Abstract
Aim
The aim of this study was to clarify the association between dietary protein intake and decline in the estimated glomerular filtration rate (eGFR) among Japanese older adults.
Methods
We used the data of the Septuagenarians, Octogenarians and Nonagenarians Investigation with Centenarians (SONIC) study, an ongoing narrow‐age range cohort study: 69–71 years, 79–81 years and 89–91 years. The outcome variable, change in eGFR, was estimated from serum creatinine measured at the baseline and 3‐year follow up, and the exposure variable, protein intake, was calculated using the brief‐type self‐administered diet history questionnaire at the baseline. Associations between eGFR change and protein intake were determined by multiple linear regression analysis.
Results
The mean eGFR change per year was −1.89 mL/min/1.73 m2. The mean protein intake was 1.50 g/kg/day. The results of this study showed that there was no significant association between protein or animal protein intake and change in eGFR per year in the entire population of participants, including the very elderly, but there was a significant positive association in those whose renal function fell into chronic kidney disease stage G3 or G4.
Conclusions
Protein intake among community‐dwelling older adults was not associated with lower eGFR, and for older chronic kidney disease patients, protein and animal protein intakes were more beneficial in maintaining eGFR. The results provide evidence that protein intake should not be restricted for older patients with chronic kidney disease, including the very elderly. Geriatr Gerontol Int 2022; 22: 286–291.
Keywords: chronic kidney disease, community‐dwelling older people, glomerular filtration rate annual decline, protein intake
Introduction
The number of chronic kidney disease (CKD) patients worldwide increased by 1.2% in terms of change in age‐adjusted prevalence between 1990 and 2017, based on a report by the Global Burden of Disease study in 2017. 1 According to estimates by the Japanese Society of Nephrology, the prevalence of CKD patients in Japan increases with age for both men and women, with the majority of patients in stage 3, accounting for 15.6% of men and 14.6% of women at age 60 years, 27.1% of men and 31.3% of women at 70 years, and 43.1% of men and 44.5% of women at 80 years. 2 Regarding lifestyle intervention for CKD patients, protein restriction is generally recommended to protect renal function, especially for CKD patients with proteinuria and advanced renal dysfunction. 3 , 4 The results of a recent meta‐analysis of randomized controlled trials reported that protein‐restricted diets for CKD patients reduce the rate of decline in renal function, but did not show a beneficial effect on all causes of death. 5 , 6 However, in contrast, a systematic review reported a negative association between protein intake and frailty in older adults, so we need to be careful about restricting protein intake, even in older CKD patients. 7 Additionally, in a cohort study of 461 older Japanese CKD patients in stages G3 to G5 with a mean age of 67.0 years and mean observation period of 3.2 years, stage 3 CKD patients aged >65 years without overt proteinuria had no end‐stage renal disease (ESRD), and the incidence of death before renal replacement therapy was 2.8/100 patient‐years. 8 Furthermore, another cohort study of 209 622 CKD patients in the USA reported a lower risk of ESRD and higher mortality with increasing age, and higher mortality than incidence of ESRD in the age group 85–100 years, regardless of baseline estimated glomerular filtration rate (eGFR) values, indicating that death is a greater risk than ESRD in very elderly CKD patients. 9 The Cardiovascular Health Study, a cohort study including older CKD patients with a mean age of 72 years, also reported no association between protein intake and decreased renal function. 10 Based on the results of these three cohort studies, protein intake restriction to reduce the risk of renal function decline and end‐stage renal failure should be carefully considered in older CKD patients, taking into account risks, such as sarcopenia. Therefore, the present study aimed to investigate the association between protein intake and protection of renal function, including very old people living in the community, for whom there is little evidence.
Methods
Study design and setting
We used the data of the Septuagenarians, Octogenarians and Nonagenarians Investigation with Centenarians (SONIC) study, an ongoing narrow‐age range cohort study: 69–71 years, 79–81 years and 89–91 years.
The SONIC study recruited participants who were living independently in four areas of both western and eastern of Japan, including urban and rural areas: Itami City, Hyogo (Western urban); Asago City, Hyogo (Western rural); Itabashi ward, Tokyo (Eastern urban); and Nishitama County, Tokyo (Eastern rural). Participants were randomly selected from the basic resident registration, and enrolled with their consent from 2010 to 2013 as the baseline survey. Detailed information was provided in a previous study. 11 We analyzed the data at the baseline and 3‐year follow up.
