Abstract
Objective
Continuous subcutaneous insulin infusion (CSII) in youths with type 1 diabetes (T1D) is often associated with lower HbA1c, lower total daily insulin dose (TDD), and lower body mass index (BMI) compared with multiple daily injections (MDI). Individual responses to CSII are diverse. The aim was to identify unique three‐variate patterns of HbA1c, BMI standard deviation score (SDS), and TDD after switching to CSII.
Methods
Five thousand one hundred and thirty‐three youths (≤20 years; 48% boys; median age at pump start 12.5 years) with T1D duration ≥3 years at CSII initiation were selected from the multicenter DPV registry. We applied group‐based multitrajectory modeling to identify groups of individuals following similar trajectories. Measurements were aggregated quarterly during a 3‐year follow‐up period. Trajectory variables were changes of HbA1c, BMI‐SDS, and TDD from baseline (delta = quarterly aggregated values at each time point [i] minus the respective baseline value).
Results
Four groups of diverging Delta‐HbA1c, Delta‐BMI‐SDS, and Delta‐TDD patterns were identified. All showed improvements in HbA1c during the first 3 months. Group 1 (12%) was characterized by modest HbA1c increase thereafter, TDD reduction, and stable BMI‐SDS. In Group 2 (39%), increasing HbA1c, decreasing BMI‐SDS, and stable TDD were found. By contrast, sustainably improved HbA1c, increasing BMI‐SDS, and stable TDD were observed in Group 3 (32%). Group 4 (17%) was characterized by increasing levels for HbA1c, BMI‐SDS, and TDD. Between‐group differences in baseline HbA1c, BMI‐SDS, TDD as well as in sex ratio, age at diabetes onset and at pump start were observed.
Conclusions
Definite trajectories of glycemic control, BMI, and TDD over 3 years after CSII initiation were identified in youths with T1D allowing a more personalized treatment recommendation.
Keywords: BMI, children and adolescents with type 1 diabetes, CSII, diabetes, DPV, GBMT, insulin pump, longitudinal patterns, multi trajectory modeling, TDD, trajectories of HbA1c, type 1 diabetes
1. INTRODUCTION
Over the past three decades, management of type 1 diabetes (T1D) has increasingly benefited from innovations in diabetes technology, such as continuous glucose monitoring (CGM) and insulin pumps, also termed continuous subcutaneous insulin infusion (CSII). 1 CSII has become standard care for children and adolescents with T1D in many parts of the world. In Western countries, pump users represent 40%–60% of the T1D population. 2 , 3
The increasing uptake of CSII over the past 20 years has resulted from improvements in pump technology and documented health benefits: on the whole, data from meta‐analyses and systematic reviews of randomized controlled trials (RCTs) including pediatric populations suggest that in T1D, the use of an insulin pump compared with the use of multiple daily injections (MDI) is associated with a modest reduction in glycated hemoglobin A1c levels (HbA1c), with lower rates of severe hypoglycemia (SH), and diabetic ketoacidosis (DKA), better quality of life and treatment satisfaction, lower insulin requirements and in some studies lower body weight. 4 , 5 This is supported by data from more recent observational studies and registries documenting sustained benefit over long periods of pump use across different populations. 6 , 7 , 8 , 9 , 10
Despite the growing evidence regarding overall superiority of CSII therapy, meeting recommended treatment targets for T1D even when switching to insulin pump remains challenging, particularly during adolescence. 11 Deterioration in glycemic control, 12 increasing insulin demands, 13 and weight gain 14 may be found in many but not in all children suggesting wide interindividual variation. Identifying groups of patients with divergent response on CSII initiation in terms of metabolic control, weight change, and insulin requirements is of major interest.
Commonly used statistical methods have focused on temporally aggregated variables, often using cross‐sectional study designs with limitations regarding the analysis of repeated measurements and trajectories of outcomes over time. 15 Innovative mathematical models have been developed and offer the potential to consider novel aspects such as the heterogeneity of chronic disorders and the concept of precision medicine. 16 Group‐based multitrajectory (GBMT) modeling is one method to identify latent groups of individuals following similar trajectories across multiple outcomes. 17 , 18 Applying single component group‐based trajectory (GBT) modeling in T1D using data from the diabetes patient follow‐up registry (Diabetes‐Patienten‐Verlaufsdokumentation—DPV), heterogeneous courses of HbA1c 12 and progression of body mass index (BMI) 14 from childhood to young adulthood were described in a large cohort of children with T1D previously. Furthermore, joint developmental trajectories of metabolic control, BMI, and daily insulin dose during puberty were identified with GBMT in the past. 15
The objective of this study was to identify latent groups in a cohort of children and adolescents with T1D from the DPV registry following similar three‐variate patterns of changes of metabolic control, age/sex‐standardized BMI (BMI‐SDS), and daily insulin requirement after switching from MDI to CSII therapy. Identifying typical responses to CSII initiation would help caregivers selecting the optimal treatment for the individual with T1D, managing patients' expectations, and providing individually tailored support.
2. METHODS
2.1. Subjects, registry, and ethics
Anonymous data were retrieved from the German/Austrian/Luxembourgian/Swiss diabetes prospective follow‐up registry DPV. 19 DPV was launched in 1995 in Germany and has been used for nationwide benchmarking and scientific analyses. Currently, demographic and clinical data are collected locally by more than 470 participating healthcare facilities using the standardized DPV electronic health record. Twice a year, centers transmit pseudonymized data to Ulm University, Ulm, Germany. Inconsistent data are reported back to the centers for confirmation/correction. Data are aggregated into a cumulative anonymized database for clinical research and quality assurance. The database covers an estimated proportion of more than 80% of all pediatric patients with diabetes in Germany, Austria, and Luxembourg. 7 The DPV registry has been approved by the Ethics Committee of Human Experimentation at Ulm University, and the data collection by local review boards.
Until March 2020, 584,066 patients with any kind of diabetes were documented in the DPV database. For the present study, patients with T1D aged 20 years and younger at their most recent visit were selected (Figure 1). Clinical visits from January 2005 to December 2019 were considered.
FIGURE 1.
