Abstract
Aim
We tested the hypothesis of a more aggressive disease process at diagnosis of type 1 diabetes during fall and winter, the colder seasons with consistently observed higher incidence of type 1 diabetes.
Methods
Seasonality in the manifestation of type 1 diabetes was examined in 4993 Finnish children and adolescents. Metabolic characteristics, beta‐cell autoantibodies and HLA class II genetics were analysed at clinical diagnosis.
Results
Significant seasonality was observed with higher number of new cases during fall and winter (n = 1353/27.1% and n = 1286/25.8%) compared with spring and summer (n = 1135/22.7% and n = 219/24.4%) (p < 0.001). The youngest children (aged 0.5–4 years) differed from the older ones (aged 5–14 years) as a minority of them were diagnosed in winter (p = 0.019) while the older children followed the same pattern as that seen in the total series. Poorer metabolic decompensation was observed during seasons with lower number of new diagnoses.
Conclusion
The heterogeneity in the seasonality of diabetes manifestation between younger and older children suggests that different environmental factors may trigger the disease at different ages. Poorer clinical condition associated with seasons with a lower number of new cases may be more likely to be due to a delay in seeking medical help than to a more aggressive autoimmunity.
Keywords: autoantibodies, clinical characteristics, HLA class II, season, type 1 diabetes
Abbreviations
- FPDR
Finnish Pediatric Diabetes Register
- GADA
antibodies to glutamic acid decarboxylase
- IA‐2A
antibodies to islet antigen 2
- IAA
insulin autoantibodies
- ICA
islet cell antibodies
- JDFU
Juvenile Diabetes Foundation unit
- RU
relative unit
- ZnT8A
zinc transporter 8 autoantibodies
Key notes.
Seasonal patterns are seen in the presentation of type 1 diabetes with the highest rate found in fall and winter.
There is heterogeneity in the seasonality of the disease manifestation between younger and older children suggesting different environmental factors contributing to the disease onset at different ages.
Poorer metabolic decompensation was observed during the seasons with fewer diagnoses but a clear link between seasonality, metabolic changes and immunology seems to be absent.
1. INTRODUCTION
Seasonal variation in the incidence of type 1 diabetes has been reported with a peak in the fall and winter months. 1 , 2 , 3 , 4 , 5 The phenomenon seems to be more pronounced in high‐incidence countries. 4 , 6 Some studies suggest seasonality to be more prominent in males and absent in the youngest children 2 , 4 , 7 while others disagree. 1 , 3 , 8 However, studies discussing the association of seasonality and the clinical characteristics of type 1 diabetes at diagnosis are sparse.
We set out to delineate the seasonal variation in the manifestation of type 1 diabetes in Finnish children and the association of seasonal timing of diagnosis with clinical presentation, markers of beta‐cell autoimmunity and HLA genetics. We also wished to analyse whether seasonal variation is dependent on sex or age. We hypothesised that the clinical presentation is more aggressive in the cold months given the higher number of new cases in that period.
2. PATIENTS AND METHODS
2.1. Study design and subjects
Between January 2003 and December 2016, 6913 children and adolescents diagnosed with type 1 diabetes under the age of 15 years were registered in the Finnish Pediatric Diabetes Register (FPDR). We excluded 1747 children because of a lack of blood samples available for the analyses of diabetes‐associated autoantibodies and HLA genotyping. The seasonal timing of the type 1 diabetes manifestation was similar between the included and excluded subjects (Table 1). Only the first diagnosed child per family who fulfilled the inclusion criteria was included as an index child. In total, we ended up with 4993 patients, aged between 6 months and 15 years (56.6% male, median age 8.2).
TABLE 1.
Comparison of age at diagnosis, sex and season of type 1 diabetes diagnosis in the study population and excluded subjects (n = 6740)
| Demographics |
Study subjects n = 4993 (74.1%) |
Excluded subjects n = 1747 (25.9%) |
p value | Adjusted p value a |
|---|---|---|---|---|
| Age at diagnosis, years, mean (SD) | 8.03 (3.89) | 7.66 (3.98) | 0.001 | |
| Sex, male, % (95% CI) | 56.6 (55.2–57.9) | 54.7 (52.4–57.1) | 0.192 | |
| Season of diagnosis, % | 0.512 | 0.493 | ||
| Spring | 22.7 | 23.4 | ||
| Summer | 24.4 | 24.3 | ||
| Fall | 27.1 | 25.4 | ||
| Winter | 25.8 | 26.9 | ||
Adjusted for sex and age at diagnosis.
The study subjects were divided into four groups by season of disease presentation. Seasons were classified according to the calendar months: spring (March–May), summer (June–August), fall (September–November) and winter (December–February).
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa. A legal caretaker gave written informed consent and participants 10–15 years of age an informed assent.
2.2. Metabolic factors
Markers of metabolic decompensation at diagnosis were analysed in the local laboratories, and these included blood pH and the levels of plasma glucose, beta‐hydroxybutyrate and HbA1c (available from 2012 onwards). Ketoacidosis was defined as a blood pH <7.30 and severe ketoacidosis as blood pH <7.10. Weight loss, level of consciousness and the puberty status at clinical diagnosis were assessed in the paediatric units and the duration of symptoms before diagnosis by interviewing the parents.
