Abstract
The objective of our study was to evaluate the survival outcome of cervical cancer patients treated using image-guided brachytherapy (IGBT). From 2008 to 2018, 341 patients with cervical cancer were treated by radical radiotherapy. IGBT (by computed tomography [CT] or transabdominal ultrasound [TAUS]) was used to treat all of these patients. The characteristic data and patient status after treatment were recorded. All data were evaluated for survival outcome analysis. From a total of 341 patients, 295 patients were analyzed and 46 patients were excluded due to data missing in the survival outcomes. At the median follow-up time of 48 months (IQR 30–80 months), The 4-year local control, progression-free survival and overall survival rates were 89.5%, 74.9% and 69.1%, respectively. For overall survival, the size (> 5 cm), pathology (non-SCCA), stage (stage III–IV by FIGO 2009), lymph node (LN) (presented) and overall treatment time (OTT) (> 56 days) showed statistical significance in univariate analysis while non-SCCA pathology, advanced stage, presented LN and longer OTT showed statistical significance in multivariate analysis. In conclusion, our analysis reports a 4-year overall survival rate of 69.1%. Non-SCCA pathology, advanced stage disease, LN presence and longer OTT showed worse prognostic factors in multivariate analysis.
Keywords: survival, outcome, cervical cancer, image-guided brachytherapy (IGBT)
INTRODUCTION
Carcinoma of cervix uteri is one of the most common female cancers in Northern Thailand [1]. Radiotherapy (external beam radiotherapy [EBRT] plus brachytherapy [BT]) with platinum-based chemotherapy is the standard treatment for locally advanced disease [2]. After developments in magnetic resonance imaging (MRI) in BT, the concepts of volume-based treatment have been utilized in routine practice since 2005 using concepts of the Groupe Européen de Curiethérapie and the European Society for Radiotherapy & Oncology (GEC-ESTRO) recommendations [3, 4]. Many clinical studies of image-guided brachytherapy (IGBT) using MRI-guided BT have been published and the latest publication of the EMBRACE-I study showed a 5-year local control and survival rate of 92% and 74%, respectively [5–9]. Computed tomography (CT)-IGBT has also been also used in many institutes. Although CT yields poorer image quality than MRI in overestimation, CT is more easily accessible and can be adapted to reduce normal tissue dose [10]. To date, CT-based guidelines have been published to support this approach [11–13]. Many studies using CT-based BT have been published to support the use of CT in BT practice [14–17]. Transabdominal ultrasound (TAUS) was developed in BT by van Dyk et al. and published clinical studies showed promising results in terms of local control and survival [18–21].
Our Institute has transformed our brachytherapy service from 2D to 3D brachytherapy in our division since 2008 with the cooperation of the University Cooperation platform with Christian-Albrechts-University, Kiel and Medical University of Vienna [22]. From 2008 to 2018, we developed two research projects in different proposal. CT-BT project was our forward project to move our department from point-based planning to volume-based planning. During the transformation, due to our workload, we could not transform all of our patients to use volume-based planning at that time. So, at that time, we looked for the technique to support the adaptive planning for point-based planning and we found that TAUS-BT was the suitable one. So, TAUS-BT project was our supported project to improve our 2D by adaptively point-based planning during transformation process. After 10 years, we fully changed to CT-based BT in 2019. We have reported our experiences in CT and TAUS in international publications [16, 17, 20, 21, 23].
After 10 years of implementation (2008–2018), evaluation of the survival outcome is one of our objectives. In this study, we evaluate the survival outcomes of IGBT (by CT and TAUS) in our Institute.
MATERIALS AND METHODS
This retrospective study evaluates the survival outcomes of cervical cancer patients treated by IGBT in our Institute. This study was approved by the institutional review board of Faculty of Medicine, Chiang Mai University with the study code of RAD-2564-08287.
