Abstract
Purpose
While the beneficial effects of medications are numerous, drug–drug interactions may lead to adverse drug reactions that are preventable causes of morbidity and mortality. Our goal was to quantify the prevalence of potential drug–drug interactions in drug prescriptions at Danish hospitals, estimate the risk of adverse outcomes associated with discouraged drug combinations, and highlight the patient types (defined by the primary diagnosis of the admission) that appear to be more affected.
Methods
This cross‐sectional (descriptive part) and cohort study (adverse outcomes part) used hospital electronic health records from two Danish regions (~2.5 million people) from January 2008 through June 2016. We included all inpatients receiving two or more medications during their admission and considered concomitant prescriptions of potentially interacting drugs as per the Danish Drug Interaction Database. We measured the prevalence of potential drug–drug interactions in general and discouraged drug pairs in particular during admissions and associations with adverse outcomes: post‐discharge all‐cause mortality rate, readmission rate and length‐of‐stay.
Results
Among 2 886 227 hospital admissions (945 475 patients; median age 62 years [IQR: 41–74]; 54% female; median number of drugs 7 [IQR: 4–11]), patients in 1 836 170 admissions were exposed to at least one potential drug–drug interaction (659 525 patients; median age 65 years [IQR: 49–77]; 54% female; median number of drugs 9 [IQR: 6–13]) and in 27 605 admissions to a discouraged drug pair (18 192 patients; median age 68 years [IQR: 58–77]; female 46%; median number of drugs 16 [IQR: 11–22]). Meropenem‐valproic acid (HR: 1.5, 95% CI: 1.1–1.9), domperidone‐fluconazole (HR: 2.5, 95% CI: 2.1–3.1), imipramine‐terbinafine (HR: 3.8, 95% CI: 1.2–12), agomelatine‐ciprofloxacin (HR: 2.6, 95% CI: 1.3–5.5), clarithromycin‐quetiapine (HR: 1.7, 95% CI: 1.1–2.7) and piroxicam‐warfarin (HR: 3.4, 95% CI: 1–11.4) were associated with elevated mortality. Confidence interval bounds of pairs associated with readmission were close to 1; length‐of‐stay results were inconclusive.
Conclusions
Well‐described potential drug–drug interactions are still missed and alerts at point of prescription may reduce the risk of harming patients; prescribing clinicians should be alert when using strong inhibitor/inducer drugs (i.e. clarithromycin, valproic acid, terbinafine) and prevalent anticoagulants (i.e. warfarin and non‐steroidal anti‐inflammatory drugs ‐ NSAIDs) due to their great potential for dangerous interactions. The most prominent CYP isoenzyme involved in mortality and readmission rates was 3A4.
Keywords: adverse outcomes, drug safety, drug–drug interactions, electronic health records, pharmacoepidemiology, propensity score
Key Points.
This combined cross‐sectional and analytic study found that discouraged pairs are often prescribed to inpatients and uncommon drugs are often part of these.
Seven discouraged pairs (six CYP‐associated) were prevalent in at least one patient type; 12 discouraged pairs (10 CYP‐associated) showed statistically significant associations with elevated mortality or readmission rates.
The CYP isoenzyme 3A4 was as the most prominent, involved in more than half of the discouraged combinations.
The most prominent drug types were anti‐infectives (azoles, carbapenems and macrolides) and anticoagulants (warfarin and NSAIDs); these are all well‐known.
Our findings emphasise that prescribing clinicians should be alert when using these drugs because of their great potential for dangerous interactions and give (more) weight to patients' comorbidities to optimise patient safety.
1. INTRODUCTION
Two drugs are said to interact when the action of one does or may affect the activity, metabolism or toxicity of the other. 1 Drug–drug interactions (DDIs) constitute a particularly important cause of adverse drug reactions (ADRs) as clinical evidence and (when known) their pharmacological mechanisms make them somewhat predictable. Many hospitalised patients take several drugs and polypharmacy 2 is estimated to affect 40–65% of hospitalised patients. 3 , 4
Although the risk of DDIs is proportional to the number of drugs taken, 5 the clinical consequences vary widely, and ADRs rarely occur. 6 Even if uncommon, serious adverse outcomes do cause harm, constitute economic losses and are to some extent preventable. At particularly elevated risk of ADRs are the elderly (often multimorbid and with reduced physiological capacity) 2 and patients with diseases in organ systems involved in drug metabolism, particularly kidneys and liver. 7 The consequences of DDIs affect both the individual patient and society as a whole: 10–20% of hospital admissions may be attributable to drug‐related problems and toxic effects of medication of i.a. DDIs, 8 , 9 and studies have linked DDIs to prolonged hospitalisation and increased healthcare costs. 10 , 11 , 12 , 13
The electronic medication management systems deployed at public hospitals in Denmark do not systematically flag problematic drug combinations. Even with such systems in place, alert fatigue is a real issue that requires tailoring to optimise their genuine utility. 14 To this end, appropriate evidence about the extent and nature of the problem is needed.
No studies to date have examined the prevalence of potential drug–drug interactions (pDDIs) in hospitals for different patient types and assessed the clinical impact of pDDIs. This study sought to fill this gap and elicit learning points for clinicians to mitigate this issue.
We used electronic health records (EHRs) to (a) elicit the prevalence of discouraged drug pairs and their expected clinical significance and documentation level, (b) identify which patient types are most affected by discouraged pairs and (c) gauge the association between discouraged pairs and three adverse outcomes: post‐discharge mortality, readmission and length‐of‐stay (LOS).
2. METHODS
2.1. Patients and data
We obtained inpatient data for admissions to twelve public hospitals in the Capital Region and Region Zealand, Denmark, from January 2008 through June 2016. The two regions comprise ~2.5 million people, about half of the Danish population. 15 Admissions of individuals using at least two drugs concomitantly were included. We defined concomitant use as temporally overlapping drug exposures as two temporally overlapping active drug prescriptions, during admission and identified all two‐way drug combinations as described in Leal et al. 16 (Figure S1). Active drug prescriptions were considered only if these were dispensed/administered at the hospital.
Information on admission timing, diagnoses and medical histories was obtained from the Danish National Patient Register (DNPR), 17 , 18 recording data for department‐specific visits. DNPR encodes diagnoses with a Danish version of the International Classification of Disease, 10th revision (ICD‐10). An admission's primary diagnoses are recorded retrospectively at discharge. Successive in‐hospital visits were combined into admissions if they were at most one day apart.