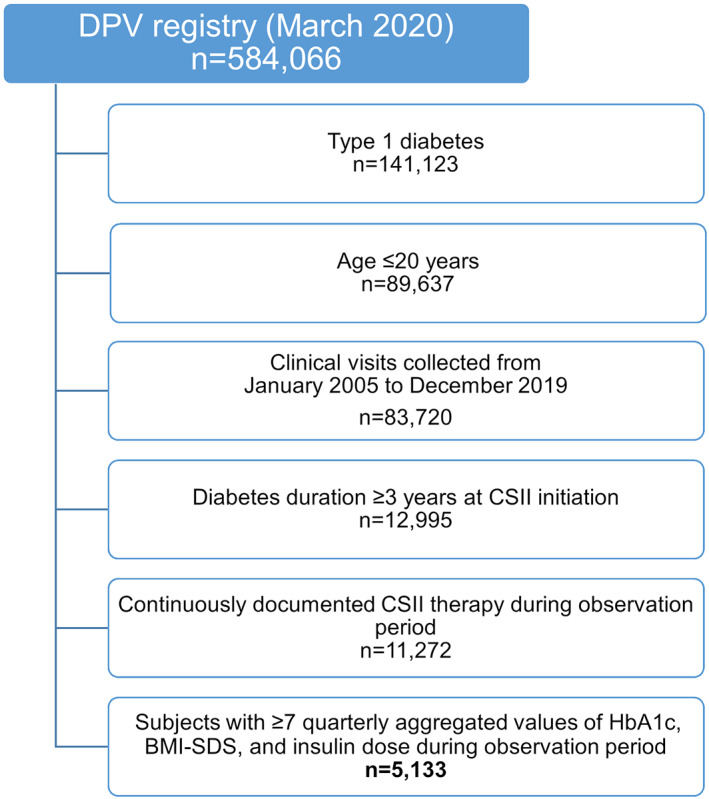
Flowchart for selection of study population from the DPV registry
Further selection criteria were a minimum diabetes duration of 3 years at initiation of insulin pump therapy and continuously documented pump use over the 3‐year observational period following pump start. For each individual, HbA1c, BMI‐z‐score, and daily insulin dose were aggregated quarterly. Baseline was defined as the start date of pump therapy and the preceding 3 months. Database records during the first 45 days after pump start were excluded from the analysis. Subjects with less than seven quarterly aggregated HbA1c, BMI, and insulin dose values during follow‐up were excluded (Figure 1).
2.2. Trajectory variables
Glycemic control was assessed by HbA1c. To adjust for between‐center differences in methods of HbA1c measurements, values were standardized to the Diabetes Control and Complications Trial reference range (20.7–42.6 mmol/mol) 20 , 21 using the multiple‐of‐the‐mean method. Age‐ and sex‐adjusted BMI‐SDS were calculated based on national pediatric reference data from the KiGGS study. 22 Total daily insulin dose (TDD) was calculated as the sum in units of premeal and basal insulin given in a day (IU/d).
Trajectory variables are presented as the differences (Delta) between quarterly aggregated values at each time point [i] and the respective baseline value, for example, Delta HbA1c [i] = quarterly aggregated HbA1c [i] ‑ (Baseline HbA1c).
2.3. Sociodemographic and clinical covariates
Sex, migratory background, age at diabetes onset, and age at start of insulin pump therapy were evaluated with respect to multitrajectory group membership. Data on clinical outcomes (SH [yes/no], diabetic ketoacidosis (DKA) [yes/no]), as well as height SDS and use of glucose monitoring devices (self‐monitoring of capillary blood glucose [SMBG]; continuous glucose monitoring [CGM] including both real‐time CGM and intermittently scanned/viewed CGM systems) were examined as clinical covariates at year 1, year 2, and year 3 following CSII initiation. Participants were classified as sensor users, if at least once sensor use had been documented in the database during the respective follow‐up year. Migratory background was defined as birthplace outside of Germany/Austria/Luxembourg/Switzerland for either patient and/or at least one parent. SH and DKA were defined in accordance with the International Society for Pediatric and Adolescent Diabetes guidelines. 23 , 24 Age‐ and sex‐adjusted height‐SDS was calculated based on the KiGGS reference tables. 22
2.4. Statistical analysis
We applied GBMT modeling, 17 a generalization of the GBT approach, 18 to identify latent groups of individuals following similar developmental curves across multiple variables over time. In this study, each multitrajectory is defined by the outcomes Delta‐HbA1c, Delta‐BMI‐SDS, and Delta‐insulin dose (K = 3). This innovative method allows the analysis of interrelationship of several outcomes and determines joint patterns over time.
The GBT is a semi‐parametric finite‐mixture modeling approach based on Nagin 18 used to analyze longitudinal data. The basic assumption of this method is that there exist J (j = 1,…,J) latent groups in the population cohort. For each group j, the outcome is modeled by a distinct polynomial function of time. Model parameters are estimated by maximum likelihood. To select the optimal number of trajectory groups and the polynomial order, the Bayesian information criterion (BIC) and sufficient group size (≥5% of subjects per group) were used. 25 The search was performed using a forward classifying process, starting with one group with polynomial function quadratic order and then adding further groups.
In the GBMT model framework, each of the trajectory groups is defined by a set of trajectories based on K multiple outcomes. 12 , 15 To find the final multitrajectory model, we first estimated trajectories for each outcome separately with varying number of groups using BIC and sufficient group size. In a second step, both a final number of multivariate trajectory groups across all three outcomes and the appropriate polynomial order for each outcome separately were determined. The statistical approach is described in a previous publication in more detail. 15
Trajectory variables were described at start of insulin pump therapy using median and quartiles (Q1; Q3). Multitrajectory groups were further characterized by examining clinical covariates across the multitrajectory groups. Results were presented as mean with 95% CI. Differences with 95% CI between estimates were calculated.
All analyses were performed with the Statistical Analysis System (SAS) 9.4, release TS1M5 (SAS Institute Inc., Cary, North CA) on a Windows MS Server 2016 mainframe. Multitrajectory analysis was performed by applying the PROC TRAJ macro. 26
3. RESULTS
Inclusion criteria were met by 5133 children, adolescents, and young adults with T1D. Forty‐eight percent of the patients were male. At the start of insulin pump therapy, median age was 12.5 years (q1; q3: 10.3; 14.4), median HbA1c was 7.7% (7.0; 8.5), patients had a median TDD of 42.00 units per day (28.00; 58.50) and a median BMI‐SDS of +0.26 (−0.32; +0.84). Median year of pump start was 2010 (2007; 2013).
3.1. Trajectory analysis
Using the GBMT approach, four unique groups had emerged (Figure 2). Across the groups, heterogeneous three‐variate patterns of Delta‐HbA1c, Delta ‐BMI‐SDS, and Delta ‐daily insulin dose were identified over the 3‐year follow‐up period. All groups showed initial improvements in HbA1c during the first 3 months after switching to CSII. Individuals with a subsequent moderate increase in HbA1c levels after initial reduction, stable BMI‐SDS over time, and steeply decreasing insulin doses during the first 3 months followed by a stable developmental curve were classified as Group 1 (red lines, n = 624, 12% of total study population). Group 2 (green lines, n = 1988, 39%) included youths with steeply increasing HbA1c as of 3 months after pump start, continuously decreasing BMI‐SDS and modestly increasing insulin doses over time. Youths in Group 3 (blue lines, n = 1644, 32%) showed considerable initial improvements in HbA1c followed by modest rise below the baseline level. BMI‐SDS levels in Group 3 were steeply increasing over the first year and plateauing at a high level thereafter, whereas insulin doses were only very modestly increasing over time. Strong increases in each of the three trajectory variables were observed in Group 4 (orange lines, n = 877, 17%), despite the initial HbA1c improvement.
FIGURE 2.
