Abstract
Aim
The protective effects of Kangaroo mother care (KMC) on the neurodevelopment of preterm infants are well established, but we do not know whether the benefits persist beyond infancy. Our aim was to determine whether providing KMC in infancy affected brain volumes in young adulthood.
Method
Standardised cognitive, memory and motor skills tests were used to determine the brain volumes of 20‐year‐old adults who had formed part of a randomised controlled trial of KMC versus incubator care. Multivariate analysis of brain volumes was conducted according to KMC exposure.
Results
The study comprised 178 adults born preterm: 97 had received KMC and 81 were incubator care controls. Bivariate analysis showed larger volumes of total grey matter, basal nuclei and cerebellum in those who had received KMC, and the white matter was better organised. This means that the volumes of the main brain structures associated with intelligence, attention, memory and coordination were larger in the KMC group. Multivariate lineal regression analysis demonstrated the direct relationship between brain volumes and duration of KMC, after controlling for potential confounders.
Conclusion
Our findings suggest that the neuroprotective effects of KMC for preterm infants persisted beyond childhood and improved their lifetime functionality and quality of life.
Keywords: grey matter, Kangaroo mother care, magnetic resonance imaging, premature infants, white matter
Abbreviations
- IQ
intelligence quotient
- KMC
Kangaroo mother care
- MRI
magnetic resonance imaging
- NHPT
Nine‐hole Peg Test
- RCT
randomised controlled trial
- WASI‐II
Wechsler Abbreviated Scale of Intelligence, Second Edition
Key Notes.
We investigated whether the protective effects of Kangaroo mother care (KMC) on the neurodevelopment of preterm infants persisted beyond infancy.
Brain scans and cognitive, memory and motor skills tests were performed on 20‐year‐old adults who had participated in a randomised controlled trial of KMC versus incubator care.
The volumes of the main brain structures associated with intelligence, attention, memory and coordination were larger in infants who had received KMC.
1. BACKGROUND
High rates of premature birth, and the increased survival of extremely preterm infants, have brought together clinicians, researchers and policy‐makers to identify priorities. Authors such as McCormick and Litt, 1 Saigal 2 and Zelkowitz 3 have realistically assessed the difficulties of studying this population and identified research priorities for prematurity.
One of the most serious problems that affects preterm infants is the impact on neurodevelopment and ulterior central nervous system functioning and performance. The central nervous system grows rapidly during the third trimester, and brain volume increases by almost five times between 26 and 40 weeks of gestation. 4 Critical neurodevelopmental processes take place during the second and third trimesters of gestation and continue beyond birth. These include neuronal migration, synaptogenesis, organisational development of cortical layers and circuitry. Prematurity disrupts key phases of brain growth, development and organisation, even when there are no specific injuries to the perinatal central nervous system. 5 Decreased brain volumes do not recover during childhood and have been associated with decreased measures of intelligence and executive functioning. 6
Kangaroo mother care (KMC) is evidence‐based technology that centres on the mother as the primary provider of heat and stimulation. It involves skin‐to‐skin contact in the Kangaroo position, Kangaroo nutrition in the form of maternal breast milk and close monitoring after early hospital discharge. 7 A growing body of evidence indicates that KMC decreases mortality and morbidity rates among preterm infants, 8 promotes breastfeeding 8 , 9 and increases mother–infant bonding and attachment. 10 It also has lasting neuroprotective effects, at least during childhood. 11 Most of the limited number of studies that have been conducted on the long‐term impact of KMC on neurodevelopmental outcomes in preterm infants have been conducted by our research group. 11
The aim of this study was to assess the long‐term impact of KMC on brain volume and its association with cognitive development. The study comprised 20‐year‐old adults who had been born preterm and had taken part in a randomised controlled trial (RCT) of KMC versus incubator care. We hypothesised that KMC would encourage better brain tissue growth, maturation and pathway formation. These have been associated with better cognitive and motor functioning in infants who received KMC after their normal in utero brain growth and development was disrupted by preterm delivery.
2. PATIENTS AND METHODS
2.1. Population and sample
This was a long‐term follow‐up study of an RCT that was conducted in Bogota, Colombia, between 1993 and 1996. The RCT studied 746 preterm and full‐term infants who were born weighing less than 2000 g. Participants were stratified into 4 categories, according to their birthweight: <1200 g, 1200–1500 g, 1501–1800 g and 1801–2000 g. Subjects in each group were randomly allocated to either KMC or the control group. The present follow‐up study involved the 433 subjects born weighing up to 1800 g, 412 of these survived up to one year of age, and 264 were traced and re‐enrolled in this study between 2012 and 2014.
In 1995, we assembled and followed a cohort of full‐term, healthy newborn infants born at the same hospital where the original RCT was performed. We traced 37 of them between 2012 and 2014, and they underwent the same neuroimaging tests as the RCT participants, in order to provide reference values for the present study.
2.2. Interventions in the original RCT
At the time of the initial study, KMC began once the infant could suck and swallow properly, without treatment. The KMC intervention had three components: exclusive or nearly exclusive breastfeeding, prolonged, continuous skin‐to‐skin contact in the Kangaroo position and early home discharge in KMC with daily follow‐up visits at an outpatient KMC clinic. These daily visits continued until the appropriate weight gain had been documented, and then, they took place weekly until the infant had reached 40 weeks of gestational age.
The preterm infants who received traditional care were kept in incubators until they achieved temperature regulation and were discharged according to hospital practice, when they weighed around 1700 g. They received the usual outpatient care that was available under social security insurance.
