Abstract
Conflicting results have been reported regarding the association between antidepressant use and out‐of‐hospital cardiac arrest (OHCA) risk. We investigated whether the use of antidepressants is associated with OHCA.
Methods
We conducted a nationwide nested case–control study to assess the association of individual antidepressant drugs within drug classes with the hazard of OHCA. Cases were defined as OHCA from presumed cardiac causes. Cox regression with time‐dependent exposure and time‐dependent covariates was conducted to calculate hazard ratios (HR) and 95% confidence intervals (95% CIs) overall and in subgroups defined by established cardiac disease and cardiovascular risk factors. Also, we studied antidepressants with and without sodium channel blocking or potassium channel blocking properties separately.
Results
During the study period from 2001 to 2015 we observed 10 987 OHCA cases, and found increased OHCA rate for high‐dose citalopram (>20 mg) and high‐dose escitalopram (>10 mg; HR:1.46 [95% CI:1.27–1.69], HR:1.43 [95% CI:1.16–1.75], respectively) among selective serotonin reuptake inhibitors (reference drug sertraline), and for high‐dose mirtazapine (>30; HR:1.59 [95% CI:1.18–2.14]) among the serotonin–norepinephrine reuptake inhibitors or noradrenergic and specific serotonergic antidepressants (reference drug duloxetine). Among tricyclic antidepressants (reference drug amitriptyline), no drug was associated with significantly increased OHCA rate. Increased OHCA rate was found for antidepressants with known potassium channel blocking properties (HR:1.14 [95% CI:1.05–1.23]), but for not those with sodium channel blocking properties. Citalopram, although not statistically significant, and mirtazapine were associated with increased OHCA rate in patients without cardiac disease and cardiovascular risk factors.
Conclusion
Our findings indicate that careful titration of citalopram, escitalopram and mirtazapine dose may have to be considered due to drug safety issues.
Keywords: antidepressants, depolarization‐blocking drugs, sudden cardiac arrest
What is already known about this subject
Untreated depression more than doubles the risk of out‐of‐hospital cardiac arrest (OHCA).
A few observational studies suggest that some antidepressants may increase OHCA risk, but the evidence from these studies is inconclusive because these studies were small, had misclassification in the definition of OHCA, and other important limitations in their design.
Given the widespread use of antidepressants in the face of the rising incidence of depression worldwide, it is of clear clinical importance to establish whether use of antidepressants is associated with OHCA.
What this study adds
The association between antidepressants and OHCA risk are studied in a large nationwide nested case–control study.
We found increased OHCA rate for high‐dose citalopram (>20 mg) and high‐dose escitalopram (>10 mg) among selective serotonin reuptake inhibitors (reference drug sertraline), and for high‐dose mirtazapine among the serotonin–norepinephrine reuptake inhibitors or noradrenergic and specific serotonergic antidepressants (reference drug duloxetine). Among tricyclic antidepressants (reference drug amitriptyline), no drug was associated with significantly increased OHCA rate.
Increased OHCA rate was found for antidepressants with known cardiac potassium channel blocking properties, but not for those with sodium channel blocking properties.
1. INTRODUCTION
Out‐of‐hospital cardiac arrest (OHCA) remains a major health problem in the affluent world, affecting 275 000 individuals per year in Europe. 1 OHCA may result from cardiac arrhythmias secondary to disruptions in cardiac electrophysiology. 2 Several antidepressants that impair cardiac repolarization and prolong the QT interval by blocking cardiac potassium channels may increase the risk of OHCA. 3 , 4 In addition, a previous study suggested that OHCA risk may be increased by antidepressants that impair cardiac depolarization by blocking cardiac sodium channels. 5 This possibility has so far received less recognition. Depolarization of the sarcolemma underlies the initiation of the cardiac action potential, and, by doing so, controls conduction velocity of the activation wavefront. 5 Accordingly, drugs that impair cardiac depolarization may result in fatal cardiac arrhythmias that underlie OHCA by slowing conduction velocity, thereby facilitating re‐entrant excitation. 5
Previous studies reported increase in cardiac arrest risk secondary to the use of some antidepressants, but these studies have important limitations, 4 , 5 , 6 , 7 in particular, inclusion of a limited number of cases, 5 the use of in‐hospital diagnoses to identify cardiac arrest events, 6 confounding by indication 4 , 7 and misclassification of the outcome by including OHCAs from noncardiac causes. 6 Furthermore, previous studies have yielded conflicting results. 4 , 5 , 6 , 7 Given the widespread use of antidepressants in the face of the rising incidence of depression worldwide, it is of clear clinical importance to establish whether use of antidepressants is associated with OHCA. Therefore, we aimed to clarify this controversy by analyzing data using nationwide registries from Denmark. We investigated whether the use of individual antidepressant drugs within drug classes is associated with OHCA. Also, we distinguished between antidepressants with and without sodium or potassium channel blocking properties to gain mechanistic insight.