From 2008 to 2018, 341 patients with cervical cancer were treated by IGBT. There were 176 patients treated by CT-based BT while 165 patients were treated by TAUS-based BT. In our hospital, 3D-BT began in 2008 with CT-based BT as a research project. Later, another project to improve the quality of 2D planning by TAUS-guided BT as 2.5D planning was initiated in 2012. All patients received external beam radiotherapy from 45 to 50.4 Gy in 23–28 fractions plus four fractions of IGBT. In presented macroscopic lymph node (LN), the boost therapy to 56–60 Gy was applied as indicated. Extended field radiotherapy (pelvic plus paraaortic fields) was used for paraaortic LN involvement. Conventional, three-dimensional conformal, or intensity-modulated radiation therapy were utilized in our patients as indicated. Weekly platinum-based regimen (cisplatin; 40 mg/m2 or carboplatin; AUC2) was prescribed concurrently during external beam radiotherapy. For BT, all patients received IGBT by CT- or TAUS-based planning techniques (Fig. 1). Pre-BT MRI did not perform in our cohort. To compensate pre-BT MRI, per vaginal examination and TAUS were utilized. Hybrid intracavitary and interstitial (IC/IS) technique was indicated in case of big or poor geometry targets (whole cervix and macroscopic tumor at BT). For pain control, oral Tylenol with codeine was prescribed for the IC approach. In patients who need IC/IS technique, intravenous pethidine/valium or spinal anesthesia will be used in our practice. The concept of dose prescriptions and cumulative doses in both techniques has been described in previous publications [16, 17, 20, 21]. For our planning aims, we keep the cumulative dose (EBRT plus BT in EQD2) to our target (cervix + macroscopic tumor extension) to be at least 80 Gy, < 90 Gy for bladder and < 75 Gy for rectum. For CT-based BT, the target was the D90 of high-risk clinical target volume (HR-CTV). For TAUS-based BT, the target was generated from the eight cervix reference points measured by TAUS.
Fig. 1.

TAUS and CT for BT.
All characteristic data (age, stage, histology, chemotherapy, cumulative dose in EQD2) were recorded. All patients were traced to the cancer registry unit to evaluate their updated status (whether dead or alive). The survival data (from start of treatment to death) were evaluated.
In statistical analysis, descriptive statistics were used to evaluate characteristic data. A Kaplan–Meier curve and a log-rank test were utilized to evaluate the overall survival rate. Univariate and multivariate analyses were performed to evaluate the prognostic factor. IBM SPSS version 22 (SPSS Inc., Chicago, Illinois, USA) was used for evaluation.
RESULTS
From 341 patients, 46 patients were excluded because their data were not recorded in the cancer registry. Consequently, 295 patients were used for the evaluation. (Fig. 2) Stage IIB (as FIGO 2009) was the most common with 51.9%. Squamous cell carcinoma (SCCA) was the most common pathology (85.1%). The median age was 57 years and 80% of patients were ≤ 65 years old. One-hundred seventy-nine patients (60.7%) were treated by conventional radiotherapy. Fifty-two patients (17.6%) had macroscopic LN and 18 patients (6.1%) had para-aortic LN involvement. Two-hundred fifteen patients (72.9%) received weekly cisplatin as concurrent chemotherapy and 198 patients (67.1%) received at least four cycles of chemotherapy. The median cumulative dose to the target was 84.8 Gy in EQD2. Twenty-seven patients (9.2%) were treated by hybrid IC/IS technique. Patient characteristic data are shown in Table 1.
Fig. 2.
Kaplan–Meier curve of local control, disease-free survival and overall survival.
Table 1.
Patient characteristic data
| Parameters | Details |
|---|---|
| Age (median; IQR) | 57 years (50–63 years) |
| Size (median; IQR) Up to 5 cm More than 5 cm |
5 cm (4–6 cm) 186 (85.1%) 103 (34.9%) |
| Pathology Squamous cell carcinoma Adenocarcinoma Others |
251 (85.1%) 40 (13.6%) 4 (1.4%) |
| Stage (FIGO 2009) I II III IV |
10 (3.4%) 164 (55.6%) 114 (38.7%) 7 (2.4%) |
| Macroscopic LN No Pelvic LN Paraaortic LN |
243 (82.4%) 34 (11.5%) 18 (6.1%) |
| IGBT CT-based TAUS-based |
149 (50.5%) 146 (49.5%) |
| Cumulative Dose in EQD2 to the target (median; IQR) | 84.8Gy (82.2–89.7 Gy) |
| Overall treatment time (median; IQR) | 56 days (50–64 days) |
At the median follow-up time of 48 months (IQR 30–80 months), The 4-year local control, progression-free survival and overall survival rates were 89.5%, 74.9% and 69.1%, respectively. Figure 2 shows the Kaplan–Meier curve of these data. For late toxicity, five patients (1.7%) developed ≥ grade 2 cystitis and 12 patients (4.1%) developed ≥ grade 2 proctitis.
