Abstract
Oxygen is a life‐saving therapy but, when given inappropriately, may also be hazardous. Therefore, in the acute medical setting, oxygen should only be given as treatment for hypoxaemia and requires appropriate prescription, monitoring and review. This update to the Thoracic Society of Australia and New Zealand (TSANZ) guidance on acute oxygen therapy is a brief and practical resource for all healthcare workers involved with administering oxygen therapy to adults in the acute medical setting. It does not apply to intubated or paediatric patients. Recommendations are made in the following six clinical areas: assessment of hypoxaemia (including use of arterial blood gases); prescription of oxygen; peripheral oxygen saturation targets; delivery, including non‐invasive ventilation and humidified high‐flow nasal cannulae; the significance of high oxygen requirements; and acute hypercapnic respiratory failure. There are three sections which provide (1) a brief summary, (2) recommendations in detail with practice points and (3) a detailed explanation of the reasoning and evidence behind the recommendations. It is anticipated that these recommendations will be disseminated widely in structured programmes across Australia and New Zealand.
Keywords: acute oxygen therapy, oxygen prescription, position statement, target oxygen saturations, titrated oxygen
A. INTRODUCTION
Purpose: The purpose of the Thoracic Society of Australia and New Zealand (TSANZ) Position Statement is to provide simple, practical evidence‐based recommendations for the acute use of oxygen in adults in clinical practice. The intended users are all health professionals responsible for the administration, and/or monitoring of oxygen therapy in the acute management of patients in clinical settings (excluding intubated, mechanically ventilated patients), and those responsible for the training of such health professionals. The Position Statement represents a clinical practice and educational initiative of the TSANZ, which was established to improve the knowledge and understanding of lung disease, to prevent respiratory illness through research and health promotion and to improve health care for people with respiratory disorders (http://www.thoracic.org.au/).
Organization of this Position Statement: The Position Statement contains three sections. Section A contains the background to this document and provides the key recommendations in six headings in Table 1. The concepts behind each of the key recommendations are listed in Table 2. Section B covers those recommendations in detail, along with ‘Practice points’, organized by the same six headings. Finally, Section C summarizes the key evidence and reasoning behind those recommendations, organized similarly under those headings.
Literature review: Targeted literature reviews were conducted by working groups of this committee, to find relevant literature since publication of the original TSANZ Guideline document in 2015. Given the absence of a formal systematic review, this document is designated as a Position Statement. As with the 2015 TSANZ Guideline document, an extensive list of references is not provided, but rather reference is made to key reviews, studies and guidelines where appropriate. The readers are referred to the 2017 British Thoracic Society (BTS) guidelines 1 for a comprehensive review of the acute oxygen therapy literature.
Grading: Grades of recommendation are presented below and are related to the National Health and Medical Research Council grading system (Table 3), based on evidence base, consistency of evidence, clinical impact, generalizability and applicability. 2
Revision Group: The Position Statement Revision Group was chaired by Gregory King, who initiated the formation of the group via the TSANZ. This group included respiratory physicians, respiratory nurses and intensive care physicians, and was formed following a general call for members by the TSANZ.
Main updates compared with the 2015 document: The evidence supporting the recommended SpO2 (arterial oxygen saturation measured by pulse oximeter) targets has been strengthened by recent publications, and the use of oxygen prescription has also been further supported by local experience with the use of various modes of prescription. There has been more recent evidence, since the 2015 document, of the efficacy of humidified nasal high‐flow oxygen (hNHF‐O2) therapy in hypoxaemic respiratory failure and so a substantial section now deals with its use to deliver oxygen. There is a large addition around non‐invasive ventilation (NIV), given its greatly increased use in ventilatory failure in Australia and New Zealand. Recent evidence on the effectiveness of early warning systems that incorporate both oxygen flow rate and SpO2 is included. Finally, the Revision Group was of the opinion that there was declining use of arterial blood gases (ABG) across Australia and New Zealand, which is detrimental to optimal clinical practice. Therefore, the recommendation to use ABG in appropriate clinical settings is strengthened.
Peer review: This statement was sent to all relevant Australian professional societies and colleges for peer review. Comments were received from the Royal Australian College of General Practitioners, the Council of Ambulance Authorities Inc. and the College of Emergency Nursing Australasia. Their comments were reviewed by the Revision Group and incorporated as appropriate, before submission to Respirology for external peer review.
Dissemination plan: The revised document will be freely available as a published open‐access document. The statement will be advertised widely via the TSANZ to relevant universities, area health services, professional bodies and societies.
Implementation: As implementation is key to practice change, recommendations and educational resources were also created and are available via the TSANZ website. Institutions should develop a long‐term implementation plan so that recommendations are used in everyday clinical practice. The limited evidence available suggests that the key recommendations from the 2015 Guideline document are not in widespread use and suggests a widespread failure of implementation. 3 , 4 We suggest that appropriate use of acute oxygen therapy should be taught at undergraduate and post‐graduate levels, and to a range of healthcare staff at all institutions which administer acute oxygen therapy. Critical factors for implementation success include: local ‘oxygen champions’ (medical and/or nursing), supporting infrastructure and processes (e.g., paper or electronic medical record prescribing), decision assist prompts and regular education programmes for both new and longer‐serving staff. It is important to ensure that knowledge and competence are updated and maintained.
Expiry date: 2027.
TABLE 1.
Key recommendations a
(1) Assess oxygenation:
|
(2) Oxygen is a drug and thus requires prescription:
|
(3) Recommended SpO2 targets:
|
(4) Delivery:
|
(5) High FiO2 to achieve target SpO2 indicates serious illness:
|
(6) Acute respiratory acidosis:
|
Abbreviations: ABG, arterial blood gases; COPD, chronic obstructive pulmonary disease; FiO2, fraction of inspired oxygen; hNHF‐O2, humidified nasal high‐flow oxygen; ICU, intensive care unit; NIV, non‐invasive ventilation; PaCO2, arterial partial pressure of carbon dioxide; SpO2, arterial oxygen saturation measured by pulse oximeter.
These recommendations apply to all patients receiving supplemental oxygen in the acute medical setting, but not to those receiving invasive mechanical ventilation.
Except in sickle cell crisis, cluster headache, carbon monoxide and paraquat poisoning and previous bleomycin exposure.
Clinician refers to medical, nursing, physiotherapy and other healthcare professionals responsible for patient care.
TABLE 2.
Concepts behind each of the key recommendations
(1) Assess oxygenation:
|
(2) Oxygen is a drug and thus requires prescription:
|
(3) Recommended SpO2 targets:
|
(4) Delivery:
|
(5) High FiO2 to achieve target SpO2 indicates serious illness:
|
(6) Acute respiratory acidosis:
|
Abbreviations: ABG, arterial blood gases; FiO2, fraction of inspired oxygen; hNHF‐O2, humidified nasal high‐flow oxygen; NIV, non‐invasive ventilation; PaO2, arterial partial pressure of oxygen; SpO2, arterial oxygen saturation measured by pulse oximeter.
TABLE 3.
Grades of recommendation
| Grade of recommendation | Description |
|---|---|
| A | Body of evidence can be trusted to guide practice |
| B | Body of evidence can be trusted to guide practice in most situations |
| C | Body of evidence provides some support for recommendation(s), but care should be taken in its application |
| D | Body of evidence is weak and recommendation must be applied with caution |
B. RECOMMENDATIONS IN DETAIL
-
1.
Assess oxygenation:
-
Pulse oximetry is a ‘vital sign’ to be considered together with other signs, including respiratory rate, and is a predictor of potentially serious clinical events. 5 Pulse oximetry should therefore be available in all clinical situations in which oxygen is used 6 (Grade C).
Practice points:- There is variable accuracy of pulse oximetry to predict SaO2 (arterial oxygen saturation [measured by arterial blood gas]) in acutely ill patients, with SpO2 measurements both over‐ and under‐estimating SaO2, with wide limits of agreement. 6 , 7 , 8 , 9 , 10 , 11 The accuracy of SpO2 may worsen with factors including disease severity and patients' physical characteristics 6 , 7 , 8 , 11 , 12 , 13 , 14 , 15 , 16 (see Section C.1.II). Clinicians need to be aware of, and take into account the variable accuracy of SpO2 in using pulse oximetry in clinical practice and measure ABGs when appropriate.
- There should always be clinical judgement when interpreting SpO2 values, and to avoid over‐reliance on pulse oximeters. In particular, when diagnosis, severity assessment and treatment are dependent on oxygen status, ABG may be necessary.
- In the immediate assessment of an acutely unwell patient, oxygen saturations should be measured by oximetry, pending the availability of ABG if required (see Point 2).
-
ABG measurement should be considered in the following situations (Grade C):
- Critically ill patients with cardiorespiratory or metabolic dysfunction.
- In patients with an SpO2 < 92% in whom hypoxaemia may be present.
- Deteriorating SpO2 requiring increased fraction of inspired oxygen (FiO2).
- Patients at risk of hypercapnia (see below).
- Patients with symptoms or signs compatible with acute respiratory disease in whom a reliable oximetry signal cannot be obtained.
Practice points:- Hypoxaemia requires investigation and treatment of the underlying cause(s), and consideration of the contribution of hypoventilation, including measurement of arterial partial pressure of carbon dioxide (PaCO2) and pH.