2.3. Autoantibodies
Insulin autoantibodies (IAA), 9 antibodies to glutamic acid decarboxylase (GADA), 10 islet antigen 2 (IA‐2A) 11 and zinc transporter 8 (ZnT8A) 12 were analysed using specific radiobinding assays, and islet cell antibodies (ICA) with indirect immunofluorescence. 13 The cut‐off limits for IAA, GADA, IA‐2A and ZnT8A positivity were 2.80 relative units (RU), 5.36 RU, 0.77 RU and 0.50 RU, respectively, based on the 99th percentiles in more than 350 Finnish nondiabetic children and adolescents. According to the Diabetes Autoantibody Standardization Programs and the Islet Autoantibody Standardization Programs between 2003 and 2016, the disease sensitivities and specificities of these assays were 42–62% and 92–99% for IAA, 64–90% and 90–98% for GADA, 62–72% and 93–100% for IA–2A and 48–70% and 97–100% for ZnT8A.
We excluded samples taken later than 30 days after diagnosis (255/4993, 5.1%) since after that the IAA assay may also detect antibodies to exogenous insulin. For calculation of the median antibody titres, only samples at or above the cut‐off limit were included in the analyses.
2.4. HLA genetics
PCR‐based amplification followed by hybridisation with lanthanide‐labelled probes and time‐resolved fluorometry detection was used for HLA typing of the major DR‐DQ haplotypes. 14 The HLA susceptibility to type 1 diabetes for each study subject was estimated based on comparison of genotype frequencies between almost 3000 children with type 1 diabetes and their affected family‐based artificial controls formed from haplotypes not transmitted to the diabetic child. The susceptibility was classified into six risk groups from strongly decreased risk (risk group 0) to high risk (risk group 5). The DQA1*05‐DQB1*02 haplotype was shortened as DR3‐DQ2 and the HLA‐DRB1*04:01/2/4/5‐DQB1*03:02 haplotype as DR4‐DQ8.
2.5. Statistics
We used IBM SPSS Statistics 24 (IBM Corp., Armonk, NY, USA) and the R Software for Statistical Computing for Windows, version 3.5.0 (R foundation, Vienna, Austria, https://cran.r‐project.org/) for statistical analyses. We compared the observed frequencies of seasons/months to the expected frequencies of the same variables considering the number of days in each season/month to account for the unequal lengths of months. A mean number of 365.25 days per year and 28.25 days in February was assumed in order to rule in the leap years. Cross‐tabulation and Pearson's χ 2 test with continuity correction when appropriate or the Fisher exact test was used for frequencies of categorical variables. Bonferroni's correction for multiple comparisons was not applied due to its overly conservative nature. Differences in levels of parametric variables were analysed with Student's t test or one‐way ANOVA and Mann–Whitney U test or Kruskal–Wallis test for nonparametric variables. Adjustment for age at diagnosis and sex was performed with logistic/ordinal/multinomial regression for dichotomous/ordinal/categorical variables and quantile regression in R (package quantreg) for nonparametric variables. A two‐tailed p value <0.05 was considered statistically significant.
3. RESULTS
A majority of the children were diagnosed in fall or winter (n = 1353/27.1% and n = 1286/25.8%) vs. in spring or summer (n = 1135/22.7% and n = 1219/24.4%) (p = 0.001). Spring was the season with the lowest number of new cases compared to the other seasons (p = 0.006). Analyses by month revealed significant seasonality, with a peak frequency of diagnoses in January followed by August and September, the lowest frequency being observed in May (p < 0.001) (Figure 1).
FIGURE 1.
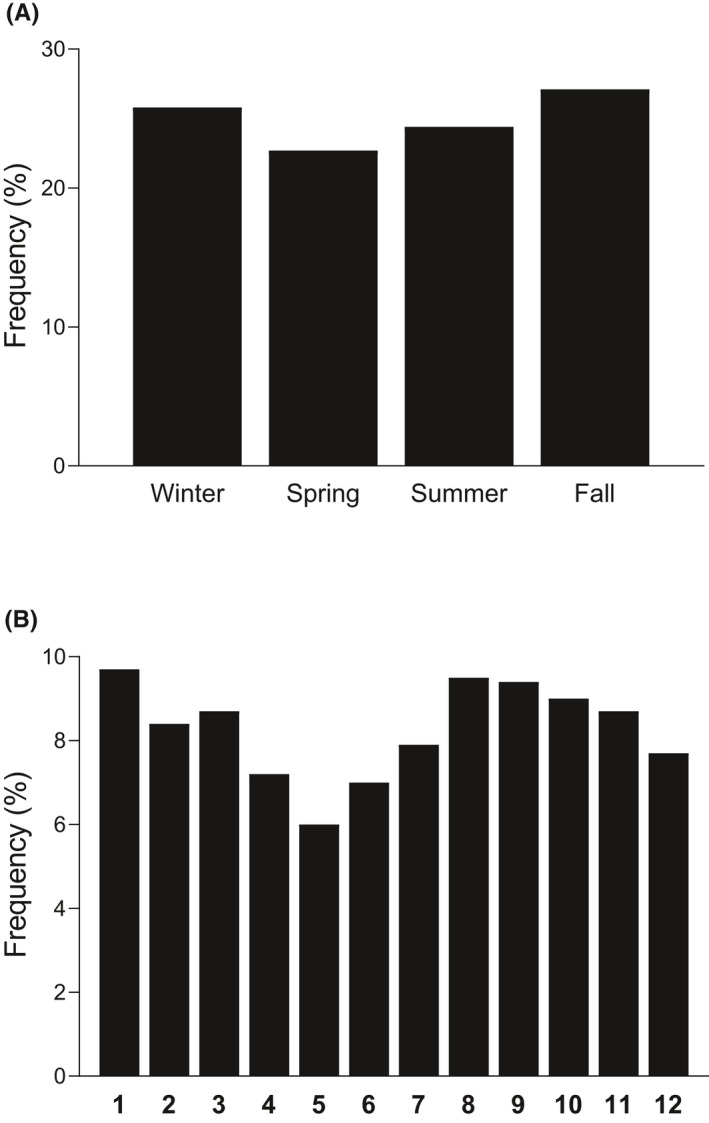
Frequencies of type 1 diabetes diagnoses according to season (A) and month (B) presented using histograms. Significant seasonality was observed both in the analyses by season (p = 0.006) and by month (p < 0.001). Significance was evaluated using the χ 2 test in R after considering the different lengths of seasons/months by comparing the observed frequencies of seasons/months to the expected frequencies of the same variables
To exclude potential changes in the environment over the years possibly contaminating the results, we looked for the difference in seasonality between those diagnosed in 2009 or earlier and 2010 or later but found no significant differences. The season of birth was not related to the time of disease manifestation.
