Abstract
As elite athletes demonstrate through the Olympic motto ‘Citius, Altius, Fortius‐ Communiter’, new performance records are driven forward by favourable skeletal muscle bioenergetics, cardiorespiratory, and endocrine system adaptations. At a recreational level, regular physical activity is an effective nonpharmacological therapy in the treatment of many endocrine conditions. However, the impact of physical exercise on endocrine function and how best to incorporate exercise therapy into clinical care are not well understood. Beyond the pursuit of an Olympic medal, elite athletes may therefore serve as role models for showcasing how exercise can help in the management of endocrine disorders and improve metabolic dysfunction. This review summarizes research evidence for clinicians who wish to understand endocrine changes in athletes who already perform high levels of activity as well as to encourage patients to exercise more safely. Herein, we detail the upper limits of athleticism to showcase the adaptability of human endocrine‐metabolic‐physiological systems. Then, we describe the growing research base that advocates the importance of understanding maladaptation to physical training and nutrition in males and females; especially the young. Finally, we explore the impact of physical activity in improving some endocrine disorders with guidance on how lessons can be taken from athletes training and incorporated into strategies to move more people more often.
Keywords: athletes, endocrine disorders, endocrinology, energy metabolism, exercise, exercise physiology
1. INTRODUCTION
One enduring characteristic of a post‐COVID pandemic is the recognition of the role of physical activity for health and well‐being. With the gradual return of fans to events such as the Tokyo 2021 Olympics, elite athletic endeavours will be witnessed by millions and the legacy of new records will inspire more people to partake in sports and exercise. Viewers of high‐performance athletes often marvel at the best of human performance. For example, the current world records for the men and women's 100 m track sprint are 9.58 and 10.49 s, respectively. The 26.2‐mile marathon world record (officially) is 2:01.39, (unofficially 1:59.40) for men and 2:14.04 for women. In the winter games, the world record for the farthest ski jump is 253.5 m for men and 200 m for women. Such remarkable feats of human endeavours are made possible by rapid skeletal muscle adenosine triphosphate degradation for energy provision and resynthesis of adenosine triphosphate by co‐ordinated metabolic, endocrine, and cardiovascular adjustment.
The endocrine system plays an important role in adaptation to acute and chronic exercise. Hormonal secretion in response to different forms of exercise are essential in mobilizing appropriate metabolic substrates for fuel supply, adjusting cardiovascular function, and managing water and electrolyte balance. Against a background of genetic potential, years of hard training result in cardiovascular, metabolic, and neuroendocrine adaptations capable of pushing the human being to new levels of sports performance. 1
On the other hand, maladaptation to chronic exercise training stress can lead to endocrine system dysfunction resulting in overtraining that negatively influences athletic performance through premature fatigue. Unfortunately, both male and female young athletes are susceptible to energy imbalances with consequences on exercise performance and long‐term clinical complications such as female triad, or male gonadal axis system dysfunction. Relative energy deficiency in sport (RED‐S) is increasingly recognized in young and adult male and female athletes and its early diagnosis is an important marker in understanding causes of under‐performance, late puberty, and growth failure. Though the impact of menstrual cycle phase on exercise performance in eumenorrheic women is unclear, 2 menstrual cycle dysfunction is an important indicator of hormone imbalance that is commonly seen in female athletes.
What can the elite athlete teach the general population about the value of physical exercise? In line with governmental guidelines, with an array of different sports and exercise patterns that can be performed alone or in social groups, the recreational exerciser can benefit from a healthier lifestyle with increased longevity and reduced incidence of many noncommunicable diseases (NCDs). Indeed, many can benefit from structuring their training in alignment with the very same principles that athletes use to safely allow their bodies to adapt to many hundreds of hours of training a year. Importantly, many of the endocrine, metabolic, and cardiovascular benefits of regular exercise are beneficial for cohorts with endocrine disorders and may attenuate the risk of various long‐term cardiometabolic complications.
Thus, it seems timely to detail in this review the role of exercise that is of clinical relevance for the management of some endocrine disorders. Athletes can be great role models and their achievements inspire the wider population to ‘move more, and more often’. The purpose of this review is to help provide greater confidence to clinicians that physical exercise can be promoted more.
2. ENERGY METABOLISM IN ATHLETES AND THE ENDOCRINE RESPONSE TO ACUTE EXERCISE
An athletes' ability to push the boundaries of modern sporting performance limits is dependent on their ability to optimize physiological adaptations from rigorous, chronic progressive training. Increased muscular energy fuel demand at the onset of, and during, exercise elicits important neuroendocrine responses, regardless of training status. The provision of different fuels for exercise (i.e., phosphocreatine, carbohydrate, and lipids) are fine‐tuned by the interactions of several endocrine hormones viz, insulin, glucagon, catecholamines, growth hormone (GH), and cortisol. 3 Volitional muscle contraction initiates parasympathetic withdrawal and sympathoadrenal activity which releases adrenaline and noradrenaline from the adrenal medulla, inhibiting pancreatic insulin secretion to below basal levels. 4 Concurrently, catecholamines and glucagon (and later GH and cortisol) increase hepatic glucose output via glycogenolysis and gluconeogenesis, in addition to facilitating lipid mobilization from adipose tissue by adrenaline‐mediated increases in hormone‐sensitive lipase activity.