2. METHODS
2.1. Data sources and definitions
A detailed description of the Danish nationwide health care registries is provided elsewhere. 8 , 9 , 10 , 11 Briefly, each resident in Denmark is assigned a unique and permanent civil registration number upon birth or immigration, which allows individual‐level linkage of information across the nationwide registries. By doing so, it is possible to follow every individual longitudinally with respect to death, immigration, drug use and all inpatient and outpatient hospital contacts. Patients with OHCA were obtained from the Danish Cardiac Arrest Registry (DANCAR), which is an ongoing nationwide observational registry of all OHCAs in Denmark. The OHCA data have been collected by the Emergency Medical Services personnel since June 2001. 8 The data collection is mandatory and consists of a case report form that provides information on important factors related to the OHCA according to Utstein recommendations. By doing so, data collection is close to complete on a nationwide level. The presumed cause of arrest was retrieved by using diagnoses codes from discharge or death certificates. OHCA with presumed cardiac cause was defined as cases with diagnosis codes containing cardiac disease, unknown disease or unexpected collapse. OHCA with presumed noncardiac cause included diagnosis codes for trauma, attempted suicide, drug overdose, drowning, violent attack and other noncardiac diseases. A detailed description of DANCAR is provided elsewhere. 8 Information on age, sex and vital status was obtained from the Civil Registration System, which is updated at regular intervals. Complete drug‐dispensing records was obtained from the National Prescription Register by using the Anatomical Therapeutic Chemical (ATC) classification system. 9 All diagnoses, procedures and operations were obtained from the Danish National Patient Registry, which contains information on all inpatient and outpatient hospital contacts and surgical procedures; discharge diagnoses are coded according to International Classification of Diseases system. 10 Finally, from the Danish Register of Causes of Death, we retrieved information about primary and contributing causes of death. 11
3. STUDY POPULATION
3.1. Study design
The main analyses of the study were conducted as nationwide nested case–control studies in the period between 1 June 2001 and 31 December 2015 as we did previously, 12 and included all patients who redeemed a prescription for an antidepressant any time between the beginning of the National Medical Prescription Register in 1995 and 31 December 2015. Cases were defined as individuals with OHCA from presumed cardiac causes.
3.2. Exposure
We defined antidepressant use as the redemption of these medications within 90 days prior to.
date of OHCA using the ATC codes. At any time during the follow up period, we classified individuals into 1 of the following mutually exclusive categories based on the current 90 day history: (i) selective serotonin reuptake inhibitor (SSRI) users; (ii) tricyclic antidepressants (TCA) users; (iii) users of serotonin–norepinephrine reuptake inhibitors (SNRI) or noradrenergic and specific serotonergic antidepressants (NaSSA) or other class of antidepressant (e.g., monoamine oxidase inhibitor); (iv) patients in treatment with 2 of the following: SSRI, TCA or SNRI/NaSSA; and (v) no users of antidepressants. Information about the potassium blocking properties of antidepressants were obtained from the clinically widely used CredibleMeds.com list (Accession Date: 2‐1‐2021). Such drugs are categorized at the CredibleMeds.com list based on their reported potential to cause torsade des pointes (TdP, a specific form of polymorphic ventricular tachycardia). 13 Information about the sodium channel blocking properties of antidepressants were obtained from www.Brugadadrugs.org (Accession Date: 2‐1‐2021). 14 Complete overview of the ATC codes of all antidepressants and their sodium or potassium blocking properties are provided in Table 1. Information regarding daily dosage was calculated from up to 5 consecutive prescriptions before the prescription of interest. Treatment duration was calculated by dividing the number the number of tablets in the prescription of interest by daily dosage, as described previously. 15
TABLE 1.
Detailed list of the included antidepressants
| Antidepressant | ATC | Sodium channel blocking antidepressants according to BrugadaDrugs list | Potassium channel blocking antidepressants and TdP risk classification according to CredibleMeds |
|---|---|---|---|
| SSRI | |||
| Sertraline | N06AB06 | No | Conditional |
| Fluoxetine | N06AB03 | Yes | Conditional |
| Citalopram | N06AB04 | No | Known |
| Paroxetine | N06AB05 | Yes | Conditional |
| Fluvoxamine | N06AB08 | Yes | Conditional |
| Escitalopram | N06AB10 | No | Known |
| TCA | |||
| Amitriptyline | N06AA09 | Yes | Conditional |
| Imipramine | N06AA02 | Yes | Possible |
| Clomipramine | N06AA04 | Yes | Conditional |
| Nortriptyline | N06AA10 | Yes | Possible |
| Doxepin | N06AA12 | Yes | Conditional |
| Maprotiline | N06AA21 | Yes | Possible |
| SNRI | |||
| Duloxetine | N06AX21 | No | Not included |
| Venlafaxine | N06AX16 | No | Possible |
| NaSSA | |||
| Mianserin | N06AX03 | No | Possible |
| Mirtazapine | N06AX11 | No | Possible |
| Other antidepressants | |||
| Isocarboxazid | N06AF01 | No | Not included |
| Moclobemid | N06AG02 | No | Not included |
| Reboxetine | N06AX18 | No | Not included |
| Agomelatine | N06AX22 | No | Not included |
ATC, Anatomical Therapeutic Chemical; NaSSA, noradrenergic and specific serotonergic antidepressants; SNRI, serotonin–norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressants; TdP, torsade des pointes
3.3. Explanatory variables
Cardiac disease and cardiovascular risk factors were considered as intermediates on the pathway between our exposure of interest and outcome. Therefore, we repeated our analyses in subgroups with the presence of cardiac disease and cardiovascular risk factors and in those without, as done previously. Cardiac disease was defined as having at least 1 of the following diagnoses up to 10 years prior to OHCA: ischaemic heart disease, congestive heart failure, cardiomyopathy or having an implantable cardioverter defibrillator. Cardiovascular risk factors were defined as having at least 1 of the following conditions: diabetes mellitus, hypertension, cerebrovascular disease, peripheral artery disease, chronic kidney disease, hyperlipidaemia, alcohol or substance abuse. Complete International Classification of Diseases and ATC codes are provided in Table S1.