The present study protocol was approved by the institutional review board of Osaka University Graduate School of Medicine, Dentistry and Human Sciences (Osaka, Japan) and Tokyo Metropolitan Institute of Gerontology (Tokyo, Japan; approval number 266, H22‐9, 22 018 and 38, respectively).
The exclusion criteria of the present study were as follows: under dialysis treatment, CKD stage 5: <eGFR15 mL/min/1.73 m2, implausible total energy intake (male: <600 kcal or >4000 kcal, female: <500 kcal or >3500 kcal), excess eGFR ±2 SD, missing data on creatinine and nutrition intake.
Variables and measurement
All data were collected at the investigation venue. Blood samples were collected using vacuum blood collection tubes. The level of serum creatinine, glycated hemoglobin, uric acid and non‐high‐density lipoprotein cholesterol were determined by biochemical examinations. Serum creatinine was assayed by an enzymatic method. Annual change in eGFR (mL/min/1.73 m2) between the baseline and follow up as the main outcome variable was calculated with an equation of the Japanese Society of Nephrology using serum creatinine: eGFR = 194 × Cr–1.094 × age–0.287 (×0.739 if female), and the annual change in eGFR between the baseline and follow‐up survey was determined as the main outcome variable. 12
Dietary intakes of protein (g/day) and other nutrients during the previous month were assessed using a brief‐type self‐administered diet history questionnaire (BDHQ) at the baseline survey. 13 , 14 BDHQ preserves the features of the self‐administered diet history questionnaire, simplifies the structure, and simplifies the answers and processing. BDHQ is a four‐page fixed‐portion questionnaire that asks about the frequency of consuming selected foods, but not about portion size, to estimate the dietary intake of 42 nutrients and 58 food items during the preceding month. Although the energy‐adjusted protein intake measured by BDHQ was significantly different from that measured by a dietary record, the ability to rank individual intakes in a population was reported in previous studies. 14 There was also a report examining the relative validity of BDHQ in individuals aged ≥80 years, and the total energy‐adjusted protein intake using the residual method was not significantly different from semi‐weighed dietary records. 15 BDHQ answered by each participant was checked by a trained research staff member during the medical examination. Protein intake calculated from BDHQ was adjusted for total energy intake by the residual method to obtain protein intake per kg of bodyweight. 16
Medical history, including hypertension, diabetes, dyslipidemia, cerebrovascular disease and cardiac disease, was obtained from the participants by doctors and nurses based on a questionnaire. Blood pressure was measured twice with the left and right arms in a sitting position while the patient was in a calm state using the auscultation method, and the average value was adopted.
Statistical analysis
We analyzed the characteristics of the participants in the baseline survey by dividing them into quartiles according to their total energy‐adjusted protein intake per bodyweight, and assessed whether there were significant differences by analysis of variance. The number of people who lost more than 4.5 kg of weight per year, one of the criteria for frailty, was analyzed by Fisher's exact test. 17 The association between total energy‐adjusted protein intake (g/day/kgBW) and the change per year in eGFR between the baseline survey and 3‐year follow up was analyzed using multiple linear regression analysis. The same analysis was then carried out for the subgroups of eGFR 60 mL/min/1.73 m2 and above and below. In addition to the total protein intake, intakes of animal and vegetable protein were also analyzed. Because eGFR has been reported to tend to be lower in the summer months when temperatures are higher, there is a risk of systematic error if the timing of measurements at baseline and follow‐up surveys are different. 18 To overcome this, the present study considered the difference in the timing of measurement at the time of both surveys in the analysis. Statistical analysis was carried out using spss Statistics version 22 (IBM Japan, Tokyo, Japan) with the significance level set at 5%. In the present study, the following were adjusted for the multivariate analysis as confounders: age, sex, season of the survey, uric acid, non‐high‐density lipoprotein cholesterol, systolic blood pressure, medical history, stroke, heart disease, hypertension, diabetes and dyslipidemia.
Results
Of the 2245 patients, 1160 were included in the analysis, excluding 1085 patients who met the exclusion criteria or could not be followed. The mean follow‐up period was 2.53 years, and the follow‐up rate was 51.7%. (Fig. 1) Table 1 shows the demographic information of the participants classified into quartiles by protein intake per kg of bodyweight. The eGFR and weight status of each quartile are described in Table 2. In terms of quartiles, there was no significant difference in eGFR, but there was a significant difference in weight in the baseline survey, follow‐up survey and annual change (Table 2). In terms of age group and sex, there were also significant differences in eGFR, and weight and its change (Table S1 in Supporting Information).
