Abstract
Ipatasertib is a highly selective small‐molecule pan‐Akt inhibitor in clinical development. Ipatasertib is predominantly eliminated by the liver, and therefore, the effect of hepatic impairment on ipatasertib pharmacokinetics (PK) was evaluated. In this phase 1 open‐label, parallel group study, the PK of ipatasertib were evaluated in subjects with hepatic impairment based on both the Child‐Pugh and the National Cancer Institute Organ Dysfunction Working Group classification for hepatic impairment. A single dose of ipatasertib at 100 mg was administered and the PK was characterized in healthy subjects with normal hepatic function or mild, moderate, and severe hepatic impairment. Based on Child‐Pugh classification, subjects with moderate and severe hepatic impairment had an ≈2‐ and 3‐fold increase in systemic exposure (area under the plasma concentration–time curve from time 0 to infinity [AUC0‐∞]) to ipatasertib, respectively, compared to subjects with normal hepatic function. Systemic exposure (AUC0‐∞) to ipatasertib in subjects with mild hepatic impairment was comparable to that in subjects with normal hepatic function. In accordance with reduced clearance capacity, subjects with mild to severe hepatic impairment showed lower systemic exposure (AUC0‐∞) of ipatasertib metabolite M1 (G‐037720). Overall results were comparable between Child‐Pugh and National Cancer Institute Organ Dysfunction Working Group classification criteria. Based on the results from this study, no dosage adjustment is required for ipatasertib when treating patients with mild hepatic impairment, whereas a dose reduction would be recommended for subjects with moderate or severe hepatic impairment. Based on real‐world data analysis, ≈2% of the intended patient population is expected to need a modified dose due to moderate or severe hepatic impairment.
Keywords: Akt inhibitor, Child‐Pugh, hepatic impairment, ipatasertib, National Cancer Institute Organ Dysfunction Working Group (NCI‐ODWG)
Ipatasertib (GDC‐0068) is a pan Akt inhibitor that is currently under development for treatment of various cancers. Ipatasertib has been shown to exert its antiproliferative effects via selective binding to the active conformation of Akt and thereby inhibiting its kinase activity. 1 Preclinical studies involving cancer cell lines as well as xenograft models show promising effects of ipatasertib against phosphatase and tensin homolog–null and phosphatidylinositol‐4,5‐biphosphate 3‐kinase–mutated tumor models. 2
Ipatasertib 400 mg administered orally once a day is clinically under evaluation in combination with other molecules in phase 2 and phase 3 trials in breast and prostate cancer. 3 , 4 , 5 The clinical dose of 400 mg once daily was determined on the basis of the exposure‐response analysis conducted in a phase 2 study 6 that exhibited optimal benefit‐risk considerations at this dose. When administered at a 400‐mg dose in patients, ipatasertib is rapidly absorbed, with a median time to maximal concentration (tmax) of 1 hour. The median half‐life (t1/2) for ipatasertib is ≈45 hours. Ipatasertib is extensively metabolized, and its major metabolite, M1 (G‐037720), formed by the cytochrome P450 3A4 (CYP3A4) enzyme, is found circulating at exposures of ≈33% to 57% in monotherapy settings. 7 Ipatasertib is also a substrate of P‐glycoprotein (P‐gp). A human mass balance study conducted using radiolabeled ipatasertib demonstrated that ipatasertib is eliminated primarily via hepatic metabolism and only ≈19% of the total administered dose (≈8% of the parent drug) was eliminated in urine (data on file). Since hepatic elimination represents the predominant path of ipatasertib elimination, liver impairment has the potential to alter ipatasertib exposures. When a 100‐mg dose of ipatasertib was coadministered with itraconazole, a strong CYP3A4 and P‐gp inhibitor, in a drug‐drug interaction (DDI) study ipatasertib exposures were increased by ≈5‐fold. 8 Because of the level of DDI observed with itraconazole, the ipatasertib 100‐mg dose was selected for this hepatic impairment study.
In this study, we present the impact of hepatic impairment on pharmacokinetics (PK) of ipatasertib and its primary M1 metabolite based on Child‐Pugh classification. As the National Cancer Institute Organ Dysfunction Working Group (NCI‐ODWG) is often used by oncologists to evaluate hepatic function, we also conducted an analysis using NCI‐ODWG criteria for classifying subjects with hepatic impairment.
Methods
Study Design and Treatment
This study was conducted according to US Food and Drug Administration (FDA) regulations, the International Conference on Harmonization E6 Guideline for Good Clinical Practice, and applicable local, state, and federal laws. This study was conducted in accordance with the applicable US Code of Federal Regulations (CFR) governing the Protection of Human Subjects (21 CFR 50), Financial Disclosure by Clinical Investigators (21 CFR 54), institutional review boards (21 CFR 56), the Investigational New Drug Application (21 CFR 312), and Applications for FDA Approval to Market a New Drug (21 CFR 314). As such, these sections of US Title 21 CFR, along with the applicable International Conference on Harmonization Guidelines, are commonly known as Good Clinical Practice, which are consistent with the Declaration of Helsinki. The subject or the subject's legally authorized representative signed the consent forms before his or her participation in the study. The case history for each subject documented the informed consent process and that written informed consent was obtained before participation in the study.
This was an open‐label, multisite, single‐dose, parallel‐group study where the primary objective was to determine the PK of ipatasertib administered to subjects with mild, moderate, or severe hepatic impairment compared to demographically matched healthy subjects with normal hepatic function (trial registration ID: NCT03341884). The secondary objective of the study was to evaluate the safety and tolerability of ipatasertib in subjects with varying degrees of impaired hepatic dysfunction. The corresponding primary end point included area under the plasma concentration–time curve from time 0 to infinity (AUC0‐∞) and maximum concentration (Cmax) of ipatasertib in plasma, and the corresponding secondary end point included the incidence of treatment‐emergent adverse events (AEs) and serious AEs (SAEs), results from clinical laboratory evaluations, vital signs, and 12‐lead electrocardiograms (ECGs).
Subjects were assigned to cohorts based on their level of hepatic function according to the Child‐Pugh classification for the primary analysis. 9 , 10 For the secondary analysis, subjects were reclassified after the recruitment was completed, based on their level of hepatic function according to the NCI‐ODWG for Hepatic Dysfunction criteria as shown in Table S1. 11
On the morning of day 1, after at least an 8‐hour fast, a single oral dose of 100‐mg ipatasertib was administered with 240 mL of room temperature water, followed by a fast for at least 4 hours after dosing. The plasma samples were collected starting before dosing on day 1 and up to day 15 to measure ipatasertib and M1 metabolite concentrations.