In local control, univariate analysis of age (up to 65 years vs > 65 years), size (up to 5 cm vs > 5 cm), pathology (SCCA vs non-SCCA), FIGO stage (stage I–II vs stage III–IV), LN (absent vs present), chemotherapy cycles (up to 3 cycles vs ≥ 4 cycles), cumulative dose to target (up to 84 Gy vs > 84 Gy), BT (CT vs TAUS) and overall treatment time (OTT; up to 56 days vs > 56 days) were evaluated. The results showed statistical significance in pathology, stage, cumulative dose to target and OTT with non-significant trends in LN. Details of univariate analysis are shown in Table 2. When we evaluated in terms of multivariate-analysis, Cox proportional regression analysis revealed a significant correlation in local control to advanced stage, non-SCCA histology, cumulative dose > 84 Gy and OTT. All evaluations are shown in Table 3.
Table 2.
Uni-variate analyses
| Variables | n | 4-year local control rate (%) | P-value* | 4-year overall survival rate (%) | P-value* |
|---|---|---|---|---|---|
| Age (years) up to 65 years More than 65 years |
238 57 |
89.1 91.2 |
0.666 | 69.3 68.4 |
0.72 |
| Size (cm) Up to 5 cm More than 5 cm |
186 103 |
91.4 86.4 |
0.134 |
76.3
55.3 |
0.004 |
| Pathology SCCA Non-SCCA |
251 40 |
91.6
77.3 |
0.002 |
70.9
59.1 |
0.007 |
| FIGO Stage Stage I–II (early) Stage III–IV (advanced) |
174 121 |
93.1 84.3 |
0.013 |
77.0 57.8 |
0.015 |
| LN Absent Present |
243 52 |
90.5 84.6 |
0.09 |
74.5 44.2 |
<0.001 |
| Cycles of chemotherapy Less than 4 cycles ≥ 4 cycles |
97 198 |
89.7 89.3 |
0.96 | 67 70.2 |
0.914 |
| Cumulative dose to target Up to 84 Gy > 84 Gy |
143 152 |
85.3 93.4 |
0.022 | 65.0 73.0 |
0.386 |
| BT CT TAUS |
149 146 |
90.6 88.4 |
0.513 | 73.2 65.1 |
0.31 |
| OTT Up to 56 days More than 56 days |
139 156 |
94.2 85.3 |
0.010 |
74.8 64.1 |
0.017 |
Note: BT = brachytherapy, CA = carcinoma, CT = computed tomography, FIGO = The International Federation of Gynecology and Obstetrics, LN = lymph node, OTT = overall treatment time, SCCA = squamous cell carcinoma, TAUS = transabdominal ultrasound; * by log-rank test
Table 3.
Multi-variate analyses
| Factor | Local control | Overall survival | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P-value | HR | 95% CI | P-value | |
| Age > 65 | 0.727 | 0.242–2.184 | 0.571 | 1.374 | 0.831–2.272 | 0.216 |
| Size > 5 cm | 1.203 | 0.558–2.594 | 0.637 | 1.461 | 0.989–2.158 | 0.057 |
| Non-SCCA | 3.986 | 1.533–10.365 | 0.005 | 2.414 | 1.440–4.046 | 0.001 |
| FIGO stage III–IV | 2.330 | 1.067–5.088 | 0.034 | 1.606 | 1.078–2.393 | 0.020 |
| Presented LN | 1.675 | 0.692–4.050 | 0.253 | 1.886 | 1.204–2.952 | 0.006 |
| ≥ 4 Cycles of chemotherapy | 0.893 | 0.346–2.306 | 0.815 | 0.942 | 0.585–1.516 | 0.806 |
| Cumulative dose to target > 84Gy | 0.33 | 0.131–0.832 | 0.019 | 0.910 | 0.555–1.493 | 0.710 |
| BT (CT vs TAUS) | 0.821 | 0.339–1.985 | 0.661 | 1.033 | 0.637–1.676 | 0.895 |
| OTT > 56 days | 2.520 | 1.063–5.973 | 0.036 | 1.557 | 1.049–2.311 | 0.028 |
Note: BT = brachytherapy, CT = computed tomography, FIGO = The International Federation of Gynecology and Obstetrics, HR = hazard ratio, LN = lymph node, OTT = overall treatment time, SCCA = squamous cell carcinoma, TAUS = transabdominal ultrasound