Both groups received standardised follow‐up care, with periodic evaluations until 1 year of corrected age. The details of the RCT have previously been published. 10 , 12 , 13
2.3. Variables of interest at 20 years of age
The outcomes of interest at 20 years of age were the cerebral volumes of grey and white matter and organisation of white matter, as determined by fractional anisotropy. Expert neuroradiologists measured segments of cortical thickness and volumes of subcortical structures, including the corpus callosum and amygdala, with diffusion tensor imaging of the cerebral tracts of white matter.
Neurological and neuropsychological tests were administered to measure performance in specific areas. Cognitive performance was measured using the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI‐II), 14 fine motor skills and coordination by the Nine‐hole Peg Test (NHPT) 15 and learning and memory by the California Verbal Learning Test, Second Edition. 16 All three tests were administered by trained psychologists following standardised protocols. The results of these tests will be analysed in depth in a later study.
Magnetic resonance imaging (MRI) and functional MRIs were performed using a Philips Achieva 3T scanner with a 16‐channel SENSE coil (Philips, Amsterdam, Netherlands). Brain structures were segmented in the FreeSurfer image analysis suite (Laboratory for Computational Neuroimaging, Harvard University, Massachusetts, USA) 17 with the aseg and aparc atlases 18 and cortical surface parcellation. 19 Diffusion tensor image post‐processing was performed with Tracula (Laboratory for Computational Neuroimaging) and FreeSurfer 20 to obtain the main white matter pathways and with Camino (Microstructure Imaging Group—University College London, UK) 21 to estimate brain fibres by mapping fractional anisotropy and mean diffusivity. Interactive visualisation of the data was performed using BRAVIZ (IMAGINE: Visual Computing Group, University of los Andes, Bogota, Colombia), 22 based on R software (R Foundation, Vienna, Austria).
The exposure variable was the original random allocation to KMC or control treatment. The duration of KMC in days was used to determine whether there was a dose–response relationship between the duration of KMC and the outcome variables. These variables were the volumes of brain structures and neurological function test scores. By definition, the control group had no days in KMC. If infants in the KMC group were re‐hospitalised without their parents, the duration of hospitalisation was subtracted from the duration of KMC. Researchers and observers were not aware of the original allocation of the participant.
2.4. Control variables
The control variables and potential confounders were the parents’ demographics, education and socioeconomic status at the time of the birth. The child's variables were their antenatal and perinatal anthropometrics and general health at birth. These included their weight, gestational age, Apgar score and intrauterine restriction, and any event before they became eligible for KMC.
2.5. Statistical analysis
More infants in the control group than KMC group died during the 20‐year follow‐up period and we had anticipated a survival cohort effect, with a possible imbalance of potential confounders. Therefore, instead of adjusting for potential individual confounders of the association between exposure and outcome, we fitted a Rasch model to estimate the overall degree of vulnerability (fragility index) attributable to the factors present before allocation 23 in the original sample of 746 participants. We selected 15 unevenly distributed binary indicators of harm during pregnancy, birth or the neonatal period before randomisation, according to individual factorial scores. This assumed that a joint latent variable measured the non‐specific fragility of an infant. Seven retrospective measures, namely binary indicators, were added to account for additional imbalances in the risk factors arising from the survival cohort effect in the 441 re‐enrolled survivors who weighed up to 1800 g. These were added to cover any damage that occurred during the perinatal period. The new items were retrospective measures that are listed in Table 1: indicators 16, 19, 20 and 22 at 1 year of age and indicators 15 and 18 at 20 years. The 22 binary items provided a score that represented a severity index. This provided a composite variable that represented the joint potential for confounding and was used as a covariate in the adjusted analyses (Table 1).
TABLE 1.
Parameters included in the severity index (two‐parameter logistic Rasch model)
| Parameter | Criterion | Difficulty | Discrimination |
|---|---|---|---|
| Multiple pregnancies | Yes | –5.20 | –0.31 |
| Preterm | <37 weeks of gestational age | –1.51 | 2.09 |
| Intrauterine growth retardation | Yes | –0.59 | –1.00 |
| Gestational age at birth | ≤34 weeks | –0.38 | 1.65 |
| Acute suffering | Yes | –0.35 | 0.32 |
| Neonatal hospital stay | Yes, with or without intensive care unit | –0.26 | 4.36 |
| Pathological jaundice | Yes | 0.42 | 4.14 |
| Aminoglycosides | Yes | 0.53 | 3.25 |
| Mechanical ventilation (days) | ≥1 day | 1.38 | 3.41 |
| Birth weight | <1500 g | 1.48 | 1.64 |
| Intensive care unit stay (days) | ≥15 days | 2.03 | 3.56 |
| Extrauterine adaptation | Not spontaneous | 2.10 | 0.74 |
| Nosocomial infection | Yes | 2.75 | 1.34 |
| Periventricular leukomalacia | Yes | 2.77 | 1.79 |
| Severe bilateral visual or auditive deficit | Yes | 3.24 | 0.87 |
| Ophthalmological examination at 1 year | No regressive retinopathy of prematurity | 3.42 | 1.76 |
| Toxaemia during pregnancy | Yes | 3.84 | 0.08 |
| Neurological examination at 20 years | Abnormal, with functional deficit | 3.84 | 0.94 |
| Cerebral palsy at 1 year | Yes | 4.28 | 1.03 |
| Neurological examination at 1 year | Abnormal | 5.26 | 0.96 |
| First child | Yes | 5.61 | 0.03 |
| Auditive examination at 1 year | Bilateral deficit | 9.84 | 0.48 |
Bivariate analyses of the distribution of the control variables in the KMC and control subjects were also conducted. Chi‐square and t tests were used to test for categorical and continuous variables, respectively.
The estimated volumes of each structure of interest were compared for KMC and control subjects by two‐way analysis of variance, with adjustment for sex.
