ABSTRACT
Globally, millions of zebrafish (Danio rerio) are used for scientific laboratory experiments for which researchers have a duty of care, with legal obligations to consider their welfare. Considering the growing use of the zebrafish as a vertebrate model for addressing a diverse range of scientific questions, optimising their laboratory conditions is of major importance for both welfare and improving scientific research. However, most guidelines for the care and breeding of zebrafish for research are concerned primarily with maximising production and minimising costs and pay little attention to the effects on welfare of the environments in which the fish are maintained, or how those conditions affect their scientific research. Here we review the physical and social conditions in which laboratory zebrafish are kept, identifying and drawing attention to factors likely to affect their welfare and experimental science. We also identify a fundamental lack knowledge of how zebrafish interact with many biotic and abiotic features in their natural environment to support ways to optimise zebrafish health and well‐being in the laboratory, and in turn the quality of scientific data produced. We advocate that the conditions under which zebrafish are maintained need to become a more integral part of research and that we understand more fully how they influence experimental outcome and in turn interpretations of the data generated.
Keywords: zebrafish, Danio rerio , welfare, physical environment, social environment, natural habitats, laboratory conditions, data quality
I. INTRODUCTION
There are several reasons why it is important to review the welfare of laboratory zebrafish (Danio rerio Hamilton). First, researchers have a duty of care for laboratory animals and in many countries are legally obliged to consider their welfare when designing experiments (e.g. UK: Home Office, 2014a; European Union: Council of the European Union, 2010; USA: National Research Council, 2011; Australia: National Health and Medical Research Council, 2013). Second, awareness and support for better welfare of research animals is increasing in the public domain and research funders are now requesting high standards of welfare as a condition of receiving research funds (NC3Rs et al., 2015). A survey by Ipsos MORI in 2018 found that 59% of the public want to know more about work to improve the welfare of research animals, a rise of 5% since 2016 (Clemence, 2018). Thirdly, good animal welfare is linked to improved quality of science (Prescott & Lidster, 2017).
The zebrafish is a key experimental model and worldwide millions are used for studies spanning toxicology, drug discovery, and the study of human diseases, but a lack of defined conditions and standards for zebrafish care have resulted in the protocols for their housing and maintenance varying among laboratories (Alestrom et al., 2019). Advances in the science of zebrafish husbandry and management and more detailed reporting of maintenance conditions of experimental fish are needed to address this issue (reviewed by Lieggi et al. 2020). Most guidelines are concerned primarily with maximising production and minimising costs and pay less attention to how aspects of the laboratory environment affect welfare or the science that they support.
We identify the science behind the conditions in which laboratory zebrafish are often kept and aim to identify and draw attention to factors likely to affect their welfare. Understanding a species’ natural biology can help to guide good welfare practice (Howell & Cheyne, 2019) and here we draw on field observations and from experiments in mesocosms and laboratory tanks, to compare and contrast the physical and social environment experienced by wild zebrafish with those of laboratory‐maintained fish. A variety of physiological and behavioural changes, including increased growth rate and reduced startle response, have been documented in zebrafish raised in a captive environment compared to fish collected from their natural habitat in India (Robison & Rowland, 2005). Captivity creates different fitness pressures compared to the fish's natural environment, potentially leading to differences in selection on various traits (Robison & Rowland, 2005). We use measures of physiology and behaviour to indicate factors likely to affect zebrafish welfare. We also identify gaps in knowledge and discrepancies among current regulatory and laboratory guidelines and illustrate some of the challenges and opportunities that confront researchers and facility managers for providing best welfare for zebrafish. We furthermore consider how these factors can affect the many fields of science that employ zebrafish as study models, including areas such as neuroscience where the recent discovery of astrocytes in zebrafish is predicted to open new research avenues into brain development, function and disease (Chen et al., 2020). Such research will likely be influenced by the environment in which the experimental fish are produced and tested. For example, temperature can affect the zebrafish nervous system, as evidenced by changes in the brain proteome and behaviour (Nonnis et al., 2021), and behavioural testing of isolated fish modifies their physiological, neuroendocrine and behavioural responses (Giacomini et al., 2015).
II. WELFARE
Optimising welfare, based on scientific evidence, is not always compatible with research methods or purpose. For example, some forms of environmental enrichment can improve welfare for some fish species (Näslund & Johnsson, 2014) but can potentially also alter features of an animal's physiology or behaviour (Killen et al., 2013), influencing research results. The benefits of refinements to housing or husbandry need to be measured against their costs and practicalities in order to make a convincing case for improvements that deliver higher welfare. This requires that welfare is quantified. Measures of fish welfare include survivorship, growth, health, reproductive performance, levels of blood/body cortisol, behaviour and affective states. These indicators of fish welfare (Table 1) along with how they are measured, examples of their use, and their advantages and disadvantages, are reviewed extensively elsewhere (Huntingford et al., 2006; Sadoul & Geffroy, 2019; Toni et al., 2019). Each of these methods reflects a particular aspect of welfare but none provide a complete evaluation, so multiple methods are often used (Huntingford & Kadri, 2008).
Table 1.
Commonly used measures of fish welfare
| Welfare indicator | Metric | Examples of use | Advantages | Disadvantages |
|---|---|---|---|---|
| Survivorship | Simple counts | Evaluate effects of processed diets (Goolish, Okutake & Lesure, 1999) and environmental enrichment (Lee, Paull & Tyler, 2019a) | Easily understood | Difficult to differentiate between underlying and immediate causes (Ellis et al., 2012a) |
| Growth | Body length, mass, body condition | Evaluate effects of social isolation (Forsatkar, Safari & Boiti, 2017), stocking density (Rabbane, Rahman & Hossain, 2016), and the use of body condition scoring to assess health and welfare (Clark et al., 2018) | Straightforward to measure | Depends upon many factors, including temperature, photoperiod, strain, diet, life stage (MacIntyre et al., 2008); optimal growth for zebrafish has yet to be established (Siccardi et al., 2009) |
| Health | Fish appearance (skin, fin, eye and gill integrity and colour) and behaviour (feeding, air‐gasping, balance, activity) (Segner et al., 2012); regular health surveillance (Harper & Lawrence, 2012); body condition (Wilson et al., 2013) | Evaluate husbandry stress on mycobacterial infections (Ramsay et al., 2009) and compare success of pathogen detection during health inspections (Marancik et al., 2019) | Assessing is pragmatic; incidence of disease relatively easy to recognise and measure | High cost of full health evaluations; retrospective results; high number of sampled fish needed to detect pathogens (Collymore, Crim & Lieggi, 2016). The link between health and welfare is complex and can be hard to interpret – although a diseased fish is in a poor state of welfare, a healthy fish is not necessarily in a good state of welfare (Segner et al., 2012) |
| Reproductive performance | Clutch size, egg viability, spawning frequency | Evaluate effects of stocking density (Castranova et al., 2011), stress (Abdollahpour et al., 2020a), and simple tank changes (Lee, Tyler & Paull, 2018) | Straightforward to measure | Counting or sizing eggs or embryos is tedious and time‐consuming. High variability among individuals and populations so high replication needed (Paull et al., 2008). |
| Cortisol levels | Whole‐body cortisol; cortisol in blood, mucus, faeces, water, scales or fins | Evaluate auditory enrichment as method of reducing stress (Barcellos et al., 2018), compare effects of handling fish with a scoop versus with a net (Brydges et al., 2009), and assess transport stress (Dhanasiri, Fernandes & Kiron, 2013) | Measuring cortisol in blood is relatively simple (Ellis et al., 2012b); non‐invasive measurements of cortisol in fish holding water is suitable for small species, such as zebrafish (Stevens et al., 2017) | May be difficult to interpret for welfare. Cortisol release is a natural reaction to challenges (Huntingford et al., 2006) but is not synonymous with suffering (Dawkins, 1998). Short‐term stress may be beneficial, chronic stress may compromise welfare (Huntingford et al., 2006) |
| Behaviour | Observation of latency, frequency or duration of behaviours such as aggression, foraging, shoaling or breeding | Impact of invasive procedures on swimming behaviour (Deakin et al., 2019; Thomson et al., 2019), and shoal cohesion as an indicator of positive welfare (Franks, Graham & von Keyserlingk, 2018) | Easy to observe, with training; ideal for daily use; can be early sign of welfare problems | Often variable over time or in response to husbandry events such as anticipation of feeding (Martins et al., 2012); may be affected by social dynamics when removing fish from social groups for individual testing (Kleinhappel, Pike & Burman, 2019); individual variance within a group; may be manipulated by parasites (Poulin, 2013); may be difficult to measure and interpret for welfare |
| Affective state | Cognitive bias, judgement bias, decision‐making, preference | Assess decision‐making after losing aggressive encounter (Rogers et al., 2020), assess preference for a range of tank enrichments (Schroeder et al., 2014), assess sensitivity to reward shifts (Tan et al., 2020) | Indicates an animal's subjective perception of internal or external stimuli; can identify positive as well as negative welfare (Jirkof, Rudeck & Lewejohann, 2019) | May be difficult to quantify; lack of established measures; judgement bias tests require training of fish but some fail to learn task so tests may systematically exclude some individuals (Rogers et al., 2020); interpretation of preference tests requires caution as choices restricted to resources provided in the test, fish preferences may change over time, or they may choose the lesser of two non‐preferred items (Maia & Volpato, 2016) |
Studies of fish welfare usually follow one of several approaches. The first, a ‘feeling‐based’ approach, sets out to prove or disprove that fish are sentient beings, i.e. that they have the capacity to suffer (Vettese, Franks & Jacquet, 2020). A second approach, ‘physiological’, attempts to measure pain and stress as negative indicators of a fish's welfare state (Daskalova, 2019). A third approach uses behavioural analyses to infer learning, preference and choice and to support arguments for fish cognition and emotions (Vila Pouca & Brown, 2017). In a fourth approach, a fish's welfare is considered good if it is able to lead a natural life and express the behaviours that it would in the wild (Huntingford & Kadri, 2009). In our opinion, all of these approaches are needed to define the welfare status of a fish most reliably. Optimising welfare is critical if researchers are to avoid variation in research results caused by changes in the physiology and behaviour of experimental animals due to compromised welfare (Prescott & Lidster, 2017).
III. PHYSICAL ENVIRONMENT
The optimum range of conditions for zebrafish, and the husbandry parameters that are most important for welfare, and therefore good science, are largely unknown (Tsang et al., 2017) with many laboratories modifying environmental conditions for zebrafish based on their own opinions, experience, and/or old literature rather than from any systematic analyses (Tsang et al., 2017). In their natural environment in India, Bangladesh and Nepal, zebrafish occupy a variety of habitats, including rice paddies, ponds, ditches, and streams, and tolerate a wide range of fluctuating temperatures and water conditions (Lee, Tyler & Paull, 2020). This adaptability accounts for the species’ ability to tolerate the wide‐ranging conditions operating among laboratories, and why ease of care is often listed as a zebrafish attribute. However, there is a need for empirical studies to evaluate the effects of environmental parameters on the health and welfare of zebrafish in order to understand which environmental factors may affect research results (Tsang et al., 2017). For example, a recent study found that temperature affects the nervous system and cognitive abilities of adult zebrafish and even short‐term variations can alter brain protein expression and behaviour (Nonnis et al., 2021). This, in turn, suggests that temperature is an important environmental factor influencing zebrafish physiology and behaviour and that the duration of thermal change may induce different responses in laboratory fish.
(1). Water quality
Water physicochemistry is hugely important for the welfare of fish but is often overlooked as a variable in zebrafish research (Hammer, 2020). Fish are sensitive to poor water quality and to contaminants (Huntingford et al., 2006) and provision of appropriate water conditions for fish maintenance does not necessarily align with the needs of the research. For example, fish used to investigate the relationship between gene function and disease may be housed in static containers during genotyping and experience a rapid decrease in water quality (Goodwin et al., 2016).
The UK Government's code of practice for the housing and care of fish used for scientific purposes (Home Office, 2014b, p. 37) states that “adequate water supply of suitable quality shall be provided” and that “water quality parameters shall be within the acceptable range that sustains normal activity and physiology for a given species”, but does not state the specific requirements for zebrafish. European legislation specifies water parameters for zebrafish embryos and larvae used for ecotoxicology studies (OECD, 2013) but recommendations for adults are vague (European Commission, 2019). United States guidelines for the care of research animals acknowledge that water quality affects aquatic animal wellbeing and that requirements vary with species, but do not define the specific needs of zebrafish (National Research Council, 2011). Given that poor water quality can directly affect fish growth and reproduction (Hammer, 2020) this lack of prescribed water parameters for breeding stock represents a significant shortfall in official guidance.
Zebrafish are widely used in (eco)toxicology (Tal, Yaghoobi & Lein, 2020), where water quality is likely to affect study outcomes through effects, for example, on the fate and behaviour of the exposure chemical (Hamm et al., 2019). It also has been shown that, during the process of fish domestication (Teletchea & Fontaine, 2014), water physicochemistry in which embryos are incubated can affect their ability to cope with laboratory conditions and such information may be passed on to future generations via epigenetic memory in the gametes (Labbé, Robles & Herraez, 2017).
