FUNDING INFORMATION
This study was supported by a Science Foundation Ireland research center grant 12/RC/2273_P2, and a Science Foundation Ireland project grant 20/COV/0158.
CONFLICT OF INTEREST
LOM is a consultant to PrecisionBiotics and has received research funding from GSK and Chiesi. LOM has participated in speaker's bureau for Nestle, Nutricia, Reckitt, and Abbott. WCA has participated in advisory boards for Pfizer, MSD, and Sanofi, with reimbursements paid to his institution. LAF and SAD are employees of Seqbiome. None of the other authors report any conflict of interest.
AUTHOR CONTRIBUTIONS
NL, WCA, NS, BB, JW, MH, CS, PWOT, and LOM contributed to study design and securing funding. WCA, NS, BB, MH, and CS were responsible for patient care, sample, and data collection. NL, LAF, SAD, JW, and LOM generated the experimental data and performed the data analysis. NL, WCA, PWOT, and LOM wrote the paper. All authors reviewed and approved the manuscript.
To the Editor,
Successful immune responses to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) usually limit viral replication, prevent tissue damage, and promote recovery, but in a subset of cases, infection with this virus can lead to severe or fatal outcomes. The most severe forms of coronavirus 2019 (COVID‐19) disease are associated with exaggerated immune responses involving a wide range of inflammatory mediators that disrupt organ homeostasis, reprogram human metabolism, drive a hypercoagulation state, and destroy cells and tissues. 1 , 2 However, the molecular triggers that underpin these uncontrolled and hyperinflammatory immune responses are not well understood.
A low level of bacterial‐derived cellular fragments is present in the systemic circulation of healthy individuals, but levels increase significantly under disease conditions where translocation of bacteria and bacterial components can initiate and intensify inflammatory cascades. 3 In this study, we tested the hypothesis that translocation of proinflammatory bacterial components across a compromised epithelial barrier into the systemic circulation occurs in those with the most severe outcomes to SARS‐CoV‐2 infection.
As a marker for bacterial translocation into the circulation, we quantified bacterial DNA levels in 351 serum samples from 171 hospitalized COVID‐19 patients, 56 serum samples from 24 Long COVID patients (longer than 4 months since hospital discharge), and 29 serum samples from healthy volunteers (obtained prior to the pandemic), using the Femto bacterial DNA quantification kit (see Appendix S1 for details). All patients or healthy volunteers signed a patient informed consent, and the study was approved by local ethics committees (EKOS 20/058 for St. Gallen, Geneva, and Ticino, and The Clinical Research Ethics Committee of the Cork Teaching Hospitals for Cork University Hospital). Baseline characteristics, underlying comorbidities, and medication use for these patients have already been described in detail and are summarized in Table S1. 