Finally, multiple linear regression models were fitted to estimate the association between the duration of KMC and the volumes of each of the target brain structures. These were adjusted using the severity index. When it came to total grey matter volume, total subcortical grey matter volume and striatum volume, WASI‐II scores were included as covariates. An additional model was fitted for neuropsychological tests related to specific anatomical areas in the available literature. For total subcortical grey matter, KMC duration was adjusted with the severity index and the total intrusions score in the California Verbal Learning Test. The NHPT scores were added to the models for the total volume of the caudate nucleus, total white matter fractional anisotropy and total cerebellar volume. SPSS, version 21 (IBM Corp, New York, USA), Stata, version 14 (StatCorp, Texas, USA), and R software were used for the analyses.
3. RESULTS
Of the 264 young adults re‐enrolled who underwent neuroimaging at 20 years of age, we excluded 18 born at term and 68 neuroimages because of the presence of dental braces or significant movement (Figure 1). This left 178 participants with reliable MRIs: 97 had received KMC and 81 were controls (Table 2).
FIGURE 1.
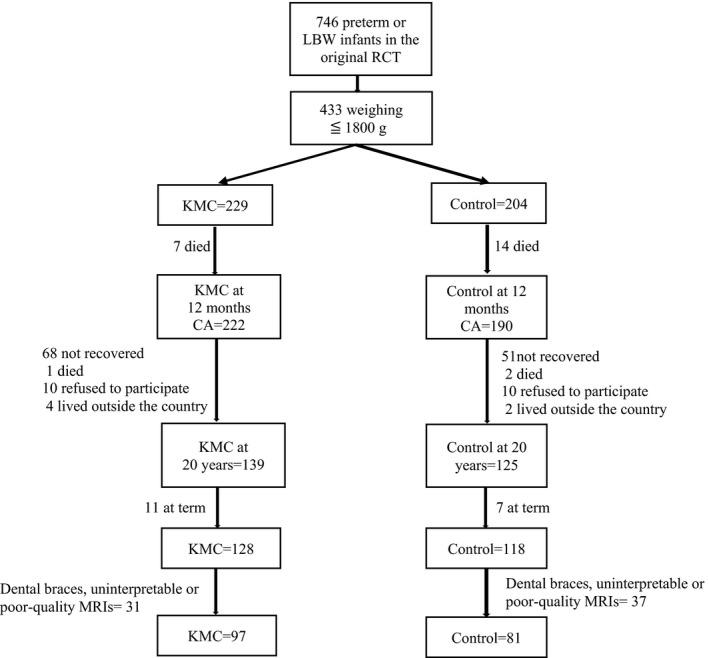
Trial flow chart
TABLE 2.
Characteristics of the overall survivor population and of the study sample
| Variable | Overall survivor population (412 survivors of the original RCT weighing <1801 g at birth) | Re‐enrolled sample (178 preterm babies with good‐quality MRI, weighing <1801 g at birth) | ||||
|---|---|---|---|---|---|---|
| KMC (222) | Control (190) | P | KMC (97) | Control (81) | P | |
| Stable couple, N (%) | 178 (80.5) | 158 (83.2) | 0.40 | 86 (88.7) | 71 (87.7) | 0.51 |
| Mother's age (years), mean (SD) | 28.1 (5.9) | 27.7 (5.7) | 0.49 | 28.4 (5.6) | 28.1 (5.3) | 0.71 |
|
Mother's educational level, N (%) Primary or less High school More than high school No data |
47 (21.2) 122 (55.0) 52 (23.4) 1 (0.4) |
43 (22.6) 101 (53.2) 45 (23.7) 1 (0.5) |
0.92 |
15 (15.5) 57 (58.7) 25 (25.8) ‐ |
12 (14.8) 49 (60.5) 20 (24.7) ‐ |
0.97 |
|
Father's educational level, N (%) Primary or less High school More than high school No data |
39 (17.5) 118 (53.2) 57 (25.7) 8 (3.6) |
33 (17.4) 116 (61.0) 35 (18.4) 6 (3.2) |
0.17 |
19 (19.6) 46 (48.0) 31 (32.0) 1 (1.0) |
11 (13.6) 51 (63.0) 18 (22.2) 1 (1.0) |
0.11 |
| Household monthly per capita income (US$), mean (SD) | 102 (66) | 102 (63) | 0.96 | 100 (68) | 104 (67) | 0.68 |
| Primiparous mother, N (%) | 91 (41.0) | 72 (37.9) | 0.30 | 42 (43.3) | 34 (42.0) | 0.86 |
| Toxaemia, N (%) | 111 (50.0) | 84 (44.2) | 0.28 | 44 (45.4) | 42 (51.9) | 0.39 |
| Multiple births, N (%) | 43 (19.5) | 25 (13.2) | 0.11 | 21 (21.6) | 12 (14.8) | 0.26 |
| Foetal distress, N (%) | 125 (56.3) | 96 (50.5) | 0.28 | 49 (50.5) | 45 (55.6) | 0.50 |
| Caesarean section, N (%) | 156 (70.9) | 123 (64.7) | 0.21 | 68 (70.1) | 58 (71.6) | 0.91 |
| Males, N (%) | 115 (51.8) | 73 (38.4) | <0.01* | 47 (48.5) | 26 (32.1) | 0.03* |
| Intrauterine growth restriction (according to Lubchenco growth charts), N (%) | 68 (30.6) | 65 (34.2) | 0.46 | 27 (27.8) | 25 (30.9) | 0.66 |
|
Neonatal resuscitation, N (%) Spontaneous Conducted Induced No data |
127 (57.2) 36 (16.2) 6 (2.7) 53 (23.9) |
111 (58.4) 29 (15.3) 3 (1.6) 47 (24.7) |
0.72 |
55 (56.7) 17 (17.5) 2 (2.1) 23 (23.7) |
42 (51.9) 11 (13.6) 1 (1.2) 27 (33.3) |
0.89 |
| Apgar score ≤7 at 5 min, N (%) | 10 (5.5) | 15 (9.8) | 0.13 | 6 (6.2) | 5 (6.2) | 0.89 |
| <33 weeks’ gestational age, N (%) | 109 (49.1) | 83 (43.7) | 0.57 | 52 (53.6) | 34 (42.0) | 0.12 |
| Birth weight (g), mean (SD) | 1548 (220) | 1580 (209) | 0.13 | 1525 (253) | 1587 (204) | 0.07 |
| Birth gestational age (weeks), mean (SD) | 32.9 (2.4) | 33.1 (2.7) | 0.24 | 32.4 (2.1) | 33.0 (2.2) | 0.11 |
| Invasive ventilation, N (%) | 36 (16.2) | 18 (9.5) | 0.04* | 20 (20.6) | 4 (4.9) | <0.01* |
