Abstract
Background and Aim
Remimazolam tosilate (RT) is under evaluation as a sedative for endoscopic procedures. Herein, we aimed to evaluate safety including cognition recovery of RT administered in elderly patients undergoing upper gastrointestinal endoscopy and assess its safety dosage.
Methods
Ninety‐nine patients presenting for upper gastrointestinal endoscopy were randomized to receive 0.1 mg/kg RT (R1) or 0.2 mg/kg RT (R2), or propofol (P). Cognitive functions (memory, attention, and executive function) were measured via neuropsychological tests conducted before sedation and 5 min after recovery to full alertness. Adverse events were also assessed.
Results
There were no statistical differences between postoperative and baseline results for R1 group and P group, whereas those for R2 group revealed worsened postoperative cognitive functions (immediate recall and short delay recall) than baseline (P < 0.05). Compared with P group, Scores demonstrated worse restoration of immediate recall in R1 group, immediate recall, short‐delayed recall, and attention function in R2 group (P < 0.05). Patients in R2 group had a longer sedation time (12.09 vs 8.27 vs 8.21 min; P < 0.001) and recovery time (6.85 vs 3.82 vs 4.33 min; P < 0.001) than that in R1 group and P group. Moreover, the incidence of hypotension was 3.0% in R1 group, whereas it was 21.2% in R2 group and 48.5% in P group (P < 0.05).
Conclusion
The addition of 0.1 mg/kg RT as an adjunct to opiate sedation for upper gastrointestinal endoscopy not only achieves more stable perioperative hemodynamics but also achieves acceptable neuropsychiatric functions in elderly patients.
Keywords: Cognitive function, Elderly patients, Endoscopic sedation, Remimazolam tosilate, Upper gastrointestinal endoscopy
Introduction
Upper gastrointestinal (GI) endoscopy has been regarded as the gold standard treatment for the diagnosis of upper GI tract diseases and is widely performed worldwide. Endoscopic procedures are invasive and may cause the patient pain and discomfort 1 ; therefore, sedation with prompt recovery including cognitive function is an important goal of postoperative care. 2
Propofol or benzodiazepine combined with analgesic agents are used widely for sedation during endoscopy. 3 Propofol is a short‐acting anesthetic agent that can be used successfully as a sedative drug during GI endoscopy. It has a favorable pharmacokinetic profile in comparison to benzodiazepine with regard to rapid induction of sedation, faster recovery, and equivalent levels of amnesia. However, concerns about cardiovascular and respiratory depression have led to the restriction of its use to anesthetists treating elderly patients and those with multiple comorbid conditions. 4 , 5 Remimazolam tosilate (HR7056, RT) is a new short‐acting benzodiazepine that was developed by HengRui Medicine Co., Ltd., in China. In 2019, the National Medical Products Administration approved its use as a new drug for use in anesthesia and sedation. It can be rapidly hydrolyzed in the body via ubiquitous tissue esterases to an inactive carboxylic acid metabolite, which results in faster‐acting onset and recovery than those of currently available short‐acting sedatives. 6 In healthy young patients undergoing upper GI endoscopy, RT was demonstrated to have a favorable safety profile and was superior to propofol. 7 In the first study of remimazolam in patients for procedural sedation, the safety profile appeared to be similar to that of midazolam, as there was a decline in cognitive function after the administration of either midazolam or remimazolam. 8 However, what is the most suitable drug dosage for elderly patients and whether the use of RT affects cognitive function remains unanswered.
In this study, we aimed to evaluate the effect of RT on the early cognitive function of elderly patients undergoing upper GI endoscopy. In addition, a suitable dose for sedation during induction was assessed.
Methods
Study design
This study was a prospective, randomized, double‐blind, positive‐controlled parallel trial comparing doses of 0.10 or 0.20 mg/kg RT (HengRui Medicine, China) to 1.0–1.5 mg/kg propofol (AstraZeneca, USA) as a sedative for patients undergoing upper GI endoscopy. Patients were recruited from the Third Xiangya Hospital in China, between August 2020 and February 2021. The trial was registered with https://www.clinicaltrials.gov (ChiCTR2100042084) and was conducted in accordance with the Declaration of Helsinki and the principles of the International Conference on Harmonization Good Clinical Practice Guideline. The protocol was approved by the Institutional Review Board of Third Xiangya Hospital. Written informed consent was obtained from each patient prior to the start of any protocol‐specified procedures.
Patient eligibility
Patients aged >60 years undergoing upper GI endoscopy who were of American Society of Anesthesiologists physical status I or II and possessed sufficient Chinese language proficiency to complete neuropsychological assessments were included in the study. Patients were excluded if they had clinically significant cardiorespiratory instability; clinically significant renal or hepatic dysfunction; or a history of drug/alcohol abuse, psychiatric illness, neurological disease, auditory, or visual disturbances that could affect the reliability of the neuropsychological assessments; pre‐existing memory or cognitive impairment; an allergy to any of the anesthetic agents; anesthesiologist refusal of the anesthetic use.