Figure 1.
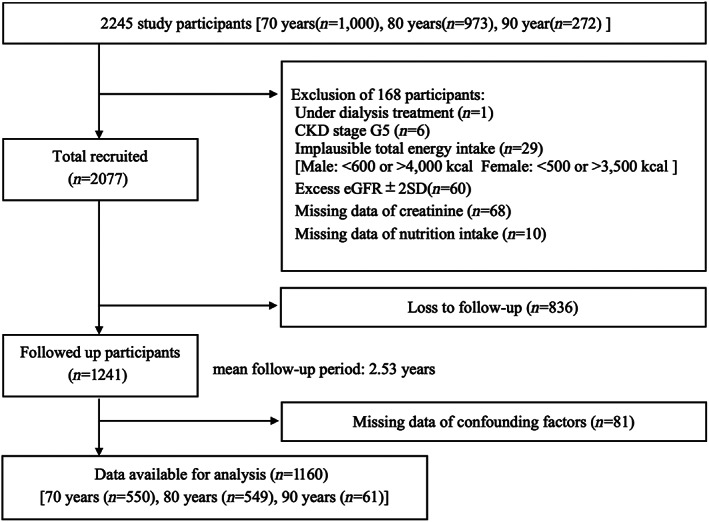
Participant recruitment and follow‐up flow diagram.
Table 1.
Participant characteristics at baseline by quartile of protein intake (g/kg bodyweight/day)
| Total | Q1 | Q2 | Q3 | Q4 | ||
|---|---|---|---|---|---|---|
| n = 1160 | n = 290 | n = 290 | n = 290 | n = 290 | P‐value | |
| Protein intake (g/kgBW/day) | 1.50 ± 0.43 | 1.01 ± 0.16 | 1.32 ± 0.07 | 1.59 ± 0.08 | 2.07 ± 0.30 | <0.01 |
| Energy intake (kcal) | 1953 ± 555 | 2170 ± 536 | 1878 ± 519 | 1842 ± 557 | 1922 ± 549 | <0.01 |
| Protein intake (g/day) | 80.3 ± 29.3 | 74.0 ± 22.0 | 73.3 ± 25.3 | 78.8 ± 28.7 | 95.1 ± 34.4 | <0.01 |
| Animal protein intake (g/day) | 47.3 ± 23.0 | 38.5 ± 15.8 | 41.1 ± 18.0 | 46.9 ± 20.7 | 62.9 ± 27.5 | <0.01 |
| Vegetable protein intake (g/day) | 32.9 ± 10.2 | 35.5 ± 10.0 | 32.2 ± 10.2 | 31.9 ± 10.1 | 32.2 ± 10.0 | <0.01 |
| BMI (kg/m2) | 22.6 ± 2.96 | 24.3 ± 2.95 | 23.1 ± 2.72 | 22.4 ± 2.51 | 20.7 ± 2.48 | <0.01 |
| Bodyweight (kg) | 55.7 ± 9.7 | 64.8 ± 8.4 | 58.1 ± 7.2 | 52.8 ± 6.4 | 47.1 ± 6.7 | <0.01 |
| Sex | <0.01 | |||||
| Male | 567 (49%) | 252 (87%) | 178 (61%) | 88 (30%) | 49 (9%) | |
| Female | 593 (51%) | 38 (13%) | 112 (39%) | 202 (70%) | 241 (91%) | |
| Age | 76.3 ± 5.9 | 74.8 ± 5.4 | 76.5 ± 5.8 | 76.5 ± 5.8 | 77.4 ± 6.3 | <0.01 |
| eGFR (mL/min/1.73 m2) | 69.15 ± 14.4 | 68.5 ± 13.5 | 68.4 ± 15.4 | 70.0 ± 13.0 | 69.7 ± 15.6 | 0.41 |
| HbA1c (%) | 5.5 ± 0.6 | 5.5 ± 0.6 | 5.6 ± 0.6 | 5.6 ± 0.6 | 5.5 ± 0.5 | 0.33 |
| Uric acid (mg/dL) | 5.2 ± 1.3 | 5.7 ± 1.2 | 5.4 ± 1.2 | 5.0 ± 1.3 | 4.7 ± 1.2 | <0.01 |
| Non‐HDL‐cholesterol (mg/dL) | 146.1 ± 32.9 | 143.0 ± 33.5 | 146.0 ± 31.4 | 147.5 ± 31.7 | 148.0 ± 34.7 | 0.26 |
| SBP (mmHg) | 110.4 ± 13.4 | 111.6 ± 13.8 | 111.0 ± 13.0 | 110.6 ± 12.9 | 108.4 ± 13.6 | 0.02 |
| Investigation season (summer) | 419 (36%) | 112 (39%) | 116 (40%) | 94 (32%) | 97 (33%) | 0.47 |
| Hypertension | 567 (49%) | 156 (54%) | 157 (54%) | 134 (46%) | 120 (41%) | <0.01 |
| Diabetes | 122 (11%) | 33 (11%) | 34 (12%) | 28 (10%) | 27 (9%) | 0.72 |
| Dyslipidemia | 405 (35%) | 101 (35%) | 96 (33%) | 112 (39%) | 96 (33%) | 0.46 |
| Stroke | 60 (5.0%) | 17 (6.0%) | 17 (5.9%) | 14 (4.8%) | 12 (4.1%) | 0.74 |
| Heart disease | 190 (16%) | 50 (17%) | 50 (17%) | 45 (16%) | 45 (16%) | 0.89 |
