Summary
Patients referred for evaluation of bleeding symptoms occasionally have a prolonged platelet function analyser (PFA) closure time, without evidence for von Willebrand disease or impaired platelet aggregation. The aim of this study was to establish a shear‐dependent platelet function defect in these patients. Patients were included based on high bleeding score and prior PFA prolongation. Common tests of von Willebrand factor (VWF) and platelet function and exome sequencing were performed. Microfluidic analysis of shear‐dependent collagen‐induced whole‐blood thrombus formation was performed. In 14 PFA‐only patients, compared to healthy volunteers, microfluidic tests showed significantly lower platelet adhesion and thrombus formation parameters. This was accompanied by lower integrin activation, phosphatidylserine exposure and P‐selectin expression. Principal components analysis indicated VWF as primary explaining variable of PFA prolongation, whereas conventional platelet aggregation primarily explained the reduced thrombus parameters under shear. In five patients with severe microfluidic abnormalities, conventional platelet aggregation was in the lowest range of normal. No causal variants in Mendelian genes known to cause bleeding or platelet disorders were identified. Multiparameter assessment of whole‐blood thrombus formation under shear indicates single or combined effects of low–normal VWF and low–normal platelet aggregation in these patients, suggesting a shear‐dependent platelet function defect, not detected by static conventional haemostatic tests.
Keywords: bleeding disorders, diagnostic haematology, flow, genetics of thrombosis and haemostasis, platelet function
INTRODUCTION
Whole‐blood assessment using a platelet function analyser (PFA) is regularly used in the diagnostic work‐up of patients suspected of a bleeding disorder. The PFA test has been developed as a proxy measurement of primary haemostasis under conditions of high wall‐shear rate. 1 , 2 , 3 , 4 However, use of the PFA test in identifying patients with a bleeding disorder has been questioned in single studies and meta‐analyses. 2 , 3 , 5 , 6
As accepted, the PFA is particularly sensitive in the detection of von Willebrand disease (VWD), especially when the level of von Willebrand factor (VWF) is below 30%. 4 , 7 , 8 Similarly, in such patients, the PFA is used for therapeutic monitoring of the effects of desmopressin or VWF supplementation. 9 , 10 , 11 Furthermore, the PFA closure time (CT) is sensitive to severe platelet function disorders (PFD), such as Glanzmann's thrombasthenia (GT) and Bernard–Soulier syndrome (BSS). 12 , 13 , 14 However, this test has only limited sensitivity for diseases with milder bleeding phenotype, 15 like primary platelet secretion syndrome, storage pool disease 16 , 17 , 18 or moderately low levels of VWF (30%–50%). 19 Accordingly, in the diagnostic work‐up for patients with bleeding symptoms, the finding of a PFA‐CT indicates that further diagnostic testing is required. 3 , 13 , 20 Often extensive laboratory testing is required before a final diagnosis can be made.
For identification of platelet function disorders, light transmission aggregometry (LTA) is the gold standard, often in combination with adenosine triphosphate (ATP) release or flow cytometry. 21 , 22 , 23 For the diagnosis of VWD, plasma levels of VWF activity and antigen are determined, if indicated in combination with testing of VWF multimer pattern, VWF–collagen binding and VWF–factor VIII binding. 24 Several reports indicate that, even after extensive testing, in a significant proportion of patients with bleeding symptoms, an ‘unexplained’ prolonged PFA‐CT is the only abnormality found. 25 , 26 These ‘PFA‐only’ patients are, like other patients with bleeding of unknown cause, provided with a treatment plan, e.g. receiving desmopressin or tranexamic acid, to prevent excessive bleeding upon surgery or tooth extraction. 27 , 28
An explanation for a prolonged PFA, as single laboratory aberration, is that this is the only test in the diagnostic work‐up for bleeding evaluation that relies on high shear forces. In primary haemostasis, initial platelet rolling is regulated by the interaction of platelet GPIb–V–IX complex and VWF bound to collagen. High shear is required to unfold VWF multimers to expose the VWF‐A1 binding site for platelet GPIb. 29 Accordingly, shear‐dependent defects of both platelets and VWF will contribute to abnormal PFA values, which are likely not detected in static tests of haemostasis. A clear limitation of the PFA, however, is that it only gives the aperture CT as a test result, not providing any information about the cause of a prolonged PFA.
Microfluidic whole‐blood flow assays are frequently used to study platelet thrombus formation in vitro. As we and others have shown previously, multiparameter measurements of thrombus formation give a wealth of information about platelet adhesion, activation and aggregation under flow conditions, especially when combined with arrays of microspots in the same flow chamber. 29 , 30 Thus, when using microspots of collagen‐I and collagen‐III (both binding plasma VWF), multiple qualitative and quantitative platelet traits have been identified, linked to genetic variation of the collagen receptor GPVI and to inherited bleeding disorders. 31 , 32 , 33 In combination with the use of brightfield and multicolour fluorescence microscopy, the microfluidic assay thus produces multiple platelet‐dependent outcome values relevant for flow conditions. 34 , 35 , 36
For the present study, our aim was to investigate whether and how multiparameter shear‐dependent microfluidic testing operating at physiological temperature can help to identify abnormalities in platelet function or VWF that explain the prolonged CTs of PFA‐only patients with a bleeding history. Furthermore, patients’ DNA was sequenced using targeted high‐throughput gene panel testing to identify genetic variants known to cause bleeding and platelet disorders.
MATERIALS AND METHODS
A detailed description of the methods is available in the Supplementary material.
Study population
Patients were selected from three observational cohort studies including adult subjects between September 2013 and November 2020. In all cases, a large panel of in‐hospital laboratory tests was performed evaluating the blood from: (i) 136 patients referred to the haematologist for bleeding evaluation (ProBe‐AHP cohort); (ii) 240 patients examined pre‐operatively (PANE cohort); and (iii) 49 patients with a prior diagnosed bleeding disorder of any kind (BEPA cohort). Detailed study designs were described previously. 37
For the present paper, patients were included if prior PFA results were abnormal (i.e. one or both cartridges showing a prolonged CT) and if this was the only aberration found in the diagnostic work‐up. All studies were approved by the local medical ethics committee. All participating patients and healthy individuals gave written informed consent according to the Helsinki declaration.
Data collection and prospective laboratory evaluation
For all patients, in order to select the PFA‐only patients, the following data were retrospectively collected: medical history; family history; medication use; International Society on Thrombosis and Haemostasis bleeding assessment tool (ISTH BAT) score; diagnosis; complete blood counts; PFA results; light transmission aggregometry (LTA) results; VWF activity and antigen; whole‐blood rotational thromboelastography (ROTEM) and plasma thrombin generation. Prospectively, medical history; ISTH BAT score, and medication use were evaluated again. Blood was taken to repeat and extend laboratory tests for VWD (including multimer pattern, VWF–FVIII binding, VWF–collagen binding) and PFD (including ATP release and flow cytometry), as described in the Supplementary material.