Randomization and masking
All eligible patients were randomized into one of three groups: RT administered in a dose of 0.10 or 0.20 mg/kg as the R1 and R2 groups, respectively, or propofol in a dose of 1.0–1.5 mg/kg as the P group based on a ratio of 1:1:1 determined by a computer‐generated coding system. We applied the double‐blind design. Anesthetists who performed sedation were aware of the treatment assignment of each participant. However, participants and primary outcome assessors were unaware of the treatment assignment throughout the study.
Procedures
On arrival to the endoscopy room, IV access was obtained and oxygen was administered at 4 L/min via a clear nasal catheter. Patients were not administered IV fluids. Routine patient monitoring included pulse oximetry, electro‐cardiograph, and noninvasive arterial blood pressure measurement. Quilts were used to keep patients warm.
All patients received 10‐g lidocaine viscous oral liquid (0.2‐g lidocaine) and butorphanol (0.01 mg/kg) before receiving the assigned investigational sedative medication. Sedative drugs were administered via IV according to the randomized group allocation (a single dose of 0.10 or 0.20 mg/kg RT; or 1.0–1.5 mg/kg propofol). When the patient was sufficiently sedated (Modified Observer's Assessment of Alertness/Sedation [MOAA/S] ≤1), the gastroscopy procedure was initiated. If sedation was deemed to be inadequate (MOAA/S > 1) or the gastroscopy procedure failed, up to a maximum of two supplemental doses administered as IV boluses (0.05 mg/kg RT for the R1 and R2 group, or 0.5 mg/kg propofol for the P group), was permitted after 1 min at the end of the initial dose, at least 1 min apart. If the initial dose and supplemental doses were not sufficient to obtain adequate sedation for scope insertion, sedative rescue medication (propofol) was to be administered at the start of the procedure at the anesthesiologist's discretion. Once the procedure was underway, to maintain the patient at an adequate sedation level (MOAA/S score of ≤1) throughout the procedure, patients were injected with supplemental doses at least 1 min apart (not to exceed a cumulative total of five supplemental doses) of sedative medication at the discretion of the anesthesiologist. If these doses were not sufficient to maintain appropriate sedation, sedative rescue medication was to be administered for the completion of the procedure and/or endoscope removal. Predefined complications (hypotension, bradycardia, hypoxia, and dreaming) were managed according to our study protocol (not shown). Patients who required rescue medication were not included in the postoperative neuropsychological assessments, which were performed within 5 min of patients becoming fully alert after the endoscopy procedure. When patients were ready for hospital discharge, they were questioned about their satisfaction with care and intraoperative awareness.
Measurements
Baseline data included patient characteristics (age, sex, education level, and body mass index), diagnosis, and preoperative comorbidities. Oxygen saturation, heart rate, and arterial blood pressure were recorded every 2 min during sedation (including pre‐operation, intra‐operation, post‐operation, and fully alert). MOAA/S scores were recorded frequently (every 30 s from 1 min to 3 min, then every 1 min) until the patient was fully alert (three consecutive MOAA/S scores of 5). Sedation time was defined as the time from administration of the last drug until patients became fully alert. Recovery time was defined as the time from removal of the endoscope until the patient became fully alert. Endoscopy time was defined as the time from insertion of the endoscope and until removal of the endoscope. Cognitive function was evaluated based on neuropsychological tests. Patient satisfaction with anesthetic care was measured using a 5‐mm visual analog scale (0 = completely dissatisfied and 5 = completely satisfied). Recall of the procedure was assessed using the Brice questionnaire after the fully alert criteria had been reached.
Neuropsychological tests
We adopted well‐established neuropsychological tests to ensure standardization of the procedure during data collection.
For the Digit Symbol Substitution Test (DSST), participants were asked to write as fast as they could symbols that were paired to Arabic numerals (1–9) on a sheet of digital array. A reference of the digit symbol pairs was offered on the same sheet. The number of symbols completed within 90 s was transformed to a scaled score. This task tests sustained attention, with higher DSST scores indicating better attention function.
The Number Connection Test (NCT) consisted of documenting the time required to sequentially connect randomly placed circles, which were labeled from 1 to 25. The total score was an index of executive function. The lower the total NCT score was, the better the executive function was rated.
The Auditory Verbal Learning Test‐Huashan (AVLT‐H) was administered by asking the patient to remember a list of 12 words that were then read out by the investigator. The patient was then asked to recall as many of the 12 words as possible immediately, which was repeated three times. A short‐delayed recall trial was conducted 5 min later. A long‐delayed recall was conducted 20 min later. This entire AVLT‐H test was performed at baseline before any study drug was administered, and then the test was performed again within 5 min of the patient becoming fully alert after the endoscopy procedure. The immediate recall score was used to assess the patient's ability to learn new information. A short‐delayed AVLT‐H score or long‐delayed AVLT‐H score demonstrated the patient's ability to memorize new information. The higher AVLT‐H score was associated with better memory ability.