- Peripheral venous blood gas (VBG) analysis is a less invasive test; however, it does not provide an accurate estimate of PaCO2 or PaO2. 1 , 16 , 17 It does, however, provide rapid clinically important information to assess acutely unwell patients, including pH, lactate, glucose, haemoglobin, sodium and potassium. A venous partial pressure of carbon dioxide (PCO2) of <40 mm Hg makes hypercapnia unlikely, but does not rule it out. Therefore, exclusion of hypercapnia, when clinically relevant, requires ABG to be measured. 16 , 17
- Arterialized capillary earlobe or fingertip blood gas measurements represent an alternative if unable to obtain ABG, recognizing that whilst providing accurate information about PaCO2 and pH, it variably underestimates PaO2 measurements 18 , 19 As a result, patient assessment can be based on pH and PCO2 levels measured from earlobe or fingertip blood gases, together with SpO2 by pulse oximetry.
- PaCO2 may rise in susceptible individuals given oxygen therapy; therefore, a repeat ABG should be considered in those individuals.
-
-
2.Oxygen is a drug and thus requires prescription:
- A specific oxygen prescription should be documented in the patient medical record and the drug chart (Grade D). 20
- A target SpO2 should be included as part of the prescription.
Practice points:- The minimum requirement for an oxygen prescription is specification of delivery device, range of flow rates that may be administered and target SpO2 range.
-
In its most detailed form, the prescription could include (considerable space on the prescription form is needed to provide such detail; see Figure 1):
- the delivery system and interface,
- the target oxygen saturation range,
- the range of flow rates that may be used for each delivery system,
- specification of SpO2 and FiO2 (or flow rate) at which clinical review should be sought and
- if hNHF‐O2 therapy is utilized, the temperature setting, flow rate and FiO2 of entrained oxygen.
- Oxygen therapy should be initiated or increased if clinically required, prior to prescription. Increasing oxygen requirement indicates underlying disease deterioration, and should result in clinical review.
-
3.Recommended SpO 2 targets (see Figure 2):
- An SpO2 target of 88%–92% is recommended in exacerbations of chronic obstructive pulmonary disease (COPD) (Grade B), 21 and other conditions associated with chronic respiratory failure (such as morbid obesity, 22 obesity hypoventilation syndrome, 23 bronchiectasis, cystic fibrosis, 24 neuromuscular disease and chest wall deformities such as severe kyphoscoliosis) (Grade C). Where there is diagnostic uncertainty as to whether COPD is the primary cause of the exacerbation, it may be preferable to titrate oxygen therapy to the 88%–92% SpO2 target range (Grade C). 21 , 25 , 26
- Oxygen can be titrated using a closed‐loop control system in which there is automated adjustment of the delivered oxygen concentration in response to continuous measurement of SpO2. This results in a greater proportion of time within a prescribed target SpO2 range compared to manual oxygen titration, in non‐ventilated adult patients with acute illnesses using standard nasal cannulae, face mask 36 , 37 , 38 or hNHF‐O2 39 , 40 (Grade B).
Practice points:-
In the presence of COPD or conditions associated with chronic respiratory failure:
- If SpO2 ≥ 88%, oxygen therapy is not initially required.
- If SpO2 < 88%, oxygen can be administered via a 24% or 28% Venturi mask, at 1–2 L/min via nasal cannulae, or via a hNHF‐O2 device, and titrated to achieve the target SpO2.
- If an SpO2 target of ≥92% is considered in such patients, ABGs are required to be measured to exclude hypercapnia, before adopting this target.
- The avoidance of inappropriate high‐concentration oxygen therapy may be facilitated by the provision of a COPD oxygen alert card, 41 bracelet or warning in the medical record.
-
In the absence of COPD or known chronic respiratory failure:
- If SpO2 ≥ 92%, oxygen therapy is not routinely required.
- If SpO2 is 85%–91%, oxygen can be initially instituted at 2–4 L/min via nasal cannulae or other suitable oxygen delivery method, and titrated to achieve the target SpO2. In many situations, this range of oxygen saturations is unlikely to be associated with risk, although oxygen is commonly administered.
- If SpO2 < 85%, oxygen can be initiated at 4 L/min via nasal cannulae, through a simple face mask at 5–10 L/min, a 100% non‐rebreather reservoir mask at 15 L/min or hNHF‐O2 device (FiO2 > 0.35). The method of oxygen administration will depend on the SpO2 level, with higher FiO2 administered in response to increasingly more severe reductions in SpO2. Oxygen is titrated to achieve the target SpO2 as soon as practically possible.
-
If oximetry is not available or reliable SpO2 cannot be determined and hypoxaemia is suspected, oxygen can be delivered at:
- 24% or 28% Venturi mask, or 1–2 L/min via nasal cannulae in patients with acute exacerbations of COPD or conditions known to be associated with chronic respiratory failure.
- 2–4 L/min oxygen via nasal cannulae in patients who are not critically ill and life‐threatening hypoxaemia is not suspected.
- 5–10 L/min via simple face mask, 15 L/min through a 100% non‐rebreather reservoir mask or high‐flow nasal cannulae (FiO2 > 0.35) in patients in whom life‐threatening hypoxaemia is suspected (see Figure 2).
-
Supplemental oxygen should not be used in normoxic patients in an attempt to protect against subsequent hypoxaemia, in the event of deterioration such as worsening gas exchange and/or alveolar ventilation:
- A high SaO2 due to supplemental oxygen obscures deterioration in SpO2.
- In this situation, there is likely to be no major change in vital signs 42 and no marked decrease in SpO2 as assessed by pulse oximetry 43 , 44 until a potentially life‐threatening situation has developed. This may potentially delay recognition of the deterioration and thus provide a false reassurance that the patient is stable. 45 , 46 At this late stage, further increasing oxygen therapy will have limited benefit while medical review and higher‐level interventions, such as ventilation and transfer to an high‐dependency unit (HDU) or intensive care unit (ICU), are undertaken.
-
4.
Delivery:
-
For most patients, standard nasal cannulae are the preferred method of oxygen delivery, with the flow rate varied to achieve the target oxygen saturation.
Practice point:
The FiO2 levels delivered by the different delivery systems may vary considerably between patients and be influenced by a number of factors, including respiratory rate and whether the patient's mouth is open or closed. 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 Approximate FiO2 values delivered by different delivery systems are:- Standard nasal cannulae can deliver an FiO2 of 0.24–0.35 at an oxygen flow of 1–4 L/min.
- Venturi masks can deliver an FiO2 of 0.24–0.60.
- hNHF‐O2 cannulae can deliver an FiO2 of 0.21–1.0.
- A simple face mask can deliver an FiO2 of 0.35–0.60 at an oxygen flow of 5‐10 L/min.
- A 100% non‐rebreather reservoir mask at 15 L/min can deliver an FiO2 of >0.60.
For simple face masks, flow rates of <5 L/min should be avoided due to the potential risk of carbon dioxide rebreathing (Grade C). 55 , 56
hNHF‐O2 devices deliver heated, humidified oxygen via wide‐bore nasal cannulae at delivered concentrations up to 100% and at high‐flow rates up to 70 L/min. The recommendations in this document only apply when oxygen is being delivered via this device and not, for example, when it is used to deliver warm, humidified air. It should be noted that there are other names used for these types of devices which perform the same function (e.g. high‐flow nasal oxygen, high‐flow nasal prong and high‐flow nasal cannula). hNHF‐O2 should be considered in selected patients with severe, hypoxaemic respiratory failure (PaO2:FiO2 < 300) (Grade B). Given the paucity of high‐quality studies, current evidence does not support the routine use of hNHF‐O2 treatment in acute respiratory acidosis. 57
Fully conscious hypoxaemic patients should be allowed to position themselves according to their preference (Grade D). In some, but not all, patients, upright posture may result in improved oxygenation. 58 , 59
-
In COPD and other conditions associated with chronic respiratory failure, if bronchodilator is required, the preferred method of administration is via metered dose inhaler (MDI) ± spacer (or via air‐driven nebulizer if considered necessary), with supplementary nasal oxygen continued as required (usually via nasal cannulae) for both modes of bronchodilator delivery (Grade B). 21 , 60
Practice points:- When hNHF‐O2 is used to treat serious illness with acute, hypoxaemic respiratory failure, ABG should be measured to exclude acute respiratory acidosis. It also allows calculation of PaO2:FiO2 ratio which is a useful indicator of severity of illness. Because hNHF‐O2 use is indicated for serious illness, recommendations below in Section B.5: ‘High FiO2 to achieve target SpO2 indicates serious illness’ should be considered.
In asthma, if bronchodilator is required, the preferred method of delivery is by MDI ± spacer. If a nebulizer is required, then it should be air‐driven (Grade C). 64 In both situations, if oxygen therapy is needed, it should continue via standard nasal cannulae during bronchodilator administration.
All aerosol‐generating procedures, for example, hNHF‐O2 and non‐invasive ventilatory support (non‐invasive ventilation [NIV] and continuous positive airway pressure [CPAP]), pose a risk of transmission of viral infection to staff and patients. While these therapies offer significant benefits to some patients, there are often alternative approaches to management that have less risk of transmitting viral infection via aerosolization. This is of particular relevance during the 2019 SARS‐CoV‐2 pandemic where protection from aerosol spread remains standard practice.
