Abstract
Objectives
We sought to evaluate the ability of the novel LA strain parameters to discriminate patients with heart failure with preserved ejection fraction (HFpEF) from individuals with risk factors of HFpEF.
Methods and results
A total of n = 389 patients with risk factors for HFpEF finally was prospectively enrolled into the study, 66 of them were diagnosed with HFpEF by the 2021 ESC HF guidelines. Fifty‐five patients were undergone left ventricular catheterization and simultaneous transthoracic echocardiography was performed, 35 of them with elevated left ventricular end‐diastolic pressure (LVEDP). Left atrial reservoir strain (LASr) was measured in all patients. LA filling index was defined as the ratio of mitral E and LASr and LA stiffness index was calculated as E/e′/LASr. Compared with the patients in the normal LVEDP subgroup, those in the elevated LVEDP subgroup showed significantly higher LA filling index, LA stiffness index, and LAVI/LASr. The receiver‐operating characteristic curve (ROC) analysis showed LASr (area under curve [AUC] .840), LA filling index (AUC .843), LA stiffness index (AUC .766), and LAVI/LASr (AUC .755) had good diagnostic accuracy for elevated LVEDP. Inter‐technique agreement analysis showed the novel algorithms with LA strain parameters had good agreement with the invasive LVEDP measurement, better than the 2016 ASE/SCAI algorithms (kappa .711 vs. .101). Furthermore, compared with patients without HFpEF, LASr was lower in HFpEF, LA filling index, LA stiffness index, and LAVI/LASr was higher in patients with HFpEF. ROC analysis showed the novel LA strain parameters with good accuracy (AUC .756 to .821) non‐inferior to conventional echocardiographic parameters could identify HFpEF, and LA stiffness index (AUC .821) was the best one.
Conclusion
The novel LA strain parameters could be of potential usefulness in estimating LVEDP and incorporated into the 2016 EACVI/ASE criteria would improve the diagnostic efficiency. The novel LA strain parameters with good accuracy non‐inferior to conventional echocardiographic parameters could discriminate HFpEF from patients with risk factors of HFpEF.
Keywords: echocardiography, heart failure with preserved ejection fraction, left atrial strain, left ventricular diastolic dysfunction
1. INTRODUCTION
About 4.9% of the general population aged ≥65 years was diagnosed with heart failure with preserved ejection fraction (HFpEF), and HFpEF accounts for more than half of all hospitalized heart failure patients. 1 Left ventricular diastolic dysfunction is an important pathophysiological process of HFpEF. 2 As left ventricular diastolic dysfunction worsens, left ventricular filling pressure (LVFP) increase, which is one of the important factors that determine the symptoms and prognosis of heart failure, 3 , 4 leading to left atrium pressure overload and left atrial abnormal function. 5 Several studies have shown that left atrial strain is negatively correlated well with LVFP or PCWP. 6 , 7 , 8 , 9 However, diastolic dysfunction per se is not tantamount to HFpEF.
Significantly impaired left atrial strain has been identified in patients with HFpEF and associated with a worse outcome. 10 , 11 In addition, the left atrial strain has the advantage that it is independent on the angle of the myocardial velocity recorded by tissue Doppler, and less affected by mitral annulus calcification and bundle branch block. 12
Therefore, we hypothesized that left atrial strain and derived index might be useful in its diagnostic evaluation. Previous studies focused more on the prognostic role of impaired left atrial strain and the relationship between left atrial strain and the pathophysiological mechanism of HFpEF, but less data regarding the evaluation of the role of left atrial strain in diagnosing HFpEF were reported. In this regard, we conducted the study to evaluate the ability of left atrial strain and derived index to discriminate patients with HFpEF from individuals with risk factors of HFpEF.
2. METHODS
2.1. Study population
Consecutive subjects with risk factors of HFpEF who were hospitalized in the First Affiliated Hospital of Soochow University were retrospectively enrolled in the study from November 2018 to December 2019. The inclusion criteria were as follows: (1) LVEF ≥ 50%; (2) no serious valvular heart disease; (3) sinus rhythm, 60–100 beats/min; and (4) patients with risk factors of HFpEF such as advanced age, obesity, diabetes, hypertension, coronary heart disease, chronic kidney disease, lack of physical activity, and abnormal electrocardiogram. 13 Exclusion criteria included as follows: (1) LVEF < 50%; (2) severe heart valve disease: any mitral or aortic valve stenosis, moderate or severer tricuspid regurgitation, moderate or severer mitral regurgitation, and experience with any valvular heart surgery or interventions; (3) atrial fibrillation; (4) insufficient echocardiographic imaging; (5) patients without risk factors of HFpEF; (6) patients with restrictive pericarditis; and (7) heart failure with reduced ejection fraction (EF) recovering EF.