Statistical analysis
The sample size was not statistically calculated but was expected to provide sufficient data for determining an appropriate dose level for further studies. Continuous data were tested for normality. Normally distributed data were summarized using the mean and standard deviation, which were compared via analysis of variance. Categorical data were presented as absolute values and percentages, which was compared among groups using the χ 2 test or Fisher's exact test. To compare the means among the three groups, a one‐way analysis of variance was performed using Tukey's post‐hoc test. All analyses were conducted using IBM SPSS Statistics 23.0 software. P < 0.05 was considered statistically significant.
Results
As depicted in Figure 1, 99 patients were randomized into the 1.0–1.5 mg/kg propofol group (P group), 0.1 mg/kg RT group (R1 group), or 0.2 mg/kg RT group (R2 group).
Figure 1.
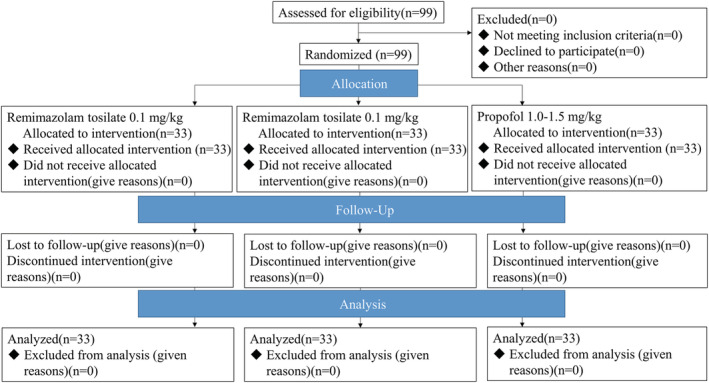
Patient disposition.
All patients completed the discharge cognitive test and were included the analysis of the primary outcome. The baseline characteristics of the three groups were similar (Table 1). No harm or unintended adverse events occurred for any of the participants.
Table 1.
Patient characteristics
| Characteristics | R1 (n = 33) | R2 (n = 33) | P (n = 33) | P value |
|---|---|---|---|---|
| Age: year, mean (SD) | 66.4 (4.8) | 65.5 (5.2) | 66.2 (5.0) | 0.721 |
| Male sex: no. (%) | 19 (57.6) | 22 (66.7) | 21 (63.6) | 0.739 |
| Height: cm, mean (SD) | 161.7 (8.2) | 160.8 (7.6) | 162.6 (7.1) | 0.658 |
| Weight: kg, mean (SD) | 59.6 (10.5) | 60.6 (12.3) | 61.5 (10.2) | 0.789 |
| BMI: kg/m2, mean (SD) | 22.7 (3.0) | 23.4 (3.9) | 23.2 (3.0) | 0.703 |
| ASA score: no. (%) | ||||
| Grade I | 18 (54.5) | 24 (72.7) | 18 (54.5) | 0.218 |
| Grade II | 15 (45.5) | 9 (27.3) | 15 (45.5) | 0.218 |
| Education degree: no. | 0.649 | |||
| University diploma and above | 8 | 7 | 7 | |
| Senior high school | 7 | 12 | 8 | |
| Junior high school | 7 | 9 | 9 | |
| Primary school and below | 11 | 5 | 9 | |
| History, yes: no. (%) | ||||
| Hypertension | 15 (45.5) | 11 (33.3) | 14 (42.4) | 0.580 |
| Diabetes | 6 (18.2) | 6 (18.1) | 5 (15.2) | 0.931 |
Primary outcome cognition
Recovery of the cognitive domain is summarized in Table 2. When considered for the entire sample for each group, performance at discharge declined significantly from baseline for immediate recall (P < 0.001) and short‐delayed recall (P = 0.005), but not long‐delayed recall only after the administration of 0.2 mg/kg RT. However, there was an increase in immediate recall (P < 0.001), short‐delayed recall (P = 0.025), and the DSS (P = 0.012) for the propofol‐treated patients.
Table 2.
Cognitive function at baseline and discharge
| R1 (n = 33) | R2 (n = 33) | P (n = 33) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | P value | Before | After | P value | Before | After | P value | ||
| Memory | Immediate recall | 16.8 ± 5.09 | 15.24 ± 5.32 | 0.075 | 17.73 ± 4.91 | 14.94 ± 4.61 | <0.001 | 16.52 ± 6.26 | 18.15 ± 5.18 | <0.001 |
| Short‐delayed recall | 6.09 ± 2.60 | 5.85 ± 2.33 | 0.516 | 6.36 ± 1.87 | 5.39 ± 2.45 | 0.005 | 5.88 ± 2.40 | 6.48 ± 2.35 | 0.025 | |
| Long‐delayed recall | 5.58 ± 2.68 | 5.45 ± 2.71 | 0.696 | 5.91 ± 2.05 | 5.48 ± 2.45 | 0.160 | 5.55 ± 2.31 | 5.76 ± 2.55 | 0.408 | |
| Attention | DSS | 28.76 ± 11.12 | 27.94 ± 9.78 | 0.360 | 33.65 ± 10.99 | 32.06 ± 9.26 | 0.172 | 26.55 ± 10.34 | 28.88 ± 12.21 | 0.012 |
| Executive function | NCT | 79.64 ± 29.20 | 74.42 ± 30.04 | 0.267 | 71.18 ± 34.28 | 78.97 ± 40.17 | 0.104 | 80.58 ± 33.08 | 80.45 ± 36.77 | 0.980 |
As for the magnitude of decline in cognitive function as assessed by the three variables described earlier, a group mean analysis performed using Tukey's honestly significant difference (HSD) test of simple‐effect comparisons (Table 3) showed that the R2 group performed worse than the P group on the immediate recall (P < 0.001), short‐delayed recall (P = 0.002), and DSS (P = 0.009) assessments. By contrast, the R1 group performed worse than the P group only on the immediate recall test (P = 0.009).