BMI, body mass index; BW, bodyweight; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; SBP, systolic blood pressure.
Table 2.
Changes of estimated glomerular filtration rate (mL/min/1.73 m2) and bodyweight between baseline and follow‐up study by quartile of protein intake
| Total | Q1 | Q2 | Q3 | Q4 | ||
|---|---|---|---|---|---|---|
| n = 1160 | n = 290 | n = 290 | n = 290 | n = 290 | P‐value | |
| Baseline | ||||||
| eGFR | 69.1 ± 14.4 | 68.5 ± 13.5 | 68.4 ± 15.4 | 70.0 ± 13.0 | 69.7 ± 15.6 | 0.41 |
| BW | 55.7 ± 9.7 | 64.8 ± 8.4 | 58.1 ± 7.2 | 52.8 ± 6.4 | 47.1 ± 6.7 | <0.01 |
| Follow up | ||||||
| eGFR | 64.6 ± 14.3 | 64.1 ± 14.5 | 63.7 ± 14.3 | 65.4 ± 13.0 | 65.0 ± 15.1 | 0.41 |
| BW | 55.4 ± 10.0 | 63.7 ± 9.2 | 56.9 ± 7.8 | 52.3 ± 7.5 | 47.8 ± 7.4 | <0.01 |
| Changes per year | ||||||
| eGFR | −1.89 ± 2.98 | −1.78 ± 2.81 | −1.90 ± 2.94 | −1.96 ± 3.11 | −1.93 ± 3.07 | 0.97 |
| BW | −0.67 ± 4.93 | −1.06 ± 5.25 | −1.26 ± 4.93 | −0.44 ± 4.71 | 0.16 ± 4.71 | 0.04 |
| BW decline 4.5 kg/year | ||||||
| 21 (100%) | 10 (47.6%) | 9 (42.9%) | 1 (4.8%) | 1 (4.8%) | <0.01 | |
BW, bodyweight; eGFR, estimated glomerular filtration rate.
Analysis of the association between protein intake and eGFR change showed that for those with eGFR of <60 mL/min/1.73 m2, protein and animal protein intakes showed significant associations with eGFR change (Table 3; Fig. 2). However, there was no difference of declining eGFR changes by amounts of protein intake both in age groups and sex (data not shown).
Table 3.
Association between baseline protein intake and changes in estimated glomerular filtration rate using linear regression †
| n | β | 95% CI | P‐value | |
|---|---|---|---|---|
| Total protein | ||||
| Total | 1160 | 0.21 | −0.27 to 0.68 | 0.39 |
| eGFR ≥60 | 869 | −0.01 | −0.57 to 0.55 | 0.98 |
| eGFR <60 | 291 | 0.98 | 0.18–1.78 | 0.02 |
| Vegetable protein | ||||
| Total | 0.73 | −0.55 to 2.01 | 0.26 | |
| eGFR ≥60 | 0.84 | −0.69 to 2.37 | 0.28 | |
| eGFR <60 | 0.16 | −1.97 to 2.29 | 0.88 | |
| Animal protein | ||||
| Total | 0.12 | −0.39 to 0.63 | 0.64 | |
| eGFR ≥60 | −0.14 | −0.75 to 0.47 | 0.65 | |
| eGFR <60 | 1.07 | 0.22 to 1.92 | 0.01 | |
Adjust for sex, age, season change, glycated hemoglobin, uric acid, systolic blood pressure, non‐high‐density lipoprotein‐cholesterol, stroke, heart failure, hypertension, diabetes and dyslipidemia.