We marshalled information on dispensed in‐hospital drug prescriptions from OPUS‐medication (OpusMed) and Electronic Patient Medication (EPM). The latter has been validated 19 and the former was used in the same manner; both use the WHO Anatomical Therapeutic Chemical (ATC) classification system. 20
As our pDDI reference we used the Danish Drug Interaction Database (DID), covering predominantly pharmacokinetic interactions based mainly on published results and maintained by specialists in clinical pharmacology under the auspices of the Danish Medicines Agency. 21
2.2. pDDI prevalence
This descriptive part was cross‐sectional. pDDIs were categorised by management recommendation (five levels), clinical significance (five levels) and documentation level (six levels); we only considered the 14 237 (from a total of 18 691) pDDIs with information on all three axes (Table 1). The quality of the documentation level is based on the evidence about the significance of the kinetic or dynamic properties.
TABLE 1.
Classification of potential drug–drug interactions based on management recommendation, clinical significance and documentation level published by the Danish Medicines Agency Drug Interaction Database.
| Management recommendation | |
| 1 | The drug combination should always be avoided (discouraged in text). |
| 2 | The drug combination can be used with dose adjustment. |
| 3 | The drug combination can be used with staggered time of ingestion. |
| 4 | The drug combination can be used under certain precautions, i.e. changing the routes of administration. Alternative agents should be considered. |
| 5 | The drug combination can be used. No action needed as the risk of adverse events appears to be small. |
| Clinical significance | |
| Major | Clinically pronounced/physiological effect with either significant altered therapeutic response or frequent occurrence of serious adverse reactions. |
| Moderate | Clinically moderate/physiological effect with either slightly altered therapeutic response, or rare occurrence of more serious side effects. Serum concentration changes, which in other experiments have been closely associated with the above‐mentioned phenomena. |
| Minor | Unchanged or not significantly altered biological response with fewer and easier side effects ‐ or serum concentration changes, which in other studies have not shown significant changes in the biological response. |
| Possible | Pharmacokinetic changes which are not accompanied by known adverse reactions or changes in the biological response. |
| None | Neither kinetic or physiological/clinical changes. |
| Undetermined | Kinetic or physiological/clinical changes that cannot be estimated based on the available documentation. |
| Documentation level | |
| Well‐documented | At least 2 (from different centres) human controlled trials and/or (before and after) trials in relevant individuals with single or multiple steady state trials in the form of either significant kinetic or dynamic changes. |
| Documented | A human controlled study and/or (before and after) study with steady state single or multiple dose trials in the form of either significant kinetic or dynamic changes. |
| Limited documented | Either more than 2 case reports with relevant during and after kinetics or dynamics or human in vitro studies with relevant cytochrome P450 (CYP) fractions and concentrations. |
| Poorly documented | 1–2 case reports. Non‐conclusive in vitro studies. |
Discouraged drug pairs were defined as prevalent when they occurred in more than 10% of admissions of at least one specific patient type, defined as the ICD‐10 chapter of the admission's primary diagnosis. We used standardised difference in proportions to compare imbalances between binary variables, taking an absolute difference above 10% to indicate substantial imbalance. 22
2.3. Adverse outcomes of exposure to discouraged combinations
In this analytic part of the study, we screened the effect of all discouraged pairs on post‐discharge all‐cause mortality rate (henceforth, post‐discharge mortality), readmission rate and LOS. Only patients' first admissions were used. We excluded patients whose exposure started outside the hospital for better‐defined exposure start. The effects on post‐discharge mortality and readmission were estimated with stratified Cox regression models assuming noninformative censoring 23 and the effects on LOS with stratified Poisson regression models, 24 with exposure to the discouraged drug pair as the sole explanatory variable. We created strata by greedy 1:5 matching on preference score, an extension of the propensity score accounting for target exposure prevalence. 25 The preference score is the probability that a patient be exposed whether this happened or not. Thus, if two patients have (almost) the same preference score but one was exposed and the other not, the exposure is a likely explanation for their difference in outcome. 26 , 27 , 28
We used Cyclops 29 to compute high‐dimensional propensity scores 27 with sparse lasso logistic regression models using up to 843 features derived from seven variables: age at admission (continuous → 1 feature), sex (binary → 1 feature), patient type (one‐hot‐encoded → 19 features), diagnoses during admission (ICD‐10 level 3, one‐hot‐encoded → 819 features), medication burden (continuous → 1 feature), whether the admission was acute or elective (binary → 1 feature), and weighted Elixhauser comorbidity score (Agency for Healthcare Research Quality 30 version, continuous → 1 feature). Seeking empirical equipoise, outcome models were fit to patients with preference scores between 0.3 and 0.7. 25 The significance level was set to 5%; power analyses were foregone. Estimates with 95% confidence intervals (CI) wider than 100 on the linear scale were omitted.
2.4. Software
We used the R statistical programming language and Python for data processing, analysis, and visualisation. The analysis workflow was built as a Snakemake pipeline 31 (Figure S2). The full analytic code is available upon request.
3. RESULTS
Among the 4 411 576 admissions of 1 481 584 patients identified, we included 2 886 227 admissions (65%) of 945 475 patients (64%) to whom two or more drugs were administered (Figure S3). Table 2 shows overall and stratified summary statistics for pertinent variables. The 538 620 (57%) women in the cohort contributed 1 551 131 admissions (54%) and 13 122 610 (54%) dispensed prescriptions. Of these, 27 605 admissions (1%) featured discouraged drug pairs and 12 655 (46%) were administrated to women. pDDIs and discouraged drug pairs were observed more frequently in older patients. Further, the median number of prescribed drugs in admissions with discouraged drug pairs (16, IQR: 11–22) was larger than any‐pDDI (9, IQR: 6–13) and no‐pDDI (4, IQR: 2–6) admissions. Patients exposed to discouraged drug pairs were more ill and had longer admissions and higher in‐hospital mortality.
TABLE 2.