Table 3.
Change in cognitive function from baseline
| R1 (n = 33) | R2 (n = 33) | P (n = 33) | P value | ||
|---|---|---|---|---|---|
| Memory | Immediate recall | −1.61 ± 5.01 † | −2.79 ± 4.04* | 1.64 ± 3.97 | <0.001 |
| Short‐delayed recall | −0.24 ± 2.12 | −0.97 ± 1.83* | 0.61 ± 1.47 | 0.003 | |
| long‐delayed recall | −0.12 ± 1.76 | −0.42 ± 1.70 | 0.21 ± 1.45 | 0.294 | |
| Attention | DSS | −0.82 ± 5.06 | −1.85 ± 6.59* | 2.33 ± 5.01 | 0.009 |
| Executive function | NCT | −5.21 ± 26.48 | 7.79 ± 26.70 | −0.12 ± 27.89 | 0.150 |
DSS, Digit Symbol Substitution; NCT, Number Connection Test.
Significant difference between R1 group and P group based on Tukey's honestly significant difference between‐group comparison (P < 0.01).
Significant difference between R2 group and P group based on Tukey's honestly significant difference between‐group comparison (P < 0.01)
Secondary outcomes
Anesthesia details and postoperative data are shown in Table 4. The success rate of sedation in each group was 100%. During the induction period, there were four patients in whom 0.05 mg/kg RT was added once and one patient in whom 0.05 mg/kg RT was added twice after the administration of 0.1 mg/kg RT. There was one patient in whom RT 0.05 mg/kg was added once after the administration of 0.2 mg/kg RT. There were no clinical differences in the endoscopy time among patients in the three groups. There were no statistically significant differences in the recovery time or sedation time between patients in the R1 group and P group, whereas the time to becoming fully alert was longer for patients in the R2 group than that in the P group, both in sedation time and recovery time (P < 0.01). The incidence of hypotension was 3.0% for the R1 group and 48.5% for the P group, which was a significant difference (P < 0.001). Patients in all treatment groups were satisfied with their procedure.
Table 4.
Intraoperative and postoperative data
| R1 (n = 33) | R2 (n = 33) | P (n = 33) | P value | |
|---|---|---|---|---|
| Endoscopy time (min) | 4.06 ± 2.49 | 5.00 ± 2.95 | 4.00 ± 2.11 | 0.205 |
| Recovery time (min) | 3.82 ± 2.49 | 6.85 ± 4.29* | 4.33 ± 2.97 | 0.001 |
| Sedation time (min) | 8.27 ± 2.49 | 12.09 ± 3.60* | 8.21 ± 2.85 | <0.001 |
| Recall no (%) | 0 (0) | 0 (0) | 1 (3.0) | 1.000 |
| Dreaming no (%) | 1 (3.0) | 2 (6.1) | 1 (3.0) | 1.000 |
| Hypotension no (%) | 1 (3.0) † | 7 (21.2) | 16 (48.5) | <0.001 |
| O2 saturation <90% no (%) | 1 (3.0) | 6 (18.2) | 7 (21.2) | 0.079 |
Significant difference between the R1 group and the P group based on Tukey's HSD between‐group comparison (P < 0.01).
Significant difference between the R2 group and the P group based on Tukey's HSD between‐group comparison (P < 0.01).
There were also no discernable differences in vital signs among patients in all three groups with respect to the mean blood pressures and preoperative heart rate. There was a slight increase in heart rate after administration of 0.1 or 0.2 mg/kg RT, which then slowly decreased to baseline after patients became fully alert. While there was a decrease in the heart rate of patients in the P group (Fig. 2), there was an initial decrease in arterial blood pressure (up to approximately 20 mmHg) in patients of all three groups after drug administration. A comparison of the maximum decreased amplitude of the mean arterial pressure of the three groups during the perioperative period revealed that the maximum reduction in the P group was 23.7% (the preoperative mean value was 88.44 mm Hg, and the minimum mean value was 67.52 mmHg), whereas the maximum reduction in the R1 group was 13.5% (the preoperative mean value was 90.18 mmHg, and the minimum mean value was 77.98 mmHg). The maximum reduction in the R2 group was 16.3% (preoperative mean value was 94.15 mm Hg, and the lowest mean value was 78.80 mmHg). Compared with P group, the fluctuation of blood pressure was smaller in the R1 group. After patients were fully alert, the mean arterial pressure of all patients showed an upward trend (Fig. 3).