CI, confidence interval; eGFR, estimated glomerular filtration rate.
Figure 2.
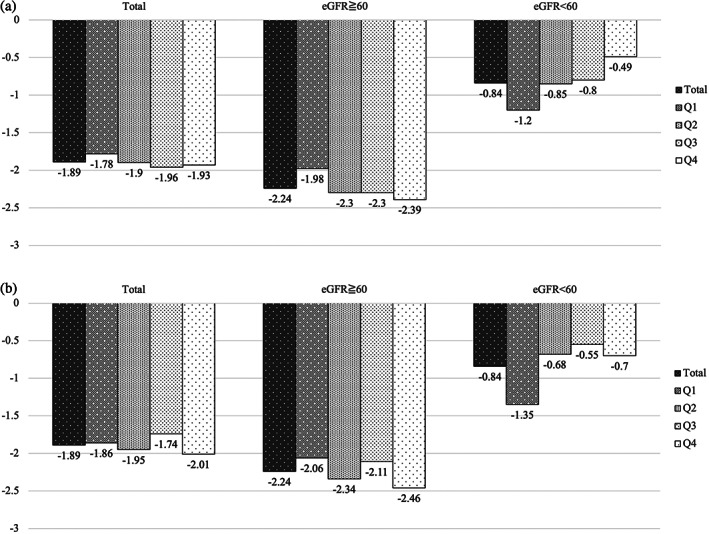
(a) Change of estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) by quartile of total protein intake. Q1, quartile 1 (0.28–1.20 g/BW/day); Q2, quartile 2 (1.20–1.45 g/BW/day); Q3, quartile 3 (1.45–1.74 g/BW/day); Q4, quartile 4 (1.74–3.41 g/BW/day). (b) Change of eGFR (mL/min/1.73 m2) by quartile of animal protein intake. Q1, quartile 1 (0.05–0.63 g/BW/day); Q2, quartile 2 (0.63–0.84 g/BW/day); Q3, quartile 3 (0.84–1.07 g/BW/day); Q4, quartile 4 (1.08–2.45 g/BW/day).
Discussion
The results of the present study showed that there was no significant association between protein or animal protein intake and change in eGFR per year in the entire population of participants, including the very elderly, but there was a significant positive association in those whose renal function fell into CKD stage G3 or G4. In addition, unintentional weight loss of >4.5 kg/year, one of the criteria for frailty, was found to occur less frequently in the group with higher protein intake. Although the Evidence‐based Clinical Practice Guideline for CKD 2018 does not recommend uniform restriction of protein intake for older CKD patients, the results of the present study also support that policy, suggesting that higher intakes of total protein and animal protein are better for preservation of renal function. 4
In participants with eGFR <60 mL/min/1.73 m2, why was the rate of decline in eGFR slower in those with high protein intake? A study of older women aged between 65 and 72 years reported that a protein intake of at least 1.1 g per kg of bodyweight prevented the onset of frailty, and that a higher intake of animal protein led to a lower prevalence of frailty. 19 Similarly, in the results of the present study, annual weight loss, one of the criteria for frailty, occurred relatively more frequently in the group with the lowest protein intake.
Cardiovascular Health Study reported that 6% of people aged ≥65 years with an eGFR of ≥60 mL/min/1.73 m2 and 15% with an eGFR of <60 mL/min/1.73 m2 are in a frail state, and that older people with conservative CKD are more likely to develop frailty. 20 Thus, the prognosis of patients with CKD in the conservative stage complicated by sarcopenia is worse than that of patients without sarcopenia, suggesting that insufficient intake of protein, especially animal protein, in patients with eGFR <60 mL/min/1.73 m2, who are at high risk of frailty, might have led to progression to frailty, and a decline in kidney function and other physical functions. 21 In addition to the prognosis, sarcopenia is associated with proteinuria and a decreased renal function in diabetes patients, according to a meta‐analysis. 22 An observational study of 336 patients with conservative CKD in the USA also showed that patients with CKD complicated by frailty had a 2.5‐fold higher risk of death or end‐stage renal failure. 23 As these studies have shown, the coexistence of sarcopenia and frailty in older patients with conservative CKD increases the risk of various physical functional declines, including renal function decline and even death.