Multiparameter thrombus formation using microfluidics
Multiparameter microfluidic assays were performed using the Maastricht flow chamber, as described previously in detail, 29 , 33 but adapted to operation at the physiological temperature of 37°C. 38
Brightfield and tri‐colour fluorescence images were taken with an EVOS‐FL microscope (Life Technologies Europe, Beringe, Belgium), every minute during an overall time of 7 min. Platelet adhesion and aggregation were assessed using the following parameters (Table 1): platelet surface area coverage: obtained from threshold, representing identified regions of all adhered platelet and thrombus structure (P1); platelet deposition: identified regions of platelet deposition and mono‐ or multi‐layered thrombi (P2); thrombus morphology score (P3); thrombus contraction score (P4) and thrombus multilayer score (P5). Fibrinogen binding was assessed to report on integrin αIIbβ3 activation (P6). Phosphatidylserine (PS) exposure (P7) was measured as a marker of platelet procoagulant activity. Platelet secretion was assessed by measuring P‐selectin expression (P8).
Table 1.
Overview of microspot surfaces (M), microfluidic parameters (P), and flow cytometry markers (C)
| Microspot surface | Platelet receptor involved | |
|---|---|---|
| M1 | Collagen type I | GP1b, GPVI, α2β1 |
| M2 | Collagen type III | GP1b, GPVI, α2β1 |
| Brightfield/Fluorescence parameters | Range | Normalized | |
|---|---|---|---|
| P1 | Platelet surface area coverage (%SAC) | 0–100 | 0–10 |
| P2 | Platelet deposition (%SAC) | 0–100 | 0–10 |
| P3 | Thrombus morphological score | 0–6 | 0–10 |
| P4 | Thrombus contraction score | 0–4 | 0–10 |
| P5 | Thrombus multilayer score | 0–4 | 0–10 |
| P6 | Integrin αIIbβ3 activation (%SAC) | 0–100 | 0–10 |
| P7 | PS exposure (%SAC) | 0–100 | 0–10 |
| P8 | P‐selectin expression (%SAC) | 0–100 | 0–10 |
| Flow cytometry markers | Platelet receptor involved | |
|---|---|---|
| C1 | CD41 | GPIIb (integrin αIIbβ3) |
| C2 | CD42a | GPIX |
| C3 | CD42b | GPIb (integrin α2) |
| C4 | CD42d | GPV |
| C5 | CD61 | GPIIIa (integrin β3) |
Abbreviations: GP, glycoprotein; PS, phosphatidylserine; SAC, surface area coverage.
Platelet adhesion and aggregation (P1–5) were assessed at a collagen‐I surface (M1) at a wall‐shear rate of 1000 s−1 (S1). Fluorescence parameters (P6–8) were assessed at a collagen‐I and a collagen‐III surface (M2) at wall‐shear rates of 1000 s−1 and 1600 s−1 (S2). Figure 1 shows representative images of the results of a healthy individual and a patient with severe (type 1) GT.
FIGURE 1.
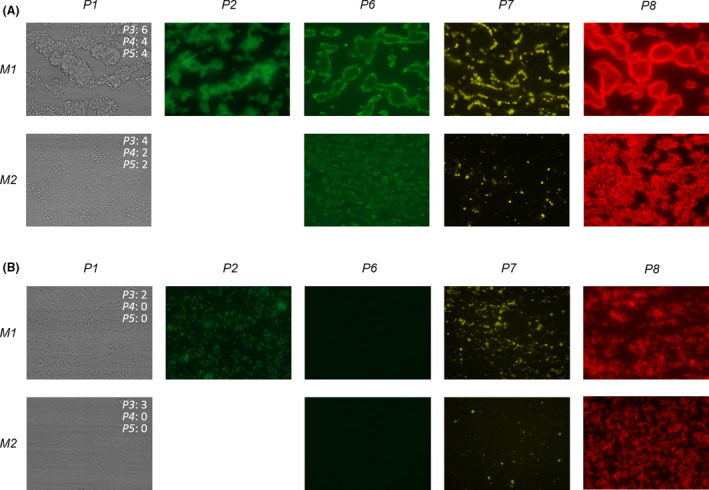
Microscopic imaging of platelet thrombus formation on two different microspots. Representative images after whole blood flow from a healthy subject (A) and a patient with type 1 Glanzmann thrombasthenia (B) over different microspots (M1, M2) at shear rate S1. For the brightfield images, score values for P3 (thrombus morphological score), P4 (thrombus contraction score) and P5 (thrombus multilayer score) at 7 min are indicated. The definition of all parameters is given in Table 1
ThromboGenomics high‐throughput sequencing (HTS)
Patients were sequenced using the ThromboGenomics HTS panel test of diagnostic‐grade genes known to harbour variants associated with rare bleeding, thrombotic, or platelet disorders. The ThromboGenomics HTS test sample preparation, sequencing protocols, tested genes, variant prioritization, interpretation and reporting, were as extensively described before. 39 , 40
RESULTS
Selection of patients and control subjects
Laboratory data from a total of 425 patients with established or suspected bleeding disorders from three earlier observational studies 37 were evaluated for the identification of ‘PFA‐only’ patients. From the 21 identified PFA‐only patients, 14 consented to be included in this study (Figure 2).
FIGURE 2.
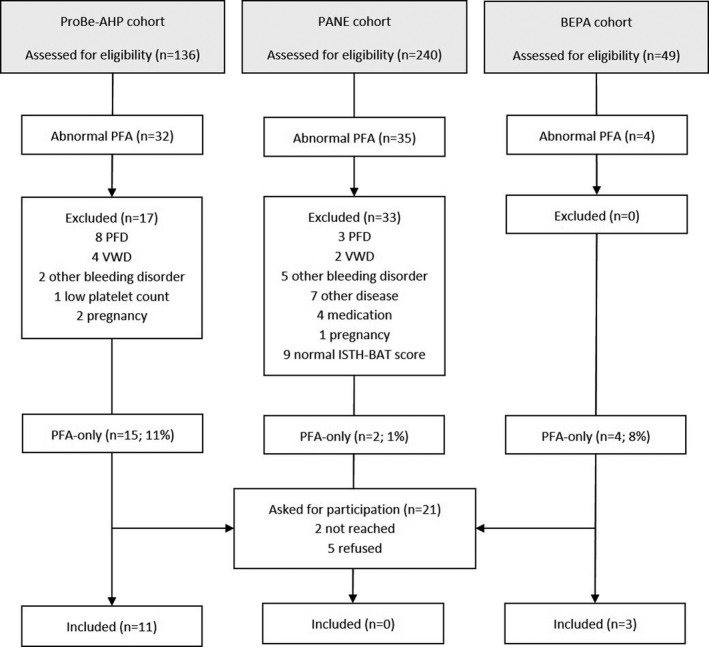
Patient Flow Diagram representing patient selection, reasons for exclusion and number of included patients from three observational studies, the ProBe‐AHP, PANE and BEPA cohorts. CT, closure time; ISTH BAT, International Society on Thrombosis and Hemostasis bleeding assessment tool; LTA, light transmission aggregometry; n, number; PFA, platelet function analyser; PFD, platelet function disorder; VWD, von Willebrand disease; VWF, von Willebrand factor
Four patients with a known bleeding disorder (VWD or GT) were selected from the BEPA study as positive controls. For the microfluidic assay, blood samples from 50 healthy controls were evaluated, in order to obtain normal values for the sets of thrombus parameters, brightfield microscopic images of shear‐dependent thrombus formation at 37°C were recorded for all 50 subjects, while parallel fluorescence images were also recorded for 23 of them and in 15 of those 23 patients, PFA results were obtained as well.