Figure 2.
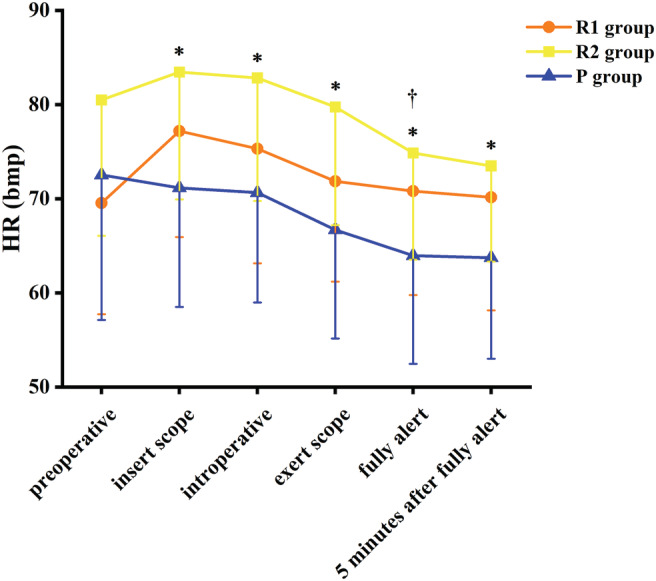
Heart rate. †Significant difference between the R1 group and the P group by Tukey honestly significant difference (HSD) between‐group comparison (P < 0.01). *Significant difference between the R2 group and the P group by Tukey HSD between‐group comparison (P < 0.01).
Figure 3.
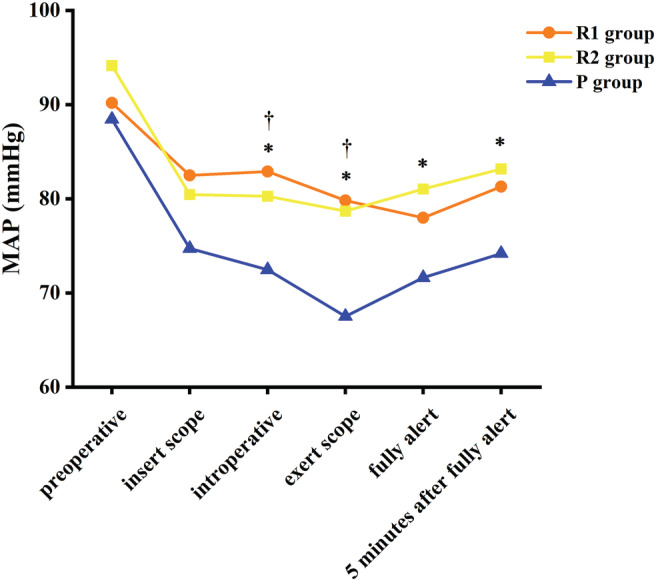
Mean arterial pressure. †Significant difference between the R1 group and the P group by Tukey honestly significant difference (HSD) between‐group comparison (P < 0.01). *Significant difference between the R2 group and the P group by Tukey HSD between‐group comparison (P < 0.01).
Discussion
The results indicated that subjects receiving 0.1 mg/kg RT exhibited significantly fewer complications such as hypotension than those receiving propofol. The clinical relevance of our study finding is that 0.1 mg/kg RT results in a lower incidence of comorbidity and safer procedures, although short cognitive impairment was observed in elderly patients. Moreover, this focus is important, since although upper GI endoscopy with sedation is increasingly performed on elderly patients, data on the outcomes and side effects of sedation are limited. 9
Fast recovery and preservation of cognitive function following endoscopic procedures are important subjects of research so that elderly patients can return to their normal lives safely and promptly. The results of this study indicate that sedation with 0.1 mg/kg RT but not propofol causes greater impairment on certain dimensions of cognitive functions. It has been shown that sedation causes deficits in cognitive function, which contribute to patients being discharged from the hospital with levels of cognitive function that contraindicate the ability to perform complex activities of daily living, so patients may be at increased risk of injury. 10 , 11 Furthermore, a large population‐based study observed an increased risk of dementia within 3–7 years of undergoing anesthesia and surgery and a shorter mean duration for the diagnosis of dementia compared to their control patients. 12 This work mainly focused on the safety profile, including cognitive symptoms. Among studies that investigated RT for endoscopy, only three made similar comparisons to this work. Vasudeven et al. performed a randomized, double‐blind study of patients aged 18 to 65 years who were undergoing sedation for elective upper GI endoscopy. By using the Hopkins Verbal Learning Test‐Revised (HVLT‐R) as a measure of memory function at baseline before administering any study drug, and again measured within 5 min of becoming fully alert, a significant decline in cognitive function was discovered after the administration of both midazolam and remimazolam, although recovery of the remimazolam‐treated patients was more pronounced. 8 Rex et al. administered sedation with remimazolam compared with midazolam and a placebo plus fentanyl in randomized patients undergoing colonoscopy, then assessed their HVLT‐R total raw scores, delayed recall, retention raw scores, and recognition discrimination at 5 min after the patients became fully alert, which demonstrated better effects with remimazolam than with the placebo and midazolam. 13 Pastis et al. also reported superior neuropsychiatric functions as a result of remimazolam compared with a placebo and midazolam in patients undergoing bronchoscopy. 14 However, the first study included a small number of participants and did not investigate the effect of remimazolam on elderly patients, whereas the latter two studies did not investigate changes in cognitive function within the same individual. None of the previous studies compared RT with other sedatives such as propofol and did not test their impacts on attention and executive function. Recently, Chen et al. reported that healthy Chinese volunteers' performance of tests of saccadic and smooth pursuit eye movement, body sway, test of choice reaction time, and word recall was significantly impaired after single‐dose midazolam and after constant rate infusion of RT. The authors recommend that health care providers perform psychomotor assessments after 2‐h conscious sedation prior to approving hospital discharge. 