Also, anemia increases the risk of frailty. In an observational study of community‐dwelling older women, the risk of frailty was 1.9‐fold higher with hemoglobin of 11.5 g/dL, and 1.5‐fold higher with hemoglobin of 12.0 g/dL than with normal hemoglobin. 24 Animal protein intake might prevent anemia by improving iron absorption, which in turn might prevent frailty. These findings suggest that high intakes of protein and animal protein prevent frailty in older people with conservative CKD, and also reduce the decline in renal function.
The strengths of the present study were that it was a prospective study of the association between protein intake and decline in renal function in community‐dwelling older adults, including the very elderly, for whom the accumulation of evidence is not yet sufficient, especially in the state of CKD, and that it used BDHQ, a validated measurement of protein intake in older adults. The results of a study comparing intakes using BDHQ and dietary records in the SONIC and TOOTH studies of older Japanese adults showed that there was no significant difference in daily intake of protein, and the result of Spearman's correlation coefficient was 0.32, which means that although the approximate intake status can be grasped, the accurate median dietary intakes might not be sufficiently estimated. Therefore, based on the results of the present study, it is not possible to make a definitive statement about the exact amount of protein intake that will reduce the decline in renal function. The present study participants were randomly selected from the community‐dwelling general population, and their protein intake was considered to be similar to the average Japanese older adult intake. According to the Japanese dietary intake standards 2020, as shown below, target amounts of protein intake are >90 g/day for men and 69 g/day for women who are active in early elderly, and >79 g/day for men and 62 g/day for women in late elderly. 25 Therefore, we would like to recommend that Japanese older adults, especially older CKD patients, consume the aforementioned target amounts.
Urinalysis was not carried out in the present study and it solely focused on eGFR with serum creatinine, so the results of this study might underestimate the impact of proteinuria. The present study did not collect detailed information on therapeutic drugs that affect renal function, such as antihypertensive drugs, and therefore could not consider their effects. Although we statistically adjusted for the effect of season on renal function, we were not able to completely eliminate the effect, so we should consider the same season for carrying out follow‐up studies. As a survival bias, the dropouts showed a relatively high protein intake per bodyweight and age, and a relatively frequent history of cardiovascular disease, hypertension and diabetes, which might have led to an underestimation of the risk of eGFR decline in the group with the higher intake. In addition, serum creatinine might not accurately show the decline in renal function, because it is affected by muscle mass and other factors. 26 , 27 Future studies should be validated with eGFR using serum cystatin C, which is not affected by muscle mass.
The present study examined the association between protein intake and the rate of eGFR decline in community‐dwelling older adults, for which there is insufficient evidence. The results showed a significant association only in CKD stage G3a or higher (eGFR <60 mL/min/1.73 m2), for which protein intake restriction is recommended. The results provide evidence that protein intake should not be restricted for older patients with conservative CKD, including the very elderly.
Disclosure statement
The authors declare no conflict of interest.
Supporting information
Table S1. Changes of estimated glomerular filtration rate (mL/min/1.73 m2) and bodyweight between baseline and follow‐up study by age group.
Acknowledgements
This study was supported in part by grants‐in‐aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KK: 2510211, 15K08910, MK: 16K12336, TS: 17K17553). In addition, this study was supported by Osaka University's International Joint Research Promotion Program Support Type A. We gratefully thank all staff members involved in the SONIC study. We sincerely appreciate all SONIC participants for their kind cooperation.