If hNHF‐O2 is used in a patient with suspected or known respiratory viral illness, including SARS‐CoV‐2, the patient must be fitted with an interface (e.g., face mask) to minimize leak and managed with the highest level of isolation available (class N‐negative pressure room is optimal, single room with door closed is adequate) with five‐piece personal protective equipment (PPE) (N95 or equivalent mask, gloves, goggles/glasses, gown, hat) precautions for healthcare personnel. Local facility infection control measures must be adopted (see https://www.health.gov.au/committees-and-groups/infection-control-expert-group-iceg and https://covid19evidence.net.au/about-the-taskforce/) (Grade D).
NIV and CPAP are delivered by a mask. Patients with suspected or proven respiratory viral illness requiring CPAP/NIV should receive this via a circuit using a non‐vented mask with a filtered external expiratory port. Staff should wear five‐piece PPE, and the patient should be in the highest available level of isolation. Local facility infection control measures must be adopted 65 (Grade D).
-
-
5.
High FiO 2 to achieve target SpO 2 indicates serious illness:
A high FiO2 to maintain adequate SpO2 is a clinical indicator of severe illness, and in this situation, there is limited capacity to increase FiO2 to avoid life‐threatening hypoxaemia should deterioration occur. Patients who need an estimated FiO2 of ≥0.40, such as ≥6 L/min via a simple face mask, to maintain an adequate SpO2, should receive senior clinician review and may require transfer to a facility such as HDU, where there are appropriate numbers of competent staff able to provide more intensive monitoring and therapy (Grade D).
-
Patients who need an estimated FiO2 of ≥0.50, such as ≥8 L/min via a simple face mask, to maintain an adequate SpO2, should receive ICU review and most will require a higher level of monitoring and supportive care which an ICU/HDU environment can provide (Grade D).
Practice points:- A reduction in SpO2 while the FiO2 is maintained, or increasing FiO2 requirements to maintain SpO2, should lead to clinical review of the patient.
- For patients whose oxygen saturations improve with oxygen therapy to above the target oxygen saturation range, oxygen therapy can be reduced or stopped. Oxygen saturation monitoring should continue to allow detection of any subsequent deterioration of the underlying condition and the requirement to increase or resume oxygen therapy.
-
Early Warning Score (EWS) systems include a number of clinical parameters which together predict in‐patient deterioration and subsequent life‐threatening adverse patient events. EWS systems recommended for use in New Zealand and across Australia vary markedly.
- It is recommended that EWS systems include scores that reflect (i) supplemental oxygen administration (i.e., scores increase as oxygen flow or FiO2 delivery increases) and (ii) patient SpO2 (i.e., scores increase as SpO2 decreases) (Grade C).
- Current evidence suggests that the Queensland Adult Deterioration Detection System (Q‐ADDS) outperforms other systems. 66
Practice point:- It is important that practitioners are familiar with the EWS system implemented in their healthcare organization. If no system is implemented, then the healthcare organization should consider using the Q‐ADDS.
-
6.
Acute respiratory acidosis:
In patients with acute respiratory acidosis, in whom ABG show a pH < 7.35 and PaCO2 > 45 mm Hg, NIV or invasive ventilation should be considered. 67 , 68 , 69 , 70 Target SpO2 during NIV for acute respiratory acidosis should be 88%–92% (Grade A). COPD patients managed with NIV require regular review to assess response to treatment or deterioration, in which case they may need intubation. Assessment should be based on clinical and biochemical parameters (e.g., O2 requirements, pH, etc.) (Grade C). 69
-
In patients in whom oxygen‐induced hypercapnia is suspected, oxygen therapy should be titrated to maintain the 88%–92% target oxygen saturation range and not be abruptly stopped due to the risk of profound rebound hypoxaemia (Grade C). 71 , 72 , 73
Practice point:- Reducing FiO2 to appropriate levels may improve the acute respiratory acidosis. This should be determined by repeat ABG.
In patients with severe cardiogenic pulmonary oedema, CPAP should be considered (Grade A). 74
While NIV is not routinely recommended in acute hypoxaemic respiratory failure, its use may be considered in certain groups such as immunosuppressed patients with pulmonary infiltrates requiring ventilatory support (Grade C). 70 , 75 , 76 , 77 , 78 , 79
It is recommended that patients receiving ventilatory support are located in a ward area such as close observation unit (COU), HDU or ICU (and may include a general ward), where it is essential that there are adequate numbers of staff experienced in ventilatory support to provide an appropriate level of monitoring, clinical expertise and titration of therapy (Grade D). 68 , 69 , 80 , 81
An individualized NIV treatment plan which covers prescription of therapy, escalation and de‐escalation of NIV/CPAP, goals of therapy and ‘ceiling of care’ should be documented (Grade D). 68 , 80
FIGURE 1.
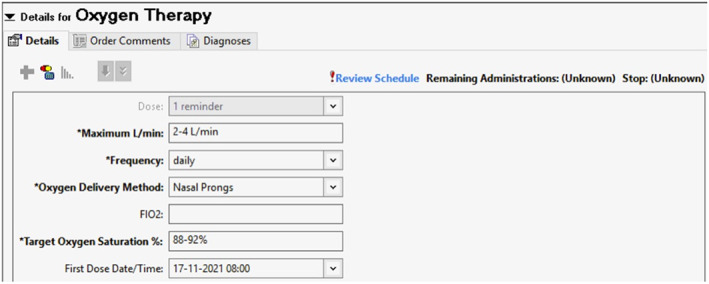
Example of oxygen prescription form (Westmead Hospital, NSW; courtesy: Jimmy Chien and Mary Roberts)
FIGURE 2.
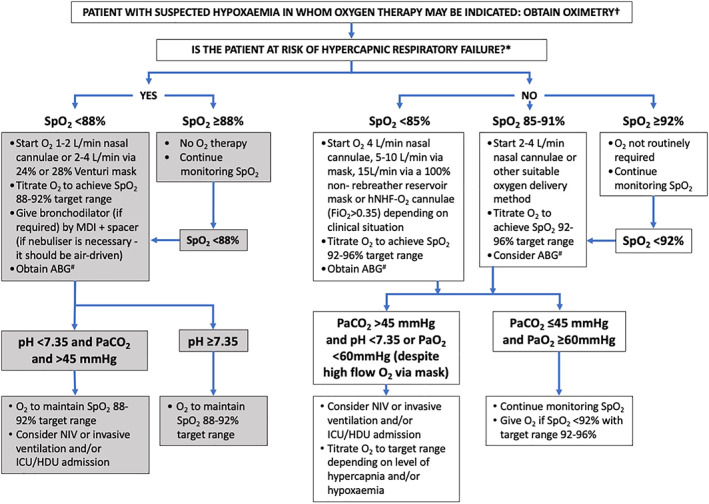
Treatment algorithm for oxygen therapy. †If oximetry is not available, or reliable oxygen saturations cannot be determined and hypoxaemia is suspected, oxygen can be delivered at: (1) 1–2 L/min via nasal cannulae or 2–4 L/min via 24% or 28% Venturi mask in patients with acute exacerbations of COPD or conditions known to be associated with chronic respiratory failure (*such as obesity hypoventilation syndrome, chest wall deformities, cystic fibrosis, bronchiectasis, neuromuscular disease and COPD); (2) 2–4 L/min via nasal cannulae in patients who are not critically ill and life‐threatening hypoxaemia is not suspected; and (3) 5–10 L/min via simple face mask or 15 L/min through a reservoir mask in patients who are critically ill or in whom life‐threatening hypoxaemia is suspected (e.g., post‐cardiac arrest or resuscitation, shock, sepsis, near drowning, anaphylaxis, major head injury or in suspected carbon monoxide poisoning). NIV or invasive ventilation and transfer to HDU or ICU should also be considered in this situation. #When administering acute O2 treatment in the community, the flow chart ends at the point of ABG, since this is not used in this setting. ABG, arterial blood gases; COPD, chronic obstructive pulmonary disease; HDU, high‐dependency unit; hNHF‐O2, humidified nasal high‐flow oxygen; ICU, intensive care unit; MDI, metered dose inhaler; NIV, non‐invasive ventilation; O2, oxygen; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; Sats, oxygen saturations; SpO2, arterial oxygen saturation measured by pulse oximeter
C. BACKGROUND EVIDENCE FOR RECOMMENDATIONS
-
Assess oxygenation:
Hypoxaemia is both a marker of risk of a poor outcome due to the severity of the underlying disease(s) that has caused hypoxaemia, and an independent risk factor of poor outcome in its own right. 82 , 83 No absolute safe lower limit of PaO2 or SaO2 can be set. The clinical effects of hypoxaemia depend on numerous factors including speed of onset and severity, duration, patient age, body temperature, disease chronicity, comorbidities such as anaemia, underlying acute conditions and their associated oxygen demand, physiological factors such as cardiovascular function which influence oxygen delivery to the tissues and individual variation in susceptibility to hypoxaemia. 84 For example, some patients with acute exacerbations of COPD and chronic respiratory failure may tolerate PaO2 values of between 20 and 40 mm Hg, equivalent to an SaO2 value of around 40%–70%, 85 and acclimatized elite mountaineers may tolerate an SaO2 of between 35% and 70% descending Mt Everest. 86 In contrast, healthy adults in simulated high‐altitude conditions may become confused at an SpO2 around 65%, progressing to imminent unconsciousness around 55%. 87 It has been proposed that a PaO2 of 50 mm Hg (6.6 kPa) can be considered as the safe lower limit of hypoxaemia in patients with COPD, 18 and that oxygen therapy which achieves a PaO2 of at least 50 mm Hg would prevent immediate death from hypoxaemia. 88
Factors that may affect the accuracy of pulse oximetry include severe hypoxaemia and hypercapnia, 11 , 12 , 16 sepsis, 11 carboxyhaemoglobin and methaemoglobin levels, 8 anaemia, dark skin, 14 low perfusion and low body temperature 13 causing SpO2 to overestimate SaO2, while excessive ambient light and nail polish cause underestimation. 6 , 15
A systematic review and meta‐analysis comparing ABG and VBG measurements 17 reported that the 95% prediction interval of bias for PaCO2 was wide, from −10.7 to +2.4 mm Hg; PaO2 was significantly higher than the venous partial pressure of oxygen (PO2) by 36.9 mmHg (95% CI 27.2–46.6 mm Hg); the pH values were similar with the arterial pH 0.03 being higher than the venous pH (95% CI 0.029–0.038). A subsequent comparison of ABG and VBG in COPD found that venous PCO2 was up to 21 mm Hg higher than arterial PCO2 (95th percentile), but could also be lower than ABG by 11 mm Hg (5th percentile). 16 Therefore, clinicians should guard against over‐reliance on venous PCO2, particularly as diagnosis of hypercapnic and metabolic acidosis has far‐reaching implications. Thus, accurate diagnosis using ABG is required in those clinical situations but is likely being under‐used in acute medical emergencies in Australia and NZ.