15 Our study confirms these results for elderly patients, but after 5 min of becoming fully alert rather than 2 h after sedation with an initial dose of 0.01 mg/kg butorphanol, a totally synthetic opioid, which exerts partial agonist and antagonist activity at the μ‐opioid receptor as well as agonist activity at the κ‐opioid receptor. Patients' performances of memory and attention skills as cognitive functions at discharge were worse than their performance of these at baseline after administration of 0.2 mg/kg RT, whereas there was no significant difference between baseline and discharge after treatment with 0.1 mg/kg RT. We did not find any deterioration in these test results after endoscopy performed under propofol sedation. Patient's tests of immediate recall, short‐delayed recall, and the DSS test showed some improvement, which was probably due to a learning effect observed in patients if these tests were performed as part of a previous trial. Change in cognitive function from baseline, which focuses on individual difference further revealed a performance decline in immediate recall, short‐delayed recall and DSS test in R2 group and a decline in immediate recall in R1 group compared with P group relatively. In fact, with propofol, cognitive function had improved. Watkins et al. randomized 96 patients into a propofol group, propofol/fentanyl group, and fentanyl/midazolam group and observed that administering propofol as a sole anesthetic caused the least cognitive disturbance at 24 and 48 h post‐procedure when compared with any other combination. 16 By using the NCT as a measure of executive function, we found no significant difference between R1 group, R2 group, and P group in the changes from baseline within individual, but there is a trend that a deterioration of NCT in R2 group (NCT changed from 71.18 ± 34.28 prior sedation to 78.97 ± 40.17 after sedation). The clinical relevance of this finding may implicate precautions in driving cars and in operating machinery. The higher doses may explain this finding of more severe cognitive impairment in patients after administration of 0.2 mg/kg RT, as age‐related pharmacokinetic changes and the presence of comorbidities and polypharmacy complicate drug therapy.
How these effects of RT relate to the adult human brain is unclear, although it has been hypothesized that the effects of centrally acting GABAA agents are dependent on the cumulative dose of the agent administered and neuronal susceptibility. 17 However, there was no deterioration in long‐delayed memory in patients, which indicated that RT distinctively impaired the acquisition stage rather than the storage and retrieval stages of the memory process. This effect was attributed to the fact that participants performed better on tests utilizing repetitively used materials. As evidenced in an earlier report, 18 benzodiazepines tended not to disrupt retention and retrieval of previously stored information. Our finding adds to existing knowledge that the acute action of RT interferes with attention ability. Moreover, although patients seem alert and vigilant in the recovery room, they might still be less capable of maintaining their focus and attention over prolonged periods of time, especially when facing distractions.
Other studies have investigated cognitive impairment after endoscopy. Padmanabhan et al. found that 18.5% of patients undergoing colonoscopy had impaired cognition at hospital discharge. The incidence of impaired cognition was not different after the addition of midazolam, but a midazolam dosage >2 mg was associated with impaired cognition. 10 Hsu et al. reported midazolam‐based light sedation in patients undergoing colonoscopy induced selective cognitive impairments and prolonged cognitive impairments in patients of advanced age. 19 Conversely, Gurunathan et al. found that the use of 0.04 mg/kg midazolam as an adjunct to propofol and opiate sedation did not show any evidence of differences in recovery of the cognitive domain of the Postoperative Quality of Recovery Scale after colonoscopy. 20 However, the time to full alertness following administration of midazolam with an opioid was up to 120 min. Midazolam is a selective substrate of CYP3A4 and CYP3A5, which causes variability in metabolic activity (approximately fivefold) and numerous drug–drug interactions. Because of these design issues and other challenges, such as the type of sedative agent and the time when cognitive assessment is performed in relation to sedation, these studies cannot be compared directly with ours.