Patients with risk factors for HFpEF finally enrolled in the study were 389 subjects, 66 of them were retrospectively diagnosed with HFpEF. Fifty‐five patients of all subjects were undergone left ventricular catheterization for dyspnea or palpitation, and the simultaneous transthoracic echocardiography was performed. This study was approved by the Ethics Committee of First Affiliated Hospital of Soochow University.
2.2. The diagnosis of HFpEF
The diagnostic criteria of HFpEF according to the new 2021 ESC HF guidelines were as follows: (1) symptoms ± signs; (2) LVEF ≥ 50%; and (3) objective evidence of cardiac structural and/or functional abnormalities consistent with the presence of LV diastolic dysfunction (LVDD)/raised LVFP, including raised natriuretic peptides. 14
2.3. Left ventricular diastolic dysfunction
The four variables to identify LVDD and their abnormal cutoff values according to the recommendations of the 2016 ASE/SCAI guideline are as follows: (1) annular e′ velocity: septal e′ < 7 cm/s, lateral e′ < 10 cm/s; (2) average E/e′ ratio > 14; (3) LA volume index (LAVI) > 34 mL/m2; and (4) peak TR velocity > 2.8 m/s. 15 The patients were diagnosed with LVDD, while > 50% of the above criteria were positive; when <50% of the aforementioned criteria were positive, LV diastolic function was considered normal; in addition, when only 50% of the criteria were positive, patients were diagnosed as having indeterminate LV diastolic function. 15
2.4. Transthoracic echocardiography
The standard transthoracic echocardiography of all subjects were performed by a GE Vivid E9 or GE Vivid E95 (Norway) 2.5 MHz transducer according to the American Society of Echocardiography guidelines. 16 The maximum or minimal volumes of the left atrium were calculated by the biplane algorithm in apical four‐ and two‐chamber views before mitral valve opening or closing for one to two frames. Left atrium maximal volume index (LAVI) was calculated as the maximum volumes/the body surface area. The maximum volume was divided by the minimal volume to obtain the left atrium empty fraction (LAEF). Relative wall thickness (RWT) calculated as twice the thickness of the posterior wall of the left ventricle divided by the end‐diastolic left ventricular diameter (LVPW*2/LVIDD). E/e′ was the ratio of early diastolic mitral inflow to mitral annular tissue velocities (the mean of septal e′ and lateral e′). Researchers were blinded to the patient's clinical characteristics.
2.5. Speckle‐tracking echocardiography
All images obtained at a frame rate of 60–80 fps were used for speckle‐tracking echocardiographic analysis. Left ventricular global longitudinal systolic strain (GLS) and left atrial strain was analyzed offline by two experienced investigators using the Echo PAC work station (GE Healthcare). GLS was calculated as the average of systolic strain obtained from all left ventricular segments in the apical 4‐chamber, apical 2‐chamber, and apical long‐axis views. 13 Left atrial reservoir strain (LASr) was measured as the mean of strain, which was from all left atrial segments in the apical 4‐chamber and apical 2‐chamber views, we took the onset of QRS complex as the zero‐reference point. LA filling index was defined as the ratio of mitral E and LASr. LA stiffness index was calculated as E/e′/LASr.
2.6. Clinical characteristics
We collected clinical data including comorbidities, body mass index, and laboratory data including N‐terminal proB‐type natriuretic peptide.
2.7. Left ventricular catheterization protocol
Left ventricular catheterization was performed through a 6Fr pigtail catheter via the right radial artery. Left ventricular end‐diastolic pressure (LVEDP) was measured at the QRS complex starting point (mean of 3–5 beats). An LVEDP value ≥ 16 mm Hg was defined as elevated LVFP. 13
2.8. Statistical analysis
Normally distributed variables are expressed as mean ± standard derivation (SD). Variables which are not normally distributed are presented as median with inter‐quartile ranges (IQRs). Categorical variables are reported as absolute numbers (%). As appropriate, between‐group differences were compared by the independent t‐test, the Mann–Whitney U test, or chi‐square test. All variables with P≤ .1 in the invasive group were included in the multiple logistic regression analysis to predict elevated LVEDP. Several models were built, for the novel LA strain parameters were analyzed separately due to LASr's multicollinearity. The C‐statistic was calculated in each model to compare between them. The accuracy of characteristic echocardiographic indexes for diagnosing HFpEF or elevated LVEDP were evaluated by receiver‐operating characteristic curve (ROC curve). P < .05 (two‐tailed) was considered statistically significant. All statistical analyses were performed by SPSS version 25.0 and MedCalc.
3. RESULTS
3.1. Characteristics of the study population
A total of n = 389 patients with risk factors for HFpEF finally was enrolled into the study, 66 of them were retrospectively diagnosed with HFpEF (Table 1). A total of n = 55 patients were undergone left ventricular catheterization for dyspnea or palpitation, the number of them with elevated LVEDP was 35 (Table 2), about 30 patients with elevated LVEDP in the invasive group were diagnosed as having indeterminate LV diastolic function or normal diastolic function according to the 2016 ASE/SCAI guideline while the symptoms or signs were highly suspected as HFpEF (Table 3), besides, 20 patients with normal LVEDP were diagnosed as normal LV diastolic function according to the recommendations of the 2016 ASE/SCAI guideline (Table 3). Thus, patients in the invasive group with smaller LAVI and E/e′ ratio (Table 4).