Baseline characteristics and alterations in PFA, platelet function and VWF in PFA‐only patients
Table 2 shows the prospective baseline characteristics of the 14 PFA‐only patients and the subgroup of 23 controls with parallel fluorescent images. Baseline characteristics, retrospective and prospective data of PFA‐only patients, controls and patients with a bleeding disorder are presented in Tables S1–S4.
Table 2.
Baseline characteristics of PFA‐only patients (n = 14) and control group (n = 23)
|
Characteristics mean ± SD, n (%) |
PFA‐only patients | Controls |
p (95% CI) |
|---|---|---|---|
| Female | 12 (86) | 16 (70) | 0.273 |
| Age (years) | 51 ± 17 | 34 ± 10 | 0.003 (6.257–26.687) |
| Blood type O | 9 (64) | ||
| ISTH BAT score a | 10 ± 4 | ||
| Haematocrit (l/l) | 0.42 ± 0.03 | 0.42 ± 0.03 | 0.848 (−0.0221 to 0.018) |
| Thrombocytes (×109/l) | 257 ± 84 | 279 ± 68 | 0.374 (−73.727 to 28.435) |
| MPV (nl) | 10.6 ± 1.3 | 9.8 ± 0.8 | 0.016 (0.165–1.495) |
| Leukocytes (×109/l) | 5.7 ± 1.1 | 6.2 ± 1.6 | 0.290 (−1.479 to 0.455) |
| PFA‐EPI (s) | 150 ± 42 | 111 ± 16 | 0.005 (13.558–63.728) |
| PFA‐ADP (s) | 118 ± 27 | 94 ± 13 | 0.007 (7.453–41.204) |
| VWF activity (%) | 93 ± 33 | ||
| VWF antigen (%) | 91 ± 32 | ||
| VWF ratio | 1.0 ± 0.1 | ||
| FVIII (%) | 125 ± 39 | ||
| Multimer pattern abnormal | 0 (0) | ||
| VWF‐collagen binding (%) | 82 ± 31 | ||
| VWF‐FVIII binding (%) | 94 ± 12 | ||
| Flow cytometry abnormal | 0 (0) |
Abbreviations: ADP, adenosine diphosphate; CI, confidence interval; EPI, epinephrine; ISTH BAT, International Society on Thrombosis and Hemostasis bleeding assessment tool; MPV, mean platelet volume; PFA, platelet function analyser; SD, standard deviation; VWF, von Willebrand factor.
An ISTH BAT score for males ≥4 and for females ≥6 was considered to be abnormal.
Compared to the controls, the PFA‐CT results of the PFA‐only patients were longer (mean PFA‐CT adenosine diphosphate (ADP): 118 ± 27 s vs 94 ± 13 s, p = 0.007; mean PFA‐CT epinephrine (EPI): 150 ± 42 s vs 111 ± 16 s, p = 0.005) and platelet volume was higher [mean platelet volume (MPV): 10.6 ± 1.3 nl vs 9.8 ± 0.8 nl, p = 0.016]. Furthermore, the patients were significantly older compared to the controls (mean age: 51 ± 17 years vs 34 ± 10 years, p = 0.003). The majority of the PFA‐only patients was female (86%), had blood type O (64%) and the mean ISTH BAT score was 10 ± 4. Extensive VWF tests, ATP release and flow cytometry were within the normal ranges in all 14 patients. Prospective LTA results showed two abnormal aggregation curves in one patient (nr. 8), fulfilling the diagnostic criteria of a platelet function disorder. 21 , 22 , 41 , 42 Patient nr. 9 used venlafaxine, a selective serotonin reuptake inhibitor (SSRI), possibly explaining the prolonged PFA‐CT.
Whole‐blood rotational thromboelastography (ROTEM) showed slightly prolonged clotting time in the INTEM and/or EXTEM in 10/14 PFA‐only patients. Plasma thrombin generation was normal in all but one patient, who showed increased thrombin generation (data presented in Tables S5 and S6).
Altered microfluidic thrombus formation in PFA‐only patients
Multiparameter microfluidic assessment of shear‐dependent thrombus formation, operating at a shear rate of 1000 s−1 and 1600 s−1 and a physiological temperature of 37°C, was performed with blood from all patients. Results are shown in Figure 3 (data presented in Table S7).
FIGURE 3.
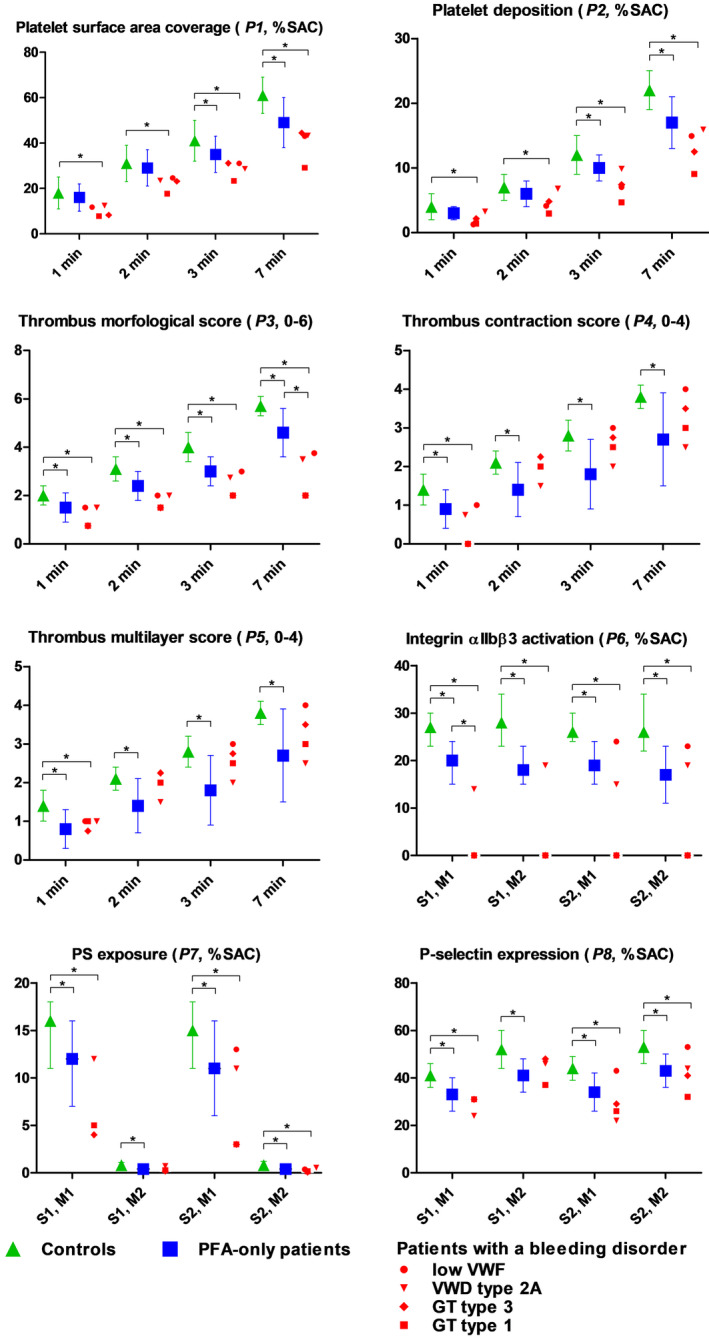
Comparison of microfluidic results of PFA‐only patients (n = 14), patients with a bleeding disorder (n = 4) and control groups (n = 50/23). Parameters P1–5 at surface M1 and shear rate S1 are compared between 50 controls (green triangle), PFA‐only patients (blue square), and patients with a bleeding disorder (in red, each symbol represents a patient). Parameters P6–8 at surface M1 and M2 and shear rate S1 and S2 are compared between PFA‐only patients, patients with a bleeding disorder and 23 controls. *alpha level of significance = p < 0.05. GT, Glanzmann thrombasthenia; PFA, platelet function analyser; PS, phosphatidylserine; SAC, surface area coverage; VWD, von Willebrand disease; VWF, von Willebrand factor. For detailed explanation of parameters (P), microspots (M) and shear rates (S) see Table 1
In the cohort of PFA‐only patients, as compared to healthy volunteers, microfluidic test results showed significantly lower platelet surface coverage (P1) and platelet deposition (P2) at 3 and 7 min (P1_7min: 49% vs 61%, p < 0.001; P2_7min: 17 vs 22, p < 0.001) and significantly lower thrombus formation scores at all time points (p < 0.008 for all comparisons). Fluorescence markers showed lower integrin activation (P6: 20% vs 27%, p = 0.001), PS exposure (P7: 12% vs 16%, p = 0.036) and P‐selectin expression (P8: 33% vs 41%, p = 0.001). No significant difference was seen in the results of PFA‐only patients compared to the results of patients with a known bleeding disorder. Thrombus contraction and multilayer score (P4, P5) were even less in PFA‐only patients when compared to patients with a bleeding disorder; however, this was not significant.