3.4. Statistical analyses
Individuals were followed from the first redeemed antidepressant prescription or 1 June 2001 (beginning of the DANCAR), whatever came last, until OHCA, death (not classified as an OHCA), date of emigration or 31 December 2015, whatever came first. Cox regression with time‐dependent exposure and time‐dependent covariates was conducted to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs). We fitted Cox regression models using a nested case–control design 16 in which each OHCA case was matched based on age at the date of OHCA, and sex with up to 10 controls from the patients at risk of OHCA to allow the baseline hazard function to vary with age and sex. 17 Based on our Cox regression model, we compared the rates of OHCA between the dynamic exposure categories and reported differences as hazard ratios. Comparison of drugs were performed within a single class considering that different antidepressants have different risk profiles and indications. 3 Therefore, we compared each SSRI with sertraline as reference, each TCA with amitriptyline and each SNRI/NaSSA antidepressant with duloxetine as reference. The main analyses included patients with a redeemed prescription for any antidepressant drug prior to OHCA date. We performed the following subgroups analyses in: (i) patients with both cardiac disease and cardiovascular risk factors at the date of OHCA; and (ii) patients with neither cardiac disease nor cardiovascular risk factors at the date of OHCA. Supplementary analyses were conducted: (i) stratifying according to sex; and (ii) adjusting for the most common used QT‐prolonging drugs with known risk of TdP. We defined the use of QT‐prolonging drugs as having a drug‐dispensing record within 180 days prior to OHCA date, except for antimicrobial (≤14 d prior to OHCA date) as these drugs are generally prescribed for shorter periods (see Table S1 for the included QT‐prolonging drugs). Next, we conducted a dose–response analysis based on the calculated doses. Lastly, we performed stratified analyses according to the presence of ischaemic heart disease or heart failure.
3.5. Ethics
This study was approved by the Danish Data Protection Agency (Ref.no. 2007‐58‐0015, local ref.no. GEH‐2014‐017, I‐Suite 0.2735). In Denmark, ethical approval is not required for observational studies where patients remain anonymous.
4. RESULTS
4.1. Main analyses
The study population consisted of 10 987 OHCA cases and 109 869 matched non‐OHCA controls (Figure 1). Characteristics of the patients at entry date are listed in Table 2. The rate of OHCA among users of TCAs or SNRIs/NaSSA was not different compared to users of SSRIs: HR 1.03 (95% CI 0.94–1.14) and HR 0.97 (95% CI 0.90–1.04), respectively (Figure 2).
FIGURE 1.
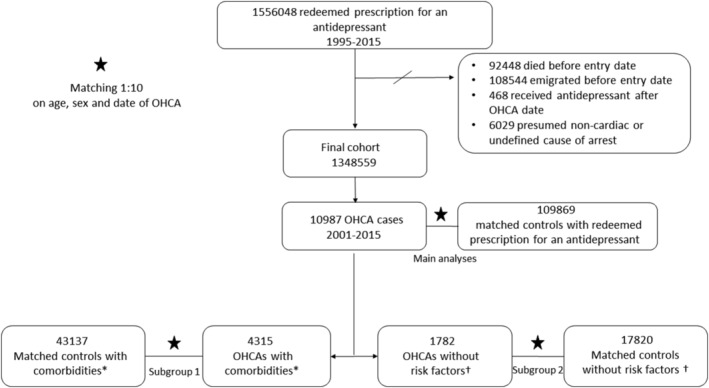
Flow chart of inclusion of out‐of‐hospital cardiac arrest (OHCA) cases and controls. *Patients with both cardiac disease and cardiovascular risk factors at the date of OHCA. † Patients with neither cardiac disease nor cardiovascular risk factors at the date of OHCA
TABLE 2.
Patient characteristics at entry date according to class of antidepressants
| No use of antidepressant within 90 d prior to entry‐date | SSRI within 90 d prior to entry‐date | TCA within 90 d prior to entry‐date | SNRI/NaSSA within 90 d prior to entry‐date | More than 1 class of antidepressants within 90 d prior to entry‐date a | |
|---|---|---|---|---|---|
| n | 247 984 | 674 849 | 149 010 | 253 336 | 23 380 |
| Age (y), median (IQR) | 53 (41–67) | 48 (31–69) | 55 (42–69) | 50 (36–67) | 57 (42–75) |
| Men, n (%) | 92 943 (37.48) | 263 854 (39.10) | 58 202 (39.06) | 116 062 (45.81) | 8931 (38.20) |
| Comorbidity, n (%) | |||||
| Cardiac disease | 18 413 (7.43) | 56 785 (8.41) | 12 665 (8.50) | 20 120 (7.94) | 2330 (9.97) |
| Cardiovascular risk factors | 48 971 (19.75) | 142 867 (21.17) | 33 728 (22.63) | 50 915 (20.10) | 6774 (28.97) |
| Antidepressant history, n (%) | |||||
| Number of patients that used earlier a different class of antidepressants | 247 984 (100) | 21 995 (3.26) | 9580 (6.43) | 19 048 (7.52) | 3465 (14.82) |
| First antidepressant prescription and time to entry‐date | |||||
| 0–30 d | 0 | 574 413 (85.12) | 121 551 (81.57) | 222 293 (87.75) | 9952 (42.57) |
| 30–90 d | 0 | 7439 (1.10) | 1349 (0.91) | 3318 (1.31) | 652 (2.79) |
| 90–360 d | 31 496 (12.70) | 17 222 (2.55) | 1937 (1.30) | 4675 (1.85) | 1491 (6.38) |
| >360 d | 216 488 (87.30) | 75 775 (11.23) | 24 173 (16.22) | 23 050 (9.10) | 11 285 (48.27) |
IQR, interquartile range; SNRI/NaSSA, serotonin–norepinephrine reuptake inhibitors/noradrenergic and specific serotonergic antidepressants; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressants.
Patients in treatment with at least 2 of the following: SSRI, TCA or SNRI/NaSSA antidepressants
FIGURE 2.