One important concern regarding sedation for endoscopy is sedation‐related complications, which can lead to significant morbidity and occasional mortality in patients. Similar to previous studies, 21 , 22 this study found that both RT and propofol could cause transient cardiovascular depression. Lower incidences of hypotension were reported for the R1 group (3.0%) compared with that of the P group (48.5%). In addition, patients' heart rate increased slightly after administration of RT. Numerous studies have demonstrated that perioperative hypotension can lead to insufficient perfusion of important organs, which results in acute and chronic irreversible organ injury and is associated with an increasing incidence of postoperative myocardial injury, ischemic stroke, postoperative delirium, acute kidney injury, and postoperative mortality, thus causing serious consequences for the patient's postoperative care and long‐term outcome. 23 , 24 , 25 The incidence of hypoxemia was also higher in the P group than in the R1 group, although the difference was not statistically significant. This advantage has been attributed to the molecular design of RT. Its ultra‐short‐acting property leads to rapid breakdown in an inactive metabolite via ubiquitous tissue esterases. Vital signs remained stable even when deep sedation occurred. Currently, RT is still considered an ideal intravenous anesthetic agent in the elderly population due to its better hemodynamic stability and use of a specific antagonist. 26 The results of this study showed that under the same surgical stimulation, sedation and recovery times of the R2 group were longer than those of the P group, whereas there were no significant differences between the R1 group and P group. Considering that the pharmacokinetics of RT are linear across escalating dosages, the degree and duration of sedation with RT are dose dependent. 27 Furthermore, older people often have to consume a variety of drugs, which can impact the bioavailability. Due to the particularity of the body condition of elderly patients, individualized and appropriate drug administration in the elderly population is critical. Our study demonstrated that the sedation success rates were 100% for both RT groups and the P group. Overall, 0.1 mg/kg RT was well‐tolerated by elderly patients and achieved a better safety profile compared with propofol.
Although this study demonstrated important findings, it also has several limitations. First, although we compared the effects of remimazolam tosilate and propofol on cognitive function, our data did not allow us to determine whether the opiate influence cognition function as all patients received opioid predication. Second, the elderly patients enrolled in our study were ASA I/II, and average age of 65, which may not fully represent the elderly patient population. The effectiveness of Remimazolam tosilate needs to be evaluated in future trials with larger sample size and patients of ASA III. Third, our study is limited to the observation of short‐term cognitive effects of sedative agents. The time to full or partial cognitive recovery has yet to be studied. Fourth, this is an exploratory study, this research can only be a suggestion that remimazolam can be a useful adjunct. Furthermore, there is a possibility of a practice effect obscuring post‐sedation impairment, given the brief test–retest interval and use of only one practice session. However, despite these limitations, the results of this study raise hypotheses and provide exploratory data for future studies.
Conclusion
In summary, RT is a safe and effective sedative for upper GI endoscopy in patients of advanced age. The addition of RT as an adjunct to opiate sedation for upper GI endoscopy can not only lead to more stable perioperative hemodynamics but also achieve acceptable neuropsychiatric functions after upper GI endoscopy in patients of advanced age. To minimize adverse impacts on cognitive function, 0.1 mg/kg RT should be considered for sedation in elderly patients undergoing upper GI endoscopy.
Acknowledgement
This work was supported by HunanHealth and Family Planning Commission Research Project (20201802), States’ KeyProject of Research and Development Plan (2018yfc2001800) and Medical ResearchFund of Hunan Medical Association (HMA202101003).
Tan, Y. , Ouyang, W. , Tang, Y. , Fang, N. , Fang, C. , and Quan, C. (2022) Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. Journal of Gastroenterology and Hepatology, 37: 576–583. 10.1111/jgh.15761.
Declaration conflict of interest: The authors declare no conflict of interest.
Financial support: This study was supported by Hunan Health and Family Planning Commission Research Project (20201802), States' Key Project of Research and Development Plan (2018yfc2001800), and Medical Research Fund of Hunan Medical Association (HMA202101003). The funding source had no control over the study concept and design, data acquisition, interpretation and analysis, manuscript writing, and publication of the study.