In their natural environment, water conditions experienced by zebrafish are highly dynamic seasonally. By contrast, water conditions in laboratories are controlled and monitored to reduce fluctuations and maintain stability throughout the year (Fig. 1; Table 2 provides tolerance ranges for water chemistry parameters in wild zebrafish, recommendations for laboratory‐maintained zebrafish, and implications for welfare). How the lack of variation in water physicochemistry and seasonality affects zebrafish is unknown, but further insight could help inform both the management of stock animals and certain areas of research, for example, how climate affects disease. As a case example, a direct link has been established between temperature during zebrafish development and cardiac anatomy (Dimitriadi et al., 2018).
Fig. 1.
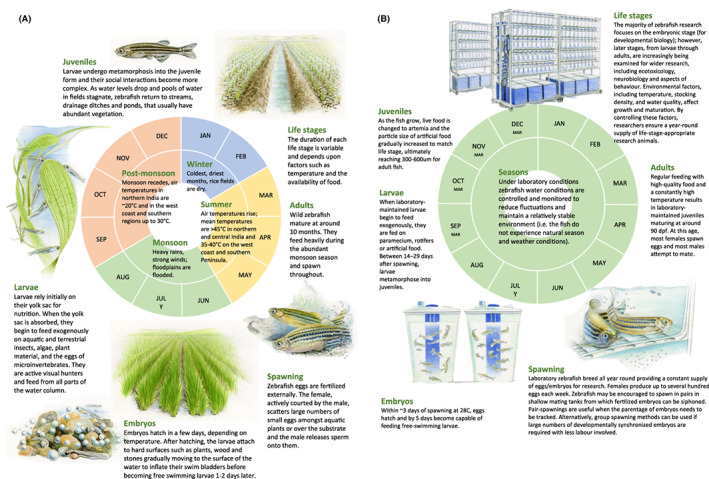
A schematic representing the life cycle and living conditions experienced by (A) wild zebrafish and (B) laboratory maintained zebrafish. Credit: Sandra Doyle.
Table 2.
Summary of water chemistry parameters reported in wild zebrafish (Danio rerio) habitats and in zebrafish facilities and their implications for welfare
| Laboratory conditions for adult zebrafish | |||||
|---|---|---|---|---|---|
| Parameter | Wild habitats | Tolerance limits | Optimal range | Recommendations | Implications for welfare |
| Dissolved oxygen | Unknown | Levels of 0.8 mg l−1 are lethal within 2 days and levels of 0.4 mg l−1 are lethal within 12 h (Rees, Sudradjat & Love, 2001) | Unknown | Range from 6 mg l−1 (Matthews, Trevarrow & Matthews, 2002) to 7.8 mg l−1 (Harper & Lawrence, 2012); with a suggested minimum of 4 mg l−1 (Lawrence & Mason, 2012) | Uneaten food, decaying solids and high fish densities can reduce levels (Hammer, 2020). Warning signs include hyperventilation, surface swimming and gulping air (Kramer, 1987); extreme depletion can damage gills, impair growth, cause immunosuppression, and lead to death. |
| Ammonia | Unknown | Levels >1.0 mg l−1 are lethal to many fish (Murray, Lains & Spagnoli, 2020) but the specific tolerance limits of zebrafish are unknown | Unknown | As close to 0 mg l−1 as possible (Hammer, 2020; Murray et al., 2020) | Highly toxic. Chronic exposure to non‐lethal levels can result in immunosuppression and reduced growth (Murray et al., 2020). Acute exposure can cause hyperexcitability, anorexia, and death (Murray et al., 2020). |
| Nitrite | Unknown | Levels of 386 mg l−1 are lethal within 4 days (Voslářová et al., 2008) | Unknown | As close to 0 mg l−1 as possible: <0.5 mg l−1 (Hammer, 2020); <1.0 mg l−1 (Murray et al., 2020) | Warning signs in fish include lethargy, remaining near water inlet, hyperventilating; chronic exposure impairs growth (Murray et al., 2020). |
| Nitrate | Unknown | Unknown | Unknown | <100 mg l−1 (Pereira et al., 2017); <50 mg l−1 (Hammer, 2020); <25 mg l−1 (Alestrom et al., 2019) | Less toxic than nitrite but may accumulate over time in recirculating systems with high fish densities (Learmonth & Carvalho, 2015). Chronic exposure can damage gills, skin, kidneys, liver and intestines (Pereira et al., 2017). |
| pH | Varies from 5.9 (Engeszer et al., 2007) to 9.8 (Arunachalam et al., 2013) | Lower and upper lethal limits: 3.0 and 12.0 respectively (Zahangir et al., 2015) | 7.4–7.5 for reproduction (Alestrom et al., 2019) | 7–8 (Hammer, 2020); 6.5–8 (Alestrom et al., 2019) | Exposure to pH near the lower or upper limits damages skin and gills, leads to loss of balance, convulsions and death (Zahangir et al., 2015). |
| Salinity | From 0.01 to 0.6 g l−1 (Spence et al., 2006) | Unknown | Unknown | 0.5–2 g l−1 (Harper & Lawrence, 2012); 0.06–0.17 g l−1 (Tsang et al., 2017); 0.08–0.93 g l−1 (Alestrom et al., 2019) | Long‐term exposure to low or high salinity may negatively affect energy expenditure and fecundity (Boisen et al., 2003). |
(2). Water flow
Zebrafish have been used to model the effects of exercise on growth (Palstra et al., 2010), ageing (Gilbert, Zerulla & Tierney, 2014), heart regeneration (Rovira et al., 2018) and anxiety (DePasquale & Leri, 2018). In these experiments, swim tunnels with adjustable flow are used to induce exercise. As repeated exercise improves the overall condition of fish (Palstra & Planas, 2011), there may be differences in the pre‐study condition of fish reared in tanks with differing flow rates, from static to high flow depending on tank system design and fish life stage. It may therefore benefit the comparison of results if flow rates in rearing tanks are standardised, or at least reported. However, none of the above‐referenced studies report rearing tank flow rates.
Wild zebrafish are found in still waters and waters that flow up to 18 cm s−1 (Spence et al., 2006, 2008; Suriyampola et al., 2015). Complex, fragmented flow patterns in natural streams and rivers are created by structures such as boulders (Branco et al., 2013), woody debris (Manga & Kirchner, 2000) and vegetation (Sukhodolov & Sukhodolova, 2010). Wild fish can move between these microhabitats as conditions or needs change. By contrast, water flows in laboratory tanks are homogeneous, with recommended flow rates of 3–5 tank volume exchanges per hour for adult zebrafish (Brand, Granato & Nüsslein‐Volhard, 2002; Varga, 2016). The flow of water through laboratory tanks varies among laboratories, and for fish at different life stages.
Water flow may benefit the welfare of zebrafish by helping to minimise mycobacteriosis (Murray et al., 2011), promote movement (Gilbert et al., 2014), and possibly stimulate the immune system (Palstra et al., 2010), but little is known about the effects of flow on other measures of welfare. Water flow may also be negative for welfare, especially in small tanks, such as the 1–10 l tanks used in commercial rack units (Lawrence & Mason, 2012), where the inability to move away from flow may increase stress and energetic costs for fish (Suriyampola et al., 2017).
Interestingly, captive zebrafish have been shown to be more active, more aggressive, and to form less‐cohesive groups in flowing, rather than still, water conditions (Suriyampola et al., 2017) but only when the areas were furnished with plants and substrate (DePasquale et al., 2019). Similarly, zebrafish in the wild are more aggressive and form less‐stable groups in flowing, rather than still, waters but, when compared to captive fish, their social behaviour is less affected by vegetation (Suriyampola et al., 2015). These studies suggest that water flow influences the social behaviour of wild and captive zebrafish and may be an important parameter to be considered in experimental/housing design.
(3). Light
Zebrafish are increasingly used as models to study light‐induced eye diseases and retina regeneration (Wan & Goldman, 2016), and light itself can act as a stressor for zebrafish in biomedical tests (Lee et al., 2019b). Despite the importance of light in the zebrafish rearing environment, in a sample of 10 published studies on eye disorders in adult zebrafish, none stated the type of light used or the light intensity (search conducted in Web of Science Core Collection, using “eye disorders adult zebrafish” and dates 2010‐01‐01 to 2020‐12‐01 as search criteria). Light is therefore a possible confounding factor among these experiments due to inconsistencies among laboratories and lack of similarity between lighting conditions in the laboratory and in the natural environment.
Light affects fish behaviour in many ways. Diel cycles of day and night shape the times at which fish feed, breed, and rest (Helfman, 1993) and seasonal light rhythms influence reproduction and spawning (Migaud, Davie & Taylor, 2010). A rhythm of activity and rest occurs in zebrafish from the first day post‐fertilisation (Dekens & Whitmore, 2008; Krylov et al., 2021), reflecting the light–dark cycle in nature. In India and Bangladesh, hours of daylight range from 10 h in December to 13 h in June (www.timebie.com/sun) with the longest day lengths coinciding with the start of the monsoon, when wild zebrafish are thought to breed (Spence et al., 2007). The sun provides up to 100000 lux of light at the Earth's surface at midday (Thorington, 1985) and sunlight covers the full spectrum from infrared to ultraviolet as it gradually changes from dawn to dusk.
In the laboratory, artificial light is provided through incandescent, fluorescent or light‐emiting diode (LED) lamps, each of which produces a different spectrum of light per wavelength. Light intensity may vary depending on the position of tanks in the rack or room, or may be reduced to inhibit algal growth (Bhargava, 2018). In addition, most artificial light fluctuates with low intensity (incandescent bulbs) or with a pronounced flicker (fluorescent and LED bulbs) (Inger et al., 2014) that can cause headaches and eye fatigue in humans (Wilkins et al., 1989), affect mate choice in captive birds (Evans, Cuthill & Bennett, 2006) and induce myopia in mice (Yu et al., 2011). Despite the potential for these factors to affect behaviour, experimental results, and reproducibility (Adatto, Krug & Zon, 2016; Sabet, van Dooren & Slabbekoorn, 2016a; Gerlai, 2018), light source and intensity are rarely reported in the literature. A cycle of 14 h of light and 10 h of darkness with phased transitions has been suggested as appropriate for laboratory zebrafish (Reed & Jennings, 2011; Tsang et al., 2017) but, across the year, this adds 1–4 h of light per day compared to the natural environment. Such an extension of day length increases growth and reproductive performance (Abdollahpour, Falahatkar & Lawrence, 2020b), but its long‐term effects on welfare have not been evaluated. In addition, biological rhythms may be disrupted by light pollution or leakage during the hours of darkness from extraneous light sources such as hallway lights and exit signs (Adatto et al., 2016). Lighting type and light cycle regime may even vary between rooms within a facility, which needs to be considered in facility design and reporting of results.
(4). Temperature
Zebrafish are increasingly used to model population sex ratios and investigate how epigenetic inheritance contributes to adaptation to new environmental conditions such as temperature exposure (Valdivieso et al., 2020). They are also used to assess the effect of temperature on cardiovascular function (Rayani et al., 2018), and to study fish diseases and infections at different temperatures (Jorgensen, 2020).
Temperature exerts a fundamental impact on all aspects of development, growth, feeding, metabolism, reproduction and behaviour in fish (Coutant, 2006). For laboratory zebrafish, rearing temperature and daily temperature cycles influence larval survival, growth, phenotype and sex determination (Schaefer & Ryan, 2006; Sfakianakis et al., 2011). However, effects of temperature on development and growth are complex.
Villamizar et al. (2014) found that a constant temperature of 24°C or 28°C resulted in male‐biased sex ratios whereas a daily thermocycle resulted in a higher proportion of females. Adults kept at 34°C were bolder and less anxious than fish kept at 26°C (Angiulli et al., 2020), a finding that could impact results and reproducibility, especially for studies of neuroscience or behaviour. Generally, the highest survival and growth rates are reported in fish maintained at a constant temperature of 28°C (Schaefer & Ryan, 2006; Sfakianakis et al., 2011), the most commonly used set point for laboratory zebrafish, or when thermocycles are set so that the transition to higher temperature coincides with dawn and the transition to lower temperature coincides with dusk (Villamizar et al., 2014).
Zebrafish are cold‐blooded (poikilothermic) and eurythermal (Nonnis et al., 2021), reflecting wide seasonal and diurnal temperature ranges reported in their natural environment, from 12°C in Arunachal Pradesh to 39°C in Orissa (Arunachalam et al., 2013). Air temperature, water depth and vegetation combine to create fine‐scale microclimates throughout a fish's home range (Welch, Jacoby & May, 1998). Riparian vegetation provides shade along the banks of streams and lakes, creating refuges that are cooler in summer and warmer in winter than similar habitats with no vegetation (Welch et al., 1998).
Zebrafish are used to investigate the effects of thermal tolerance on aquatic ectotherms (Schaefer & Ryan, 2006), however, evidence of differences in thermal tolerance between wild and laboratory‐reared zebrafish (Morgan et al., 2019) suggests that the tolerance of wild populations could be overestimated if reliance is placed solely on the results of laboratory experiments. Understanding species’ thermal limits is central to understanding how they will respond to climate change (Bennett et al., 2021).
Temperature is a potentially useful tool for the husbandry and health management of zebrafish. When allowed to move freely across a gradient of 18–35°C, fish infected with a virus spent most of their time at a temperature of 29°C and showed temperature‐dependent changes in the brain transcriptome higher than those of fish with limited thermal choices (Boltaña et al., 2013). A comparable experiment with larval zebrafish produced similar results (Rey et al., 2017). Thermal choice and, conversely, thermal restriction may therefore impact upon a fish's health and welfare.
