Abstract
The impact of the 2,4-diacetylphloroglucinol-producing biocontrol agent Pseudomonas fluorescens F113Rif on the diversity of the resident community of culturable fluorescent pseudomonads associated with the roots of field-grown sugar beet seedlings was evaluated. At 19 days after sowing, the seed inoculant F113Rif had replaced some of the resident culturable fluorescent pseudomonads at the rhizoplane but had no effect on the number of these bacteria in the rhizosphere. A total of 498 isolates of resident fluorescent pseudomonads were obtained and characterized by molecular means at the level of broad phylogenetic groups (by amplified ribosomal DNA restriction analysis) and at the strain level (with random amplified polymorphic DNA markers) as well as phenotypically (55 physiological tests). The introduced pseudomonad induced a major shift in the composition of the resident culturable fluorescent Pseudomonas community, as the percentage of rhizoplane isolates capable of growing on three carbon substrates (erythritol, adonitol, and l-tryptophan) not assimilated by the inoculant was increased from less than 10% to more than 40%. However, the pseudomonads selected did not display enhanced resistance to 2,4-diacetylphloroglucinol. The shift in the resident populations, which was spatially limited to the surface of the root (i.e., the rhizoplane), took place without affecting the relative proportions of phylogenetic groups or the high level of strain diversity of the resident culturable fluorescent Pseudomonas community. These results suggest that the root-associated Pseudomonas community of sugar beet seedlings is resilient to the perturbation that may be caused by a taxonomically related inoculant.
Biological control of diseases and pests of crops using microbial inoculants is receiving increased attention as an environmentally friendly alternative to the use of chemical pesticides (6, 14, 45). For biocontrol strains of fluorescent Pseudomonas spp., the production of antimicrobial secondary metabolites often represents a key factor in their ability to protect plant roots from fungal soilborne diseases (12, 21, 40). One promising secondary metabolite is 2,4-diacetylphloroglucinol (Phl), a polyketide synthesized by a diverse array of biocontrol pseudomonads (22, 45). Indeed, genetic enhancement of Phl-producing ability in pseudomonads can lead to higher Phl levels in the rhizosphere (2, 27) and increased plant protection against fungal root diseases (27, 40, 41).
Efficient biocontrol requires that large numbers of microbial cells be released into the environment, and issues relating to the biosafety of this technology have been highlighted, regardless of whether wild-type or genetically modified strains are considered (6, 9, 48). Often, the impact of bacterial inoculants on nontarget populations has been assessed quantitatively, i.e., with respect to the population sizes of particular microbial groups defined on the basis of taxonomy or physiological function (4, 11, 16, 33, 37, 47). However, this approach is limited by the fact that important ecological impacts may take place in terms of the composition of a particular microbial group without modification of its population size.
Indirect evidence of perturbations caused by bacterial inoculants on the indigenous bacterial community of the rhizosphere has been obtained by studying community-level catabolic profiles on BIOLOG plates, microbial enzymatic activities, profiles of fatty acid methyl esters extracted from the rhizosphere, and/or the distribution of r/K strategists on plates (10, 11, 34, 37, 46). The impact of biocontrol pseudomonads on the diversity of the bacterial community has been investigated at the level of the genus and/or species (26, 37), and it can be expected that this type of work will benefit from current developments in molecular, culture-independent approaches (25, 38). However, lower taxonomic levels (i.e., below the species level) have been neglected so far. Because of niche overlap (33, 36), a Pseudomonas inoculant will be expected to interact with fellow fluorescent pseudomonads indigenous to the soil for colonization of the rhizosphere. The community of resident culturable fluorescent pseudomonads (RCFP) in the rhizosphere plays a key role in the functioning of the ecosystem through its contribution to plant health, nutrient cycling, and soil fertility (8). It is therefore important to understand how Phl-producing Pseudomonas biocontrol inoculants can influence this nontarget community.
The objective of the current work was to assess, under field conditions, the impact of the Phl-producing biocontrol agent Pseudomonas fluorescens F113Rif on the community of RCFP associated with the roots of sugar beet seedlings. This objective was achieved by comparing the intraspecific diversities of rhizosphere and rhizoplane RCFP obtained from uninoculated sugar beet seedlings and seedlings inoculated with strain F113Rif.
MATERIALS AND METHODS
Inoculation of seeds and field experiment.
P. fluorescens F113, which was isolated from the roots of sugar beets (42), can protect sugar beets from Pythium damping-off disease. Pythium spp. infect the plant shortly after sowing (13). Strain F113Rif is a spontaneous rifampin-resistant mutant of F113 that grows and produces Phl like the wild-type strain in vitro (4) and whose disease-suppressive ability has been demonstrated (13, 32).
Strain F113Rif was delivered at 6.0 log CFU per sugar beet seed (cultivar Accord) using a biocontrol-compatible (32) seed-pelleting formulation (30). The experiment was carried out in 1994, near Bandon (County Cork, Ireland), at a site where the ecological impact of F113Rif on ecosystem functioning has been studied using a combination of different approaches (30, 31, 35). The field site, soil characteristics (brown podzolic soil), and farming conditions have been described in detail elsewhere (30). Damping-off disease of sugar beets often takes place at this site, but there was no disease pressure in 1994 (probably for climatic reasons), so that potential nontarget effects of the inoculant could not be compensated for by its positive biocontrol effect. Four plots (adjacent to plots I-1, III-1, V-1, and VII-1 defined by Moënne-Loccoz et al. [30]) located along a 72-m transect and comprising furrows of uninoculated sugar beet seeds and furrows of F113Rif-inoculated seeds were studied. The distance between the centers of two consecutive plots was 24 m.
Sampling and colony counts.
At 19 days after sowing, three adjacent inoculated sugar beets (spaced 17 cm apart on the row), three adjacent uninoculated plants, and a 100-g bulk soil sample (taken from the surface soil horizon halfway between two uninoculated rows) were collected at the center of each plot. Two neighboring rows (distant by 56 cm) were used to sample the plants (one row with inoculated sugar beets and the other with uninoculated sugar beets). Plant shoots were 4 to 6 cm high, and roots (about 10 to 12 cm long) were all located within the 22-cm-deep loamy surface soil horizon.
Each sample was processed individually as follows. Bulk soil (1 g) was transferred into 10 ml of one-quarter-strength Ringer's solution (Oxoid, Hampshire, United Kingdom) in a McCartney bottle, and the bottles were vortexed for 5 min. Loosely adhering soil was detached from the roots by shaking and was discarded. The soil remaining on the roots (i.e., closely adhering soil) was considered rhizosphere soil. A diligent effort was made to remove this soil adhering closely to the roots by using a spatula and then by dipping the root system for 2 s in 10 ml of one-quarter-strength Ringer's solution in a McCartney bottle. The rhizosphere soil removed with the spatula was subsequently added to those bottles, which were vortexed for 5 min. The shoots were excised. Each soil-free root system was transferred to a new bottle containing 10 ml of one-quarter-strength Ringer's solution and was extracted by vortexing the bottle for 5 min. The extract was used to recover rhizoplane bacteria.