In Figure 4, the results of the microfluidic parameters are visualized per individual patient. In all patients abnormalities in microfluidic parameters were found, indicating reduced thrombus formation, compared to the group of controls. In PFA‐only patients 8, 9, 12, 13 and 14, severe abnormalities in thrombus formation were found, comparable to patients with VWD or thrombopathy.
FIGURE 4.
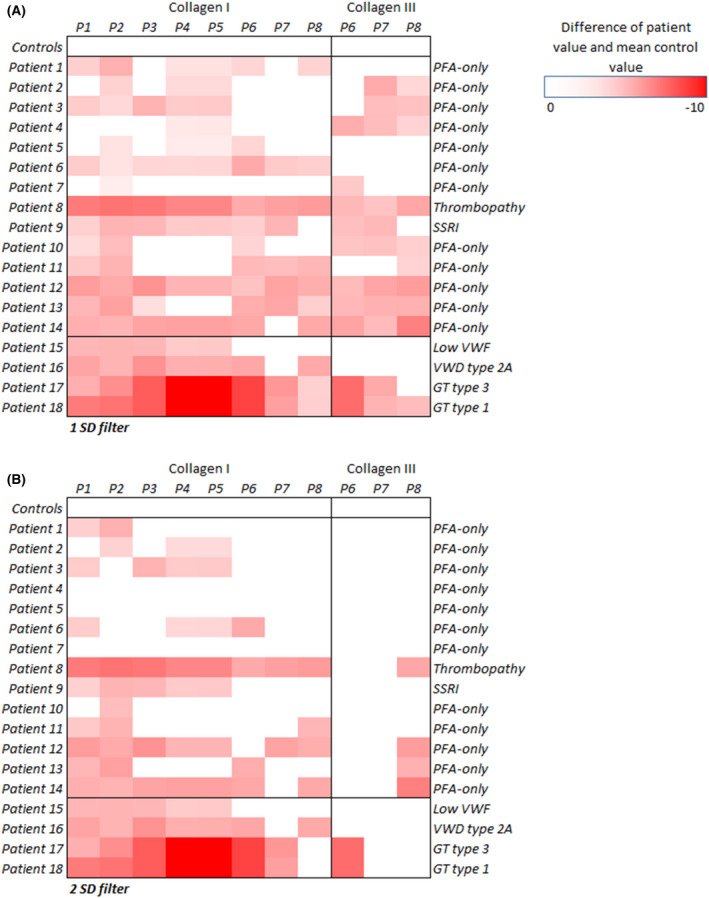
Heatmaps of microfluidic results per individual patient compared to the group of controls with 1 SD (A) and 2 SD filter (B). A darker red colour indicates that the patient's result was lower than the average result of the control group. GT, Glanzmann thrombasthenia; PFA, platelet function analyser; SD, standard deviation; SSRI, selective serotonin re‐uptake inhibitor; VWD, von Willebrand disease; VWF, von Willebrand factor
Integration of data sets
For a systemic evaluation of the characteristics, correlation analysis were performed for the laboratory results and microfluidic parameters in the 14 PFA‐only patients. Integrated results of the correlation coefficients R and p values are visualized in Figure 5A,B, respectively (results of all subjects are presented in Figure S1). The PFA‐CT results correlated moderately with VWF activity (R = −0.58, p < 0.031) and with PS exposure (P7) at M2, S2 (R = −0,62, p = 0.022) but not with other microfluidic parameters. Moderate to strong correlations were seen between microfluidic parameters and LTA aggregation curves, but not with ATP release. Furthermore, both LTA results and microfluidic parameters correlated with haematocrit (R range 0.40–0.77, p < 0.05).
FIGURE 5.
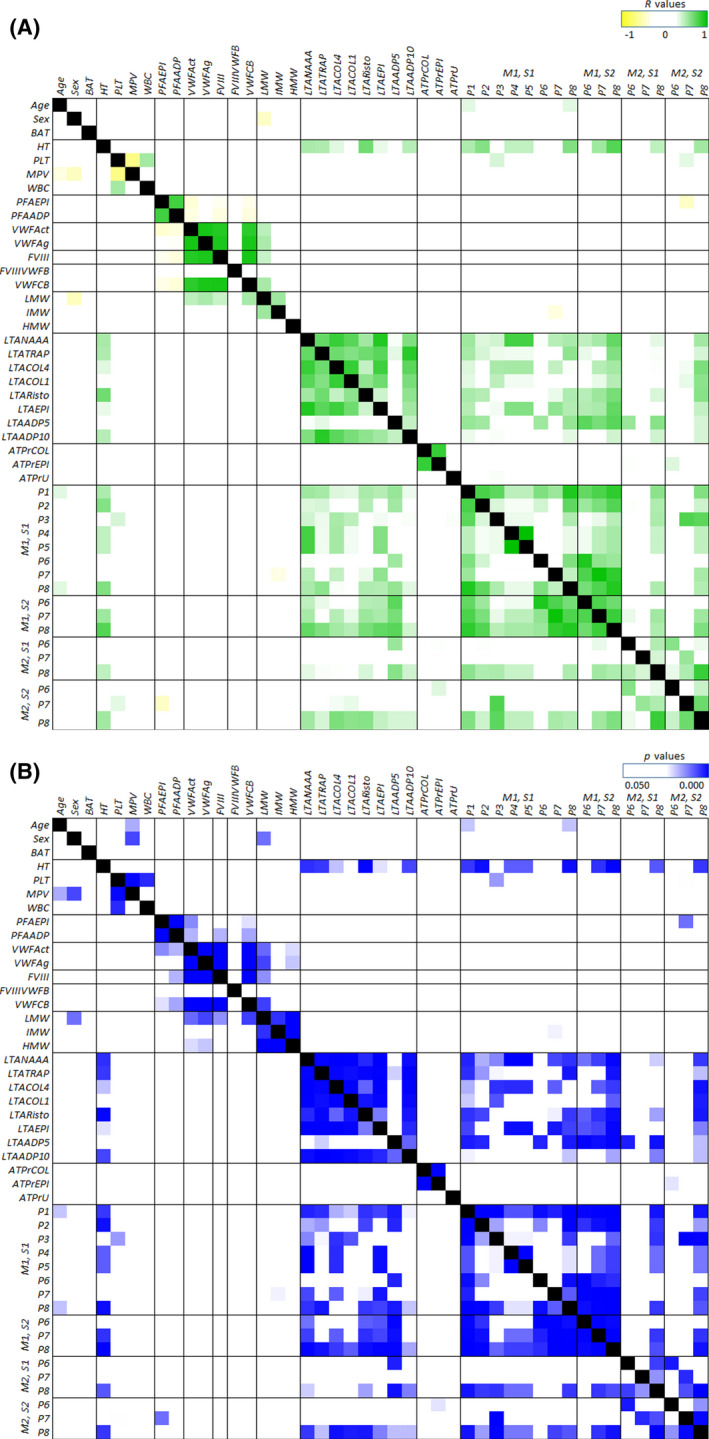
Heatmapped correlations (R) with p values of microfluidic parameters and haematology, PFA, platelet function tests, age, sex, and BAT score in the 14 PFA‐only patients. (A) Heatmapped Pearson correlation coefficients R: a darker colour indicates a stronger negative (yellow) or positive (green) correlation. (B) Heatmapped p values in which a darker colour (blue) indicates a highly significant correlation (white offset at p = 0.05). ADP, adenosine diphosphate; ATPr, adenosine triphosphate release; BAT, bleeding assessment tool; COL, collagen; EPI, epinephrine; HT, haematocrit; LTA, light transmission aggregometry; MPV, mean platelet volume; NAAA, arachidonic acid; PFA; platelet function analyser; PLT, platelet count; R, correlation coefficient; Risto, ristocetin; TRAP, thrombin receptor activatable peptide aggregometry; PFA, platelet function analyser; WBC, white blood cell count; VWFact, von Willebrand factor activity; VWFag, von Willebrand factor antigen. For detailed explanation of parameters (P), microspots (M) and shear rates (S) see Table 1