Catecholamines stimulate hepatic and skeletal muscle glycogen phosphorylase activity towards glycogenolytic reactions, opposing insulin‐driven glycogenesis. As a result of chronic exercise training, endurance athletes possess greater intramuscular glycogen stores (see Hearris et al. 5 ) which are relatively distributed towards intramyofibrillar regions (in type 1 ‘endurance’ muscle fibres) compared with nonathlete individuals. 6 , 7 Similarly, intramuscular lipids are distributed as smaller droplets and are more concentrated in the intramyofibrillar compartment in endurance athletes compared with subsarcolemmal spaces in untrained individuals which are further from muscle contractile proteins. 8 Furthermore, the droplets are in closer proximity to mitochondria, which themselves have a higher volume density after training. 9 Accordingly a greater capacity for energy production is enabled and a shift in metabolic fuel preference applies a greater reliance on fat, rather than carbohydrate, metabolism. 9 This is favourable in the avoidance of glycogen depletion and subsequent fatigue‐induced reduction in force output. 10
Several factors can influence the neuroendocrine system response to an acute bout of exercise including, but not limited to, manipulations to acute programme variables (e.g., exercise intensity, duration, and volume), environmental factors (e.g., temperature and altitude), and individual demographics (e.g., age, gender, and training history). 11 During acute exercise, blood catecholamine concentrations can increase in an intensity‐dependent manner to over tenfold basal concentrations (e.g., adrenaline >5 nmol.L−1 and noradrenaline >20 nmol.L−1 after repeated sprints) in well‐trained athletes, resulting in augmented hepatic glycogenolysis and raised blood glucose. 12 , 13
Significant resistance exercise also induces a rise in catecholamines, as well as raised testosterone, GH, and insulin‐like growth factor‐1 (IGF‐1) (‘anabolic’ hormones) concentrations, creating a milieu for maximizing strength and muscle mass gains. 14 Conversely, exercise programmes that elicit the greatest acute GH response also elicit the greatest cortisol response—the primary protein ‘catabolic’ hormone. 15 , 16 This reflects the dual process of tissue remodelling, consisting of an initial phase of breakdown before a period of growth and repair. 17
3. ENDOCRINOLOGICAL ADAPTATIONS IN ATHLETES
Structured and planned regular training can be thought of as repeated exposure to acute exercise ‘stress’. As part of an adaptive process, long‐term adherence to exercise training potentiates alterations in several neuroendocrine responses to subsequent stressors (exercise or otherwise). 11 , 18 Many endocrine hormones are essential in initiating and regulating the training‐induced adaptations that occur in various organs and readers are directed to some excellent early references for detailed appraisals (SeeGalbo and Bunt 19 , 20 ).
Exercise training typically attenuates the magnitude of the plasma hormonal response to any given submaximal absolute workload; resulting in lower plasma hormone concentrations in trained compared with untrained individuals (Table 1). The influence of training status on the exercise‐stimulated release of gonadotropins, prolactin, and gonadal hormones is ambiguous within literature and discussed in detail elsewhere. 19 , 21 Trained athletes may present with lower secretion of aldosterone and vasopressin (hormones involved in the maintenance of body fluid and electrolyte balance) during exercise, 22 possibly reflecting the influence of training on plasma volume shifts (i.e., hypervolaemia): an important early adaptation to endurance training. 23 In pancreatic hormones, trained individuals experience a lesser decline in plasma insulin levels during exercise (resulting in higher relative circulating concentrations than in those who are untrained) while the glucagon response is attenuated. 24
Table 1.
The endocrine response to acute and chronic endurance and resistance exercise in healthy individuals
| Endocrine gland (Hormone secreted) | Endurance | Resistance | |||
|---|---|---|---|---|---|
| Duration | Intensity | Training | Acute resistance exercise | Training | |
| Adrenal cortex | |||||
| Cortisol | ↑ | ↑ | ↓ | ↑ | ↓ |
| Adrenal medulla | |||||
| Epinephrine | ↑ | ↑ | ↓ | ↑ | ↓ |
| Norepinephrine | ↑ | ↑ | ↓ | ↑ | ↓ |
| Pancreas | |||||
| Glucagon | ↑ | ↑ | ↓ | ↑ | ↓ |
| Insulin | ↓ | ↓ | ↑ | ↓ | ↑ |
| Pituitary | |||||
| ACTH | ↑ | ↑ | ↓ | ↑ | ↓ |
| GH | ