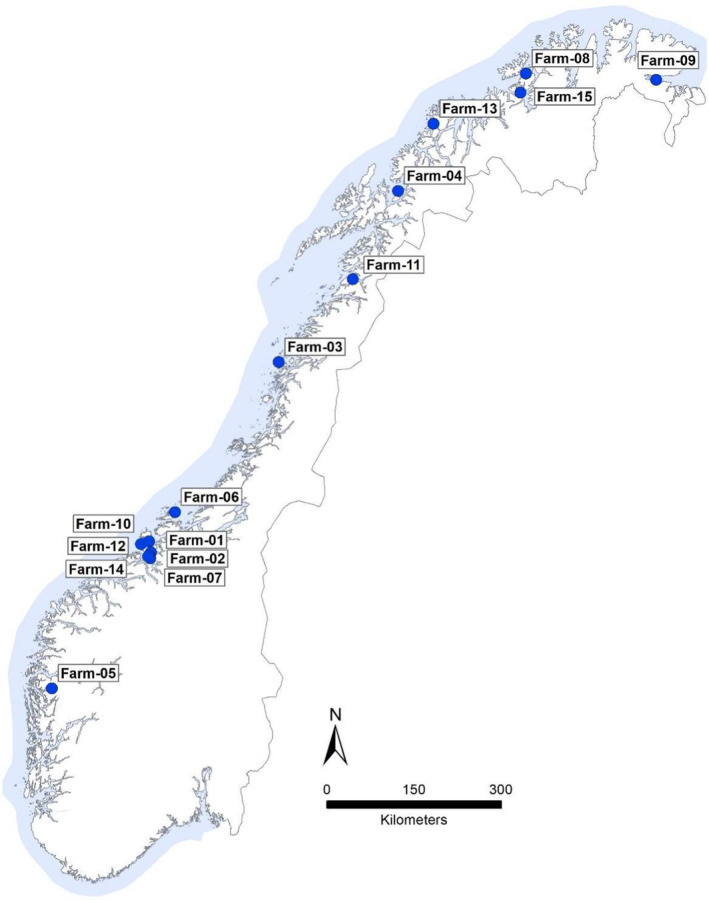
Location of sampled fish (n = 15) farms plotted on a map of Norway. Samples were received from farms with suspected outbreaks of tenacibaculosis along most of the Norwegian coast
3.1. Macropathology
Of the 78 investigated fish, head lesions alone were observed in 44 fish, 26 displayed visible lesions involving both head and flank and flank lesions alone were observed in 8 fish.
3.2. Culture
Isolates confirmed as members of genus Tenacibaculum dominated the bacteriological findings in all 15 cases (Figure 2 and Table S1). While Tenacibaculum spp. dominated cultures from external lesions, in most cases other bacterial species were also identified. Moritella viscosa and Aliivibrio wodanis were identified in 3 and 11 outbreaks, respectively, but in relatively few fish. Kidney‐based culture resulted in the growth of Tenacibaculum spp. from only three fish (in a single site).
FIGURE 2.
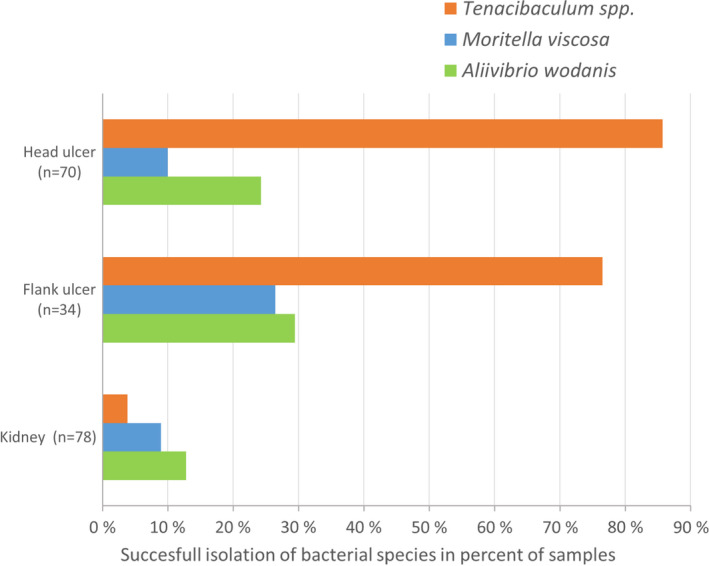
Tenacibaculum spp., Moritella viscosa and Aliivibrio wodanis cultivated from samples of head ulcers, flank ulcers and kidney of the 78 investigated fish. Values are calculated as percent of successful isolation of bacterial species relative to number of samples
Fish with macroscopically normal skin status were bacteriologically sampled from neighbouring cages in eight of the affected farms. Tenacibaculum spp. were identified in sparse to moderate amounts in four of these cages, while M. viscosa and A. wodanis were found in three and five of these cages, respectively (Table S1). Tenacibaculum spp. were not recovered from skin swabs taken from fish in unaffected control sites.
3.3. Species determination
Each genome was assembled as described in materials and methods (see Table S2 for details). Species identification was achieved by ANI analysis, utilizing the speciation threshold (95%–96% ANI) proposed by Richter and Rossello‐Mora (2009). MALDI‐TOF analysis separated all of the studied isolates to species and genomovar level in full agreement with ANI analysis. For all included taxa, intra‐species and intra‐genomovar ANI values were ≥98.0% and MALDI‐TOF scores >2.0 (Table S3). The majority of the isolates (97%) were identified as T. finnmarkense, with the two remaining isolates identified as T. dicentrarchi. The T. finnmarkense isolates were then subdivided utilizing an ANI similarity threshold of 97.5%, into two lineages consistent with genomovars finnmarkense and ulcerans, respectively, as recently described by Olsen et al. (2020). T. finnmarkense gv. finnmarkense was found in 13 of the 15 outbreaks, while gv. ulcerans was identified in 7.
3.4. Core genome MLST
A core genome of 1802 genes within a pangenome of 5112 genes was identified from the 76 T. finnmarkense gv. finnmarkense, T. finnmarkense gv. ulcerans and T. dicentrarchi genome assemblies generated in the present study and reference sequences. Overall, UPGMA clustering (Figure 3) of cgMLST data resulted in a tree topology consistent with ANI and MALDI‐TOF MS speciation, with each bacterial species and genomovar forming a distinct lineage. Clustering patterns and branch lengths show considerable genetic diversity within T. finnmarkense gv. ulcerans, with isolates appearing as distinct singletons or in small clusters. While several smaller closely related clusters also occur within T. finnmarkense gv. finnmarkense, the majority (39/57) of these isolates belong to a single, low‐diversity cluster. The allelic distances within this genomovar were on average 71.7 with a maximum of 181 in the low diversity cluster, while the remaining assemblies in the study displayed an average distance of 1592.
FIGURE 3.
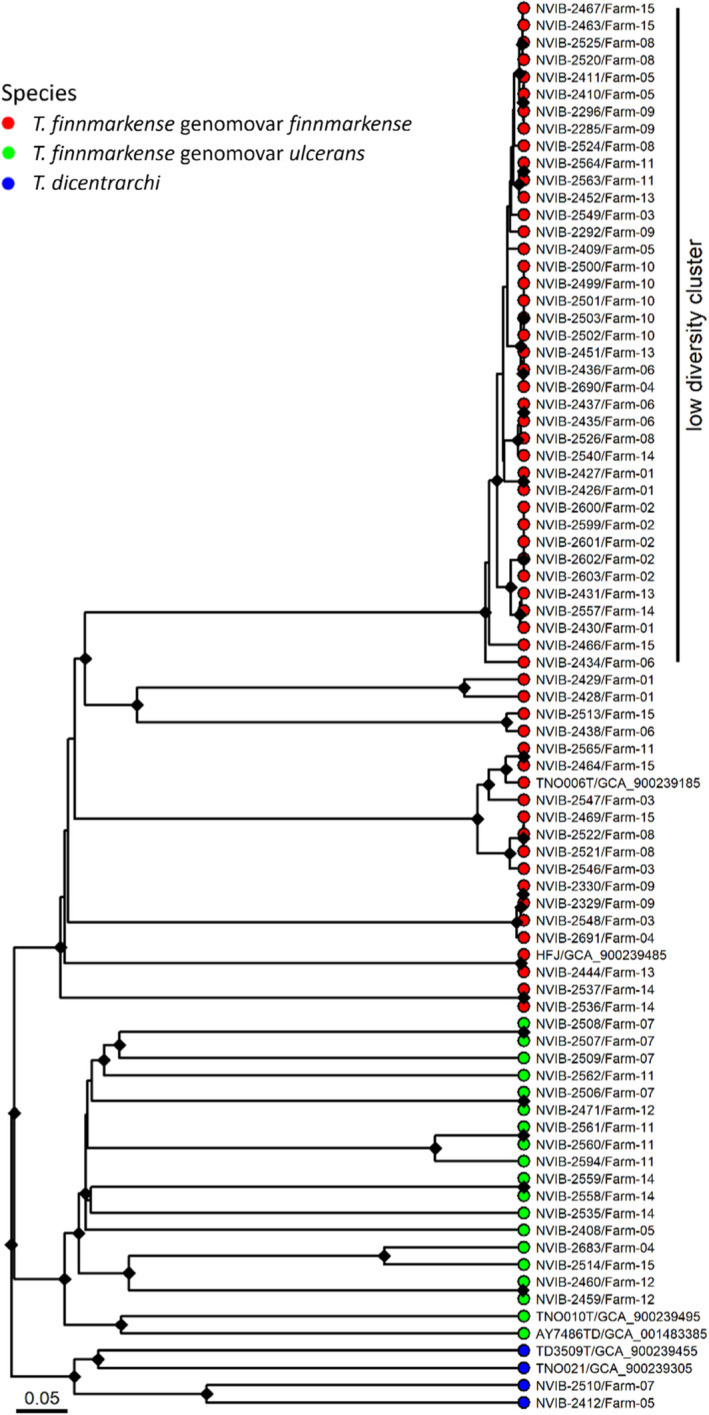
Relationships between the sequenced strains and NCBI reference strains analysed by core genome MLST. The low‐diversity cluster is labelled. The different species in the dendrograms are visualized with different tip point colours; T. finnmarkense genomovar finnmarkense in red, T. finnmarkense genomovar ulcerans in green and T. dicentrarchi in blue. The references are labelled with strain designations and accession numbers. The cgMLST dendrogram is based on hierarchical clustering of alleles of 1802 core genes for each strain using chewBBACA. Bootstrapping was performed with 1000 replicates and nodes with bootstrap support above 95% are labelled with a black diamond. The bar describes normalized UPGMA distance (0.05 roughly equals 90 alleles difference)
Members of this dominant gv. finnmarkense cluster (Figure 3) were recovered from 13 of the 15 farms investigated (Figure 1) and were isolated over a large geographical area including the southernmost (farm‐05) and northernmost (farm‐09) farms studied, that is, spanning a distance of more than 2000 km. Although these closely related isolates were identified in the majority of farms investigated, a range of other Tenacibaculum variants were recovered concurrently from most farms.
Of 25 assemblies generated from isolates collected in 2019, 21 were identified as T. finnmarkense gv. finnmarkense, 2 as T. finnmarkense gv. ulcerans and 2 as T. piscium. A cgMLST analysis revealed the same pattern as for the 2018 isolates, with 18 out of the 25 isolates clustering in the low diversity cluster (Figure S1).
For detailed study of the phylogeny within T. finnmarkense gv. finnmarkense, and the low diversity cluster in particular, a SNP‐based analysis limited to members of this genomovar isolated in 2018 and 2019, was generated. The underlying multiple sequence alignment constituted 2,923,232 base pairs and contained 46,610 polymorphic sites. The average alignment fraction was 89.2% of each genome. A SNP tree in which putative recombination events are removed is shown in Figure 4 and a tree where these are retained is shown in Figure S2. The two trees show identical clustering, consistent also with that demonstrated by cgMLST.
FIGURE 4.
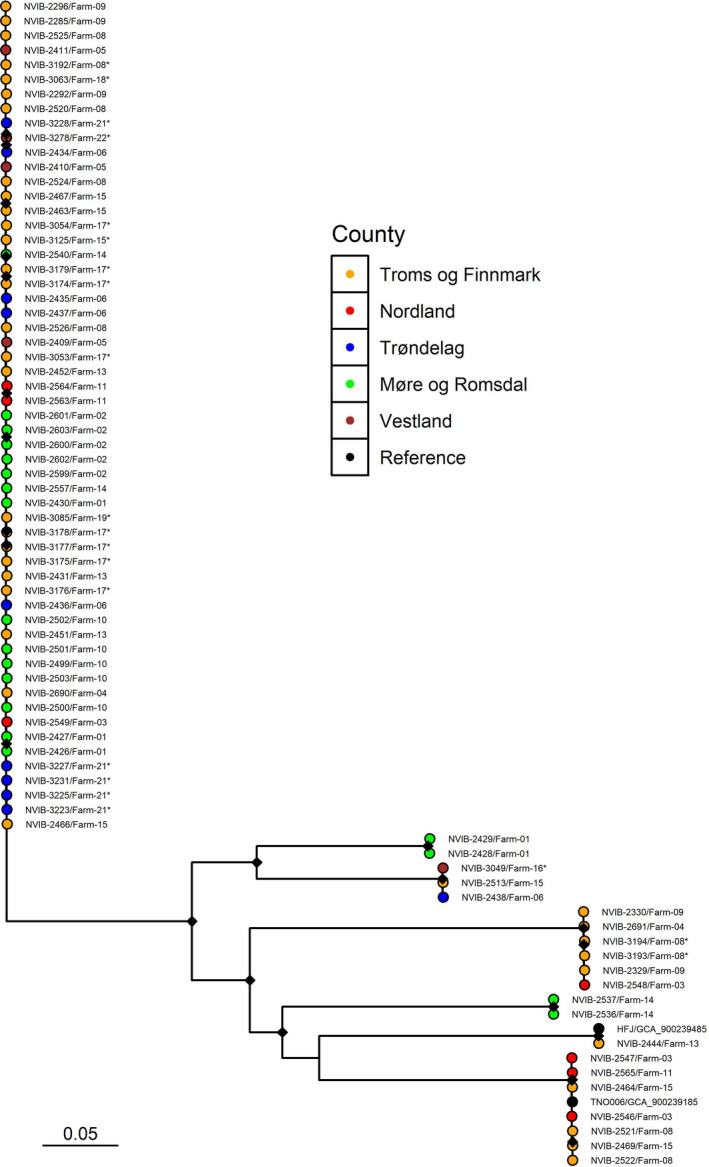
Maximum likelihood SNP tree showing the relationship between the T. finnmarkense genomovar finnmarkense strains isolated in 2018 and 2019 and references. The tree is based on a multiple sequence alignment covering 82.0% of the reference genome (Strain: TNO006T, GCF_900239185.1) and was generated using a generalized time reversible (GTR) model and ascertainment bias correction (ASC) with putative recombination events removed. The tip point colours denote the county of origin as specified in the legend. Strains isolated in 2019 are labelled with an asterisk. Bootstrapping was performed with 1000 replicates and nodes with bootstrap support above 95% are labelled with a black diamond
Before recombination removal, 1618 pairwise SNPs and/or indels were on average identified within the low‐diversity cluster versus 23,101 among the remaining gv. finnmarkense isolates (Figure 4). Following exclusion of putative recombination events, these averages were reduced to 34 SNPs/indels within the low‐diversity cluster versus 9354 among the remaining isolates.
The two T. dicentrarchi isolates from two different farms do not appear to be epidemiologically closely related (Figure 3).
3.5. Gross and histopathological studies
Seventy‐eight fish with skin lesions were histopathologically investigated. Gill, pseudobranch, internal organs, intact skin and skeletal muscle were sampled from all fish. The studied material included 70 tissue sections from head lesions and 34 from flank lesions.
Affected fish typically displayed tissue loss and exposure of jaw bones and cartilage in head and snout lesions (Figure 5). In some individuals, uni‐ and bilateral ocular lesions were observed, some involving rupture of the cornea. In the most severe cases, large areas of the head were eroded and the cranium was visible. Some fish displayed abdominal lesions, typically with a non‐scaled border around exposed muscle tissue. Frayed caudal fins were also observed.
FIGURE 5.

