Abstract
Aims
Little information is available on sex differences in coronary microvascular dysfunction (CMD) in heart failure with preserved ejection fraction (HFpEF). We investigated sex‐specific proteomic profiles associated with CMD in patients with HFpEF.
Methods and results
Using the prospective multinational PROMIS‐HFpEF study (Prevalence of Microvascular Dysfunction in HFpEF; n = 182; 54.6% women), we compared clinical and biomarker correlates of CMD (defined as coronary flow reserve [CFR] <2.5) between men and women with HFpEF. We used lasso penalized regression to analyse 242 biomarkers from high‐throughput proximity extension assays, adjusting for age, body mass index, creatinine, smoking and study site. The prevalence of CMD was similarly high in men and women with HFpEF (77% vs. 70%; p = 0.27). Proteomic correlates of CFR differed by sex, with 10 versus 16 non‐overlapping biomarkers independently associated with CFR in men versus women, respectively. In men, proteomic correlates of CFR included chemokine ligand 20, brain natriuretic peptide, proteinase 3, transglutaminase 2, pregnancy‐associated plasma protein A and tumour necrosis factor receptor superfamily member 14. Among women, the strongest proteomic correlates with CFR were insulin‐like growth factor‐binding protein 1, phage shock protein D, CUB domain‐containing protein 1, prostasin, decorin, FMS‐like tyrosine kinase 3, ligand growth differentiation factor 15, spondin‐1, delta/notch‐like epidermal growth factor‐related receptor and tumour necrosis factor receptor superfamily member 13B. Pathway analyses suggested that CMD was related to the inflammation‐mediated chemokine and cytokine signalling pathway among men with HFpEF, and the P13‐kinase and transforming growth factor‐beta signalling pathway among women with HFpEF.
Conclusion
While the prevalence of CMD among men and women with HFpEF is similar, the drivers of microvascular dysfunction may differ by sex. The current inflammatory paradigm of CMD in HFpEF potentially predominates in men, while derangement in ventricular remodelling and fibrosis may play a more important role in women.
Keywords: Women, Coronary microvascular dysfunction, Sex differences, Heart failure with preserved ejection fraction
Introduction
Coronary microvascular dysfunction (CMD) is common in heart failure with preserved ejection fraction (HFpEF) and associated with greater severity of heart failure and systemic endothelial dysfunction. 1 Sex differences in heart failure are well‐known, with women outnumbering men with HFpEF, 2 and prominent sex‐by‐treatment interaction found in the largest HFpEF outcome trial to date. 3 Yet, sex differences in CMD in HFpEF have not been well‐studied. We hypothesized that circulating proteomic correlates of CMD may differ by sex, and illustrate potential sex‐specific pathophysiologic mechanisms underlying CMD in HFpEF. We compared the clinical and circulating proteomic correlates of CMD between men and women with HFpEF.
Methods
Among men and women with a validated diagnosis of HFpEF (left ventricular ejection fraction ≥50% in the absence of unrevascularized epicardial coronary artery disease) in the prospective multinational PROMIS‐HFpEF study (Prevalence of Microvascular Dysfunction in HFpEF), coronary flow reserve (CFR, adenosine‐induced hyperaemic flow velocity divided by resting coronary flow velocity) was measured with adenosine stress transthoracic echocardiography, as described previously. 1 Doppler echocardiography for assessment of CFR has been validated, is reproducible and is endorsed by the European Society of Cardiology. 4 , 5 CMD was defined as CFR < 2.5. Among 182 patients with available blood samples, 265 proteins were measured using high‐throughput proximity extension assays (Olink Proseek Multiplex cardiovascular II and III, and inflammation 96 × 96 kits) at a central Olink Proteomics laboratory in Sweden. We excluded 23 proteins with values below the assay detection limit (<15%). Deidentified biomarker data were subsequently merged with patients' clinical data for analysis. To assess the association between CFR and proteins, we used lasso penalized regression analyses including 242 biomarkers adjusting for age, body mass index, creatinine, smoking and study site (to reflect regional variation in HFpEF phenotype). Analyses were performed with R version 3.4.0. A 2‐sided p‐value of < 0.05 was considered statistically significant. We corrected for multiple testing using the Benjamini–Hochberg method. We identified the functional classifications of differentially expressed proteins using their gene names as input for the PANTHER annotation tool in men and women separately. Written informed consent from all study participants and ethics approval from institutional review boards at participating sites were obtained. The PROMIS‐HFpEF study complies with the Declaration of Helsinki.