Inclusion/Exclusion Criteria
Men (sterile or agreed to use contraception) and women (postmenopausal, surgically sterile, or agreed to use contraception) between 18 and 74 years of age, inclusive, considered to have mild, moderate, or severe hepatic impairment (clinically stable for at least 1 month before screening) classified by Child‐Pugh score (Table S1) of 5 to 6 (mild), 7 to 9 (moderate), or 10 to 15 (severe) were eligible for the study. Healthy subjects with normal hepatic function were recruited to match the subject participants with mild, moderate, or severe hepatic impairment in sex, age (±10 years), and body weight (±15%).
Key exclusion criteria included history of stomach or intestinal surgery or resection that would have potentially altered absorption and/or excretion of orally administered drugs; history or presence of an abnormal ECG; history of unstable diabetes mellitus (as evidenced by hemoglobin A1c ≥8.5% at screening); prior treatment with ipatasertib; or participation in any other investigational study drug trial in which receipt of an investigational study drug occurred within 5 half‐lives or 30 days, whichever was longer, before the first scheduled visit. Use of potent CYP3A4 inducers and inhibitors were not permitted within 14 days before the first scheduled visit and for the duration of the study. Similarly, use of any over‐the‐counter, nonprescription medication; alcohol‐, grapefruit‐, or caffeine‐containing foods or beverages; and nicotine‐containing products were restricted from use.
Additional exclusion criteria for hepatically impaired subjects included evidence of progressive liver disease that had worsened or was worsening within 1 month before the screening visit; evidence of hepatorenal syndrome; ascites that required paracentesis (within 3 months before the first scheduled visit, with the exception of diuretics); treatment for gastrointestinal bleeding within 12 months before the first scheduled visit; additional medication for hepatic encephalopathy within the 12 months (6 months for severe hepatic impairment) before the first scheduled visit; or total bilirubin levels >6 mg/dL (levels >6 mg/dL allowed at the discretion of the investigator in discussion with the sponsor and medical monitor).
Safety Plan
Safety assessments consisted of monitoring and recording AEs, including SAEs and AEs of special interest, laboratory assessments, vital signs, and ECGs.
Pharmacokinetic Sampling and Analysis
Plasma samples were collected before dosing and at 0.167, 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 36, 48, 72, 96, 120, 168, 216, and 336 hours after dosing. Plasma concentrations were determined by the Bioanalytical Department of Covance Laboratories, Inc. (Madison, Wisconsin) using a validated liquid chromatography–tandem mass spectrometry assay with lower limit of quantification of 0.463 and upper limit of quantification of 371 ng/mL for ipatasertib and lower limit of quantification of 0.500 and upper limit of quantification of 400 ng/mL for M1 metabolite. Stable labeled internal standards were used for both analytes. PK calculations were performed using commercial software Phoenix WinNonlin version 8.1 (Certara USA, Inc., Princeton, New Jersey).
Statistical Methodology
The primary analysis was to evaluate the PK of ipatasertib after a single dose in subjects with mild, moderate, or severe hepatic impairment (test), compared to subjects with normal hepatic function (reference). The primary PK parameters for ipatasertib and its M1 metabolite, AUC0‐∞, and Cmax, were log‐transformed and then analyzed using a mixed‐model analysis of variance, with hepatic impairment cohort assignment as a fixed factor. The least squares means of test and reference treatments obtained from the mixed model was back‐transformed to give geometric least squares means and 90% confidence intervals (CIs). All calculations were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina). Descriptive statistics (mean, median, minimum, maximum, standard deviation, geometric mean, and geometric coefficient of variation) were calculated for all PK parameters.
Hepatic Impairment in Routine Clinical Practice—Real World Data Analysis
To assess the potential prevalence of hepatic impairment in the real‐world setting, we conducted retrospective secondary‐data analyses using Flatiron Health electronic health records data, which includes data from ≈280 cancer clinics across the United States. The Flatiron Health database is a longitudinal database, comprising deidentified patient‐level structured and unstructured data, curated via technology‐enabled abstraction. 12 , 13 The majority of patients in the database originate from community oncology settings; relative community/academic proportions may vary depending on the study cohort. The aim was to quantify the prevalence of hepatic impairment prior to first‐line therapy in metastatic castrate‐resistant prostate cancer (mCRPC) and hormone receptor (HR)+/human epidermal growth factor receptor 2 (HER2)– metastatic breast cancer (mBC) indications.
All patients included in the metastatic prostate cancer and mBC cohorts were included. Patients were required to have been diagnosed with mCRPC between January 1, 2013, and May 31, 2020, to be included in the analysis cohort, and were included in the mBC cohort if they had been diagnosed with mBC between January 1, 2011, and May 31, 2020. Furthermore, HR+/HER2– breast cancer subtype was classified for patients who had a positive result for estrogen receptor and progesterone receptor and negative or equivocal result for HER2 within a 90‐day window before and after their metastatic diagnosis or within 90 days of their primary diagnosis if no results from the metastatic diagnosis window were available.
To assess hepatic impairment, patients were required to have bilirubin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) laboratory results, which were conducted and recorded as part of routine clinical practice, available 90 days before the start of first‐line therapy. For all patients with eligible laboratory values, hepatic impairment was classified according to the NCI‐ODWG hepatic dysfunction criteria as normal, mild, moderate, and severe (see Table S1). AST and ALT cutoffs were considered as 2.5 times the upper limit of normal (ULN), where ULN was defined as 40 U/L for AST and 50 U/L for ALT. High bilirubin was classified as >1.2 mg/dL (ie, ULN). Results, patient characteristics, and hepatic impairment prevalence estimates are presented as n (%) and mean (standard deviation), where applicable.
Results
Subjects
A total of 36 subjects were enrolled in the study, which included 13 healthy subjects with normal hepatic function, 8 subjects with mild hepatic impairment, 8 subjects with moderate hepatic impairment, and 7 subjects with severe hepatic impairment. The impairment status was based on the Child‐Pugh scores at screening. The demographics and baseline characteristics are listed in Table 1. Overall, the demographics and baseline characteristics were similar among the 3 hepatic impairment groups (mild, moderate, or severe) when compared to healthy subjects.