TABLE 1.
Echocardiographic variables of HFpEF and non‐HF patients in whole cohort
| HFpEF (n = 66) | non‐HF (n = 323) | P | |
|---|---|---|---|
| LV structure and function | |||
| LV end‐diastolic dimension (mm) | 50 ± 1 | 48 ± 1 | .050 |
| LVMI (g/m2) | 141 ± 5 | 111 ± 2 | <.001 |
| RWT | .41 ± .01 | .41 ± .01 | .822 |
| LVEF (%) | 61 ± 1 | 65 ± 1 | .002 |
| GLS (%) | 17.6 ± .6 | 20.0 ± .2 | <.001 |
| Doppler | |||
| Mitral E (IQR, cm/s) | 98(80,109) | 73(61,90) | <.001 |
| Mitral A (IQR, cm/s) | 94(72, 107) | 85(70.5,100) | .285 |
| E/A ratio | 1.3 ± .1 | .9 ± .6 | .004 |
| TR peak velocity (m/s) | 2.8 ± .1 | 2.5 ± .1 | <.001 |
| Septal e′ velocity (cm/s) | 5.1 ± .2 | 6.7 ± .1 | <.001 |
| Lateral e′ velocity (cm/s) | 6.9 ± .3 | 9.3 ± .2 | <.001 |
| E/e′ ratio | 16.4 ± .8 | 10.4 ± .3 | <.001 |
| LA structure and function | |||
| LAVI (mL/m2) | 39 ± 2 | 30 ± 1 | <.001 |
| LAEF (%) | 45 ± 2 | 55 ± 1 | <.001 |
| LASr (%) | 19.8 ± 1.1 | 28.7 ± .6 | <.001 |
| LA strain derived index | |||
| LA filling index (%) | 6.1 ± .1 | 3.0 ± .1 | <.001 |
| LA stiffness index | 1.15 ± .15 | .44 ± .02 | <.001 |
| LAVI/LASr | 2.7 ± .3 | 1.3 ± .1 | <.001 |
GLS, left ventricular global longitudinal systolic strain; HFpEF, heart failure with preserved ejection fraction; LAEF, left atrial empty fraction; LASr, left atrium reservoir strain; LAVI, left atrium maximal volume index; LV, left ventricle; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; RWT, relative wall thickness; TR, tricuspid regurgitation.
TABLE 2.
Characteristics of patients underwent left ventricular catheterization
| LVEDP < 16 mm Hg (n = 20) | LVEDP ≥ 16 mm Hg (n = 35) | P | |
|---|---|---|---|
| Male sex | 5(25%) | 14(34%) | .473 |
| Age (year) | 64 ± 2 | 62 ± 2 | .626 |
| Heart rate (beat/min) | 64 ± 2 | 68 ± 2 | .098 |
| Body mass index (kg/m2) | 24.1 ± .7 | 24.6 ± .5 | .543 |
| Medical history | |||
| Hypertension | 12(60%) | 29(83%) | .061 |
| Coronary disease | 9(45%) | 11(31%) | .314 |
| CKD(stage ≥ 3)or ERSD | 1(5%) | 1(3%) | 1.000 |
| COPD | 1(5%) | 1(3%) | 1.000 |
| Diabetes | 4(20%) | 10(29%) | .483 |
| Medication, n (%) | |||
| β‐blockers | 5(25%) | 18(51%) | .056 |
| ACEI | 5(25%) | 8(23%) | .857 |
| ARBs | 4(20%) | 12(34%) | .262 |
| Calcium blocker | 5(25%) | 15(43%) | .185 |
| Diuretic | 2(10%) | 3(9%) | .859 |
| Laboratory | |||
| NT‐proBNP (pg/mL) | 71 ± 10 | 400 ± 124 | <.001 |
| Echocardiographic parameters | |||
| LV structure and function | |||
| LV end‐diastolic dimension (mm) | 47 ± 1 | 47 ± 1 | .253 |
| LVMI (g/m2) | 114 ± 4 | 122 ± 7 | .297 |
| RWT | .43 ± .2 | .42 ± .1 | .538 |
| LVEF(%) | 68 ± 2 | 64 ± 2 | .344 |
| GLS (%) | 20.0 ± .7 | 17.7 ± .7 | .044 |
| Doppler | |||
| Mitral E (cm/s) | 66 ± 2 | 69 ± 2 | .468 |
| Mitral A (cm/s) | 77 ± 4 | 87 ± 4 | .072 |
| E/A ratio | .8(.7,1.0) | .8(.6,.9) | .192 |
| TR peak velocity (IQR, m/s) | 2.4(2.1,2.5) | 2.3(2.2,2.6) | .850 |
| Septal e′ velocity (IQR, cm/s) | 6.7 ± .5 | 6.2 ± ± .3 | .367 |
| Lateral e′ velocity (cm/s) | 10.0(7.5,11.0) | 8.0(6.5,10.2) | .224 |
| E/e′ ratio (IQR) | 8.5(6.9,10.7) | 9.8(8.0,10.9) | .270 |
| LA structure and function | |||
| LAVI (mL/m2) | 15.0 ± 1.6 | 17.2 ± 1.2 | .297 |