-
Oxygen is a drug and thus requires prescription:
Oxygen is used to treat hypoxaemia, not breathlessness. Oxygen therapy does not relieve breathlessness in the absence of hypoxaemia. For example, there is no clinical benefit with short‐burst oxygen therapy in COPD patients with breathlessness, 89 , 90 or with the use of oxygen over room air via nasal cannulae for patients with COPD who do not have severe resting hypoxaemia. Similarly, there is no additional symptomatic benefit in the use of daily oxygen over room air via nasal cannulae for refractory breathlessness in the palliative setting. 91 Thus, in the absence of hypoxaemia, oxygen therapy is not indicated except in carbon monoxide poisoning, cluster headaches or sickle cell crisis.
The specification of a range of O2 flow rates that can be administered with a particular delivery device is to avoid flow rates being increased to maintain SpO2 within the specified target range, without considering potential underlying clinical deterioration and need for urgent clinical review. Hence, if SpO2 cannot be maintained within the specified target SpO2 range using the specified range of O2 flow rates, then this requires clinical review.
The potential risks due to hyperoxaemia with high‐concentration oxygen therapy include respiratory (increased PaCO2, absorption atelectasis and direct pulmonary toxicity), cardiovascular (increased systemic vascular resistance and blood pressure, reduced coronary artery blood flow, reduced cardiac output), cerebrovascular (reduced cerebral blood flow) effects and increased reperfusion injury due to increased reactive oxygen species. 91 , 92 , 93 , 94 , 95 , 96
The physiological response of an increase in PaCO2 due to high‐concentration oxygen therapy has been demonstrated not only in stable and acute exacerbations of COPD, 85 but also in severe asthma, 27 , 97 community‐acquired pneumonia, 28 morbid obesity 22 and obesity hypoventilation syndrome. 23 Proposed mechanisms for oxygen‐induced hypercapnia include increased ventilation perfusion mismatch due to reduced hypoxic pulmonary vasoconstriction, reduced ventilatory drive, atelectasis and the Haldane effect (increased PaCO2 due to displacement from haemoglobin by O2), with the contribution of each likely to depend on the clinical situation. 1
-
Recommended SpO 2 targets:
-
A target SpO2 range of 88%–92% is recommended in the treatment of COPD and other conditions associated with chronic respiratory failure due to demonstration of:
A greater than two‐fold reduction in mortality with pre‐hospital oxygen therapy titrated to this target, compared with high‐concentration oxygen therapy in patients with an acute exacerbation of COPD. 21
In hospitalized COPD patients receiving supplemental oxygen during an exacerbation, the risk of mortality is greater in those within the 93%–96% oxygen saturation range, compared within the 88%–92% range, when data are adjusted for baseline mortality risk. 98
A clinically significant increase in PaCO2 results from several minutes of 100% oxygen therapy in patients with chronic respiratory failure due to obesity hypoventilation syndrome. 23
A general target SpO2 range of 92%–96% in acute medical conditions, excluding invasively ventilated patients, has been recommended, based on the evidence from the systematic review and meta‐analysis of all randomized controlled trials comparing liberal versus conservative oxygen therapy in critically ill adults. 99 A total of 25 randomized controlled trials enrolled 16,037 patients with sepsis, critical illness, stroke, trauma, myocardial infarction or cardiac arrest, and patients who had emergency surgery. Compared with a conservative oxygen strategy, a liberal oxygen strategy (median baseline SpO2 across trials, 96% [range 94%–99%, IQR 96–98]) was associated with an increased risk of mortality in‐hospital (relative risk [RR] 1.21, 95% CI 1.03–1.43). Morbidity outcomes were similar between groups. Findings were robust to trial sequential, subgroup and sensitivity analyses.
An international expert panel report, which used the Lancet review 99 to inform guidelines, made a strong recommendation for maintaining an SpO2 of no more than 96% in acutely unwell patients (upper limit). 100 The panel suggested that patients with acute stroke or myocardial infarction and an SpO2 ≥ 90% not receive supplemental oxygen (a weak recommendation if SpO2 is 90%–92% and a strong recommendation if SpO2 is 93%–100%). The findings from two recent studies of mechanically ventilated patients suggest that there is no benefit of conservative versus liberal oxygen therapy in this setting, 101 , 102 and thus there are potential differences in how oxygen should be used between mechanically ventilated and other acute medical patients.
-
Physiological support for the suggested target range:
An SpO2 of 92% is a practical lower threshold to rule out hypoxaemia, defined as an SaO2 < 90% 8 or a PaO2 < 60 mm Hg (8 kPa). 7
There is no known risk of hypoxic tissue injury at an SaO2 of 90%.
Older healthy subjects have SaO2 levels to this lower level of 90%. 103 , 104
Healthy subjects have a mean nadir SpO2 of around 90% during sleep. 105
Subjects with sleep‐disordered breathing commonly tolerate SpO2 levels between 70% and 90% for prolonged periods. 105
Adults with comorbidities tolerate SpO2 levels between 80% and 90% during long‐distance travel. 106
In adults with coronary artery disease, anaerobic metabolism indicative of myocardial ischaemia is observed in some patients with SaO2 between 70% and 85%, suggesting a ‘safe’ lower limit of oxygen saturation of 90%. 107
There is a significant variability in SpO2 targets recommended in national and international guidelines in acute cardiac and medical emergencies. For example, the National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand recommend supplemental oxygen therapy in patients suffering from acute coronary syndrome or heart failure when oxygen saturations are <94%, while recognizing that those with comorbid COPD should have target SpO2 of between 88% and 92%. 108 , 109 In comparison, the American College of Cardiology/American Heart Association recommend supplemental oxygen in patients suffering from non‐ST elevation acute coronary syndrome when oxygen saturations are <90%, are in respiratory distress or have high‐risk features of hypoxaemia. 110 The European Society of Cardiology guidelines for the management of acute heart failure recommend giving supplemental oxygen to patients with oxygen saturations <90% or have a PaO2 of <60 mm Hg. 111
-
A recommended target SpO2 range of 85% in patients with prior exposure to bleomycin or in paraquat poisoning is due to the demonstration of:
The evidence that oxygenation above target oxygen saturations delays deterioration of SpO2 comes from physiological studies of hypoventilation 43 , 44 and from modelling of rapidly increasing right to left shunt. 46 These studies show that there is clinically significant delay in SpO2 falling, despite halving of ventilation and increasing alveolar partial pressure of CO2. Although this has direct implications to patients receiving sedating drugs, SpO2 may respond differently in other acute medical emergencies, where higher than the recommended target ranges for SpO2 are achieved with supplemental oxygen.
-
-
Delivery:
-
The potential advantages of conventional nasal cannulae as an initial method of delivering oxygen therapy are:
Ability to give bronchodilator by MDI ± spacer or air‐driven nebulizer at the same time as oxygen is administered.
Oxygen can be prescribed by variable flows to achieve a target saturation range rather than a fixed FiO2, although oxygenation may be maintained better with Venturi mask. 47
Comfort, ease of use and low cost.
Less likely to be taken off to eat or speak, and less likely to fall off.
No risk of rebreathing of carbon dioxide.
However, skin damage from pressure areas and nasal drying may occur.
hNHF‐O2 delivers oxygen therapy at higher flow rates and FiO2 than conventional oxygen therapy (simple face mask, nasal cannulae or Venturi mask) and, therefore, is a way to administer greater respiratory support in seriously ill patients. The higher flow rates (up to 70 L/min) can be titrated to provide sufficient flow according to the patients' minute ventilation, provide positive end‐expiratory pressure (PEEP) > 5 cm H2O depending on flow rate and other factors 112 , 113 and reduce upper airway dead‐space by washing out CO2.