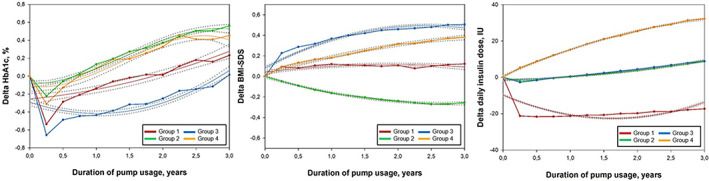
Multitrajectories of change of glycemic control (HbA1c), age‐ and sex‐adjusted BMI (BMI‐SDS), and daily insulin dose with 95% CIs over 3 years following insulin pump therapy initiation. Trajectory variables are presented as the differences (Delta) between quarterly aggregated values at each time point [i] and the respective baseline value, for example, Delta HbA1c [i] = HbA1c [i] ‐ (Baseline HbA1c). A positive value indicates that the measurement was higher at the specific follow up time interval than it was at baseline. Multitrajectories are displayed as solid lines with 95% confidence intervals in gray dotted lines. Estimated mean values were presented as points and were linked to each other. Four trajectory groups were identified: Group 1 (12%, red lines), group 2 (39%, green lines), group 3 (32%, blue lines), and group 4 (17%, orange lines). Age‐ and sex‐adjusted BMI‐SDS was calculated based on national pediatric reference data from the KiGGS study
For girls and boys separately, the multitrajectory analysis was performed starting with one group and adding further groups to identify unique multi‐trajectories for K = 3 outcomes (Figure 3). Again, four unique groups were identified for both sexes with group sizes and trajectories being very similar compared to the overall cohort (Figure 2). Overall, HbA1c deterioration was greater in boys compared to girls after initial improvements (first 3 months). Regarding BMI‐SDS changes, higher BMI‐SDS levels were found in girls. Delta daily insulin dose was similar in girls and boys, except in the groups with the highest increases over time.
FIGURE 3.
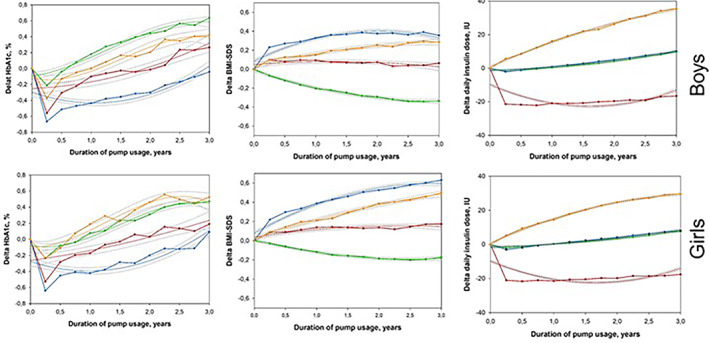
Multitrajectories of changes of glycemic control (HbA1c), age‐ and sex‐adjusted BMI (BMI‐SDS), and daily insulin dose with 95% CIs over 3 years following insulin pump therapy initiation for boys and girls separately. Trajectory variables are presented as the differences (Delta) between quarterly aggregated values at each time point [i] and the respective baseline value, for example, Delta HbA1c [i] = HbA1c [i]—(Baseline HbA1c). A positive value indicates that the measurement was higher at the specific follow‐up time interval than it was at baseline. Multitrajectories are displayed as solid lines with 95% CIs in gray dotted lines. Estimated mean values were presented as points and were linked to each other. Four trajectory groups were identified for both sexes: boys (figures at top): Group B1 (12% red lines), Group B2 (38%, green lines), Group B3 (33%, blue lines), and Group B4 (17%, orange lines); girls (figures at bottom): Group G1 (13%, red line), Group G2 (39%, green line), Group G3 (31%, blue line), and Group G4 (17%, orange line). Age‐ and sex‐adjusted BMI‐SDS was calculated based on national pediatric reference data from the KiGGS study
Additionally in line with the sex‐specific analysis, we conducted the multitrajectory methodology separately for individuals for whom prepubertal status was assumed (age at CSII initiation ≤8 years) and for youths most likely in puberty or entering puberty during the follow‐up period (age at CSII initiation 12–15 years). For the pubertal cohort (n = 1939), again four unique groups were determined (Groups S1 to S4; Figure S1). The identified patterns were relatively similar to the overall cohort's trajectories, particularly regarding changes in Delta‐BMI‐SDS and Delta‐insulin dose. With respect to Delta‐HbA1c, initial HbA1c improvement was observed in all groups; however, of lower extent compared to the overall cohort. Thereafter, one group was identified with steeply increasing HbA1c on a higher level, whereas the other groups experience HbA1c deterioration slightly above or even below the baseline level over time. Unfortunately, for the prepubertal subgroup the multitrajectory analysis could not be performed as the sample size was too small (n = 451).
3.2. Comparison of demographics and baseline characteristics
Patient characteristics of each group are depicted in Table 1. In Group 4, all trajectory variables showed increasing levels over time. Due to these regular developmental patterns of change of all outcomes, Group 4 was defined as reference.
TABLE 1.
Demographics and baseline outcomes across the multitrajectory groups
| Name N (%) | Group 1 (red lines) 624 (12) | Group 2 (green lines) 1988 (39) | Group 3 (blue lines) 1644 (32) | Group 4 (orange lines) 877 (17) |
|---|---|---|---|---|
| Boys, % |
44.1 (40.2; 48.0) −7.4 (−12.5; −2.2) |
53.4 (51.2; 55.6) 2.0 (−1.9; 6.0) |
42.7 (40.1; 44.9) −9.0 (−13.1; −4.9) |
51.4 (48.1; 54.7) Ref. |
| Migratory background, % |
17.3 (14.3; 20.3) −1.3 (−5.2; 2.7) |
18.4 (16.7; 20.1) −0.2 (−3.3; 2.9) |
16.1 (14.3; 17.8) −2.5 (−5.7; 0.6) |
18.6 (16.0; 21.2) Ref. |
| Age at diabetes onset, years |
7.5 (7.3; 7.8) 1.0 (0.7; 1.3) |
5.5 (5.3; 5.6) −1.0 (−1.3; −0.8) |
6.2 (6.1; 6.4) −0.3 (−0.5; −0.0) |
6.5 (6.3; 6.7) Ref. |
| Age at start of pump therapy, years |
14.2 (14.0; 14.5) 1.4 (1.3; 1.8) |
11.3 (11.2; 11.4) −1.4 (−1.6; −1.2) |
12.5 (12.3; 12.6) −0.2 (−0.5; −0.0) |
12.7 (12.5; 12.9) Ref |
| Baseline HbA1c, % |
8.0 (7.9; 8.1) 0.1 (−0.1; 0.2) |
7.5 (7.5; 7.6) −0.4 (−0.5; −0.3) |
8.1 (8.0; 8.2) 0.2 (0.1; 0.3) |
7.9 (7.9; 8.0) Ref. |
| Baseline HbA1c, mmol/mol |
64 (63; 65) 1 (−1; 2) |
58 (58; 60) −4 (−5; −3) |
65 (64; 66) 2 (1; 3) |
63 (63; 64) Ref. |
| Baseline BMI‐SDS |
0.46 (0.39; 0.53) 0.20 (0.11; 0.28) |
0.38 (0.35; 0.42) 0.12 (0.05; 0.19) |
−0.02 (−0.06; 0.02) −0.29 (−0.36; −0.22) |
0.27 (0.21; 0.32) Ref. |
| Baseline daily insulin dose, IU |
74.5 (73.0; 76.1) 33.5 (31.5; 35.5) |
38.2 (37.3; 39.0) −2.9 (−4.4; −1.3) |
44.9 (44.0; 45.9) 3.9 (2.3; 5.5) |
41.0 (39.8; 42.3) Ref. |
Note: Outcomes and clinical covariates are presented as mean with 95% CI. Differences between estimates with 95% CI were calculated (second line in each cell). Significant differences are marked in bold.