When examining the whole cohort, there were no differences in the seasonality between sexes. Because of a more apparent seasonality previously observed in older children (aged ≥5 years) than among younger children (aged <5 years), 2 , 4 we further divided the study population into two groups by age at diagnosis (aged 0.5–4 years and 5–14 years). Within‐group analyses were performed by both season and month, and we observed significant seasonality in the subgroup of older children (p = 0.004 and p < 0.001 respectively) but not in the subgroup of younger children (p = 0.254 and p = 0.395). Between‐group analysis showed a clear difference as a minority of the youngest children were affected in winter while the peak of diagnoses was from fall to winter among the older children (Figure 2). Moreover, this observation was significant in boys but not in girls.
FIGURE 2.
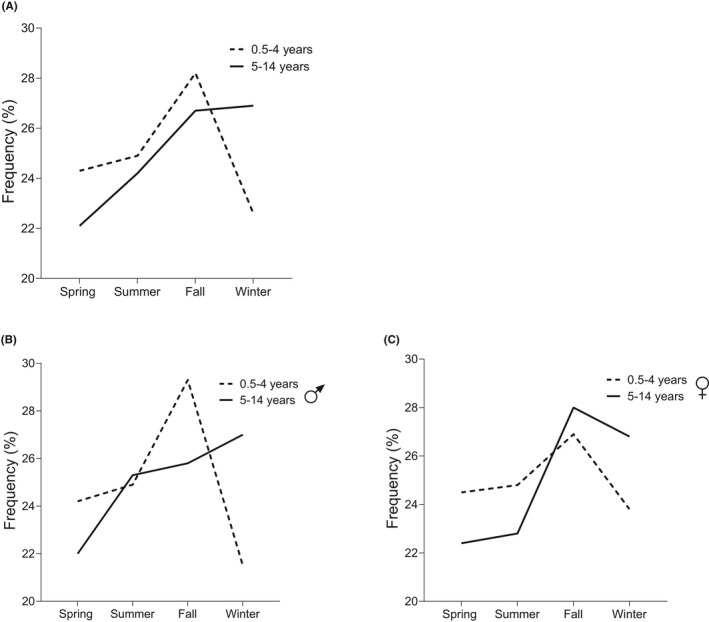
Seasonal variation in diabetes onset among children aged 0.5–4 and 5–14 years. The between‐group analysis showed a significant difference in the distribution of diagnosis frequencies by season. There was a peak in the frequency of diagnoses during fall and a drop‐down during winter among the younger children while the older children were most often diagnosed in fall followed by winter (p = 0.019) (A). The phenomenon was more pronounced in the subgroup of boys (p = 0.020) (B) and not seen in the subgroup of girls (p = 0.347) (C). Significance was evaluated using cross‐tabulation and the χ 2 test
All the analyses were then adjusted for age at diagnosis and sex. Those diagnosed in spring or summer suffered from ketoacidosis more often than those diagnosed in fall (Table 2). Furthermore, weight loss at diagnoses was the highest in summer.
TABLE 2.