| Invasive ventilation (days), mean (SD) | 4.3 (3.0) | 5.6 (3.0) | 0.01* | 2.7 (2.0) | 1.6 (2.8) | 0.20 |
| Hospitalized at least 1 day in NCU, N (%) | 177 (79.7) | 128 (67.4) | <0.01* | 83 (85.6) | 55 (67.9) | <0.01* |
| Total days in NCU, median (min–max) | 16.0 (1–64) | 22.5 (1–65) | 0.01* | 15 (1–64) | 20 (1–57) | 0.01* |
| Hospitalized in NICU, N (%) | 45 (20.3) | 36 (19.9) | 0.74 | 24 (24.7) | 11 (13.6) | 0.06 |
| Gestational age at discharge (weeks), mean (SD) | 34.9 (2.0) | 35.5 (2.0) | 0.01* | 34.8 (1.7) | 35.4 (2.0) | 0.04* |
| Weight at discharge (g), mean (SD) | 1560 (169) | 1645 (145) | <0.01* | 1538 (183) | 1629 (137) | <0.01* |
| Gestational age at eligibility (weeks), mean (SD) | 34.5 (2.0) | 34.7 (2.0) | 0.34 | 34.3 (1.8) | 34.6 (1.9) | 0.30 |
| Weight at eligibility (g), mean (SD) | 1551 (179) | 1577 (182) | 0.16 | 1520 (201) | 1572 (163) | 0.06 |
| Total time on oxygen (days), mean (SD) | 8.8 (7.9) | 10.8 (9.0) | 0.06 | 7.58 (8.7) | 10.1 (9.9) | 0.13 |
| Fragility index (before randomization), mean (SD) | 0.21(0.56) | 0.13(0.55) | 0.15 | 0.21 (0.54) | 0.15 (0.45) | 0.41 |
| Severity index (re‐enrolled cohort), mean (SD) | NA | NA | NA | 0.54 (0.72) | 0.25 (0.70) | <0.01* |
Abbreviations: NA, not available; NCU, neonatal care unit; NICU, neonatal intensive care unit.
statistically significant.
Table 2 compares all the study subjects who survived with the sample recruited at 20 years and shows that they were similar. However, a higher proportion of those who received KMC were male, had received invasive ventilation, had been hospitalised in a neonatal intensive care unit and had a higher severity index. They also had a shorter median hospital stay and lower gestational age and weight at discharge than the control group.
Table 3 compares the brain and cerebellar measurements between the 178 preterm young adults with reliable MRIs and the reference group. The preterm subjects had smaller volumes in all structures, except for the cerebral amygdala, and smaller fractional anisotropy, length and total white matter fibre counts.
TABLE 3.
Brain structure measurements in patients born pre‐term (study cohort) and those born at term (reference group)
| Brain structure |
Born at term N = 37 Mean (SD) |
Born pre‐term N = 178 Mean (SD) |
Difference Mean (SD) |
Difference 95% CI |
P (Student t test) | ||
|---|---|---|---|---|---|---|---|
| Grey matter (volume, mm3) | Total | 607 794.2 (60 934.5) | 571 772.5 (57, 944) | 36 021.7 (10 562.5) | (15 201.2, 56 842.2) | <0.01 | |
| Cortical | 547 009.2 (56 354.1) | 515 630.0 (53 919.1) | 31 379.1 (9817.8) | (12 026.7, 50 731.7) | <0.01 | ||
| Subcortical | Total volume | 60 785 (5419.7) | 56 142.5 (5432.1) | 4642.5 (981.1) | (2708.6, 6576.4) | <0.01 | |
| Caudate nucleus | 7785.5 (1012.1) | 7123.0 (992.3) | 662.5 (179.9) | (307.9, 1017.1) | <0.01 | ||
| Putamen | 13 112.0 (1453) | 12 303.0 (1329) | 808.9 (244.1) | (327.8, 1290) | <0.01 | ||
| Globus pallidus | 3430.0 (604.7) | 3140.3 (506.3) | 289.6 (94.7) | (102.9, 476.3) | <0.01 | ||
| Accumbens nucleus | 1617.1 (220.4) | 1484.9 (235.0) | 132.1 (42.0) | (49.3, 215) | <0.01 | ||
| Striatum | 25 944.5 (2629.6) | 24 051.3 (2542.5) | 1893.2 (462.1) | (928.3, 2804) | <0.01 | ||
| Amygdala nuclei | 3485.7 (438.0) | 3318.8 (482.0) | 167 (85.8) | (–2.15, 336.1) | 0.05 | ||
| Hippocampus | 7880.8 (865.0) | 7322.4 (863.6) | 558.4 (156.1) | (250.8, 866.1) | <0.01 | ||
| Thalamus | 14 203.7 (1472.8) | 12 872.1 (1522.6) | 1331.6 (273.6) | (792.3, 1871) | <0.01 | ||
| Cerebellum | 87 633.2 (9493.2) | 82 289.3 (9526.3) | 5344 (1720.2) | (1953.1, 8734.7) | <0.01 | ||
| White matter | Total volume (mm3) | 356 273.6 (48 042.2) | 320 761.3 (48 042.2) | 35 512.2 (9034.4) | (–53 320.5, –17 704.0) | <0.01 | |
| Total volume of corpus callosum (mm3) | 3088.6 (480.1) | 2811.6 (461.0) | 277.1 (83.9) | (111.7, 442.4) | <0.01 | ||
| Total volume of corticospinal tract (mm3) | 2328.1 (397.4) | 1988.6 (428.0) | 339.5 (76.5) | (188.8, 490.3) | <0.01 | ||
