Abstract
Secretory breast carcinoma is a rare neoplasm, histologically well‐characterized, and secondary to ETV6‐NTRK3 gene fusion, whose cytological features are scarcely described in the literature. We report the case of a woman with a history of secretory breast carcinoma 8 years before, who presented a periareolar nodule. A recurrence was diagnosed by fine‐needle aspiration based on the cytomorphological features and pan‐TRK immunocytochemistry on the cell block, and the patient underwent a mastectomy. The histology and molecular studies performed on the surgical specimen (immunohistochemistry, FISH and NGS) confirmed the diagnosis. Cytological smears showed abundant epithelial cellularity, in groups and single cells. These cells showed moderate atypia, with abundant cytoplasm. We observed intracytoplasmic inclusions and extracellular metachromatic globules. Immunocytochemical and immunohistochemical studies showed a triple negative breast tumour. NTRK overexpression was demonstrated with immunocytochemistry against pan‐TRK on the cell block, as well as with immunohistochemistry in the surgical specimen. NTRK3 rearrangement was proved by FISH. In the primary tumour and in the recurrence, we demonstrated ETV6‐NTRK3 fusion by NGS. After conducting a literature review, we have found 26 articles describing the cytological features of secretory breast carcinoma in 33 patients. The smears were described as groups of epithelial cells with vacuolated cytoplasm, single signet ring cells and a globular extracellular secretion. In only two cases molecular confirmation of the diagnosis with ETV6‐NTRK3 fusion was proven, although not in the cytological specimen, but in the subsequent biopsy. The distinct cytological features of secretory breast carcinoma can help in its diagnosis, thus guiding the molecular studies. This is the first reported case that proves TRK overexpression, as a fusion surrogate, in the cytological sample.
Keywords: molecular diagnosis, NGS, NTRK, secretory breast carcinoma
1. INTRODUCTION
Secretory breast carcinoma (SBC) is a very rare but distinct breast neoplasm that accounts for <0.05% of all breast cancers. 1 It predominantly affects women, with a mean patient age of 53 years, 1 although it was first described in children. 2
Histologically, it is composed of epithelial cells with intracytoplasmic vacuoles and an extracellular eosinophilic bubbly secretion, both of which are PAS positive and diastase‐resistant. 3 Regarding immunohistochemistry, secretory carcinoma usually exhibits a triple negative profile, without expression of oestrogen receptor, progesterone receptor nor human epidermal growth factor receptor 2 (HER2). It normally expresses GATA3, SOX10 and S100. 4 In spite of portraying a triple negative profile, SBC is associated with a favourable prognosis. 5
SBC is well characterized molecularly by the pathognomonic ETV6‐NTRK3 gene fusion, 6 with oncogenic activity and susceptible of treatment with TRK inhibitors. 7 This fusion can be detected by fluorescent in situ hybridization (FISH), reverse transcription polymerase chain reaction (RT‐PCR) and next generation sequencing (NGS) techniques. 1 Additionally, it is possible to suggest the presence of this fusion using immunohistochemistry with a pan‐TRK antibody. With this technique, NTRK3‐fused cases characteristically tend to show nuclear staining. 3
Despite these distinct histomorphological and molecular features, SBC cytological features are scarcely described in the literature.
2. CASE REPORT
We report the case of a 73 year‐old woman with a history of a pT1cN0M0 secretory breast carcinoma (diagnosis based only on morphological criteria), treated with a lumpectomy with sentinel lymph node biopsy, and adjuvant radiotherapy (40 Gy) in 2012. Eight years later, she presented with a periareolar nodule.
On physical examination, a 1.5 cm firm palpable subcutaneous nodule, next to the surgical scar, was evident. A recurrence was diagnosed by fine needle aspiration. Cytological smears of the periareolar nodule showed abundant epithelial cellularity, arranged in groups and single cells. They showed moderate nuclear atypia, with prominent nucleoli and abundant dense polygonal cytoplasm, resembling a squamous neoplasm. We observed dense and metachromatic material, located as intracytoplasmic drops and as extracellular globules (Figure 1). The patient was treated with a mastectomy and axillary lymph node dissection. Given the favourable histology, she did not receive subsequent adjuvant chemotherapy.