Each extract (from individual samples of bulk soil, rhizosphere soil, and rhizoplane) was serially diluted in one-quarter-strength Ringer's solution and spread plated. Colony counts of F113Rif were determined on Luria-Bertani (LB) (39) agar containing 100 μg of rifampin/ml (i.e., Rif100) and the antifungal compound cycloheximide (100 μg/ml). Colonies derived from inoculated plants and resistant to Rif100 displayed a random amplified polymorphic DNA (RAPD) profile (29) identical to that of F113Rif (30). A few Rif100-resistant colonies were obtained from bulk soil or uninoculated plants, but their RAPD profiles differed from that of F113Rif (data not shown).
The total culturable fluorescent pseudomonads and the total culturable aerobic bacteria were recovered on S1 agar (17) and LB agar as described by Carroll et al. (4) and Moënne-Loccoz et al. (30), respectively. S1 agar is a selective medium for fluorescent pseudomonads (17), and colony counts on S1 agar were shown to be in agreement with 16S ribosomal DNA (rDNA) quantitative direct PCR data for enumeration of root-associated pseudomonads (20). In addition, Pseudomonas diversity is higher on S1 agar than on King's B agar (19), which is the medium traditionally used to recover fluorescent pseudomonads, and S1 has become the medium of choice for these bacteria (19, 36, 47, 49). All plates were incubated at room temperature for 3 to 7 days prior to counting of colonies.
Isolation of resident fluorescent pseudomonads.
Colonies on S1 agar were chosen at random, purified by subculturing, and replica plated on LB Rif100 agar to distinguish resident bacteria from F113Rif (and to determine the percentage of colonies on S1 agar that corresponded to the inoculant). Colonies resistant to Rif100 were discarded for all treatments, and a replica of the others was checked for fluorescence under UV light. Thirty RCFP from bulk soil were obtained from each of the four plots (for a total of 120 isolates). Totals of 30 rhizoplane isolates and 30 rhizosphere isolates were obtained from uninoculated sugar beets as well as from F113Rif-inoculated sugar beets from each of the four plots. The 600 RCFP were stored at −80°C in glycerol solutions and kept on LB agar at 4°C for short-term maintenance. About 17% of the isolates were lost during the study, and characterization was completed using the 498 remaining isolates.
Genotypic and phenotypic characterizations.
Strain F113Rif and all 498 RCFP isolates were studied by amplified 16S rDNA restriction analysis (ARDRA) and RAPD analysis. ARDRA was performed using TaqI and AluI as described previously (50). Analysis of RAPD markers was done using primer DAF-4 (52). Banding patterns were generated using an automated laser fluorescent sequencer (Amersham Pharmacia Biotech, Freiburg, Germany) and compared with the use of WinCam2.2 software (Cybertech, Berlin, Germany).
For phenotypic characterization, 55 physiological attributes (see Tables 4 and 5) were studied by replica plating using actively growing colonies from LB plates. Gelatin liquefaction was determined as described previously (15). Growth on single carbon sources was studied with a low-potassium minimal medium (3) containing 0.05% yeast extract and 15 mM carbon source and solidified with purified agar (Oxoid). Growth of F113Rif and the isolates did not take place on this medium without a carbon source. To study the growth on seed exudates, sugar beet seeds (250 g) were added to 500 ml of sterile distilled water, and the flasks were shaken at 100 rpm for 3 h. The solution was filtered successively through a series of five Millipore membranes 5, 3, 1.2, 0.45, and 0.20 μm in pore diameter. The seed exudate solution was mixed with a 3% agar solution in a 1:1 ratio and poured into plates. Growth in the presence of antibiotics (see Table 5) was assessed using LB plates amended with the antibiotics. Synthetic Phl was obtained from the Chemistry Department, National University of Ireland, Cork. Plates were scored (growth or no growth) after 3 to 7 days of incubation at 28°C. The ability to produce Phl was investigated using the Phl-sensitive indicator bacterium Bacillus subtilis A1 (14), and RCFP that inhibited A1 growth were studied further by high-pressure liquid chromatography analysis (43).
TABLE 4.
Phenotypic properties of RCFP following inoculation of seeds with the biocontrol agent P. fluorescens F113Rif
| Testa | % (± SD) of isolates positive for the indicated test in the following treatmentsb:
|
F113Rifc | ||||
|---|---|---|---|---|---|---|
| Bulk soil | Sugar beet
|
|||||
| Rhizosphere
|
Rhizoplane
|
|||||
| No inoculation | Inoculation with F113Rif | No inoculation | Inoculation with F113Rif | |||
| Sugar beet seed exudates | 58.0 (19.4) ab | 68.6 (7.5) a | 44.2 (10.1) b | 76.8 (10.1) a | 94.3 (4.4) c | + |
| d-Xylose | 58.0 (19.4) a | 90.1 (6.3) b | 86.1 (4.1) b | 87.5 (10.0) b | 78.3 (13.8) ab | + |
| Trehalose | 74.7 (10.0) a | 67.6 (12.7) a | 81.0 (11.3) a | 80.4 (5.1) a | 93.0 (8.1) b | − |
| Erythritol | 1.1 (2.3) a | 6.1 (5.2) ab | 13.1 (10.4) b | 5.5 (2.4) ab | 47.9 (21.1) c | − |
| Adonitol | 8.6 (4.2) ab | 1.7 (3.4) a | 17.8 (15.2) b | 6.6 (4.2) ab | 61.3 (16.6) c | − |
| l-Tryptophan | 25.8 (20.0) a | 6.9 (6.2) b | 32.0 (11.1) a | 19.6 (13.8) ab | 69.5 (16.8) c | − |
Growth at the expense of sugar beet seed exudates and assimilation of carbon sources.
All 498 isolates and F113Rif utilized d-ribose, mannitol, glucose, d-mannose, d-fructose, acetate, succinate, malonate, dl-malate, lactate, citrate, glycerate, glycerol, l-proline, d-alanine, l-serine, l-leucine, l-valine, and l-lysine as sole carbon sources. The 498 isolates and F113Rif were unable to utilize maltose, cellobiose, d-arabinose, sorbose, and pantothenate as sole carbon sources. Treatments had no influence on the percentages of RCFP capable of assimilating d-galactose (97.2%), l-rhamnose (9.4%), sucrose (60.2%), raffinose (2.4%), galacturonate (95.0%), glycolate (1.4%), l-tartrate (26.1%), nicotinate (11.4%), benzoate (70.5%), ethanol (27.5%), sorbitol (16.5%), glycine (40.4%), l-ornithine (85.7%), or l-phenylalanine (97.0%) or displaying gelatinase activity (66.3%). Data that were statistically different (P < 0.05) between treatments are indicated by lowercase letters within each row.