Little is known about the temperature preferences of zebrafish. When allowed to choose between water temperatures of 20°C and 24°C, zebrafish showed a consistent preference for the higher temperature (López‐Olmeda & Sánchez‐Vázquez, 2009). In addition, there is evidence that fish personality may affect temperature preference, with bold, aggressive zebrafish preferring warmer waters than shy, passive fish (Rey, Digka & Mackenzie, 2015). More research is needed to define the preferences of unstressed fish, increase understanding of the effects of temperature, diurnal cycles, and thermal gradients on zebrafish, and evaluate the potential benefits of temperature choice on welfare and fitness.
(5). Physical space
Tank size is a potential confounding factor for studies of fish cognition and spatial learning (Salena et al., 2021). Like rodents and birds, fish use geometry to solve spatial tasks and differences in the size and shape of the tanks in which they are reared can affect their subsequent performance in spatial tests (Brown, Spetch & Hurd, 2007; Carbia & Brown, 2019; Salena et al., 2021). Tank size can also affect studies of the growth rates of Atlantic salmon (Salmo salar; Espmark et al., 2017) and aggression and welfare in the Midas cichlid (Amphilophus citrinellus; Oldfield, 2011). However, few experiments have measured zebrafish responses to different tank sizes and little attention has been paid to the small amount of space a laboratory zebrafish has compared to what it may use as a free‐living animal.
Laboratory housing often involves confining fish at high densities, with no opportunity for dispersal or exploration of novel space, and in groups differing in size and social structure from those found in nature, where shoals are dynamic and individuals interact in an ever‐changing social network (Krause et al., 2000). One consequence is an effect on mating strategy. Group spawning accounted for 72% of spawning events when fish were held at high densities in 17 l tanks, whereas most fish spawned in pairs when housed at low densities in 450–1000 l tanks, suggesting that group spawning may result from mating pairs being unable to escape from conspecifics (Hutter et al., 2010). Undoubtedly, the movement and behaviour of laboratory zebrafish is greatly restricted compared to that of wild fish, with consequences for research into genetic architecture and the evolution of behavioural traits (Wright et al., 2006a). Fish may be routinely and repeatedly moved from their home tanks to smaller spawning chambers with the potential for additional stress due to handling and confinement, and to changes in water conditions and flow, social structure and density, room position, lighting, etc. In addition, visual health checks are more challenging in small tanks as sick fish cannot easily isolate from the group and behavioural signs of disease, such as changes in swimming activity or social behaviour (Kirsten et al., 2018), are more easily missed.
There are no data‐driven standards for the size and shape of tanks used to house zebrafish. Neither the UK Home Office Code of Practice for the housing and care of animals used for scientific purposes (Home Office, 2014a) nor the European Directive on the protection of animals used for scientific purposes (Council of the European Union, 2010) give guidance on tank dimensions. RSPCA guidelines state simply that “tanks need to be of sufficient size to accommodate the physical and behavioural needs of zebrafish and to allow appropriate social interactions. The necessary dimensions depend on the size and age of the fish” (Reed & Jennings, 2011). For this advice to be of practical use, the physical and behavioural preferences of zebrafish at different life stages need to be determined and defined and the number of fish in the tank taken into account. Unsurprisingly, given the lack of published standards, tank sizes and shapes and stocking densities vary considerably among facilities. Tanks reportedly used for rearing larvae range in volume from 1.3 l housing 50 larvae (38 larvae l−1) to 0.75 l for 100 larvae (133 larvae l−1) while tanks for adult fish range from 1.8 l for 18 fish (10 fish l−1) to 75 l for 300 fish (4 fish l−1) (Harper & Lawrence, 2012; Lawrence & Mason, 2012; Varga, 2016). Commercially available rack units that hold tanks, typically from 1 to 10 l, are used by many research establishments to house zebrafish (Lawrence & Mason, 2012) as they enable a large number of fish to be held in a relatively small space.
The size and depth of water bodies change drastically between wet and dry seasons in ponds, rice fields and streams throughout zebrafish natural habitats and are linked to different life‐history stages (Lee et al., 2020). This seismic shift in water volume is often mimicked for captive zebrafish during their first few days of life when they are kept in shallow water until swim bladder inflation, at which point they are transferred to shallow tanks which are filled gradually, over a period of a few hours to a day, often to the depth at which they will remain for their adult lives. The size of water bodies in which wild zebrafish have been found range from puddles of 1 m2 to lakes of 3.4 ha, with depths from 5 cm in irrigation channels to 1 m in rivers and lakes (Spence et al., 2007; Arunachalam et al., 2013; Suriyampola et al., 2015; Sundin et al., 2019). Even the smallest water body in nature offers more physical space than typical laboratory conditions, which may impact the responses that zebrafish give to cognition, learning and other tests. Careful reporting of tank size and shape during rearing and testing is therefore recommended.
(6). Environmental enrichment
Despite growing interest among neuroscientists in how environmental enrichment influences brain processes and behaviours, its role in zebrafish models is poorly understood (Volgin et al., 2018). Enrichment improves reproducibility of rodent studies by increasing animal well‐being and positively influencing brain physiology and behaviour (Volgin et al., 2018). It stimulates several brain regions, promotes neuroprotection and neurogenesis, and protects against the effects of unpredictable chronic stress (Marcon et al., 2018). In fish species, enrichment has been found to affect several aspects of biology, from aggression and stress to disease susceptibility, but these differ depending upon the species and life stage (reviewed by Näslund & Johnsson, 2014). In zebrafish, enrichment appears to reduce anxiety and provide positive neurological stimulation, but more studies are needed to determine its influence fully (Volgin et al., 2018).
Zebrafish live in diverse environments within their natural range, where substrates and vegetation are varied and structurally complex. By contrast, laboratory fish are almost always housed in bare tanks with few stimuli. Such barren housing may be associated with poor welfare even when animals are otherwise well provisioned (Schroeder & Mocho, 2014).
Environmental enrichment for laboratory fish involves increasing the complexity of the tank environment, usually by the addition of substrates or plants, in order to improve welfare and minimise unwanted behaviour such as aggression (Näslund & Johnsson, 2014). The goal when designing enrichment is to identify elements of the natural environment that can be modified to provide measurable welfare benefits without compromising research (Johnsson, Brockmark & Näslund, 2014), and can be accommodated in small laboratory tanks and practically maintained by animal care staff. Preference tests can be informative since they allow fish to choose what they want. However, the interpretation of such tests requires caution as choices are restricted to the resources provided in the test, fish preferences may change over time, or they may choose the lesser of two non‐preferred items that are presented to them (Maia & Volpato, 2016).
Physical items placed in the tank, such as gravel, plants and other objects, are often seen as desirable enrichment features, however they may not be compatible with research studies. For example in (eco)toxicology, these objects add surfaces for adherence of the test chemical and establishment of microbe biofilms that may increase the rate of degradation of the test chemical (Wilkes et al., 2012). Such inclusions can also serve to harbour pathogens as well as restrict viewing of the fish, affecting the likelihood of early detection of disease and/or other health‐related problems (Williams, Readman & Owen, 2009). In some case the provision of these enrichment features is not feasible, and even for laboratories where it is, the ambiguity surrounding their benefits, the cost of enrichment objects and the time required for their day‐to‐day management are concerns (Lidster et al., 2017).
Despite the importance of the sensory system for the zebrafish's view of the world (Moorman, 2001), few studies have examined the effects of sensory enrichment for zebrafish. Tank background colour affects anxiety‐like behaviour and cortisol stress response but effects change with age (de Abreu et al., 2020). Facilities’ tanks are usually blue, green or clear depending on the manufacturer, but little work has been done on the importance of tank colour to help define the most suitable tank design for welfare and to guide commercial companies as to what to manufacture based on scientific evidence. Zebrafish display preference for images of gravel beneath their tanks and this preference is almost as high as for the actual substrate (Schroeder et al., 2014). By contrast, visual enrichment in the form of seascape backing paper, along with an artificial plant and upturned pot, increased aggression, but the effect, if any, of the backing paper is unknown (Woodward, Winder & Watt, 2019). Classical music as auditory enrichment resulted in zebrafish appearing less anxious in behavioural tests and had a positive effect on immune gene expression (Barcellos et al., 2018). Together, these results suggest that auditory and visual stimulation may have potential as enrichment, although further research is needed to determine their merits and long‐term impacts. There is a growing body of work comparing soundscapes in zebrafish natural habitats and the laboratory and the possible impact that sound could have on research outcomes and reproducibility (Sabet et al., 2016b; Lara & Vasconcelos, 2019, 2021).
Environmental enrichment in various forms has varied effects on zebrafish. Some effects are positive from the perspective of welfare (Basquill & Grant, 1998), some are negative (Hamilton & Dill, 2002), while others show inconsistent responses (Kistler et al., 2011). Understanding and comparing study results is hampered by confounding variables such as the age and provenance of experimental fish, the environment in which they were reared, the social context before and during the experiment, tank size and shape, and different types of enrichment offered. Measures used to assess the effects of enrichment, and whether time points used for behavioural assessments were appropriate to avoid confounding by other husbandry practices, may also affect comparison of results (see Table 3 for examples illustrating differences in study design that hinder comparison of the effects of various forms of environmental enrichment). A more systematic approach is needed to tease apart the influence of different elements of enrichment on zebrafish at different life stages, and the effects on welfare of variable, stable and different amounts of enrichment (Stevens, Reed & Hawkins, 2021).
Table 3.
A representative sample of reports investigating the effects on zebrafish (Danio rerio) of various forms of environmental enrichment (EE) to illustrate differences in study design that hinder comparison of results and replication of studies
| Enrichment | Rearing environment | Social context | Age of fish | Measures | Results | Study | |
|---|---|---|---|---|---|---|---|
| Before study | During study | ||||||
| Gravel, sand, image of gravel or sand, artificial plants (floating and submerged), air stone | Barren tanks | Mixed‐sex groups of 10 | Pairs and groups of 8 | 9 months | Preference for enrichments and combinations of enrichments | Pairs preferred substrate over barren tanks. Groups preferred substrates and plants over barren areas; strong preference for gravel and images of gravel; males preferred floating plants to submerged plants; air stones not preferred | Schroeder et al. (2014) |
| Plastic plants, water flow | 3 populations: wild fish from still waters; wild fish from flowing waters; laboratory‐reared in bare tanks | Groups of 15–20 | Groups of 6 | Unreported | Latency to feed, aggression, shoal distance | Plastic plants increased aggression; water flow decreased latency to feed | Bhat, Greulich & Martins (2015) |
| Plastic plants | Bare tanks | 10 fish per 2.5 l tank | Single fish or groups of 5 fish per 2.5 l tank | 6 months | Novel‐tank, light–dark, and place‐preference tests | Single housing in barren tank increased anxiety. Single‐housed in barren tanks and group‐housed with or without EE spent more time with conspecifics than with artificial plants; single‐housed fish with EE showed no preference for conspecifics or artificial plant | Collymore, Tolwani & Rasmussen (2015) |
| Plastic plants | Bare tanks | 5 fish l−1 | Single males; mixed sex pairs | 5 months | Survival; cortisol levels | EE prevented deaths from fighting; higher cortisol levels among pairs on day 5 but lower levels by day 10 so no overall reduction in cortisol | Keck et al. (2015) |
| Sand, artificial plant, artificial rock formation | Barren or enriched housing from hatching | 20 fish in bare tank; 24 fish in enriched tank | Single fish | 6, 12, and 24 months | Black and white preference test; inhibitory avoidance test; expression of neuroplasticity; gene expression | EE decreased anxiety‐like behaviour and increased exploration; reduced inhibitory avoidance in 6‐ and 12‐month‐old fish, but not in 24‐month‐old fish; differences in gene expression at 6 months but not at 24 months; delayed inhibitory avoidance at 24 months compared with 6 months. | Manuel et al. (2015) |
| Plastic grass, plastic leaves | Bare tanks | Groups of 30 | Pairs | 90–180 dpf; 0–6 dpf | Fertility, fecundity, survivorship | More eggs spawned on plastic grass than on plastic leaves; no effect on fry survivorship | Wafer et al. (2016) |
| Flowing water, plastic plants | Wild‐caught fish held in bare tanks for 3 months prior to study | Groups of 6 (3 males, 3 females) for 1 month prior to study | Groups of 6 (3 males, 3 females) | Unreported | Shoal cohesion, aggression, activity level | Flowing water resulted in less cohesive, more aggressive, more active groups. Effect not exaggerated by turbulence. Fish more active and aggressive in complex tank. | Suriyampola et al. (2017) |
| Auditory enrichment (classical music) | Unreported | Unreported | Groups of 3 in 3 l tanks | 1 year | Novel tank and light–dark tests; whole‐body cortisol; gene expression | Fish with EE were less anxious, less active; decreased levels of pro‐inflammatory cytokines and increased activity of some CNS genes; no effect on whole‐body cortisol | Barcellos et al. (2018) |
| Gravel, plastic object, plastic plants | Unknown; fish obtained from pet shop | Group of 96 in 16 l tank for 15 days prior to study | Single fish | Adults | Effects of unpredictable chronic stress | EE reduced effects of stress on behaviour and cortisol, and prevented effects on reactive oxygen species levels | Marcon et al. (2018) |
| Seascape backing paper, artificial plant, plant pot | Bare tanks | Not reported | Groups of 10 (5 males, 5 females) | Adults | Aggression, fertilisation success, growth | EE increased aggression over time; EE fish were shorter; no effect on mass or fertilisation success | Woodward et al. (2019) |
| Above‐tank shade; plastic plants | Tanks enriched with gravel and plants | Mixed‐sex groups of 36 in 54 l tanks | Unsexed groups of 3 from same housing tank | Adults | Preference | No preference for shade or plants | Jones et al. (2019) |
| Plastic plants, sand substrate, flowing water | Unknown; fish obtained from pet shop | Groups of 8 (4 males, 4 females) for 2 weeks prior to study | Groups of 8 (4 males, 4 females) | 12 months | Preference | Fish preferred EE plus flowing water, avoided flowing water only and plain zones, EE plus exercise more important than either factor alone | DePasquale et al. (2019) |
| Plastic plants, marbles, mesh strips, PVC pipe, various images, mirrored paper, sight of conspecifics | Not reported | Group housed at density of 5 fish l−1 | Single fish | 6–12 months | Preference | Fish preferred mirrored paper and sight of conspecifics | Krueger et al. (2020) |
CNS, central nervous system; dpf, days post‐fertilisation; PVC, polyvinyl chloride.