Overall and stratified summary statistics of included admissions
| Overall | No pDDIs | pDDIs | Discouraged drug pairs | |
|---|---|---|---|---|
| Admissions | 2 886 227 | 1 050 057 (36%) | 1 836 170 (64%) | 27 605 (1%) |
| Women | 1 551 131 (54%) | 565 697 (54%) | 985 434 (54%) | 12 655 (46%) |
| Patients | 945 475 | 553 612 | 659 525 | 18 192 |
| No. prescriptions | 9 (5–15) | 5 (3–8) | 12 (7–19) | 22 (14–36) |
| In women | 8 (4–15) | 4 (3–7) | 12 (7–19) | 22 (14–35) |
| No. unique prescribed drugs | 7 (4–11) | 4 (2–6) | 9 (6–13) | 16 (11–22) |
| Unique prescribed drugs | ||||
| 2–4 drugs | 888 934 (31%) | 629 786 (60%) | 259 148 (14%) | 520 (2%) |
| 5–9 drugs | 1 042 023 (36%) | 347 943 (33%) | 694 080 (38%) | 4408 (16%) |
| ≥ 10 drugs | 955 270 (33%) | 72 328 (7%) | 882 942 (48%) | 22 677 (82%) |
| Age in years | 62 (41–74) | 51 (30–69) | 65 (49–77) | 68 (58–77) |
| Age group | ||||
| < 18 years | 203 125 (7%) | 148 043 (14%) | 55 082 (3%) | 492 (2%) |
| 18–44 years | 619 540 (22%) | 293 005 (28%) | 326 535 (18%) | 2359 (9%) |
| 45–64 years | 773 558 (27%) | 269 693 (26%) | 503 865 (27%) | 7790 (28%) |
| 65–74 years | 577 389 (20%) | 162 494 (16%) | 414 895 (23%) | 8166 (30%) |
| 75–84 years | 461 247 (16%) | 114 094 (11%) | 347 153 (19%) | 6588 (24%) |
| ≥ 85 years | 251 368 (9%) | 62 728 (6%) | 188 640 (10%) | 2210 (8%) |
| pDDIs per patient | 1 (0–3) | 0 (0–0) | 2 (1–5) | 9 (5–15) |
| Length of stay in days | 3 (1–6) | 2 (1–4) | 3 (2–7) | 7 (3–15) |
| Acute admission | 2 107 774 (73%) | 765 816 (73%) | 1 341 958 (73%) | 19 746 (72%) |
| In‐hospital mortality | 62 830 (2%) | 14 397 (1%) | 48 433 (3%) | 1252 (5%) |
| Low eGFR (<30 mL/min/1.73m2) | 109 907 (4%) | 15 198 (1%) | 94 709 (5%) | 2660 (10%) |
| Elixhauser index (AHQR) | ||||
| <0 | 646 561 (22%) | 230 311 (22%) | 416 250 (23%) | 4893 (18%) |
| 0 | 854 868 (30%) | 399 526 (38%) | 455 342 (25%) | 3838 (14%) |
| 1–4 | 297 174 (10%) | 104 072 (10%) | 193 102 (11%) | 3157 (11%) |
| ≥5 | 1 087 624 (40%) | 316 148 (30%) | 771 476 (42%) | 15 717 (57%) |
| Most common drug classes (ATC level 3) | ||||
| Other analgesics and antipyretics (N02B) | 1 334 677 (63%) | 501 208 (48%) | 1 334 677 (73%) | 22 024 (80%) |
| Antithrombotic agents (B01A) | 1 038 880 (43%) | 211 944 (20%) | 1 038 880 (57%) | 21 890 (79%) |
| Opioids (N02A) | 917 092 (43%) | 318 180 (30%) | 917 092 (50%) | 18 098 (66%) |
| Anti‐inflammatory and antirheumatic products, non‐steroids (M01A) | 663 518 (29%) | 178 152 (17%) | 663 518 (36%) | 14 900 (54%) |
| Drugs for peptic ulcer and gastro‐oesophageal reflux disease (GORD) (A02B) | 642 650 (27%) | 141 344 (13%) | 642 650 (35%) | 15 429 (56%) |
| Beta‐lactam antibacterials, penicillins (J01C) | 686 899 (24%) | 206 305 (20%) | 480 594 (26%) | 10 796 (39%) |
| Loop (high‐ceiling) diuretics (C03C) | 518 342 (18%) | 52 487 (5%) | 465 855 (25%) | 131,98 (48%) |
| Most common primary diagnosis | ||||
| Abdominal and pelvic pain (R10) | 63 574 (2%) | 26 387 (3%) | 37 187 (2%) | 448 (2%) |
| Pneumonia, organism unspecified (J18) | 60 237 (2%) | 21 369 (2%) | 38 868 (2%) | 1057 (4%) |
| Atrial fibrillation and flutter (I48) | 55 405 (2%) | 14 422 (1%) | 40 983 (2%) | 667 (2%) |
| Mental and behavioural disorders due to use of alcohol (F10) | 51 347 (2%) | 29 206 (3%) | 22 141 (1%) | 135 (0%) |
| Other chronic obstructive pulmonary disease (J44) | 45 919 (2%) | 16 039 (2%) | 29 880 (2%) | 474 (2%) |
| Nonrheumatic aortic valve disorders (I35) | 10 244 (0%) | 1784 (0%) | 8646 (0%) | 1439 (5%) |
| Acute myocardial infarction (I21) | 30 250 (1%) | 983 (0%) | 29 267 (2%) | 244 (1%) |
| Angina pectoris (I20) | 31 664 (1%) | 5366 (1%) | 26 298 (1%) | 190 (1%) |
| Bacterial pneumonia, NOC (J15) | 24 045 (1%) | 8420 (1%) | 15 625 (1%) | 528 (2%) |
| Other sepsis (A41) | 28 401 (1%) | 7348 (1%) | 21 053 (1%) | 482 (2%) |
Note: Values are N (%) and median (interquartile range).
Abbreviations: AHRQ, Agency for Healthcare Research Quality; pDDI, potential drug–drug interaction.