FIGURE 1.

(A) May‐Grünwald Giemsa. Cluster of atypical epithelioid cells with moderate nuclear atypia, prominent nucleoli and abundant squamous‐like cytoplasm. A mitotic figure is shown (arrow). (B) Papanicolaou. Atypical cells with signet‐ring features and intracytoplasmic drops (arrows). (C) May‐Grünwald Giemsa. Atypical cells, one of them with signet ring features and an intracytoplasmic metachromatic drop (arrow). (D) May‐Grünwald Giemsa. Atypical epithelioid cells next to an extracellular metachromatic secretion
A year later the patient developed elevated skin lesions, occupying a large area of the right chest wall, which on histologic examination demonstrated dermic and lymphatic infiltration by secretory carcinoma. At this time, the patient was included in an open‐label, single arm, phase 2 clinical trial (NCT04408118) with atezolizumab in combination with paclitaxel and bevacizumab, showing partial response after 5 cycles.
Molecular studies were carried out with immunocytochemistry on the cell block (formalin fixed and paraffin embedded) and with immunohistochemistry, FISH and NGS on the mastectomy specimen.
In the mastectomy specimen, a 2.2 cm firm poorly‐delimited white mass was found. In the histopathological evaluation, we could observe that it was composed of epithelial cells arranged in a microcytic pattern with eosinophilic secretions in a sclerotic stroma. The cells were polygonal with eosinophilic cytoplasm and mild nuclear atypia (Figure 2A–C).
FIGURE 2.

(A) Mastectomy specimen, showing a 2.2 cm ill‐defined mass. (B) Panoramic view of the tumour. (C) Epithelial cells arranged in a microcytic pattern with eosinophilic secretions in a sclerotic stroma. (D) Pan‐TRK immunohistochemistry showing strong nuclear and weak cytoplasm staining
Immunocytochemical and immunohistochemical studies showed a triple negative breast tumour, negative for oestrogen receptor (EP1 clone, Agilent) (Figure 3C), progesterone receptor (PgR 1294 clone, Agilent) and HER2 (Herceptest, Agilent); and positive for GATA3 (L50‐823, Roche) and Sox10 (5P267, Roche). The proliferation index (Ki67 [MIB1, Agilent]) in the surgical specimen reached 20%.
FIGURE 3.
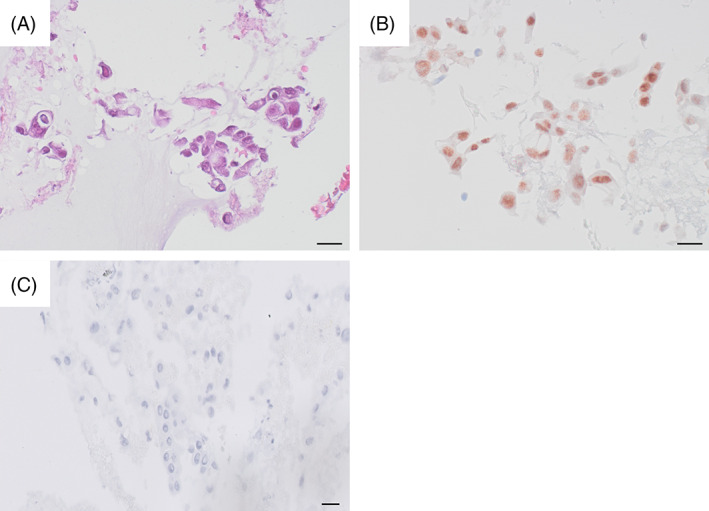
(A) Haematoxylin eosin, cell block. Atypical cells with signet‐ring features and intracytoplasmic drops. Scale bar: 20 μm. (B) Pan‐TRK immunocytochemistry, cell block. Strong nuclear staining. Scale bar: 20 μm. (C) Oestrogen receptor immunocytochemistry, cell block. Negative staining. Scale bar: 20 μm
NTRK overexpression was demonstrated with immunocytochemistry against pan‐TRK (1‐2‐3) (EPR17341, Roche) on the cell block, as well as with immunohistochemistry in the mastectomy specimen. In both cases, a striking nuclear positivity was noted (Figures 2D and 3B).