| Total fractional anisotropy (0–1) | 0.58 (0.01) | 0.59 (0.01) | –0.004 (0.002) | (–0.01, 0.00) | 0.07 | ||
| Total fibre length (cm) | 73.7 (2.6) | 72.9 (3.7) | 0.86 (0.78) | (–0.67, 2.39) | 0.27 | ||
| Total fibre count (no.) | 17 725.7 (3499.6) | 16 401.3 (3818.7) | 1324.4 (808.5) | (270.0, 2918.8) | 0.10 | ||
| Cerebellar white matter volume (mm3) | 26 999.4 (3519.9) | 24 714.6 (3314.9) | 2284.9 (605.4) | (1091.6, 3478.1) | <0.01 | ||
Table 4 compares the brain and cerebellar measurements in young adults who received KMC and those who had received conventional incubator care. The KMC group had larger volumes of total grey matter and cortical grey matter and subcortical grey matter in the striatum, caudate and putamen nuclei. We found no difference in the volumes of the accumbens nucleus, globus pallidum, amygdala nuclei, hippocampus, thalamus or cerebellar grey matter. With regard to white matter, the KMC group had a significantly higher volume of corticospinal tracts. No differences were found in total fractional anisotropy, the total fibre length or the total fibre count.
TABLE 4.
Brain measurements in patients born preterm according to intervention: KMC or traditional care (control group)
| Brain Structure |
KMC N = 97 Mean (SD) |
Traditionalcare N = 81 Mean (SD) |
Difference Mean (SD) |
Difference 95% CI |
P (Student's t test) | ||
|---|---|---|---|---|---|---|---|
| Grey matter (volume, mm3) | Total | 580 600.5 (55 508.8) | 561 200.8 (59 357.9) | 19 399.7 (8623.1) | (2381.7, 36 417.7) | 0.03 | |
| Cortical | 441 539.9 (47 465.6) | 425 275.9 (51 069.4) | 16 263.9 (7395.8) | (1668.1, 30 859.8) | 0.03 | ||
| Subcortical | Total volume | 56 782.0 (4889.7) | 55 376.7 (5958.6) | 1405.4 (813.1) | (–199.2, 3010.0) | 0.09 | |
| Caudate nucleus | 7280.5 (924.7) | 6934.4 (1042.2) | 346.2 (147.5) | (55.1, 637.2) | 0.02 | ||
| Putamen | 12 510.5 (1269.7) | 12 054.6 (1363.4) | 455.8 (197.6) | (65.8, 845.9) | 0.02 | ||
| Globus pallidus | 3149.5 (505.6) | 3129.3 (510.0) | 20.2 (76.4) | (–130.6, 171.0) | 0.79 | ||
| Accumbens | 1486.4 (208.4) | 1483.1 (264.7) | 3.28 (35.5) | (–66.7, 73.3) | 0.93 | ||
| Striatum | 24 427.0 (2,322.1) | 23 601.5 (2730.4) | 825.5 (378.7) | (78.2, 1572.9) | 0.03 | ||
| Amygdala nuclei | 3358.1 (484.9) | 3271.7 (477.2) | 86.4 (72.5) | (–56.6, 229.4) | 0.24 | ||
| Hippocampus | 7386.1 (766.8) | 7246.0 (966.4) | 140.1 (129.9) | (–116.3, 396.5) | 0.28 | ||
| Thalamus | 12 929.0 (1378.4) | 12 756.0 (1680.6) | 213.1 (229.3) | (–239.4, 665.5) | 0.35 | ||
| Cerebellum | 83 043.9 (9853.2) | 81 385.7 (9097.6) | 1658.2 (1432.5) | (–1,168.8, 4485.3) | 0.25 | ||
| White matter | Total volume (mm3) | 322 405.9 (44 532.2) | 318 792.0 (52 150.0) | 3614.0 (7246.5) | (–10 687.2, 17 915.2) | 0.62 | |
| Total volume of corpus callosum (mm3) | 2787.9 (412.5) | 2839.9 (514.2) | 52.0 (69.5) | (–189.1, 85.1) | 0.46 | ||
| Total volume of corticospinal tract (mm3) | 2056.1 (402.8) | 1909.3 (445.2) | 146.7 (63.9) | (20.5, 273.0) | 0.02 | ||
| Total fractional anisotropy (0–1) | 0.60 (0.01) | 0.59 (0.01) | 0.003 (0.002) | (0.002, –0.001) | 0.11 | ||
| Total fibre length (cm) | 73.0 (3.3) | 72.7 (4.2) | 0.35 (0.57) | (–0.78, 1.47) | 0.54 | ||
| Total fibre count (no.) | 16 496.5 (3492.7) | 16 288.3 (4192.8) | 208.2 (580.9) | (–938.4, 1354.9) | 0.72 | ||
| Cerebellar white matter volume (mm3) | 24 733.8 (3360.4) | 24,691.5 (3280.3) | 42.3 (500.3) | (–945.2, 1029.7) | 0.93 | ||
3.1. Cognitive function
Analysis of the total brain grey matter, total cortical grey matter and the WASI‐II test of intelligence quotient (IQ) showed an association between the duration of KMC and total brain grey matter. For each day of KMC, the volume of total grey matter increased by 0.15 mm3 after adjustment for the severity index (fragility Rasch 441) and the WASI‐II full‐scale composite score (p = 0.04). A similar association was observed for the volume of total subcortical grey matter, which increased by 0.15 mm3 per day of KMC, after adjustment for the severity index (p = 0.03) (Figure 2).