Of 344 489 unique drug pairs administered in‐hospital, 5646 (2%) were pDDIs; 1 836 170 admissions (64%) of 659 525 patients (70%) featured at least one of these 5646 pDDIs. In 27 605 admissions (1%) of 18 192 patients (2%) at least one of the 146 (3%) discouraged drug pairs was used, most with expected major (71%) and moderate (21%) clinical significance (Tables 3 and S1). The most prescribed drugs involved in discouraged drug pairs were, in descending order of number of users, pantoprazole (nine admissions [0.0%] of five patients [0.0%] exposed to discouraged drug pairs of 570 440 admissions of 224 002 pantoprazole users), ibuprofen (9982 admissions [1.8%] of 7368 patients [2.0%] of 569 223 admissions of 365 302 users), simvastatin (5048 admissions [1.1%] of 3887 patients [3.6%] of 442 545 admissions of 148 579 users), metoprolol (1191 admissions [0.3%] of 399 patients [0.3%] exposed of 379 785 admissions of 127 237 users) and diclofenac (1917 admissions [1.1%] of 1326 patients [1.1%] exposed of 177 928 admissions of 120 256 users) affecting up to 3% of the hospitalised patients receiving the drugs (Table S2). In contrast, more uncommon drugs (used by less than 1% of hospitalised patients), e.g. erythromycin (1573 admissions [27.8%] of 1461 patients [29.2%] exposed out of 5665 admissions of 5001 users), rifabutin (25 admissions [24.8%] of 10 patients [21.7%] exposed out of 101 admissions of 46 users), ketoconazole (644 admissions [20.4%] of 320 patients [21.2%] exposed out of 3158 admissions of 1513 users), warfarin (12 570 admissions [10.3%] of 8791 patients [20.9%] exposed out of 121 653 admissions of 42 101 users) and domperidone (2872 admissions [12.4%] of 2028 patients [19.2%] exposed out of 23 213 admissions of 10 571 users) were more often given as part of discouraged pairs (Tables S2 and S3, Figure S4).
TABLE 3.
Unique drug combinations (upper cells) and prevalence (lower cells) of pDDIs by management recommendation and clinical significance
| Recommendation level | Clinical significance | ||||||
|---|---|---|---|---|---|---|---|
| Major | Moderate | Minor | Possible | None | Un‐determined | Total | |
| 1: Discouraged | 104 (71) | 31 (21) | ‐ | 8 (5) | ‐ | 3 (2) | 146 (3) |
| 16 339 (90) | 1293 (7) | ‐ | 1206 (7) | ‐ | 24 (0) | 18 192 (3) | |
| 2: Dose adjustment | 164 (16) | 457 (45) | 48 (5) | 279 (28) | 1 (0) | 56 (6) | 1005 (18) |
| 40 718 (27) | 91 264 (61) | 12 606 (8) | 58 622 (39) | 25 (0) | 4953 (3) | 148 455 (23) | |
| 3: Staggered ingestion | 53 (23) | 100 (44) | 9 (4) | 47 (21) | ‐ | 17 (8) | 226 (4) |
| 14 339 (16) | 38 544 (43) | 244 (0) | 12 264 (14) | ‐ | 43 776 (48) | 90 662 (14) | |
| 4: Precautions | 300 (17) | 602 (35) | 86 (5) | 601 (35) | 30 (2) | 123 (7) | 1742 (31) |
| 45 221 (8) | 459 717 (86) | 82 611 (16) | 249 539 (47) | 12 214 (2) | 106 406 (20) | 532 066 (81) | |
| 5: No action needed | 6 (0) | 82 (3) | 311 (12) | 206 (8) | 1648 (65) | 274 (11) | 2527 (45) |
| 165 (0) | 97 285 (21) | 189 797 (40) | 116 185 (25) | 399 935 (85) | 191 767 (41) | 470 956 (71) | |
| Total | 627 (11) | 1272 (23) | 454 (8) | 1141 (20) | 1679 (30) | 473 (8) | 5646 |
| 92 167 (14) | 517 599 (78) | 225 512 (34) | 1679 (0) | 400 935 (61) | 264 711 (40) | 659 525 | |
Note: Values are N (%).
Overall, patients admitted with cardiovascular diseases (ICD‐10 chapter IX); endocrine, nutritional and metabolic diseases (chapter IV); and respiratory diseases (chapter X) were more frequently exposed to discouraged drug pairs unlike obstetrical patients (chapter XV) and patients admitted for other reasons (chapter XXI) (Figures 1A and S5). Discouraged pairs varied among the remaining patient types, but within the ±0.1 threshold indicative of negligible imbalance (Figure 1A). In contrast, most drugs were more frequently prescribed in admissions with discouraged pairs with many above the 0.1 threshold except misoprostol and oxytocin (Figure 1B).
FIGURE 1.
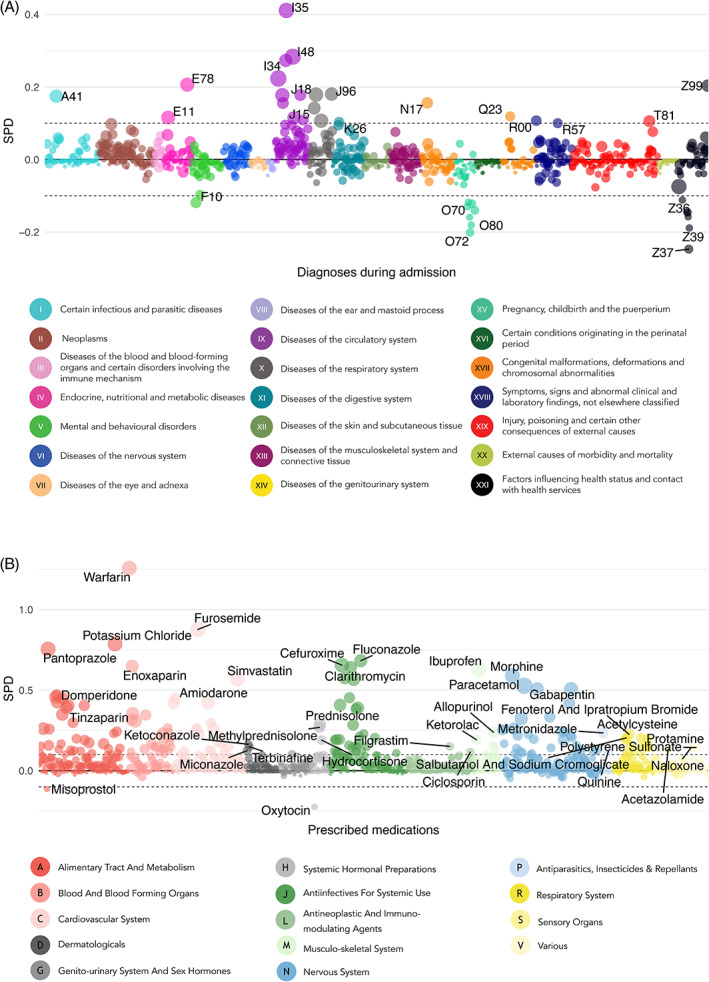
Standardised differences in proportions (i.e. discouraged drug pairs initiated versus not) of diagnoses (A) and prescribed drugs during admissions (B), respectively. The colour represents ICD‐10 chapter and anatomical ATC level, respectively, and the size is the prevalence in patients exposed to discouraged drug pairs. The top three diagnoses and drugs are labelled [Colour figure can be viewed at wileyonlinelibrary.com]
In the 65 discouraged drug pairs (45%) prescribed to five patients or more (Table S4), seven were prevalently (>10% of admissions) prescribed during hospital admissions (Figure 2). The most prominent pair was warfarin‐ibuprofen, prevalent in all patient types except three (chapters X, XVI and XX). The second‐most prominent was simvastatin‐clarithromycin, prevalent in six patient types (I, III, IV and X‐XII); the third‐most was domperidone‐fluconazole, prevalent in five patient types (II‐IV, VXIII and XXI). The other four were warfarin‐diclofenac (XIV, XV, XVII), fluoxetine‐venlafaxine (V), meropenem‐valproic acid (VI) and erythromycin‐fluconazole (XI). Figures S5–S7 show the prevalence of each drug and each diagnosis in patients exposed versus non‐exposed to discouraged drug pairs.