NTRK3 and ETV6 rearrangement was proved by FISH (ZytoLight SPEC NTRK3 Dual Color Break Apart Probe) (Zytovision) and LSI ETV6 Break Apart Rearrangement Probe (Vysis); respectively). Both in the primary tumour and in the recurrence, we demonstrated ETV6‐NTRK3 (ETV6‐NTRK3.E5N15.COSF571.1) fusion by NGS (OncomineTM Focus Assay (ThermoFisher Scientific), with 62,459 and 49,699 reads counts, respectively (Figure 4). FoundationOne CDx (Roche) was also performed on the mastectomy specimen, showing the ETV6‐NTRK3 fusion once again, as well as a TERT promoter substitution mutation (−124C>T) and a frameshift mutation on DNMT3A (D658fs*47). We also found variants of unknown significance on FANCA (R825G), RAD54L (P433L), IKBKE (E148_G149>DW), FGFR1 (R822C), NOTCH3 (P2209L), BRCA1 (P142H) and ALK (R806C).
FIGURE 4.
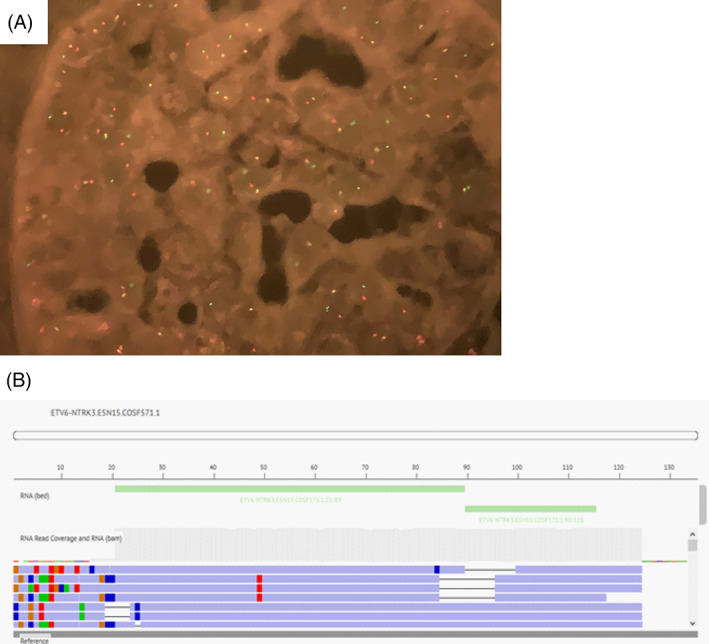
(A) NTRK3 FISH, showing the break‐apart pattern of the rearrangement. (B) ETV6‐NTRK3 gene fusion proved by NGS
3. DISCUSSION
Secretory breast carcinoma is recognized as a distinct morpho‐molecular entity due to its characteristic morphology and the presence of ETV6‐NTRK3 gene fusion.
We conducted an extensive literature review on the cytological features of secretory breast carcinoma, and we found 24 articles that described its cytological features, with a total of 33 cases. However, only in 22 studies including 25 patients, we were able to retrieve clinicopathological data. These studies included 22 women (88%) and 3 men (12%), with a mean age of 53.5 years (min 12 years; max 91 years). The tumour measurement was provided in 17 cases, with a mean size of 3 cm (min 1.5 cm; max 6.5 cm), and the final treatment was recorded in 16 patients, with mastectomy in 13 cases and lumpectomy in 3 cases.