Tests in which F113Rif scored positive are indicated by +.
TABLE 5.
Resistance to Phl of RCFP following inoculation of seeds with the Phl-producing biocontrol agent P. fluorescens F113Rifa
| Phl concn (μg/ml) | % (± SD) of isolates resistant to Phl in the following treatmentsb:
|
F113Rifc | ||||
|---|---|---|---|---|---|---|
| Bulk soil | Sugar beet
|
|||||
| Rhizosphere
|
Rhizoplane
|
|||||
| No inoculation | Inoculation with F113Rif | No inoculation | Inoculation with F113Rif | |||
| 200 | 53.9 (23.1) | 63.0 (16.8) | 73.1 (10.5) | 56.2 (10.2) | 76.1 (13.5) | + |
| 300 | 21.7 (18.9) | 29.2 (5.5) | 26.9 (11.0) | 38.2 (6.2) | 29.3 (28.9) | + |
Commercial antibiotics were also tested. All 498 isolates and F113Rif were resistant to ampicillin (100 μg/ml) and spectinomycin (25 μg/ml). Treatments had no effect on the percentages of RCFP resistant to rifampin at 10 μg/ml (81.7%), chloramphenicol at 50 μg/ml (46.4%), gentamicin at 2 μg/ml (33.7%), kanamycin at 15 μg/ml (4.8%), streptomycin at 20 μg/ml (7.2%), and tetracycline at 2.5 μg/ml (29.3%).
There was no statistical difference (P < 0.05) between treatments at either of the two Phl concentrations. F113Rif can grow in the presence of Phl at 500 μg/ml, and the percentage of RCFP isolates resistant to 500 μg of Phl/ml was less than 5% in each of the five treatments.
Growth of F113Rif is indicated by +.
Indices of strain diversity.
Strains were defined based on either phenotypic profiles (yielding phenotypically defined strains) or RAPD profiles (yielding genotypically defined strains) as follows. Isolates sharing the same phenotypic or RAPD profile were considered to belong to the same phenotypically or genotypically defined strain, respectively. The genotypic (RAPD) and phenotypic diversities of the RCFP were evaluated with regard to the number of strains identified (i.e., strain richness) using Shannon's H′ index (44) and the distribution of isolates among those strains (i.e., strain evenness) using Shannon's E index (44). Strain evenness was computed from H′ and the total number of strains (S) as follows: E = H′/ln S.
Statistics.
Colony counts obtained from individual plants were log transformed. The effect of inoculation on the numbers of RCFP and resident culturable aerobic bacteria was assessed for the rhizosphere (12 replications) and for the rhizoplane (12 replications). This assessment was done by analyses of variance (P < 0.05), and Systat 5.05 was used (SPSS Science, Chicago, Ill.).
Three statistical approaches were used to study the composition of the RCFP community at Bandon. In the first one, analyses of variance were carried out, followed (when appropriate) by Fisher's least-significant-difference tests (P < 0.05; Systat 5.05). Each of the four plots was considered a replication (i.e., results for isolates from each set of three adjacent plants were pooled), and arcsine-transformed values of the square root of percentages were used in the analyses. Two limitations need to be kept in mind with these types of analyses. First, there is always a small number of isolates that die during a biodiversity study that focuses on environmental bacteria (16); consequently, treatments do not contain exactly the same numbers of isolates. In this work, fluctuations in the final number of isolates from one treatment to the next were relatively modest (from 80 to 110 isolates per treatment), and statistical analyses gave the same results when a total of 80 randomly chosen isolates were used for each treatment. The second issue corresponds to the normality of the data which, considering the experimental design, could not be established formally in this work.
Therefore, the statistical relationship between treatments was confirmed using procedures of the general linearized model (GLIM) as described by Crawley (7). In this situation, error type is binomial when the exact number of isolates in each treatment is considered. For each variable studied, the proportion of isolates scoring positively was analyzed according to a first factor corresponding to the distance to the root (i.e., rhizosphere versus rhizoplane) and a second factor corresponding to inoculation (i.e., inoculation with F113Rif versus no inoculation). The significance of each simple factor and the interaction between those factors were studied using t tests with GLIM parameters (P < 0.05) as described previously (7). This statistical approach is interesting when one is considering the differences in the number of isolates from one treatment to the next and the issue of normality, but the fifth treatment (i.e., bulk soil) is excluded from the analyses. However, both statistical approaches gave similar results for the four root-associated treatments in most instances. Therefore, the statistical analyses presented in this report are those obtained with the first approach (36), which were confirmed by GLIM analysis.
The third statistical approach was based on χ2 tests (P < 0.05) and focused on resident isolates obtained from the rhizoplane of inoculated plants, with the objective of comparing the subpopulation of RCFP isolates that could assimilate the substrates erythritol, adonitol, and l-tryptophan with the other isolates from the same treatment. Data expressed as the numbers of isolates were used in all χ2 tests.
RESULTS AND DISCUSSION
The biocontrol inoculant had replaced some of the root-associated RCFP at 19 days.
At 19 days after sowing, the biocontrol inoculant P. fluorescens F113Rif was found at 5.61 ± 0.35 log CFU/root system in the rhizosphere of sugar beet seedlings. The presence of the inoculant in the rhizosphere had no effect on the number of RCFP or that of the total culturable fluorescent pseudomonads (Table 1), despite the fact that F113Rif represented as much as 63% of the latter in the rhizosphere of inoculated sugar beets. The inoculant was recovered at 5.29 ± 0.41 log CFU/root system at the rhizoplane, and the number of RCFP at the rhizoplane of inoculated sugar beets was lower than that for uninoculated plants (Table 1). This lower value was due to the presence of F113Rif, as the numbers of total culturable fluorescent pseudomonads at the rhizoplane did not differ statistically for inoculated and uninoculated sugar beets. The inoculant represented 75% of the total culturable fluorescent pseudomonads at the rhizoplane of inoculated sugar beets.
TABLE 1.
Effect of the biocontrol seed inoculant P. fluorescens F113Rif on populations of culturable fluorescent pseudomonads and culturable aerobic bacteria associated with roots of field-grown sugar beet seedlings at 19 days
| Root-associated habitata | Bacterial group | log CFU (± SD) of bacteria per root systemb
|
|
|---|---|---|---|
| Uninoculated | F113Rif inoculated | ||
| Rhizosphere | RCFP | 5.54 (0.44) | 5.40 (0.21) |
| Total culturable fluorescent pseudomonads | 5.54 (0.44) | 5.82 (0.19) | |
| Resident culturable aerobic bacteria | 6.06 (0.21) | 6.22 (0.13) | |
| Total culturable aerobic bacteria | 6.06 (0.21) | 6.23 (0.28) | |
| Rhizoplane | RCFP | 5.07 (0.37) a | 4.64 (0.52) b |
| Total culturable fluorescent pseudomonads | 5.07 (0.37) | 5.24 (0.50) | |
| Resident culturable aerobic bacteria | 5.48 (0.31) | 5.58 (0.35) | |
| Total culturable aerobic bacteria | 5.48 (0.31) | 5.72 (0.36) | |
RCFP were recovered at 6.07 ± 0.10 (mean ± standard deviation) log CFU/g of soil.