FIGURE 2.
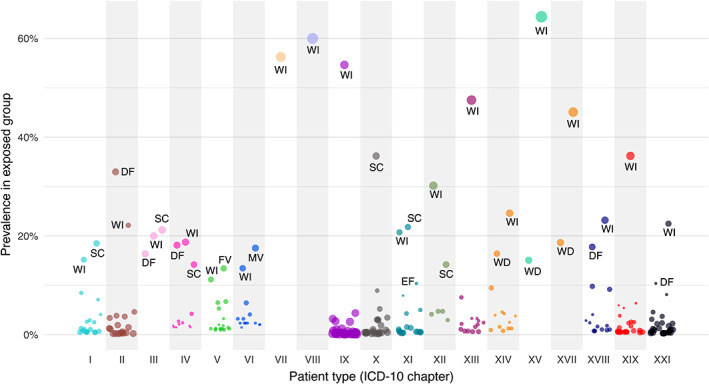
Prevalence of discouraged drug pairs by patient type. Each point represents one discouraged drug pair, and size the absolute value of the standardised difference in proportions using as reference admissions during which treatment with any discouraged pair was initiated. DF (N = 5): Domperidone (A03FA03) + Fluconazole (J02AC01); WD (N = 3): Warfarin (B01AA03) + Diclofenac (M01AB05, systemic); WI (N = 18): Warfarin (B01AA03) + Ibuprofen (M01AE01); SC (N = 6): Simvastatin (C10AA01) + Clarithromycin (J01FA09); MV (N = 1): Meropenem (J01DH02) + Valproic acid (N03AG01); EF (N = 1): Erythromycin (J01FA01) + Fluconazole (J02AC01); FV (N = 1): Fluoxetine (N06AB03) + Venlafaxine (N06AX16) [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3 shows the estimated effects of exposure on mortality rate, readmission rate and LOS; Table S5 contains the numerical estimates. Six discouraged drug pairs were significantly associated with increased mortality rate, of which particularly the 95% CIs of meropenem‐valproic acid, domperidone‐fluconazole, imipramine‐terbinafine and agomelatine‐ciprofloxacin are relatively far from 1. Ertapenem‐fluconazole, amitriptyline‐terbinafine as well as clarithromycin with ticagrelor, tacrolimus and everolimus, respectively, were associated with substantially elevated readmission rates albeit with CI bounds near 1. Many discouraged pairs were associated with longer or shorter hospital stays with most effect sizes within ~±1 day.
FIGURE 3.
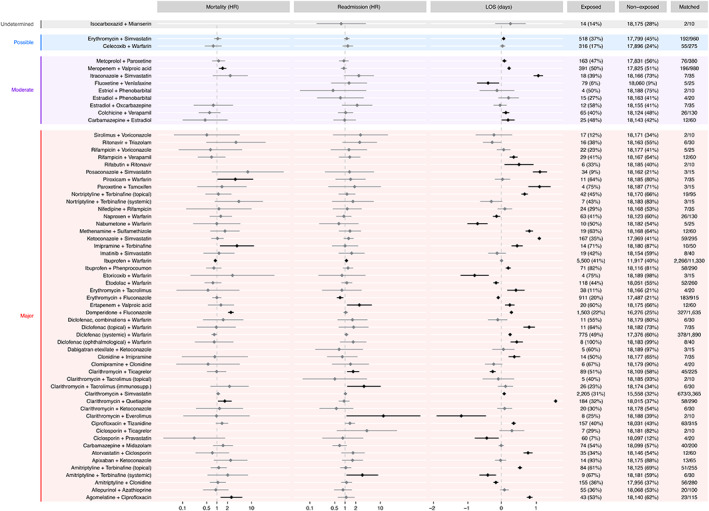
Estimate effect sizes of exposure to discouraged drug pairs and post‐discharge mortality rate (hazard ratio, HR), readmission rate (HR) and length‐of‐stay (change in days). Diamonds show point estimates of the effect sizes, horisontal lines the 95% confidence intervals. The exposed and non‐exposed columns show count (empirical equipoise) and the matched column shows the number of exposed/non‐exposed used to estimate the effects of that pair [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
We found that 1 836 170 admissions (64%) of 659 525 patients (70%) featured at least one pDDI and that during 27 605 admissions (1%) of 18 192 patients (2%) at least one discouraged drug pair was administered. Seven discouraged pairs were prevalent, most notably warfarin‐ibuprofen (18 patient types), simvastatin‐clarithromycin (six patient types) and domperidone‐fluconazole (five patient types). Of the prevalent discouraged pairs, domperidone‐fluconazole and meropenem‐valproic acid (one patient type) were significantly associated with elevated mortality. The prevalent pair warfarin‐ibuprofen was just statistically significantly associated with elevated readmission rates and three of five discouraged pairs associated with elevated readmission rates involved clarithromycin. LOS results were inconclusive.
The increasing availability of longitudinal patient data and growing access to databases with DDI information facilitate comparative, data‐driven approaches to identify, anticipate and explain DDIs. 32 Indeed, in this study we used comprehensive phenotypic in‐hospital data to detail the landscape of in‐hospital pDDIs with particular focus on discouraged drug pairs to elicit their associations with potentially preventable adverse outcomes. Although propensity (and, by extension, preference) scores underpin causal inference, 33 we used them mainly to qualify the strengths of the association instead of using e.g. unadjusted estimates.