-
Potential advantages of hNHF‐O2 compared with standard O2 therapy include:
Reduced risk of endotracheal intubation in patients with hypoxaemic respiratory failure (although it is not associated with reduced mortality). 57 , 79 , 114 , 115
Reduced respiratory rate and level of dyspnoea, and improved gas exchange (PaO2:FiO2 ratio) with reduced accessory muscle activation.
Heated and humidified gas may facilitate greater comfort and airway secretion clearance.
Preservation of upper airway function (e.g., speech, cough and swallowing).
-
Potential disadvantages of hNHF‐O2 devices include:
Risk of complacency if a high FiO2 requirement is not recognized to represent life‐threatening illness requiring more than correction of hypoxaemia.
Role in severe exacerbations of COPD and asthma has not been clarified, with studies ongoing.
The efficacy of hNHF‐O2 has been studied in a variety of patients suffering from acute, severe, hypoxaemic respiratory failure. 79 , 115 , 116 The inclusion criteria for patients varied between studies and mostly included patients with pneumonia and sepsis. In most studies, patients with reduced Glasgow Coma Scale (GCS) and/or a suspected inability to maintain the upper airway were excluded. Use of hNHF‐O2 in conditions such as asthma or COPD exacerbations with acute respiratory acidosis, and cardiogenic pulmonary oedema has not been established. Therefore, the use of hNHF‐O2 should be carefully considered to ensure that it is used in appropriate clinical settings.
The risk of spread of infectious diseases from aerosol‐generating procedures, which includes all delivery devices discussed in this document, remains uncertain. Although the oxygen delivery devices generate aerosols which potentially spread infectious agents, the absolute risk and RRs (compared with talking and coughing) are not currently able to be quantified. However, aerosol spread (hence the risk of spread of viral infection) may be considerably less than previously thought. 65 , 117 , 118 , 119 , 120
-
-
High FiO 2 to achieve target SpO 2 indicates serious illness:
A comparison of 33 different EWS systems 121 showed those assigning scores to oxygen saturation (from pulse oximetry) and oxygen administration outperformed systems that did not include these parameters for predicting in‐patient deterioration and subsequent adverse patient events. The best performing of these 33 systems was the National Early Warning Score (NEWS), introduced in the UK in 2012. Since then, numerous EWS systems have been developed. Those used in Australia and New Zealand vary markedly, including significant variation in how oxygen supplementation and pulse oximetry components are scored. The largest validation of Australian systems to date 66 compared the performance of statewide systems in New South Wales (Between The Flags, BTF) and Queensland (Q‐ADDS), and two other international systems (including NEWS). This showed Q‐ADDS outperformed BTF in predicting adverse outcomes.
It is recommended that EWS systems include both scores that reflect supplemental oxygen administration (that increase with higher oxygen flow or percentage delivered) and patient oxygen saturation (that increase as saturation decreases). However, assigning scores for hyperoxia in EWS for patients at risk of type 2 respiratory failure receiving supplemental oxygen was not shown to improve system performance and therefore, the current evidence base does not support this approach. 122
Increasing respiratory rate is a highly sensitive marker of clinical deterioration and high respiratory rates are strong predictors of serious events, such as cardiac arrest. Although respiratory rate measurement is common to all EWS (and thus does not contribute to differences in performance), it is nevertheless an important measure of the severity of underlying illness and predictor of poor outcome. 5
Patients who fail hNHF‐O2 therapy and require intubation may be predicted with an index derived from FiO2, SpO2 and respiratory rate. 123 However, this score requires a more complex calculation to be performed and has only been validated in patients with respiratory failure from pneumonia or pneumonitis. EWS systems improve the detection of deterioration irrespective of the underlying pathology. 124
-
6.
Acute respiratory acidosis:
-
V.
There are local, national and international guidelines on the use of NIV and CPAP in acute medical emergencies, which provide concise and practical information on appropriate use. The reader is directed to those guidelines and statements for in‐depth reviews that support those recommendations. 67 , 68 , 69 , 80 , 125
-
VI.
NIV and CPAP may be used to deliver mechanical respiratory support via a nasal or oro‐nasal mask in clinically indicated scenarios, when oxygen therapy alone is insufficient. NIV provides higher inspiratory and lower expiratory pressures, thus providing ventilatory support, while CPAP maintains the same airway pressure during the entire respiratory cycle. Higher FiO2 concentrations can also be delivered via nasal or oro‐nasal masks compared to nasal cannulae or open face mask. There are variations in delivery circuits for NIV and CPAP to suit the clinical environments and applications (e.g., single limb filtered circuits used during the COVID‐19 pandemic or dual limb circuits).
-
VII.
NIV and CPAP require specific expertise to administer and to monitor response. Therefore, patients should be clinically managed in environments which provide sufficient technical support for administration, continuous monitoring, sufficient nursing expertise and nursing ratios (1:2 or 1:3) and appropriate medical support. In most situations, this would be in an ICU, HDU, COU or a medical ward with high level of experienced staffing, continuous monitoring and support. 68 , 69 , 80
-
VIII.
NIV may be indicated for acute respiratory acidosis, in conditions such as COPD, non‐cystic fibrosis and cystic fibrosis related bronchiectasis, neuromuscular and chest wall disease and obesity hypoventilation syndrome. 68 , 69 , 80 Measurement of ABG is necessary prior to or, as soon as practical, after the initiation of NIV to confirm and measure the severity of respiratory acidosis and to monitor treatment response.
-
IX.
NIV is not routinely indicated in acute hypoxaemic respiratory failure without acidosis. Results from clinical trials and observational studies have provided mixed results for various patient groups, 70 , 76 , 126 , 127 , 128 , 129 while weak evidence from a recent meta‐analysis suggests that NIV may reduce mortality and the risk of intubation. 79 There may be a role for NIV, if clinically indicated in immunocompromised patients with pulmonary infiltrates. 70 , 75 , 78 , 127 , 129 Should NIV be given in acute hypoxaemic respiratory failure without acidosis, it must be carefully considered as more severe illness (i.e., higher respiratory and heart rates, worse acidosis and hypoxaemia and impaired consciousness) predicts NIV failure. Delaying intubation and NIV failure are associated with increased mortality. Therefore, it must be delivered by suitably experienced clinicians and be regularly reviewed, ideally in ICU/HDU given the need for intubation should NIV fail. 130
-
X.
Helmet NIV is at least as efficacious as oro‐nasal delivery in hypoxaemic and hypercapnic respiratory failure. There may be advantages in application (e.g., improved comfort and possibly improved outcomes) compared with oro‐nasal administration or hNHF‐O2. However, there is little/no experience of its use in Australia and NZ and further studies to establish benefit are needed. 79 , 130 , 131
-
XI.
Asthma is highly heterogeneous; it may have a clinical appearance similar to COPD with long‐standing, irreversible airways obstruction. In contrast, other patients may have acute, severe bronchospasm but have normal lung function at other times. There is little evidence to support the use of NIV in acute severe asthma, either with or without acute respiratory acidosis (there has only been a single trial of NIV in the former 132 ). As such, use of NIV in asthma should be carefully considered and should not delay intubation and mechanical ventilation which result in excellent outcomes when administered. 69
-
XII.
In acute pulmonary oedema and acutely decompensated chronic heart failure, NIV or CPAP may reduce intubation and mortality compared with O2 therapy, with little evidence of benefit of NIV over CPAP. However, first‐line treatment for these patients is pharmacological therapy, with escalation to non‐invasive ventilatory support if clinically required. Caution should be exercised in acute cardiac failure with hypotension where NIV may reduce mean arterial blood pressure further.
-
V.
CONFLICT OF INTEREST
Gregory King, Catherine Buchan, Jimmy Chien, Claude S. Farah, Christine F. McDonald, Belinda Miller, Maitri Munsif, Alex Psirides, Lynette Reid, Mary Roberts, Natasha Smallwood and Sheree Smith declared no conflicts of interest with regard to the topic of this paper. Richard Beasley has received research funding from Fisher and Paykel Healthcare.
Supporting information
Video Abstract. Summary of the Thoracic Society of Australia and New Zealand Position Statement on Acute Oxygen Use in Adults, presented by Gregory King.
Barnett A, Beasley R, Buchan C, Chien J, Farah CS, King G, et al. Thoracic Society of Australia and New Zealand Position Statement on Acute Oxygen Use in Adults: ‘Swimming between the flags’. Respirology. 2022;27:262–276. 10.1111/resp.14218
Associate Editor: Chi Chiu Leung; Senior Editor: Philip Bardin
This document has been endorsed by the Thoracic Society of Australia and New Zealand Board on 18 February 2022. It is due for review in 2027. The authors are listed in alphabetical order.