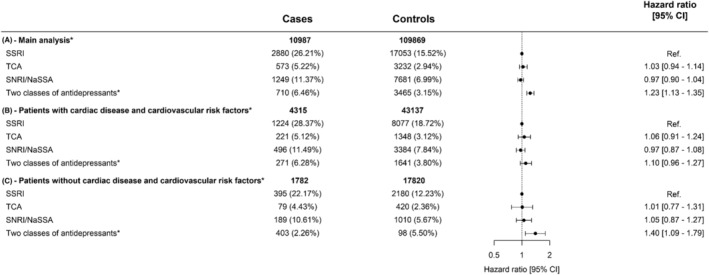
Hazard ratio of out‐of‐hospital cardiac arrest (OHCA) following treatment with antidepressants in (A) overall population who redeemed prescription for antidepressants between 1995 and 2015, (B) patients both cardiac disease and cardiovascular risk factors and (C) patients without cardiac disease and cardiovascular risk factors. Not included in the table: cases (%) and controls (%) of nonusers of antidepressants 90 days before case index: (A) 5575 (50.74%)/78 438 (71.39%), (B) 2103 (48.74%)/28 687 (66.50%), (C) 1021 (57.30%)/13 807(77.48%). * Patients in treatment with 2 of SSRI, TCA or SNRI/NaSSA/other antidepressants. SNRI/NaSSA, serotonin–norepinephrine reuptake inhibitor/noradrenergic and specific serotonergic antidepressant; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant
When we studied individual antidepressants within the same drug class, we found that, among SSRIs (reference drug sertraline), the rate of OHCA was increased for citalopram (HR 1.20 [95% CI 1.06–1.36], Figure 3). In our dose–response analyses, we found that, the rate of OHCA was increased for high‐dose citalopram (>20 mg) and high‐dose escitalopram (>10 mg; HR 1.46 [95% CI 1.27–1.69], HR 1.43 [95% CI 1.16–1.75], respectively, Table 3). In the class of SNRIs/NaSSA antidepressants (reference drug duloxetine), the rate of OHCA was increased for high‐dose mirtazapine (>30 mg; HR 1.59 [95% CI 1.18–2.14], Table 3). In the class of TCAs (reference drug amitriptyline), no drug was associated with increased rate of OHCA (Table 3). In the subanalyses in men and women, citalopram (HR 1.31 [95%‐CI:1.10–1.56], Table S2) and escitalopram (HR 1.33 [95% CI:1.06–1.66], Table S2) were associated with OHCA in men but not in women. Antidepressants with sodium channel blocking properties showed no higher rate of OHCA compared to antidepressants without this property (HR 0.96 [95% CI 0.89–1.05], Figure 4). Our analyses of antidepressants with potassium channel blocking properties and TdP risk revealed that drugs with known risk of TdP were associated with higher rate of OHCA compared with antidepressants with no or conditional risk of TdP (HR 1.14[95% CI:1.05–1.23], Figure 4). In our sensitivity analysis, the estimates for the association between individual antidepressant drugs and OHCA rate did not change significantly when we adjusted for the concomitant use of other QT‐prolonging drugs (Table S3).
FIGURE 3.
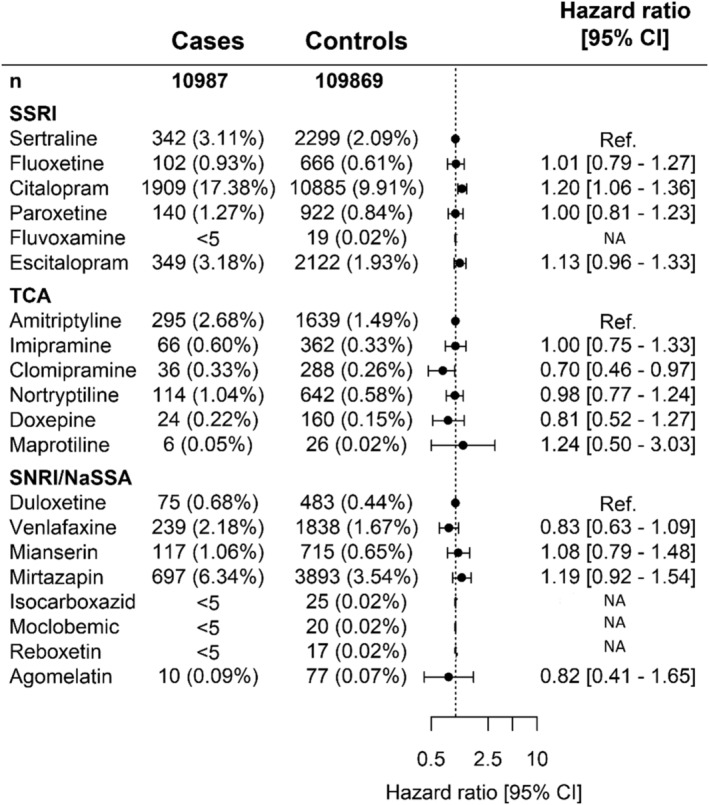
Hazard ratio of out‐of‐hospital cardiac arrest (OHCA) following treatment with specific antidepressants in overall population who redeemed prescription for antidepressants between 1995–2015. Not included in the table: cases (%) and controls (%) of nonusers of antidepressants 90 days before case index or users of >2 antidepressants: (A) 5575(50.74%)/78 438(71.39%), 881 (8.02%)/4333 (3.94%). SNRI/NaSSA, serotonin–norepinephrine reuptake inhibitor/noradrenergic and specific serotonergic antidepressant; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant
TABLE 3.