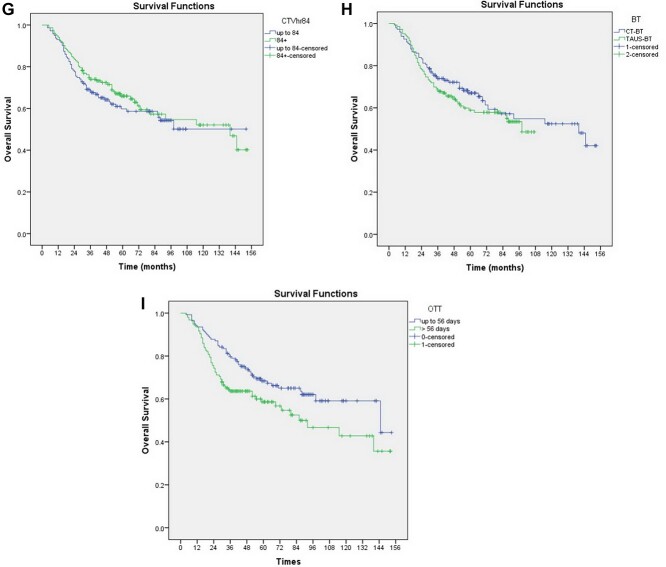
In overall survival, the same nine parameters were evaluated. In terms of univariate analysis, the results showed statistical significance in size, pathology, stage, LN and OTT. Details of univariate analysis are shown in Table 2. The Kaplan–Meier curve of age, size, pathology, stage, LN status, chemotherapy cycles, cumulative dose to target, BT and OTT were showed in Fig. 3a–3i. When we evaluated in terms of multivariate-analysis, Cox proportional regression analysis revealed a significant correlation in overall survival to non-SCCA histology, advanced stage, presented LN and longer OTT. All evaluations are shown in Table 3.
Fig. 3.
Kaplan–Meier curves of overall survival according to: a) age, b) size, c) pathology, d) stage, e) LN status, f) chemotherapy cycles, g) cumulative dose to target, h) BT technique, and i) OTT.
DISCUSSION
Nowadays, radiotherapy for cervical cancer has changed a lot in the technology of treatment, especially in BT. After the first clinical study was published in 2007 by Pötter et al. [5]. The use of IGBT has developed rapidly using MRI-, CT- or ultrasound-based planning [6, 9, 14, 19, 24]. The latest publications of the EMBRACE-I study showed promising results of MRI-guided BT with a 5-year local control and overall survival rates of 92% and 74%, respectively [8]. This result showed an improvement from the previous report of the retro-EMBRACE study that showed 5-year overall survival rate of 65% [7]. From the literature, the survival rate for cervical cancer treated by IGBT ranged from 58 to 86% over 3–5 years. (Table 4).
Table 4.
Selected studies of IGBT for cervical cancer
| Study | n | Image | Local control rate (%) | Survival rate (%) |
|---|---|---|---|---|
| Pötter et al. [8] | 1341 | MRI | 92% (5 yr) | 74% (5 yr) |
| Möller et al. [25] | 138 | MRI/CT | 94.2% (5 yr) | 65% (5 yr) |
| Sturdza et al. [7] | 731 | MRI/CT | 89%(5 yr) | 65% (5 yr) |
| Narayan et al. [19] | 292 | TAUS | 87.5% (5 yr) | 65% (5 yr) |
| Castelnau-Marchand et al. [26] | 225 | MRI/CT | 86.4% (3 yr) | 76.1% (3 yr) |
| Lindegaard et al. [24] | 140 | MRI | 91%(3 yr) | 79% (3 yr) |
| Pötter et al. [6] | 156 | MRI | 95%(3 yr) | 68% (3 yr) |
| Pötter et al. [5] | 145 | MRI | 85%(3 yr) | 58% (3 yr) |
| Ribeiro et al. [27] | 170 | MRI/CT | 96%(3 yr) | 65% (5 yr) |
| Ohno et al. [14] | 80 | CT | 94% (5 yr) | 86% (5 yr) |
| Our study | 295 | CT/TAUS | 89.5% (4 yr) | 69.1% (4 yr) |
Note: CT = computed tomography, MRI = magnetic resonance imaging, TAUS = transabdominal ultrasound, yr = year
Our study reports experiences of IGBT by CT or TAUS in our Institute from 2008 to 2018. For our reported data, the 4-year local control, progression-free survival and overall survival rates were 89.5%, 74.9% and 69.1%, respectively. For local control, our report were close to the results from retro-EMBRACE study (89%) and Narayan et al. (87.5%) but inferior to EMBRACE-I study (92%) [7, 19]. For overall survival, our results are still close to the results from the retro-EMBRACE study (65%), Möller et al. (65%), Ribeiro et al. (65%) and Narayan et al. (65%) but show inferior results to the EMBRACE-I study (74%) [7, 8, 19, 25, 27].