Table 1.
Summary of Demographics and Baseline Characteristics of Subjects Enrolled in the Hepatic Impairment Study
| Parameter | Category/Statistic | Healthy (N = 13) | Mild (N = 8) | Moderate (N = 8) | Severe (N = 7) |
|---|---|---|---|---|---|
| Sex, n (%) | Male | 8 (61.5) | 5 (62.5) | 5 (62.5) | 5 (71.4) |
| Female | 5 (38.5) | 3 (37.5) | 3 (37.5) | 2 (28.6) | |
| Race, n (%) | Black or African American | 2 (15.4) | 2 (25.0) | … | … |
| White | 11 (84.6) | 6 (75.0) | 7 (87.5) | 7 (100) | |
| Multiple | … | 1 (12.5) | … | … | |
| Ethnicity, n (%) | Hispanic or Latino | 5 (38.5) | 4 (50.0) | 1 (12.5) | 5 (71.4) |
| Not Hispanic or Latino | 8 (61.5) | 4 (50.0) | 7 (87.5) | 2 (28.6) | |
| Age, y | Mean (range) | 55 (44‐71) | 58 (49‐67) | 56 (43‐68) | 56 (46‐66) |
| Weight, kg | Mean (range) | 85.4 (65.1‐107.5) | 83.5 (57.0‐102.0) | 83.0 (66.0‐104.0) | 84.6 (69.6‐98.3) |
| Height, cm | Mean (range) | 172.0 (160.0‐190.0) | 169.7 (153.0‐182.0) | 166.4 (155.0‐176.5) | 169.3 (155.0‐185.0) |
| BMI, kg/m2 | Mean (range) | 28.8 (21.3‐35.2) | 29.1 (20.4‐35.0) | 29.8 (25.9‐34.3) | 29.8 (22.7‐37.5) |
BMI, body mass index.
Ipatasertib and M1 Pharmacokinetics
The mean ipatasertib and M1 metabolite plasma concentration–time profiles for the 3 hepatic impairment groups and healthy subjects based on Child‐Pugh scores are presented in Figure 1. Tables 2 and 3 provide a summary of ipatasertib and M1 PK parameters and the statistical analysis of the effect of hepatic impairment on PK parameters for ipatasertib, respectively.
Figure 1.
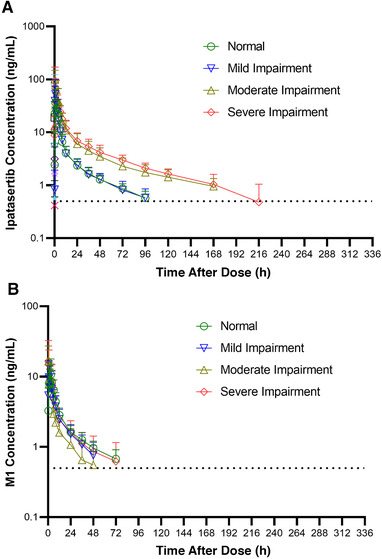
Mean plasma concentration vs time profiles of (A) ipatasertib and (B) M1 following a single oral dose of 100‐mg ipatasertib administered to normal subjects and subjects with hepatic impairment based on Child‐Pugh scores. Mean standard deviation expressed in the error bars and dotted lines represent the lower limit of quantification for plasma concentrations.
Table 2.
Summary of Ipatasertib and M1 Pharmacokinetic Parameters Following a Single Oral Dose of 100‐mg Ipatasertib Administered to Normal Subjects and Subjects With Hepatic Impairment Based on Child‐Pugh Scores
| Dose of 100 mg Ipatasertib | ||||
|---|---|---|---|---|
| Analyte Parameter (Units) | Healthy (N = 13) | Mild (N = 8) | Moderate (N = 8) | Severe (N = 7) |
| Ipatasertib | ||||
| AUC0‐t, ng • h/mL | 277 (28.3) | 284 (45.7) | 726 (60.7) b | 843 (30.9) c |
| AUC0‐72, ng • h/mL | 253 (26.9) | 261 (40.1) | 555 (56.9) | 632 (25.7) |
| AUC0‐∞, ng • h/mL | 318 (25.2) | 323 (43.9) | 808 (48.3) | 920 (28.2) |
| Cmax, ng/mL | 45.3 (52.4) | 53.0 (72.4) | 89.1 (60.7) | 80.0 (78.4) |
| tmax e (h) | 1.0 (0.5‐6.0) | 1.0 (0.5‐4.0) | 0.5 (0.5‐6.0) | 1.0 (0.5‐1.0) |
| tlast, e h | 120.0 (72.0‐120.4) | 120.0 (48.0‐168.0) | 216.0 (146.5‐334.5) b | 192.2 (167.7‐336.0) c |
| t1/2, f h | 49.8 (11.2) | 48.6 (16.8) | 83.1 (28.7) | 75.4 (38.8) |
| CL/F, L/h | 314 (25.2) | 310 (43.9) | 124 (48.3) | 109 (28.2) |
| Vz/F, L | 21900 (37.7) | 20200 (34.0) | 14000 (71.2) | 10600 (52.0) |
| M1 | ||||
| AUC0‐t, ng • h/mL | 148 (42.4) | 113 (61.6) | 65.1 (150.8) | 140 (73.5) |
| AUC0‐72, ng • h/mL | 139 (35.1) | 114 (47.2) | 99.8 (52.6) b | 131 (47.6) |
| AUC0‐∞, ng • h/mL | 199 (33.6) a | 143 (63.0) b | 146 (53.7) d | 177 (75.4) |
| Cmax, ng/mL | 11.5 (69.8) | 10.4 (55.6) | 13.4 (138.2) | 18.2 (65.1) |
| tmax, e h | 3.0 (1.0‐6.0) | 1.0 (0.5‐4.0) | 0.75 (0.5‐6.0) | 1.0 (0.5‐1.0) |
| tlast, e h | 95.5 (48.0‐120.0) | 72.0 (36.0‐120.0) | 48.0 (12.0‐96.0) | 72.0 (36.0‐216.0) |
| t1/2, f h | 48.0 (12.2) | 43.2 (20.7) | 34.9 (15.4) b | 56.7 (46.3) |
| MRAUC | 0.662 (27.1) a | 0.467 (25.1) b | 0.156 (29.4) d | 0.212 (54.3) |
| MRAUC0‐72 | 0.607 (21.1) | 0.478 (17.1) | 0.173 (17.2) b | 0.228 (44.2) |
AUC0‐∞, area under the plasma concentration‐time curve from time 0 to infinity; Cmax, maximum observed concentration; CL/F, apparent systemic clearance; MRAUC, metabolite ratio based on AUC0‐∞; t1/2, apparent terminal elimination half‐life; tlast, time to last measurable concentration; tmax, time to maximum observed concentration; VZ/F, apparent volume of distribution during the terminal phase.