Principle components analysis (PCA) was performed to find the gross relationships between the microfluidic parameters at S1, M1 and the subject parameters in the PFA‐only patients (Figure 6A,B). Values of all subjects are shown in Figure S2. Markedly, Figure 6A shows the extent of clustering of microfluidic parameters P1–8 and haematocrit in component 1 (42%). Neither age, sex nor BAT contributed to this component. In component 2 (22%) the other haematology parameters clustered together. Figure 6B shows the extent of clustering of microfluidic parameters, LTA aggregation curves and haematocrit in component 1 (52%), while PFA with VWF and to a lesser extent P6 clustered in component 2 (18%).
FIGURE 6.
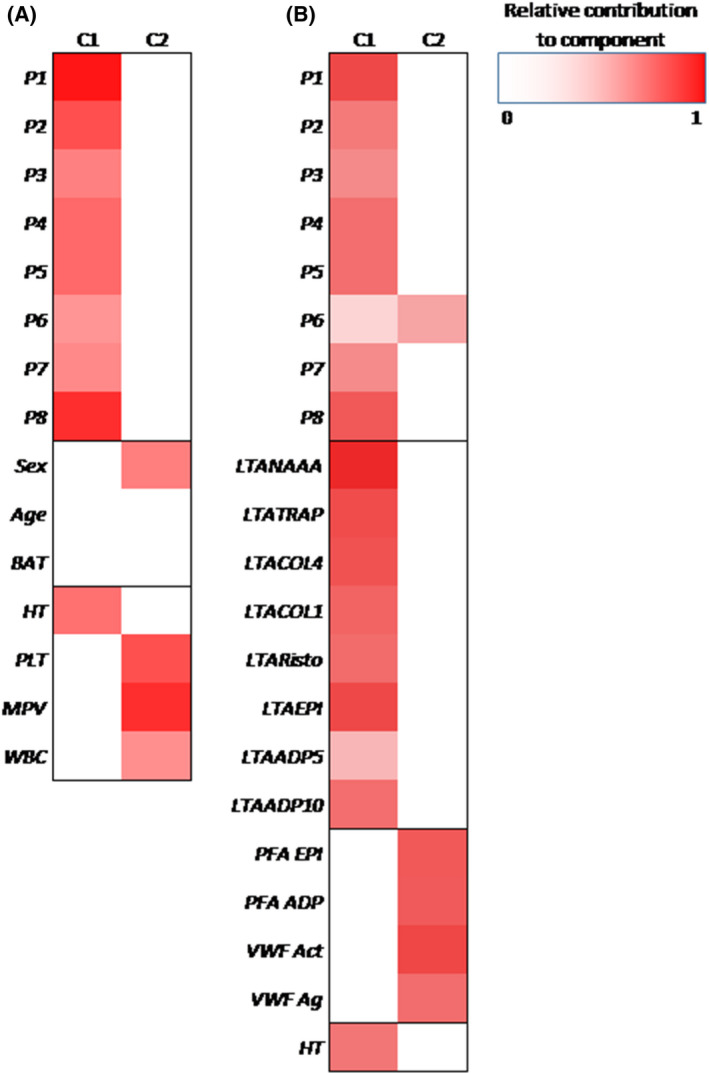
Principal components analysis of microfluidic parameters P1–8 at surface M1 at shear rate S1 and (A) sex, age, ISTH BAT score, HT, PLT, MPV and WBC score, and (B) LTA aggregation curves, PFA, VWF and HT in PFA‐only patients (n = 14). Darker (red) colours indicate the parameters that tend to cluster together per component when compared to other sets of parameters. ADP, adenosine diphosphate; BAT, bleeding assessment tool score; COL, collagen; EPI, epinephrine; HT, haematocrit; ISTH BAT, International Society on Thrombosis and Hemostasis bleeding assessment tool; LTA, light transmission aggregometry; MPV, mean platelet volume; NAAA, arachidonic acid; PFA, platelet function analyser; PLT, platelet count; RISTO, ristocetin; TRAP, thrombin receptor activatable peptide aggregometry; WBC, white blood cell count; VWFact, von Willebrand factor activity; VWFag, von Willebrand factor antigen. For detailed coding of parameters (P), microspots (M) and shear rates (S) see Table 1
To further investigate the parameters in the PFA‐only patients, comparative analysis was performed in subgroups stratified for results below respectively above the mean value of all patients, for PFA (total of PFA‐CT EPI and ADP), VWF activity, scaled microfluidic results, and haematocrit. Results are shown in Table S8. Patients with more prolonged PFA results (7/14), had less integrin activation (P6: 17 ± 3% vs 23 ± 4%, p = 0.011) in thrombus formation, and a trend towards a lower VWF activity (76 ± 13% vs 109 ± 39%, p = 0.070), compared to patients with higher PFA results. Patients with lower VWF activity (8/14) had significantly prolonged PFA‐CT EPI compared to patients with higher VWF (PFA‐CT EPI: 169 ± 38 s vs 124 ± 33 s, p = 0.038), but no differences were found in microfluidic test results.