REFERENCES
- 1. O'Driscoll BR, Howard LS, Earis J, Mak V. British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ Open Respir Res. 2017;4(1):e000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Health and Medical Research Council . 2016 NHMRC Standards for Guidelines. 2016. [Accessed: December, 2021]. Available from: https://www.nhmrc.gov.au/guidelinesforguidelines/standards
- 3. Cousins JL, Wark PA, McDonald VM. Acute oxygen therapy: a review of prescribing and delivery practices. Int J Chron Obstruct Pulmon Dis. 2016;11:1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cousins JL, Wark PAB, Hiles SA, McDonald VM. Understanding clinicians' perceived barriers and facilitators to optimal use of acute oxygen therapy in adults. Int J Chron Obstruct Pulmon Dis. 2020;15:2275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cretikos MA, Bellomo R, Hillman K, Chen J, Finfer S, Flabouris A. Respiratory rate: the neglected vital sign. Med J Aust. 2008;188(11):657–9. [DOI] [PubMed] [Google Scholar]
- 6. Pretto JJ, Roebuck T, Beckert L, Hamilton G. Clinical use of pulse oximetry: official guidelines from the Thoracic Society of Australia and New Zealand. Respirology. 2014;19(1):38–46. [DOI] [PubMed] [Google Scholar]
- 7. Kelly AM, McAlpine R, Kyle E. How accurate are pulse oximeters in patients with acute exacerbations of chronic obstructive airways disease? Respir Med. 2001;95(5):336–40. [DOI] [PubMed] [Google Scholar]
- 8. Lee WW, Mayberry K, Crapo R, Jensen RL. The accuracy of pulse oximetry in the emergency department. Am J Emerg Med. 2000;18(4):427–31. [DOI] [PubMed] [Google Scholar]
- 9. Modica R, Rizzo A. Accuracy and response time of a portable pulse oximeter. The Pulsox‐7 with a finger probe. Respiration. 1991;58(3–4):155–7. [DOI] [PubMed] [Google Scholar]
- 10. Perkins GD, McAuley DF, Giles S, Routledge H, Gao F. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit Care. 2003;7(4):R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson BJ, Cowan HJ, Lord JA, Zuege DJ, Zygun DA. The accuracy of pulse oximetry in emergency department patients with severe sepsis and septic shock: a retrospective cohort study. BMC Emerg Med. 2010;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muñoz X, Torres F, Sampol G, Rios J, Martí S, Escrich E. Accuracy and reliability of pulse oximetry at different arterial carbon dioxide pressure levels. Eur Respir J. 2008;32(4):1053–9. [DOI] [PubMed] [Google Scholar]
- 13. Ebmeier SJ, Barker M, Bacon M, Beasley RC, Bellomo R, Knee Chong C, et al. A two centre observational study of simultaneous pulse oximetry and arterial oxygen saturation recordings in intensive care unit patients. Anaesth Intensive Care. 2018;46(3):297–303. [DOI] [PubMed] [Google Scholar]
- 14. Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. N Engl J Med. 2020;383(25):2477–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coté CJ, Goldstein EA, Fuchsman WH, Hoaglin DC. The effect of nail polish on pulse oximetry. Anesth Analg. 1988;67(7):683–6. [PubMed] [Google Scholar]
- 16. McKeever TM, Hearson G, Housley G, Reynolds C, Kinnear W, Harrison TW, et al. Using venous blood gas analysis in the assessment of COPD exacerbations: a prospective cohort study. Thorax. 2016;71(3):210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Byrne AL, Bennett M, Chatterji R, Symons R, Pace NL, Thomas PS. Peripheral venous and arterial blood gas analysis in adults: are they comparable? A systematic review and meta‐analysis. Respirology. 2014;19:168–75. [DOI] [PubMed] [Google Scholar]
- 18. Murphy R, Thethy S, Raby S, Beckley J, Terrace J, Fiddler C, et al. Capillary blood gases in acute exacerbations of COPD. Respir Med. 2006;100(4):682–6. [DOI] [PubMed] [Google Scholar]
- 19. Zavorsky GS, Cao J, Mayo NE, Gabbay R, Murias JM. Arterial versus capillary blood gases: a meta‐analysis. Respir Physiol Neurobiol. 2007;155(3):268–79. [DOI] [PubMed] [Google Scholar]
- 20. Dodd ME, Kellet F, Davis A, Simpson JC, Webb AK, Haworth CS, et al. Audit of oxygen prescribing before and after the introduction of a prescription chart. BMJ. 2000;321(7265):864–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Austin MA, Wills KE, Blizzard L, Walters EH, Wood‐Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pilcher J, Richards M, Eastlake L, McKinstry SJ, Bardsley G, Jefferies S, et al. High flow or titrated oxygen for obese medical inpatients: a randomised crossover trial. Med J Aust. 2017;207(10):430–4. [DOI] [PubMed] [Google Scholar]
- 23. Wijesinghe M, Williams M, Perrin K, Weatherall M, Beasley R. The effect of supplemental oxygen on hypercapnia in subjects with obesity‐associated hypoventilation: a randomized, crossover, clinical study. Chest. 2011;139(5):1018–24. [DOI] [PubMed] [Google Scholar]
- 24. Gozal D. Nocturnal ventilatory support in patients with cystic fibrosis: comparison with supplemental oxygen. Eur Respir J. 1997;10(9):1999–2003. [DOI] [PubMed] [Google Scholar]
- 25. Denniston AK, O'Brien C, Stableforth D. The use of oxygen in acute exacerbations of chronic obstructive pulmonary disease: a prospective audit of pre‐hospital and hospital emergency management. Clin Med (Lond). 2002;2(5):449–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hale KE, Gavin C, O'Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25(11):773–6. [DOI] [PubMed] [Google Scholar]
- 27. Perrin K, Wijesinghe M, Healy B, Wadsworth K, Bowditch R, Bibby S, et al. Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax. 2011;66(11):937–41. [DOI] [PubMed] [Google Scholar]
- 28. Wijesinghe M, Perrin K, Healy B, Weatherall M, Beasley R. Randomized controlled trial of high concentration oxygen in suspected community‐acquired pneumonia. J R Soc Med. 2012;105(5):208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bismuth C, Garnier R, Dally S, Fournier PE, Scherrmann JM. Prognosis and treatment of paraquat poisoning: a review of 28 cases. J Toxicol Clin Toxicol. 1982;19(5):461–74. [DOI] [PubMed] [Google Scholar]
- 30. Cersosimo RJ, Matthews SJ, Hong WK. Bleomycin pneumonitis potentiated by oxygen administration. Drug Intell Clin Pharm. 1985;19(12):921–3. [DOI] [PubMed] [Google Scholar]
- 31. Fairshter RD, Rosen SM, Smith WR, Glauser FL, McRae DM, Wilson AF. Paraquat poisoning: new aspects of therapy. Q J Med. 1976;45(4):551–65. [PubMed] [Google Scholar]
- 32. Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72(5):745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weaver LK. Carbon monoxide poisoning. N Engl J Med. 2009;360(12):1217–25. [DOI] [PubMed] [Google Scholar]
- 34. Weaver LK. Carbon monoxide poisoning. Undersea Hyperb Med. 2020;47(1):151–69. [PubMed] [Google Scholar]
- 35. Tetzlaff K, Shank ES, Muth CM. Evaluation and management of decompression illness—an intensivist's perspective. Intensive Care Med. 2003;29(12):2128–36. [DOI] [PubMed] [Google Scholar]
- 36. L'Her E, Dias P, Gouillou M, Riou A, Souquiere L, Paleiron N, et al. Automatic versus manual oxygen administration in the emergency department. Eur Respir J. 2017;50(1):1602552. [DOI] [PubMed] [Google Scholar]
- 37. L'Her E, Jaber S, Verzilli D, Jacob C, Huiban B, Futier E, et al. Automated closed‐loop versus standard manual oxygen administration after major abdominal or thoracic surgery: an international multicentre randomised controlled study. Eur Respir J. 2020;57:2000182. [DOI] [PubMed] [Google Scholar]
- 38. Lellouche F, Bouchard P‐A, Roberge M, Simard S, L'Her E, Maltais F, et al. Automated oxygen titration and weaning with FreeO2 in patients with acute exacerbation of COPD: a pilot randomized trial. Int J Chron Obstruct Pulmon Dis. 2016;11(1):1983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harper JC, Kearns NA, Maijers I, Bird GE, Braithwaite I, Shortt NP, et al. Closed‐loop oxygen control using a novel nasal high‐flow device: a randomized crossover trial. Respir Care. 2020;66(3):416–24. [DOI] [PubMed] [Google Scholar]
- 40. Harper J, Kearns N, Bird G, Braithwaite I, Eathorne A, Shortt N, et al. Automatic versus manual oxygen titration using a novel nasal high‐flow device in medical inpatients with an acute illness: a randomised controlled trial. BMJ Open Respir Res. 2021;8:e000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gooptu B, Ward L, Ansari SO, Eraut CD, Law D, Davison AG. Oxygen alert cards and controlled oxygen: preventing emergency admissions at risk of hypercapnic acidosis receiving high inspired oxygen concentrations in ambulances and A&E departments. Emerg Med J. 2006;23(8):636–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thrush DN, Downs JB, Hodges M, Smith RA. Does significant arterial hypoxemia alter vital signs? J Clin Anesth. 1997;9(5):355–7. [DOI] [PubMed] [Google Scholar]
- 43. Niesters M, Mahajan RP, Aarts L, Dahan A. High‐inspired oxygen concentration further impairs opioid‐induced respiratory depression. Br J Anaesth. 2013;110(5):837–41. [DOI] [PubMed] [Google Scholar]
- 44. Fu ES, Downs JB, Schweiger JW, Miguel RV, Smith RA. Supplemental oxygen impairs detection of hypoventilation by pulse oximetry. Chest. 2004;126(5):1552–8. [DOI] [PubMed] [Google Scholar]