Hazard ratio of out‐of‐hospital cardiac arrest following treatment with antidepressant according to low and high dose in the overall population
| Cases (n = 10 987) | Controls (n = 109 869) | Hazard ratio [95% CI] | |
|---|---|---|---|
| SSRI, n (%) | |||
| Sertraline | 342 (3.11) | 2299 (2.09) | Reference |
| Fluoxetine low dose ≤20 | 32 (0.29) | 267 (0.24) | 0.80 [0.54–1.17] |
| Fluoxetine high dose >20 | 70 (0.64) | 399 (0.36) | 1.15 [0.87–1.52] |
| Citalopram low dose ≤20 | 1294 (11.78) | 8057 (7.33) | 1.10 [0.97–1.26] |
| Citalopram high dose >20 | 615 (5.60) | 2828 (2.57) | 1.46 [1.27–1.69] |
| Paroxetine low dose ≤20 | 77 (0.70) | 579 (0.53) | 0.88 [0.68–1.15] |
| Paroxetine high dose >20 | 63 (0.57) | 343 (0.31) | 1.20 [0.90–1.61] |
| Fluvoxamine low dose ≤75 | <5 | <5 | NA |
| Fluvoxamine high dose >75 | <5 | 17 (0.02) | NA |
| Escitalopram low dose ≤10 | 189 (1.72) | 1356 (1.23) | 0.96 [0.79–1.16] |
| Escitalopram high dose >10 | 160 (1.46) | 766 (0.70) | 1.43 [1.16–1.75] |
| TCA, n (%) | |||
| Amitriptyline | 295 (2.68) | 1639 (1.49) | Reference |
| Imipramine low dose ≤100 | 58 (0.53) | 339 (0.31) | 0.94 [0.69–1.27] |
| Imipramine high dose >100 | 8 (0.07) | 23 (0.02) | 1.90 [0.84–4.29] |
| Clomipramine low dose ≤50 | 16 (0.15) | 143 (0.13) | 0.61 [0.36–1.04] |
| Clomipramine high dose >50 (15) | 20 (0.18) | 145 (0.13) | 0.73 [0.45–1.18] |
| Nortriptyline low dose ≤75 | 64 (0.58) | 392 (0.36) | 0.91 [0.68–1.22] |
| Nortriptyline high dose >75 | 50 (0.46) | 250 (0.23) | 1.09 [0.78–1.51] |
| Doxepin low dose ≤75 | 18 (0.16) | 125 (0.11) | 0.79 [0.47–1.31] |
| Doxepin high dose >75 | 6 (0.05) | 35 (0.03) | 0.91 [0.38–2.16] |
| Maprotiline low dose ≤100 | <5 | 16 (0.01) | NA |
| Maprotiline high dose >100 | <5 | 10 (0.01) | NA |
| SNRI/NaSSA, n (%) | |||
| Duloxetine | 75 (0.68) | 483 (0.44) | Reference |
| Venlafaxin low dose ≤75 | 56 (0.51) | 555 (0.51) | 0.65 [0.45–0.94] |
| Venlafaxin high dose >75 | 183 (1.67) | 1283 (1.17) | 0.90 [0.68–1.20] |
| Mianserin low dose ≤50 | 107 (0.97) | 669 (0.61) | 1.06 [0.77–1.45] |
| Mianserin high dose >50 | 10 (0.09) | 46 (0.04) | 1.40 [0.68–2.90] |
| Mirtazepin low dose ≤30 | 539 (4.91) | 3241 (2.95) | 1.11 [0.85–1.44] |
| Mirtazapin high dose >30 | 158 (1.44) | 652 (0.59) | 1.59 [1.18–2.14] |
| Isocarboxazid low dose ≤30 | <5 | 17 (0.02) | NA |
| Isocarboxazid high dose >30 | <5 | 8 (0.01) | NA |
| Moclobemid low dose ≤300 | <5 | 8 (0.01) | NA |
| Moclobemid high dose >300 | <5 | 12 (0.01) | NA |
| Reboxetin low dose ≤5 | <5 | <5 | NA |
| Reboxetin high dose >5 | <5 | 13 (0.01) | NA |
| Agomelatin low dose ≤25 | <5 | 32 (0.03) | NA |
| Agomelatin high dose >25 | 7 (0.06) | 45 (0.04) | 0.99 [0.43–2.28] |
Not included in the table cases (%)/controls (%) of nonusers of antidepressants in the period of 90 days before case index: 5575(50.74)/78 438(71.39) or users of >2 antidepressants: 881(8.02)/4333(3.94). The dosage is in mg.
CI, confidence interval; SNRI/NaSSA, serotonin–norepinephrine reuptake inhibitors/noradrenergic and specific serotonergic antidepressants; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressants.
FIGURE 4.