IV. SOCIAL ENVIRONMENT
The social environment is an important consideration when designing and maintaining housing and husbandry that best suit zebrafish. Zebrafish show a strong preference to associate with conspecifics, experience isolation stress when separated from the group, and recover better from an aversive event when housed in a group rather than as pairs or individuals (White et al., 2017). Zebrafish pay attention to social interactions between conspecifics. They learn to assess risk by observing the behaviour of others, and use social information to adjust their behaviour (Nunes et al., 2017). Adults exhibit a rich repertoire of social behaviour from shoaling and courtship to aggression. They react to social stimuli, such as the sight and smell of conspecifics, and consistently recognise familiar conspecifics (Spence & Smith, 2006; Oliveira, 2013; Madeira & Oliveira, 2017) while larvae as young as 6 days post‐fertilisation (dpf) can discriminate between kin and non‐kin (Hinz et al., 2013).
(1). Sociodynamics
The zebrafish's sociality, capacity for cognitive processing and decision making, and similar brain architecture to that of humans, makes them a powerful model for neuroscience (Kalueff, Echevarria & Stewart, 2014), psychiatric disorders such as schizophrenia (Gawel et al., 2019) and autism (Meshalkina et al., 2018), and the impact of social interaction and isolation on the young, developing brain (Tunbak et al., 2020). But zebrafish social behaviour is plastic and dependent upon their surroundings and the presence, number, age, sex ratio, and relatedness of conspecifics. Even a short period of isolation evokes changes in behaviour and brain activity (Tunbak et al., 2020) yet most behavioural tests are conducted on isolated fish (Pagnussat et al., 2013), in contrast to how zebrafish live in nature. This discrepancy could affect experimental results and lead to flawed conclusions or reproducibility problems.
The limited space in laboratory tanks restricts behaviours such as courtship, aggression and territoriality (Hutter et al., 2010), and prevents the fission and fusion dynamics seen in wild shoals. A typical laboratory tank lacks spawning sites and places to hide from aggressive conspecifics. Lack of variability in the age and size of fish in the tank restricts an individual's choice of shoal mates (Wright et al., 2006b). In addition, inadvertent and natural selection on laboratory fish may change their social behaviour compared to wild fish, decreasing the stimulus required to provoke agonistic responses and so increasing aggression (Ruzzante, 1994). Whether such changes matter to science depends on the type of research the fish are used for. An ecologist, for example, might study the richness of natural behaviour and how a species adapts to diverse environments, whereas a neuroscientist may concentrate on a single aspect of behaviour under artificial conditions. In both cases, the brain evolved to generate behaviour in complex natural environments, and behaviours of interest may not be captured under reduced conditions (Orger & de Polavieja, 2017).
In the wild, zebrafish aggregate in shoals, compete for food and spawning sites, cooperate in predator inspection, and reproduce with each other in a dynamic network of social interactions (Nunes et al., 2017; Fig. 1). In natural systems, zebrafish have been recorded in groups of up to 300 individuals (Suriyampola et al., 2015). It is likely that individuals transfer between shoals frequently and that shoal sizes change rapidly, as in other freshwater species (Krause et al., 2000). Simple social decisions drive the movements of individuals and determine which conspecifics an individual encounters (He, Maldonado‐Chaparro & Farine, 2019). Factors affecting such decisions include habitat structure and the spatial distribution of resources, predators, mates, and competitors (He et al., 2019). These factors are dynamic and create temporal variation in the social structure of wild populations (He et al., 2019).
Zebrafish are usually reared in social groups, but may then be housed in pairs for breeding or individually for behavioural tests or for procedures such as genotyping. Stress levels, as evidenced by water‐borne and whole body cortisol levels, are higher in fish housed singly compared to fish housed in groups (White et al., 2017), with isolated individuals taking longer to recover from stressful events, probably due to increased base‐level stress caused by social isolation (White et al., 2017). Pair‐housed fish show an even greater response to stressors, possibly because of the additional strain imposed by dominance or subservience in the housing situation (White et al., 2017). Overall, zebrafish are less stressed when housed in groups, suggesting that social housing may hasten recovery from stressors and enhance welfare and should be considered in experimental and housing design.
(2). Sex
Sex ratio in experimental zebrafish is often unreported, possibly because its ascertainment from external morphology is subjective and requires expertise. But there is growing evidence that zebrafish behaviour and responses to pharmacological treatments and pollutants are influenced by sex (Genario et al., 2020a). For example, when a novel tank test was used to measure behavioural reactions to two common anxiolytic drugs, both sexes responded to melatonin, but only males responded to diazepam (Genario et al., 2020b). Sex is therefore an important variable. Improved data reporting, including methods used to determine the sex of fish, could increase the replicability and reproducibility of experiments performed with zebrafish. The traditional method of determining the sex of zebrafish relies on visually identifying differences in colour, shape and behaviour between the sexes (Paull et al., 2008) and is highly subjective. Other methods include examination of the pectoral fins to reveal the presence or absence of breeding tubercle clusters in male fish (Dai et al., 2021); microscopic examination of dissected gonad tissue (Abozaid, Wessels & Hörstgen‐Schwark, 2011), which requires the fish to be killed; and machine learning strategies to identify the sex of an individual accurately based on body colour and pattern and caudal fin colour (Hosseini et al., 2019). Recent studies have looked for sex‐specific genetic markers in zebrafish, similar to those found in roach (Rutilus rutilus) (Lange et al., 2020), that will allow non‐destructive polymerase chain reaction (PCR) determination of sex from skin swabs or fin clips and improve sex determination in juvenile zebrafish (King, Gut & Zenker, 2020). However, given that genetic sex determination in zebrafish is dependent upon the fish line, and some lines have lost the sex chromosome, it is possible that a sex‐specific marker may not be attainable for some zebrafish lines.
Male and female zebrafish differ in their motivation, preferences and behaviour, driven by the potential fitness consequences of being male or female (Magurran & Garcia, 2000). Studies investigating female and male preferences for shoal size and for members of the opposite sex have not provided any consensus in outcomes (Delaney et al., 2002; Pyron, 2003; Ruhl & McRobert, 2005; Spence & Smith, 2006; Hutter et al., 2010; Paull et al., 2010), likely due to differences in study design. For example, experimental tank sizes ranged ftom 17 l to 1100 l; some tanks were furnished with plastic plants, others were not; study length varied from 10 min to 5 days; and focal fish were tagged in one experiment.
Sex ratios are highly variable in zebrafish (Wilson et al., 2014). The ratio in wild populations appears to be 1:1 (Spence et al., 2007), but stressful environmental conditions, increased temperature and hypoxia may alter the ratio in favour of males (Shang, Yu & Wu, 2006). In research facilities, there is evidence that rearing zebrafish at high densities increases the number of males in the population (Ribas et al., 2017) and that male‐bias increases aggression (Spence & Smith, 2005) with associated welfare implications, so most laboratories aim for a 1:1 ratio for long‐term maintenance and for practical reasons such as spawning requirements. Female bias can result in females becoming egg‐bound as the oviduct becomes blocked with degenerating eggs (Stevens et al., 2021), and females held in single‐sex groups prior to spawning have been found to produce fewer eggs (Osborne et al., 2016). For this information to be of practical use, further studies are needed to determine (i) how sex ratio mediates breeding strategy, reproductive output and social hierarchies, (ii) how these factors are affected by laboratory housing and experimental design, and (iii) how they interact to influence welfare and affect research results.
(3). Kinship
There is growing interest in using zebrafish to study a diverse range of social interactions, including altruism, group‐living, and self‐organisation (Robinson et al., 2019). The concept of kinship is central to understanding the evolution of social behaviours and new ways to estimate kinship in natural and domestic animal populations (Goudet, Kay & Weir, 2018) will be useful for understanding how zebrafish compete with conspecifics for access to resources.
Shoaling with kin can reduce competition among relatives, increase stability of dominance hierarchies, and improve foraging performance (Frommen & Bakker, 2004). In addition, females may use kin recognition to avoid mating with related males (Gerlach & Lysiak, 2006). Little is known about the social structure or genetic relatedness of wild zebrafish, due to the practical difficulties of such studies, and so kin‐biased behaviour of wild fish is inferred from laboratory studies. Zebrafish represent one of the best‐documented examples of kin recognition in vertebrates (Gerlach & Wullimann, 2021). There is strong evidence that zebrafish prefer to associate with kin, and do so by using olfactory and visual cues that develop by imprinting at 6 dpf (Hinz et al., 2013). As fish mature, their preference for kin changes: adult females prefer unrelated males while adult males show no preference for related or unrelated females (Gerlach & Wullimann, 2021).
Larvae reared in kin groups have been shown to grow significantly faster than larvae reared with non‐kin (Gerlach et al., 2007). In that study, similar shoaling behaviour and rates of aggression were observed in both groups, and the authors suggested that the difference in weight gain was due to increased stress levels in the larvae housed with non‐kin (Gerlach et al., 2007). Given that young zebrafish prefer to associate with kin (Gerlach & Lysiak, 2006) and have reduced growth (a proven indicator of stress in fish) when housed with non‐kin (Gerlach et al., 2007), the welfare of juvenile zebrafish might be improved by housing them in kin groups until sexual maturity, which occurs from 2 to 4 months depending on husbandry conditions.
(4). Stocking density
Zebrafish stocking density should be considered when interpreting study results. For example, intermittent crowding of adult zebrafish has been shown to increase cortisol secretion and reduce their capacity to renew heart tissue after injury (Sallin & Jaźwińska, 2016), with ramifications for studies of stress responses and tissue regeneration. Temporary crowding has been reported to improve zebrafish working memory (Fontana et al., 2021) with implications for neurobiology, spatial learning and memory research. Further, crowding embryos may lead to hypoxia, which has been shown to result in a higher percentage of malformations and a male‐biased F1 generation (Wu, 2009).
Recommended stocking densities from. research studies range from 4 to 10 fish per litre (Lawrence & Mason, 2012; Alestrom et al., 2019; Cockington, 2020) while manufacturers of zebrafish housing systems recommend 6–15 fish per litre (Castranova et al., 2011). Respondents to an international survey of husbandry practices in research laboratories revealed that stocking densities in their establishments range from <1 to >5 fish per litre (Lidster et al., 2017). Density has been found to affect various aspects of physiology and behaviour in laboratory zebrafish and low as well as high stocking density can have a negative effect on fish welfare (Turnbull et al., 2005). Increased density has been shown to reduce survival and growth (Ribas et al., 2017) and egg batch size (Spence & Smith, 2005) but not in all cases (Castranova et al., 2011). Stress levels have been shown to be elevated by both high and low stocking densities (Spagnoli, Lawrence & Kent, 2016). Furthermore, high stocking density causes invasion of individuals’ space and a reduction in the duration of sleep, with negative implications for welfare (Ali & Nicholson, 2018). Changes in aggression, breeding behaviour and foraging behaviour have been linked with stocking density. Aggression increases when zebrafish are held in small groups of six or less (Paull et al., 2010). Density also interacts with tank size and availability of spawning sites to affect courtship and breeding behaviour (Spence & Smith, 2005; Hutter et al., 2010). Density too affects foraging behaviour by reducing the effectiveness of aggression in excluding competitors from food patches (Gillis & Kramer, 1987). Increased density furthermore creates practical welfare issues. At high densities, it becomes increasingly difficult to observe and manage the health of fish or to remove sick fish. Further investigations into optimum stocking densities are needed to define the components of stocking density and social hierarchy that affect welfare, provide insight for researchers, and guide improvements to welfare.
V. FUTURE DIRECTIONS
Past and present drivers for housing laboratory fish are maximising growth and reproduction in a small space. Except for water quality and diet, other factors that may affect welfare have been more of an afterthought rather than a major consideration in tank and facility design. As we learn more about husbandry‐related effects on science, welfare is set to become more integral to research needs. We have optimised the production of zebrafish from eggs to breeding adults in the least amount of time and managed our fish to produce as many eggs as possible, but we have not engaged with aspects of welfare to help inform, for example, tank and facility design to best fit scientific needs.
The use of zebrafish as a research model to study human disease, develop new therapies, understand environmental health and assess the safety of chemicals continues to grow (https://apps.webofknowledge.com) with additional fields of research, such as neuroscience, behavioural science, and epigenetics, also now widely employing embryonic, larval and adult zebrafish as study models. Research questions using zebrafish include those requiring accurate measurements of subtle and sensitive endpoints which are likely to be influenced by the housing and social environments in which experimental zebrafish are produced and maintained. Challenges for future research to help ensure consistent, high‐quality experimental research with zebrafish requires that they are maintained under the best possible practicable conditions for their welfare and this in turn will help reduce the numbers of animals required for any given purpose as experimental repeatability will inevitably be improved.