Seasonal variation in demographic, clinical and metabolic characteristics in type 1 diabetes affected children diagnosed in spring, summer, fall and winter
| n | 1. Spring, n = 1135 (22.7%) | 2. Summer, n = 1219 (24.4%) | 3. Fall, n = 1353 (27.1%) | 4. Winter, n = 1286 (25.8%) | p value | Adjusted p value a | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Sex, male, % (95% CI) | 4993 | 56.0 (53.1–58.9) | 58.4 (55.6–61.2) | 55.7 (53.0–58.3) | 56.2 (53.5–58.9) | 0.506 | |
| Age, median (range) | 4993 | 8.04 (0.52–14.98) | 7.84 (0.59–14.99) | 7.99 (0.82–14.99) | 8.49 (0.53–14.98) | 0.174 | |
| Familial, % (95% CI) | 4993 | 10.1 (8.4–11.9) | 11.2 (9.4–12.9) | 9.5 (8.0–11.1) | 10.8 (9.1–12.5) | 0.541 | 0.531 |
| Pubertal, % (95% CI) | 3764 | 17.8 (15.3–20.3) | 16.4 (14.0–18.7) | 17.6 (15.3–20.0) | 16.8 (14.4–19.1) | 0.819 | 0.279 |
| Metabolic decompensation at diagnosis | |||||||
| Duration of symptoms, % | 4614 | 0.067 | 0.041 | ||||
| No symptoms | 1.5 | 0.5 | 1.0 | 0.8 | |||
| <1 week | 21.0 | 22.8 | 23.1 | 23.2 | |||
| 1–4 weeks | 60.2 | 55.7 | 55.8 | 58.5 | |||
| >4 weeks | 17.4 | 20.9 | 20.2 | 17.5 | |||
| 1 vs. 2: <0.050 | |||||||
| Impaired consciousness, % (95% CI) | 4784 | 5.2 (3.9–6.6) | 6.5 (5.1–7.9) | 4.9 (3.7–6.1) | 5.2 (4.0–6.4) | 0.331 | 0.327 |
| Ketoacidosis, % (95% CI) | 4817 | 18.8 (16.5–21.2) | 19.9 (17.6–22.2) | 15.8 (13.9–17.8) | 17.5 (15.4–19.6) | 0.051 | 0.036 |
| 1 vs. 3: <0.050 | |||||||
| 2 vs. 3: <0.050 | |||||||
| Severe ketoacidosis, % (95% CI) | 4817 | 3.8 (2.7–5.0) | 5.6 (4.3–7.0) | 4.4 (3.3–5.5) | 4.6 (3.4–5.7) | 0.212 | 0.183 |
| Weight loss, %, median (range) | 4610 | 4.9 (0–33.2) | 6.0 (0–32.3) | 4.7 (0–40.0) | 5.4 (0–28.3) | <0.001 | <0.001 |
| 1 vs. 2:0.017 | |||||||
| 2 vs. 3: <0.001 | |||||||
| 3 vs. 4:0.003 | |||||||
| pH, median (range) | 4817 | 7.38 (6.82–7.53) | 7.38 (6.82–7.54) | 7.38 (6.82–7.53) | 7.38 (6.72–7.57) | 0.122 | 0.112 |
| Beta‐hydroxybutyrate, mmol/L, median (range) | 4384 | 1.6 (0–18.4) | 1.9 (0–17.4) | 1.7 (0–27.0) | 1.7 (0–23.5) | 0.234 | 0.330 |
| Plasma glucose, mmol/L, median (range) | 4869 | 24.2 (3.2–93.2) | 24.5 (3.6–83.2) | 23.4 (3.5–97.6) | 23.5 (3.6–94.6) | 0.079 | 0.070 |
| HbA1c, mmol/mol, median (range) | 841 | 93.2 (40.0–176.0) | 91.0 (38.0–164.0) | 90.0 (36.0–189.0) | 89.0 (37.7–171.0) | 0.700 | 0.427 |
| HbA1c, %, median (range) | 841 | 10.7 (5.8–18.3) | 10.5 (5.6–17.2) | 10.4 (5.4–19.4) | 10.3 (5.6–17.8) | 0.696 | 0.392 |
In case of significant differences in the analyses between the four groups of season, paired comparisons by groups were also performed. Only significant p values are presented from the paired analyses.
Adjusted for sex and age at diagnosis.
After adjustment, the ICA titres were slightly higher if type 1 diabetes was diagnosed in fall compared with the other seasons (65 Juvenile Diabetes Foundation units [JDFU] vs. 49–64 JDFU, p = 0.002). No other differences were found in autoantibody profiles at diagnoses (Table 3). The season was not associated with HLA class II genetics (Table 4).
TABLE 3.
Comparison of autoantibody positivity, levels of autoantibodies in positive samples and the number of positive autoantibodies in children diagnosed with type 1 diabetes in spring, summer, fall and winter
| Autoantibodies | n | 1. Spring, n = 1135 (22.7%) | 2. Summer, n = 1219 (24.4%) | 3. Fall, n = 1353 (27.1%) | 4. Winter, n = 1286 (25.8%) | p value | Adjusted p value a |
|---|---|---|---|---|---|---|---|
| ICA, % (95% CI) | 4738 | 91.7 (90.0–93.3) | 92.1 (90.6–93.7) | 90.8 (89.2–92.4) | 92.4 (90.9–93.9) | 0.500 | 0.434 |
| ICA, JDFU, median (range) | 4347 | 49.0 (3.0–4096.0) | 64.0 (3.0–2049.0) | 65.0 (3.0–5120.0) | 49.0 (3.0–2049.0) | 0.335 | 0.002 |
| 1 vs. 3: 0.007 | |||||||
| 2 vs. 3: 0.008 | |||||||