Results
Among 182 patients with HFpEF (mean age 74.2 ± 8.8 years, 57% women), men and women had similar prevalence of CMD (77 vs. 70%, p = 0.27; online supplementary Table S1 ). Hyperaemic flow rates were similar between the two sexes, but mean baseline flow velocity was lower in women than men with HFpEF (8.8 ± 3.1 vs. 9.8 ± 3.3 m/s, p = 0.041). Presence of CMD was associated with higher troponin T levels (adjusted odds ratio [OR] 1.04 per ng/ml increase; 95% confidence interval [CI] 1.00–1.08) and atrial fibrillation (adjusted OR 2.26; 95% CI 1.04–4.92) in both men and women (p interaction >0.1 for both). Smoking was associated with CMD in men (adjusted OR 11.9; 95% CI 2.76–51.9) but not in women (adjusted OR 1.77; 95% CI 0.65–4.82) (p interaction = 0.015), after adjusting for age, body mass index, atrial fibrillation, diabetes, revascularized coronary artery disease, troponin T levels and left ventricular mass. Proteomic correlates of CFR differed by sex, with 10 versus 16 non‐overlapping biomarkers independently associated with CFR in men versus women, respectively, in lasso penalized regression models (Figure 1 , penalized β > 0). In men, chemokine ligand 20, proteinase 3, pregnancy‐associated plasma protein A (PAPP‐A), brain natriuretic peptide, transglutaminase 2, interleukin‐6, elafin, tumour necrosis factor receptor superfamily member 14, cluster of differentiation 93 and epithelial cell adhesion molecule were significantly associated with CFR. In contrast, the strongest proteomic correlates of CFR among women were insulin‐like growth factor‐binding protein 1, phage shock protein D, CUB domain‐containing protein 1, prostasin, decorin, FMS‐like tyrosine kinase 3 ligand, growth differentiation factor 15, spondin‐1, delta/notch‐like epidermal growth factor‐related receptor and tumour necrosis factor receptor superfamily member 13B. Functional classification analyses suggested that proteins related to CMD in men were associated with the inflammation‐mediated chemokine and cytokine signalling pathways (Figure 1C ). In contrast, proteins associated with CMD in women were related to the P13‐kinase and transforming growth factor‐beta signalling pathway (Figure 1D ).
Figure 1.
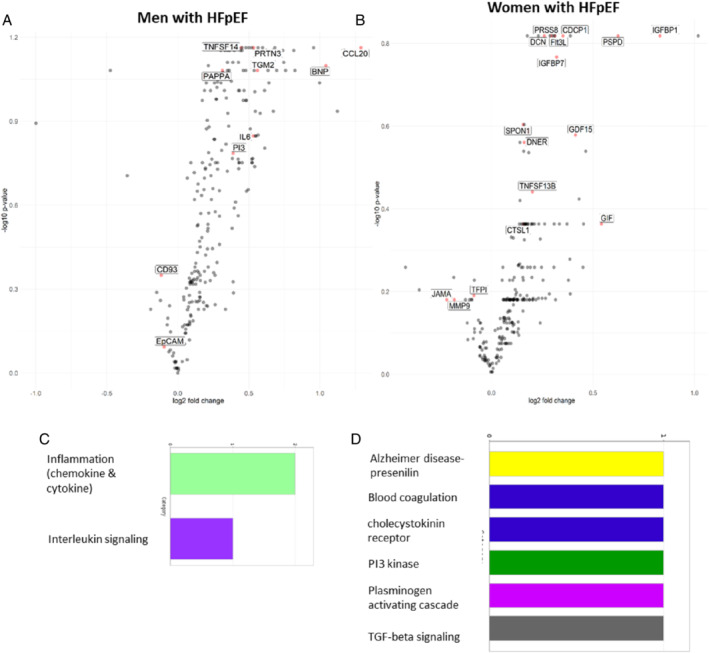
Volcano plots in men (A) and women (B) with heart failure and preserved ejection fraction (HFpEF) showing biomarkers (labelled) selected to be associated with coronary microvascular dysfunction (CMD) (coronary flow reserve [CFR] < 2.5) by lasso penalized regression analyses. The y‐axis shows the −log10 of the false discovery rate‐corrected p‐values for the associations of individual biomarkers with CMD (CFR <2.5) versus no CMD. The x‐axis shows the log2 of the fold changes of the respective biomarker differences between individuals with and without CMD. Bar graphs show functional classification analyses of differentially expressed proteins in men (C) and women (D) with HFpEF. BNP, brain natriuretic peptide; CCL20, chemokine ligand 20; CD93, cluster of differentiation 93; CDCP1, CUB domain‐containing protein 1; CTSL1, cathepsin L1; DCN, decorin; DNER, delta/notch‐like epidermal growth factor‐related receptor; EpCAM, epithelial cell adhesion molecule; Flt3L, FMS‐like tyrosine kinase 3 ligand; GDF15, growth differentiation factor 15; GIF, gastric intrinsic factor; IGFBP1, insulin‐like growth factor‐binding protein 1; IGFBP7, insulin‐like growth factor binding protein 7; IL6, interleukin‐6; JAM‐A, junctional adhesion molecule A; KIM1, kidney injury molecule 1; MMP9, metalloproteinase‐9; PAPP‐A, pregnancy‐associated plasma protein A; PI3, elafin; PRSS8, prostasin; PRTN3, proteinase 3; PSPD, phage shock protein D; SPON1, spondin‐1; TFPI, tissue factor pathway inhibitor; TGM2, transglutaminase 2; TNFSF13B, tumour necrosis factor receptor superfamily member 13B; TNFSF14, tumour necrosis factor receptor superfamily member 14.