VI. CONCLUSIONS
Good animal welfare is widely accepted as being important for high‐quality science, but understanding and evaluating the welfare of zebrafish is challenging. Legislation and guidelines for their care differ from country to country, and there is no single litmus‐test measure of fish welfare. Indeed, in some cases it is unclear whether a particular measure is reliable.
To promote welfare for zebrafish, it is essential that housing and social conditions are tailored to the needs of the species and that features of its life history and ecology are considered when designing housing systems and interpreting study results.
This review identifies, in particular, the need for more fundamental knowledge of how zebrafish interact with the biotic and abiotic features of their natural environment and which of these features are most important for their welfare.
More focus is needed on what captive zebrafish need by way of housing and social interaction to ensure they are physiologically and behaviourally ‘best suited’ for the research they are purposed for. Improving zebrafish welfare is not a passive process. It needs to be continuously assessed, revised and implemented, while taking into account the competing priorities of researchers and animal care staff.
As a research commmunity using zebrafish as an experimental model we also have a duty to increase our knowledge and understanding of welfare to enable evidence‐based decisions on how best to improve the quality of life for laboratory zebrafish.
VII. ACKNOWLEDGEMENTS
This research was funded by the University of Exeter.
REFERENCES
- Abdollahpour, H. , Falahatkar, B. , Jafari, N. & Lawrence, C. (2020a). Effect of stress severity on zebrafish (Danio rerio) growth, gonadal development and reproductive performance: do females and males respond differently? Aquaculture 522, 1–8. [Google Scholar]
- Abdollahpour, H. , Falahatkar, B. & Lawrence, C. (2020b). The effect of photoperiod on growth and spawning performance of zebrafish, Danio rerio . Aquaculture Reports 17, 1–5. [Google Scholar]
- Abozaid, H. , Wessels, S. & Hörstgen‐Schwark, G. (2011). Effect of rearing temperatures during embryonic development on the phenotypic sex in zebrafish (Danio rerio). Sexual Development 5, 259–265. [DOI] [PubMed] [Google Scholar]
- Adatto, I. , Krug, L. & Zon, L. I. (2016). The red light district and its effects on zebrafish reproduction. Zebrafish 13, 226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alestrom, P. , D'Angelo, L. , Midtlyng, P. J. , Schorderet, D. F. , Schulte‐Merker, S. , Sohm, F. & Warner, S. (2019). Zebrafish: housing and husbandry recommendations. Laboratory Animals 0, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, M. K. & Nicholson, H. L. (2018). Increasing zebrafish (Danio rerio) numbers in a limited tank space reduces night‐time fish sleep‐like state and and induces aggressive behaviour. World Journal of Depression and Anxiety 1, 1–6. [Google Scholar]
- Angiulli, E. , Pagliara, V. , Cioni, C. , Frabetti, F. , Pizzetti, F. , Alleva, E. & Toni, M. (2020). Increase in environmental temperature affects exploratory behaviour, anxiety and social preference in Danio rerio . Scientific Reports 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam, M. , Raja, M. , Vijayakumar, C. , Malaiammal, P. & Mayden, R. L. (2013). Natural history of zebrafish (Danio rerio) in India. Zebrafish 10, 1–14. [DOI] [PubMed] [Google Scholar]
- Barcellos, H. H. A. , Koakoski, G. , Chaulet, F. , Kirsten, K. S. , Kreutz, L. C. , Kalueff, A. V. & Barcellos, L. J. G. (2018). The effects of auditory enrichment on zebrafish behavior and physiology. PeerJ 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basquill, S. P. & Grant, J. W. A. (1998). An increase in habitat complexity reduces aggression and monopolization of food by zebra fish (Danio rerio). Canadian Journal of Zoology 76, 770–772. [Google Scholar]
- Bennett, J. M. , Sunday, J. , Calosi, P. , Villalobos, F. , Martínez, B. , Molina‐Venegas, R. , Araújo, M. B. , Algar, A. C. , Clusella‐Trullas, S. , Hawkins, B. A. , Keith, S. A. , Kühn, I. , Rahbek, C. , Rodríguez, L. , Singer, A. , et al. (2021). Evolution of critical thermal limits of life on Earth. Nature Communications 12, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava, Y. (2018). Open‐design recirculating systems for zebrafish culture. Aquacultural Engineering 81, 71–79. [Google Scholar]
- Bhat, A. , Greulich, M. M. & Martins, E. P. (2015). Behavioral plasticity in response to environmental manipulation among zebrafish (Danio rerio) populations. PLoS One 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisen, A. M. Z. , Amstrup, J. , Novak, I. & Grosell, M. (2003). Sodium and chloride transport in soft water and hard water acclimated zebrafish (Danio rerio). Biochimica et Biophysica Acta 1618, 207–218. [DOI] [PubMed] [Google Scholar]
- Boltaña, S. , Rey, S. , Roher, N. , Vargas, R. , Huerta, M. , Huntingford, F. A. , Goetz, F. W. , Moore, J. , Garcia‐Valtanen, P. , Estepa, A. & MacKenzie, S. (2013). Behavioural fever is a synergic signal amplifying the innate immune response. Proceedings of the Royal Society B: Biological Sciences 280, e20131381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco, P. , Boavida, I. , Santos, J. M. , Pinheiro, A. & Ferreira, M. T. (2013). Boulders as building blocks: improving habitat and river connectivity for stream fish. Ecohydrology 6, 627–634. [Google Scholar]
- Brand, M. , Granato, M. & Nüsslein‐Volhard, C. (2002). Keeping and raising zebrafish. In Zebrafish: A Practical Approach (eds Nüsslein‐Volhard C. and Dahm R.), pp. 7–38. Oxford University Press, Oxford. [Google Scholar]
- Brown, A. A. , Spetch, M. L. & Hurd, P. L. (2007). Growing in circles: rearing environment alters spatial navigation in fish. Psychological Science 18, 569–573. [DOI] [PubMed] [Google Scholar]
- Brydges, N. M. , Boulcott, P. , Ellis, T. & Braithwaite, V. A. (2009). Quantifying stress responses induced by different handling methods in three species of fish. Applied Animal Behaviour Science 116, 295–301. [Google Scholar]
- Carbia, P. S. & Brown, C. (2019). Environmental enrichment influences spatial learning ability in captive‐reared intertidal gobies (Bathygobius cocosensis). Animal Cognition 22, 89–98. [DOI] [PubMed] [Google Scholar]
- Castranova, D. , Lawton, A. , Lawrence, C. , Baumann, D. P. , Best, J. , Coscolla, J. , Doherty, A. , Ramos, J. , Hakkesteeg, J. , Wang, C. , Wilson, C. , Malley, J. & Weinstein, B. M. (2011). The effect of stocking densities on reproductive performance in laboratory zebrafish (Danio rerio). Zebrafish 8, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Poskanzer, K. E. , Freeman, M. R. & Monk, K. R. (2020). Live‐imaging of astrocyte morphogenesis and function in zebrafish neural circuits. Nature Neuroscience 23, 1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, T. S. , Pandolfo, L. M. , Marshall, C. M. , Mitra, A. K. & Schech, J. M. (2018). Body condition scoring for adult zebrafish (Danio rerio). Journal of the American Association for Laboratory Animal Science 57, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemence, M. (2018). Public Attitudes to Animal Research in 2018. London: Ipsos/Department for Business, Energy and Industrial Strategy. [Google Scholar]
- Cockington, J. (2020). Aquatic housing. In The Zebrafish in Biomedical Research (eds Cartner S. C., Eisen J. S., Farmer S. C., Guillemin K. J., Kent M. L. and Sanders G. E.), pp. 279–298. Academic Press, London. [Google Scholar]
- Collymore, C. , Crim, M. J. & Lieggi, C. (2016). Recommendations for health monitoring and reporting for zebrafish research facilities. Zebrafish 13, S138–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collymore, C. , Tolwani, R. J. & Rasmussen, S. (2015). The behavioral effects of single housing and environmental enrichment on adult zebrafish (Danio rerio). Journal of the American Association for Laboratory Animal Science 54, 280–285. [PMC free article] [PubMed] [Google Scholar]
- Council of the European Union (2010). Council Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union L276, 33–79. [Google Scholar]
- Coutant, C. C. (2006). Thermal effects on fish ecology. In Encyclopedia of Science and Engineering, Vol. I (eds Pfafflin J. R. and Ziegler E. N.), pp. 1146–1151. Taylor & Francis, Boca Raton. [Google Scholar]
- Dai, X. , Pu, D. , Wang, L. , Cheng, X. , Liu, X. , Yin, Z. & Wang, Z. (2021). Emergence of breeding tubercles and puberty onset in male zebrafish: evidence for a dependence on body growth. Journal of Fish Biology 99, 1–8. [DOI] [PubMed] [Google Scholar]
- Daskalova, A. (2019). Farmed fish welfare: stress, post‐mortem muscle metabolism, and stress‐related meat quality changes. International Aquatic Research 11, 113–124. [Google Scholar]
- Dawkins, M. S. (1998). Evolution and animal welfare. The Quarterly Review of Biology 73, 305–328. [DOI] [PubMed] [Google Scholar]
- de Abreu, M. S. , Giacomini, A. C. V. V. , Genario, R. , dos Santos, B. E. , Marcon, L. , Demin, K. A. & Kalueff, A. V. (2020). The impact of housing environment color on zebrafish anxiety‐like behavioral and physiological (cortisol) responses. General and Comparative Endocrinology 294, 113499. [DOI] [PubMed] [Google Scholar]
- Deakin, A. G. , Buckley, J. , AlZu'bi, H. S. , Cossins, A. R. , Spencer, J. W. , Al'Nuaimy, W. , Young, I. S. , Thomson, J. S. & Sneddon, L. U. (2019). Automated monitoring of behaviour in zebrafish after invasive procedures. Scientific Reports 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekens, M. P. S. & Whitmore, D. (2008). Autonomous onset of the circadian clock in the zebrafish embryo. EMBO Journal 27, 2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, M. , Follet, C. , Ryan, N. , Hanney, N. , Lusk‐Yablick, J. & Gerlach, G. (2002). Social interaction and distribution of female zebrafish (Danio rerio) in a large aquarium. Biological Bulletin 203, 240–241. [DOI] [PubMed] [Google Scholar]
- DePasquale, C. , Fettrow, S. , Sturgill, J. & Braithwaite, V. A. (2019). The impact of flow and physical enrichment on preferences in zebrafish. Applied Animal Behaviour Science 215, 77–81. [Google Scholar]
- DePasquale, C. & Leri, J. (2018). The influence of exercise on anxiety‐like behavior in zebrafish (Danio rerio). Behavioural Processes 157, 638–644. [DOI] [PubMed] [Google Scholar]
- Dhanasiri, A. K. S. , Fernandes, J. M. O. & Kiron, V. (2013). Acclimation of zebrafish to transport stress. Zebrafish 10, 87–98. [DOI] [PubMed] [Google Scholar]
- Dimitriadi, A. , Beis, D. , Arvanitidis, C. , Adriaens, D. & Koumoundouros, G. (2018). Developmental temperature has persistent, sexually dimorphic effects on zebrafish cardiac anatomy. Scientific Reports 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, T. , Berrill, I. , Lines, J. , Turnbull, J. F. & Knowles, T. G. (2012a). Mortality and fish welfare. Fish Physiology and Biochemistry 38, 189–199. [DOI] [PubMed] [Google Scholar]
- Ellis, T. , Yildiz, H. Y. , López‐Olmeda, J. , Spedicato, M. T. , Tort, L. , Overli, O. & Martins, C. I. M. (2012b). Cortisol and finfish welfare. Fish Physiology and Biochemistry 38, 163–188. [DOI] [PubMed] [Google Scholar]
- Engeszer, R. E. , Patterson, L. B. , Rao, A. A. & Parichy, D. M. (2007). Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 4, 21–38. [DOI] [PubMed] [Google Scholar]
- Espmark, A. M. , Kolarevic, J. , Asgard, T. & Terjesen, B. F. (2017). Tank size and fish management history matters in experimental design. Aquaculture Research 48, 2876–2894. [Google Scholar]
- European commission, directorate‐general for environment Caring for animals aiming for better science: Directive 2010/63/EU on protection of animals used for scientific purposes: education and training framework. Publications Office, Brussels
- Evans, J. E. , Cuthill, I. C. & Bennett, A. T. D. (2006). The effect of flicker from fluorescent lights on mate choice in captive birds. Animal Behaviour 72, 393–400. [Google Scholar]
- Fontana, B. D. , Gibbon, A. J. , Cleal, M. , Sudwarts, A. , Pritchett, D. , Miletto Petrazzini, M. E. , Brennan, C. H. & Parker, M. O. (2021). Moderate early life stress improves adult zebrafish (Danio rerio) working memory but does not affect social and anxiety‐like responses. Developmental Psychobiology 63, 54–64. [DOI] [PubMed] [Google Scholar]