Atlantic salmon with ulcerative lesions around the mouth and exposed upper and lower jaw bones
The histopathological findings were consistent with a previous description of Tenacibaculum infection in Atlantic salmon (Olsen et al., 2011). Characteristic features in both abdominal and cranial lesions included loss of epidermis and the presence of varying numbers of filamentous bacterial rods in necrotic dermis. In affected areas, large quantities of bacteria becoming gradually more dispersed were observed in underlying connective tissues. When present, bacteria were also observed in the endomysium as well as within degenerated muscle cells (Figure 6a, b). In cranial lesions, complete loss of skin tissues was common and dense layers of long bacterial rods, intimately associated with necrotic cartilage, were observed (Figure 6c, d). Also, connective tissues covering bones and present in the interface between bone and cartilage commonly contained numerous filamentous bacterial rods (Figure 6e, f). Typically, inflammatory cells were sparse or absent in tissues invaded by the filamentous bacteria, although leucocytes and bleedings could be observed to some extent in adjacent tissues. A more distinct inflammatory response, involving shorter bacterial cells alongside long, filamentous rods, was occasionally observed. Older lesions in the process of healing, comprising granulation tissues with fibrinous deposition in the presence of inflammatory cells, were noted in a few cases. Only minor and varied histopathological findings were identified in the gills, pseudobranch and internal organs of some of the sampled fish, and none could be consistently related to the obvious gross skin pathology observed.
FIGURE 6.
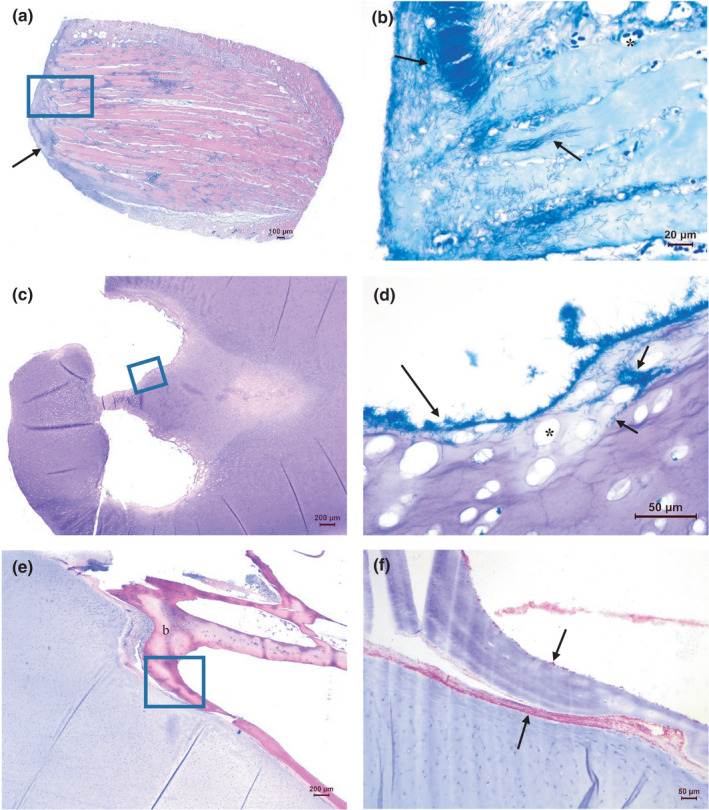
Histopathology of ulcers on the head. (a, b) Skin and muscle tissues with filamentous rods, especially numerous towards not only on the surface but also present in endomysium between muscle cells, as well as within myocytes (arrows). Lack of inflammatory cells is evident. Dilated vessels as a sign of hyperaemia are seen (asterisk). (a: H&E, b: May‐Grünwald‐Giemsa (MGG) of a new section covering approximately the boxed area in a). (c, d) Mandibular cartilage with long filamentous rods present on irregular eroded surfaces of luminal structures (long arrow). Bacteria are also present within the cartilage (short arrows). The cartilage matrix is degenerated with enlarged lacunae devoid of chondrocytes (asterisk). (c: H&E, d: MGG of an area approximately covering the boxed area in c). (e, f) Numerous filamentous rods present in connective tissue in the interface between the mandibular cartilage and adjacent bone. Bacteria are also seen on the surface of the bone (b), probably associated with the fibrous layer covering the bone (arrows). (e: H&E, f: IHC of an area approximately covering the boxed area in e)
3.6. Immunohistochemistry
Immunohistochemical investigation using antiserum raised against T. finnmarkense resulted in positive marking in all ulcers tested. A positive reaction with antiserum raised against M. viscosa was seen in a few foci in 4/43 samples (3/11 farms). No reaction was detected when the samples were tested with antiserum raised against A. wodanis (results not shown).
3.7. Production related epidemiology
Of the 15 farms from which Tenacibaculum was isolated and sequenced in 2018, 13 involved relatively small fish (average weight 142–369 g), 1–4.7 months after sea transfer, while two cases involved larger fish, respectively, 7.2 (~800 g) and 8.5 (~1800 g) months after sea transfer. No cases were submitted to the project from the southernmost salmon farming regions Rogaland and Vest‐Agder.
The timelines from the first month of sea cage production to the time when samples were taken from each site, as well as the seawater temperature at the time of sampling, are presented in Figure S3. There was a significant degree of variation in monthly site‐level mortality among affected sites, with individual farms losing between 0.44% and 33.89% of stocks (median 2.43%, mean 8.24%) in the month of sampling (data not shown). Tenacibaculum spp. were the dominating agent present in all cases, confirmed by culture and histopathological investigation. As other bacteria considered to contribute to similar pathologies were identified (in addition to Tenacibaculum) in only a minority of cases (Table S1), and histopathological investigation did not indicate involvement of other diseases or infectious agents, it was considered reasonable to assume that tenacibaculosis was responsible for a significant proportion of the registered mortality.