Geometric mean (geometric coefficient of variation) data are presented unless otherwise indicated.
N = 12.
N = 7.
N = 6.
N = 5.
Median (min‐max).
Arithmetic mean (standard deviation).
Table 3.
Summary of the Statistical Analysis to Assess Effect of Hepatic Impairment on Pharmacokinetic Parameters for Ipatasertib Based on Child‐Pugh Scores
| Comparison (Test vs Reference) | Parameter (Units) | n a | Test Geometric Mean b | n a | Reference Geometric Mean b | Test/Reference c (%) | 90%CI c (%) | P Value d |
|---|---|---|---|---|---|---|---|---|
| Mild vs normal | Cmax, ng/mL | 8 | 53.0 | 8 | 53.7 | 98.6 | 52.8‐184.0 | .9662 |
| AUC0‐∞, ng • h/mL | 8 | 322.5 | 8 | 360.5 | 89.5 | 65.1‐123.0 | .5291 | |
| Moderate vs normal | Cmax, ng/mL | 8 | 89.1 | 8 | 48.4 | 184.1 | 112.2‐302.0 | .0523 |
| AUC0‐∞, ng • h/mL | 8 | 808.2 | 8 | 309.1 | 261.5 | 191.2‐357.5 | .0007 | |
| Severe versus normal | Cmax, ng/mL | 7 | 80.0 | 7 | 48.9 | 163.8 | 86.4‐310.3 | .1843 |
| AUC0‐∞, ng • h/mL | 7 | 920.0 | 7 | 308.7 | 298.1 | 228.4‐389.0 | .0002 |
AUC0‐∞, area under the concentration‐time curve extrapolated to infinity; Cmax, maximum observed concentration; CI, confidence interval.
Hepatic impairment groups (normal, mild, moderate, severe) were classified using the Child‐Pugh at screening.
n is the number of observations in each group used for the comparison.
Geometric means, calculated by transforming the natural log means back to the linear scale.
The ratio and corresponding CI were back‐transformed from the mean difference and its CI, which were calculated on the log scale from the paired t test.
The P value was obtained from the paired t test and assesses the difference between mild/moderate/severe hepatic impairment groups against the normal hepatic group. The P values were compared against a significance level of .1.
The mean ipatasertib plasma concentrations were higher in subjects with moderate and severe hepatic impairment compared to subjects with normal hepatic function (Figure 1). The ipatasertib plasma concentrations were similar between subjects with mild hepatic impairment and normal hepatic function.
Ipatasertib was rapidly absorbed upon the administration of a single oral 100‐mg dose of ipatasertib. Median tmax was achieved within 0.5 to 1 hour across all the groups (Table 2, Figure 1). While the mean elimination t1/2 was similar between subjects with mild hepatic impairment and normal hepatic function, it was ≈1.7‐ and 1.5‐fold longer in subjects with moderate and severe hepatic impairment, respectively, compared to subjects with normal hepatic function. Similarly, apparent plasma clearance and apparent volume of distribution values were similar across subjects with mild hepatic impairment and normal hepatic function; however, both apparent plasma clearance and apparent volume of distribution values were ≈2.5‐ and 1.6‐fold lower in subjects with moderate hepatic impairment and ≈2.9‐ and 2.1‐fold lower in subjects with severe hepatic impairment when compared to subjects with normal hepatic function (Table 2). Based on the primary statistical analysis using Child‐Pugh scores, administration of ipatasertib to subjects with mild hepatic impairment resulted in no appreciable difference in the systemic exposure of ipatasertib, whereas subjects with moderate and severe hepatic impairment showed an increase in the systemic exposure of ipatasertib by ≈2‐ to 3‐fold compared to subjects with normal hepatic function (Table 3). Geometric mean ratios (90%CIs) for Cmax, expressed as percentages, comparing subjects with mild, moderate, or severe hepatic impairment to subjects with normal hepatic function were 98.6 (52.8‐184.0), 184.1 (112.2‐302.0), and 163.8 (86.4‐310.3), respectively. Geometric mean ratios (90% CIs) for AUC0‐∞, expressed as percentages, comparing subjects with mild, moderate, or severe hepatic impairment to subjects with normal hepatic function were 89.5 (65.1‐123.2), 261.5 (191.2‐357.5), and 298.1 (228.4‐389.0), respectively.
M1, a major metabolite of ipatasertib was also measured in this study. M1 was detected in plasma immediately after the administration of a 100‐mg dose of ipatasertib (Figure 1). The median tmax ranged from 0.75 to 3 hours for all the patient groups. In contrast to ipatasertib, the t1/2 for M1 was similar in subjects with hepatic impairment compared to subjects with normal hepatic function (Table 2). Cmax for M1 increased by ≈1.1‐ to 1.5‐fold when ipatasertib was administered to subjects with moderate or severe hepatic impairment, respectively, and was slightly decreased (by ≈32.8%) in subjects with mild hepatic impairment compared to subjects with normal hepatic function. However, AUC0‐∞ for M1 decreased ≈7.9% to 35.3% when ipatasertib was administered to subjects with mild, moderate, or severe hepatic impairment compared to administration in subjects with normal hepatic function (Table S2). Geometric mean metabolite ratios of AUC0‐∞ of M1 to ipatasertib (MRAUC) decreased by 29%, 76%, and 68% in subjects with mild, moderate, and severe hepatic impairment, respectively, compared to healthy subjects with normal hepatic function (Table 2).