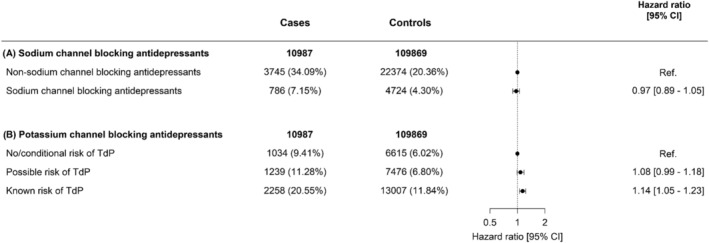
Hazard ratio of out‐of‐hospital cardiac arrest (OHCA) following treatment with antidepressants with and without sodium channel blocking or potassium channel blocking properties. Not included in the table: cases (%) and controls (%) of nonusers of antidepressants 90 days before case index or users of >2 antidepressants: (A) 5575 (50.74%)/78 438 (71.39%), 881 (8.02%)/4333 (3.94%); (B) 5575 (50.74%)/78 438 (71.39%), 881 (8.02%)/4333 (3.94%). TdP, torsade des pointes
4.2. Subgroup analyses
From all OHCAs included in the main analyses, we identified 4315 OHCAs with both cardiac disease and cardiovascular risk factors and matched them with 43 137 controls with these conditions. In this subgroup, we found no increased rate of OHCA among any of the studied antidepressants (Figure S1). We identified 1782 OHCAs without cardiac disease nor cardiovascular risk factors and matched them with 17 820 controls without these conditions. In this subgroup, the rate of OHCA among SSRIs was nonsignificantly increased for citalopram (HR 1.32 [95% CI 0.95–1.84]), while it was increased among SNRIs/NaSSA antidepressants for mianserin (HR 3.00 [95% CI 1.07–8.34]) and mirtazapine (HR 2.80 [95% CI 1.10–7.13]); among TCAs, no drug was associated with increased OHCA risk (Figure S1). Finally, our stratified analyses according to the presence of ischaemic heart disease or heart failure showed no increased OHCA rate for any of the studied individual antidepressants in patients with ischaemic heart disease or heart failure, while citalopram was associated with higher OHCA rate in patients without ischaemic heart disease and heart failure (Table S4).
5. DISCUSSION
In this nationwide nested case–control study, we found that, relative to sertraline from the group of SSRIs, high‐dose citalopram (>20 mg) and high‐dose escitalopram (>10 mg) were associated with increased OHCA rate. Relative to duloxetine from the group of SNRI/NaSSA antidepressants, high‐dose mirtazapine (>30 mg) was associated with increased OHCA rate. Compared to amitriptyline from the group of TCAs, no TCA was associated with increased OHCA rate. The use of antidepressants with known potassium channel blocking properties and TdP risk, but not antidepressants with sodium channel blocking properties, was associated with increased rate of OHCA.
Previous studies have investigated the association between use of antidepressants and sudden cardiac arrest but obtained conflicting results. 4 , 5 , 6 , 7 A previous study by Weeke et al. found that use of citalopram was associated with an increased risk of OHCA. 4 Since we used the same OHCA registry, cases analysed by Weeke et al. were also included in our study. However, Weeke et al. performed a case–time–control design, which may result in persistent user bias when used to study medication exposures that do not vary over time, e.g., antidepressant use. 18 A retrospective cohort study by Leonard et al. found no evidence that risk of cardiac arrest for citalopram differed significantly from that of paroxetine. 6 However, that study solely relied on emergency department and inpatient diagnoses, which may cause important inclusion bias since the majority of sudden cardiac deaths occurs outside the hospital. Our OHCA registry resolved this limitation due to our collaboration with all EMS departments in Denmark. By doing so, we could enrol both patients who died before hospital arrival and those who survived to hospital arrival. While we observed an increased rate of OHCA among citalopram, escitalopram and mirtazapine in our main analyses, this increase in OHCA rate only occurred at high‐dose, and were small to modest. Our findings are in line with a previous study that investigated the effects of antidepressants on QT interval in which was reported that QT prolongation among SSRIs were largely caused by citalopram and escitalopram (the effects of mirtazapine was not studied). 19 The individual antidepressants differ substantially between each other in their affinity and selectivity for the distinct cardiac ion channels. 20 For instance, previous studies revealed that certain SSRIs, e.g., citalopram and escitalopram, block the cardiac potassium channels in a concentration‐dependent manner, 21 whereas certain TCAs, e.g., nortriptyline, block the cardiac sodium channels. 5 Accordingly, a previous clinical study demonstrated that nortriptyline may also increase the risk of OHCA by blocking cardiac sodium channels. 5 Bardai et al. found that nortriptyline was associated with a 4.5‐fold increase in the risk for OHCA compared with no use of nortriptyline. 5 However, the small number of exposed OHCAs with nortriptyline included in the study (n = 4) limits the validity of these results. Similarly, Weeke et al. found a 5‐fold increase in the risk for OHCA upon use of nortriptyline compared with no use of nortriptyline. 4 In both studies, confounding by indication may play an important role since nortriptyline was compared with no use of nortriptyline. Our study tried to minimize confounding by indication by including only patients who redeemed antidepressants and comparing antidepressants within the same drug classes, aiming to make the groups comparable with respect to underlying condition and disease severity.
Our analyses according to antidepressants with and without sodium or potassium channel blocking properties revealed no increase of OHCA rate in subjects using antidepressants with sodium channel blocking properties, while we observed a modest increase in rate of OHCA with known potassium channel blocking properties. This suggests that it is less likely that sodium channel blocking properties of antidepressants account for the association between certain antidepressants and OHCA.
In our subgroup analyses including only patients with known cardiac disease and cardiovascular risk factors, we observed no higher OHCA hazard for any of the studied individual antidepressants. One possibility might be that physicians who treat these patients may have become more prudent in prescribing antidepressants with QT prolonging properties because of the attention for the potential OHCA risk of antidepressants with QT‐prolonging properties in the past decades. For instance, patients with additional risk factors (e.g., antiarrhythmic drugs) may be excluded from receiving antidepressants with QT prolonging properties. Moreover, in the summary of product characteristics of the drugs that we found associated with increased OHCA rate (high‐dose of citalopram, escitalopram, mirtazapine), it is reported that the risk of QT‐prolongation and thereby cardiac arrhythmias is particularly high in patients with pre‐existing QT‐prolongation or with known cardiovascular disease. An alternative explanation could be that patients with established cardiovascular disease are at higher risk of cardiac arrhythmias and thereby OHCA, regardless the use of antidepressants. In the subgroup analyses including only patients without known risk factors for OHCA, we observed statistically significantly higher OHCA rate for mirtazapine and mianserin, while citalopram showed a trend towards increased OHCA rate. Although studies indicated that mianserin influence cardiac electrophysiology by modulating cardiac potassium channels, 22 our findings regarding mianserin should be interpreted with caution, since our estimates were based on a limited number of events (mianserin n = 21), which is also reflected by the wide confidence interval.