FIGURE 2.
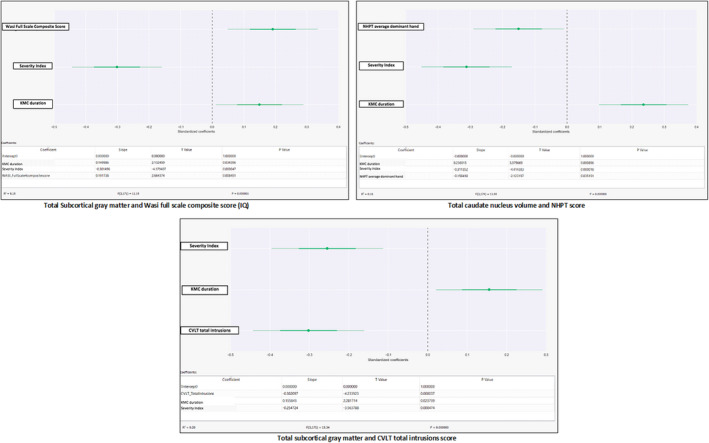
Subcortical grey matter and WASI or CVLT(KMC)
Multiple linear regression showed that, for each day of KMC, the total volume of the caudate nucleus, putamen and accumbens nucleus increased by 0.19 mm3, after the data were controlled for the severity index and the WASI‐II full‐scale composite score (p < 0.01).
3.2. Memory and attention
For each day of KMC, the volume of the total subcortical grey matter increased by 0.16 mm3, after it was controlled for the severity index and the California Verbal Learning Test, Second Edition score for total intrusions on memory and attention (p = 0.02) (Figure 2).
3.3. Fine motor skills and coordination
We also used multiple linear regression to show the impact that each day of KMC had on fine motor skills and coordination. Our results showed that the total volume of the caudate nucleus increased by 0.24 mm3, after it was controlled for the severity index and the NHPT score (p < 0.01) (Figure 2). They also showed that the total cerebellar volume increased by 0.16 mm3, after adjustment for the severity index (fractional anisotropy) (Figure 3). Finally, total fractional anisotropy increased by 0.23 mm3, after it was controlled for the severity index and average NHPT score (p < 0.01) (Figure 4).
FIGURE 3.
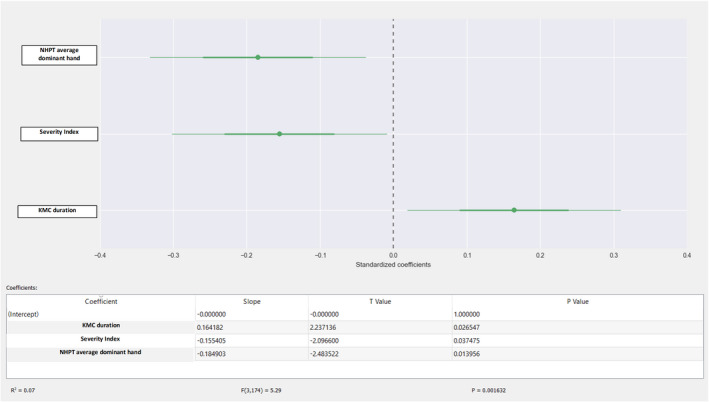
Total cerebellar volume and NHPT
FIGURE 4.
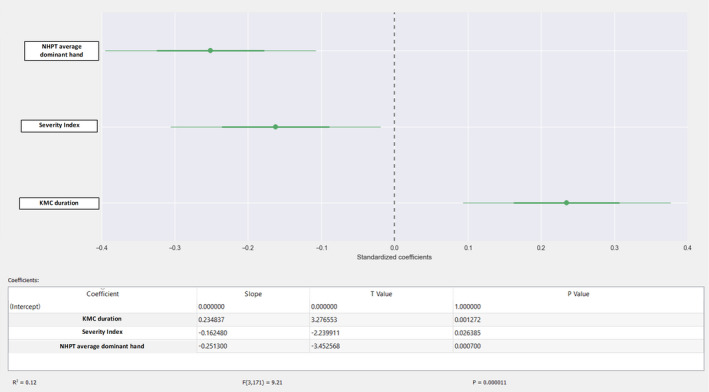
FA and NHPT
4. DISCUSSION
These results, obtained 20 years after the initial KMC trial, showed that KMC had significant independent effects on selected brain areas known to be associated with cognitive function. It also showed that volumes increased as the duration of KMC increased, including significant improvements in fractional anisotropy of total brain white matter. These findings indicated a dose–response relationship between the duration of KMC and neuroprotective effects. They support a causal association with KMC.
Infants who receive routine post‐neonatal or intensive care remain in hospital until their medical status is stable, and they can be discharged home. Hospitalisation is generally viewed as stressful for infants, as it prevents normal interaction with their parents. KMC shortens this sub‐optimal period and allows parents to play an active role, as in developmental care programmes. KMC is currently implemented as soon as possible in hospitals around the world. The World Health Organization has been involved in studying the effect of immediate KMC on neonatal mortality and has produced some interesting results. 24 , 25
We suggest two possible mechanisms for the effect of KMC on neuromotor, sensorial and psychomotor development in premature infants: a neurological mechanism and a social mechanism.