A possible reason of different results from EMBRACE I study might be that the percentage of stage III patients in the EMBRACE-I study was 15.2% in comparison to 38.7% in our study [8]. However, in our cohort, only FIGO 2009 was evaluated. Additionally, 43% of patients in the EMBRACE-I study used combined IC/IS BT to get dose escalation [8]. From our analysis, 9.2% (27 patients) of our patients were treated by hybrid IC/IS technique at that time. Although the utilization of IC/IS technique in our study was still lower than retro-EMBRACE study (23%), our study showed approximate results in terms of local control and overall survival [7]. After installation of CT in BT unit, we aim that the use of IC/IS technique will increase and this will improve our results in the future.
In our study, nine prognostic parameters (age, size, pathology, stage, presented LN, chemotherapy cycles, cumulative dose to the target, technique of IGBT and OTT) were evaluated in univariate and multivariate analyses. When we evaluated local control, in univariate analysis, our results showed statistical significances in pathology (non-SCCA) stage (III–IV), cumulative dose to target (> 84 Gy) and OTT (> 56 days) with trends in LN (present) (Table 2) For multivariate-analysis, Cox proportional regression analysis revealed a significant correlation in local control to non-SCCA histology, advanced stage, cumulative dose > 84 Gy and OTT > 56 days (Table 3). In overall survival, size (> 5 cm), non-SCCA histology, advanced stage (III–IV), LN status (presented LN) and OTT (> 56 days) showed statistical significance in univariate analysis (Table 2). When we evaluated using multivariate analysis, non-SCCA histology, advanced stage, presented LN and longer OTT than 56 days were of statistical significance (Table 3). Our analysis is similar to that of Möller et al. which showed that FIGO stage and LN metastases were prognostic factors for cancer-specific survival rates [25].
When we focused on SCCA group (251 patients), the 4-year local control, disease-free survival and overall survival rates were 91.6%, 78.1% and 70.9%, respectively. For overall survival, presented LN showed a significant correlation in overall survival in our analysis (P = 0.045; HR 1.544; 95% CI = 1.011–2.787) while no statistical significance in size (P = 0.055; HR 1.544; 95% CI = 0.991–2.404) and clinical stage (P = 0.158; HR 1.371; 95% CI = 0.885–2.124) was observed.
From our analysis, non-SCCA histology, advanced stage and presented LN were the factors which patients presented before treatment, in contrast to cumulative dose to the target and OTT that we can manage. According to the latest publication by Korenaga et al., completing chemoradiotherapy (standard of care) in the recommended 8 weeks was associated with a superior overall survival in cervical cancer [28]. Consequently, to keep the OTT to be as less as possible is very important to achieve favorable results. Previously, one fraction per week was routinely used in our practice and it caused some patients who had treatment break during EBRT got high tendency to treat longer than 8 weeks. Nowadays, twice-a-week schedule is currently utilized in our hospital and we expect that this will improve our results in the future.
Nonetheless, workload is still important obstacle of twice-a-week schedule. For the cumulative dose to target, cumulative dose (> 84 Gy in EQD2) showed statistical significance in local control in our analysis (P = 0.019) but, unfortunately, not in overall survival (P = 0.710). The plan of our practice to increase cumulative dose to > 84 Gy by hybrid IC/IS technique was designed after installation of CT in the BT unit.
For the technique of BT, no difference in techniques of IGBT (CT vs TAUS) was observed in terms of local control and survival. However, the trends of BT for cervical cancer shift to hybrid IC/IS technique according to the retro-EMBRACE and EMBRACE I studies [7, 8]. Hybrid IC/IS utilization should increase in practice and the further study of CT-based IGBT vs TAUS-based IGBT in hybrid IC/IS approach is interesting. Nevertheless, the planning system for TAUS-based BT in hybrid approaches is during development [29].