Analysis Based on NCI‐ODWG Criteria
Oncology patients are often classified for hepatic function using the NCI‐ODWG criteria; therefore, in this study, PK analyses were conducted based on Child‐Pugh and NCI classification. All subjects classified with normal hepatic function using the Child‐Pugh classification were also classified as normal under the NCI‐ODWG criteria. Eight subjects were classified with mild hepatic impairment using the Child‐Pugh criteria; of those 8 subjects, 3 were reclassified with mild hepatic impairment and 5 with normal hepatic function using the NCI‐ODWG criteria. Eight subjects were classified with moderate hepatic impairment using the Child‐Pugh criteria; of those 8 subjects, 2 were reclassified with moderate, 3 with normal, 2 with mild, and 1 with severe hepatic impairment using the NCI‐ODWG criteria. Seven subjects were classified with severe hepatic impairment using the Child‐Pugh criteria; of those 7 subjects, 4 subjects were reclassified with severe and 3 with moderate hepatic impairment using the NCI‐ODWG criteria (Table S4).
The mean ipatasertib and M1 plasma concentration–time profiles for subjects with normal hepatic function and subjects with different degrees of hepatic impairment based on NCI‐ODWG criteria are presented in Figure 2. Tables 4 and 5 provide a summary of ipatasertib and M1 PK parameters and the statistical analysis of the effect of hepatic impairment on PK parameters for ipatasertib, respectively, based on NCI‐ODWG criteria.
Figure 2.
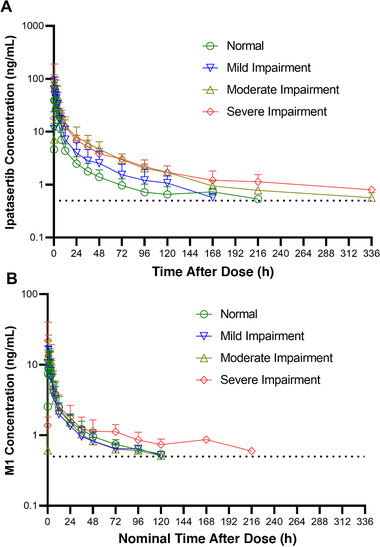
Mean plasma concentration versus time profiles of (A) ipatasertib and (B) M1 following a single oral dose of 100‐mg ipatasertib administered to normal subjects and subjects with hepatic impairment based on National Cancer Institute Organ Dysfunction Working Group criteria. Mean standard deviation expressed in the error bars and dotted lines represent the lower limit of quantification for plasma concentrations.
Table 4.
Summary of Ipatasertib and M1 Pharmacokinetic Parameters Following a Single Oral Dose of 100‐mg Ipatasertib Administered to Normal Subjects and Subjects With Hepatic Impairment Based on NCI‐ODWG Criteria
| Dose of 100‐mg Ipatasertib | ||||
|---|---|---|---|---|
| Analyte Parameter (Units) | Healthy (N = 21) | Mild (N = 5) | Moderate (N = 5) | Severe (N = 5) |
| Ipatasertib | ||||
| AUC0‐t, ng • h/mL | 295 (39.3) | 414 (77.0) c | 985 (20.0) c | 859 (39.5) |
| AUC0‐72, ng • h/mL | 263 (33.5) | 398 (65.0) | 729 (30.1) | 627 (30.8) |
| AUC0‐∞, ng • h/mL | 337 (36.7) | 522 (64.9) | 1030 (15.6) | 943 (40.5) |
| Cmax, ng/mL | 50.9 (60.2) | 63.3 (95.8) | 97.1 (23.6) | 78.5 (100.7) |
| tmax, d h | 1.0 (0.5‐6.0) | 1.0 (0.5‐4.0) | 1.0 (0.5‐3.0) | 1.0 (0.5‐1.0) |
| tlast, d h | 120.0 (48.0‐240.0) | 144.1 (96.0‐168.0)c | 240.8 (168.0‐334.5)c | 216.4 (167.7‐336.0) |
| t1/2, e h | 53.1 (18.3) | 59.9 (16.6) | 76.6 (45.8) | 86.1 (34.1) |
| CL/F, L/h | 297 (36.7) | 191 (64.9) | 97.1 (15.6) | 106 (40.5) |
| Vz/F, L | 21300 (37.5) | 16000 (68.1) | 9270 (79.7) | 12300 (14.4) |
| M1 | ||||
| AUC0‐t, ng • h/mL | 107 (100.7) | 104 (61.2) | 137 (36.8) | 146 (91.6) |
| AUC0‐72, ng • h/mL | 121 (46.2)a | 108 (46.0) | 136 (28.7) | 134 (57.9) |
| AUC0‐∞, ng • h/mL | 172 (50.4)b | 153 (56.7) c | 168 (35.5) | 187 (93.5) |
| Cmax, ng/mL | 10.6 (86.3) | 12.6 (77.2) | 20.0 (36.7) | 17.9 (75.8) |
| tmax,d h | 3.0 (0.5‐6.0) | 1.0 (0.5‐4.0) | 1.0 (0.50‐4.0) | 0.5 (0.5‐1.0) |
| tlast,d h | 72.0 (12.0‐120.0) | 72.0 (48.0‐120.0) | 72.0 (48.0‐120.0) | 96.0 (36.0‐216.0) |
| t1/2,e h | 42.8 (17.0) a | 48.6 (14.2) | 40.0 (19.0) | 62.2 (53.4) |
| MRAUC | 0.552 (56.7) b | 0.361 (46.8) c | 0.180 (29.8) | 0.218 (61.5) |
| MRAUC0‐72 | 0.502 (50.1) a | 0.298 (59.2) | 0.205 (29.8) b | 0.235 (44.8) |
AUC0‐∞, area under the plasma concentration–time curve from time 0 to infinity; Cmax, maximum observed concentration; CL/F, apparent systemic clearance; MRAUC, metabolite ratio based on AUC0‐∞; t1/2, apparent terminal elimination half‐life; tmax, time to maximum observed concentration; tlast, time to last measureable concentration; VZ/F, apparent volume of distribution during the terminal phase.
Geometric mean (geometric coefficient of variation) data are presented unless otherwise indicated.
N = 20.
N = 17.
N = 4.
Median (min‐max).
Arithmetic mean (standard deviation).
Table 5.