Data that were statistically different (P < 0.05) are indicated by lowercase letters.
Inoculation of soil or seeds with pseudomonads for biocontrol purposes implies the release of cells in large numbers (often higher than the number of RCFP), which can cause a transient increase in the number of total culturable fluorescent pseudomonads (1, 49) and even sometimes in that of total culturable aerobic bacteria (4, 49). At 19 days after sowing, the number of total culturable fluorescent pseudomonads at the rhizoplane was not affected, but that of RCFP was reduced (Table 1), similar to the results of other studies (10, 36, 47). This suggests that sampling took place after this transient situation and that the seed inoculant interacted with RCFP while colonizing the roots of sugar beet seedlings.
The biocontrol inoculant had no impact on the structure of the community of root-associated RCFP at 19 days.
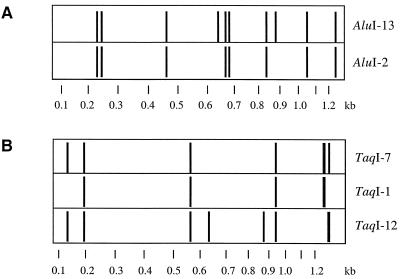
A total of 498 RCFP isolates were characterized. No colony was found on S1 agar when uninoculated seeds were studied (as described by Moënne-Loccoz et al. [32]) before sowing, which means that for the four root-associated treatments, the RCFP sampled were unlikely to have originated from populations of naturally occurring seed-borne pseudomonads. Four phylogenetic groups were identified by ARDRA for 496 of the 498 RCFP isolates (i.e., groups AluI-2/TaqI-1, AluI-13/TaqI-1, AluI-13/TaqI-7, and AluI-13/TaqI-12) (Fig. 1). The percentage of resident isolates within each of these four ARDRA groups was not influenced by the presence of roots or inoculation with F113Rif (Table 2). Similarly, inoculation of a pseudomonad did not alter the community structure of the culturable aerobic bacteria colonizing cucumber roots (studied at the genus level) (26) or the 16S rDNA denaturing gradient gel electrophoretic patterns of bacteria in the potato rhizosphere (25). In summary, the biocontrol inoculant F113Rif had no impact on the structure of the RCFP community associated with the roots of sugar beet seedlings.
FIG. 1.
Molecular analysis of P. fluorescens F113Rif and the 498 isolates of RCFP by ARDRA. The DNA fragments obtained by ARDRA were separated by electrophoresis using an automated laser fluorescent sequencer. The resulting traces were then incorporated into image analysis software (WinCam2.2) and converted into the banding patterns shown. Four ARDRA groups were identified for 496 of the 498 RCFP isolates when restriction analysis of amplified 16S rDNA was done using AluI and TaqI as follows. With AluI, the isolates yielded profile AluI-2 or AluI-13 (A). With TaqI, isolates with profile AluI-13 displayed profile TaqI-1, TaqI-7, or TaqI-12, and those with profile AluI-2 yielded profile TaqI-1 (B). The biocontrol inoculant F113Rif displayed profiles AluI-13 and TaqI-1.
TABLE 2.
Frequency of ARDRA groups AluI-2/TaqI-1, AluI-13/TaqI-1, AluI-13/TaqI-7, and AluI-13/TaqI-12 in RCFP following inoculation of sugar beet seeds with the biocontrol agent P. fluorescens F113Rif
| ARDRA groupa | % (± SD) of ARDRA group in the following treatmentsb:
|
F113Rifc | ||||
|---|---|---|---|---|---|---|
| Bulk soil | Sugar beet
|
|||||
| Rhizosphere
|
Rhizoplane
|
|||||
| No inoculation | Inoculation with F113Rif | No inoculation | Inoculation with F113Rif | |||
| AluI-2/TaqI-1 | 12.5 (11.9) | 4.5 (5.3) | 18.1 (12.4) | 7.2 (7.2) | 19.0 (17.0) | − |
| AluI-13/TaqI-1 | 27.4 (18.0) | 40.0 (7.0) | 32.3 (17.2) | 44.6 (16.9) | 27.3 (15.7) | + |
| AluI-13/TaqI-7 | 41.3 (7.2) | 42.0 (3.8) | 35.9 (23.6) | 36.9 (5.2) | 34.4 (14.9) | − |
| AluI-13/TaqI-12 | 17.6 (2.2) | 13.5 (7.0) | 13.7 (8.9) | 10.3 (9.1) | 17.6 (13.3) | − |
A total of 496 of 498 isolates belonged to one of the four ARDRA groups.
There was no statistical difference (P < 0.05) between treatments for any of the ARDRA groups.
ARDRA group membership for F113Rif is indicated by +.
The biocontrol inoculant had no impact on strain distribution patterns within the community of root-associated RCFP at 19 days.
The most frequent RAPD profile was shared by 27 of the 498 RCFP isolates, which in turn displayed a total of 24 different phenotypic profiles. One of the rhizoplane isolates displayed a RAPD profile identical to that of F113Rif, but the isolate was phenotypically different from the inoculant based on several properties in addition to Rif100 sensitivity. A striking feature of the collection of 498 RCFP isolates sampled in the experiment was its very high level of strain diversity, regardless of whether strains were defined by molecular (RAPD analysis) (Fig. 2) or phenotypic (based on 55 independent tests) characterization. Indeed, RAPD and phenotypic determinations identified as many as 310 and 442 different strains, respectively, from the 498 isolates. Plasmids are infrequent in fluorescent pseudomonads isolated from soil or roots (23) and thus were unlikely to account for the high level of strain diversity found in these isolates. Not surprisingly, only small percentages (usually less than 10%) of the RAPD profiles and phenotypic profiles observed with a given treatment also were found with another treatment. In parallel, the level of strain diversity (Shannon's H′ index) was high and strain evenness (Shannon's E index) was close to 1, regardless of whether phenotypic or RAPD profiles were considered (Table 3). These results suggest that the disturbance caused to soil RCFP by the plant and/or the inoculant was small, if it existed at all.
FIG. 2.
Diversity of RAPD profiles for 16 isolates randomly chosen from the 498 RCFP isolates (lanes 3 to 23) and for strain F113Rif (lane 1). PCR fragments generated by the RAPD technique were separated by electrophoresis, and the photograph was taken after silver staining of the gel. The size marker ΦX174-RF-DNA (HaeIII digest; Amersham Pharmacia Biotech) is included in lane 2 and shows bands (from top to bottom) of 1,358, 1,078, 872, 603, 310 to 271 (in fact, three bands too close to each other to be distinguished), 234, 194, and 118 bp (a 72-bp band is too faint to be seen). Isolates X109, X116, and X117 (lanes 14, 21, and 22, respectively) exhibited the same RAPD profile, as did isolates X113 and X115 (lanes 18 and 20, respectively). The reproducibility of the RAPD procedure is illustrated by duplicate assays of isolates X103 (in lanes 8 and 9) and X110 (in lanes 15 and 16).