The major limitations of the study are as follows. First, it was a retrospective analysis performed over a relatively long period of time (10 years). Second, short median follow-up time was observed (48 months) because some patients were lost to follow-up at our Institution, especially in this COVID-19 era. However, the strength of the present study is that our cohort contains cervical cancer patients who were treated by IGBT from a single institution and that data could be traced in the cancer registry database. The results of our data (4-year overall survival rate of 69.1%) showed close results to the retro-EMBRACE study (5-year overall survival rate of 65%) and Narayan et al. (5-year overall survival rate of 65%) [7, 19].
Consequently, our analysis showed that 4-year local control, progression-free survival and overall survival rates were 89.5%, 74.9% and 69.1%, respectively. The prognostic factors for local control were non-SCCA histology, advanced stage, cumulative dose > 84 Gy and OTT > 56 days. The prognostic factors for overall survival were non-SCCA histology, advanced stage, presented LN and longer OTT. To increase the cumulative dose by hybrid IC/IS technique and reduce OTT by strictly ‘twice a week’ schedule will be our plan to improve our treatment outcomes in the future.
CONCLUSION
The 4-year overall survival from our experiences was 69.1% closed to the retro-EMBRACE study. Non-SCCA histology, advanced stage, presented LN and longer OTT effected the overall survival in multivariate analysis.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.
Contributor Information
Ekkasit Tharavichitkul, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Bongkot Jia-Mahasap, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Pooriwat Muangwong, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Somvilai Chakrabandhu, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Pitchayaponne Klunklin, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Wimrak Onchan, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Damrongsak Tippanya, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Wannapa Nobnop, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Anirut Watcharawipha, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Kittikun Kittidachanan, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
Ravan M Galalae, MedAustron Ion Therapy Center, Wiener Neustadt 2700, Austria; Faculty of Medicine, Christian-Albrechts-University, Kiel 24118, Germany.
Imjai Chitapanarux, The Division of Radiation Oncology, Department of Radiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand; Northern Thailand Radiation Oncology Group, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
References
- 1. Kamnerdsupaphon P, Srisukho S, Sumitsawan Y et al. Cancers in Northern Thailand. Biomed Imaging Interv J 2008;4:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatla N, Aoki D, Sharma DN et al. Cancer of the cervix uteri. Int J Gynecol Obstet 2018;143:22–36. [DOI] [PubMed] [Google Scholar]
- 3. Haie-Meder C, Pötter R, Van Limbergen E et al. Recommendations from gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–45. [DOI] [PubMed] [Google Scholar]
- 4. Pötter R, Haie-Meder C, Van Limbergen E et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy - 3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiolo. Radiother Oncol 2006;78:67–77. [DOI] [PubMed] [Google Scholar]
- 5. Pötter R, Dimopoulos J, Georg P et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol 2007;83:148–55. [DOI] [PubMed] [Google Scholar]
- 6. Pötter R, Georg P, Dimopoulos JCA et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011;100:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sturdza A, Pötter R, Fokdal LU et al. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol 2016;120:428–33. [DOI] [PubMed] [Google Scholar]
- 8. Pötter R, Tanderup K, Schmid MP et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol 2021;22:538–47. [DOI] [PubMed] [Google Scholar]
- 9. Mahantshetty U, Krishnatry R, Hande V et al. Magnetic resonance image guided adaptive brachytherapy in locally advanced cervical cancer: an experience from a tertiary cancer center in a low and middle income countries setting. Int J Radiat Oncol Biol Phys 2017;99:608–17. [DOI] [PubMed] [Google Scholar]
- 10. Viswanathan AN, Dimopoulos J, Kirisits C et al. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol 2007;68:491–8. [DOI] [PubMed] [Google Scholar]