- Forsatkar, M. N. , Safari, O. & Boiti, C. (2017). Effects of social isolation on growth, stress response, and immunity of zebrafish. Acta Ethologica 20, 255–261. [Google Scholar]
- Franks, B. , Graham, C. & von Keyserlingk, M. A. G. (2018). Is heightened‐shoaling a good candidate for positive emotional behavior in zebrafish? Animals 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommen, J. G. & Bakker, T. C. M. (2004). Adult three‐spined sticklebacks prefer to shoal with familiar kin. Behaviour 141, 1401–1409. [Google Scholar]
- Gawel, K. , Banono, N. S. , Michalak, A. & Esguerra, C. V. (2019). A critical review of zebrafish schizophrenia models: time for validation? Neuroscience and Biobehavioral Reviews 107, 6–22. [DOI] [PubMed] [Google Scholar]
- Genario, R. , de Abreu, M. S. , Giacomini, A. C. V. V. , Demin, K. A. & Kalueff, A. V. (2020a). Sex differences in behavior and neuropharmacology of zebrafish. European Journal of Neuroscience 52, 2586–2603. [DOI] [PubMed] [Google Scholar]
- Genario, R. , Giacomini, A. C. V. V. , de Abreu, M. S. , Marcon, L. , Demin, K. A. & Kalueff, A. V. (2020b). Sex differences in adult zebrafish anxiolytic‐like responses to diazepam and melatonin. Neuroscience Letters 714, e134548. [DOI] [PubMed] [Google Scholar]
- Gerlach, G. , Hodgins‐Davis, A. , MacDonald, B. & Hannah, R. C. (2007). Benefits of kin association: related and familiar zebrafish larvae (Danio rerio) show improved growth. Behavioral Ecology and Sociobiology 61, 1765–1770. [Google Scholar]
- Gerlach, G. & Lysiak, N. (2006). Kin recognition and inbreeding avoidance in zebrafish, Danio rerio, is based on phenotype matching. Animal Behaviour 71, 1371–1377. [Google Scholar]
- Gerlach, G. & Wullimann, M. F. (2021). Neural pathways of olfactory kin imprinting and kin recognition in zebrafish. Cell and Tissue Research 383, 271–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai, R. (2018). Reproducibility and replicability in zebrafish behavioral neuroscience research. Pharmacology Biochemistry and Behavior 178, 30–38. [DOI] [PubMed] [Google Scholar]
- Giacomini, A. C. V. V. , de Abreu, M. S. , Koakoski, G. , Idalencio, R. , Kalichak, F. , Oliveira, T. A. , da Rosa, J. G. S. & Gusso, D. (2015). My stress, our stress: blunted cortisol response to stress in isolated housed zebrafish. Physiology & Behavior 139, 182–187. [DOI] [PubMed] [Google Scholar]
- Gilbert, M. J. H. , Zerulla, T. C. & Tierney, K. B. (2014). Zebrafish (Danio rerio) as a model for the study of aging and exercise: physical ability and trainability decrease with age. Experimental Gerontology 50, 106–113. [DOI] [PubMed] [Google Scholar]
- Gillis, D. M. & Kramer, D. L. (1987). Ideal interference distributions: population density and patch use by zebrafish. Animal Behaviour 35, 1875–1882. [Google Scholar]
- Goodwin, N. , Westall, L. , Karp, N. A. , Hazlehurst, D. , Kovacs, C. , Keeble, R. , Thompson, P. , Collins, R. & Bussell, J. (2016). Evaluating and optimizing fish health and welfare during experimental procedures. Zebrafish 13, S127–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goolish, E. M. , Okutake, K. & Lesure, S. (1999). Growth and survivorship of larval zebrafish Danio rerio on processed diets. North American Journal of Aquaculture 61, 189–198. [Google Scholar]
- Goudet, J. , Kay, T. & Weir, B. S. (2018). How to estimate kinship. Molecular Ecology 27, 4121–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, I. M. & Dill, L. M. (2002). Monopolization of food by zebrafish (Danio rerio) increases in risky habitats. Canadian Journal of Zoology 80, 2164–2169. [Google Scholar]
- Hamm, J. T. , Ceger, P. , Allen, D. , Stout, M. , Maull, E. A. , Baker, G. , Zmarowski, A. , Padilla, S. , Perkins, E. , Planchart, A. , Stedman, D. , Tal, T. , Tanguay, R. L. , Volz, D. C. , Wilbanks, M. S. , et al. (2019). Characterizing sources of variability in zebrafish embryo screening protocols. Altex 36, 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer, H. S. (2020). Water quality for zebrafish culture. In The Zebrafish in Biomedical Research (eds Cartner S. C., Eisen J. S., Farmer S. C., Guillemin K. J., Kent M. L. and Sanders G. E.), pp. 321–335, First Edition. London: Academic Press. [Google Scholar]
- Harper, C. & Lawrence, C. (2012). The Laboratory Zebrafish. CRC Press, Boca Raton. [Google Scholar]
- He, P. , Maldonado‐Chaparro, A. A. & Farine, D. R. (2019). The role of habitat configuration in shaping social structure: a gap in studies of animal social complexity. Behavioral Ecology and Sociobiology 73, 1–14. [Google Scholar]
- Helfman, G. S. (1993). Fish behaviour by day, night and twilight. In Behaviour of Teleost Fishes (ed. Pitcher T. J.), pp. 479–512, Second Edition. Chapman & Hall, London. [Google Scholar]
- Hinz, C. , Kobbenbring, S. , Kress, S. , Sigman, L. , Müller, A. & Gerlach, G. (2013). Kin recognition in zebrafish, Danio rerio, is based on imprinting on olfactory and visual stimuli. Animal Behaviour 85, 925–930. [Google Scholar]
- Home Office (2014a). Code of practice for the housing and care of animals bred, supplied or used for scientific purposes.
- Home Office (2014b). Guidance on the operation of the Animals (Scientific Procedures) Act 1986.
- Hosseini, S. , Simianer, H. , Tetens, J. , Brenig, B. , Herzog, S. & Sharifi, A. R. (2019). Efficient phenotypic sex classification of zebrafish using machine learning methods. Ecology and Evolution 9, 13332–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, C. P. & Cheyne, S. M. (2019). Complexities of using wild versus captive activity budget comparisons for assessing captive primate welfare. Journal of Applied Animal Welfare Science 22, 78–96. [DOI] [PubMed] [Google Scholar]
- Huntingford, F. A. , Adams, C. , Braithwaite, V. A. , Kadri, S. , Pottinger, T. G. , Spandoe, P. & Turnbull, J. F. (2006). Current issues in fish welfare. Journal of Fish Biology 68, 332–372. [Google Scholar]
- Huntingford, F. A. & Kadri, S. (2008). Welfare and fish. In Fish Welfare (ed. Branson E. J.), pp. 19–29. Blackwell Publishing, Oxford. [Google Scholar]
- Huntingford, F. A. & Kadri, S. (2009). Taking account of fish welfare: lessons from aquaculture. Journal of Fish Biology 75, 2862–2867. [DOI] [PubMed] [Google Scholar]
- Hutter, S. , Penn, D. J. , Magee, S. & Zala, S. M. (2010). Reproductive behaviour of wild zebrafish (Danio rerio) in large tanks. Behaviour 147, 641–660. [Google Scholar]
- Inger, R. , Bennie, J. , Davies, T. W. & Gaston, K. J. (2014). Potential biological and ecological effects of flickering artificial light. PLoS One 9, e98631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirkof, P. , Rudeck, J. & Lewejohann, L. (2019). Assessing affective state in laboratory rodents to promote animal welfare—what is the progress in applied refinement research? Animals 9, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson, J. I. , Brockmark, S. & Näslund, J. (2014). Environmental effects on behavioural development consequences for fitness of captive‐reared fishes in the wild. Journal of Fish Biology 85, 1946–1971. [DOI] [PubMed] [Google Scholar]
- Jones, N. A. R. , Spence, R. , Jones, F. A. M. & Spence‐Jones, H. C. (2019). Shade as enrichment: testing preferences for shelter in two model fish species. Journal of Fish Biology 95, 1161–1165. [DOI] [PubMed] [Google Scholar]
- Jorgensen, L. G. (2020). Zebrafish as a model for fish diseases in aquaculture. Pathogens 9, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff, A. V. , Echevarria, D. J. & Stewart, A. M. (2014). Gaining translational momentum: more zebrafish models for neuroscience research. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 55, 1–6. [DOI] [PubMed] [Google Scholar]
- Keck, V. A. , Edgerton, D. S. , Hajizadeh, S. , Swift, L. L. , Dupont, W. D. , Lawrence, C. & Boyd, K. L. (2015). Effects of habitat complexity on pair‐housed zebrafish. Journal of the American Association for Laboratory Animal Science 54, 378–383. [PMC free article] [PubMed] [Google Scholar]
- Killen, S. S. , Marras, S. , Metcalfe, N. B. , McKenzie, D. J. & Domenici, P. (2013). Environmental stressors alter relationships between physiology and behaviour. Trends in Ecology and Evolution 28, 651–658. [DOI] [PubMed] [Google Scholar]
- King, A. C. , Gut, M. & Zenker, A. K. (2020). Shedding new light on early sex determination in zebrafish. Archives of Toxicology 94, 4143–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten, K. , Soares, S. M. , Koakoski, G. , Kreutz, L. C. & Barcellos, L. J. G. (2018). Characterization of sickness behavior in zebrafish. Brain, Behavior, and Immunity 73, 596–602. [DOI] [PubMed] [Google Scholar]
- Kistler, C. , Hegglin, D. , Würbel, H. & König, B. (2011). Preference for structured environment in zebrafish (Danio rerio) and checker barbs (Puntius oligolepis). Applied Animal Behaviour Science 135, 318–327. [Google Scholar]
- Kleinhappel, T. K. , Pike, T. W. & Burman, O. H. P. (2019). Stress‐induced changes in group behaviour. Scientific Reports 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, D. L. (1987). Dissolved oxygen and fish behavior. Environmental Biology of Fishes 18, 81–92. [Google Scholar]
- Krause, J. , Hoare, D. J. , Croft, D. , Lawrence, J. , Ward, A. , Ruxton, G. D. , Godin, J.‐G. J. & James, R. (2000). Fish shoal composition: mechanism and constraints. Proceedings of the Royal Society B: Biological Sciences 267, 2011–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger, L. D. , Thurston, S. E. , Kirk, J. , Elsaeidi, F. , Freeman, Z. T. , Goldman, D. , Lofgren, J. L. & Keller, J. M. (2020). Enrichment preferences of singly housed zebrafish (Danio rerio). Journal of the American Association for Laboratory Animal Science 59, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylov, V. V. , Izvekov, E. I. , Pavlova, V. V. , Pankova, N. A. & Osipova, E. A. (2021). Circadian rhythms in zebrafish (Danio rerio) behaviour and the sources of their variability. Biological Reviews 96, 785–797. [DOI] [PubMed] [Google Scholar]
- Labbé, C. , Robles, V. & Herraez, M. P. (2017). Epigenetics in fish gametes and early embryo. Aquaculture 472, 93–106. [Google Scholar]
- Lange, A. , Paris, J. R. , Gharbi, K. , Cézard, T. , Miyagawa, S. , Iguchi, T. , Studholme, D. J. & Tyler, C. R. (2020). A newly developed genetic sex marker and its application to understanding chemically induced feminisation in roach (Rutilus rutilus). Molecular Ecology Resources 20, 1007–1022. [DOI] [PubMed] [Google Scholar]
- Lara, R. A. & Vasconcelos, R. O. (2019). Characterization of the natural soundscape of zebrafish and comparison with the captive noise conditions. Zebrafish 16, 152–164. [DOI] [PubMed] [Google Scholar]
- Lara, R. A. & Vasconcelos, R. O. (2021). Impact of noise on development, physiological stress and behavioural patterns in larval zebrafish. Scientific Reports 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, C. & Mason, T. (2012). Zebrafish housing systems: a review of basic operating principles and considerations for design and functionality. ILAR Journal 53, 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learmonth, C. & Carvalho, A. P. (2015). Acute and chronic toxicity of nitrate to early life stages of zebrafish—setting nitrate safety levels for zebrafish rearing. Zebrafish 12, 305–311. [DOI] [PubMed] [Google Scholar]
- Lee, C. J. , Paull, G. C. & Tyler, C. R. (2019a). Effects of environmental enrichment on survivorship, growth, sex ratio and behaviour in laboratory maintained zebrafish Danio rerio . Journal of Fish Biology 94, 86–95. [DOI] [PubMed] [Google Scholar]
- Lee, C. J. , Tyler, C. R. & Paull, G. C. (2018). Can simple tank changes benefit the welfare of laboratory zebrafish Danio rerio? Journal of Fish Biology 92, 653–659. [DOI] [PubMed] [Google Scholar]
- Lee, C. J. , Tyler, C. R. & Paull, G. C. (2020). Geographic range and natural distribution. In The Zebrafish in Biomedical Research (eds Cartner S., Eisen J., Farmer S., Guillemin K., Kent M. and Sanders G.), pp. 41–56, First Edition. London: Academic Press. [Google Scholar]