| LAEF (%) | 54.5 ± 2.0 | 55 ± 2.0 | .772 |
| LASr (%) | 32.5 ± 1.4 | 23.5 ± 1.1 | <.001 |
| LA strain derived index | |||
| E/LASr (%) | 2.0 ± .1 | 3.1 ± .2 | <.001 |
| LASr/(E/e') | 4.1 ± .4 | 2.7 ± .2 | .001 |
| LAVi/LASr | .8 ± .1 | 1.2 ± .1 | .002 |
| LVMI/LASr | 3.5 ± .2 | 5.5 ± .6 | .003 |
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end‐stage renal disease; GLS, left ventricular global longitudinal systolic strain; IQR, inter‐quartile ranges, LAEF, left atrial empty fraction; LASr, left atrium reservoir strain.; LAVI, left atrium maximal volume index; LV, left ventricle; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; RWT, relative wall thickness; TR, tricuspid regurgitation.
TABLE 3.
Contingency table of agreement among the novel LA strain parameters incorporated into the 2016 ASE Diastolic Guideline
| Kappa= | .711 | The novel criterion | |
|---|---|---|---|
| Normal or indeterminate | Elevated | ||
| Invasive | Normal | 20 | 0 |
| Elevated | 8 | 27 | |
TABLE 4.
Characteristics of the study population
| Invasive group (n = 55) | The whole cohort (n = 389) | P | |
|---|---|---|---|
| Age (IQR, year) | 64 (5670.5) | 64 (5572) | .922 |
| Male sex | 17 (30.9%) | 187(48.1%) | .017 |
| Body mass index (kg/m2) | 24.3 ± .4 | 24.2 ± .2 | .542 |
| Medical history | |||
| Smoker | 25(45.5%) | 69(17.7%) | <.001 |
| Alcoholism | 9(16.4%) | 26(6.7%) | .013 |
| Hypertension | 41(74.5%) | 237 (60.9%) | .051 |
| Coronary disease | 14(25.5%) | 90(23.1%) | .701 |
| Diabetes | 20 (36.4%) | 89 (22.9%) | .030 |
| COPD | 2 (3.6%) | 10 (2.6%) | .648 |
| CKD (Stage ≥ 3) or ESRD | 2 (3.6%) | 26 (6.7%) | .384 |
| Laboratory | |||
| NT‐proBNP (pg/mL) | 302 ± 94 | 871 ± 163 | .002 |
| Echocardiographic parameters | |||
| LV structure and function | |||
| LV end‐diastolic dimension (mm) | 48 ± 1 | 48 ± 1 | .217 |
| LVMI (g/m2) | 118 ± 5 | 117 ± 2 | .718 |
| RWT | .43 ± .01 | .41 ± .01 | .072 |
| LVEF (%) | 66 ± 1 | 64 ± 1 | .351 |
| GLS (%) | 18.6 ± .6 | 19.5 ± .2 | .091 |
| Doppler | |||
| Mitral E (IQR, cm/s) | 67 (6077) | 75 (6290.5) | .004 |
| Mitral A (IQR, cm/s) | 81 (6996.5) | 85 (70 101) | .436 |
| E/A ratio | .9 ± .1 | 1.0 ± .1 | .005 |
| TR peak velocity (m/s) | 2.4 ± .1 | 2.5 ± .1 | <.001 |
| Septal e′ velocity (cm/s) | 6.3 ± .3 | 6.4 ± .1 | .860 |
| Lateral e′ velocity (cm/s) | 9.0 ± .4 | 8.9 ± .2 | .787 |
| E/e′ ratio | 9.4 ± .5 | 11.4 ± .3 | .001 |
| LA structure and function | |||
| LAVI (mL/m2) | 27.5 ± 1.4 | 32.1 ± .7 | .011 |
| LAEF (%) | 55 ± 1 | 54 ± 1 | .426 |
| LASr (%) | 27.4 ± 1.1 | 27.3 ± .6 | .823 |
| LA strain derived index | |||
| LA filling index (%) | 2.6 ± .1 | 3.4 ± .1 | <.001 |
| LA stiffness index | .38 ± .03 | .56 ± .04 | <.001 |
| LAVI/LASr | 1.06 ± .06 | 1.54 ± .08 | <.001 |
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end‐stage renal disease; GLS, left ventricular global longitudinal systolic strain; IQR, inter‐quartile ranges, LAEF, left atrial empty fraction; LASr, left atrium reservoir strain.; LAVI, left atrium maximal volume index; LV, left ventricle; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; RWT, relative wall thickness; TR, tricuspid regurgitation.