4. DISCUSSION
Tenacibaculum species have for many years been associated with fish disease and mortality in Norwegian aquaculture (Olsen et al., 2011, 2017). However, due to the diversity among isolates recovered and apparent lack of host specificity (Olsen et al., 2017, 2020), their mode of transmission and precise role in specific pathologies have remained relatively poorly understood. The present study seeks to explain the aetiology behind ulcerative skin disease of the jaw and head in sea‐farmed Atlantic salmon observed during the late winter and spring.
Farms were sampled by fish health practitioners on suspicion of tenacibaculosis. While most cases submitted to this study presented similar clinical histories, that is, relatively small fish, recently transferred to sea at (relatively) low water temperatures, some cases, particularly those sampled during 2019, involved larger fish. Histopathological study of fish displaying cranial lesions revealed a very similar pathological picture in all cases, suggesting a common aetiology. Numerous bacterial cells, morphologically consistent with Tenacibaculum were associated with the pathological findings, predominantly necrosis, and in some cases inflammation and haemorrhage. Tenacibaculum species pathogenic for fish are recognized to have an affinity for collagen‐containing tissues (Olsen et al., 2011). The presence of Tenacibaculum cells in the collagen‐rich connective tissues of the head and jaws in the present study is, therefore, consistent with invasion of these types of tissue as important in pathogenesis of tenacibaculosis. The low numbers of Tenacibaculum cultured from intact skin versus open lesions may further indicate the need for an initial break in the mucus/epidermal barrier to allow colonization and multiplication within these collagen‐rich tissues. The actual mode of invasion into the underlying tissues needs further research.
Isolation of Tenacibaculum spp. as the dominating bacterial flora in all 15 cases studied in 2018 provided further support for consideration of Tenacibaculum as the main aetiological agent(s) underlying the observed lesions. Although a mixed bacterial flora including Moritella viscosa and Aliivibrio wodanis from both kidney and skin tissues was identified in a few fish in a few outbreaks, the involvement of these taxa in manifestation of cranial lesions was considered minor.
A 100% agreement between MALDI‐TOF MS and ANI for classification to species and genomovar levels demonstrates the utility of MALDI‐TOF for rapid and reliable identification of T. finnmarkense to sub‐species level, and provides further support for use of MALDI in subtyping bacterial pathogens (Croxatto et al., 2012). As cold‐water Tenacibaculum species are relatively slow growing, often have highly similar colony morphologies and are relatively unreactive in many phenotypical tests, diagnostic confirmation of their identity by classical methods is often a lengthy affair. MALDI‐TOF MS thereby promises to be an easy, rapid, cost efficient and precise tool in future Tenacibaculum diagnostics.
A variety of Tenacibaculum spp. have previously been isolated from various diseased fish species sampled at various times of the year (Olsen et al., 2017). A lower degree of diversity was identified in the present study, in which sampling was limited to late winter and spring, with almost all isolates recovered from the 15 sites identified as T. finnmarkense, with the majority belonging to a particularly closely related cluster of gv. finnmarkense. This strongly suggests a link between this species/genomovar and development of clinical tenacibaculosis in sea‐farmed Norwegian salmon in the late winter and spring.
Core genome MLST analysis revealed strikingly differing population structures among disease‐associated T. finnmarkense gv. finnmarkense and ulcerans. While all T. finnmarkense gv. finnmarkense isolates clustered into genetically distinct, conserved sub‐clusters, the T. finnmarkense gv. ulcerans population consisted of (comparatively) unrelated singletons or pairs of isolates (Figure 3). Although a mixed Tenacibaculum flora was identified in most cases, members of the dominating gv. finnmarkense low‐diversity cluster were identified in 13 of the 15 outbreaks investigated. As bacterial culture was performed on open, exposed lesions, colonization by a variety of Tenacibaculum possibly less involved in initial lesion development cannot be discounted. Similarly, as isolates were randomly selected for sequencing, the presence of the low diversity gv. finnmarkense variant in the two remaining outbreaks, is neither unlikely. The high degree of genetic conservation in the low diversity cluster is supported by SNP, cgMLST and ANI analyses, clearly indicating a clonal population structure within this cluster of isolates.
Little is known of the demographics of environmental Tenacibaculum spp. And all isolates studied here were isolated from farmed fish. Whether the clonal complex dominating among diseased fish identified here also dominates the environmental population in Norwegian coastal waters is unknown. The wide geographical distribution combined with its clonal nature is, however, consistent with purifying evolutionary forces constraining genetic change (Cvijović et al., 2018). For whatever reason, these forces appear to have produced a bacterium which thrives in skin lesions of farmed salmon.
Tenacibaculum finnmarkense has a wide geographical distribution, having been identified both in Chile (gv. ulcerans; Bridel et al., 2018) and in the present study along large parts of the Norwegian coastline. Both T. finnmarkense genomovars further appear to have a shared geographical range in Norway, as both were isolated in the far north and far south of the study area. There are no indications of geographical separation as the low‐diversity cluster was present in all counties sampled. Bacteriological swabbing of environmental substrates (kelp and rocks) collected from the same geographical regions, but distant from affected salmon farms, onto marine agar containing 5 µg/ml kanamycin (Frisch et al. 2017) identified (MALDI‐TOF MS) representatives of T. finnmarkense gv. finnmarkense, T. finnmarkense gv. ulcerans and T. piscium (data not shown) among an otherwise diverse bacterial flora. Thus, while the dominance of a clonal strain of T. finnmarkense gv. finnmarkense in the present study indicates an aetiological role for this genomovar in the disease observed, the intra‐outbreak involvement of multiple Tenacibaculum taxa, in addition to their environmental presence, suggests that colonization of individual fish directly from sea water is perhaps more common than fish‐to‐fish transmission.