| 3 vs. 4: 0.002 | |||||||
| IAA, % (95% CI) | 4738 | 42.8 (39.8–45.7) | 44.4 (41.6–47.3) | 41.8 (39.1–44.5) | 43.1 (40.3–45.9) | 0.611 | 0.322 |
| IAA, RU, median (range) | 2037 | 10.7 (2.8–7809.0) | 10.3 (2.9–829.8) | 10.8 (2.9–343.5) | 9.1 (2.8–484.9) | 0.123 | 0.916 |
| IA2A, % (95% CI) | 4738 | 74.8 (72.2–77.4) | 75.0 (72.5–77.5) | 74.4 (72.0–76.8) | 76.0 (73.6–78.4) | 0.817 | 0.847 |
| IA2A, RU, median (range) | 3556 | 102.0 (0.8–453.7) | 110.0 (1.0–254.1) | 107.2 (0.9–240.9) | 104.1 (0.8–553.3) | 0.634 | 0.082 |
| GADA, % (95% CI) | 4738 | 67.6 (64.8–70.4) | 66.4 (63.7–69.2) | 67.3 (64.7–69.8) | 64.2 (61.6–66.9) | 0.299 | 0.248 |
| GADA, RU, median (range) | 3144 | 38.0 (5.5–2675.1) | 32.8 (5.4–3051.4) | 36.0 (5.4–24849.0) | 37.8 (5.5–812.4) | 0.570 | 0.275 |
| Zn8TA, % (95% CI) | 4738 | 68.5 (65.8–71.3) | 68.8 (66.3–71.6) | 68.6 (66.0–71.1) | 71.5 (69.0–74.1) | 0.322 | 0.414 |
| Zn8TA, RU median (range) | 3289 | 13.2 (0.5–209.3) | 11.3 (0.5–177.7) | 11.8 (0.5–1201.9) | 11.9 (0.5–247.5) | 0.214 | 0.798 |
| Number of positive biochemical antibodies, median (mean) | 4738 | 3 (2.54) | 3 (2.55) | 3 (2.52) | 3 (2.55) | 0.856 | 0.980 |
| Number of positive antibodies, median (mean) | 4738 | 4 (3.45) | 4 (3.47) | 4 (3.43) | 4 (3.47) | 0.759 | 0.954 |
| Autoantibody negative, % (95% CI) | 4738 | 2.5 (96.5–98.4) | 2.0 (97.2–98.8) | 2.2 (97.0–98.6) | 2.5 (96.7–98.4) | 0.810 | 0.836 |
| Positivity for multiple (≥2) autoantibodies, % (95% CI) | 4738 | 92.5 (91.0–94.1) | 92.8 (91.3–94.2) | 91.7 (90.2–93.2) | 93.0 (91.6–94.5) | 0.599 | 0.518 |
In case of significant differences in the analyses between the four groups of season, paired comparisons by groups were also performed. Only significant p values are presented from the paired analyses.
Abbreviations: GADA, antibodies to glutamic acid decarboxylase; IA‐2A, antibodies to islet antigen 2; IAA, insulin autoantibodies; ICA, islet cell antibodies; JDFU, Juvenile Diabetes Foundation unit; RU, relative unit; ZnT8A, zinc transporter 8 autoantibodies.
Adjusted for sex and age at diagnosis.
TABLE 4.
Frequencies of HLA risk genotypes and haplotypes in children diagnosed in spring, summer, autumn and winter (n = 4993)
| Genetics, % (95% CI) | 1. Spring, n = 1135 (22.7%) | 2. Summer, n = 1219 (24.4%) | 3. Autumn, n = 1353 (27.1%) | 4. Winter, n = 1286 (25.8%) | p value | Adjusted p value a |
|---|---|---|---|---|---|---|
| DR3‐DQ2/DR4‐DQ8 | 20.9 (18.5–23.2) | 21.2 (19.0–23.5) | 21.1 (19.0–23.3) | 22.0 (19.7–24.3) | 0.914 | 0.869 |
| DR3‐DQ2/x b | 16.4 (14.2–18.5) | 15.5 (13.5–17.5) | 15.7 (13.7–17.6) | 13.8 (12.0–15.7) | 0.344 | 0.336 |
| DR4‐DQ8/y c | 47.9 (45.0–50.8) | 48.5 (45.7–51.3) | 47.7 (45.0–50.3) | 47.7 (45.0–50.5) | 0.977 | 0.971 |
| x b /y c | 14.8 (12.7–16.9) | 14.8 (12.8–16.8) | 15.5 (13.6–17.5) | 16.4 (14.4–18.4) | 0.636 | 0.676 |
| DR3‐DQ2 | 37.3 (34.5–40.1) | 36.8 (34.0–39.5) | 36.8 (34.2–39.4) | 35.8 (33.2–38.5) | 0.905 | 0.930 |
| DR4‐DQ8 | 68.8 (66.1–71.5) | 69.7 (67.2–72.3) | 68.8 (66.3–71.3) | 69.7 (67.2–72.2) | 0.926 | 0.905 |
| DR3‐DQ2 homozygote | 2.3 (1.4–3.2) | 2.5 (1.6–3.3) | 3.5 (2.6–4.5) | 3.6 (2.6–4.6) | 0.110 | 0.112 |
| DR4‐DQ8 homozygote | 8.6 (7.0–10.3) | 7.3 (5.8–8.8) | 8.0 (6.5–9.4) | 8.6 (7.1–10.2) | 0.577 | 0.627 |
| Risk group | 0.080 | 0.065 | ||||
| 0 | 0.7 | 0.7 | 1.0 | 0.9 | ||
| 1 | 1.6 | 2.7 | 1.1 | 2.8 | ||
| 2 | 15.6 | 15.0 | 16.6 | 16.3 | ||
| 3 | 24.9 | 22.1 | 23.7 | 20.3 | ||
| 4 | 36.1 | 38.2 | 36.5 | 37.6 | ||
| 5 | 21.1 | 21.2 | 21.1 | 22.1 | ||
In case of significant differences in the analyses between the four groups of season, paired comparisons by groups were also performed. Only significant p values are presented from the paired analyses.
Adjusted for sex and age at diagnosis.
x ≠ DR4‐DQ8.
y ≠ DR3‐DQ2.