Compared to the whole cohort, NT‐proBNP levels were lower in the invasive group, in addition, the invasive group had a higher prevalence of smoker and alcoholism (Table 4).
3.2. Cardiac structure and function
There were no significant differences in LV structure and function indicators such as LV end‐diastolic dimension, LVMI, RWT, E/e′ ratio, LVEF, GLS, and so on, and the conventional LA structure and function indicators such as LAVI and LAEF, between the normal LVEDP and elevated LVEDP subgroup (Table 2).
On the other hand, compared patients without HFpEF, LVEF, and GLS were lower in patients with HFpEF. LVMI was greater in HFpEF, but LAEF was lower. LV diastolic function was impaired in HFpEF patients with larger left atrium volume, higher E/e′ ratio, and lower septal or lateral e′ (Table 1).
3.3. Left atrial strain parameters
Compared with the patients in the normal LVEDP subgroup, those in the elevated LVEDP subgroup showed significantly higher LA filling index, LA stiffness index, and LAVI/LASr, and LASr was significantly impaired (Table 2).
Furthermore, compared with patients without HFpEF, LASr (28.7 ± .6% vs. 19.8 ± 1.1%, P<.001) was lower in HFpEF, LA filling index, LA stiffness index, and LAVI/LASr were higher in patients with HFpEF (Table 1).
3.4. Logistic regression analysis and prediction models indicating elevated LVEDP
Multivariate logistic regression analyses showed that LASr, LA filling index, LA stiffness index, and LAVI/LASr were still the predictors of elevated LVEDP in their respective models after adjusted for hypertension, heart rate, β‐blockers, mitral A, and GLS (Table 5).
TABLE 5.
Multivariate logistics regression analysis of the novel LA strain parameters indicating elevated LVEDP after adjusted by hypertension, heart rate, β‐blockers, mitral A, and GLS (P<.1)
| OR | 95%CI | P | C‐statistic | |
|---|---|---|---|---|
| LASr | .719 | .584,.884 | .002 | .945 |
| LA filling index | 31.111 | 2.858, 338.614 | .005 | .919 |
| LA stiffness index | 631.949 | 100.653, 3967.696 | .003 | .912 |
| LAVI/LASr | 68.136 | 3.147, 1475.168 | .001 | .898 |
CI, confidence interval; LASr, left atrium reservoir strain; LAVI, left atrium maximal volume index; OR, odds ratio.
3.5. Diagnostic performance of the novel left atrial strain parameters to determine elevated LVEDP
LASr (area under curve [AUC] .840, cutoff value 24.4%, sensitivity 65.7%, specificity 95.0%), LA filling index, LA stiffness index, and LAVI/LASr had good diagnostic accuracy for elevated LVEDP (Table 6).
TABLE 6.
The diagnostic performance of the novel LA strain parameters to determine elevated LVEDP
| AUC | 95%CI | P | Cut‐off value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| LASr | .840 | .716, .925 | <.001 | 24.4 | 65.7% | 95.0% |
| LA filling index | .843 | .720, .927 | <.001 | 2.6 | 65.8% | 95.0% |
| LA stiffness index | .766 | .632, .869 | <.001 | .43 | 51.0% | 100% |
| LAVI/LASr | .755 | .614, .864 | <.001 | .82 | 90.6% | 63.0% |
AUC, area under the curve; CI, confidence interval; LASr, left atrium reservoir strain; LAVI, left atrium maximal volume index.
3.6. Consistency of echocardiographic and invasive techniques
The novel LA strain parameters were incorporated into 2016 ASE/SCAI algorithms as follows: (1) septal e′ < 7 cm/s, lateral e′ < 10 cm/s; (2) average E/e′ ratio > 14 or LA stiffness index >.43; (3) LAVI > 34 mL/m2 or LAVI/LASr >.82; and (4) peak TR velocity > 2.8 m/s or LASr <24.4% or LA filling index >2.6. The patients were diagnosed with LVDD, while > 50% of the above criteria were positive; when <50% of the aforementioned criteria were positive, LV diastolic function was considered normal. In addition, when only 50% of the criteria were positive, patients were diagnosed as having indeterminate LV diastolic function (Figure 1).
FIGURE 1.