While T. finnmarkense was the main bacteriological finding in the present study, it is clear that other Tenacibaculum species may also be associated with disease in Atlantic salmon farmed in sea water. Among others, T. dicentrarchi has been described in association with skin lesions in seawater‐farmed Atlantic salmon in both Chile (Avandaño‐Herrera et al., 2016) and Norway (Habib et al., 2014; Klakegg et al., 2019). Interestingly, it was speculated by Klakegg et al. (2019) that T. dicentrarchi may also have been associated with a number of outbreaks of tenacibaculosis which occurred in north‐west Norway during 2018, coinciding with the sampling period of the present study. Our results suggest, however, that T. dicentrarchi probably only plays a minor role in tenacibaculosis in Norwegian Atlantic salmon farmed in open sea cages when water temperatures are low. There are notable differences between the single outbreaks described by Avandaño‐Herrera et al. (2016) and Klakegg et al. (2019), and those investigated in the present study. Firstly, both of the previous studies involving T. dicentrarchi described disease in salmon held in enclosed facilities at water temperatures of 18 and 12°C, respectively, while outbreaks scrutinized in the current study involved fish held in open sea cages at water temperatures of 4–6°C. Furthermore, both Avandaño‐Herrera et al. (2016) and Klakegg et al. (2019) described the major clinical findings associated with T. dicentrarchi infection as ulceration of the abdomen and ‘fin‐rot’, while in the present study nearly all outbreaks were dominated by lesions of the head/jaw. T. dicentrarchi has also been previously linked to disease in wrasse species used as cleaner fish in Norwegian salmon aquaculture (Olsen et al., 2017). As wrasse, commonly wild‐caught, are normally stocked and monitored at relatively high water temperatures, it is tempting to speculate that T. dicentrarchi‐associated tenacibaculosis may be linked to water temperatures above those recorded in the present study.
The majority of outbreaks investigated during the current project were related to populations of recently sea‐transferred smolts during a relatively limited time window, characterized by low water temperatures. It would seem highly likely that these factors are of epidemiological importance. Furthermore, the disease most commonly affects only a limited number of cages on each farm, thus indicating that batch‐specific factors relating to the exposed salmon population may be of importance. Recreation of tenacibaculosis in salmon under controlled laboratory circumstances has not been straightforward, and has depended upon lengthy exposure to either large numbers of bacteria or preexposure scarification (Olsen et al., 2011; Småge et al., 2018). Although not examined in the present survey, anecdotal reports indicate that outbreaks of tenacibaculosis may occasionally be associated with a panic reaction among the fish during cage transfer. Physical damage to the nose/jaw area by rubbing against the net walls may thus provide an opportunity for Tenacibaculum colonization. Overall, it seems likely that development of tenacibaculosis in newly sea‐transferred Atlantic salmon is dependent on a number of factors, including the predisposing prior presence of a cutaneous injury combined with exposure to large numbers of environmental Tenacibaculum finnmarkense gv. finnmarkense at low water temperatures. Whether low temperatures contribute to the situation by exerting an immunosuppressive effect on the salmon, by inducing increased virulence or favouring particular genetic variants of the bacterium, and/or by some other mechanism, is not known.
In conclusion, we identified the dominating presence of T. finnmarkense in association with severe cranial lesion development in recently sea‐transferred Atlantic salmon smolts, held in open sea cages over a wide geographical range in Norway during the late winter and spring of 2018. The highly consistent clinical, histopathological and bacteriological picture, combined with the close physical association between bacterial cells and the observed pathological findings, strongly indicates T. finnmarkense, and T. finnmarkense gv. finnmarkense in particular, as the major aetiological agent in all cases investigated. While identification of a particular aetiologically relevant bacterial species is undoubtedly an important step towards eventual vaccine development, initial testing of a trial T. finnmarkense vaccine was unsuccessful (Småge et al., 2018). In addition to a successful vaccination strategy, production‐related parameters, such as smolt quality and time of sea transfer in relation to water temperature and natural infection pressure, as well as avoidance of factors disturbing the fish skin barrier, should be investigated as potential means of preventing tenacibaculosis related losses.
PATIENT CONSENT STATEMENT
Not applicable.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
CLINICAL TRIAL REGISTRATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
ETHICS APPROVAL STATEMENT
The described study was based on material taken under routine health investigations and therefore did not require approval from the national experimental animal committee.
Supporting information
Fig S1
Fig S2
Fig S3
Table S1
Table S2
Table S3
ACKNOWLEDGEMENTS
Eve Marie Louise Zeyl Fiskebeck, Håkon Kaspersen and Attila Tarpai of the Norwegian Veterinary Institute are gratefully acknowledged for assistance with plotting trees in R and preparation of Figure 1. The contribution by the technical staff of the Norwegian Veterinary Institute is also much appreciated, as is the contribution made by the many fish health service personnel and farming companies who submitted samples. Photograph of fish with ulcers: Morten Styrvold.