4. DISCUSSION
In our nationwide cohort, children were diagnosed with type 1 diabetes more often in the cold seasons, as 52.9% of cases were diagnosed in fall or winter. The frequency is in line with a report from Sweden (53%) 5 while in general, comparisons with previous studies may be challenging because of differences in the research designs and methods used. We did not observe any difference in the seasonality of diabetes manifestation between the sexes. There is a male‐to‐female excess in patients with type 1 diabetes, 15 and the reason for a more pronounced seasonality in males observed in some reports might reflect a stronger statistical power.
We observed significant seasonality in the group of older children (aged 5–14 years) but not in the group of younger children (aged 0–4 years). A previous report by Weets et al. agree with that finding, although they compared children aged <10 and ≥10 years. 7 Between‐group analysis showed a clear difference as the youngest children had a peak of disease presentation in fall and a nadir in winter in contrast to the older children with peaks both in fall and in winter. Early life infections may contribute to the disease pathogenesis. 16 In particular, a link between certain enteroviruses and progression to clinical type 1 diabetes has been implicated (reviewed in 17 ). There is evidence that enteroviruses may function as a trigger of beta‐cell autoimmunity but may also be the last inducer of clinical type 1 diabetes. Enteroviruses more often affect younger children, and in Finland, more than 80% of enterovirus infections are diagnosed between August and December. 18 This may explain the peak of type 1 diabetes during fall and the low frequency of disease presentation in winter among the younger children. Some seasonal cycling exists also in the serum 25(OH)D concentrations, as in Finnish children, they are observed to be decreased in winter in parallel with the decreased amount of sunlight. 19 Moreover, decreased 25(OH)D concentrations were associated with islet autoimmunity and multipositivity for diabetes‐related autoantibodies, especially in carriers of certain genotypes of the vitamin D receptor gene. 20 Compared with the older children, the vitamin D intake in the younger children might be more closely controlled by the parents, as the families also contact the professionals in the child health clinic more frequently during early childhood. However, based on the results from other large prospective studies, no clear causal link can be confirmed between vitamin D intake or 25(OH)D concentrations and type 1 diabetes. 21 , 22 The recent observations about the decreasing trend in the incidence rate of the disease only among youngest children 23 , 24 support the theory of two different endotypes of type 1 diabetes in younger and older children. Our results together with these observations suggest that environmental factors triggering type 1 diabetes in early life may have changed over time and differ from the factors that contribute to the disease progression in older children.
Most studies considering type 1 diabetes seasonality are focused on incidence trends by season whereas we wanted to evaluate the association of seasonality and the disease characteristics at diagnosis. Contrary to our hypothesis, we observed higher frequency of ketoacidosis in spring and in summer and weight loss at diagnosis was the highest if diagnosed in summer. Hanberger et al. reported similar findings of HbA1c as the levels peaked in late spring and summer when the number of children diagnosed with type 1 diabetes was the lowest. 5 The results might be due to the higher risk of dehydration or the masking of increased thirst during the seasons with higher temperature leading to more severe metabolic decompensation, or due to the delay of diagnosis during summer holidays and poorer availability of health services. Similarly, the COVID‐19 pandemic has reduced access to medical services resulting in an increased frequency of diabetic ketoacidosis and need for intensive care in children with newly diagnosed type 1 diabetes, 25 , 26 although underlying causes may be multifactorial.
Interestingly, a few studies have reported a positive correlation between high‐incidence seasons and C‐peptide levels at diagnosis, suggesting variation in the secretory capability of remaining beta cells by season. 27 , 28 As classical symptoms and weight loss present less often in children with higher C‐peptide concentrations at diagnosis, 28 possibly this could explain our observation of a less severe clinical condition in fall and winter. Unfortunately, we were unable to test this hypothesis, as the FPDR does not provide information on the C‐peptide concentrations at diagnosis. Nevertheless, the above‐mentioned observations might let us to expect that there should be milder signs of autoimmunity during the high‐incidence seasons reflecting heterogeneity on the pathogenic process according to the season of diagnosis. However, we failed to show seasonality in the number of positive autoantibodies at diagnosis and the only notable relationship between season of disease presentation and autoantibodies was higher ICA titres observed in fall. In a small Slovakian study, IA‐2A positivity at diagnosis showed seasonal cycling with a peak in fall. 29 Similar seasonality has not been observed in IAA 29 or GADA at diagnosis. 29 , 30 Thus, results concerning seasonality in the autoantibody profile at diagnosis are sparse and inconsistent. In a Belgian cohort, seasonality in the diagnosis of type 1 diabetes was restricted to HLA DR3/DR4‐negative males. 7 However, we did not find any differences in the HLA genetics between the seasons.
4.1. Strengths and limitations
A strength of this cross‐sectional observational study is the large sample derived from a nationwide register including more than 90% of all children diagnosed with diabetes. The retrospective nature of this study is a limitation. There is a possibility of selection bias as 1747 children were excluded because of the lack of samples for analyses. However, the frequencies of seasons of disease presentation did not differ between the included and the excluded children.
5. CONCLUSION
In conclusion, our results from the country with the highest incidence of type 1 diabetes confirmed the seasonality of type 1 diabetes manifestation with peaks in fall and winter. Seasonality of disease presentation was similar in both sexes. Younger children were most seldom diagnosed in winter whereas the peak of the diagnoses continued from fall to winter in older children, suggesting heterogeneity in environmental factors contributing to type 1 diabetes manifestation in children of different ages. Signs of a poorer metabolic status was observed during the seasons with fewer diagnoses, in spring and summer. However, based on the results from this and previous studies, no clear link can be discerned between seasonality, metabolic decompensation and beta‐cell autoimmunity at diagnosis.