4 , 5 Bacterial DNA levels in serum were significantly higher in patients hospitalized for COVID‐19 (Figure 1A). These high levels of bacterial DNA in serum declined over time and were no longer elevated relative to controls within 3–4 weeks. Serum bacterial DNA levels in Long COVID patients (greater than 4 months following hospitalization) were similar to those in healthy volunteers, suggesting that increased translocation of bacterial cells/ DNA does not persist beyond the acute phase of COVID‐19 infection (Figure 1B).
FIGURE 1.
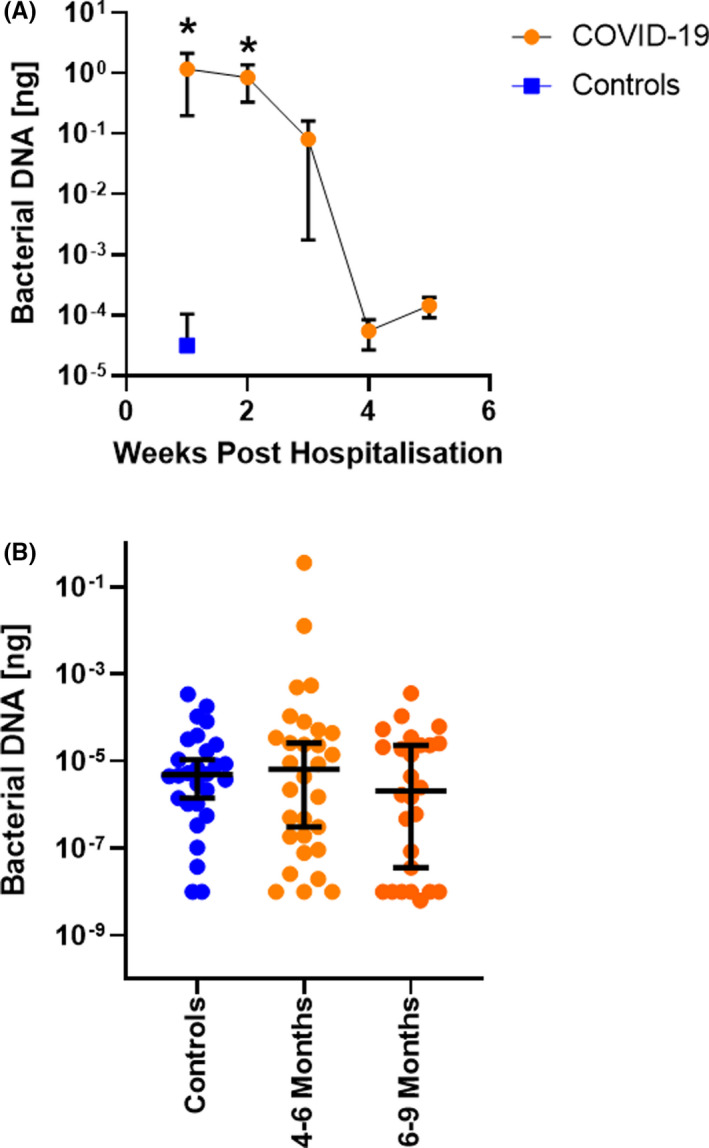
Bacterial DNA levels in serum. (A) Bacterial DNA levels were quantified in serum samples obtained during week 1 (n = 207 samples from 171 patients), week 2 (n = 82 samples from 82 patients), week 3 (n = 38 samples from 38 patients), week 4 (n = 13 samples from 13 patients), or week 5 (n = 11 samples from 11 patients) following hospitalization for COVID‐19. Serum samples from healthy volunteers (controls, n = 29 samples from 29 healthy volunteers) were included for comparison. (B) Bacterial DNA was quantified in serum samples from healthy volunteers (n = 29 samples from 29 healthy volunteers), Long COVID patients 4–6 months (n = 30 samples from 24 patients), or 6–9 months (n = 26 samples from 24 patients) following hospital discharge. *p < .05 ANOVA analysis
We next examined if bacterial DNA levels in the earliest serum sample obtained following study enrollment after admission to the intensive care unit (ICU) or the hospital ward was associated with disease severity. Patients were categorized to have mild disease if there were no radiographic indications of pneumonia, and moderate disease if pneumonia with fever and respiratory tract symptoms were present. Severe disease was defined as a respiratory rate ≥30 breaths per minute, oxygen saturation ≤93% when breathing ambient air or PaO2/FiO2 ≤300 mm Hg, or anyone that required mechanical ventilation. Only those that died during their hospital stay were recorded as a SARS‐CoV‐2‐related death in this study. Bacterial DNA levels were highest in those patients with severe disease who had a fatal outcome (Figure 2). Bacterial DNA levels were higher than the highest level observed in healthy volunteers for 0%, 21%, 21%, or 39% of patients with mild COVID‐19, moderate COVID‐19, severe COVID‐19 that recovered, or severe COVID‐19 with a fatal outcome, respectively (Figure 2). Bacterial DNA levels in serum were not associated with gender, BMI, or age (Figure S1). In addition, pre‐existing comorbidities such as hypertension, dyslipidemia, diabetes, or a respiratory inflammatory disease (asthma or COPD) did not significantly associate with bacterial DNA levels in serum of hospitalized COVID‐19 patients (Figure S2).
FIGURE 2.