- 11. Ohno T, Wakatsuki M, Toita T et al. Recommendations for high-risk clinical target volume definition with computed tomography for three-dimensional image-guided brachytherapy in cervical cancer patients. J Radiat Res 2017;58:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahantshetty U, Poetter R, Beriwal S et al. IBS-GEC ESTRO-ABS recommendations for CT based contouring in image guided adaptive brachytherapy for cervical cancer. Radiother Oncol 2021;160:273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viswanathan AN, Erickson B, Gaffney DK et al. Comparison and consensus guidelines for delineation of clinical target volume for CT- and MR-based brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol 2014;90:320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohno T, Noda SE, Okonogi N et al. In-room computed tomography-based brachytherapy for uterine cervical cancer: results of a 5-year retrospective study. J Radiat Res 2017;58:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murakami N, Kobayashi K, Shima S et al. A hybrid technique of intracavitary and interstitial brachytherapy for locally advanced cervical cancer: initial outcomes of a single-institute experience. BMC Cancer 2019;19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tharavichitkul E, Mayurasakorn S, Lorvidhaya V et al. Preliminary results of conformal computed tomography (CT)-based intracavitary brachytherapy (ICBT) for locally advanced cervical cancer: a single institution’s experience. J Radiat Res 2011;52:634–40. [DOI] [PubMed] [Google Scholar]
- 17. Tharavichitkul E, Chakrabandhu S, Wanwilairat S et al. Intermediate-term results of image-guided brachytherapy and high-technology external beam radiotherapy in cervical cancer: Chiang Mai University experience. Gynecol Oncol 2013;130:81–5. [DOI] [PubMed] [Google Scholar]
- 18. Van Dyk S, Bernshaw D. Ultrasound-based conformal planning for gynaecological brachytherapy. J Med Imaging Radiat Oncol 2008;52:77–84. [DOI] [PubMed] [Google Scholar]
- 19. Narayan K, van Dyk S, Bernshaw D et al. Ultrasound guided conformal brachytherapy of cervix cancer: Survival, patterns of failure, and late complications. J Gynecol Oncol 2014;25:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tharavichitkul E, Chakrabandhu S, Klunklin P et al. Intermediate-term results of trans-abdominal ultrasound (TAUS)-guided brachytherapy in cervical cancer. Gynecol Oncol 2018;148:468–73. [DOI] [PubMed] [Google Scholar]
- 21. Tharavichitkul E, Tippanya D, Jayavasti R et al. Two-year results of transabdominal ultrasound-guided brachytherapy for cervical cancer. Brachytherapy 2015;14:238–44. [DOI] [PubMed] [Google Scholar]
- 22. Galalae R, Tharavichitkul E, Wanwilairat S et al. University Cooperation Platform (UCP) between Christian-Albrechts-University Kiel (Germany) and Chiang Mai University (Thailand): Implementation of image-guided gynecological brachytherapy. J Contemp Brachytherapy 2015;7:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tharavichitkul E, Muangwong P, Chakrabandhu S et al. Comparison of clinical outcomes achieved with image-guided adaptive brachytherapy for cervix cancer using CT or transabdominal ultrasound. Brachytherapy 2021;20:543–9. [DOI] [PubMed] [Google Scholar]
- 24. Lindegaard JC, Fokdal LU, Nielsen SK et al. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol (Madr) 2013;52:1510–9. [DOI] [PubMed] [Google Scholar]
- 25. Möller S, Mordhorst LB, Hermansson RS et al. Combined external pelvic chemoradiotherapy and image-guided adaptive brachytherapy in treatment of advanced cervical carcinoma: experience from a single institution. J Contemp Brachytherapy 2020;12:356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castelnau-Marchand P, Chargari C, Maroun P et al. Clinical outcomes of definitive chemoradiation followed by intracavitary pulsed-dose rate image-guided adaptive brachytherapy in locally advanced cervical cancer. Gynecol Oncol 2015;139:288–94. [DOI] [PubMed] [Google Scholar]
- 27. Ribeiro I, Janssen H, De Brabandere M et al. Long term experience with 3D image guided brachytherapy and clinical outcome in cervical cancer patients. Radiother Oncol 2016;120:447–54. [DOI] [PubMed] [Google Scholar]
- 28. Korenaga TRK, Yoshida EJ, Pierson W et al. Better late than never: brachytherapy is more important than timing in treatment of locally advanced cervical cancer. Gynecol Oncol 2022;164:348–56. [DOI] [PubMed] [Google Scholar]
- 29. van Dyk S, Khaw P, Lin MY et al. Ultrasound-guided brachytherapy for cervix cancer. Clin Oncol 2021;33:e403–11. [DOI] [PubMed] [Google Scholar]