The use of antidepressants is fundamental for the control of symptoms in depression and untreated depression more than doubles the risk of OHCA. 23 Therefore, our findings that most of the antidepressants are not associated with OHCA are of clinical importance and indicate that careful titration may have to be considered when citalopram, escitalopram and mirtazapine is prescribed.
5.1. Strengths and limitations
A major strength of our study is the population‐based real‐world design of our DANCAR‐registry, which minimizes selection bias by including every OHCA, rendering our findings representative for the community at large. However, as with any observational study, residual confounding remains possible since data on several important features such as body mass index, smoking and alcohol use were not available. Therefore, we could only detect associations without proving causality. Furthermore, we had no information regarding the exact cause of OHCA, thus raising the possibility of misclassification of the outcome. 24 Another limitation is that, although drug‐dispensing records were complete, we had no information whether claimed medications were actually taken. However, we expect that a possible misclassification arising from this was probably similarly distributed between cases and controls. Finally, we had no information regarding the indication for the antidepressant prescription.
6. CONCLUSION
Users of high‐dose citalopram (>20 mg) and high‐dose escitalopram (>10 mg) had a higher rate of OHCA compared to sertraline users, and users of high‐dose mirtazapine (>30 mg) had a higher rate of OHCA compared to duloxetine users. Careful titration of citalopram, escitalopram and mirtazapine dose may have to be considered in order to warrant drug safety.
COMPETING INTERESTS
L.V.K has for 3 years been a consultant for Lundbeck and Teva. N.Z. has received funding from the European Union's Horizon 2020 research and innovation program ESCAPE‐NET and Helsefonden. All other authors have no interests to declare.
CONTRIBUTORS
T.E.E. conceived the study idea, designed the research, performed the statistical analyses and wrote the manuscript. C.A.B. helped with designing the research and with the data analyses. G.H.G. helped with designing the research. All authors critically revised and approved the manuscript.
Supporting information
TABLE S1 Detailed list of ICD codes to define comorbidities
TABLE S2a Hazard ratio of out‐of‐hospital cardiac arrest stratified following treatment with antidepressants according to sex
TABLE S2b Hazard ratio of out‐of‐hospital cardiac arrest following treatment with specific antidepressants in woman
TABLE S2c Hazard ratio of out‐of‐hospital cardiac arrest following treatment with specific antidepressants in men
FIGURE S1 Hazard ratio of out‐of‐hospital cardiac arrest (OHCA) following treatment with specific antidepressants in (A) patients with cardiac disease and cardiovascular risk factors and in (B) patients without cardiac disease and cardiovascular risk factors who redeemed prescription for antidepressants between 1995 and 2015
TABLE S3a Hazard ratio of out‐of‐hospital cardiac arrest following treatment with antidepressants adjusted for the use of QT‐prolonging drugs
TABLE S3b Hazard ratio of out‐of‐hospital cardiac arrest following treatment with specific antidepressants adjusted for the use of QT‐prolonging drugs
TABLE S3c Hazard ratio of out‐of‐hospital cardiac arrest (OHCA) following treatment with antidepressant according to low and high dose adjusted for the use of QT‐prolonging drugs
TABLE S4a Hazard ratio of out‐of‐hospital cardiac arrest following treatment with specific antidepressants in patients with ischaemic heart disease or heart failure
TABLE S4b Hazard ratio of out‐of‐hospital cardiac arrest following treatment with specific antidepressants in patients without ischaemic heart disease or heart failure
ACKNOWLEDGEMENTS
The authors greatly appreciate the contributions of all participating regional ambulance services and fire brigades in the study region for their contribution and support.
This work was supported by the European Union's Horizon 2020 research and innovation programme under the acronym ESCAPE‐NET, registered under grant agreement No 733381 (T.E.E., H.L.T.), and the COST Action PARQ (grant agreement No CA19137) supported by COST (European Cooperation in Science and Technology). The funders were not involved in designing the study, collecting and analysing the data, preparing the manuscript, or decision to publish. The funders were not involved in designing the study, collecting and analysing the data, preparing the manuscript, or decision to publish.
Eroglu TE, Barcella CA, Gerds TA, et al. Risk of out‐of‐hospital cardiac arrest in antidepressant drug users. Br J Clin Pharmacol. 2022;88(7):3162-3171. doi: 10.1111/bcp.15224
The authors confirm that the PI for this paper is Gunnar H. Gislason.
Funding information COST Action PARQ, Grant/Award Number: CA19137; European Union’s Horizon 2020, Grant/Award Number: 733381
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly due to ethical/privacy reasons.