TABLE 3.
Effect of the biocontrol seed inoculant P. fluorescens F113Rif on strain diversity of RCFPa
| Characteristic | Value for the following treatmentsb:
|
||||
|---|---|---|---|---|---|
| Bulk soil (n = 80) | Rhizosphere
|
Rhizoplane
|
|||
| No inoculation (n = 105) | Inoculation with F113Rif (n = 105) | No inoculation (n = 110) | Inoculation with F113Rif (n = 98) | ||
| H′ for RAPD profilesc | 2.83 (2.69–2.91) | 3.05 (2.70–3.22) | 2.95 (2.75–3.12) | 3.04 (2.93–3.15) | 2.83 (2.69–3.09) |
| H′ for phenotypic profilesc | 2.96 (2.77–3.09) | 3.18 (2.94–3.29) | 3.25 (3.09–3.40) | 3.24 (3.12–3.36) | 3.22 (2.87–3.40) |
| Ed for RAPD profilese | 0.99 (0.01) | 0.98 (0.01) | 1.00 | 0.98 (0.01) | 0.99 (0.01) |
| E for phenotypic profilese | 0.99 (0.01) | 0.98 (0.01) | 0.98 (0.01) | 0.98 (0.01) | 0.97 (0.01) |
Strains were defined from the 498 isolates based on a comparison of RAPD profiles or of phenotypic profiles.
n, number of isolates.
Values in parentheses are ranges.
E ranges from 0 to 1 (i.e., maximal evenness of strain distribution).
Values in parentheses are standard deviations.
In summary, the strain diversity of RCFP at 19 days was high and did not appear to have been influenced by the presence of sugar beet roots or inoculation with F113Rif. This finding contrasts with the reduced diversity levels observed for RCFP colonizing tomato or flax in mesocosms (24), as well as for other resident bacteria (Paenibacillus polymyxa) at the rhizoplane of wheat compared with bulk soil in microcosms (28), and suggests that the community of root-associated RCFP of field-grown sugar beet seedlings may be particularly resilient to ecological perturbation in terms of strain diversity.
The biocontrol inoculant had a major impact on the composition of root-associated RCFP at 19 days.
In the current work, specific catabolic traits were used to compare the 498 isolates of RCFP, as was done for resident culturable aerobic bacteria from soybeans inoculated with Bacillus cereus UW85 (16). A large proportion of the carbon substrates tested have been detected in the exudates of plants (including sugar beets) but under laboratory conditions (5, 8, 18, 51). The capacity to assimilate d-xylose, a monomer of several plant cell polymers that is present in sugar beet seed exudates (5), was less frequent in RCFP from bulk soil than in those from three of the four treatments associated with roots (Table 4); this result indicates possible selection by the roots of d-xylose-assimilating strains. d-Xylose was not assimilated by any RCFP associated with flax roots, whereas this compound could be used by a large percentage of RCFP isolated from tomato roots (23). In the current work, however, the frequency of the other catabolic traits in the resident isolates was not influenced by the presence of roots, indicating that the perturbation caused to RCFP by the plant was small. This may be a particular feature of young sugar beet plants and/or of crop rotation systems, as suggested by the moderate (tomato) and strong (flax) perturbations caused to RCFP in greenhouse mesocosms under monoculture conditions (23, 24).
In contrast, the introduced biocontrol agent had a major impact on RCFP. This conclusion is indicated by the fact that the percentages of isolates assimilating trehalose, erythritol, adonitol, or l-tryptophan, which F113Rif cannot assimilate, were higher at the rhizoplane of F113Rif-inoculated plants than with the other four treatments (Table 4). In parallel, the percentage of RCFP from the rhizoplane capable of growing on sugar beet seed exudates was statistically higher for inoculated plants than for uninoculated sugar beets. Whether these four compounds were actually present in the vicinity of sugar beet roots is unknown, but this result suggests that RCFP displaying catabolic abilities different from those of F113Rif (and thus more likely to secure carbon substrates released by the root and not used by the inoculant) were favored over RCFP placed in more direct competition with F113Rif for growth substrates derived from the plant. This notion is also suggested by the observation that the percentage of root-associated RCFP isolates capable of assimilating l-tryptophan was lower for cucumber grown in soil inoculated with the Phl-producing biocontrol strain P. fluorescens CHA0 (which can assimilate this amino acid) than for cucumber grown in uninoculated soil (36). In the rhizosphere, however, the percentage of RCFP capable of growing on sugar beet seed exudates was statistically lower for inoculated plants than for uninoculated sugar beets in the current work (Table 4), a result which suggests movements of resident pseudomonads from one root compartment (rhizosphere) to the other (rhizoplane) for inoculated plants.
The major impact of the biocontrol inoculant on root-associated RCFP at 19 days was unlikely to be mediated by Phl inhibition of the RCFP.
Synthetic Phl is inhibitory at low levels to various microorganisms in vitro (21, 42). Here, however, the impact of F113Rif did not result from Phl-resistant RCFP enrichment, as treatments had no influence on the percentages of RCFP capable of growing in the presence of Phl at various concentrations (Table 5). This finding is consistent with the fact that a Phl-negative mutant of strain F113 (lacking the ability to suppress Pythium damping-off disease) (14) colonized the roots of sugar beets in a manner similar to that of its Phl-positive counterpart (4). In fact, a majority of isolates were resistant to rather high levels of Phl, confirming previous results (36). In contrast, the trifolitoxin-producing strain Rhizobium etli CE3(pT2TFXK) [but not the trifolitoxin-negative nearly isogenic mutant CE3(pT2TX3K)] had a negative impact on taxonomically related bacteria indigenous to the rhizosphere of field-grown beans, but these indigenous bacteria were trifolitoxin sensitive (38).
In the current work, the resistance of RCFP to Phl was unlikely to have resulted from previous exposure to Phl released by pseudomonads indigenous to the site, since none of the 498 RCFP isolates studied could produce Phl, as indicated by inhibition tests of Phl-sensitive indicator bacterium B. subtilis A1 on plates and high-pressure liquid chromatography analysis. Determination of the patterns of resistance of RCFP to commercial antibiotics has been proposed as an efficient phenotypic approach to distinguish between different root-associated bacteria (16). This was confirmed here, but the method failed to identify an effect of the inoculant on RCFP (Table 5). In summary, Phl inhibition of RCFP was not an important factor in the ecological perturbation to the RCFP community that followed the inoculation of Phl-producing strain F113Rif.
Characterization of RCFP favored at the rhizoplane in the presence of the biocontrol inoculant.