Spilsberg, B. , Nilsen, H. K. , Tavornpanich, S. , Gulla, S. , Jansen, M. D. , Lagesen, K. , Colquhoun, D. J. , & Olsen, A.‐B. (2022). Tenacibaculosis in Norwegian Atlantic salmon (Salmo salar) cage‐farmed in cold sea water is primarily associated with Tenacibaculum finnmarkense genomovar finnmarkense . Journal of Fish Diseases, 45, 523–534. 10.1111/jfd.13577
Funding information
This study was financed by the Norwegian Seafood Research Fund under project FHF 901434 ‘Tenacibaculum spp. as the aetiological agent of atypical winter ulcer in Norwegian farmed salmon’. "The computations were performed on the Saga Cluster provided by UNINETT Sigma2 ‐ the National Infrastructure for High Performance Computing and Data Storage in Norway"
DATA AVAILABILITY STATEMENT
The genome assemblies have been deposited at DDBJ/ENA/GenBank under accession numbers JAAGBE000000000 – JAAGEB000000000 and JAFMUA000000000 – JAFMUT000000000, as described in Table S2.
REFERENCES
- Avendaño‐Herrera, R. , Irgang, R. , Sandoval, C. , Moreno‐Lira, P. , Houel, A. , Duchaud, E. , Poblete‐Morales, P. , Nicolas, P. , & Ilardi, P. (2016). Isolation, characterization and virulence potential of Tenacibaculum dicentrarchi in salmonid cultures in Chile. Transboundary and Emerging Diseases, 63(2), 121–126. 10.1111/tbed.12464 [DOI] [PubMed] [Google Scholar]
- Bankevich, A. , Nurk, S. , Antipov, D. , Gurevich, A. A. , Dvorkin, M. , Kulikov, A. S. , Lesin, V. M. , Nikolenko, S. I. , Pham, S. , Prjibelski, A. D. , Pyshkin, A. V. , Sirotkin, A. V. , Vyahhi, N. , Tesler, G. , Alekseyev, M. A. , & Pevzner, P. A. (2012). SPAdes: A new genome assembly algorithm and its applications to single‐cell sequencing. Journal of Computational Biology, 19(5), 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger, A. M. , Lohse, M. , & Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics, 30(15), 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridel, S. , Olsen, A. B. , Nilsen, H. , Bernardet, J. F. , Achaz, G. , Avendaño‐Herrera, R. , & Duchaud, E. (2018). Comparative genomics of Tenacibaculum dicentrarchi and “Tenacibaculum finnmarkense” highlights intricate evolution of fish‐pathogenic species. Genome Biology and Evolution, 10(2), 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxatto, A. , Prod'hom, G. , & Greub, G. (2012). Applications of MALDI‐TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiology Reviews, 36(2), 380–407. 10.1111/j.1574-6976.2011.00298.x [DOI] [PubMed] [Google Scholar]
- Cvijović, I. , Good, B. H. , & Desai, M. M. (2018). The effect of strong purifying selection on genetic diversity. Genetics, 209(4), 1235–1278. 10.1534/genetics.118.301058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch, K. , Småge, S. B. , Vallestad, C. , Duesund, H. , Brevik, Ø. J. , Klevan, A. , Olsen, R. H. , Sjaatil, S. T. , Gauthier, D. , Brudeseth, B. , & Nylund, A. (2018). Experimental induction of mouthrot in Atlantic salmon smolts using Tenacibaculum maritimum from Western Canada. Journal of Fish Diseases, 41(8), 1247–1258. 10.1111/jfd.12818 [DOI] [PubMed] [Google Scholar]
- Habib, C. , Houel, A. , Lunazzi, A. , Bernardet, J. F. , Olsen, A. B. , Nilsen, H. , Toranzo, A. E. , Castro, N. , Nicolas, P. , & Duchaud, E. (2014). Multilocus sequence analysis of the marine bacterial genus Tenacibaculum suggests parallel evolution of fish pathogenicity and endemic colonization of aquaculture systems. Applied and Environment Microbiology, 80(17), 5503–5514. 10.1128/AEM.01177-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, C. , Rodriguez‐R, L. M. , Phillippy, A. M. , Konstantinidis, K. T. , & Aluru, S. (2018). High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nature Communications, 9(1), 1–8. 10.1038/s41467-018-07641-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen, C. , Thorarinsson, R. , Wallace, C. , Salonius, K. , & Midtlyng, P. J. (2017). Atlantic salmon winter‐ulcer disease: Combining mortality and skin ulcer development as clinical efficacy criteria against Moritella viscosa infection. Aquaculture, 473, 538–544. 10.1016/j.aquaculture.2017.01.035 [DOI] [Google Scholar]
- Karlsen, C. , Vanberg, C. , Mikkelsen, H. , & Sørum, H. (2014). Co‐infection of Atlantic salmon (Salmo salar), by Moritella viscosa and Aliivibrio wodanis, development of disease and host colonization. Veterinary Microbiology, 171(1–2), 112–121. 10.1016/j.vetmic.2014.03.011 [DOI] [PubMed] [Google Scholar]
- Klakegg, Ø. , Abayneh, T. , Fauske, A. K. , Fülberth, M. , & Sørum, H. (2019). An outbreak of acute disease and mortality in Atlantic salmon (Salmo salar) post‐smolts in Norway caused by Tenacibaculum dicentrarchi . Journal of Fish Diseases, 42(6), 789–807. 10.1111/jfd.12982 [DOI] [PubMed] [Google Scholar]