- Lee, H. B. , Schwab, T. L. , Sigafoos, A. N. , Gauerke, J. L. , Krug, R. G. , Serres, M. R. , Jacobs, D. C. , Cotter, R. P. , Das, B. , Petersen, M. O. , Daby, C. L. , Urban, R. M. , Berry, B. C. & Clark, K. J. (2019b). Novel zebrafish behavioral assay to identify modifiers of the rapid, nongenomic stress response. Genes, Brain and Behavior 18, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidster, K. , Readman, G. D. , Prescott, M. J. & Owen, S. F. (2017). International survey on the use and welfare of zebrafish Danio rerio in research. Journal of Fish Biology 90, 1891–1905. [DOI] [PubMed] [Google Scholar]
- Lieggi, C. , Kalueff, A. V. , Lawrence, C. & Collymore, C. (2020). The influence of behavioral, social, and environmental factors on reproducibility and replicability in aquatic animal models. ILAR Journal 60, 270–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Olmeda, J. F. & Sánchez‐Vázquez, F. J. (2009). Zebrafish temperature selection and synchronization of locomotor activity circadian rhythm to ahemeral cycles of light and temperature. Chronobiology International 26, 200–218. [DOI] [PubMed] [Google Scholar]
- MacIntyre, C. M. , Ellis, T. , North, B. P. & Turnbull, J. F. (2008). The influences of water quality on the welfare of farmed rainbow trout: a review. In Fish Welfare (ed. Branson E. J.), pp. 150–184. Blackwell Publishing, Oxford. [Google Scholar]
- Madeira, N. & Oliveira, R. F. (2017). Long‐term social recognition memory in zebrafish. Zebrafish 14, 305–310. [DOI] [PubMed] [Google Scholar]
- Magurran, A. E. & Garcia, C. M. (2000). Sex differences in behaviour as an indirect consequence of mating system. Journal of Fish Biology 57, 839–857. [Google Scholar]
- Maia, C. M. & Volpato, G. L. (2016). A history‐based method to estimate animal preference. Scientific Reports 6, e28328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manga, M. & Kirchner, J. W. (2000). Stress partitioning in streams by large woody debris. Water Resources Research 36, 2373–2379. [Google Scholar]
- Manuel, R. , Gorissen, M. , Stokkermans, M. , Zethof, J. , Ebbesson, L. O. E. , van de Vis, H. , Flik, G. & van den Bos, R. (2015). The effects of environmental enrichment and age‐related differences on inhibitory avoidance in zebrafish (Danio rerio Hamilton). Zebrafish 12, 152–165. [DOI] [PubMed] [Google Scholar]
- Marancik, D. , Collins, J. , Afema, J. & Lawrence, C. (2019). Exploring the advantages and limitations of sampling methods commonly used in research facilities for zebrafish health inspections. Laboratory Animals 53, 373–385. [DOI] [PubMed] [Google Scholar]
- Marcon, M. , Mocelin, R. , Benvenutti, R. , Costa, T. , Herrmann, A. P. , de Oliveira, D. L. , Koakoski, G. , Barcellos, L. J. G. & Piato, A. (2018). Environmental enrichment modulates the response to chronic stress in zebrafish. Journal of Experimental Biology 221, 1–7. [DOI] [PubMed] [Google Scholar]
- Martins, C. I. M. , Galhardo, L. , Noble, C. , Damsgård, B. , Spedicato, M. T. , Zupa, W. , Beauchaud, M. , Kulczykowska, E. , Massabuau, J. C. , Carter, T. , Planellas, S. R. & Kristiansen, T. (2012). Behavioural indicators of welfare in farmed fish. Fish Physiology and Biochemistry 38, 17–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, M. , Trevarrow, B. & Matthews, J. (2002). A virtual tour of the Guide for zebrafish users. Lab Animal 31, 34–40. [DOI] [PubMed] [Google Scholar]
- Meshalkina, D. A. , Kizlyk, M. N. , Kysil, E. V. , Collier, A. D. , Echevarria, D. J. , Abreu, M. S. , Barcellos, L. J. G. , Song, C. , Warnick, J. E. , Kyzar, E. J. & Kalueff, A. V. (2018). Zebrafish models of autism spectrum disorder. Experimental Neurology 299, 207–216. [DOI] [PubMed] [Google Scholar]
- Migaud, H. , Davie, A. & Taylor, J. F. (2010). Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. Journal of Fish Biology 76, 27–68. [DOI] [PubMed] [Google Scholar]
- Moorman, S. J. (2001). Development of sensory systems in zebrafish (Danio rerio). ILAR Journal 42, 292–298. [DOI] [PubMed] [Google Scholar]
- Morgan, R. , Sundin, J. , Finnøen, M. H. , Dresler, G. , Vendrell, M. M. , Dey, A. , Sarkar, K. & Jutfelt, F. (2019). Are model organisms representative for climate change research? Testing thermal tolerance in wild and laboratory zebrafish populations. Conservation Physiology 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, K. N. , Bauer, J. , Tallen, A. , Matthews, J. L. , Westerfield, M. & Varga, Z. M. (2011). Characterization and management of asymptomatic Mycobacterium infections at the Zebrafish International Resource Center. Journal of the American Association for Laboratory Animal Science 50, 675–679. [PMC free article] [PubMed] [Google Scholar]
- Murray, K. N. , Lains, D. & Spagnoli, S. T. (2020). Water quality and idiopathic diseases of laboratory zebrafish. In The Zebrafish in Biomedical Research (eds Cartner S. C., Eisen J. S., Farmer S. C., Guillemin K. J., Kent M. L. and Sanders G. E.), pp. 463–477, First Edition. London: Academic Press. [Google Scholar]
- Näslund, J. & Johnsson, J. I. (2014). Environmental enrichment for fish in captive environments: effects of physical structures and substrates. Fish and Fisheries 17, 1–30. [Google Scholar]
- National Health and Medical Research Council (2013). Australian Code for the Care and Use of Animals for Scientific Purposes, Eighth Edition. National Health and Medical Research Council, Canberra. [Google Scholar]
- National Research Council (2011). Guide for the Care and Use of Laboratory Animals, Eighth Edition. National Academies Press, Washington, DC. [Google Scholar]
- NC3Rs , BBSRC , Defra , MCR , NERC & Wellcome Trust (2015). Responsibility in the use of animals in bioscience research: expectations of the major research council and charitable funding bodies.
- Nonnis, S. , Angiulli, E. , Maffioli, E. , Frabetti, F. , Negri, A. , Cioni, C. , Alleva, E. , Romeo, V. , Tedeschi, G. & Toni, M. (2021). Acute environmental temperature variation affects brain protein expression, anxiety and explorative behaviour in adult zebrafish. Scientific Reports 11, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes, A. R. , Ruhl, N. , Winberg, S. & Oliveira, R. F. (2017). Social phenotypes in zebrafish. In The Rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish (ed. Kalueff A. V.), pp. 95–130, First Edition. Springer International Publishing, Cham. [Google Scholar]
- OECD (2013). Test No. 236: fish embryo acute toxicity (FET) test. In OECD Guidelines for the Testing of Chemicals, Section 2, pp. 1–22. OECD Publishing, Paris. [Google Scholar]
- Oldfield, R. G. (2011). Aggression and welfare in a common aquarium fish, the Midas cichlid. Journal of Applied Animal Welfare Science 14, 340–360. [DOI] [PubMed] [Google Scholar]
- Oliveira, R. F. (2013). Mind the fish: zebrafish as a model in cognitive social neuroscience. Frontiers in Neural Circuits 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orger, M. B. & de Polavieja, G. G. (2017). Zebrafish behavior: opportunities and challenges. Annual Review of Neuroscience 40, 125–147. [DOI] [PubMed] [Google Scholar]
- Osborne, N. , Paull, G. C. , Grierson, A. , Dunford, K. , Busch‐Nentwich, E. M. , Sneddon, L. U. , Wren, N. , Higgins, J. & Hawkins, P. (2016). Report of a meeting on contemporary topics in zebrafish husbandry and care. Zebrafish 13, 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat, N. , Piato, A. L. , Schaefer, I. C. , Blank, M. , Tamborski, A. R. , Guerim, L. D. , Bonan, C. D. , Vianna, M. R. M. & Lara, D. R. (2013). One for all and all for one: the importance of shoaling on behavioral and stress responses in zebrafish. Zebrafish 10, 338–342. [DOI] [PubMed] [Google Scholar]
- Palstra, A. P. & Planas, J. V. (2011). Fish under exercise. Fish Physiology and Biochemistry 37, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palstra, A. P. , Tudorache, C. , Rovira, M. , Brittijn, S. A. , Burgerhout, E. , van den Thillart, G. E. E. J. M. , Spaink, H. P. & Planas, J. V. (2010). Establishing zebrafish as a novel exercise model: swimming economy, swimming‐enhanced growth and muscle growth marker gene expression. PLoS Biology 5, e14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull, G. C. , Filby, A. L. , Giddins, H. G. , Coe, T. S. , Hamilton, P. B. & Tyler, C. R. (2010). Dominance hierarchies in zebrafish (Danio rerio) and their relationship with reproductive success. Zebrafish 7, 109–117. [DOI] [PubMed] [Google Scholar]
- Paull, G. C. , van Look, K. J. W. , Santos, E. M. , Filby, A. L. , Gray, D. M. , Nash, J. P. & Tyler, C. R. (2008). Variability in measures of reproductive success in laboratory‐kept colonies of zebrafish and implications for studies addressing population‐level effects of environmental chemicals. Aquatic Toxicology 87, 115–126. [DOI] [PubMed] [Google Scholar]
- Pereira, A. , Carvalho, A. P. , Cruz, C. & Saraiva, A. (2017). Histopathological changes and zootechnical performance in juvenile zebrafish (Danio rerio) under chronic exposure to nitrate. Aquaculture 473, 197–205. [Google Scholar]
- Poulin, R. (2013). Parasite manipulation of host personality and behavioural syndromes. Journal of Experimental Biology 216, 18–26. [DOI] [PubMed] [Google Scholar]
- Prescott, M. J. & Lidster, K. (2017). Improving quality of science through better animal welfare: the NC3Rs strategy. Lab Animal 46, 152–156. [DOI] [PubMed] [Google Scholar]
- Pyron, M. (2003). Female preferences and male‐male interactions in zebrafish.pdf. Canadian Journal of Fisheries and Aquatic Sciences 81, 122–125. [Google Scholar]
- Rabbane, M. G. , Rahman, M. M. & Hossain, M. A. (2016). Effects of stocking density on growth of zebrafish (Danio rerio, Hamilton, 1822). Bangladesh Journal of Zoology 44, 209. [Google Scholar]
- Ramsay, J. M. , Watral, V. , Schreck, C. B. & Kent, M. L. (2009). Husbandry stress exacerbates mycobacterial infection in adult zebrafish, Danio rerio (Hamilton). Journal of Fish Diseases 32, 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayani, K. , Lin, E. , Craig, C. , Lamothe, M. , Shafaattalab, S. , Gunawan, M. , Li, A. Y. , Hove‐Madsen, L. & Tibbits, G. F. (2018). Zebrafish as a model of mammalian cardiac function: optically mapping the interplay of temperature and rate on voltage and calcium dynamics. Progress in Biophysics and Molecular Biology 138, 69–90. [DOI] [PubMed] [Google Scholar]
- Reed, B. & Jennings, M. (2011). Guidance on the Housing and Care of Zebrafish Danio rerio. RSPCA, Horsham. [Google Scholar]
- Rees, B. B. , Sudradjat, F. A. & Love, J. W. (2001). Acclimation to hypoxia increases survival time of zebrafish, Danio rerio, during lethal hypoxia. Journal of Experimental Zoology 289, 266–272. [DOI] [PubMed] [Google Scholar]
- Rey, S. , Digka, N. & Mackenzie, S. (2015). Animal personality relates to thermal preference in wild‐type zebrafish, Danio rerio . Zebrafish 12, 243–249. [DOI] [PubMed] [Google Scholar]
- Rey, S. , Moiche, V. , Boltaña, S. , Teles, M. & MacKenzie, S. (2017). Behavioural fever in zebrafish larvae. Developmental and Comparative Immunology 67, 287–292. [DOI] [PubMed] [Google Scholar]
- Ribas, L. , Valdivieso, A. , Díaz, N. & Piferrer, F. (2017). Appropriate rearing density in domesticated zebrafish to avoid masculinization: links with the stress response. Journal of Experimental Biology 220, 1056–1064. [DOI] [PubMed] [Google Scholar]
- Robinson, K. J. , Bosch, O. J. , Levkowitz, G. , Busch, K. E. , Jarman, A. P. & Ludwig, M. (2019). Social creatures: model animal systems for studying the neuroendocrine mechanisms of social behaviour. Journal of Neuroendocrinology 31, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison, B. D. & Rowland, W. (2005). A potential model system for studying the genetics of domestication: behavioral variation among wild and domesticated strains of zebra danio (Danio rerio). Canadian Journal of Fisheries and Aquatic Sciences 62, 2046–2054. [Google Scholar]
- Rogers, L. , Sales, E. , Shamsi, S. , Kopf, R. K. & Freire, R. (2020). Aggressive encounters lead to negative affective state in fish. PLoS One 15, e0231330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira, M. , Borràs, D. M. , Marques, I. J. , Puig, C. & Planas, J. V. (2018). Physiological responses to swimming‐induced exercise in the adult zebrafish regenerating heart. Frontiers in Physiology 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl, N. & McRobert, S. P. (2005). The effect of sex and shoal size on shoaling behaviour in Danio rerio . Journal of Fish Biology 67, 1318–1326. [Google Scholar]