When we considered together the phenotypic properties of the resident strains from the rhizoplane of inoculated sugar beets, it appeared that the ability to assimilate erythritol, adonitol, and l-tryptophan was shared by 42.9% of the RCFP (i.e., 42 of 98 isolates) and that the latter could also grow on trehalose and seed exudates (Table 6). This percentage of 42.9% was statistically higher than that found with the other treatments (0% in bulk soil and 7.6% or less with the three other root-associated treatments). An ecological impact of this magnitude caused by a Pseudomonas biocontrol inoculant on RCFP was not detected before (36), perhaps for methodological reasons. The impact of F113Rif was larger than that of Burkholderia cepacia MCI 7 on resident Burkholderia populations associated with maize roots (33).
TABLE 6.
Characterization of the 42 RCFP that were capable of assimilating the three compounds erythritol, adonitol, and l-tryptophan and that were obtained from the rhizoplane of sugar beets inoculated with the biocontrol agent P. fluorescens F113Rif
| Parameter | Group, test, or concn | Result fora:
|
||
|---|---|---|---|---|
| RCFP that
|
F113Rif | |||
| Assimilate the three compounds (n = 42) | Do not assimilate the three compounds (n = 56) | |||
| ARDRA groupb | AluI-2/TaqI-1 | 24.4 | 16.1 | − |
| AluI-13/TaqI-1 | 29.3 | 26.8 | + | |
| AluI-13/TaqI-7 | 34.1 | 35.7 | − | |
| AluI-13/TaqI-12 | 9.8 | 23.2 | − | |
| Catabolic propertiesc | Sugar beet seed exudates | 100 | 89.3 | + |
| d-Xylose | 78.0 | 78.6 | + | |
| Trehalose | 100 | 87.5 | − | |
| Erythritol | 100 a | 8.9 b | − | |
| Adonitol | 100 a | 32.1 b | − | |
| l-Tryptophan | 100 a | 46.4 b | − | |
| Resistance to Phld | 200 μg/ml | 70.7 | 80.4 | + |
| 300 μg/ml | 22.0 | 35.7 | + | |
Data for RCFP are reported as percentages. Data for F113Rif are reported with + when scored positively. Data that were statistically different (P < 0.05) between the two groups of isolates are indicated by lowercase letters within each row.
One of the 42 isolates did not yield a clear ARDRA profile.
The results of the other catabolic tests for the 42 isolates were as follows (percent positive): d-galactose, 97.6%; l-rhamnose, 0%; sucrose, 63.4%; raffinose, 12.0%; galacturonate, 92.7%; glycolate, 0%; l-tartrate, 26.8%; nicotinate, 7.3%; benzoate, 75.6%; ethanol, 26.8%; sorbitol, 26.8%; glycine, 34.1%; l-ornithine, 85.4%; l-phenylalanine, 92.7%; and gelatinase activity, 72.5%.
The results of the other resistance tests for the 42 isolates were as follows (percent resistant): rifampin, 90.2%; chloramphenicol, 53.7%; gentamicin, 29.3%; kanamycin, 12.2%; streptomycin, 29.3%; and tetracycline, 19.3%.
Considering the impact of F113Rif on the RCFP at the rhizoplane, it may seem unexpected that a high level of strain diversity was maintained with this treatment (Table 3). In fact, RAPD and phenotypic analyses distinguished as many as 32 and 39 strains, respectively, from the 42 isolates from the rhizoplane of inoculated sugar beets with the ability to assimilate the carbon compounds erythritol, adonitol, and l-tryptophan. The high number of phenotypic profiles is explained by the diversity of responses of the 42 isolates in the other 52 phenotypic tests (Table 6). In addition, these isolates were distributed over the four main ARDRA groups (Table 6). Therefore, when one is considering the meaning of the shift caused by F113Rif at the strain level, it appears that (i) this shift took place in each phylogenetic group and (ii) in each of these groups several different strains with particular catabolic traits in common were selected, so that the level of strain diversity of the RCFP community at the rhizoplane of F113Rif-inoculated plants remained high (Table 3).
Ecological significance.
In this study, the interactions between the Phl-producing inoculant P. fluorescens F113Rif and RCFP appeared essentially to have involved phenomena other than Phl-mediated antagonism (probably microbial competition), and these interactions did not result in a modification of the structure or of the high level of strain diversity of the RCFP community. Despite its impact on RCFP, the inoculant had no effect on key aspects of ecosystem functioning (30, 31, 35). Overall, the results indicate that certain nontarget resident bacterial communities (here the community of RCFP) may have the capacity to buffer the ecological impact to which they are subjected following the introduction of taxonomically related bacterial inoculants. Further work is needed to assess whether these findings are specific to the pioneer community colonizing seedlings or can be extended to the RCFP community associated with roots of older plants. Nevertheless, they establish novel baseline information for biosafety research and will be important to consider in characterizing the fate of genetically modified inoculants (e.g., Phl-overproducing Pseudomonas strains displaying improved biocontrol activity) released into the soil environment.
ACKNOWLEDGMENTS
We thank P. Higgins and J. McCarthy (BIOMERIT Research Centre, National University of Ireland, Cork) for technical assistance and F. Gourbière (UMR CNRS Ecologie Microbienne, Lyon 1, Villeurbanne) for help and discussions regarding statistics. Y.M.-L. was a visiting professor at the BIOMERIT Research Centre during part of this study.
This work was supported by grants from the Biotechnology Programme (BIO2-CT93-0053 [IMPACT Project], BIO2-CT93-0196, BIO4-CT96-0027 [IMPACT 2 Project], BIO4-CT96-0181, BIO4-CT97-2227, and BIO4-CT98-0254) and the TMR Programme (FMRX-CT96-0039) of the European Commission as well as by grants awarded by Enterprise Ireland (SC/98/261 and SC/98/306).