Summary of the Statistical Analysis to Assess Effect of Hepatic Impairment on Pharmacokinetic Parameters for Ipatasertib Based on NCI‐ODWG Criteria
| Test | Reference | |||||||
|---|---|---|---|---|---|---|---|---|
| Comparison (Test vs Reference) | Parameter (Units) | n a | Geometric Mean b | n a | Geometric Mean b | Test/Reference c (%) | 90%CI c (%) | P Value d |
| Mild vs normal | Cmax, ng/mL | 5 | 63.3 | 5 | 62.6 | 101.1 | 35.9‐284.8 | .9824 |
| AUC0‐∞, ng • h/mL | 5 | 522.4 | 5 | 340.0 | 153.6 | 87.7‐269.2 | .1780 | |
| Moderate vs normal | Cmax, ng/mL | 5 | 97.1 | 5 | 49.0 | 198.1 | 109.5‐358.4 | .0699 |
| AUC0‐∞, ng • h/mL | 5 | 1030.0 | 5 | 303.8 | 339.1 | 241.7‐475.6 | .0015 | |
| Severe vs normal | Cmax, ng/mL | 5 | 78.5 | 5 | 50.6 | 155.2 | 70.7–340.9 | .2995 |
| AUC0‐∞, ng • h/mL | 5 | 942.7 | 5 | 353.1 | 267.0 | 195.6‐364.3 | .0025 | |
AUC0‐inf, area under the concentration‐time curve extrapolated to infinity; Cmax, maximum observed concentration; CI, confidence interval.
Hepatic impairment groups (normal, mild, moderate, severe) were classified using the National Cancer Institute Organ Dysfunction Working Group criteria. The worst hepatic impairment severity recorded for bilirubin and aspartate aminotransferase at screening was used.
n is the number of observations in each group used for the comparison.
Geometric means, calculated by transforming the natural log means back to the linear scale.
The ratio and corresponding confidence interval were back‐transformed from the mean difference and its confidence interval, which were calculated on the log scale from the paired t test.
The P value was obtained from the paired t test and assesses the difference between mild/moderate/severe hepatic impairment groups against the normal hepatic group. The P values were compared against a significance level of .1.
The effect of hepatic impairment on the PK of ipatasertib showed similar trends from the statistical analysis based on NCI‐ODWG criteria as that from the statistical analysis using the Child‐Pugh scoring. The statistical analysis conducted using the NCI‐ODWG classification showed that the systemic exposure to ipatasertib increased by ≈1.6‐ to 3.4‐fold when ipatasertib was administered to subjects with moderate or severe hepatic impairment compared to that for healthy subjects with normal hepatic function (Table 5). In subjects with mild hepatic impairment compared to healthy subjects with normal hepatic function, ipatasertib Cmax was similar, while ipatasertib AUC0‐∞ was ≈1.5‐fold higher. Geometric mean ratios (90%CIs) for ipatasertib Cmax comparing subjects with mild, moderate, or severe hepatic impairment to subjects with normal hepatic function were 101.1 (35.9‐284.8), 198.1 (109.5‐358.4), and 155.2 (70.7‐340.9), respectively. Geometric mean ratios (90%CIs) for ipatasertib AUC0‐∞ comparing subjects with mild, moderate, or severe hepatic impairment to subjects with normal hepatic function were 153.6 (87.7‐269.2), 339.1 (241.7‐475.6), and 267.0 (195.6‐364.3), respectively.
Based on the statistical analysis, Cmax for M1 increased by ≈1.4‐ to 1.5‐fold when ipatasertib was administered to subjects with moderate or severe hepatic impairment compared to that for healthy subjects with normal hepatic function (Table 5) and was slightly decreased (by ≈21.8%) in subjects with mild hepatic impairment. AUC0‐∞ for M1 on the other hand decreased by ≈11.9% to 30.3% when ipatasertib was administered to subjects with mild, moderate, or severe hepatic impairment compared to administration in subjects with normal hepatic function (Table S3). The MRAUC decreased by 35%, 67%, and 60% in subjects with mild, moderate, and severe hepatic impairment, respectively, compared to healthy subjects with normal hepatic function (Table 4).
Safety
Ipatasertib administered as a single oral dose of 100 mg was generally safe and well tolerated in subjects with normal hepatic function and subjects with hepatic impairment. A total of 12 AEs was reported in 9 subjects (25%) with 3 AEs in 2 subjects with mild hepatic impairment, 3 AEs in 3 subjects with moderate hepatic impairment, 5 AEs in 3 subjects with severe hepatic impairment (25%), and 1 AE in 1 subject with normal hepatic function. The majority of the AEs were mild (grade 1) in intensity and resolved without any treatment intervention by the end of the study. One subject with moderate hepatic impairment had 1 AE of orthostatic hypotension that was assessed by the investigator as severe (grade 3) in intensity. There were no deaths or SAEs, and no subjects were discontinued from the study due to AEs.
No clinically significant changes or findings in vital signs, laboratory values, or ECG measurements were observed during the study.
Hepatic Impairment in Routine Clinical Practice—Real‐World Data Analysis
A total of 12 669 patients with metastatic prostate cancer were included in the analyses, of which 4477 (35.3%) were diagnosed with mCRPC, and had laboratory results recorded within 90 days before the first‐line start. The HR+/HER2– mBC cohort included 12 198 patients, of which 6337 (52.0%) had laboratory values of interest recorded. Mean ages at diagnosis were 73.0 in the mCRPC cohort and 64.1 in the HR+/HER2– mBC cohort (data not shown). There was no difference in year of diagnosis and group stage at diagnosis between the overall cohort and those patients who had eligible laboratory values available.
Using the NCI‐ODWG classification in patients with available laboratory results, 12.4% of the mCRPC and 18.8% of the HR+/HER2– mBC cohorts were determined to have mild hepatic impairment as a comorbidity (Table 6). The proportion of patients with moderate to severe hepatic impairment was 0.7% in patients with mCRPC and 1.4% in patients with HR+/HER2– mBC (Table 6).
Table 6.
Summary of Real‐World Data Analysis Where Subjects With mCRPC and HR+/HER2– mBC Were Classified Based on Their Level of Hepatic Function Using the NCI‐ODWG Criteria
| Categorization of Hepatic Impairment Based on NCI‐ODWG Criteria | mCRPC Subjects (N = 4477), n (%) | HR+/HER2– mBC Subjects (N = 6337), n (%) |
|---|---|---|
| Normal | 3890 (86.9) | 5057 (79.8) |
| Mild B1 | 484 (10.8) | 1097 (17.3) |
| Mild B2 | 72 (1.6) | 95 (1.5) |
| Moderate | 25 (0.6) | 50 (0.8) |
| Severe | 6 (0.1) | 38 (0.6) |
HR+/HER2–, hormone receptor positive/human epidermal growth factor negative; mBC, metastatic breast cancer; mCRPC, metastatic castrate‐resistant prostate cancer; NCI‐ODWG, National Cancer Institute‐Organ Dysfunction Working Group.