- Ruzzante, D. E. (1994). Domestication effects on aggressive and schooling behavior in fish. Aquaculture 120, 1–24. [Google Scholar]
- Sabet, S. S. , van Dooren, D. & Slabbekoorn, H. (2016a). Son et lumière: sound and light effects on spatial distribution and swimming behavior in captive zebrafish. Environmental Pollution 212, 480–488. [DOI] [PubMed] [Google Scholar]
- Sabet, S. S. , Wesdorp, K. , van Dooren, D. & Slabbekoorn, H. (2016b). Sound affects behavior of captive zebrafish: always consider the potential for acoustic effects on your laboratory tests. Proceedings of Meetings on Acoustics 27, 10010–10024. [Google Scholar]
- Sadoul, B. & Geffroy, B. (2019). Measuring cortisol, the major stress hormone in fishes. Journal of Fish Biology 94, 540–555. [DOI] [PubMed] [Google Scholar]
- Salena, M. G. , Turko, A. J. , Singh, A. , Pathak, A. , Hughes, E. , Brown, C. & Balshine, S. (2021). Understanding fish cognition: a review and appraisal of current practices. Animal Cognition 24, 395–406. [DOI] [PubMed] [Google Scholar]
- Sallin, P. & Jaźwińska, A. (2016). Acute stress is detrimental to heart regeneration in zebrafish. Open Biology 6, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, J. & Ryan, A. (2006). Developmental plasticity in the thermal tolerance of zebrafish Danio rerio . Journal of Fish Biology 69, 722–734. [Google Scholar]
- Schroeder, P. , Jones, S. , Young, I. S. & Sneddon, L. U. (2014). What do zebrafish want? Impact of social grouping, dominance and gender on preference for enrichment. Laboratory Animals 48, 328–337. [DOI] [PubMed] [Google Scholar]
- Schroeder, P. & Mocho, J. P. (2014). A veterinary perspective on laboratory zebrafish welfare. Fish Veterinary Journal 14, 34–43. [Google Scholar]
- Segner, H. , Sundh, H. , Buchmann, K. , Douxfils, J. , Sundell, K. S. , Mathieu, C. , Ruane, N. , Jutfelt, F. , Toften, H. & Vaughan, L. (2012). Health of farmed fish: its relation to fish welfare and its utility as welfare indicator. Fish Physiology and Biochemistry 38, 85–105. [DOI] [PubMed] [Google Scholar]
- Sfakianakis, D. G. , Leris, I. , Laggis, A. & Kentouri, M. (2011). The effect of rearing temperature on body shape and meristic characters in zebrafish (Danio rerio) juveniles. Environmental Biology of Fishes 92, 197–205. [Google Scholar]
- Shang, E. H. H. , Yu, R. M. K. & Wu, R. S. S. (2006). Hypoxia affects sex differentiation and development, leading to a male‐dominated population in zebrafish (Danio rerio). Environmental Science and Technology 40, 3118–3122. [DOI] [PubMed] [Google Scholar]
- Siccardi, A. J. , Garris, H. W. , Jones, W. T. , Moseley, D. B. , D'Abramo, L. R. & Watts, S. A. (2009). Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish 6, 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli, S. , Lawrence, C. & Kent, M. L. (2016). Stress in fish as model organisms. In Biology of Stress in Fish ‐ Fish Physiology (eds Schreck C. B., Tort L. and Brauner C. J.), pp. 541–564. Academic Press, London. [Google Scholar]
- Spence, R. , Fatema, M. K. , Ellis, S. , Ahmed, Z. F. & Smith, C. (2007). Diet, growth and recruitment of wild zebrafish in Bangladesh. Journal of Fish Biology 71, 304–309. [Google Scholar]
- Spence, R. , Fatema, M. K. , Reichard, M. , Huq, K. A. , Wahab, M. A. , Ahmed, Z. F. & Smith, C. (2006). The distribution and habitat preferences of the zebrafish in Bangladesh. Journal of Fish Biology 69, 1435–1448. [Google Scholar]
- Spence, R. , Gerlach, G. , Lawrence, C. & Smith, C. (2008). The behaviour and ecology of the zebrafish, Danio rerio . Biological Reviews 83, 13–34. [DOI] [PubMed] [Google Scholar]
- Spence, R. & Smith, C. (2005). Male territoriality mediates density and sex ratio effects on oviposition in the zebrafish, Danio rerio . Animal Behaviour 69, 1317–1323. [Google Scholar]
- Spence, R. & Smith, C. (2006). Mating preference of female zebrafish, Danio rerio, in relation to male dominance. Behavioral Ecology 17, 779–783. [Google Scholar]
- Stevens, C. H. , Croft, D. P. , Paull, G. C. & Tyler, C. R. (2017). Stress and welfare in ornamental fishes: what can be learned from aquaculture? Journal of Fish Biology 44, 1–20. [DOI] [PubMed] [Google Scholar]
- Stevens, C. H. , Reed, B. T. & Hawkins, P. (2021). Enrichment for laboratory zebrafish ‐ a review of the evidence and the challenges. Animals 11, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolov, A. N. & Sukhodolova, T. A. (2010). Effect of submerged aquatic plants on turbulence structure in a lowland river. Journal of Hydraulic Engineering 136, 434–446. [Google Scholar]
- Sundin, J. , Morgan, R. , Finnøen, M. H. , Dey, A. , Sarkar, K. & Jutfelt, F. (2019). On the observation of wild zebrafish (Danio rerio) in India. Zebrafish 16, 546–553. [DOI] [PubMed] [Google Scholar]
- Suriyampola, P. S. , Shelton, D. S. , Shukla, R. , Roy, T. , Bhat, A. & Martins, E. P. (2015). Zebrafish social behavior in the wild. Zebrafish 13, 1–8. [DOI] [PubMed] [Google Scholar]
- Suriyampola, P. S. , Sykes, D. J. , Khemka, A. & Shelton, D. S. (2017). Water flow impacts group behavior in zebrafish (Danio rerio). Behavioral Ecology 28, 94–100. [Google Scholar]
- Tal, T. , Yaghoobi, B. & Lein, P. J. (2020). Translational toxicology in zebrafish. Current Opinion in Toxicology 23–24, 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, S. L. T. , Handasyde, K. A. , Rault, J. L. & Mendl, M. (2020). Insensitivity to reward shifts in zebrafish (Danio rerio) and implications for assessing affective states. Animal Cognition 23, 87–100. [DOI] [PubMed] [Google Scholar]
- Teletchea, F. & Fontaine, P. (2014). Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish and Fisheries 15, 181–195. [Google Scholar]
- Thomson, J. S. , Al‐Temeemy, A. A. , Isted, H. , Spencer, J. W. & Sneddon, L. U. (2019). Assessment of behaviour in groups of zebrafish (Danio rerio) using an intelligent software monitoring tool, the chromatic fish analyser. Journal of Neuroscience Methods 328, 108433. [DOI] [PubMed] [Google Scholar]
- Thorington, L. (1985). Spectral, irradiance, and temporal aspects of natural and artificial light. Annals of the New York Academy of Sciences 453, 28–54. [DOI] [PubMed] [Google Scholar]
- Toni, M. , Manciocco, A. , Angiulli, E. , Alleva, E. , Cioni, C. & Malavasi, S. (2019). Review: assessing fish welfare in research and aquaculture, with a focus on European directives. Animal 13, 161–170. [DOI] [PubMed] [Google Scholar]
- Tsang, B. , Zahid, H. , Ansari, R. , Lee, R. , Partap, A. & Gerlai, R. (2017). Breeding zebrafish: a review of different methods and a discussion on standardization. Zebrafish 14, 561–573. [DOI] [PubMed] [Google Scholar]
- Tunbak, H. , Vazquez‐Prada, M. , Ryan, T. M. , Kampff, A. R. & Dreosti, E. (2020). Whole‐brain mapping of socially isolated zebrafish reveals that lonely fish are not loners. eLife 9, e55863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull, J. , Bell, A. , Adams, C. , Bron, J. & Huntingford, F. (2005). Stocking density and welfare of cage farmed Atlantic salmon: application of a multivariate analysis. Aquaculture 243, 121–132. [Google Scholar]
- Valdivieso, A. , Ribas, L. , Monleón‐Getino, A. , Orbán, L. & Piferrer, F. (2020). Exposure of zebrafish to elevated temperature induces sex ratio shifts and alterations in the testicular epigenome of unexposed offspring. Environmental Research 186, 109601. [DOI] [PubMed] [Google Scholar]
- Varga, Z. M. (2016). Aquaculture, husbandry, and shipping at the Zebrafish International Resource Center. In The Zebrafish: Genetics, Genomics and Transcriptomics (eds Detrich H. W., Westerfield M. and Zon L. I.), pp. 510–527, Fourth Edition. Academic Press, Cambridge. [DOI] [PubMed] [Google Scholar]
- Vettese, T. , Franks, B. & Jacquet, J. (2020). The great fish pain debate. Issues in Science and Technology 36, 49–53. [Google Scholar]
- Vila Pouca, C. & Brown, C. (2017). Contemporary topics in fish cognition and behaviour. Current Opinion in Behavioral Sciences 16, 46–52. [Google Scholar]
- Villamizar, N. , Vera, L. M. , Foulkes, N. S. & Sánchez‐Vázquez, F. J. (2014). Effect of lighting conditions on zebrafish growth and development. Zebrafish 11, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgin, A. D. , Yakovlev, O. V. , Demin, K. A. , de Abreu, M. S. , Rosemberg, D. B. , Meshalkina, D. A. , Alekseeva, P. A. , Friend, A. J. , Amstislavskaya, T. G. & Kalueff, A. V. (2018). Understanding the role of environmental enrichment in zebrafish neurobehavioral models. Zebrafish 15, 425–432. [DOI] [PubMed] [Google Scholar]
- Voslářová, E. , Pištěková, V. , Svobodová, Z. & Bedánová, I. (2008). Nitrite toxicity to Danio rerio: effects of subchronic exposure on fish growth. Acta Veterinaria Brunensis 77, 455–460. [PubMed] [Google Scholar]
- Wafer, L. N. , Jensen, V. B. , Whitney, J. C. , Gomez, T. H. , Flores, R. & Goodwin, B. S. (2016). Effects of evironmental enrichment on the fertility and fecundity of zebrafish (Danio rerio). Journal of the American Association for Laboratory Animal Science 55, 291–294. [PMC free article] [PubMed] [Google Scholar]
- Wan, J. & Goldman, D. (2016). Retina regeneration in zebrafish. Current Opinion in Genetics and Development 40, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch, E. B. , Jacoby, J. M. & May, C. W. (1998). Stream quality. In River Ecology and Management: Lessons From the Pacific Coastal Ecoregion (eds Naiman R. J. and Bilby R. E.), pp. 69–95, First Edition. Springer‐Verlag, New York. [Google Scholar]
- White, L. J. , Thomson, J. S. , Pounder, K. C. , Coleman, R. C. & Sneddon, L. U. (2017). The impact of social context on behaviour and the recovery from welfare challenges in zebrafish, Danio rerio . Animal Behaviour 132, 189–199. [Google Scholar]
- Wilkes, L. , Owen, S. F. , Readman, G. D. , Sloman, K. A. & Wilson, R. W. (2012). Does structural enrichment for toxicology studies improve zebrafish welfare? Applied Animal Behaviour Science 139, 143–150. [Google Scholar]
- Wilkins, A. J. , Nimmo‐Smith, I. , Slater, A. I. & Bedocs, L. (1989). Fluorescent lighting, headaches and eyestrain. Lighting Research & Technology 21, 11–18. [Google Scholar]
- Williams, T. D. , Readman, G. D. & Owen, S. F. (2009). Key issues concerning environmental enrichment for laboratory‐held fish species. Laboratory Animals 43, 107–120. [DOI] [PubMed] [Google Scholar]
- Wilson, C. , Dunford, K. , Nichols, C. , Callaway, H. , Hakkesteeg, J. & Wicks, M. (2013). Body condition scoring for laboratory zebrafish. Animal Technology and Welfare 12, 1–7. [Google Scholar]
- Wilson, C. A. , High, S. K. , McCluskey, B. M. , Amores, A. , Yan, Y. L. , Titus, T. A. , Anderson, J. L. , Batzel, P. , Carvan, M. J. , Schartl, M. & Postlethwait, J. H. (2014). Wild sex in zebrafish: loss of the natural sex determinant in domesticated strains. Genetics 198, 1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward, M. A. , Winder, L. A. & Watt, P. J. (2019). Enrichment increases aggression in zebrafish. Fishes 4, 1–13. [Google Scholar]
- Wright, D. , Nakamichi, R. , Krause, J. & Butlin, R. K. (2006a). QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio). Behavior Genetics 36, 271–284. [DOI] [PubMed] [Google Scholar]
- Wright, D. , Ward, A. J. W. , Croft, D. P. & Krause, J. (2006b). Social organization, grouping, and domestication in fish. Zebrafish 3, 141–155. [DOI] [PubMed] [Google Scholar]
- Wu, R. S. S. (2009). Effects of hypoxia on fish reproduction and development. In Fish Physiology (eds Richards J. G., Farrell A. P. and Brauner C. J.), pp. 79–141, First Edition. Academic Press, London. [Google Scholar]
- Yu, Y. , Chen, H. , Tuo, J. & Zhu, Y. (2011). Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthalmic Research 46, 80–87. [DOI] [PubMed] [Google Scholar]
- Zahangir, M. , Haque, F. , Mostakim, G. M. & Islam, M. S. (2015). Secondary stress responses of zebrafish to different pH: evaluation in a seasonal manner. Aquaculture Reports 2, 91–96. [Google Scholar]