We were able to retrieve cytological descriptions from the abstracts and/or full texts in 17 cases. Most of the smears are described as groups of epithelial cells with vacuolated cytoplasm, single cells with signet ring cell morphology and an extracellular secretion resembling globules. In just two cases ETV6‐NTRK3 gene fusion was demonstrated, although not in the cytological specimen, but in the subsequent biopsy (Table 1).
TABLE 1.
Clinicopathological features of the cases included in the reviewed articles
| Article | Sex | Age (years) | Size (cm) | Cytological description | Molecular testing |
|---|---|---|---|---|---|
| Craig 8 | Woman | 63 | NA | Solid and papillary | NA |
| D'Amore et al. 9 | Woman | 48 | NA | Sheets | NA |
| Nguyen and Neifer 10 | Woman | 73 | 2 | Sheets | NA |
| Richard et al. 11 | Woman | 37 | 1.5 | NA | NA |
| Domínguez et al. 12 | Woman | 63 | NA | Sheets and clusters | NA |
| Gupta et al. 13 | NA | NA | NA | NA | NA |
| Shinagawa et al. 14 | NA | NA | NA | Clusters | NA |
| de la Cruz et al. 15 | NA | NA | NA | NA | NA |
| Pohar‐Marinsek and Golough 16 | Man | 20 | NA | NA | NA |
| Shinagawa et al. 17 | 5 cases | NA | NA | Sheets and clusters | NA |
| Hou et al. 18 | Woman | 35 | NA | NA | NA |
| Nonomura et al. 19 | Woman | 12 | NA | Sheets and clusters | NA |
| Jayaram et al. 20 | Woman | 39 | 1.5 | Clusters | NA |
| Furugaki et al. 21 | Woman | 73 | 2.3 | NA | NA |
| Vesoulis et al. 22 | Man | 33 | NA | NA | NA |
| Gupta et al. 23 | Woman | 91 | 3 | Sheets | NA |
| Gupta et al. 23 | Woman | 83 | 4.5 | Sheets | NA |
| Gupta et al. 23 | Woman | 55 | 3 | Sheets | NA |
| Gupta et al. 23 | Woman | 69 | 6.5 | Sheets | NA |
| Izumi et al. 24 | Woman | 61 | 2.8 | Single cells | NA |
| Alenda et al. 25 | Man | 79 | 3 | Sheets | NA |
| Oh et al. 26 | Woman | 42 | 2 | NA | NA |
| Sukpan et al. 27 | Woman | 57 | 3.5 | Sheets and clusters | NA |
| Mardi and Sharma 28 | Woman | 52 | NA | NA | NA |
| Jena and Shariff 29 | Woman | 24 | 4 | Clusters | NA |
| Jena and Shariff 29 | Woman | 40 | 5 | Clusters | NA |
| Shanthi et al. 30 | Woman | 62 | 5 | Clusters | NA |
| Gupta and Gupta 31 | Woman | 66 | 2.5 | Clusters and papillae | In mastectomy specimen, technique not specified |
| Shukla et al. 32 | Woman | 60 | 2.5 | Clusters and papillae | ETV6‐NTRK PCR in mastectomy specimen |
Abbreviation: NA, not available.
The ETV6‐NTRK3 gene fusion was first described in secretory breast carcinoma in 2002 6 and most of the articles we found describing its cytological features are previous to this year. Thus, to our knowledge, this is the first reported case that proves TRK overexpression, as a fusion surrogate, in the cytological sample.