- 45. Beasley R, Aldington S, Robinson G. Is it time to change the approach to oxygen therapy in the breathless patient? Thorax. 2007;62(10):840–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Downs JB, Smith RA. Increased inspired oxygen concentration may delay diagnosis and treatment of significant deterioration in pulmonary function. Crit Care Med. 1999;27(12):2844–6. [DOI] [PubMed] [Google Scholar]
- 47. Bazuaye EA, Stone TN, Corris PA, Gibson GJ. Variability of inspired oxygen concentration with nasal cannulas. Thorax. 1992;47(8):609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boumphrey SM, Morris EA, Kinsella SM. 100% inspired oxygen from a Hudson mask – a realistic goal? Resuscitation. 2003;57(1):69–72. [DOI] [PubMed] [Google Scholar]
- 49. Garcia JA, Gardner D, Vines D, Shelledy D, Wettstein R, Peters J. The oxygen concentrations delivered by different oxygen therapy systems. Chest. 2005;128(4):389S. [Google Scholar]
- 50. Jeffrey AA, Warren PM. Should we judge a mask by its cover? Thorax. 1992;47(7):543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marino P, Sutin K. The ICU book. Philadelphia: Lippincott, Williams & Wilkins; 2007. [Google Scholar]
- 52. Waldau T, Larsen VH, Bonde J. Evaluation of five oxygen delivery devices in spontaneously breathing subjects by oxygraphy. Anaesthesia. 1998;53(3):256–63. [DOI] [PubMed] [Google Scholar]
- 53. Walls R, Murphy M. Manual of emergency airway management. Philadelphia: Lippincott, Williams & Wilkins; 2012. [Google Scholar]
- 54. Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low‐flow and high‐flow nasal cannulas. Respir Care. 2005;50(5):604–9. [PubMed] [Google Scholar]
- 55. Bethune DW, Collis JM. An evaluation of oxygen therapy equipment. Experimental study of various devices on the human subject. Thorax. 1967;22(3):221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jensen AG, Johnson A, Sandstedt S. Rebreathing during oxygen treatment with face mask. The effect of oxygen flow rates on ventilation. Acta Anaesthesiol Scand. 1991;35(4):289–92. [DOI] [PubMed] [Google Scholar]
- 57. Huang Y, Lei W, Zhang W, Huang J‐a. High‐flow nasal cannula in hypercapnic respiratory failure: a systematic review and meta‐analysis. Can Respir J. 2020;2020:7406457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hardie JA, Mørkve O, Ellingsen I. Effect of body position on arterial oxygen tension in the elderly. Respiration. 2002;69(2):123–8. [DOI] [PubMed] [Google Scholar]
- 59. Tyson S, Nightingale P. The effects of position on oxygen saturation in acute stroke: a systematic review. Clin Rehabil. 2004;18:863–71. [DOI] [PubMed] [Google Scholar]
- 60. Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess. 2001;5(26):1–149. [DOI] [PubMed] [Google Scholar]
- 61. Bardsley G, Pilcher J, McKinstry S, Shirtcliffe P, Berry J, Fingleton J, et al. Oxygen versus air‐driven nebulisers for exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. BMC Pulm Med. 2018;18(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Edwards L, Perrin K, Williams M, Weatherall M, Beasley R. Randomised controlled crossover trial of the effect on PtCO2 of oxygen‐driven versus air‐driven nebulisers in severe chronic obstructive pulmonary disease. Emerg Med J. 2012;29(11):894–8. [DOI] [PubMed] [Google Scholar]
- 63. Gunawardena KA, Patel B, Campbell IA, MacDonald JB, Smith AP. Oxygen as a driving gas for nebulisers: safe or dangerous? Br Med J (Clin Res Ed). 1984;288(6413):272–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta‐agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;2013(9):CD000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Landry SA, Barr JJ, MacDonald MI, Subedi D, Mansfield D, Hamilton GS, et al. Viable virus aerosol propagation by positive airway pressure (PAP) circuit leak and mitigation with a ventilated patient hood. Eur Respir J. 2020;57:2003666. [DOI] [PubMed] [Google Scholar]
- 66. Campbell V, Conway R, Carey K, Tran K, Visser A, Gifford S, et al. Predicting clinical deterioration with Q‐ADDS compared to NEWS, Between the Flags, and eCART track and trigger tools. Resuscitation. 2020;153:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. [DOI] [PubMed] [Google Scholar]
- 68. Sanchez D, Smith G, Piper A, Rolls K. Non–invasive ventilation guidelines for adult patients with acute respiratory failure: a clinical practice guideline. Chatswood NSW: Agency for Clinical Innovation NSW Government Version 1; 2014. ISBN:978‐1‐74187‐954‐4. https://www.aci.health.nsw.gov.au/networks/icnsw/intensive‐care‐manual/statewide‐guidelines/non‐invasive‐ventilation‐guidelines [Google Scholar]
- 69. Davidson AC, Banham S, Elliott M, Kennedy D, Gelder C, Glossop A, et al. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. 2016;71(Suppl 2):ii1–ii35. [DOI] [PubMed] [Google Scholar]
- 70. Nava S, Hill N. Non‐invasive ventilation in acute respiratory failure. Lancet. 2009;374(9685):250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Campbell EJ. Respiratory failure: the relation between oxygen concentrations of inspired air and arterial blood. Lancet. 1960;2(7140):10–1. [DOI] [PubMed] [Google Scholar]
- 72. Kane B, Turkington PM, Howard LS, Davison AG, Gibson GJ, O'Driscoll BR. Rebound hypoxaemia after administration of oxygen in an acute exacerbation of chronic obstructive pulmonary disease. BMJ. 2011;342:d1557. [DOI] [PubMed] [Google Scholar]
- 73. Rudolf M, Turner JAM, Harrison BDW, Riordan JF, Saunders KB. Changes in arterial blood gases during and after a period of oxygen breathing in patients with chronic hypercapnic respiratory failure and in patients with asthma. Clin Sci. 1979;57(5):389–96. [DOI] [PubMed] [Google Scholar]
- 74. Peter JV, Moran JL, Phillips‐Hughes J, Graham P, Bersten AD. Effect of non‐invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta‐analysis. Lancet. 2006;367(9517):1155–63. [DOI] [PubMed] [Google Scholar]
- 75. Antonelli M, Conti G, Bufi M, Costa MG, Lappa A, Rocco M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000;283(2):235–41. [DOI] [PubMed] [Google Scholar]
- 76. Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto Meduri G. Acute respiratory failure in patients with severe community‐acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1585–91. [DOI] [PubMed] [Google Scholar]
- 77. Ferrer M, Esquinas A, Leon M, Gonzalez G, Alarcon A, Torres A. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med. 2003;168(12):1438–44. [DOI] [PubMed] [Google Scholar]
- 78. Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi‐Benissan G, Dupon M, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344(7):481–7. [DOI] [PubMed] [Google Scholar]
- 79. Ferreyro BL, Angriman F, Munshi L, Del Sorbo L, Ferguson ND, Rochwerg B, et al. Association of noninvasive oxygenation strategies with all‐cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta‐analysis. JAMA. 2020;324(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Davies M, Allen M, Bentley A, Bourke SC, Creagh‐Brown B, D'Oliveiro R, et al. British Thoracic Society Quality Standards for acute non‐invasive ventilation in adults. BMJ Open Respir Res. 2018;5(1):e000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Osadnik CR, Tee VS, Carson‐Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non‐invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7(7):CD004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bowton DL, Scuderi PE, Haponik EF. The incidence and effect on outcome of hypoxemia in hospitalized medical patients. Am J Med. 1994;97(1):38–46. [DOI] [PubMed] [Google Scholar]
- 83. Cameron L, Pilcher J, Weatherall M, Beasley R, Perrin K. The risk of serious adverse outcomes associated with hypoxaemia and hyperoxaemia in acute exacerbations of COPD. Postgrad Med J. 2012;88:684–9. [DOI] [PubMed] [Google Scholar]
- 84. Beasley R, Mackle D, Young P. Oxygen: a new look at an old therapy. J R Soc N Z. 2019;49(2):126–42. [Google Scholar]
- 85. Murphy R, Driscoll P, O'Driscoll R. Emergency oxygen therapy for the COPD patient. Emerg Med J. 2001;18(5):333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Grocott MPW, Martin DS, Levett DZH, McMorrow R, Windsor J, Montgomery HE. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009;360(2):140–9. [DOI] [PubMed] [Google Scholar]
- 87. Hoffman CE, Clark RT Jr, Brown EB Jr. Blood oxygen saturations and duration of consciousness in anoxia at high altitudes. Am J Physiol. 1946;145:685–92. [DOI] [PubMed] [Google Scholar]
- 88. Hutchison DC, Flenley DC, Donald KW. Controlled oxygen therapy in respiratory failure. Br Med J. 1964;2(5418):1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. O'Neill B, Bradley JM, Heaney L, O'Neill C, MacMAHON J. Short burst oxygen therapy in chronic obstructive pulmonary disease: a patient survey and cost analysis. Int J Clin Pract. 2005;59(7):751–3. [DOI] [PubMed] [Google Scholar]