Youths in Group 2 were youngest at diabetes onset and youngest at insulin pump start, while members of Group 1 were oldest at diagnosis and oldest at pump initiation. There was a preponderance of female members in Groups 1 and 3. People with migratory background were evenly distributed across the groups.
Mean HbA1c levels at baseline were above recommended targets in all groups with the lowest baseline levels seen in Group 2. Members of group 1 had highest BMI‐SDS and considerably higher baseline daily insulin doses. Lowest insulin doses at baseline were observed in Group 2, while Group 3 had lowest baseline BMI‐SDS levels.
3.3. Comparison of clinical covariates during follow‐up
The interrelationship between HbA1c, BMI‐SDS, and insulin dose related to clinical covariates were also analyzed during CSII therapy (Table S1). CGM usage was highest in Group 4 and Group 1. Conversely, high frequency of daily SMBG was observed in Group 2 and Group 3 throughout the whole follow‐up period. Lower height‐SDS was found in Group 2. Frequency of severe hypoglycemic episodes and DKA event rates were low in all groups and did not differ significantly (data not shown).
4. CONCLUSIONS
This longitudinal study identified four distinct joint trajectories of changes of metabolic control, BMI‐SDS, and insulin doses in children, adolescents, and young adults with T1D during the first 3 years after switching from MDI to insulin pump therapy. The analysis represents a first attempt using large observational real‐life data to cluster patients regarding their response to CSII by applying an innovative statistical method.
All identified groups showed improvements in HbA1c levels during the first 3 months following pump start. However, initial improvements were followed by subsequent deteriorations of HbA1c levels of varying degrees indicating that sustaining favorable HbA1c trajectories over a longer period of time is challenging even with insulin pump therapy.
The heterogeneity in response to pump therapy is particularly evident when focusing on the two largest groups of the cohort, that is, group 2 (39% of the cohort) and Group 3 (32% of the cohort). While Group 3 showed sustained improvements in glycemic control, we observed most pronounced deterioration of HbA1c levels in Group 2. Of note, the highest baseline HbA1c levels were found in Group 3. Greater reductions in HbA1c for individuals with worse glycemic control at pump start were previously reported in RCTs and observational studies. 4 , 27 More advantageous HbA1c trajectories, however, might be associated with substantial increases in BMI‐SDS (Group 3), particularly in females. Female sex had been associated with higher BMI‐SDS during puberty in individuals with T1D in the past. 28 , 29 Unfortunately, frequency of non‐SH—a risk factor for weight gain in T1D 30 —was not assessed in this cohort.
Individuals with significant deterioration of glycemic control after pump start, that is, Groups 2 and 4 both with a preponderance of male individuals, either showed declining BMI‐SDS levels if insulin doses were barely altered (Group 2) or rising BMI‐SDS levels along with rising TDD (Group 4). Trajectories for Group 4 are consistent with results from previous studies applying single‐component GBT modeling, where higher HbA1c increase during puberty was characterized by higher insulin dose at 16 years of age. 12 This is also in agreement with previous findings linking worse glycemic control during puberty with comparably higher and increasing BMI‐SDS. 29 , 31 Additionally, highest increments in height‐SDS were seen in Group 4 (Table S1). Most likely, the identified trajectories reflect a period with the highest insulin resistance and impaired insulin action which might be linked to an increased amplitude of growth hormone pulses during growth spurt. 13
Perhaps counter‐intuitively, insulin doses in Group 2 remained relatively stable over the observational period despite significantly increasing HbA1c levels indicating that members of Group 2 might have been relatively under insulinized. Insufficient insulin dosing leads to higher HbA1c levels 32 and might per se cause weight loss. Consequently, BMI‐SDS in Group 2 might have dropped and we found declining height‐SDS during the follow‐up period (Table S1). Overall decline in height‐SDS has been associated with higher HbA1c levels during puberty. 31 Additionally, volitional omission of insulin to control for weight is quite common in adolescence. 33 Unfortunately, bolus frequency and risk and/or prevalence of psychiatric comorbidities including eating disorders were not evaluated in this cohort.
Sex‐specific disparities in response to CSII initiation were evident. Even in groups following similar overall patterns (Figure 3), that is, male Group B2 and female Group G2, differences were found, particularly as regards the magnitude of changes with boys showing greater deterioration in HbA1c and girls showing higher Delta‐BMI‐SDS. In boys, previous analysis using DPV data had shown the highest HbA1c increases between 12 and 14 years of age, 29 which coincides with Group B2's period of significantly deteriorating HbA1c levels. Girls usually show the highest mean increases in HbA1c later during puberty 29 which corresponds well with Group G2 girls' weaker and less pronounced increase in HbA1c.
Group 1 (12% of the cohort) was characterized by oldest age at diagnosis and at pump start with highest baseline insulin requirements, highest baseline BMI‐SDS and declining SDS for height, all indicating advanced pubertal or postpubertal development. In this group, HbA1c showed moderate progression, insulin requirements were decreasing over a 3‐year window, and accordingly, BMI‐SDS did not further deteriorate. Similar longitudinal patterns for HbA1c, BMI‐SDS, and insulin dose across all identified trajectory groups were found in this particular age range in a previous three‐variate analysis applying the GBMT approach in a cohort including MDI and CSII users. 15 Still, more favorable trajectories were previously observed with insulin pump compared to MDI therapy in this age group. 7 , 12 , 34 Even when considering almost adult circumstances, rising HbA1c levels in the long‐term following pump initiation is a common phenomenon seen in adults well beyond puberty, too. 35
Taking into account that this cohort's median age at insulin pump start was 12.5 years, and given the 3‐year follow‐up period, puberty as a potential confounder could not be excluded. A separate sub‐analysis was performed for individuals aged 12–15 years to minimize the cohort's heterogeneity regarding age and between‐group differences in pubertal development (Figure S1). Again, four groups had emerged with similar trajectories regarding Delta‐BMI‐SDS and Delta‐insulin dose compared with the overall cohort. Despite the groups' heterogeneous HbA1c trajectories, it seemed as if for most of the pubertal cohort (i.e., Groups S1, S3, and S4; 73% of individuals in total), the impact on HbA1c following pump start was more favorable compared to the overall cohort. However, 27% of pubertal individuals (Group S2) showed even more pronounced increases in HbA1c levels.
With SMBG frequency being highest in Group 2 and usage of CGM being highest in Group 4, that is, in groups with most pronounced increases in HbA1c levels, adherence to glucose monitoring did not seem to be linked to more favorable HbA1c trajectories as one would expect based on existing literature. 1 However, these findings need to be interpreted with caution: Participants for whom sensor use was at least once documented per quarter irrespective of actual sensor use per day were classified as sensor users. Second, the median year of pump start in our cohort was 2010, a time at which CGM uptake was relatively low compared to more recent years. 3 In countries participating in the DPV registry, CGM usage has rapidly increased as of 2015, 3 which coincided with the introduction of newer generation CGM devices, changes in reimbursement policies, and coverage by public health care systems. Hence, the biggest impact of CGM technology on overall glycemic control and other parameters of interest might not lie within our analysis' observation period. In this respect, further analysis focusing on more recent data are warranted.