Prevalence patterns and effects of pDDIs are elusive because many potentially interacting drug combinations offer genuine clinical utility if used consciously by alert physicians. Teasing apart these dynamics is difficult on a large scale. Indeed, studies of pDDI prevalence in hospitalised patients often use relatively small samples from sub‐populations such as critically ill or oncological patients. 34 , 35 , 36 Our approach was different seeking to conduct a large‐scale screening of hospitalised patients, focusing on outright discouraged drug pairs because their clinical benefits unlikely outweigh their potential harm.
A recent systematic review of clinically manifested DDIs 37 found prevalence estimates up to 26% in not‐critically‐ill hospitalised patients. 38 Further, the number of drugs used concomitantly has been shown to be a significant risk factor for interactions at hospitals and in primary care. 39 , 40 , 41 , 42 We also observed widespread polypharmacy among patients exposed to pDDIs especially when exposed to discouraged drug pairs. However, unlike for diagnoses, no drugs involved in pDDIs emerged as neither particularly frequent nor infrequent except misoprostol and oxytocin. Thus, perceiving the effect of polypharmacy solely in terms of the association between number of concomitant drugs and pDDIs is arguably of limited use as it tells us little about the nature of the association. Instead, other phenotypic factors such as comorbidities may be of greater utility to the prescribing physician at point of care.
A Danish study of 167 232 patients from 1998 on the island of Funen found that 4.4% of all inhabitants of age above 70 were prescribed drug combinations with a high risk of severe interactions. 43 A recent Brazilian study with ~340 000 patients from primary‐ and secondary‐care hospitals arrived at a similar figure. 44 These estimates are substantially lower than our 14% patients prescribed pDDIs with expected major clinical significance (Table 3), likely because our data are newer than those in the former and include also tertiary hospitals unlike both those studies.
Another study from Denmark published in 2005 found that pDDIs are prevalent but mostly clinically insignificant. 45 Our results agree with this notion: six discouraged combinations featured substantial and statistically significant associations with elevated mortality, of which only two were prevalent in particular patient types (meropenem‐valproic acid, domperidone‐fluconazole). This was the case for only warfarin‐ibuprofen with respect to readmission rates.
Rarely used drugs are more often involved in potentially dangerous DDIs perhaps due to prescribers' lack of specific knowledge on these drugs; consider three examples. First, using meropenem (or ertapenem) with valproic acid elevates the risk of seizures (unknown mechanism) and meropenem consumption is increasingly prescribed at emergency departments, often by junior doctors. Second, the cardiac risks of domperidone and erythromycin (prolonged QT and Torsades‐de‐Pointes) are aggravated by concurrent use of fluconazole (or any conazole) because the latter impedes their metabolism by inhibiting CYP3A4. 46 Third, concurrent use of agomelatine and ciprofloxacin increases the exposure of the former because the later inhibits CYP1A2. Causes of death in deceased exposed to these drug pairs did not suggest unexpected patterns (Figure S8).
Some active ingredients involved in discouraged drug pairs have several ATC codes (terbinafine, diclofenac and tacrolimus) and the somewhat agreeing effect estimates on mortality and readmission rates add confidence to these findings. Interestingly, the effects on LOS were not consistent across ATC codes for the same active ingredient prompting cautious interpretation. Indeed, LOS is elusive: for example, a short admission can end with discharge to home or death. To arrive at meaningful conclusions on lengths‐of‐stays, one would need to use for example drug administrations allowing for time‐to‐event analyses, something not possible with these data.
4.1. Strengths and limitations
This study features a range of strengths. First, this is the largest study assessing the prevalence of discouraged drug pairs and their effects on adverse outcomes among hospitalised patients. Second, we used unfiltered data from a heterogeneous population of almost one million hospitalised patients over an eight‐year period. Third, detailed and reliable register data allow for detailed phenotyping, both with respect to diagnoses and medication use. Fourth, such deep phenotyping underpins the use of high‐dimensional preference scores to obtain approximate empirical equipoise when studying the associations between exposure and adverse outcomes. Fifth, the risk of selection bias and loss to follow‐up was minimal.
Nonetheless, there are potential weaknesses. First, hospital data may be subject to recall and information bias. This is likely not an issue for this study because we rely on near‐objective data (e.g. validated source of medication data) used also for administrative and billing purposes. Bias by indication could be a problem but the use of high‐dimensional propensity scores should, at least in part, counter this. Carry‐over effects of drugs with long half‐lives will elude our analysis and accounting for this would require considering pharmacokinetic profiles to the individual drugs. Owing to our use of drug prescription periods, exposure for pro necessitate prescriptions may be inaccurate, something that might be remedied using timestamps of the actual administrations and, potentially, pharmacokinetic data for these drugs. Second, we only considered two‐way pDDIs. Large‐scale screening for N‐drug interactions is difficult due to combinatorial explosion in the number of possibilities and difficulties in defining a proper reference to which the results should be compared. Instead, targeted investigations would be meaningful, e.g. on triple whammy and its effect on kidney function. Third, different pDDI databases (perhaps most notably from different countries or healthcare settings) likely feature discrepancies regarding management recommendations and clinical significance, and the DID covers primarily pharmacokinetic interactions. Further, DID allows different levels of evidence: for older drugs only pDDIs supported by published evidence are considered, whereas for newer drugs also pDDIs from summaries of product characteristics not published elsewhere are included. This database, nonetheless, is well‐known among Danish physicians and used in daily practice to guide medicinal treatment and, as such, makes for a natural gold standard against which to compare real‐life prescriptions in Denmark. Fourth, the pDDIs involving antibiotics and systemic antifungals and associated with elevated mortality are used to treat serious infections. Thus, exposure to these combinations could be proxies for serious clinical conditions, themselves associated with high mortality. If so, physicians could have deemed it worthwhile to use a discouraged drug pair due to bleak prognoses. Fifth, despite a large dataset we had relatively few patients exposed to several discouraged drug pairs, making it difficult to rule out effects of these exposures on mortality and readmission rates even though we did not find any.