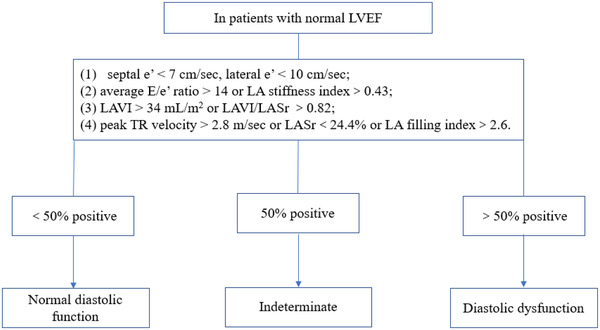
The novel LA strain parameters was incorporated into 2016 ASE/SCAI algorithms. LASr, left atrium reservoir strain; LAVI, left atrium maximal volume index
After we classified the patients with indeterminate condition or normal diastolic function into the normal or indeterminate LV diastolic function subgroup, the agreement between the 2016 ASE/SCAI algorithms and the invasive technique in our study population was slightly low, the kappa coefficient was only .101 (Table 7). While the novel algorithms with LA strain parameters had higher kappa coefficient (.711) than the 2016 ASE/SCAI algorithms, and showed good agreement with the invasive technique (Table 3).
TABLE 7.
Contingency table of agreement among the 2016 ASE Diastolic Guidelines
| Kappa= | .101 | Guideline | |
|---|---|---|---|
| Normal or indeterminate | Elevated | ||
| Invasive | Normal | 20 | 0 |
| Elevated | 30 | 5 | |
3.7. Diagnostic performance of the novel left atrial strain parameters to identify HFpEF
LASr (AUC .756), LA filling index (AUC .788), and LAVI/LASr (AUC .785) had good diagnostic accuracy for HFpEF, LA stiffness index (AUC .821) had the highest discriminatory ability for HFpEF (Table 8). LASr, LA filling index, LAVI/LASr, and LA stiffness index with higher AUC were superior to conventional echocardiographic measures of diagnosing HFpEF except for E/e′ ratio (Table 9).
TABLE 8.
The performance of characteristic echocardiography parameters diagnosis of HFpEF
| AUC | 95%CI | P | Cut‐off value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| LVMI | .724 | .676, .768 | <.001 | 134 | 56.1% | 82.8% |
| LVEF | .625 | .575, .673 | .001 | 64 | 68.2% | 52.6% |
| GLS | .648 | .595, .698 | <.001 | 18.2 | 55.6% | 76.5% |
| Mitral E | .693 | .645, .739 | <.001 | 97 | 43.9% | 90.4% |
| E/A ratio | .604 | .553, .653 | .010 | 1.48 | 25.8% | 94.1% |
| TR peak velocity | .728 | .679, .774 | <.001 | 2.7 | 67.2% | 76.3% |
| Septal e′ velocity | .716 | .669, .760 | <.001 | 5 | 69.7% | 67.2% |
| Lateral e′ velocity | .731 | .684, .774 | <.001 | 7 | 61.2% | 67.8% |
| E/e′ ratio | .795 | .751, .834 | <.001 | 10.7 | 83.3% | 64.0% |
| LAVI | .728 | .680, .771 | <.001 | 33 | 73.9% | 65.3% |
| LAEF | .625 | .575, .673 | .001 | 64 | 68.2% | 52.6% |
AUC, area under the curve; CI, confidence interval; GLS, left ventricular global longitudinal systolic strain; HFpEF, heart failure with preserved ejection fraction; LAEF, left atrial empty fraction; LASr, left atrium reservoir strain, LAVI, left atrium maximal volume index; LV, left ventricle; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; TR, tricuspid regurgitation.
TABLE 9.
The performance of the novel LA strain parameters to discriminate HFpEF
| AUC | 95%CI | P | Cut‐off value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| LASr | .756 | .710, .798 | <.001 | 24.4 | 77.3% | 66.6% |
| LA filling index | .788 | .744, .828 | <.001 | 2.6 | 84.9% | 53.3% |
| LA stiffness index | .821 | .780, .858 | <.001 | .43 | 84.9% | 66.3% |
| LAVI/LASr | .785 | .741, .825 | <.001 | .82 | 96.9% | 36.3% |
AUC, area under the curve; CI, confidence interval; HFpEF, heart failure with preserved ejection fraction; LASr, left atrium reservoir strain, LAVI, left atrium maximal volume index.
4. DISCUSSION
In this study, we evaluated the diagnostic accuracy of LA strain parameters in patients with the risk factors of HFpEF. We found that HFpEF or elevated LVEDP could be accurately discriminated from patients with risk factors of HFpEF by the novel left atrial strain parameters. In addition, LASr, LA filling index, LAVI/LASr, and LA stiffness index outperformed other echocardiographic measures that have been used in the evaluation LVFP. Furthermore, the novel algorithms built by adding the novel LA strain parameters into the 2016 ASE/SCAI algorithms showed better agreement with the invasive technique than the 2016 ASE/SCAI algorithms, which suggested the novel LA strain parameters could improve the diagnostic performance of the 2016 EACVI/ASE criteria to determine elevated LVFP.