In previous analyses from the DPV registry including youth with T1D on both CSII and MDI, migratory background has been associated with less beneficial longitudinal trajectories for glycemic control, 12 unfavorable diabetes outcomes, 36 and higher risk of DKA events. 37 In our analysis, however, migratory background was evenly distributed across all identified groups indicating that in our cohort, migratory background per se might have no impact, positive or negative, on trajectories of changes in HbA1c, BMI‐SDS, and insulin dose following pump start.
Our findings underline the need for personalized diabetes care based on patient characteristics. When counseling people with T1D showing similar characteristics as found in our groups, future trajectories of outcomes might be predictable, which is also useful in managing patients' expectations. Our results might offer guidance on parameters to be monitored in conjunction with HbA1c during follow‐up. People at risk for unfavorable trajectories of glycemic control, weight, and insulin dose might be identified, and prevention strategies (e.g., individualized training sessions) might be tailored to individual needs.
This study was conducted using a large cohort of pediatric patients derived from the population‐based multicenter DPV database. A further strength was the innovative GBMT technique which was applied to analyze three‐variate patterns of Delta HbA1c, Delta BMI‐SDS, and Delta insulin dose over a 3‐year observation period. Additionally, the DPV database provides detailed information on patients' characteristics that allows for the examination of multiple factors associated with HbA1c, BMI‐SDS, and insulin dose trajectories. One limitation might be that HbA1c was not measured in a central laboratory. However, HbA1c levels were mathematically standardized to reduce variation between laboratories. Of note, this analysis focused on people switching from MDI to CSII. Individuals who are put on a pump at diabetes onset—which has become common practice in many pediatric centers, particularly in preschool children 38 —were precluded from this analysis. No sub‐analysis was performed with respect to the specific pump models used nor with respect to glucose‐responsive insulin delivery features of pumps (e.g., low glucose suspension, predictive low glucose suspension). Only a limited amount of group characteristics was explored in the present analysis without any claim of completeness. Hence, parts of this discussion remained predominantly descriptive leaving room for future analyses.
One limitation of the methodology might be that the GBMT approach cannot represent large short‐term changes in the slope of the trajectories. In the present study, group mean values and developmental curves partly differ in the first 3 months. To clarify this, we also included group mean values in the figures. In the GBT modeling approach, missing data are assumed to be missing at random, and model parameters are estimated by using all available observations. Hence, our data provide sufficient information. As per exclusion criteria, however, a considerable number of patients with less than seven quarterly aggregated data points for HbA1c, BMI, and insulin dose during follow‐up were not included in this analysis (Figure 1). Though baseline characteristics between included and excluded subjects were similar (data not shown), a potential selection bias cannot be ruled out with any certainty. Notably, insulin requirements were expressed as total insulin dose per day, and not as insulin dose per kilogram body weight per day. Since delta values were used for trajectory variables rather than absolute values, the calculation of the delta for insulin dose per kilogram body weight would have resulted in exceedingly small numbers. The GBMT approach would not work with such small and similar numbers.
In conclusion, the three‐variate trajectories of Delta‐HbA1c, Delta‐BMI‐SDS, and Delta‐insulin doses in children, adolescents, and young adults with T1D in response to CSII initiation were very heterogeneous. All in common was a positive impact on HbA1c seen during the first months on CSII followed by a deterioration of varying degrees over the 3‐year follow‐up. Individual patient characteristics including baseline HbA1c, sex, and age at pump start might be key factors responsible for diverging trajectories.
CONFLICT OF INTEREST
Martin Tauschmann, Anke Schwandt, Nicole Prinz, Melanie Hess, Martin Holder, Beate Karges, Andrea Näke, Oliver Kuss, and Reinhard W. Holl have no competing interests to disclose. Marianne Becker reports having received Advisory Board honoraria from Novo Nordisk. Torben Biester reports lecture fees from AstraZeneca, DexCom, Medtronic, Novo Nordisk, Roche, Sanofi, Ypsomed, Advisory Board honoraria from AstraZeneca, DexCom, Medtronic. Simone von Sengbusch. reports being a consultant for Abbott, Dexcom, Lilly, Novo Nordisk, and Medtronic and receiving lecture fees from Abbott, Berlin‐Chemie, Infectopharm, Lilly, Novo Nordisk, Merck, Medtronic, and Sanofi.
AUTHOR CONTRIBUTIONS
Martin Tauschmann and Anke Schwandt wrote the manuscript. Martin Tauschmann., Anke Schwandt, Nicole Prinz, and Reinhard W. Holl designed the analysis and interpreted the results. Anke Schwandt analyzed the data and created figures and tables. Nicole Prinz and Reinhard W. Holl reviewed drafts, contributed to discussion, and edited the manuscript. Marianne Becker, Torben Biester, Melanie Hess, Martin Holder, Beate Karges, Andrea Näke, Oliver Kuss, Simone von Sengbusch critically reviewed and commented on the manuscript. Reinhard W. Holl and Anke Schwandt are the guarantor of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13320.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
The authors acknowledge all 295 participating centers in Germany, Luxembourg, Switzerland, and Austria that contributed data to this analysis (Germany: 267, Luxembourg: 1, Switzerland: 2, Austria: 25). The authors also thank A. Hungele and R. Ranz for support and the development of the DPV documentation software and K. Fink and E. Bollow for the DPV data management (clinical data managers and software developers, Ulm University). The authors are grateful to all investigators participating in the DPV initiative.
APPENDIX A.