REFERENCES
- 1.Bolton H, Jr, Fredrickson J K, Thomas J M, Li S W, Workman D J, Bentjen S A, Smith J L. Field calibration of soil-core microcosms: ecosystem structural and functional comparisons. Microb Ecol. 1991;21:175–189. doi: 10.1007/BF02539152. [DOI] [PubMed] [Google Scholar]
- 2.Bonsall R F, Weller D M, Thomashow L S. Quantification of 2,4-diacetylphloroglucinol produced by fluorescent Pseudomonas spp. in vitro and in the rhizosphere of wheat. Appl Environ Microbiol. 1997;63:951–955. doi: 10.1128/aem.63.3.951-955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brazil G M, Kenefick L, Callanan M, Haro A, de Lorenzo V, Dowling D N, O'Gara F. Construction of a rhizosphere pseudomonad with potential to degrade polychlorinated biphenyls and detection of bph gene expression in the rhizosphere. Appl Environ Microbiol. 1995;61:1946–1952. doi: 10.1128/aem.61.5.1946-1952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll H, Moënne-Loccoz Y, Dowling D N, O'Gara F. Mutational disruption of the biosynthesis genes coding for the antifungal metabolite 2,4-diacetylphloroglucinol does not influence the ecological fitness of Pseudomonas fluorescens F113 in the rhizosphere of sugar beets. Appl Environ Microbiol. 1995;61:3002–3007. doi: 10.1128/aem.61.8.3002-3007.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey C E, O'Sullivan O B, O'Gara F, Glennon J D. Ion chromatographic analysis of nutrients in seed exudate for microbial colonisation. J Chromatogr A. 1998;804:311–318. [Google Scholar]
- 6.Cook R J. Assuring the safe use of microbial biocontrol agents: a need for policy based on real rather than perceived risks. Can J Plant Pathol. 1996;18:439–445. [Google Scholar]
- 7.Crawley M J. GLIM for ecologists. Oxford, United Kingdom: Blackwell Science Ltd.; 1993. [Google Scholar]
- 8.Curl E A, Truelove B. The rhizosphere. Berlin, Germany: Springer-Verlag KG; 1986. [Google Scholar]
- 9.Défago G, Keel C, Moënne-Loccoz Y. Fate of released Pseudomonas bacteria in the soil profile: implications for the use of genetically-modified microbial inoculants. In: Zelikoff J T, Lynch J M, Shepers J, editors. EcoToxicology: responses, biomarkers and risk assessment. Fair Haven, N.J: SOS Publications; 1997. pp. 403–418. [Google Scholar]
- 10.de Leij F A A M, Sutton E J, Whipps J M, Lynch J M. Effect of a genetically modified Pseudomonas aureofaciens on indigenous microbial populations of wheat. FEMS Microbiol Ecol. 1994;13:249–258. [Google Scholar]
- 11.de Leij F A A M, Sutton E J, Whipps J M, Fenlon J S, Lynch J M. Impact of field release of genetically modified Pseudomonas fluorescens on indigenous microbial populations of wheat. Appl Environ Microbiol. 1995;61:3443–3453. doi: 10.1128/aem.61.9.3443-3453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling D N, O'Gara F. Metabolites of Pseudomonas involved in the biocontrol of plant disease. Trends Biotechnol. 1994;12:133–141. [Google Scholar]
- 13.Dunne C, Moënne-Loccoz Y, McCarthy J, Higgins P, Powell J, Dowling D N, O'Gara F. Combining proteolytic and phloroglucinol-producing bacteria for improved biocontrol of Pythium-mediated damping-off of sugar beet. Plant Pathol. 1998;47:299–307. [Google Scholar]
- 14.Fenton A M, Stephens P M, Crowley J, O'Callaghan M, O'Gara F. Exploitation of a gene(s) involved in 2,4-diacetylphloroglucinol biosynthesis to confer a new biocontrol capability to a Pseudomonas strain. Appl Environ Microbiol. 1992;58:3873–3878. doi: 10.1128/aem.58.12.3873-3878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C.: American Society for Microbiology; 1981. [Google Scholar]
- 16.Gilbert G S, Parke J L, Clayton M K, Handelsman J. Effects of an introduced bacterium on bacterial communities on roots. Ecology. 1993;74:840–854. [Google Scholar]
- 17.Gould W D, Hagedorn C, Bardinelli T R, Zablotowicz R M. New selective media for enumeration and recovery of fluorescent pseudomonads from various habitats. Appl Environ Microbiol. 1985;49:28–32. doi: 10.1128/aem.49.1.28-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale M G, Moore L D, Griffin G J. Root exudates and exudation. In: Dommergues Y R, Krupa S V, editors. Interactions between non-pathogenic soil microorganisms and plants. Developments in agricultural and managed-forest ecology. Vol. 4. Amsterdam, The Netherlands: Elsevier; 1978. pp. 163–203. [Google Scholar]
- 19.Johnsen K, Nielsen P. Diversity of Pseudomonas strains isolated with King's B and Gould's S1 agar determined by repetitive extragenic palindromic-polymerase chain reaction, 16S rDNA sequencing and Fourier transform infrared spectroscopy characterisation. FEMS Microbiol Lett. 1999;173:155–162. doi: 10.1111/j.1574-6968.1999.tb13497.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnsen K, Enger O, Jacobsen C S, Thirup L, Torsvik V. Quantitative selective PCR of 16S ribosomal DNA correlates well with selective agar plating in describing population dynamics of indigenous Pseudomonas spp. in soil hot spots. Appl Environ Microbiol. 1999;65:1786–1789. doi: 10.1128/aem.65.4.1786-1788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keel C, Schnider U, Maurhofer M, Voisard C, Laville J, Burger U, Wirthner P, Haas D, Défago G. Suppression of root diseases by Pseudomonas fluorescens strain CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Mol Plant-Microbe Interact. 1992;5:4–13. [Google Scholar]
- 22.Keel C, Weller D M, Natsch A, Défago G, Cook R J, Thomashow L S. Conservation of the 2,4-diacetylphloroglucinol biosynthesis locus among fluorescent Pseudomonas strains from diverse geographic locations. Appl Environ Microbiol. 1996;62:552–563. doi: 10.1128/aem.62.2.552-563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latour X, Corberand T, Laguerre G, Allard F, Lemanceau P. The composition of fluorescent pseudomonad populations associated with roots is influenced by plant and soil type. Appl Environ Microbiol. 1996;62:2449–2456. doi: 10.1128/aem.62.7.2449-2456.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemanceau P, Corberand T, Gardan L, Latour X, Laguerre G, Boeufgras J-M, Alabouvette C. Effect of two plant species, flax (Linum usitatissimum L.) and tomato (Lycopersicon esculentum Mill.), on the diversity of soilborne populations of fluorescent pseudomonads. Appl Environ Microbiol. 1995;61:1004–1012. doi: 10.1128/aem.61.3.1004-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lottmann J, Heuer H, de Vries J, Mahn A, Düring K, Wackernagel W, Smalla K, Berg G. Establishment of introduced antagonistic bacteria in the rhizosphere of transgenic potatoes and their effect on the bacterial community. FEMS Microbiol Ecol. 2000;33:41–49. doi: 10.1111/j.1574-6941.2000.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 26.Mahaffee W F, Kloepper J W. Bacterial communities of the rhizosphere and endorhiza associated with field-grown cucumber plants inoculated with a plant growth-promoting rhizobacterium or its genetically modified derivative. Can J Microbiol. 1997;43:344–353. doi: 10.1139/m97-048. [DOI] [PubMed] [Google Scholar]