The expression of NTRK by immunocytochemistry has been previously described in secretory carcinoma of the salivary gland. 33 This tumour was previously named “mammary analogue secretory carcinoma” due to its resemblance to secretory breast carcinoma, including the ETV6‐NTRK3 fusion. 34
SBC is associated with a favourable prognosis. However, our case has shown an atypical course, with multiple recurrences; and previous studies have reported SBC recurrences. 35 Moreover, metastatic SBC harbouring TERT promoter mutation, like our case, has been previously described, 36 and it has also been reported in metastatic and locally recurrent NTRK‐rearranged thyroid carcinoma 37 and in high grade gliomas harbouring NTRK fusions. 38 Additionally, TERT promoter mutation has been the most commonly found co‐alteration in a cohort of TRK fusion cancers. 39 Regarding DNMT3A alterations, they have been described in metastatic breast cancer 40 and are associated with poor prognosis in papillary thyroid carcinoma. 41
Although SBC is very rare, its correct diagnosis has a major impact on the management of patients with this tumour. As previously stated, it usually has a good prognosis despite showing a triple negative profile. The cytological sample might be the first diagnosis material; thus, recognition of its distinct cytological features and early demonstration of ETV6‐NTRK3 gene fusion can avoid an aggressive management. Nevertheless, the cases that do not progress well are eligible for systemic treatment with TRK inhibitors. 42
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Irene Carretero‐Barrio, Amparo Benito. Data curation: Irene Carretero‐Barrio, Tamara Caniego Casas, Elena López Miranda, Maria Eugenia Reguero‐Callejas. Funding acquisition: José Palacios. Investigation: Almudena Santón. Supervision: Belén Pérez Mies, Amparo Benito, José Palacios. Writing – Original draft preparation: Irene Carretero‐Barrio, Elena López Miranda, Belén Pérez Mies, Amparo Benito, José Palacios. Writing – Review & editing: Irene Carretero‐Barrio, Almudena Santón, Tamara Caniego Casas, Maria Eugenia Reguero‐Callejas, Belén Pérez Mies, Amparo Benito, José Palacios.
Carretero‐Barrio I, Santón A, Caniego Casas T, et al. Cytological and molecular characterization of secretory breast carcinoma. Diagnostic Cytopathology. 2022;50(7):E174‐E180. doi: 10.1002/dc.24945
Funding information Centro de Investigación Biomédica en Red de Cáncer; European Regional Development Fund; Instituto de Salud Carlos III
Contributor Information
Amparo Benito, Email: amparo.benito@salud.madrid.org.
José Palacios, Email: jose.palacios@salud.madrid.org.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the Table 1 of this article. Further information can be requested to the corresponding authors.
REFERENCES
- 1. Lokuhetty D, White VA, Watanabe R, Cree IA, WHO, Classification of Tumours Editorial Board . Breast Tumours. International Agency for Research on Cancer; 2019. [Google Scholar]
- 2. McDivitt RW, Stewart FW. Breast carcinoma in children. JAMA. 1966;195(5):388‐390. [PubMed] [Google Scholar]
- 3. Harrison BT, Fowler E, Krings G, et al. Pan‐TRK immunohistochemistry: a useful diagnostic adjunct for secretory carcinoma of the breast. Am J Surg Pathol. 2019;43(12):1693‐1700. [DOI] [PubMed] [Google Scholar]
- 4. Li D, Xiao X, Yang W, et al. Secretory breast carcinoma: a clinicopathological and immunophenotypic study of 15 cases with a review of the literature. Mod Pathol. 2012;25(4):567‐575. [DOI] [PubMed] [Google Scholar]
- 5. Horowitz DP, Sharma CS, Connolly E, Gidea‐Addeo D, Deutsch I. Secretory carcinoma of the breast: results from the survival, epidemiology and end results database. The Breast. 2012;21(3):350‐353. [DOI] [PubMed] [Google Scholar]