List of participating DPV centers contributing anonymized data to the present study:
Augsburg Uni‐Kinderklinik, Aachen ‐ Uni‐Kinderklinik RWTH, Ahlen St. Franziskus Kinderklinik, Aue Helios Kinderklink, Aurich Kinderklinik, Wien Uni‐Kinderklinik, Weingarten Kinderarztpraxis, Berlin Lichtenberg ‐ Kinderklinik, Berlin Virchow‐Kinderklinik, Bad Aibling Internist. Praxis, Bremerhaven Kinderklinik, Bielefeld Kinderklinik Gilead, Bonn Uni‐Kinderklinik, Hinrichsegen‐Bruckmühl Diabetikerjugendhaus, Chemnitz Kinderklinik, Coesfeld Kinderklinik, Düsseldorf Uni‐Kinderklinik, Darmstadt Kinderklinik Prinz. Margaret, Deggendorf Pädiatrie‐Praxis, Düren‐Birkesdorf Kinderklinik, Delmenhorst Kinderklinik, Detmold Kinderklinik, Dortmund Kinderklinik, Dresden Uni‐Kinderklinik, Datteln Vestische Kinderklinik, Essen Uni‐Kinderklinik, Erlangen Uni‐Kinderklinik, Erfurt Kinderklinik, Esslingen Klinik für Kinder und Jugendliche, Eutin Kinderklinik, Frankfurt Uni‐Kinderklinik, Offenbach/Main Kinderklinik, Freiburg Uni‐Kinderklinik, Friedrichshafen Kinderklinik, Fürth Kinderklinik, Fulda Kinderklinik, Gaissach Fachklinik der Deutschen Rentenversicherung Bayern Süd, Garmisch‐Partenkirchen Kinderklinik, Gießen Uni‐Kinderklinik, Göppingen Kinderklinik am Eichert, Gelsenkirchen Kinderklinik Marienhospital, Göttingen Uni‐Kinderklinik, Hannover Kinderklinik MHH, Hannover Kinderklinik auf der Bult, Halle Uni‐Kinderklinik, Hachenburg Kinderpraxis, Hamm Kinderklinik, Bremen Zentralkrankenhaus Kinderklinik, Bremen ‐ Kinderklinik Nord, Heidelberg Uni‐Kinderklinik, Heidenheim Kinderklinik, Herford Klinikum Kinder & Jugendliche, Bad Hersfeld Kinderklinik, Hermeskeil Kinderpraxis, Hagen Kinderklinik, Hamburg Altonaer Kinderklinik, Hamburg Kinderklinik Wilhelmstift, Hamburg‐Nord Kinder‐MVZ, Hildesheim Kinderarztpraxis, Lübeck Uni‐Kinderklinik, Homburg Uni‐Kinderklinik Saarland, Hanau Kinderklinik, Itzehoe Kinderklinik, Jena Uni‐Kinderklinik, Köln Uni‐Kinderklinik, Karlsruhe Städtische Kinderklinik, Kaiserslautern‐Westpfalzklinikum Kinderklinik, Karlsburg Klinik für Diabetes & Stoffwechsel, Kiel Städtische Kinderklinik, Koblenz Kinderklinik Kemperhof, Kassel Klinikum Kinder‐ und Jugendmedizin, Leipzig Uni‐Kinderklinik, Ludwigsburg Kinderklinik, Landshut Kinderklink, Lingen Kinderklinik St. Bonifatius, Lippstadt Evangelische Kinderklinik, Ludwigshafen Kinderklinik St.Anna‐Stift, Lüdenscheid Märkische Kliniken ‐ Kinder & Jugendmedizin, München von Haunersche Kinderklinik, München‐Harlaching Kinderklinik, Mannheim Uni‐Kinderklinik, Mechernich Kinderklinik, Minden Kinderklinik, Moers Kinderklinik, Münster pädiat. Schwerpunktpraxis, Münster Uni‐Kinderklinik, Mutterstadt Kinderarztpraxis, Nürnberg Uniklinik Zentrum f Neugeb./Kinder & Jugendl., Neuwied Kinderklinik Elisabeth, Neunkirchen Marienhausklinik Kohlhof Kinderklinik, Nürnberg Cnopfsche Kinderklinik, Oberhausen Kinderklinik, Oldenburg Kinderklinik, Osnabrück Christliches Kinderhospital, Bad Oeynhausen Herz‐und Diabeteszentrum NRW, Paderborn St. Vincenz Kinderklinik, Pforzheim Kinderklinik, Regensburg Kinderklinik St. Hedwig, Remscheid Kinderklinik, Mönchengladbach Kinderklinik Rheydt Elisabethkrankenhaus, Rendsburg Kinderklinik, Rosenheim Kinderklinik, Rastatt Gemeinschaftspraxis, Ravensburg Kinderklink St. Nikolaus, Rotenburg/Wümme Agaplesion Diakonieklinikum Kinderabteilung, Stuttgart Olgahospital Kinderklinik, Saalfeld Thüringenklinik Kinderklinik, Saarlouis Kinderklinik, Saarbrücken Kinderklinik Winterberg, Schw. Gmünd Stauferklinik Kinderklinik, Suhl Kinderklinik, Siegen Kinderklinik, Singen ‐ Hegauklinik Kinderklinik, Spaichingen Innere, Stade Kinderklinik, Sylt Rehaklinik, Trier Kinderklinik der Borromäerinnen, Ulm Uni‐Kinderklinik, Vechta Kinderklinik, Viersen Kinderkrankenhaus St. Nikolaus, Weiden Kinderklinik, Wiesbaden Kinderklinik DKD, Wiesbaden Helios Horst‐Schmidt‐Kinderkliniken, Herdecke Kinderklinik, Waldshut‐Tiengen Kinderpraxis Biberbau, Winnenden Rems‐Murr Kinderklinik, Worms Kinderklinik, Wuppertal Universitäts‐Kinderklinik, Kassel Städtische Kinderklinik, Magdeburg Uni‐Kinderklinik, Schweinfurt Kinderklinik, Hildesheim GmbH ‐ Innere, Saaldorf‐Surheim Diabetespraxis, Neuss Lukaskrankenhaus Kinderklinik, München‐Schwabing Kinderklinik, Passau Kinderklinik, Neuburg Kinderklinik, Memmingen Kinderklinik, Innsbruck Uni‐Kinderklinik, Bad Kösen Median Kinderklinik, Tübingen Uni‐Kinderklinik, Heringsdorf Inselklinik, Gelnhausen Kinderklinik, Bad Reichenhall Kreisklinik Innere Med., Stolberg Kinderklinik, Münster St. Franziskus Kinderklinik, Passau Kinderarztpraxis, Leverkusen Kinderklinik, Dornbirn Kinderklinik, Eberswalde Klinikum Barnim Werner Forßmann ‐ Innere, Offenburg Kinderklinik, Kiel Universitäts‐Kinderklinik, Rostock Uni‐Kinderklinik, Bocholt Kinderklinik, Oberhausen Kinderpraxis, Schwerin Kinderklinik, Rheine Mathiasspital Kinderklinik, Essen Elisabeth Kinderklinik, Mainz Uni‐Kinderklinik, Traunstein diabetol. Schwerpunktpraxis, Herford Kinderarztpraxis, München‐Gauting Kinderarztzentrum, Magdeburg Städtisches Klinikum Innere, Papenburg Marienkrankenhaus Kinderklinik, Wilhelmshaven Klinikum