CONFLICT OF INTEREST
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
ACKNOWLEDGEMENTS
The authors are grateful to all participants in the FPDR, to the participating hospitals and their staff. We thank P. Bergman (Biostatistics consulting, Department of Public Health, University of Helsinki and Helsinki University Hospital) for her statistical consulting. The Finnish Pediatric Diabetes Register (FPDR) comprises the following investigators: Principal Investigator: M. Knip. Steering Committee: P‐H. Groop, J. Ilonen, T. Otonkoski, R. Veijola. Locally responsible investigators: A. Abram, H. Aito, I. Arkhipov, E. Blanco‐Sequeiros, J. Bondestam, M. Granholm, M. Haapalehto‐Ikonen, T. Horn, H. Huopio, J. Janer, C. Johansson, L. Kalliokoski, P. Keskinen, A. Kinnala, M. Korteniemi, H. Laakkonen, J. Lähde, P. Miettinen, P. Nykänen, E. Popov, M. Pulkkinen, M. Salonen, P. Salonen, J. Sankala, V. Sidoroff, A‐M. Suomi, T. Tiainen, R. Veijola.
Turtinen M, Härkönen T, Ilonen J, Parkkola A, Knip M. Seasonality in the manifestation of type 1 diabetes varies according to age at diagnosis in Finnish children. Acta Paediatr. 2022;111:1061–1069. doi: 10.1111/apa.16282
A complete list of the investigators for the Finnish Pediatric Diabetes Register and Sample Repository are found in the Acknowledgements.
REFERENCES
- 1. Gerasimidi Vazeou A, Kordonouri O, Witsch M, et al. Seasonality at the clinical onset of type 1 diabetes‐Lessons from the SWEET database. Pediatr Diabetes. 2016;17(Suppl):32‐37. doi: 10.1111/pedi.12433 [DOI] [PubMed] [Google Scholar]
- 2. Patterson CC, Gyürüs E, Rosenbauer J, et al. Seasonal variation in month of diagnosis in children with type 1 diabetes registered in 23 European centers during 1989–2008: little short‐term influence of sunshine hours or average temperature. Pediatr Diabetes. 2015;16(8):573‐580. doi: 10.1111/pedi.12227 [DOI] [PubMed] [Google Scholar]
- 3. Lévy‐Marchal C, Patterson C, Green A. Variation by age group and seasonality at diagnosis of childhood IDDM in Europe. The EURODIAB ACE Study Group. Diabetologia. 1995;38(7):823‐830. doi: 10.1007/s001250050359 [DOI] [PubMed] [Google Scholar]
- 4. Moltchanova EV, Schreier N, Lammi N, Karvonen M. Seasonal variation of diagnosis of type 1 diabetes mellitus in children worldwide. Diabet Med. 2009;26(7):673‐678. doi: 10.1111/j.1464-5491.2009.02743.x [DOI] [PubMed] [Google Scholar]
- 5. Hanberger L, Akesson K, Samuelsson U. Glycated haemoglobin variations in paediatric type 1 diabetes: the impact of season, gender and age. Acta Paediatr. 2014;103(4):398‐403. doi: 10.1111/j.1464-5491.2009.02743.x [DOI] [PubMed] [Google Scholar]
- 6. Padaiga Z, Tuomilehto J, Karvonen M, et al. Seasonal variation in the incidence of type 1 diabetes mellitus during 1983 to 1992 in the countries around the Baltic Sea. Diabet Med. 1999;16(9):736‐743. doi: 10.1046/j.1464-5491.1999.00140.x [DOI] [PubMed] [Google Scholar]
- 7. Weets I, Kaufman L, Van der Auwera B, et al. Seasonality in clinical onset of type 1 diabetes in belgian patients above the age of 10 is restricted to HLA‐DQ2/DQ8‐negative males, which explains the male to female excess in incidence. Diabetologia. 2004;47(4):614‐621. doi: 10.1007/s00125-004-1369-8 [DOI] [PubMed] [Google Scholar]
- 8. Dahlquist G, Blom L, Holmgren G, et al. The epidemiology of diabetes in Swedish children 0–14 years–a six‐year prospective study. Diabetologia. 1985;28(11):802‐808. doi: 10.1007/BF00291068 [DOI] [PubMed] [Google Scholar]
- 9. Ronkainen MS, Hämäläinen AM, Koskela P, Akerblom HK, Knip M, Finnish Trigr Study Group . Pregnancy induces nonimmunoglobulin insulin‐binding activity in both maternal and cord blood serum. Clin Exp Immunol. 2001;124(2):190‐196. doi: 10.1046/j.1365-2249.2001.01506.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Savola K, Sabbah E, Kulmala P, Vähäsalo P, Ilonen J, Knip M. Autoantibodies associated with type I diabetes mellitus persist after diagnosis in children. Diabetologia. 1998;41(11):1293‐1297. doi: 10.1007/s001250051067 [DOI] [PubMed] [Google Scholar]
- 11. Savola K, Bonifacio E, Sabbah E, et al. IA‐2 antibodies–a sensitive marker of IDDM with clinical onset in childhood and adolescence. Childhood diabetes in Finland study group. Diabetologia. 1998;41(4):424‐429. doi: 10.1007/s001250050925 [DOI] [PubMed] [Google Scholar]
- 12. Salonen KM, Ryhänen S, Härkönen T, Ilonen J, Knip M, Finnish Pediatric Diabetes Register . Autoantibodies against zinc transporter 8 are related to age, metabolic state and HLA DR genotype in children with newly diagnosed type 1 diabetes. Diabetes Metab Res Rev. 2013;29(8):646‐654. doi: 10.1002/dmrr.2440 [DOI] [PubMed] [Google Scholar]