Discussion
Ipatasertib is extensively metabolized primarily by CYP3A4 and is also a substrate of P‐gp. As such, hepatic elimination is expected to play a major role in ipatasertib disposition. Based on the real‐world data, hepatic impairment is a relatively common comorbidity in patients with prostate and breast cancer, with mild impairment being more prevalent as compared to moderate and severe impairment. Thus, this study was conducted to evaluate the PK of ipatasertib in patients with hepatic impairment to enable effective use of ipatasertib in this special population.
In patients with solid tumors, 400 mg once daily was determined to be the maximum tolerated dose for ipatasertib in a combination setting, and this dose is currently being evaluated for clinical efficacy in several phase 1‐3 studies. In a DDI study, itraconazole, a strong CYP3A4 and P‐gp inhibitor, increased exposure of ipatasertib (100 mg) by ≈5‐fold. Given that hepatic elimination of ipatasertib via CYP3A4 can underlie this DDI, dose selection was an important consideration for this study as metabolic capability is often reduced in the patients with hepatic impairment. 14 , 15 Following a single dose of ipatasertib, close to dose proportionality has been observed between doses of 100 to 800 mg in a dose escalation study. 7 Given the level of DDI observed with itraconazole, a 100‐mg dose was selected for the hepatic impairment study, to ensure sufficient safety margins. In this study, the PK of ipatasertib were evaluated following a single 100‐mg oral dose in subjects with normal hepatic function and subjects with mild, moderate, and severe hepatic impairment based upon Child‐Pugh as well as NCI‐ODWG criteria of classification. As ipatasertib exhibits low human plasma protein binding, protein binding was not assessed in this study and only total exposures for ipatasertib have been reported. The overall mean percentage of unbound ipatasertib and unbound M1 metabolite in human plasma were 63.6% and 59.3%, respectively. The exposures observed in subjects with normal hepatic function in this study are comparable to those observed in previous studies in patients with cancer, 7 and as such, the PK observations from this study are directly applicable to the intended oncology population for ipatasertib. By either method of classification of hepatic impairment, the systemic exposure to ipatasertib, as assessed by Cmax and AUC0‐∞ was comparable between subjects with normal hepatic function and subjects with mild hepatic impairment. For subjects with both moderate or severe hepatic impairment, on the other hand, the Cmax and AUC0‐∞ increased by ≈2‐ and 3‐fold, respectively, when compared to subjects with normal hepatic function. M1 being pharmacologically several‐fold less effective than ipatasertib, it is not expected to contribute significantly toward efficacy based on its potency to inhibit Akt. Thus, changes in M1 exposure are not considered in the dose adjustment decisions for patients with impaired hepatic function.
Anticancer drugs such as venetoclax, 16 dacomitinib, 17 sunitinib, 18 cobimetinib, 19 and several others 20 demonstrate comparable exposure in subjects with normal and mildly impaired hepatic function, similar to that observed with ipatasertib. The increase in exposure with increasing impairment is also consistent with potential loss of metabolic capacity. CYP3A4 activity has shown to be correlated with Child‐Pugh scores in studies with midazolam, a sensitive substrate of CYP3A4. 21 Ipatasertib is also a sensitive substrate of CYP3A4, and the results of this study demonstrate a marked contribution of liver in ipatasertib clearance.
The study was conducted in accordance with the FDA and EMA guidance that recommends using Child‐Pugh classification for enrollment and analysis for hepatic impairment studies. 14 , 15 The classification as shown in Table S1 assesses hepatic function by grouping subjects based on 5 parameters: hepatic encephalopathy, ascites, serum bilirubin, serum albumin, and international normalized ratio. While Child‐Pugh classification provides dosing recommendations for most oncology as well as nononcology drugs per regulatory guidance, a simpler NCI‐ODWG criterion that is based on only 2 laboratory parameters—total bilirubin and AST—is most commonly employed by oncologists to evaluate their patients’ hepatic function. Prior reports have advocated use of this criterion as changes in liver function due to liver metastases or prior therapies are often not reflected in the Child‐Pugh method. 22 Because hepatic impairment is evaluated by clinicians based on NCI‐ODWG criteria, it was deemed important to explore the results based on this criterion in addition to the Child‐Pugh method and compare the outcomes. Elmeliegy et al 23 recently conducted a study where they evaluated the discordance between Child‐Pugh and NCI‐ODWG criteria and its impact on PK analysis and dosing decisions. Of the 5 studies evaluated, they noted that a significant number of subjects were classified in at least 1 lower hepatic impairment category when evaluated using NCI‐ODWG criteria compared to Child‐Pugh scores. Our study shows trends that are consistent with this report. Despite the differences in the number of subjects with hepatic impairment between the 2 classification systems, the trends in systemic exposure for ipatasertib and M1 were similar between subjects classified with Child‐Pugh and NCI‐ODWG criteria (Tables 3 and 5).
Ipatasertib 400 mg once daily is clinically under evaluation in patients with breast and prostate cancers. Based on the observed ipatasertib exposures, no dose adjustment is needed for patients with mild hepatic impairment, whereas a dose reduction is likely needed for patients with moderate and severe hepatic impairment to limit exposures to those generally observed in patients without hepatic impairment. According to the real‐world data, although hepatic impairment is a relatively common comorbidity in patients with prostate and breast cancer, prevalence of moderate and severe impairment is low. Although the real‐world data results are based on electronic health records data that were not initially collected for the purpose of research, and as such may be prone to some underreporting and biases, the generally lower prevalence indicates that the dose reductions will likely affect a relatively smaller number of patients.
Conclusions
Overall, this PK study has provided important information for the safe use of ipatasertib in subjects with impaired hepatic function. Ipatasertib PK in subjects with hepatic impairment were similar when evaluated using either Child‐Pugh or NCI‐ODWG classification. Therefore, regardless of the hepatic impairment classification method employed by the oncologists in the clinical setting, the PK results from this study can be adequately leveraged for dosing recommendations.