- 6. Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6‐NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2(5):367‐376. [DOI] [PubMed] [Google Scholar]
- 7. Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol. 2019;32(1):147‐153. [DOI] [PubMed] [Google Scholar]
- 8. Craig JP. Secretory carcinoma of the breast in an adult. Correlation of aspiration cytology and histology on the biopsy specimen. Acta Cytol. 1985;29(4):589‐592. [PubMed] [Google Scholar]
- 9. d'Amore ES, Maisto L, Gatteschi MB, Toma S, Canavese G. Secretory carcinoma of the breast. Report of a case with fine needle aspiration biopsy. Acta Cytol. 1986;30(3):309‐312. [PubMed] [Google Scholar]
- 10. Nguyen G‐K, Neifer R. Aspiration biopsy cytology of secretory carcinoma of the breast. Diagn Cytopathol. 1987;3(3):234‐237. [DOI] [PubMed] [Google Scholar]
- 11. Richard G, Hawk JC, Baker AS, Austin RM. Multicentric adult secretory breast carcinoma: DNA flow cytometric findings, prognostic features, and review of the world literature. J Surg Oncol. 1990;44(4):238‐244. [DOI] [PubMed] [Google Scholar]
- 12. Domínguez F, Riera JR, Junco P, Sampedro A. Secretory carcinoma of the breast. Report of a case with diagnosis by fine needle aspiration. Acta Cytol. 1992;36(4):507‐510. [PubMed] [Google Scholar]
- 13. Gupta RK, Lallu SD, Fauck R, Simpson JS, Wakefield SJ. Needle aspiration cytology, immunocytochemistry, and electron microscopy in a rare case of secretory carcinoma of the breast in an elderly woman. Diagn Cytopathol. 1992;8(4):388‐391. [DOI] [PubMed] [Google Scholar]
- 14. Shinagawa T, Tadokoro M, Takeuchi E, Oikawa K, Kanasugi K, Kataba Y. Aspiration biopsy cytology of secretory carcinoma of the breast. A case report. Acta Cytol. 1992;36(2):189‐193. [PubMed] [Google Scholar]
- 15. de la Cruz MA, de la Cruz ME, Leston JS, de Agustin de Agustín P. Secretory carcinoma of the breast. Acta Cytol. 1994;38(6):968‐969. [PubMed] [Google Scholar]
- 16. Pohar‐Marinsek Z, Golouh R. Secretory breast carcinoma in a man diagnosed by fine needle aspiration biopsy. A case report. Acta Cytol. 1994;38(3):446‐450. [PubMed] [Google Scholar]
- 17. Shinagawa T, Tadokoro M, Kitamura H, Mizuguchi K, Kushima M. Secretory carcinoma of the breast. Correlation of aspiration cytology and histology. Acta Cytol. 1994;38(6):909‐914. [PubMed] [Google Scholar]
- 18. Hou MF, Chai CY, Wuu JR, Shen YY, Lin HJ, Huang TJ. Secretory carcinoma of the breast—a case report. Gaoxiong Yi Xue Ke Xue Za Zhi. 1995;11(9):546‐551. [PubMed] [Google Scholar]
- 19. Nonomura A, Kimura A, Mizukami Y, et al. Secretory carcinoma of the breast associated with juvenile papillomatosis in a 12‐year‐old girl. A case report. Acta Cytol. 1995;39(3):569‐576. [PubMed] [Google Scholar]
- 20. Jayaram G, Looi LM, Yip CH. Fine needle aspiration cytology of secretory carcinoma of breast: a case report. Malays J Pathol. 1997;19(1):69‐73. [PubMed] [Google Scholar]
- 21. Furugaki K, Nagai E, Shinohara M, et al. Secretory carcinoma of the breast in an elderly woman: report of a case. Surg Today. 1998;28(2):219‐222. [DOI] [PubMed] [Google Scholar]
- 22. Vesoulis Z, Kashkari S. Fine needle aspiration of secretory breast carcinoma resembling lactational changes. A case report. Acta Cytol. 1998;42(4):1032‐1036. [DOI] [PubMed] [Google Scholar]
- 23. Gupta RK, Kenwright D, Naran S, Lallu S, Fauck R. Fine needle aspiration cytodiagnosis of secretory carcinoma of the breast. Cytopathol off J Br Soc Clin Cytol. 2000;11(6):496‐502. [DOI] [PubMed] [Google Scholar]
- 24. Izumi J, Komaki K, Hirokawa M, Masuda E, Monden Y. Secretory carcinoma of the breast with a cystically dilated intraductal component: report of a case. Surg Today. 2003;33(2):110‐113. [DOI] [PubMed] [Google Scholar]