4.2. Conclusion
Discouraged drug pairs are common in hospitalised patients at large and so are potentially problematic drug pairs, notably, combinations of warfarin and non‐steroidal anti‐inflammatory drugs (NSAIDs) and with antiinfectives (especially, azoles, carbapenems and macrolides). The meropenem‐valproic acid and domperidone‐fluconazole combination, both prevalent in at least one patient type, were significantly associated with elevated post‐discharge mortality rate. This study elicited unfortunate prescription patterns with potentially detrimental effects in hospitalised patients and the CYP3A4 isoenzyme was involved in more than half the discouraged pairs associated with elevated mortality or readmission rates. Moreover, our findings provided quantitative evidence about the prevalence and risk of adverse outcomes associated with distinct patient types that clinicians should consider when pondering initiation of therapies and give (more) weight to patients' comorbidities to optimise patient safety.
CONFLICT OF INTEREST
Søren Brunak reports ownerships in Intomics A/S, Hoba Therapeutics Aps, Novo Nordisk A/S, Lundbeck A/S and managing board memberships in Intomics A/S outside the submitted work. All other authors report no competing interests.
AUTHOR CONTRIBUTIONS
Robert Eriksson proposed the idea. Cristina Leal Rodríguez and Benjamin Skov Kaas‐Hansen designed and conducted the study. Søren Brunak and Stig Ejdrup Andersen co‐created the project that made available and curated the EHR data. Søren Brunak and Stig Ejdrup Andersen obtained funding. Cristina Leal Rodríguez, Benjamin Skov Kaas‐Hansen and Jorge Hernansanz Biel performed pre‐processing of the data. Benjamin Skov Kaas‐Hansen extracted eGFR measurements, and calculated comorbidity scores; Jorge Hernansanz Biel extracted the information from Danish interaction database under the supervision of Cristina Leal Rodríguez. Cristina Leal Rodríguez pre‐processed the clinical and medication data, calculated the treatment exposures and treatment overlaps. Cristina Leal Rodríguez and Benjamin Skov Kaas‐Hansen performed the computational and statistical analysis. Benjamin Skov Kaas‐Hansen did the adverse outcome modelling. Cristina Leal Rodríguez and Benjamin Skov Kaas‐Hansen interpreted data and provided critical intellectual content, under guidance by Stig Ejdrup Andersen. Cristina Leal Rodríguez performed the literature search. Cristina Leal Rodríguez and Benjamin Skov Kaas‐Hansen wrote the initial draft. All authors have contributed to and approved the final manuscript.
CODE AVAILABILITY
The full analytic code is available upon request.
ETHICS STATEMENT
Data were stored and analysed on a secure cloud in Denmark. Registry data access was approved by the Danish Health Data Authority (FSEID‐00003092, FSEID‐00004491, FSEID‐00003724) and the Danish Patient Safety Authority, which at the time was the competent body for approvals regarding research in EHRs, approved journal access and the purpose for the study (3–3013–1731‐1).This article observes relevant items in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. 47
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGEMENTS
This work was supported by the Novo Nordisk Foundation (grants NNF14CC0001 and NNF17OC0027594) and the Danish Innovation Fund (grant 5153‐00002B). The funding bodies had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. Cristina Leal Rodríguez and Benjamin Skov Kaas‐Hansen had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Leal Rodríguez C, Kaas‐Hansen BS, Eriksson R, et al. Drug interactions in hospital prescriptions in Denmark: Prevalence and associations with adverse outcomes. Pharmacoepidemiol Drug Saf. 2022;31(6):632‐642. doi: 10.1002/pds.5415
Cristina Leal Rodríguez and Benjamin Skov Kaas‐Hansen are joint first authors.
This article has been published as preprint in MedRxiv doi: https://doi.org/10.1101/2021.05.27.21257764.
Funding information Innovationsfonden; Novo Nordisk Fonden; Danish Innovation Fund, Grant/Award Number: 5153‐00002B; Novo Nordisk Foundation, Grant/Award Numbers: NNF17OC0027594, NNF14CC0001
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Baxter K, Preston CL. Stockley's Drug Interactions. Vol 495. Pharmaceutical Press; 2010. [Google Scholar]
- 2. Guthrie B, Makubate B, Hernandez‐Santiago V, Dreischulte T. The rising tide of polypharmacy and drug–drug interactions: population database analysis 1995–2010. BMC Med. 2015;13(1):74‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doucet J, Chassagne P, Trivalle C, et al. Drug–drug interactions related to hospital admissions in older adults: a prospective study of 1000 patients. J. Am. Geriatr. Soc. 1996;44(8):944‐948. [DOI] [PubMed] [Google Scholar]
- 4. Egger SS, Drewe J, Schlienger RG. Potential drug–drug interactions in the medication of medical patients at hospital discharge. Eur. J. Clin. Pharmacol. 2003;58(11):773‐778. [DOI] [PubMed] [Google Scholar]
- 5. Hansten PD, Horn JR. Hansten and Horn's drug interactions analysis and management: a clinical perspective and analysis of current developments. Appl. Therap. Incorp. 2004. [Google Scholar]
- 6. Jankel CA, Speedie SM. Detecting drug interactions: a review of the literature. DICP. 1990;24(10):982‐989. [DOI] [PubMed] [Google Scholar]
- 7. McInnes GT, Brodie MJ. Drug interactions that matter. Drugs. 1988;36(1):83‐110. [DOI] [PubMed] [Google Scholar]
- 8. Einarson TR. Drug‐related hospital admissions. Ann Pharmacother; 1993;27(7‐9):832–40. [DOI] [PubMed] [Google Scholar]
- 9. Bjerrum L, Søgaard J, Hallas J, Kragstrup J. Polypharmacy in general practice: differences between practitioners. Br. J. Gen. Pract. 1999;49(440):195‐198. [PMC free article] [PubMed] [Google Scholar]
- 10. Reis AMM, Cassiani SHDB. Prevalence of potential drug interactions in patients in an intensive care unit of a university hospital in Brazil. Clinics. 2011;66(1):9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moura CS, Acurcio FA, Belo NO. Drug–drug interactions associated with length of stay and cost of hospitalization. J. Pharm. Pharm. Sci. 2009;12(3):266‐272. [DOI] [PubMed] [Google Scholar]
- 12. Shad MU, Marsh C, Preskorn SH. The economic consequences of a drug–drug interaction. J. Clin. Psychopharmacol. 2001;21(1):119‐120. [DOI] [PubMed] [Google Scholar]
- 13. Bucşa C, Farcaş A, Cazacu I, et al. How many potential drug–drug interactions cause adverse drug reactions in hospitalized patients? Eur. J. Intern. Med. 2013;24(1):27‐33. [DOI] [PubMed] [Google Scholar]
- 14. Kuperman GJ, Bobb A, Payne TH, et al. Medication‐related clinical decision support in computerized provider order entry systems: a review. J. Am. Med. Inform. Assoc. 2007;14(1):29‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. FOLK1A: Population at the first day of the quartemarital status. Statistics Denmark. Accessed March 24, 2021. www.statbank.dk/FOLK1A.