Compared with tissue Doppler measurement of mitral valve annulus velocity, LA strain has the advantage of being independent of angle and less affected by mitral valve pathology. 12 On the other hand, left atrial strain evaluates the systolic and diastolic function of the left atrium during the whole cardiac cycle, rather than the functional state of a certain instant in a cardiac cycle. 12
Left ventricular diastolic dysfunction is one of the major pathophysiological processes of HFpEF. 2 In the early stage of LVDD, the left atrium enhances its pump function to compensate for the obstruction of left ventricular filling caused by reduced left ventricular compliance. With the prolonged and worse LVDD, the diastolic and compliance of the left atrium gradually impaired, which results in a decrease in the reserve of the left atrium, eventually leading to the enlargement and failure of the left atrium. 17 , 18 Therefore, left atrial strain reflects the cumulative adverse impact of chronically left ventricular diastolic dysfunction impaired on the left atrium. 5 , 19
Recently, several studies have shown that left atrial strain is impaired in patients with left ventricular diastolic dysfunction, 10 and left atrial strain is well correlated with LVFP or PCWP.6–9 Thus, the LA filling index, the ratio of the mitral E velocity over LASr, could reflect the LA functional changes (LASr) and current LV filling status (mitral E velocity). 20 LA stiffness index comprised of the best LA strain parameter and a preferable estimated LVFP parameter (E/e′) would reveal the LA compliance influenced by LV diastolic function and the LV function affected by LVFP. 21 LAVI/LASr combines LA structure (LAVI) and LA function parameters (LASr) which influenced by LV diastolic function. Therefore, LA filling index, LA stiffness index, and LAVI/LASr could be the potentially indexes in detecting elevated LVFP.
Braunauer et al. study showed that LA filling index (AUC .82) had a good diagnostic performance to determine elevated LVFP, 20 and Lin et al. study found that LA stiffness index (AUC .83) was the most accurate parameter in identifying elevated LVEDP patients from those with coronary disease. 21 In this study, we confirmed and expanded the results of prior studies. LASr, LA filling index, LAVI/LASr, and LA stiffness index discriminating elevated LVEDP with good accuracy were superior to conventional echocardiographic parameters used to identify LVDD. As we know, in the early stages of LVDD, LVEDP could be the only elevated pressure, while LA pressure and mean PCWP remain normal, 16 and LA cavity could remain normal. In agreement with these pathophysiological considerations, LAVI was normal in the most of the patients (n = 30) with elevated LVEDP in this study, besides, E/e′ between 8 and 15 identifies elevated LVFP with poor accuracy, 22 and the number of patients with E/e′ ratio between 8 and 15 in the invasive group was 34 (61.8%) in our study. Therefore, this study showed LAVI and E/e′ failed to discriminate elevated LVEDP from the invasive group.
Although the 2016 ASE/SCAI guideline makes it more convenient than previous versions, the diagnostic quandary of “indeterminate” status or clinical situations where the acquisition of Doppler parameters such as not‐measurable TR, tachycardia obscuring mitral annular tissue Doppler tracing and so on is difficult, is still unsolved. While the novel LA strain parameters added into the 2016 EACVI/ASE criteria (Figure 1) could improve the diagnostic performance of the algorithms to determine elevated LVFP. Which suggested that LA stiffness index would play an important role while E/e′ was in gray area, and LA filling index or LASr could take place of peak TR velocity when the peak TR velocity was not measurable.
However, left ventricular diastolic dysfunction itself is not equivalent to HFpEF. Much less studies have evaluated the role of left atrial strain in the diagnosis of HFpEF. Kurt et al. study showed that left atrial strain could accurately discriminated HFpEF (n = 20) from the patients with hypertension, and LA stiffness index had better diagnostic accuracy for HFpEF (AUC .85). 9 Obokata et al. found that the left atrial strain could distinguish HFpEF from the hypertension control group at rest. Furthermore, after increasing the preload by raising the leg, the accuracy of the left atrial strain (AUC .95) in the diagnosis of HFpEF would be significantly improved. 23 Reddy et al. study has shown that LASr (AUC .719), LASr/(E/e′) (AUC .772), and LAVI/LASr (AUC .750) have good accuracy in diagnosing HFpEF (n = 238) from patients presenting with unexplained dyspnea, where HFpEF was definitively confirmed or refuted using invasive exercise testing. 12 In addition, LASr, LA stiffness index, and LAVI/LASr were superior to conventional echocardiographic measures used to diagnose HFpEF. 12 The results of this study further confirmed the prior studies, that LA stiffness index outperformed other echocardiographic measures that have been used in the evaluation HFpEF.