- Lunder, T. , Evensen, Ø. , Holstad, G. , & Håstein, T. (1995). 'Winter ulcer' in the Atlantic salmon Salmo salar. Pathological and bacteriological investigations and transmission experiments. Diseases of Aquatic Organisms, 23(1), 39–49. 10.3354/dao023039 [DOI] [Google Scholar]
- Olsen, A. B. , Gulla, S. , Steinum, T. , Colquhoun, D. J. , Nilsen, H. K. , & Duchaud, E. (2017). Multilocus sequence analysis reveals extensive genetic variety within Tenacibaculum spp. associated with ulcers in sea‐farmed fish in Norway. Veterinary Microbiology, 205, 39–45. 10.1016/j.vetmic.2017.04.028 [DOI] [PubMed] [Google Scholar]
- Olsen, A. B. , Nilsen, H. , Sandlund, N. , Mikkelsen, H. , Sørum, H. , & Colquhoun, D. J. (2011). Tenacibaculum sp. associated with winter ulcers in sea‐reared Atlantic salmon Salmo salar . Diseases of Aquatic Organisms, 94(3), 189–199. 10.3354/dao02324 [DOI] [PubMed] [Google Scholar]
- Olsen, A. B. , Spilsberg, B. , Nilsen, H. K. , Lagesen, K. , Gulla, S. , Avendaño‐Herrera, R. , Irgang, R. , Duchaud, E. , & Colquhoun, D. J. (2020). Tenacibaculum piscium sp. nov., isolated from skin ulcers of sea‐farmed fish, and description of Tenacibaculum finnmarkense sp. nov. with subdivision into genomovars finnmarkense and ulcerans . International Journal of Systematic and Evolutionary Microbiology, 70(12), 6079–6090. 10.1099/ijsem.0.004501 [DOI] [PubMed] [Google Scholar]
- Richter, M. , & Rosselló‐Móra, R. (2009). Shifting the genomic gold standard for the prokaryotic species definition. Proceedings of the National Academy of Sciences, 106(45), 19126–19131. 10.1073/pnas.0906412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, M. , Machado, M. P. , Silva, D. N. , Rossi, M. , Moran‐Gilad, J. , Santos, S. , Ramirez, M. , & Carriço, J. A. (2018). chewBBACA: A complete suite for gene‐by‐gene schema creation and strain identification. Microbial Genomics, 4(3). 10.1099/mgen.0.000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Småge, S. B. , Brevik, Ø. J. , Duesund, H. , Ottem, K. F. , Watanabe, K. , & Nylund, A. (2016). Tenacibaculum finnmarkense sp. nov., a fish pathogenic bacterium of the family Flavobacteriaceae isolated from Atlantic salmon. Antonie van Leeuwenhoek, 109(2), 273–285. 10.1007/s10482-015-0630-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Småge, S. B. , Brevik, Ø. J. , Frisch, K. , Watanabe, K. , Duesund, H. , & Nylund, A. (2017). Concurrent jellyfish blooms and tenacibaculosis outbreaks in Northern Norwegian Atlantic salmon (Salmo salar) farms. PLoS One, 12(11), e0187476. 10.1371/journal.pone.0187476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Småge, S. B. , Frisch, K. , Brevik, Ø. J. , Watanabe, K. , & Nylund, A. (2016). First isolation, identification and characterisation of Tenacibaculum maritimum in Norway, isolated from diseased farmed sea lice cleaner fish Cyclopterus lumpus L. Aquacult, 464, 178–184. 10.1016/j.aquaculture.2016.06.030 [DOI] [Google Scholar]
- Småge, S. B. , Frisch, K. , Vold, V. , Duesund, H. , Brevik, Ø. J. , Olsen, R. H. , Sjaatil, S. T. , Klevan, A. , Brudeseth, B. , Watanabe, K. , & Nylund, A. (2018). Induction of tenacibaculosis in Atlantic salmon smolts using Tenacibaculum finnmarkense and the evaluation of a whole cell inactivated vaccine. Aquacult, 495, 10.1016/j.aquaculture.2018.06.063 [DOI] [Google Scholar]
- Sommerset, I. , Bang, J. B. , Bornø, G. , Haukass, A. , & Brun, E. (2020). (In Norwegian) Fiskehelserapporten 2020. Norwegian Veterinary Institute Report. ASSN 1893‐1480. https://www.vetinst.no/rapporter‐og‐publikasjoner/rapporter/2021/fiskehelserapporten‐2020
- Takle, H. R. , Ytteborg, E. , Nielsen, K. V. , Karlsen, C. R. , Nilsen, H. K. , Sveen, L. , Colquhoun, D. , Olsen, A. B. , Sørum, H. , & Nilsen, A. (2015). In Norwegian. Sårproblematikk og hudhelse i laks‐og regnbueørrettoppdrett. Nofima AS 2015 (ISBN 978‐82‐8296‐260‐5) 108, p. Nofima report series (5/2015)
- Walker, B. J. , Abeel, T. , Shea, T. , Priest, M. , Abouelliel, A. , Sakthikumar, S. , Cuomo, C. A. , Zeng, Q. , Wortman, J. , Young, S. K. , & Earl, A. M. (2014). Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One, 9(11), e112963. 10.1371/journal.pone.0112963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. Springer‐Verlag New York. ISBN 978‐3‐319‐24277‐4, https://ggplot2.tidyverse.org
- Yu, G. , Smith, D. K. , Zhu, H. , Guan, Y. , & Lam, T. T. Y. (2017). ggtree: An R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods in Ecology and Evolution, 8(1), 28–36. 10.1111/2041-210X.12628 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1
Table S2
Table S3
Data Availability Statement
The genome assemblies have been deposited at DDBJ/ENA/GenBank under accession numbers JAAGBE000000000 – JAAGEB000000000 and JAFMUA000000000 – JAFMUT000000000, as described in Table S2.