Patients with severe abnormal microfluidic results (5/14), had lower LTA aggregation curves (although not considered as diagnostically relevant) and less ATP release on epinephrine compared to patients with less severe abnormalities [e.g. LTA arachidonic acid (NAAA): 72 ± 7% vs 85 ± 4%, p = 0.001; LTA ADP 5: 63 ± 11% vs 81 ± 4%, p = 0.001; ATPrEPI: 1.19 ± 0.09 nmol vs 1.54 ± 0.41 nmol, p = 0.032], there was no significant difference in VWF activity. Finally, patients with lower haematocrit (6/14) had lower LTA aggregation curves and more abnormal microfluidic test results (e.g. LTA NAAA: 74 ± 8% vs 84 ± 5%, p = 0.014; platelet deposition P2: 14 ± 3% vs 20 ± 2%, p = 0.001).
Results of platelet addition experiments
To see if the reduction in thrombus formation could be rescued, blood of patients 12 and 14 as well as two healthy controls was supplemented with either 80 μl HEPES buffer pH 7.45 or washed platelets, in order to increase the platelet count of the patients by 20% or 40%. Results displayed in Figure S3 in the Supplementary material, showed that an addition of 20% healthy donor platelets did not increase the amount or size of platelet aggregates formed [end‐point multilayer surface area coverage (ML SAC)]. In patient nr. 12, the platelet count was then increased with 40%, which resulted in a significant increase in platelet aggregate size (ML SAC: 3% vs 6%, p = 0.010). However, platelet aggregate size remained much lower compared to the healthy control (ML SAC: 22%). A further increase in platelet count was not possible, because this caused to much dilution of the samples.
Results of high‐throughput sequencing of PFA‐only patients
The ThromboGenomics HTS gene panel test results were available for all PFA‐only patients. The patients were tested with the ThromboGenomics version 3 (TG.V3), including 96 Mendelian genes causing coagulation, thrombotic, or platelet disorders and probes for 10 000 common single‐nucleotide variants (SNVs) to estimate relatedness and ancestry. 39 No genetic variants (SNV or structural variants) that could explain the coagulation or platelet abnormality were identified.
DISCUSSION
In this study we investigated whether and how the multiparameter shear‐dependent microfluidic assay could help to identify abnormalities in platelets or VWF that can explain the prolonged closure times of PFA‐only patients with a bleeding history.
We found that in PFA‐only patients: (i) most microfluidic test parameters were impaired compared to healthy controls. Abnormalities reached to levels seen in patients with established bleeding disorders, such as GT and VWD; (ii) PCA analysis indicated VWF and platelet activation as co‐variables of PFA results; (iii) LTA aggregation results and haematocrit were indicated as co‐variables of microfluidic parameters; (iv) in patients with the most severe microfluidic abnormalities, LTA aggregation results were in the lowest range of normal; (v) patients with lower haematocrit had more abnormal microfluidic test results; and (vi) exome sequencing did not reveal a monogenic explanation for the abnormalities found.
Our results show that an abnormal PFA as a single aberrant test in patients referred for bleeding evaluation, has to be considered for further evaluation. We found that PFA‐only patients exhibited abnormal multiparameter microfluidic test results, and analysis of the specific microfluidic outcome parameters indicated impaired thrombus formation and less platelet activation in these patients. In some PFA‐only patients abnormalities were even comparable to those in patients with previously established VWD or a PFD. PFA prolongation was associated with low–normal VWF, while microfluidic abnormalities were also associated with low–normal LTA aggregation curves and low haematocrit.
Aberrations in microfluidic assays have been described in patients with mild to severe platelet function defects like Gray platelet syndrome, storage pool disease, Bernard–Soulier and Glanzmann thrombasthenia, 29 , 30 but also in patients with VWD type 1 and low VWF. 30 , 43 The correlations of microfluidic test results with LTA aggregation results, found in our study, indicate that the abnormalities found in these patients could reflect a shear‐dependent platelet activation defect, that is not detected using the current thresholds for normality in the static conventional platelet function tests.
Although addition of healthy platelets, in vitro, did not fully rescue the reduction in thrombus formation, an increase in platelet aggregate size was observed when platelet count was increased by 40% by addition of healthy platelets. This result suggests that substantial platelet transfusion will be needed to normalize thrombus formation and to prevent bleeding in PFA‐only patients. In‐vivo experiments, before and after platelet transfusion in PFA‐only patients, are needed to investigated this in detail.
Both the PFA‐100 and the microfluidic test rely on combined processes of shear‐dependent primary (collagen) and secondary (autocrine) platelet activation along with VWF function. Exome sequencing of a large panel of genes associated with coagulation, thrombotic, or platelet disorders, did not reveal a monogenic explanation (e.g., a TIER1 mutation) for the abnormalities found in the PFA‐only patients. This does not exclude genetic variations in unknown genes involved in the VWF–platelet interactions, or the accumulative effects of a number of common variants, 39 which might result in the abnormalities found (Tier‐2/3 mutations were not tested, since there are no prior data on how these affect platelet traits under high‐shear conditions). Furthermore, for the majority of patients we are not aware of a familial history of bleeding, which includes the possibility of an acquired or somatic mutation effect. Some of the patients had low–normal VWF. We hypothesize that the bleeding disorder in PFA‐only patients is multifactorial, and includes combinations of low VWF, Tier‐2/3 mutations of platelet function genes, reduced autocrine processes and acquired or somatic platelet defects. Furthermore, there may be a relation with the novel shear‐dependent receptor GRP56, but nothing is known about the expression variability of this receptor. 44
In the ROTEM test the activation and acceleration of coagulation relies on platelet phospholipids, since no phospholipids are added to the samples. Although the shear stress in this method is only low, these results might reflect a problem in the flip‐flop mechanism, phospholipid expression and procoagulant activity of the platelets.
In the PFA‐only patients, but not in all subjects, haematocrit correlated with the microfluidic test results. Patients with low–normal haematocrit had more abnormal microfluidic test results. Haematocrit is known to influence platelet interaction with the vessel wall, but the effect is limited to levels below 0.25 l/l. 45 A previous study of a microfluidic assay in healthy individuals, found no association between haematocrit and the microfluidic assay results. 46 However, in PFA‐only patients, reduced red blood cell‐dependent platelet migration towards the vessel wall might aggravate the abnormalities of platelet function found in the microfluidic assay.
Based on the present analysis, we propose that, in all patients with an unexplained abnormal PFA, microfluidic testing should be considered in the diagnostic work‐up for mild bleeding tendency as an additional test of shear‐dependent VWF or platelet function. Our results also indicate that it would be interesting to perform microfluidic testing in all patients with bleeding of unknown cause (BUC). Only one study investigated the results of a microfluidic assay in a group of ten BUC patients and found no significant differences compared to the healthy controls. 43 However, the microfluidic assay used in this study differed greatly from our assay. This study is limited by the small number of PFA‐only patients, of which two patients prospectively had an explanation for the prolongation of PFA‐CT. This prevented us from performing a robust regression analysis and therefore, our results should be interpreted with care. However, we still believe that our findings add important information to the discussion about BUC patients with an unexplained abnormal PFA and give directions for further research.
In conclusion, our study showed that an abnormal PFA as a single aberrant test in bleeding patients should be further evaluated. The multiparameter microfluidic test is able to detect abnormalities in these patients, also detecting low–normal LTA aggregation results, suggesting a shear‐dependent platelet function defect, not detected by the static conventional platelet function tests. To further develop of this assay, an alpha‐version prototype 38 has been placed in the diagnostics laboratory of our hospital, and is currently used to evaluate blood samples from patients with bleeding alongside the routine platelet functions tests. Based on the outcome of this routine analysis, beta versions will be placed in other laboratories.