Sekiguchi T, Kabayama M, Ryuno H, et al. Association between protein intake and changes in renal function among Japanese community‐dwelling older people: The SONIC study. Geriatr. Gerontol. Int. 2022;22:286–291. 10.1111/ggi.14355
Data availability statement
Author elects to not share data
References
- 1. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Japanese Society of Nephrology . Clinical Practice Guidebook for Diagnosis and Treatment of Chronic Kidney Disease 2012. Tokyo: Tokyo igakusya, 2012. (In Japanese.). [Google Scholar]
- 3. Kidney Disease: improving Global Outcomes CKD Work Group . KDIGO2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; Suppl 3: 1–150. [Google Scholar]
- 4. Japanese Society of Nephrology . Evidence‐Based Clinical Practice Guideline for CKD 2018. Tokyo: Tokyo igakusya, 2018. (In Japanese.). [Google Scholar]
- 5. Yan B, Su X, Xu B, Qiao X, Wang L. Effect of diet protein restriction on progression of chronic kidney disease: a systematic review and meta‐analysis. PLoS One 2018; 13: e0206134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng J, Xiaoyan Z, Lichuan Y, Zi L, Wei Q. Effect of restricted protein diet supplemented with keto analogues in chronic kidney disease: a systematic review and meta‐analysis. Int Urol Nephrol 2016; 48: 409–418. [DOI] [PubMed] [Google Scholar]
- 7. Hélio JC, Bruno R, Uhida M, Emanuele M. Low protein intake is associated with frailty in older adults: a systematic review and meta‐analysis of observational studies. Nutrients 2018; 10: 1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Obi Y, Kimura T, Nagasawa Y et al. Impact of age and overt proteinuria on outcomes of stage 3 to 5 chronic kidney disease in a referred cohort. Clin J Am Soc Nephrol 2010; 5: 1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ann MO, Andy IC, Daniel B et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 2007; 18: 2758–2765. [DOI] [PubMed] [Google Scholar]
- 10. Jeannette MB, Ronit K, Michael S, Dena ER, David S, Robert K. Dietary protein intake and change in estimated GFR in the cardiovascular health study. Nutrition 2014; 30: 794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gondo Y, Masui Y, Kamide K et al. SONIC study: a longitudinal cohort study of the older people as part of a centenarian study. In: Pachana NA, ed. Encyclopedia of Geropsychology. Singapore: Springer Press, 2016; 1–10. [Google Scholar]
- 12. Matsuo S, Imai E, Horio M et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi S, Murakami K, Sasaki S et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief‐type self‐administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr 2011; 14: 1200–1211. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi S, Honda S, Murakami K et al. Both comprehensive and brief self‐administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol 2012; 22: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobayashi S, Xiaoyi Y, Sasaki S et al. Relative validity of brief‐type self‐administered diet history questionnaire among very old Japanese aged 80 years or older. Public Health Nutr 2019; 22: 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walter CW, Geoffrey RH, Lawrence HK. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997; 65: 1220–1228. [DOI] [PubMed] [Google Scholar]
- 17. Linda PF, Catherine MT, Jeremy W et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 18. Masugata H, Senda S, Inukai M et al. Seasonal variation in estimated glomerular filtration rate based on serum creatinine levels in hypertensive patients. Tohoku J Exp Med 2011; 224: 137–142. [DOI] [PubMed] [Google Scholar]
- 19. Masoud I, Joonas S, Toni R et al. Higher protein intake is associated with a lower likelihood of frailty among older women, Kuopio OSTPRE‐fracture prevention study. Eur J Nutr 2020; 59: 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michael GS, Catherine SB, Linda FF et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis 2004; 43: 561–867. [DOI] [PubMed] [Google Scholar]
- 21. Raíssa AP, Antonio CC, Carla MA et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant 2015; 30: 1718–1725. [DOI] [PubMed] [Google Scholar]
- 22. Ida S, Kaneko R, Imataka K, Murata K. Association between sarcopenia and renal function in patients with diabetes: a systematic review and meta‐analysis. J Diabetes Res 2019; 2019:1365189. [Cited 14 Nov 2021] Available from URL: 10.1155/2019/1365189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baback R, Minesh K, Cassianne RC et al. A prospective study of frailty in nephrology‐referred patients with CKD. Am J Kidney Dis 2012; 60: 912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paulo HMC, Richard DS, Sean XL et al. Impact of anemia and cardiovascular disease on frailty status of community‐dwelling older women: the Women's health and aging studies I and II. J Gerontol A Biol Sci Med Sci 2005; 60: 729–735. [DOI] [PubMed] [Google Scholar]
- 25. Ministry of Health, Labour and Welfare . Dietary Reference Intakes for Japanese 2020. [monograph on the internet]. (2020) [Cited 5 Jan 2022]. Available from URL: https://www.mhlw.go.jp/content/10904750/000586553.pdf. (in Japanese.)
- 26. Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis 2013; 61: 197–203. [DOI] [PubMed] [Google Scholar]
- 27. Michael GS, Mark JS, Ronit K et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352: 2049–2060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Changes of estimated glomerular filtration rate (mL/min/1.73 m2) and bodyweight between baseline and follow‐up study by age group.
Data Availability Statement
Author elects to not share data