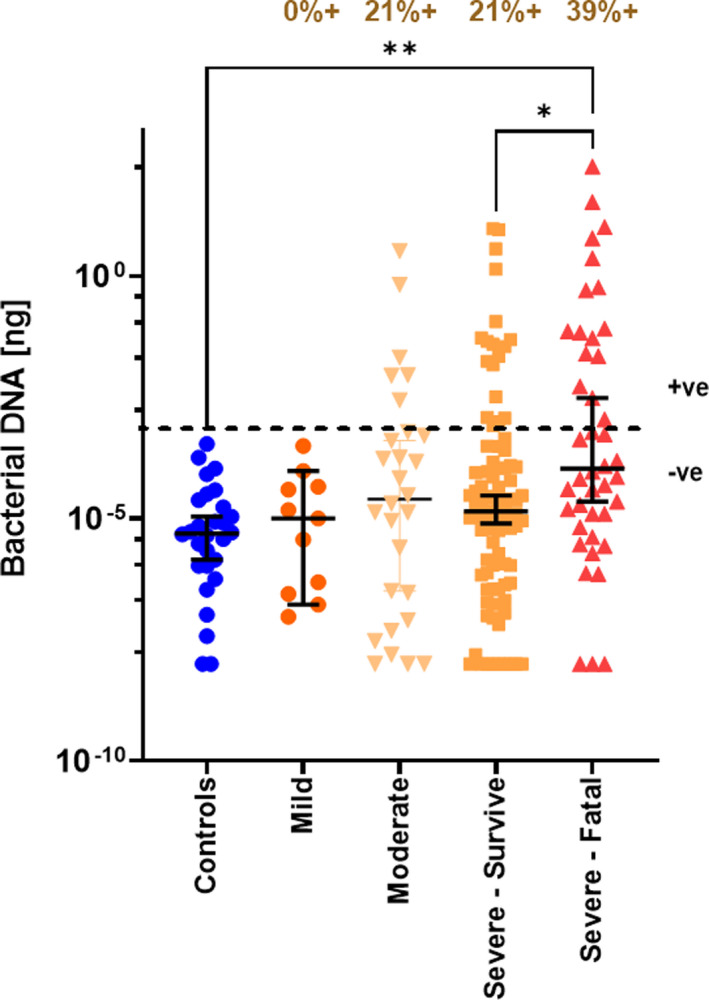
Serum bacterial DNA levels correlate with severity. Bacterial DNA levels were quantified in serum samples from healthy controls (n = 29), and the first serum sample obtained following hospitalization for patients with mild COVID‐19 (n = 11), moderate COVID‐19 (n = 28), patients with severe COVID‐19 who survived (n = 90), and patients with severe COVID‐19 that died (n = 42). The dotted line indicates the cut‐off value for the highest bacterial DNA level in serum from healthy volunteers. Bacterial DNA levels were higher than the highest level observed in healthy volunteers for 0%, 21%, 21%, or 39% of patients with mild COVID‐19, moderate COVID‐19, severe COVID‐19 that recovered, or severe COVID‐19 with a fatal outcome, respectively. Statistical significance was determined using the Kruskal‐Wallis test and Dunn's multiple comparison test
This study provides additional evidence for a loss of epithelial barrier function in patients with severe COVID‐19. 6 These data are also consistent with the epithelial barrier hypothesis, 3 which highlights the importance of immune responses to microbes that cross‐epithelial barriers damaged by external environmental factors such as pollutants, toxins, or infections. Mechanisms may include the direct consequences of viral infection in epithelial cells or impaired barrier function may be indirectly caused by infection‐related inflammatory mediators, oxidative stress, or microvascular thrombosis. Higher levels of bacterial DNA would likely be accompanied by higher levels of bacterial cell wall components such as lipopolysaccharide and peptidoglycan, which have potent immunostimulatory properties. However, we do not know the origin of the bacterial DNA, or if it came from live, senescent, or fragmented bacteria. In addition, we cannot determine a cause‐and‐effect relationship from our data, as a pre‐existing deficit in the epithelial barrier may contribute to pathology and severity, or the barrier may have become compromised only following SARS‐CoV‐2 infection. Nevertheless, early detection of elevated bacterial DNA levels in serum could be beneficial for planning clinical management of patients with COVID‐19, while interventions designed to improve host epithelial barriers as preventative or therapeutic strategies should be further explored to mitigate the most serious outcomes of SARS‐CoV‐2 infection.
Supporting information
App S1
ACKNOWLEDGEMENTS
This study was supported by a Science Foundation Ireland research center grant 12/RC/2273_P2 and a Science Foundation Ireland project grant 20/COV/0158. Open access funding enabled and organized by IRel.
REFERENCES
- 1. Sokolowska M, Lukasik ZM, Agache I, et al. Immunology of COVID‐19: mechanisms, clinical outcome, diagnostics, and perspectives‐A report of the European Academy of Allergy and Clinical Immunology (EAACI). Allergy. 2020;75(10):2445‐2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75(7):1564‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739‐751. [DOI] [PubMed] [Google Scholar]
- 4. Ahearn‐Ford S, Lunjani N, McSharry B, et al. Long‐term disruption of cytokine signalling networks is evident in patients who required hospitalization for SARS‐CoV‐2 infection. Allergy. 2021;76(9):2910‐2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albrich WC, Ghosh TS, Ahearn‐Ford S, et al. A high‐risk gut microbiota configuration associates with hyperinflammatory immune and metabolic responses to acute SARS‐CoV‐2 infection. Preprint: https://biorxiv.org/cgi/content/short/2021.10.26.465865v1. Accessed December 15, 2021. [DOI] [PMC free article] [PubMed]
- 6. Giron LB, Dweep H, Yin X, et al. Plasma markers of disrupted gut permeability in severe COVID‐19 patients. Front Immunol. 2021;9(12):686240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