Conflicts of Interest
All authors are past or present employees of Genentech or Roche and stockholder for Roche Holdings AG.
Author Contributions
R.S., V.M., and D.S. wrote the manuscript. R.S., V.M., E.C., P.T., A.H., and L.M. designed and performed research. R.S., V.M., D.S., C.C., J.W., and L.M. analyzed the data.
Funding
This work was supported by Genentech Inc.
Data Accessibility Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.html).
Supporting information
Supporting Information
Acknowledgments
The authors thank the patients who participated in this study, the clinical study site personnel, and Covance for their assistance in the operational aspects of this study. We would also like to thank the investigators Dr Thomas Marbury, Dr Kenneth Lasseter, Dr Eric Lawitz, and Dr William Smith for their contribution to this clinical study. Editorial assistance was provided by Anshin BioSolutions Corp.
References
- 1. Lin K, Lin J, Wu WI, et al. An ATP‐site on‐off switch that restricts phosphatase accessibility of Akt. Sci Signal. 2012;5:ra37. [DOI] [PubMed] [Google Scholar]
- 2. Lin J, Sampath D, Nannini MA, et al. Targeting activated Akt with GDC‐0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res. 2013;19:1760‐1772. [DOI] [PubMed] [Google Scholar]
- 3. de Bono JS, De Giorgi U, Rodrigues DN, et al. Randomized phase II study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res. 2019;25:928‐936. [DOI] [PubMed] [Google Scholar]
- 4. Kim SB, Dent R, Im SA, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first‐line therapy for metastatic triple‐negative breast cancer (LOTUS): a multicentre, randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Oncol. 2017;18:1360‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oliveira M, Saura C, Nuciforo P, et al. FAIRLANE, a double‐blind placebo‐controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple‐negative breast cancer. Ann Oncol. 2019;30:1289‐1297. [DOI] [PubMed] [Google Scholar]
- 6. Zhu R, Poland B, Wada R, et al. Exposure‐response‐based product profile‐driven clinical utility index for ipatasertib dose selection in prostate cancer. CPT Pharmacometrics Syst Pharmacol. 2019;8:240‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saura C, Roda D, Rosello S, et al. A first‐in‐human phase I study of the ATP‐competitive AKT inhibitor ipatasertib demonstrates robust and safe targeting of AKT in patients with solid tumors. Cancer Discov. 2017;7:102‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sane RS, Plise E, Malhi V, Chen B, Musib L. Evaluation of ipatasertib interaction with a strong CYP3A4 inhibitor, itraconazole and endogenous substrate of OATP1B1/1B3 substrate coproporphyrin I and III in one drug‐drug interaction study. Poster No. 029. 2019 Annual Meeting American College of Clinical Pharmacology(R). Clin Pharmacol Drug Dev. 2019;8(Suppl 1):1‐101.31432623 [Google Scholar]
- 9. Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1‐85. [PubMed] [Google Scholar]
- 10. Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646‐649. [DOI] [PubMed] [Google Scholar]
- 11. Patel H, Egorin MJ, Remick SC, et al. Comparison of Child‐Pugh (CP) criteria and NCI organ dysfunction working group (NCI‐ODWG) criteria for Hepatic Dysfunction (HD): implications for chemotherapy dosing. J Clin Oncology. 2004;22:6051‐6051. [Google Scholar]
- 12. Birnbaum B, Nussbaum N, Seidl‐Rathkopf K, et al. Model‐assisted cohort selection with bias analysis for generating large‐scale cohorts from the EHR for oncology research. https://arxiv.org/abs/2001.09765. Accessed June 22, 2021.
- 13. Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of Population Characteristics in Real‐World Clinical Oncology Databases in the US: Flatiron Health, SEER, and NPCR. https://www.medrxiv.org/content/10.1101/2020.03.16.20037143v2.full.pdf. Accessed June 22, 2021. [Google Scholar]
- 14. EMEA . Guideline on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients With Impaired Hepatic Function. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-productspatients-impaired-hepatic-function_en.pdf. Accessed June 22, 2021. [Google Scholar]
- 15. FDA . Guidance For Industry. Pharmacokinetics in Patients With Impaired Hepatic Function: Study Design, Data Analysis and Impact on Dosing and Labeling. https://www.fda.gov/media/71311/download. Accessed June 22, 2021. [Google Scholar]
- 16. Salem AH, Dave N, Marbury T, et al. Pharmacokinetics of the BCL‐2 inhibitor venetoclax in subjects with hepatic impairment. Clin Pharmacokinet. 2019;58:1091‐1100. [DOI] [PubMed] [Google Scholar]
- 17. Giri N, Masters JC, Plotka A, et al. Investigation of the impact of hepatic impairment on the pharmacokinetics of dacomitinib. Invest New Drugs. 2015;33:931‐941. [DOI] [PubMed] [Google Scholar]
- 18. Bello CL, Garrett M, Sherman L, Smeraglia J, Ryan B, Toh M. Pharmacokinetics of sunitinib malate in subjects with hepatic impairment. Cancer Chemother Pharmacol. 2010;66:699‐707. [DOI] [PubMed] [Google Scholar]
- 19. Cheeti S, Deng Y, Chang I, et al. Effect of hepatic impairment on cobimetinib pharmacokinetics: the complex interplay between physiological changes and drug characteristics. Clin Pharmacol Drug Dev. 2021;10:144‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krens SD, Lassche G, Jansman FGA, et al. Dose recommendations for anticancer drugs in patients with renal or hepatic impairment. Lancet Oncol. 2019;20:e200‐e207. [DOI] [PubMed] [Google Scholar]
- 21. Albarmawi A, Czock D, Gauss A, et al. CYP3A activity in severe liver cirrhosis correlates with child‐pugh and model for end‐stage liver disease (MELD) scores. Br J Clin Pharmacol. 2014;77:160‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palmieri C, Macpherson I. Use of the child‐pugh score in anticancer drug dosing decision making: proceed with caution. Lancet Oncol. 2019;20:e289. [DOI] [PubMed] [Google Scholar]
- 23. Elmeliegy M, Yang DZ, Salama E, Parivar K, Wang DD. Discordance between child‐pugh and national cancer institute classifications for hepatic dysfunction: implications on dosing recommendations for oncology compounds. J Clin Pharmacol. 2021;61:105‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.html).