- 90. Moore RP, Berlowitz DJ, Denehy L, Pretto JJ, Brazzale DJ, Sharpe K, et al. A randomised trial of domiciliary, ambulatory oxygen in patients with COPD and dyspnoea but without resting hypoxaemia. Thorax. 2011;66(1):32–7. [DOI] [PubMed] [Google Scholar]
- 91. Abernethy AP, McDonald CF, Frith PA, Clark K, Herndon JE 2nd, Marcello J, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double‐blind, randomised controlled trial. Lancet. 2010;376(9743):784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Beasley R, McNaughton A, Robinson G. New look at the oxyhaemoglobin dissociation curve. Lancet. 2006;367(9517):1124–6. [DOI] [PubMed] [Google Scholar]
- 93. McHugh G, Freebairn R. Optimal oxygen therapy in the critically ill patient with respiratory failure. Curr Respir Med Rev. 2010;6(4):229–37. [Google Scholar]
- 94. Ridler N, Plumb J, Grocott M. Oxygen therapy in critical illness: friend or foe? A review of oxygen therapy in selected acute illnesses. J Intensive Care Soc. 2014;15(3):190–8. [Google Scholar]
- 95. Sjöberg F, Singer M. The medical use of oxygen: a time for critical reappraisal. J Intern Med. 2013;274(6):505–28. [DOI] [PubMed] [Google Scholar]
- 96. Thomson AJ, Webb DJ, Maxwell SRJ, Grant IS. Oxygen therapy in acute medical care. BMJ. 2002;324(7351):1406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rodrigo GJ, Verde MR, Peregalli V, Rodrigo C. Effects of short‐term 28% and 100% oxygen on PaCO2 and peak expiratory flow rate in acute asthma: a randomized trial. Chest. 2003;124(4):1312–7. [DOI] [PubMed] [Google Scholar]
- 98. Echevarria C, Steer J, Wason J, Bourke S. Oxygen therapy and inpatient mortality in COPD exacerbation. Emerg Med J. 2021;38(3):170–7. [DOI] [PubMed] [Google Scholar]
- 99. Chu DK, Kim LHY, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta‐analysis. Lancet. 2018;391(10131):1693–705. [DOI] [PubMed] [Google Scholar]
- 100. Siemieniuk RAC, Chu DK, Kim LH‐Y, Güell‐Rous M‐R, Alhazzani W, Soccal PM, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. [DOI] [PubMed] [Google Scholar]
- 101. Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382(11):999–1008. [DOI] [PubMed] [Google Scholar]
- 102. The ICU‐ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group . Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2019;382(11):989–98. [DOI] [PubMed] [Google Scholar]
- 103. Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1525–31. [DOI] [PubMed] [Google Scholar]
- 104. Hardie JA, Vollmer WM, Buist AS, Ellingsen I, Mørkve O. Reference values for arterial blood gases in the elderly. Chest. 2004;125(6):2053–60. [DOI] [PubMed] [Google Scholar]
- 105. Gries RE, Brooks LJ. Normal oxyhemoglobin saturation during sleep: how low does it go? Chest. 1996;110(6):1489–92. [DOI] [PubMed] [Google Scholar]
- 106. Akerø A, Christensen CC, Edvardsen A, Skjønsberg OH. Hypoxaemia in chronic obstructive pulmonary disease patients during a commercial flight. Eur Respir J. 2005;25(4):725–30. [DOI] [PubMed] [Google Scholar]
- 107. Neill WA. Effects of arterial hypoxemia and hyperoxia on oxygen availability for myocardial metabolism: patients with and without coronary heart disease. Am J Cardiol. 1969;24(2):166–71. [DOI] [PubMed] [Google Scholar]
- 108. Atherton JJ, Sindone A, De Pasquale CG, Driscoll A, MacDonald PS, Hopper I, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ. 2018;27(10):1123–208. [DOI] [PubMed] [Google Scholar]
- 109. Chew DP, Scott IA, Cullen L, French JK, Briffa TG, Tideman PA, et al. National Heart Foundation of Australia & Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes 2016. Heart Lung Circ. 2016;25(9):895–951. [DOI] [PubMed] [Google Scholar]
- 110. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC guideline for the management of patients with non–ST‐elevation acute coronary syndromes. Circulation. 2014;130(25):e344–426. [DOI] [PubMed] [Google Scholar]
- 111. Ponikowski P, Voors AA, Anker SD, Bueno H, JGF C, AJS C, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. [DOI] [PubMed] [Google Scholar]
- 112. Okuda M, Tanaka N, Naito K, Kumada T, Fukuda K, Kato Y, et al. Evaluation by various methods of the physiological mechanism of a high‐flow nasal cannula (HFNC) in healthy volunteers. BMJ Open Respir Res. 2017;4(1):e000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high‐flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest. 2015;148(1):253–61. [DOI] [PubMed] [Google Scholar]
- 114. Rochwerg B, Einav S, Chaudhuri D, Mancebo J, Mauri T, Helviz Y, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46(12):2226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rochwerg B, Granton D, Wang DX, Helviz Y, Einav S, Frat JP, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta‐analysis. Intensive Care Med. 2019;45(5):563–72. [DOI] [PubMed] [Google Scholar]
- 116. Baldomero AK, Melzer A, Greer N, Majeski BN, Macdonald R, Wilt TJ. Effectiveness and harms of high‐flow nasal oxygen (HFNO) for acute respiratory failure: a systematic review protocol. BMJ Open. 2020;10(2):e034956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hamilton F, Arnold D, Bzdek BR, Dodd J, White C, Murray J, et al. Aerosol generating procedures: are they of relevance for transmission of SARS‐CoV‐2? Lancet Respir Med. 2021;9(7):687–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hui DS, Chow BK, Lo T, Tsang OTY, Ko FW, Ng SS, et al. Exhaled air dispersion during high‐flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53(4):1802339. [DOI] [PubMed] [Google Scholar]
- 119. Ip M, Tang JW, Hui DSC, Wong ALN, Chan MTV, Joynt GM, et al. Airflow and droplet spreading around oxygen masks: a simulation model for infection control research. Am J Infect Control. 2007;35(10):684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wilson NM, Marks GB, Eckhardt A, Clarke AM, Young FP, Garden FL, et al. The effect of respiratory activity, non‐invasive respiratory support and facemasks on aerosol generation and its relevance to COVID‐19. Anaesthesia. 2021;76(11):1465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Smith GB, Prytherch DR, Schmidt PE, Featherstone PI. Review and performance evaluation of aggregate weighted 'track and trigger' systems. Resuscitation. 2008;77(2):170–9. [DOI] [PubMed] [Google Scholar]
- 122. Pimentel MAF, Redfern OC, Gerry S, Collins GS, Malycha J, Prytherch D, et al. A comparison of the ability of the National Early Warning Score and the National Early Warning Score 2 to identify patients at risk of in‐hospital mortality: a multi‐centre database study. Resuscitation. 2019;134:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high‐flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368–76. [DOI] [PubMed] [Google Scholar]
- 124. Prower E, Grant D, Bisquera A, Breen CP, Camporota L, Gavrilovski M, et al. The ROX index has greater predictive validity than NEWS2 for deterioration in Covid‐19. EClinicalMedicine. 2021;35:100828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. National Institute for Health and Care Excellence Chronic obstructive pulmonary disease in over 16s: diagnosis and management. London: National Institute for Health and Care Excellence (NICE); 2019 Jul. PMID: 31211541. [PubMed]
- 126. Murad A, Li PZ, Dial S, Shahin J. The role of noninvasive positive pressure ventilation in community‐acquired pneumonia. J Crit Care. 2015;30(1):49–54. [DOI] [PubMed] [Google Scholar]
- 127. Keenan SP, Sinuff T, Burns KE, Muscedere J, Kutsogiannis J, Mehta S, et al. Clinical practice guidelines for the use of noninvasive positive‐pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011;183(3):E195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lim WS, Smith DL, Wise MP, Welham SA. British Thoracic Society community acquired pneumonia guideline and the NICE pneumonia guideline: how they fit together. Thorax. 2015;70(7):698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. AlYami MA, AlAhmari MD, Alotaibi H, AlRabeeah S, AlBalawi I, Mubasher M. Evaluation of efficacy of non‐invasive ventilation in non‐COPD and non‐trauma patients with acute hypoxemic respiratory failure: a systematic review and meta‐analysis. Ann Thorac Med. 2015;10(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Piraino T. Noninvasive respiratory support in acute hypoxemic respiratory failure. Respir Care. 2019;64(6):638–46. [DOI] [PubMed] [Google Scholar]
- 131. Chaudhuri Dipayan, Jinah Rehman, Burns Karen E.A., Angriman Federico, Ferreyro Bruno, Munshi Laveena, Goligher Ewan, Scales Damon, Cook Deborah J., Mauri Tommaso, Rochwerg Bram Helmet non‐invasive ventilation compared to facemask non‐invasive ventilation and high flow nasal cannula in acute respiratory failure: a systematic review and meta‐analysis. European Respiratory Journal. 2021;2101269. 10.1183/13993003.01269-2021 [DOI] [PubMed] [Google Scholar]
- 132. Meduri GU, Cook TR, Turner RE, Cohen M, Leeper KV. Noninvasive positive pressure ventilation in status asthmaticus. Chest. 1996;110(3):767–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Abstract. Summary of the Thoracic Society of Australia and New Zealand Position Statement on Acute Oxygen Use in Adults, presented by Gregory King.