Kinderklinik, Bochum Universitätskinderklinik St. Josef, Köln Kinderklinik Amsterdamerstrasse, Heilbronn Kinderklinik, Graz Uni‐Kinderklinik, Krefeld Kinderklinik, Rosenheim Schwerpunktpraxis, Bad Waldsee Kinderarztpraxis, Aalen Kinderklinik, Kirchen DRK Krankenhaus Kinderklinik, Berlin DRK‐Kliniken Pädiatrie, München 3. Orden Kinderklinik, Bautzen Oberlausitz KK, Freiburg Uni Innere, Rüsselsheim Kinderklinik, Trostberg Innere, Oy‐Mittelberg Hochgebirgsklinik Kinder‐Reha, Berchtesgaden CJD, St. Augustin Kinderklinik, Tettnang Innere Medizin, Frankenthal Kinderarztpraxis, Dresden Neustadt Kinderklinik, Haren Kinderarztpraxis, Bad Mergentheim ‐ Kinderdiabetologische Praxis, Konstanz Kinderklinik, Waldshut Kinderpraxis, Gera Kinderklinik, Reutlingen Kinderarztpraxis, Arnsberg‐Hüsten Karolinenhosp. Kinderabteilung, Schwäbisch Hall Diakonie Kinderklinik, Oldenburg Schwerpunktpraxis Pädiatrie, Hof Kinderklinik, Kreischa‐Zscheckwitz Klinik Bavaria, Linz Krankenhaus der Barmherzigen Schwestern Kinderklinik, Nauen Havellandklinik, Hameln Kinderklinik, Heide Kinderklinik, München Kinderarztpraxis diabet. SPP, Iserlohn Innere Medizin, Ulm Endokrinologikum, Bad Orb Spessart Klinik Reha, Frankfurt Diabeteszentrum Rhein‐Main‐Erwachsenendiabetologie (Bürgerhospital), Wittenberg Kinderklinik, Mödling Kinderklinik, St. Pölten Universitäts‐Kinderklinik, Braunschweig Kinderarztpraxis, Berlin Endokrinologikum, Böblingen Kinderklinik, Gießen Ev. Krankenhaus Mittelhessen, Plauen Vogtlandklinikum, Bad Salzungen Kinderklinik, Bad Mergentheim ‐ Diabetesfachklinik, Reutlingen Kinderklinik, Villach Kinderklinik, Frankfurt Diabeteszentrum Rhein‐Main‐pädiat. Diabetologie (Clementine‐Hospital), Forchheim Diabeteszentrum SPP, Salzburg Universitäts‐Kinderklinik, Wien Uni Innere Med III, Scheidegg Prinzregent Luitpold, Wien Preyersches Kinderspital, Kaiserslautern Kinderarztpraxis, Leoben LKH Kinderklinik, Wien SMZ Ost Donauspital, Wien KH Nord‐Klinik Floridsdorf, Worms ‐ Weierhof, Linz KUK MedCampus IV Kinderklinik, Wels Klinikum Pädiatrie, Duisburg Sana Kinderklinik, Villingen‐Schwenningen Schwarzwald Baar Klinikum Kinderklinik, Freiburg St. Josef Kinderklinik, Feldkirch Kinderklinik, Lappersdorf Kinderarztpraxis, Ludwigshafen diabetol. SPP, Dessau Kinderklinik, Luxembourg ‐ Centre Hospitalier, Bad Orb Spessart Klinik, Bad Kreuznach‐Viktoriastift, Lienz Diabetesschwerpunktpraxis für Kinder und Jugendliche, Olpe pädiatrische Gemeinschaftspraxis, Waren‐Müritz Kinderklinik, Freiburg Kinder‐MVZ, Duisburg‐St. Johannes Helios, Essen Diabetes‐Schwerpunktpraxis, Leer Klinikum ‐ Klinik Kinder & Jugendmedizin, Singen Kinderarztpraxis, Kempten Oberallgäu Kinderklinik, Halberstadt Kinderklinik AMEOS, Bruchweiler Edelsteinklinik Kinder‐Reha, Coburg Kinderklinik, Essen Kinderarztpraxis, Filderstadt Kinderklinik, Meissen Kinderklinik Elblandklinikum, Greifswald Uni‐Kinderklinik, Ried Innkreis Barmherzige Schwestern, Traunstein Kinderklinik, Augsburg Josefinum Kinderklinik, Amberg Kinderklinik St. Marien, Zweibrücken Kinderarztpraxis, Neuruppin Kinderklinik, Bochum Universitäts St. Josef, Marburg Uni‐Kinderklinik, Bielefeld Kinderarztpraxis, Münsterlingen Kinderklinik, Reutte Tirol BKH Kinderklinik, Graz Uni Innere, Wesel Marienhospital Kinderklinik, St. Johann Tirol Kinderklinik, Vöcklabruck Kinderklinik, Neuss Lukas‐Krankenhaus Kinderklinik, Memmingen Internistische Praxis, Hohenmölsen Diabeteszentrum, Basel Uni‐Kinderspital beider Basel (UKBB), Neunkirchen Gemeinschaftspraxis Kinderheilkunde, Zams Kinderklinik, Jena Kinderarztpraxis, Gummersbach Oberbergklinikum, München Praxiszentrum Saarstrasse, Kaufbeuren Kinderklinik, Witten Kinderarztpraxis, Magdeburg Ki‐Klinik St. Marienstift, Bad Kreuznach Diakonie Kikli, Schleswig Heliosklinik Kinderklinik, Klagenfurt Kinderklinik, Berchtesgaden CJD‐Beruf.REHA, Hildesheim Bernward Krks Kinderheilkunde, Hanau diabetol. Schwerpunktpraxis, Rüsselsheim MVZ, Kamen MKK ‐ Medizinisches Kompetenzkollegium, Lindlar DM‐Zentrum, Karlsruhe Schwerpunktpraxis, Idar Oberstein Schwerpunktpraxis,
Tauschmann M, Schwandt A, Prinz N, et al. Three‐variate trajectories of metabolic control, body mass index, and insulin dose: Heterogeneous response to initiation of pump therapy in youth with type 1 diabetes. Pediatr Diabetes. 2022;23(3):330‐340. doi: 10.1111/pedi.13320
Funding informationThe DPV was supported through the German Federal Ministry for Education and Research within the German Center for Diabetes Research (DZD, grant number: 82DZD14A02). The work described in this paper was supported by the Innovative Medicines Initiative of the European Union (no. 875534—SOPHIA). Further financial support was received by the German Robert Koch Institute (RKI) and the German Diabetes Association (DDG). The sponsors had no role in study design, collection, analysis, and interpretation of data, writing of the report, and the decision to submit the manuscript for publication.
List of participating DPV centers contributing anonymized data to the present study given in Appendix A.
Martin Tauschmann and Anke Schwandt contributed equally to this manuscript.