- 16. Rodríguez CL, Mazzoni G, Haue AD, et al. Polypharmacy and drug dosage modifications: a longitudinal analysis of 3.5 million electronic health records. Forthcoming 2022.
- 17. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin. Epidemiol. 2015;7:449‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish Health Care System and Epidemiological Research: from Health Care Contacts to Database Records. Vol 11. Dove Press; 2019:563‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen TB, Jimenez‐Solem E, Cortes R, et al. Content and validation of the electronic patient medication module (EPM)—the administrative in‐hospital drug use database in the Capital Region of Denmark. Scand. J. Public Health. 2020;48(1):43‐48. [DOI] [PubMed] [Google Scholar]
- 20. Oslo N. ATC Classification Index with DDDs. WHO Collaborating Centre for Drug Statistics Methodology; 2017. [Google Scholar]
- 21. Aagaard L, Kristensen M. The national drug interactions database. Ugeskr. Laeger. 2005;167(35):3283‐3286. [PubMed] [Google Scholar]
- 22. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. Simul. Comput. 2009;38(6):1228‐1234. [Google Scholar]
- 23. Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 24. Sloane D, Morgan SP. An introduction to categorical data analysis. Annu. Rev. Sociol. 1996;22(1):351‐375. [Google Scholar]
- 25. Walker AM, Patrick AR, Lauer MS, et al. A tool for assessing the feasibility of comparative effectiveness research. Comp Eff Res. 2013;2013(3):11‐20. [Google Scholar]
- 26. Stürmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J. Clin. Epidemiol. 2006;59(5):e431‐e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High‐dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20(4):512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raghunathan K, Layton JB, Ohnuma T, Shaw AD. Observational research using propensity scores. Adv. Chronic Kidney Dis. 2016;23(6):367‐372. [DOI] [PubMed] [Google Scholar]
- 29. Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans. Model. Computer Simul. 2013;23(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in‐hospital mortality using hospital administrative data. Med Care. 2017;55(7):698‐705. [DOI] [PubMed] [Google Scholar]
- 31. Köster J, Rahmann S. Snakemake—a scalable bioinformatics workflow engine. Bioinformatics. 2012;28(19):2520‐2522. [DOI] [PubMed] [Google Scholar]
- 32. Percha B, Altman RB. Informatics confronts drug–drug interactions. Trends Pharmacol. Sci. 2013;34(3):178‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hernán MA, Robins JM. Causal Inference: What if. Chapman & Hall/CRC; 2020. [Google Scholar]
- 34. Hines LE, Murphy JE. Potentially harmful drug–drug interactions in the elderly: a review. Am. J. Geriatr. Pharmacother. 2011;9(6):364‐377. [DOI] [PubMed] [Google Scholar]
- 35. Chiatti C, Bustacchini S, Furneri G, et al. The economic burden of inappropriate drug prescribing, lack of adherence and compliance, adverse drug events in older people. Drug Saf. 2012;35(1):73‐87. [DOI] [PubMed] [Google Scholar]
- 36. Gnjidic D, Johnell K. Clinical implications from drug–drug and drug‐disease interactions in older people. Clin. Exp. Pharmacol. Physiol. 2013;40(5):320‐325. [DOI] [PubMed] [Google Scholar]
- 37. de Andrade G, Santos TN, da Cruz M, Macieira G, Cardoso Sodré Alves BM, et al. Prevalence of clinically manifested drug interactions in hospitalized patients: a systematic review and meta‐analysis. PLoS One. 2020;15(7):e0235353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muñoz‐Torrero JFS, Barquilla P, Velasco R, et al. Adverse drug reactions in internal medicine units and associated risk factors. Eur. J. Clin. Pharmacol. 2010;66(12):1257‐1264. [DOI] [PubMed] [Google Scholar]
- 39. Cruciol‐Souza JM, Thomson JC. Prevalence of potential drug–drug interactions and its associated factors in a Brazilian teaching hospital. J. Pharm. Pharm. Sci. 2006;9(3):427‐433. [PubMed] [Google Scholar]
- 40. Koenig Á, Adrieno G. Potential drug interactions prevalence in intensive care units. Rev Bras Ter Intens. 2008;20(4):349‐354. [PubMed] [Google Scholar]
- 41. Straubhaar B, Krähenbühl S, Schlienger RG. The prevalence of potential drug–drug interactions in patients with heart failure at hospital discharge. Drug Saf. 2006;29(1):79‐90. [DOI] [PubMed] [Google Scholar]
- 42. Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH. Hospitalisations and emergency department visits due to drug–drug interactions: a literature review. Pharmacoepidemiol. Drug Saf. 2007;16(6):641‐651. [DOI] [PubMed] [Google Scholar]
- 43. Rosholm J‐U, Bjerrum L, Hallas J, Worm J, Gram LF. Polypharmacy and the risk of drug–drug interactions among Danish elderly. A prescription database study. Dan. Med. Bull. 1998;45(2):210‐213. [PubMed] [Google Scholar]
- 44. Brattig Correia R, de Araújo Kohler LP, Mattos MM, Rocha LM. City‐wide electronic health records reveal gender and age biases in administration of known drug–drug interactions. NPJ Digital Med. 2019;2(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Glintborg B, Andersen SE, Dalhoff K. Drug–drug interactions among recently hospitalised patients–frequent but mostly clinically insignificant. Eur. J. Clin. Pharmacol. 2005;61(9):675‐681. [DOI] [PubMed] [Google Scholar]
- 46. Gibbs MA, Thummel KE, Shen DD, Kunze KL. Inhibition of cytochrome P‐450 3A (CYP3A) in human intestinal and liver microsomes: comparison of K i values and impact of CYP3A5 expression. Drug Metab. Dispos. 1999;27(2):180‐187. [PubMed] [Google Scholar]
- 47. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern. Med. 2007;147(8):573‐577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
Not applicable.