- 27.Maurhofer M, Keel C, Haas D, Défago G. Influence of plant species on disease suppression by Pseudomonas fluorescens CHA0 with enhanced antibiotic production. Plant Pathol. 1995;44:44–50. [Google Scholar]
- 28.Mavingui P, Laguerre G, Berge O, Heulin T. Genetic and phenotypic diversity of Bacillus polymyxa in soil and in the wheat rhizosphere. Appl Environ Microbiol. 1992;58:1894–1903. doi: 10.1128/aem.58.6.1894-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moënne-Loccoz Y, McHugh B, Stephens P M, McConnell F I, Glennon J D, Dowling D N, O'Gara F. Rhizosphere competence of fluorescent Pseudomonas sp. B24 genetically modified to utilise additional ferric siderophores. FEMS Microbiol Ecol. 1996;19:215–225. [Google Scholar]
- 30.Moënne-Loccoz Y, Powell J, Higgins P, McCarthy J, O'Gara F. An investigation of the impact of biocontrol Pseudomonas fluorescens F113 on the growth of sugarbeet and the performance of subsequent clover-Rhizobium symbiosis. Appl Soil Ecol. 1998;7:225–237. [Google Scholar]
- 31.Moënne-Loccoz Y, Powell J, Higgins P, Britton J, O'Gara F. Effect of the biocontrol agent Pseudomonas fluorescens F113 released as sugarbeet inoculant on the nutrient contents of soil and foliage of a red clover rotation crop. Biol Fertil Soils. 1998;27:380–385. [Google Scholar]
- 32.Moënne-Loccoz Y, Naughton M, Higgins P, Powell J, O'Connor B, O'Gara F. Effect of inoculum preparation and formulation on survival and biocontrol efficacy of Pseudomonas fluorescens F113. J Appl Microbiol. 1999;86:108–116. [Google Scholar]
- 33.Nacamulli C, Bevivino A, Dalmastri C, Tabacchioni S, Chiarini L. Perturbation of maize rhizosphere microflora following seed bacterization with Burkholderia cepacia MCI 7. FEMS Microbiol Ecol. 1997;23:183–193. [Google Scholar]
- 34.Naseby D C, Lynch J M. Impact of wild-type and genetically modified Pseudomonas fluorescens on soil enzyme activities and microbial population structure in the rhizosphere of pea. Mol Ecol. 1998;7:617–625. [Google Scholar]
- 35.Naseby D C, Moënne-Loccoz Y, Powell J, O'Gara F, Lynch J M. Soil enzyme activities in the rhizosphere of field-grown sugar beet inoculated with the biocontrol agent Pseudomonas fluorescens F113. Biol Fertil Soils. 1998;27:39–43. [Google Scholar]
- 36.Natsch A, Keel C, Hebecker N, Laasik E, Défago G. Influence of the biocontrol strain of Pseudomonas fluorescens and its antibiotic overproducing derivative on the diversity of resident root colonizing pseudomonads. FEMS Microbiol Ecol. 1997;23:341–352. [Google Scholar]
- 37.Natsch A, Keel C, Hebecker N, Laasik E, Défago G. Impact of Pseudomonas fluorescens strain CHA0 and a derivative with improved biocontrol activity on the culturable resident bacterial community on cucumber roots. FEMS Microbiol Ecol. 1998;27:365–380. [Google Scholar]
- 38.Robleto E A, Borneman J, Triplett E W. Effects of bacterial antibiotic production on rhizosphere microbial communities from a culture-independent perspective. Appl Environ Microbiol. 1998;64:5020–5022. doi: 10.1128/aem.64.12.5020-5022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Sarniguet A, Kraus J, Henkels M D, Muehlchen A M, Loper J E. The sigma factor ςs affects antibiotic production and biological control activity of Pseudomonas fluorescens Pf-5. Proc Natl Acad Sci USA. 1995;92:12255–12259. doi: 10.1073/pnas.92.26.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnider U, Keel C, Blumer C, Troxler J, Défago G, Haas D. Amplification of the housekeeping sigma factor in Pseudomonas fluorescens CHA0 enhances antibiotic production and improves biocontrol abilities. J Bacteriol. 1995;177:5387–5392. doi: 10.1128/jb.177.18.5387-5392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shanahan P, Glennon D J, Crowley J J, Donnelly D F, O'Gara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol. 1992;58:353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shanahan P, Glennon J D, Crowley J J, Donnelly D F, O'Gara F. Liquid chromatographic assay of microbially derived phloroglucinol antibiotics for establishing the biosynthetic route to production, and the factors affecting their regulation. Anal Chim Acta. 1993;272:271–277. [Google Scholar]
- 44.Shannon C E, Weaver W. The mathematical theory of communication. Urbana: University of Illinois Press; 1949. [Google Scholar]
- 45.Sharifi-Tehrani A, Zala M, Natsch A, Moënne-Loccoz Y, Défago G. Biocontrol of soil-borne fungal plant diseases by 2,4-diacetylphloroglucinol-producing fluorescent pseudomonads with different restriction profiles of amplified 16S rDNA. Eur J Plant Pathol. 1998;104:631–643. [Google Scholar]
- 46.Siciliano S D, Germida J J. BIOLOG analysis and fatty acid methyl ester profiles indicate that pseudomonad inoculants that promote phytoremediation alter the root-associated microbial community of Bromus biebersteinii. Soil Biol Biochem. 1998;30:1717–1723. [Google Scholar]
- 47.Thirup L, Johnsen K, Winding A. Succession of indigenous Pseudomonas spp. and actinomycetes on barley roots affected by the antagonistic strain Pseudomonas fluorescens DR54 and the fungicide imazalil. Appl Environ Microbiol. 2001;67:1147–1153. doi: 10.1128/AEM.67.3.1147-1153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiedje J M, Colwell R K, Grossman Y L, Hodson R E, Lenski R E, Mack R N, Regal P J. The planned introduction of genetically engineered organisms: Ecological considerations and recommendations. Ecology. 1989;70:298–315. [Google Scholar]
- 49.Troxler J, Zala M, Moënne-Loccoz Y, Keel C, Défago G. Predominance of nonculturable cells of the biocontrol strain Pseudomonas fluorescens CHA0 in the surface horizon of large outdoor lysimeters. Appl Environ Microbiol. 1997;63:3776–3782. doi: 10.1128/aem.63.10.3776-3782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaneechoutte M, Rossau R, De Vos P, Gillis M, Janssens D, Paepe N, De Rouck A, Fiers T, Claeys G, Kersters K. Rapid identification of bacteria of the Comamonadaceae with amplified ribosomal DNA-restriction analysis (ARDRA) FEMS Microbiol Lett. 1992;93:227–234. doi: 10.1111/j.1574-6968.1992.tb05102.x. [DOI] [PubMed] [Google Scholar]
- 51.Waisel Y, Eshel A, Kafkafi U. Plant roots: the hidden half. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1996. [Google Scholar]
- 52.Wiedmann-Al-Ahmad M, Tichy H-V, Schön G. Characterization of Acinetobacter type strains and isolates obtained from wastewater treatment plants by PCR fingerprinting. Appl Environ Microbiol. 1994;60:4066–4071. doi: 10.1128/aem.60.11.4066-4071.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]