- 13. Bottazzo GF, Florin‐Christensen A, Doniach D. Islet‐cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2(7892):1279‐1283. [DOI] [PubMed] [Google Scholar]
- 14. Ilonen J, Kiviniemi M, Lempainen J, et al. Genetic susceptibility to type 1 diabetes in childhood ‐ estimation of HLA class II associated disease risk and class II effect in various phases of islet autoimmunity. Pediatr Diabetes. 2016;17(Suppl):8‐16. doi: 10.1111/pedi.12327 [DOI] [PubMed] [Google Scholar]
- 15. Turtinen M, Härkönen T, Parkkola A, Ilonen J, Knip M, Finnish Pediatric Diabetes Register . Sex as a determinant of type 1 diabetes at diagnosis. Pediatr Diabetes. 2018;19(7):1221‐1228. doi: 10.1111/pedi.12697 [DOI] [PubMed] [Google Scholar]
- 16. Mustonen N, Siljander H, Peet A, et al. Early childhood infections precede development of beta‐cell autoimmunity and type 1 diabetes in children with HLA‐conferred disease risk. Pediatr Diabetes. 2018;19(2):293‐299. doi: 10.1111/pedi.12547 [DOI] [PubMed] [Google Scholar]
- 17. Hyöty H, Leon F, Knip M. Developing a vaccine for type 1 diabetes by targeting Coxsackievirus B. Expert Rev Vaccines. 2018;17(12):1071‐1083. doi: 10.1080/14760584.2018.1548281 [DOI] [PubMed] [Google Scholar]
- 18. Jaakola S, Rimhanen‐Finne R, Lyytikäinen O, et al. In: Salminen M, editor. Respiratory infections. Infectious Diseases in Finland 2016. THL; 2016:7‐16. [Google Scholar]
- 19. Miettinen ME, Niinistö S, Erlund I, et al. Serum 25‐hydroxyvitamin D concentration in childhood and risk of islet autoimmunity and type 1 diabetes: the TRIGR nested case‐control ancillary study. Diabetologia. 2020;63(4):780‐787. doi: 10.1007/s00125-019-05077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norris JM, Lee HS, Frederiksen B, et al. Plasma 25‐hydroxyvitamin D concentration and risk of islet autoimmunity. Diabetes. 2018;67(1):146‐154. doi: 10.2337/db17-0802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simpson M, Brady H, Yin X, et al. No association of vitamin D intake or 25‐hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia. 2011;54(11):2779‐2788. doi: 10.1007/s00125-011-2278-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mäkinen M, Mykkänen J, Koskinen M, et al. Serum 25‐hydroxyvitamin D concentrations in children progressing to autoimmunity and clinical type 1 diabetes. J Clin Endocrinol Metab. 2016;101(2):723‐729. doi: 10.1210/jc.2015-3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayer‐Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419‐1429. doi: 10.1056/NEJMoa1610187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parviainen A, But A, Siljander H, Knip M, Finnish Pediatric Diabetes Register . Decreased incidence of type 1 diabetes in young finnish children. Diabetes Care. 2020;43(12):2953‐2958. doi: 10.2337/dc20-0604 [DOI] [PubMed] [Google Scholar]
- 25. Kamrath C, Mönkemöller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID‐19 pandemic in Germany. JAMA. 2020;324(8):801‐804. doi: 10.1001/jama.2020.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salmi H, Heinonen S, Hästbacka J, et al. New‐onset type 1 diabetes in Finnish children during the COVID‐19 pandemic [published online ahead of print May 27, 2021]. Arch Dis Child. 2022;107(2):180‐185. 10.1136/archdischild-2020-321220 [DOI] [PubMed] [Google Scholar]
- 27. Weets I, Truyen I, Verschraegen I, et al. Sex‐ and season‐dependent differences in C‐peptide levels at diagnosis of immune‐mediated type 1 diabetes. Diabetologia. 2006;49(6):1158‐1162. doi: 10.1007/s00125-006-0191-x [DOI] [PubMed] [Google Scholar]
- 28. Samuelsson U, Lindblad B, Carlsson A, et al. Residual beta cell function at diagnosis of type 1 diabetes in children and adolescents varies with gender and season. Diabetes Metab Res Rev. 2013;29(1):85‐89. doi: 10.1002/dmrr.2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michalková D, Mikulecký M, Tomecková E. IA‐2A positivity rate at manifestation of type 1 diabetes mellitus in Slovak children culminates in September. J Pediatr Endocrinol Metab. 2003;16(9):1263‐1265. doi: 10.1515/jpem.2003.16.9.1263 [DOI] [PubMed] [Google Scholar]
- 30. Hagopian WA, Sanjeevi CB, Kockum I, et al. Glutamate decarboxylase‐, insulin‐, and islet cell‐antibodies and HLA typing to detect diabetes in a general population‐based study of Swedish children. J Clin Invest. 1995;95(4):1505‐1511. doi: 10.1172/JCI117822 [DOI] [PMC free article] [PubMed] [Google Scholar]