5. LIMITATIONS
This study has some limitations. First, this study used the 2021 ESC diagnostic criteria for the diagnosis of HFpEF, rather than the gold standard that invasive testing demonstrates a high LV filling pressure at rest or exercise for diagnosis of HFpEF. Second, the sample size of the study is limited, and increasing the number of subjects may improve the reliability of the results. Third, this study focused on the performance of left atrial strain in the diagnosis of HFpEF in patients with sinus rhythm; however, atrial fibrillation is one of the main risk factors for HFpEF, further study is needed to evaluate the diagnostic effect of left atrial strain in the diagnosis of HFpEF with atrial fibrillation. Forth, further study is needed to explore the best way how to incorporate the novel LA strain parameters with conventional parameters to identify elevated LVEDP.
6. CONCLUSIONS
The novel LA parameters could be of potential usefulness in estimating LVFP and incorporated into the 2016 EACVI/ASE criteria would improve the diagnostic efficiency. The novel may discriminate HFpEF from patients with risk factors of HFpEF, whose accuracy was non‐inferior to conventional echocardiographic parameters of diagnosing HFpEF.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interests.
Ma C‐S, Liao Y‐P, Fan J‐L, Zhao X, Su Bo, Zhou B‐Y. The novel left atrial strain parameters in diagnosing of heart failure with preserved ejection fraction. Echocardiography. 2022;39:416–425. 10.1111/echo.15304
Chang‐Sheng Ma, Yu‐Ping Liao, Jia‐Li Fan, and Xin Zhao are the first coauthors and contributed equally to this work.
Contributor Information
Bo Su, Email: 980463051@qq.com.
Bing‐Yuan Zhou, Email: zhoubinyuan@sina.com.
REFERENCES
- 1. van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016;18(3):242–252. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. [DOI] [PubMed] [Google Scholar]
- 3. Stevenson LW, Tillisch JH, Hamilton M, et al. Importance of hemodynamic response to therapy in predicting survival with ejection fraction less than or equal to 20% secondary to ischemic or nonischemic dilated cardiomyopathy. Am J Cardiol. 1990;66(19):1348–1354. [DOI] [PubMed] [Google Scholar]
- 4. Steimle AE, Stevenson LW, Fonarow GC, Hamilton MA, Moriguchi JD. Prediction of improvement in recent onset cardiomyopathy after referral for heart transplantation. J Am Coll Cardiol. 1994;23(3):553–559. [DOI] [PubMed] [Google Scholar]
- 5. Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. 1999;100(4):427–436. [DOI] [PubMed] [Google Scholar]
- 6. Fan JL, Su B, Zhao X, et al. Correlation of left atrial strain with left ventricular end‐diastolic pressure in patients with normal left ventricular ejection fraction. Int J Cardiovasc Imaging. 2020;36(9):1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Y, Zhao CM, Shen ZY, Zhao X, Zhou BY. Mitral early‐diastolic inflow peak velocity (E)‐to‐left atrial strain ratio as a novel index for predicting elevated left ventricular filling pressures in patients with preserved left ventricular ejection fraction. Cardiovasc Ultrasound. 2021;19(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cameli M, Lisi M, Mondillo S, et al. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound. 2010;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kurt M, Wang J, Torre‐Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2(1):10–15. [DOI] [PubMed] [Google Scholar]
- 10. Morris DA, Gailani M, Vaz Perez A, et al. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24(6):651–662. [DOI] [PubMed] [Google Scholar]
- 11. Freed BH, Daruwalla V, Cheng JY, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9(3):e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reddy YNV, Obokata M, Egbe A, et al. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21(7):891–900. [DOI] [PubMed] [Google Scholar]
- 13. Pieske B, Tschope C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297–3317. [DOI] [PubMed] [Google Scholar]
- 14. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. [DOI] [PubMed] [Google Scholar]
- 15. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–1360. [DOI] [PubMed] [Google Scholar]
- 16. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. [DOI] [PubMed] [Google Scholar]
- 17. Freed BH, Shah SJ. Stepping out of the left ventricle's shadow: time to focus on the left atrium in heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2017;10(4):e006267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dernellis JM, Stefanadis CI, Zacharoulis AA, Toutouzas PK. Left atrial mechanical adaptation to long‐standing hemodynamic loads based on pressure‐volume relations. Am J Cardiol. 1998;81(9):1138–1143. [DOI] [PubMed] [Google Scholar]
- 19. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive‐echocardiographic study. Circulation. 2017;135(9):825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braunauer K, Dungen HD, Belyavskiy E, et al. Potential usefulness and clinical relevance of a novel left atrial filling index to estimate left ventricular filling pressures in patients with preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging. 2020;21(3):260–269. [DOI] [PubMed] [Google Scholar]
- 21. Lin J, Ma H, Gao L, et al. Left atrial reservoir strain combined with E/E' as a better single measure to predict elevated LV filling pressures in patients with coronary artery disease. Cardiovasc Ultrasound. 2020;18(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler‐catheterization study. Circulation. 2000;102(15):1788–1794. [DOI] [PubMed] [Google Scholar]
- 23. Obokata M, Negishi K, Kurosawa K, et al. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6(7):749–758. [DOI] [PubMed] [Google Scholar]