The neurological mechanism involves maturation and regulation of the brain organisation and anatomical structures. The brain has extraordinary potential to adapt to insults and postnatal brain plasticity is even more impressive, with specific environmental inputs to normal brain development. Cell differentiation and synaptogenesis enable the removal of aberrant pathways and strengthen functional pathways, as shown in experiments with rats and preterm monkeys. 26 The potential of the brain for anatomical refinement could be how KMC resets maturation of the basal nucleus and the cerebral cortex.
The volume of the cerebral cortex increases five times during the third trimester and by three to four times in postnatal life. The growth is determined by interactions between established genetic programming and environmental modifications. There are critical periods when the capacity for plasticity and functionality are highly sensitive to external influences. Brain plasticity at this stage is due to factors that include postnatal persistence of neurogenesis in certain parts of the brain, selective depletion of neurons by apoptosis, proliferation and pruning of synapses and activity‐dependent refinement of synaptic connections. These factors have been associated with improvements in functional capacity, such as learning and memory. This means that the immature brain has increased capacity to develop in response to environmental stimuli, although it is also more sensitive to deprivation. 27
During KMC, the mother's womb is replaced by the parents’ bodies. It shields the infant from a disruptive extrauterine environment and provides, and filters, the flow of information and stimuli necessary for development of the sensorimotor nervous system. All the senses are properly stimulated during KMC. These are the vestibular system through the mother's chest movements, touch through skin‐to‐skin contact, smell through exposure to the mother's body odour and milk, vision through eye contact with the mother during feeding and KMC and hearing the mother's voice, breathing and heartbeat. Further proprioceptive stimulation is provided by the position the baby is held in, which simulates foetal restraint in the womb. Holding the infant in a skin‐to‐skin position may potentiate neurobiologically programmed development of the brain during the last few months of gestation, including maturation of the brain. The findings from this study provide evidence that these long‐term results persist into young adulthood, suggesting that the protective role of KMC on brain maturation has long‐lasting effects.
With regard to the social mechanism, a vital component of the KMC intervention is the involvement and empowerment of parents. KMC strengthens the connection between the infants and the mother or father who is holding them, and each becomes more sensitive to the other. We suggest that KMC enhances the macro‐environment, by creating a climate in which the parents become progressively more aware of the child and more prone to sensitive caring. This optimises the infant's long‐term development. 28 , 29
Another paper by our team on the 20‐year follow‐up study reported that parents in the KMC group were more protective and nurturing. 30 This was reflected by less school absenteeism (p = 0.006) and a more stimulating home environment (p = 0.00014), resulting in higher IQs in the most fragile groups (SD = 0.5; p = 0.009; d = 0.657). KMC had a greater impact on the quality of the home environment when the mother had a lower level of education, and the family had a lower socioeconomic status. Young adults who were premature and received KMC were less hyperactive and less aggressive and showed less internalisation, externalisation and socially deviant conduct. 30 The effects were sizeable. Furthermore, parents who were involved in KMC knew their children better.
This 20‐year‐long study represents the first comprehensive quantitative analysis of the physical, social and behavioural impacts of KMC from preterm birth to early adult life. Our work demonstrates that timely discharge from hospital to receive KMC at home empowers parents to take care of their child. It allows them to bond with their infant and build a stimulating social family home environment, which prepares them for both the immediate and long‐term needs of their child. Over the past 20 years of this study, KMC has transitioned from a practice that was only at the time of hospital discharge, after stabilisation to one implemented soon after birth in the neonatal unit. The original RCT in discussion reviewed the effect of such earlier discharge in KMC compared with the remaining in a hospital minimal care unit. While today's implementation may occur sooner but in a hospital setting, the results from this long‐term study demonstrate that KMC is effective and that early home discharge is a crucial component of KMC’s long‐term success.
The cohort in this study were examined 20 years after the KMC intervention, and many additional factors may have affected their neurodevelopment. That is why careful statistical analyses were performed to establish patients who took part at 20 years of age had similar characteristics to those who took part in the original experiment. This showed a slight imbalance against the KMC group (Table 2). Despite this, we found more favourable effects in the KMC group when they were compared with the conventional care control group.
5. CONCLUSION
The results of this study suggest that the protective, nurturing neuroprotective effect of KMC persisted into young adulthood. An independent positive association was found between the duration of KMC and the volumes of total grey matter, basal nuclei and the cerebellum and the organisation of white matter. These structures are involved in both minor and severe sequelae that are considered typical of prematurity, such as IQ deficits, and problems with attention, memory, fine and gross motor skills and coordination. KMC is not just an alternative to a lack of incubators or an initiative that is used to improve mother–infant bonding and promote breastfeeding. It can also induce a long‐lasting protective effect against the deleterious neurodevelopmental consequences of leaving the womb too soon.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
ACKNOWLEDGEMENTS
We thank the research team and the coordinator, Stanislas Teillaud, for collecting the data and Dr Jean Leblond, statistician at the University of Laval, Quebec, Canada, who analysed the results. We also thank the participants and their families for taking part in the study.
Charpak N, Tessier R, Ruiz JG, et al. Kangaroo mother care had a protective effect on the volume of brain structures in young adults born preterm. Acta Paediatr. 2022;111:1004–1014. doi: 10.1111/apa.16265
Funding information
Grand Challenge Canada.