CLINICAL TRIAL REGISTRY INFORMATION
Netherlands Trial Register: NL3873 (NTR4070), https://www.trialregister.nl/trial/3873
CONFLICTS OF INTEREST
All authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest. NJJ is registered in a joined PhD program of the Universities of Maastricht and Birmingham and received funding from the European Union's Horizon 2020 research and innovation program under Marie Sklodowska‐Curie grant agreement TAPAS 766118. PM and JWMH are cofounders and JWMH is share‐holder of FlowChamber b.v. FCJIH‐M, SLNB, LB, RJHW, PWMV, KM, KD, EAMB and YMCH declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
FCJIH‐M contributed to the concept and study design, included patients, managed the database, performed statistical analysis and wrote the manuscript. SLNB performed statistical analysis and contributed to critical writing, and approved the final version to be published. LH performed laboratory analysis, contributed to critical writing and approved the final version to be published. LB included patients, managed the database, contributed to critical writing and approved the final version to be published. NJJ performed additional laboratory analysis for the revised version of the manuscript and approved the final version to be published. RJHW performed laboratory analysis and contributed to critical writing, and approved the final version to be published. PWMV performed laboratory analysis, contributed to critical writing and approved the final version to be published. PM approved the final version to be published. KM performed and interpreted the ThromboGenomics HTS test, contributed to critical writing and approved the final version to be published. KD performed and interpreted the ThromboGenomics HTS test, contributed to critical writing and approved the final version to be published. JWMH contributed to the concept and study design, contributed to critical writing and revising the intellectual content, and approved the final version to be published. EAMB contributed to the concept and study design, contributed to critical writing and revising the intellectual content, and approved the final version to be published. YMCH ontributed to the concept and study design, contributed to critical writing and revising the intellectual content, and approved the final version to be published.
Supporting information
Supplementary Material
Heubel‐Moenen FCJI, Brouns SLN, Herfs L, Boerenkamp LS, Jooss NJ, Wetzels RJH, et al. Multiparameter platelet function analysis of bleeding patients with a prolonged platelet function analyser closure time. Br J Haematol. 2022;196:1388–1400. 10.1111/bjh.18003
Funding information
NJJ received funding from the European Union's Horizon 2020 research and innovation programme under Marie Sklodowska‐Curie grant agreement TAPAS 766118. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication
References
- 1. Kratzer MA, Born GV. Simulation of primary haemostasis in vitro. Haemostasis. 1985;15(6):357–62. [DOI] [PubMed] [Google Scholar]
- 2. Favaloro EJ. Clinical utility of closure times using the platelet function analyzer‐100/200. Am J Hematol. 2017;92(4):398–404. [DOI] [PubMed] [Google Scholar]
- 3. Harrison P. The role of PFA‐100 testing in the investigation and management of haemostatic defects in children and adults. Brit J Haematol. 2005;130(1):3–10. [DOI] [PubMed] [Google Scholar]
- 4. Fressinaud E, Veyradier Agnès, Truchaud F, Martin I, Boyer‐Neumann C, Trossaert M, et al. Screening for von Willebrand disease with a new analyzer using high shear stress: a study of 60 cases. Blood. 1998;91(4):1325–31. [PubMed] [Google Scholar]
- 5. Karger R, Donner‐Banzhoff N, Muller HH, Kretschmer V, Hunink M. Diagnostic performance of the platelet function analyzer (PFA‐100 (R)) for the detection of disorders of primary haemostasis in patients with a bleeding history ‐ a systematic review and meta‐analysis. Platelets. 2007;18(4):249–60. [DOI] [PubMed] [Google Scholar]
- 6. Cattaneo M. Are the bleeding time and PFA‐100R useful in the initial screening of patients with mucocutaneous bleedings of hereditary nature? J Thromb Haemost. 2004;2(6):890–1. [DOI] [PubMed] [Google Scholar]
- 7. Ardillon L, Ternisien C, Fouassier M, Sigaud M, Lefrançois A, Pacault M, et al. Platelet function analyser (PFA‐100) results and von Willebrand factor deficiency: a 16‐year ‘real‐world’ experience. Haemophilia. 2015;21(5):646–52. [DOI] [PubMed] [Google Scholar]
- 8. Favaloro EJ. Utility of the platelet function analyser (PFA‐100/200) for exclusion or detection of von Willebrand disease: a study 22 years in the making. Thromb Res. 2020;188:17–24. [DOI] [PubMed] [Google Scholar]
- 9. Cattaneo M, Federici AB, Lecchi A, Agati B, Lombardi R, Stabile F, et al. Evaluation of the PFA‐100 system in the diagnosis and therapeutic monitoring of patients with von Willebrand disease. Thromb Haemost. 1999;82(1):35–9. [PubMed] [Google Scholar]
- 10. Favaloro EJ, Pasalic L, Curnow J. monitoring therapy during treatment of von Willebrand disease. Semin Thromb Hemost. 2017;43(3):338–54. [DOI] [PubMed] [Google Scholar]
- 11. van Vliet HH, Kappers‐Klunne MC, Leebeek FW, Michiels JJ. PFA‐100 monitoring of von Willebrand factor (VWF) responses to desmopressin (DDAVP) and factor VIII/VWF concentrate substitution in von Willebrand disease type 1 and 2. Thromb Haemost. 2008;100(3):462–8. [PubMed] [Google Scholar]
- 12. Cattaneo M, Lecchi A, Agati B, Lombardi R, Zighetti ML. Evaluation of platelet function with the PFA‐100 system in patients with congenital defects of platelet secretion. Thromb Res. 1999;96(3):213–7. [DOI] [PubMed] [Google Scholar]
- 13. Moenen FCJI, Vries MJA, Nelemans PJ, van Rooy KJM, Vranken JRRA, Verhezen PWM, et al. Screening for platelet function disorders with multiplate and platelet function analyzer. Platelets. 2019;30(1):81–7. [DOI] [PubMed] [Google Scholar]
- 14. Kerényi A, Schlammadinger Á, Ajzner É, Szegedi I, Kiss C, Pap Z, et al. Comparison of PFA‐100 closure time and template bleeding time of patients with inherited disorders causing defective platelet function. Thromb Res. 1999;96(6):487–92. [DOI] [PubMed] [Google Scholar]
- 15. Podda GM, Bucciarelli P, Lussana F, Lecchi A, Cattaneo M. Usefulness of PFA‐100 (R) testing in the diagnostic screening of patients with suspected abnormalities of hemostasis: comparison with the bleeding time. J Thromb Haemost. 2007;5(12):2393–8. [DOI] [PubMed] [Google Scholar]
- 16. Norman J, Westbury S, Jones M, Mumford A. How should we test for nonsevere heritable platelet function disorders? Int J Lab Hematol. 2014;36(3):326–33. [DOI] [PubMed] [Google Scholar]
- 17. Hayward C, Harrison P, Cattaneo M, Ortel T, Rao A. Platelet function analyzer (PFA)‐100 closure time in the evaluation of platelet disorders and platelet function. J Thromb Haemost. 2006;4(2):312–9. [DOI] [PubMed] [Google Scholar]