- 25. Alenda C, Aranda FI, Seguí FJ, Laforga J. Secretory carcinoma of the male breast: correlation of aspiration cytology and pathology. Diagn Cytopathol. 2005;32(1):47‐50. [DOI] [PubMed] [Google Scholar]
- 26. Oh Y‐H, Jang KS, Song YS, Paik SS, Park YW, Chon S‐H. Secretory carcinoma of the breast diagnosed by fine needle aspiration. Acta Cytol. 2005;49(3):343‐344. [DOI] [PubMed] [Google Scholar]
- 27. Sukpan K, Chanmuenwai W, Khunamornpong S. Secretory carcinoma of the breast: a case report with cytologic and histologic findings. Chiang Mai Med Bull. 2005;56:161‐166. [Google Scholar]
- 28. Mardi K, Sharma J. A rare case of secretory breast carcinoma in an elderly woman: correlation of aspiration cytology and histology. Indian J Pathol Microbiol. 2007;50(4):865‐867. [PubMed] [Google Scholar]
- 29. Jena M, Shariff S. Cytodiagnosis of secretory carcinoma of the breast: a report on two cases. Diagn Cytopathol. 2010;38(12):921‐924. [DOI] [PubMed] [Google Scholar]
- 30. Shanthi V, Rama Krishna BA, Rao NM, Sujatha C. Cytodiagnosis of secretory carcinoma of the breast. J Cytol Indian Acad Cytol. 2012;29(1):63‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta S, Gupta P. Secretory carcinoma breast—the characteristic cytological features in diagnosis of this rare carcinoma. J Cytol. 2020;37(1):63‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shukla A, Arshad F, Naseem I. Secretory carcinoma of breast: a diagnostic dilemma. Indian J Pathol Microbiol. 2020;63(Supplement):S143‐S145. [DOI] [PubMed] [Google Scholar]
- 33. Ito H, Ishida M, Ebisu Y, et al. Utility of an immunocytochemical analysis for pan‐Trk in the cytodiagnosis of secretory carcinoma of the salivary gland. Diagn Cytopathol. 2021;49(8):E329‐E335. [DOI] [PubMed] [Google Scholar]
- 34. Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6‐NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599‐608. [DOI] [PubMed] [Google Scholar]
- 35. Li L, Wu N, Li F, Li L, Wei L, Liu J. Clinicopathologic and molecular characteristics of 44 patients with pure secretory breast carcinoma. Cancer Biol Med. 2019;16(1):139‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoda RS, Brogi E, Pareja F, et al. Secretory carcinoma of the breast: Clinicopathologic profile of 14 cases emphasizing distant metastatic potential. Histopathology. 2019;75(2):213‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chu Y‐H, Dias‐Santagata D, Farahani AA, et al. Clinicopathologic and molecular characterization of NTRK‐rearranged thyroid carcinoma (NRTC). Mod Pathol Off J U S Can Acad Pathol Inc. 2020;33(11):2186‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Torre M, Vasudevaraja V, Serrano J, et al. Molecular and clinicopathologic features of gliomas harboring NTRK fusions. Acta Neuropathol Commun. 2020;8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosen EY, Goldman DA, Hechtman JF, et al. TRK fusions are enriched in cancers with uncommon histologies and the absence of canonical driver mutations. Clin Cancer Res. 2020;26(7):1624‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rong G, Yi Z, Ma F, et al. DNA damage response as a prognostic indicator in metastatic breast cancer via mutational analysis. Ann Transl Med. 2021;9(3):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siraj AK, Pratheeshkumar P, Parvathareddy SK, et al. Prognostic significance of DNMT3A alterations in middle eastern papillary thyroid carcinoma. Eur J Cancer. 2019;117:133‐144. [DOI] [PubMed] [Google Scholar]
- 42. Shukla N, Roberts SS, Baki MO, et al. Successful targeted therapy of refractory pediatric ETV6‐NTRK3 fusion‐positive secretory breast carcinoma. JCO Precis Oncol. 2017;2017:PO.17.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available in the Table 1 of this article. Further information can be requested to the corresponding authors.