REFERENCES
- 1. Atwood C, Eisenberg MS, Herlitz J, Rea TD. Incidence of EMS‐treated out‐of‐hospital cardiac arrest in Europe. Resuscitation. 2005;67(1):75‐80. [DOI] [PubMed] [Google Scholar]
- 2. Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345(20):1473‐1482. [DOI] [PubMed] [Google Scholar]
- 3. Fanoe S, Kristensen D, Fink‐Jensen A, et al. Risk of arrhythmia induced by psychotropic medications: a proposal for clinical management. Eur Heart J. 2014;35(20):1306‐1315. [DOI] [PubMed] [Google Scholar]
- 4. Weeke P, Jensen A, Folke F, et al. Antidepressant use and risk of out‐of‐hospital cardiac arrest: a nationwide case‐time‐control study. Clin Pharmacol Ther. 2012;92:72‐79. [DOI] [PubMed] [Google Scholar]
- 5. Bardai A, Amin AS, Blom MT, et al. Sudden cardiac arrest associated with use of a non‐cardiac drug that reduces cardiac excitability: evidence from bench, bedside, and community. Eur Heart J. 2013;34(20):1506‐1516. [DOI] [PubMed] [Google Scholar]
- 6. Leonard CE, Bilker WB, Newcomb C, Kimmel SE, Hennessy S. Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf. 2011;20(9):903‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2004;75(3):234‐241. [DOI] [PubMed] [Google Scholar]
- 8. Wissenberg M, Hansen CM, Folke F, et al. Survival after out‐of‐hospital cardiac arrest in relation to sex: a nationwide registry‐based study. Resuscitation. 2014;85:1212‐1218. [DOI] [PubMed] [Google Scholar]
- 9. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38‐41. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Helweg‐Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39:26‐29. [DOI] [PubMed] [Google Scholar]
- 12. Barcella CA, Mohr G, Kragholm K, et al. Risk of out‐of‐hospital cardiac arrest in patients with bipolar disorder or schizophrenia. Heart. 2021;107:1544‐1551. [DOI] [PubMed] [Google Scholar]
- 13. Woosley RL , Heise CW , Gallo T , Tate J , Woosley D, Romero KA. www.CredibleMeds.org, QTdrugs List, [Accession Date: 2‐1‐2021], AZCERT, Inc. 1822 Innovation Park Dr., Oro Valley, AZ 85755.
- 14. Postema PG, Wolpert C, Amin AS, et al. Drugs and Brugada syndrome patients: review of the literature, recommendations and an up‐to‐date website (www.brugadadrugs.org). Heart Rhythm. 2009;6:1335‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gislason GH, Rasmussen JN, Abildstrøm SZ, et al. Long‐term compliance with beta‐blockers, angiotensin‐converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27:1153‐1158. [DOI] [PubMed] [Google Scholar]
- 16. Ernster VL. Nested case‐control studies. Prev Med. 1994;23(5):587‐590. [DOI] [PubMed] [Google Scholar]
- 17. Borgan Ø, Samuelsen SO. Nested case‐control and case‐cohort studies. In: Handbook of Survival Analysis; 2013:343‐367. [Google Scholar]
- 18. Hallas J, Pottegård A, Wang S, Schneeweiss S, Gagne JJ. Persistent user bias in case‐crossover studies in pharmacoepidemiology. Am J Epidemiol. 2016;1‐9. [DOI] [PubMed] [Google Scholar]
- 19. Beach SR, Kostis WJ, Celano CM, et al. Meta‐analysis of selective serotonin reuptake inhibitor–associated QTc prolongation. J Clin Psychiatry. 2014;75(5):441‐449. [DOI] [PubMed] [Google Scholar]
- 20. Sicouri S, Antzelevitch C. Mechanisms underlying the actions of antidepressant and antipsychotic drugs that cause sudden cardiac arrest. Arrhythmia Electrophysiol Rev. 2018;7(3):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chae YJ, Jeon JH, Lee HJ, et al. Escitalopram block of hERG potassium channels. Naunyn Schmiedebergs Arch Pharmacol. 2014;387(1):23‐32. [DOI] [PubMed] [Google Scholar]
- 22. Scherer D, von Löwenstern K, Zitron E, et al. Inhibition of cardiac hERG potassium channels by tetracyclic antidepressant mianserin. Naunyn Schmiedebergs Arch Pharmacol. 2008;378(1):73‐83. [DOI] [PubMed] [Google Scholar]
- 23. Empana JP, Jouven X, Lemaitre RN, et al. Clinical depression and risk of out‐of‐hospital cardiac arrest. Arch Intern Med. 2006;166:195‐200. [DOI] [PubMed] [Google Scholar]
- 24. Tseng ZH, Olgin JE, Vittinghoff E, et al. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation. 2018;137(25):2689‐2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Detailed list of ICD codes to define comorbidities
TABLE S2a Hazard ratio of out‐of‐hospital cardiac arrest stratified following treatment with antidepressants according to sex
TABLE S2b Hazard ratio of out‐of‐hospital cardiac arrest following treatment with specific antidepressants in woman
TABLE S2c Hazard ratio of out‐of‐hospital cardiac arrest following treatment with specific antidepressants in men
FIGURE S1 Hazard ratio of out‐of‐hospital cardiac arrest (OHCA) following treatment with specific antidepressants in (A) patients with cardiac disease and cardiovascular risk factors and in (B) patients without cardiac disease and cardiovascular risk factors who redeemed prescription for antidepressants between 1995 and 2015
TABLE S3a Hazard ratio of out‐of‐hospital cardiac arrest following treatment with antidepressants adjusted for the use of QT‐prolonging drugs
TABLE S3b Hazard ratio of out‐of‐hospital cardiac arrest following treatment with specific antidepressants adjusted for the use of QT‐prolonging drugs
TABLE S3c Hazard ratio of out‐of‐hospital cardiac arrest (OHCA) following treatment with antidepressant according to low and high dose adjusted for the use of QT‐prolonging drugs
TABLE S4a Hazard ratio of out‐of‐hospital cardiac arrest following treatment with specific antidepressants in patients with ischaemic heart disease or heart failure
TABLE S4b Hazard ratio of out‐of‐hospital cardiac arrest following treatment with specific antidepressants in patients without ischaemic heart disease or heart failure
Data Availability Statement
The data underlying this article cannot be shared publicly due to ethical/privacy reasons.