References
- 1. Early DS, Lightdale JR, Vargo JN et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest. Endosc. 2018; 87: 327–337. [DOI] [PubMed] [Google Scholar]
- 2. Allampati S, Wen S, Liu F, Kupec JT. Recovery of cognitive function after sedation with propofol for outpatient gastrointestinal endoscopy. Saudi J. Gastroenterol. 2019; 25: 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Triantafillidis JK, Merikas E, Nikolakis D, Papalois AE. Sedation in gastrointestinal endoscopy: current issues. World J. Gastroenterol. 2013; 19: 463–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goudra B, Nuzat A, Singh PM, Borle A, Carlin A, Gouda G. Association between type of sedation and the adverse events associated with gastrointestinal endoscopy: an analysis of 5 years' data from a tertiary center in the USA. Clin. Endosc. 2017; 50: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruiz‐Curiel RE, Bonilla HY, Baptista A, Bronstein M. Sedation with propofol in digestive endoscopy administered by gastroenterologists. Experience in a Venezuelan hospital. Rev. Esp. Enferm. Dig. 2018; 110: 246–249. [DOI] [PubMed] [Google Scholar]
- 6. Zhou J, Leonowens C, Ivaturi VD et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J. Clin. Anesth. 2020; 66: 109899. [DOI] [PubMed] [Google Scholar]
- 7. Chen SH, Yuan TM, Zhang J et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non‐inferiority, phase III trial. J GASTROEN HEPATOL. 2021; 36: 474–481. [DOI] [PubMed] [Google Scholar]
- 8. Borkett KM, Riff DS, Schwartz HI et al. A phase IIa, randomized, double‐blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth. Anal. 2015; 120: 771–780. [DOI] [PubMed] [Google Scholar]
- 9. Veitch AM, Uedo N, Yao K, East JE. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat. Rev. Gastroenterol. Hepatol. 2015; 12: 660–667. [DOI] [PubMed] [Google Scholar]
- 10. Padmanabhan U, Leslie K, Eer AS, Maruff P, Silbert BS. Early cognitive impairment after sedation for colonoscopy: the effect of adding midazolam and/or fentanyl to propofol. Anesth. Analg. 2009; 109: 1448–1455. [DOI] [PubMed] [Google Scholar]
- 11. Riphaus A, Gstettenbauer T, Frenz MB, Wehrmann T. Quality of psychomotor recovery after propofol sedation for routine endoscopy: a randomized and controlled study. Endoscopy 2006; 38: 677–683. [DOI] [PubMed] [Google Scholar]
- 12. Kim CT, Myung W, Lewis M et al. Exposure to general anesthesia and risk of dementia: a nationwide population‐based cohort study. J. Alzheimers Dis. 2018; 63: 395–405. [DOI] [PubMed] [Google Scholar]
- 13. Rex DK, Bhandari R, Desta T et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest. Endosc. 2018; 88: 427–437. [DOI] [PubMed] [Google Scholar]
- 14. Pastis NJ, Yarmus LB, Schippers F et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest 2019; 155: 137–146. [DOI] [PubMed] [Google Scholar]
- 15. Chen X, Sang N, Song K et al. Psychomotor recovery following remimazolam‐induced sedation and the effectiveness of flumazenil as an antidote. Clin. Ther. 2020; 42: 614–624. [DOI] [PubMed] [Google Scholar]
- 16. Watkins TJ, Bonds RL, Hodges K, Goettle BB, Dobson DA, Maye JP. Evaluation of postprocedure cognitive function using 3 distinct standard sedation regimens for endoscopic procedures. AANA J. 2014; 82: 133–139. [PubMed] [Google Scholar]
- 17. Maloney SE, Creeley CE, Hartman RE et al. Using animal models to evaluate the functional consequences of anesthesia during early neurodevelopment. Neurobiol. Learn. Mem. 2019; 165: 106834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghoneim MM, Block RI, Ping ST, El‐Zahaby HM, Hinrichs JV. The interactions of midazolam and flumazenil on human memory and cognition. Anesthesiology 1993; 79: 1183–1192. [DOI] [PubMed] [Google Scholar]
- 19. Hsu YH, Lin FS, Yang CC, Lin CP, Hua MS, Sun WZ. Evident cognitive impairments in seemingly recovered patients after midazolam‐based light sedation during diagnostic endoscopy. J. Formos. Med. Assoc. 2015; 114: 489–497. [DOI] [PubMed] [Google Scholar]
- 20. Gurunathan U, Rahman T, Williams Z et al. Effect of midazolam in addition to propofol and opiate sedation on the quality of recovery after colonoscopy: a randomized clinical trial. Anesth. Analg. 2020; 131: 741–750. [DOI] [PubMed] [Google Scholar]
- 21. Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single‐blind, randomized, parallel‐group, phase IIb/III trial. J. Anesth. 2020; 34: 543–553. [DOI] [PubMed] [Google Scholar]
- 22. Chen S, Wang J, Xu X et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive‐controlled, phase III clinical trial. Am J Transl Res. 2020; 12: 4594–4603. [PMC free article] [PubMed] [Google Scholar]
- 23. Monk TG, Bronsert MR, Henderson WG et al. Association between intraoperative hypotension and hypertension and 30‐day postoperative mortality in noncardiac surgery. Anesthesiology 2015; 123: 307–319. [DOI] [PubMed] [Google Scholar]
- 24. Hallqvist L, Granath F, Huldt E, Bell M. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery: an observational study. Eur. J. Anaesthesiol. 2018; 35: 273–279. [DOI] [PubMed] [Google Scholar]
- 25. Sessler DI, Meyhoff CS, Zimmerman NM et al. Period‐dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death: a substudy of the POISE‐2 Trial. Anesthesiology 2018; 128: 317–327. [DOI] [PubMed] [Google Scholar]
- 26. Goudra BG, Singh PM. Remimazolam: the future of its sedative potential. Saudi J Anaesth. 2014; 8: 388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy 2016; 36: 1021–1027. [DOI] [PubMed] [Google Scholar]