Discussion
The prevalence of CMD was similarly high in men and women with HFpEF in the absence of unrevascularized macrovascular coronary artery disease. Clinical correlates of CMD were broadly similar between the sexes except for smoking. Yet, biomarkers associated with CMD were notably different, with a predominance of inflammatory biomarkers in men and fibrotic biomarkers in women.
Of note, while the hyperaemic coronary flow rates were similar between the sexes, mean baseline coronary flow rate was lower in women than men with HFpEF, resulting in higher CFR (i.e. ratio of hyperaemic to baseline flow rates) in women than men. Our findings extend prior reports of sex differences in invasive measures of CMD among patients with angina in the absence of obstructive coronary artery disease, 6 as well as sex differences in myocardial blood flow assessed by positron emission tomography myocardial perfusion imaging among patients referred for suspected coronary artery disease. 7
Our previous report on circulating biomarkers associated with CMD in HFpEF identified PAPP‐A as a critical biomarker of reduced CFR. 8 This study elucidates the significant association of PAPP‐A with CMD in men but not women with HFpEF. PAPP‐A is a zinc binding metalloproteinase, identified as a marker for coronary artery disease and acute coronary syndrome. PAPP‐A is elevated in unstable atherosclerosis and found to be associated with higher risk of ischaemic events. 9 In the current study, men with HFpEF had higher prevalence of prior revascularized coronary artery disease (31.9% vs. 9.0%) and more inflammatory biomarker profile than women with HFpEF.
To our knowledge, this is the first study to investigate sex differences in biomarkers associated with CMD among patients with HFpEF. Study limitations include inferences that are limited to circulating protein biomarkers, which were pre‐selected cardiovascular and inflammation markers in the assay panels. Specific inflammatory biomarkers such as erythrocyte sedimentation rate and procalcitonin were not available. Absolute biomarker levels were not quantified in this study.
While the prevalence of CMD in men and women with HFpEF is similar, the drivers of microvascular dysfunction appear to differ by sex. The current inflammatory paradigm of CMD in HFpEF potentially predominates in men, who may therefore be less responsive to neurohormonal modulation. On the other hand, derangement in ventricular remodelling and fibrosis may play a more important role in the pathogenesis of CMD among women with HFpEF, who may accordingly be more responsive to reverse remodelling by neurohormonal modulation. 3 The hypothesis‐generating findings from this explorative analysis warrant further investigation in future studies to elucidate the sex‐specific modulations of each identified target.
Conflict of interest: C.S.P.L. reports grants from National Medical Research Council Singapore; non‐financial support from Boston Scientific, Thermofisher, Vifor Pharma; non‐financial support and other from Bayer; and other from Takeda, Merck, AstraZeneca, Janssen Research & Development, LLC, Menarini, Boehringer Ingelheim, Abbott Diagnostics, DC Devices, PCT/SG2016/050217 Patent pending, outside the submitted work. All other authors have nothing to disclose.
Supporting information
Table S1. Supplementary Table.
References
- 1. Shah SJ, Lam CS, Svedlund S, Saraste A, Hage C, Tan RS, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS‐HFpEF. Eur Heart J. 2018;39:3439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lam CS, Arnott C, Beale AL, Chandramouli C, Hilfiker‐Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J. 2019;40:3859–68c. [DOI] [PubMed] [Google Scholar]
- 3. Solomon SD, McMurray JJ, Anand IS, Ge J, Lam CS, Maggioni AP, et al.; PARAGON‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–20. [DOI] [PubMed] [Google Scholar]
- 4. Olsen RH, Pedersen LR, Snoer M, Christensen TE, Ghotbi AA, Hasbak P, et al. Coronary flow velocity reserve by echocardiography: feasibility, reproducibility and agreement with PET in overweight and obese patients with stable and revascularized coronary artery disease. Cardiovasc Ultrasound. 2015;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC Guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi Y, Fearon WF, Honda Y, Tanaka S, Pargaonkar V, Fitzgerald PJ, et al. Effect of sex differences on invasive measures of coronary microvascular dysfunction in patients with angina in the absence of obstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tromp J, Hage C, Ouwerkerk W, Sanders‐van Wijk S, Svedlund S, Saraste A, et al. Biomarker correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction. Circulation. 2019;140:1359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bayes‐Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR Jr, et al. Pregnancy‐associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345:1022–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Supplementary Table.