REFERENCES
- 1. McCormick MC, Litt JS. The outcomes of very preterm infants: Is it time to ask different questions? Pediatrics. 2017;139(1):e20161694. [DOI] [PubMed] [Google Scholar]
- 2. Saigal S. In their own words: Life at adulthood after very premature birth. Semin Perinatol. 2016;40:578‐583. [DOI] [PubMed] [Google Scholar]
- 3. Zellkowitz P. Prematurity and its impact on psychosocial and emotional development in children. In: Tremblay RE, Barr RG, Peters R, eds. Encyclopedia old early childhood development. Centre of Excellence for Early Childhood Decelopment; 2006:11‐15. [Google Scholar]
- 4. Hüppi P, Warfield S, Kikinis R, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224‐235. [DOI] [PubMed] [Google Scholar]
- 5. Ortinau C, Neil J. The neuroanatomy of prematurity: Normal brain development and the impact of preterm birth. Clin Anat. 2015;28:168‐183. [DOI] [PubMed] [Google Scholar]
- 6. Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939‐948. [DOI] [PubMed] [Google Scholar]
- 7. Charpak N, Ruiz‐Pelaez JG, Charpak Y. Rey‐Martinez Kangaroo Mother Program: an alternative way of caring for low birth weight infants? One year mortality in a two cohort study. Pediatrics. 1994;94:804‐810. [PubMed] [Google Scholar]
- 8. Conde‐Agudelo A, Díaz‐Rossello J. Kangaroo mother care to reduce morbidity and mortality in low‐birth‐weight infants. Cochrane Database Syst Rev. 2017;2017(2):1‐148. [DOI] [PubMed] [Google Scholar]
- 9. Boundy EO, Dastjerdi R, Spiegelman D, et al. Kangaroo mother care and neonatal outcomes: A meta‐analysis. Rev Artic Pediatr. 2016;137(1):CD002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tessier R, Cristo M, Velez S, et al. Kangaroo mother care and the bonding hypothesis. Pediatrics. 1998;102:e17. [DOI] [PubMed] [Google Scholar]
- 11. Head LM. The effect of Kangaroo care on neurodevelopmental outcomes in preterm infants. J Perinat Neonatal Nurs. 2014;28:290‐299. [DOI] [PubMed] [Google Scholar]
- 12. Charpak N, Ruiz‐Peláez JG, de Figueroa CZ, Charpak Y. Kangaroo mother versus traditional care for newborn infants ≤2000 grams: A randomized, controlled trial. Pediatrics. 1997;100:682‐688. [DOI] [PubMed] [Google Scholar]
- 13. Charpak N, Ruiz‐Peláez JG, de Figueroa CZ, Charpak Y. A randomized, controlled trial of kangaroo mother care: results of follow‐up at 1 year of corrected age. Pediatrics. 2001;108:1072‐1079. [DOI] [PubMed] [Google Scholar]
- 14. Irby SM, Floyd RG. Test Review: Wechsler Abbreviated Scale of Intelligence, Second Edition. Canadian J School Psychol. 2013;28(3):295‐299. [Google Scholar]
- 15. Wang YC, Magasi SR, Bohannon RW, et al. Assessing dexterity function: a comparison of two alternatives for the NIH Toolbox. J Hand Ther. 2011;24:313‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donders J. A Confirmatory Factor Analysis of the California Verbal Learning Test—Second Edition (CVLT‐II) in the Standardization Sample. Assessment. 2008;15(2):123‐131. [DOI] [PubMed] [Google Scholar]
- 17. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341‐355. [DOI] [PubMed] [Google Scholar]
- 18. Tadel F, Mosher JC. Volume source estimation [Internet]. Brainstorm. Available from: https://neuroimage.usc.edu/brainstorm/Tutorials/TutVolSource#Volume_atlases
- 19. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968‐980. [DOI] [PubMed] [Google Scholar]
- 20. Yendiki A, Panneck P, Srinivasan P, et al. Automated probabilistic reconstruction of white‐matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform. 2011;5:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cook PA, Bai Y, Nedjati‐Gilani S, Seunarine KK, Hall MG, Camino PGJ, et al. Camino: Open‐source diffusion‐MRI reconstruction and processing. In: 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine Seattle, WA, USA; 2006. p. 2759.
- 22. Angulo DA, Schneider C, Oliver JH, Charpak N, Hernandez JT. A multi‐facetted visual analytics tool for exploratory analysis of human brain and function datasets. Front Neuroinform. 2016;10:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rizopoulos D. An R package for latent variable modeling and item response theory analyses. J Stat Softw. 2006;17:1‐25. [Google Scholar]
- 24. WHO Immediate KMC Study Group . Immediate “Kangaroo Mother Care” and survival of infants with low birth weight. NEJM. 2021;384:2028‐2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Linnér A, Westrup B, Lode‐Kolz K, et al. Immediate parent‐infant skin‐to‐skin study (IPISTOSS): study protocol of a randomised controlled trial on very preterm infants cared for in skin‐to‐skin contact immediately after birth and potential physiological, epigenetic, psychological and neurodevelo. BMJ Open. 2020;10:e038938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rakic P, Bourgeois JP, Goldman‐Rakic PS. Synaptic development of the cerebral cortex: Implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227‐243. [DOI] [PubMed] [Google Scholar]
- 27. Borsani E, Della Vedova AM, Rezzani R, Rodella LF, Cristini C. Correlation between human nervous system development and acquisition of fetal skills: An overview. Brain Dev. 2019;41:225‐233. [DOI] [PubMed] [Google Scholar]
- 28. Tessier R, Charpak N, Girón M, et al. Kangaroo Mother Care, home environment and father involvement in the first year of life: a randomized controlled study. Acta Paediatr. 2009;98:1444‐1450. [DOI] [PubMed] [Google Scholar]
- 29. Lawn JE, Mwansa‐Kambafwile J, Horta BL, Barros FC, Cousens S. “Kangaroo mother care” to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol. 2010;39(Suppl 1):i144‐i154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Charpak N, Tessier R, Ruiz JG, et al. Twenty‐year follow‐up of Kangaroo mother care versus traditional care. Pediatrics. 2017;139(1):e20162063. [DOI] [PubMed] [Google Scholar]