- 18. Harrison P, Robinson M, Liesner R, Khair K, Cohen H, Mackie I, et al. The PFA‐100: a potential rapid screening tool for the assessment of platelet dysfunction. Clin Lab Haematol. 2002;24(4):225–32. [DOI] [PubMed] [Google Scholar]
- 19. Favaloro EJ. The utility of the PFA‐100 in the identification of von Willebrand disease: a concise review. Semin Thromb Hemost. 2006;32(5):537–45. [DOI] [PubMed] [Google Scholar]
- 20. Favaloro EJ. Clinical utility of the PFA‐100. Semin Thromb Hemost. 2008;34(8):709–33. [DOI] [PubMed] [Google Scholar]
- 21. Gresele P, Physiology SP. Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13(2):314–22. [DOI] [PubMed] [Google Scholar]
- 22. Dawood BB, Lowe GC, Lordkipanidzé M, Bem D, Daly ME, Makris M, et al. Evaluation of participants with suspected heritable platelet function disorders including recommendation and validation of a streamlined agonist panel. Blood. 2012;120(25):5041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pai M, Wang G, Moffat KA, Liu Y, Seecharan J, Webert K, et al. Diagnostic usefulness of a lumi‐aggregometer adenosine triphosphate release assay for the assessment of platelet function disorders. Am J Clin Pathol. 2011;136(3):350–8. [DOI] [PubMed] [Google Scholar]
- 24. Sharma R, Haberichter SL. New advances in the diagnosis of von Willebrand disease. Hematology Am Soc Hematol Educ Program. 2019;2019(1):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quiroga T, Goycoolea M, Panes O, Aranda E, Martinez C, Belmont S, et al. High prevalence of bleeders of unknown cause among patients with inherited mucocutaneous bleeding. A prospective study of 280 patients and 299 controls. Haematologica. 2007;92(3):357–65. [DOI] [PubMed] [Google Scholar]
- 26. Posan E, McBane RD, Grill DE, Motsko CL, Nichols WL. Comparison of PFA‐100 testing and bleeding time for detecting platelet hypofunction and von Willebrand disease in clinical practice. Thromb Haemost. 2003;90(3):483–90. [DOI] [PubMed] [Google Scholar]
- 27. MacDonald S, Wright A, Beuche F, Downes K, Besser M, Symington E, et al. Characterization of a large cohort of patients with unclassified bleeding disorder; clinical features, management of haemostatic challenges and use of global haemostatic assessment with proposed recommendations for diagnosis and treatment. Int J Lab Hematol. 2019;42(2):116–25. [DOI] [PubMed] [Google Scholar]
- 28. Obaji S, Alikhan R, Rayment R, Carter P, Macartney N, Collins P. Unclassified bleeding disorders: outcome of haemostatic challenges following tranexamic acid and/or desmopressin. Haemophilia. 2016;22(2):285–91. [DOI] [PubMed] [Google Scholar]
- 29. de Witt SM, Swieringa F, Cavill R, Lamers MME, van Kruchten R, Mastenbroek T, et al. Identification of platelet function defects by multi‐parameter assessment of thrombus formation. Nat Commun. 2014;5:4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brouns SLN, van Geffen JP, Heemskerk JWM. High‐throughput measurement of human platelet aggregation under flow: application in hemostasis and beyond. Platelets. 2018;29(7):662–9. [DOI] [PubMed] [Google Scholar]
- 31. Petersen R, Lambourne JJ, Javierre BM, Grassi L, Kreuzhuber R, Ruklisa D, et al. Platelet function is modified by common sequence variation in megakaryocyte super enhancers. Nat Commun. 2017;8:16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagy M, Mastenbroek TG, Mattheij NJA, de Witt S, Clemetson KJ, Kirschner J, et al. Variable impairment of platelet functions in patients with severe, genetically linked immune deficiencies. Haematologica. 2018;103(3):540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Geffen JP, Brouns SLN, Batista J, McKinney H, Kempster C, Nagy M, et al. High‐throughput elucidation of thrombus formation reveals sources of platelet function variability. Haematologica. 2019;104(6):1256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roest M, Reininger A, Zwaginga JJ, King MR, Heemskerk JWM, Isth BSS. Flow chamber‐based assays to measure thrombus formation in vitro: requirements for standardization. J Thromb Haemost. 2011;9(11):2322–4. [DOI] [PubMed] [Google Scholar]
- 35. Van Kruchten R, Cosemans JM, Heemskerk JW. Measurement of whole blood thrombus formation using parallel‐plate flow chambers–a practical guide. Platelets. 2012;23(3):229–42. [DOI] [PubMed] [Google Scholar]
- 36. Heemskerk JW, Sakariassen KS, Zwaginga JJ, Brass LF, Jackson SP, Farndale RW. Collagen surfaces to measure thrombus formation under flow: possibilities for standardization. J Thromb Haemost. 2011;9(4):856–8. [DOI] [PubMed] [Google Scholar]
- 37. Moenen F, Nelemans PJ, Schols SEM, Schouten HC, Henskens YMC, Beckers EAM. The diagnostic accuracy of bleeding assessment tools for the identification of patients with mild bleeding disorders: a systematic review. Haemophilia. 2018;24(4):525–35. [DOI] [PubMed] [Google Scholar]
- 38. Herfs L, Swieringa F, Jooss N, Kozlowski M, Heubel‐Moenen FCJ, van Oerle R, et al. Multiparameter microfluidics assay of thrombus formation reveals increased sensitivity to contraction and antiplatelet agents at physiological temperature. Thromb Res. 2021;203:46–56. [DOI] [PubMed] [Google Scholar]
- 39. Downes K, Megy K, Duarte D, Vries M, Gebhart J, Hofer S, et al. Diagnostic high‐throughput sequencing of 2396 patients with bleeding, thrombotic, and platelet disorders. Blood. 2019;134(23):2082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Megy K, Downes K, Simeoni I, Bury L, Morales J, Mapeta R, et al. Curated disease‐causing genes for bleeding, thrombotic, and platelet disorders: Communication from the SSC of the ISTH. J Thromb Haemost. 2019;17(8):1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hayward CP. Diagnostic evaluation of platelet function disorders. Blood Rev. 2011;25(4):169–73. [DOI] [PubMed] [Google Scholar]
- 42. Cattaneo M, Cerletti C, Harrison P, Hayward CPM, Kenny D, Nugent D, et al. Recommendations for the Standardization of Light Transmission Aggregometry: a consensus of the working party from the Platelet Physiology Subcommittee of SSC/ISTH. J Thromb Haemost. 2013;11(6):1183–9. [DOI] [PubMed] [Google Scholar]
- 43. Lehmann M, Ashworth K, Manco‐Johnson M, Di Paola J, Neeves KB, Ng CJ. Evaluation of a microfluidic flow assay to screen for von Willebrand disease and low von Willebrand factor levels. J Thromb Haemost. 2018;16(1):104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yeung J, Adili R, Stringham EN, Luo R, Vizurraga A, Rosselli‐Murai LK, et al. GPR56/ADGRG1 is a platelet collagen‐responsive GPCR and hemostatic sensor of shear force. Proc Natl Acad Sci U S A. 2020;117(45):28275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weisel JW, Litvinov RI. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost. 2019;17(2):271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neeves KB, Onasoga AA, Hansen RR, Lilly JJ, Venckunaite D, Sumner MB, et al. Sources of variability in platelet accumulation on type 1 fibrillar collagen in microfluidic flow assays. PLoS One. 2013;8(1):e54680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