Funding information German Diabetes Association (DDG); German Federal Ministry for Education and Research within the German Center for Diabetes Research (DZD), Grant/Award Number: 82DZD14A02; German Robert Koch Institute (RKI); Innovative Medicines Initiative of the European Union, Grant/Award Number: 875534 ‐ SOPHIA; Ulm University
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Tauschmann M, Hovorka R. Technology in the management of type 1 diabetes mellitus ‐ current status and future prospects. Nat Rev Endocrinol. 2018;14(8):464‐475. [DOI] [PubMed] [Google Scholar]
- 2. Szypowska A, Schwandt A, Svensson J, et al. Insulin pump therapy in children with type 1 diabetes: analysis of data from the SWEET registry. Pediatr Diabetes. 2016;17(Suppl 23):38‐45. [DOI] [PubMed] [Google Scholar]
- 3. van den Boom L, Karges B, Auzanneau M, et al. Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care. 2019;42(11):2050‐2056. [DOI] [PubMed] [Google Scholar]
- 4. Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta‐analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25(7):765‐774. [DOI] [PubMed] [Google Scholar]
- 5. Pańkowska E, Błazik M, Dziechciarz P, Szypowska A, Szajewska H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta‐analysis of randomized control trials. Pediatr Diabetes. 2009;10(1):52‐58. [DOI] [PubMed] [Google Scholar]
- 6. Johnson SR, Cooper MN, Jones TW, Davis EA. Long‐term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population‐based case‐control study. Diabetologia. 2013;56(11):2392‐2400. [DOI] [PubMed] [Google Scholar]
- 7. Karges B, Schwandt A, Heidtmann B, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318(14):1358‐1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beato‐Víbora P, Yeoh E, Rogers H, Hopkins D, Amiel SA, Choudhary P. Sustained benefit of continuous subcutaneous insulin infusion on glycaemic control and hypoglycaemia in adults with type 1 diabetes. Diabet Med. 2015;32(11):1453‐1459. [DOI] [PubMed] [Google Scholar]
- 9. Steineck I, Cederholm J, Eliasson B, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ. 2015;350:h3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mameli C, Scaramuzza AE, Ho J, Cardona‐Hernandez R, Suarez‐Ortega L, Zuccotti GV. A 7‐year follow‐up retrospective, international, multicenter study of insulin pump therapy in children and adolescents with type 1 diabetes. Acta Diabetol. 2014;51(2):205‐210. [DOI] [PubMed] [Google Scholar]
- 11. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016‐2018. Diabetes Technol Ther. 2019;21(2):66‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwandt A, Hermann JM, Rosenbauer J, et al. Longitudinal trajectories of metabolic Control from childhood to young adulthood in type 1 diabetes from a large German/Austrian registry: a group‐based modeling approach. Diabetes Care. 2017;40(3):309‐316. [DOI] [PubMed] [Google Scholar]
- 13. Acerini CL, Williams RM, Dunger DB. Metabolic impact of puberty on the course of type 1 diabetes. Diabetes Metab. 2001;27(4 Pt 2):S19‐S25. [PubMed] [Google Scholar]
- 14. Prinz N, Schwandt A, Becker M, et al. Trajectories of body mass index from childhood to young adulthood among patients with type 1 diabetes‐a longitudinal group‐based modeling approach based on the DPV registry. J Pediatr. 2018;201:78‐85.e4. [DOI] [PubMed] [Google Scholar]
- 15. Schwandt A, Kuss O, Dunstheimer D, et al. Three‐variate longitudinal patterns of metabolic control, body mass index, and insulin dose during puberty in a type 1 diabetes cohort: a group‐based multitrajectory analysis. J Pediatr. 2020;218:64‐71.e3. [DOI] [PubMed] [Google Scholar]
- 16. Radder JE, Shapiro SD, Berndt A. Personalized medicine for chronic, complex diseases: chronic obstructive pulmonary disease as an example. Pers Med. 2014;11(7):669‐679. [DOI] [PubMed] [Google Scholar]
- 17. Nagin DS, Jones BL, Passos VL, Tremblay RE. Group‐based multi‐trajectory modeling. Stat Methods Med Res. 2018;27(7):2015‐2023. [DOI] [PubMed] [Google Scholar]
- 18. Nagin D. Group‐Based Modeling of Development. Harvard University Press; 2005. [Google Scholar]
- 19. Hofer SE, Schwandt A, Holl RW, Initiative AGD. Standardized documentation in pediatric Diabetology: experience from Austria and Germany. J Diabetes Sci Technol. 2016;10(5):1042‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Diabetes Association, European Association for the Study of diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, international diabetes federation. Consensus statement on the worldwide standardisation of the HbA1c measurement. Diabetologia. 2007;50(10):2042‐2043. [DOI] [PubMed] [Google Scholar]
- 21. Diabetes Control , Complications Trial Research Group , Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The diabetes control and complications trial research group. N Engl J Med. 1993;329(14):977‐986. [DOI] [PubMed] [Google Scholar]
- 22. Rosario AS, Kurth BM, Stolzenberg H, Ellert U, Neuhauser H. Body mass index percentiles for children and adolescents in Germany based on a nationally representative sample (KiGGS 2003‐2006). Eur J Clin Nutr. 2010;64(4):341‐349. [DOI] [PubMed] [Google Scholar]
- 23. Jones TW, Abraham MB, Naranjo D, et al. Defining relevant hypoglycemia measures in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2018;19(3):354‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155‐177. [DOI] [PubMed] [Google Scholar]
- 25. Twisk J, Hoekstra T. Classifying developmental trajectories over time should be done with great caution: a comparison between methods. J Clin Epidemiol. 2012;65(10):1078‐1087. [DOI] [PubMed] [Google Scholar]
- 26. Jones BL. Traj: group‐based modeling of longitudinal data. Last amended. 2018. http://www.andrew.cmu.edu/user/bjones/index.htm.
- 27. Clements M, Matuleviciene V, Attvall S, et al. Predicting the effectiveness of insulin pump therapy on glycemic control in clinical practice: a retrospective study of patients with type 1 diabetes from 10 outpatient diabetes clinics in Sweden over 5 years. Diabetes Technol Ther. 2015;17(1):21‐28. [DOI] [PubMed] [Google Scholar]
- 28. Fröhlich‐Reiterer EE, Rosenbauer J, Bechtold‐Dalla Pozza S, et al. Predictors of increasing BMI during the course of diabetes in children and adolescents with type 1 diabetes: data from the German/Austrian DPV multicentre survey. Arch Dis Child. 2014;99(8):738‐743. [DOI] [PubMed] [Google Scholar]
- 29. Plamper M, Gohlke B, Woelfle J, et al. Interaction of pubertal development and metabolic Control in adolescents with type 1 diabetes mellitus. J Diabetes Res. 2017;2017:8615769‐8615768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bumbu A, Moutairou A, Matar O, et al. Non‐severe hypoglycaemia is associated with weight gain in patients with type 1 diabetes: results from the diabetes Control and complication trial. Diabetes Obes Metab. 2018;20(5):1289‐1292. [DOI] [PubMed] [Google Scholar]
- 31. Marcovecchio ML, Heywood JJ, Dalton RN, Dunger DB. The contribution of glycemic control to impaired growth during puberty in young people with type 1 diabetes and microalbuminuria. Pediatr Diabetes. 2014;15(4):303‐308. [DOI] [PubMed] [Google Scholar]
- 32. Burdick J, Chase HP, Slover RH, et al. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113(3 Pt 1):e221‐e224. [DOI] [PubMed] [Google Scholar]
- 33. Hanlan ME, Griffith J, Patel N, Jaser SS. Eating disorders and disordered eating in type 1 diabetes: prevalence, screening, and treatment options. Curr Diab Rep. 2013;13:909‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clements MA, Foster NC, Maahs DM, et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes. 2016;17(5):327‐336. [DOI] [PubMed] [Google Scholar]
- 35. Nixon R, Folwell R, Pickup JC. Variations in the quality and sustainability of long‐term glycaemic control with continuous subcutaneous insulin infusion. Diabet Med. 2014;31(10):1174‐1177. [DOI] [PubMed] [Google Scholar]
- 36. Mönkemöller K, Müller‐Godeffroy E, Lilienthal E, et al. The association between socio‐economic status and diabetes care and outcome in children with diabetes type 1 in Germany: the DIAS study (diabetes and social disparities). Pediatr Diabetes. 2019;20(5):637‐644. [DOI] [PubMed] [Google Scholar]
- 37. Hammersen J, Tittel SR, Warncke K, et al. Previous diabetic ketoacidosis as a risk factor for recurrence in a large prospective contemporary pediatric cohort: results from the DPV initiative. Pediatr Diabetes. 2021;22(3):455‐462. [DOI] [PubMed] [Google Scholar]
- 38. Danne T, Phillip M, Buckingham BA, et al. ISPAD clinical practice consensus guidelines 2018: insulin treatment in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):115‐135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
Data available on request from the authors.


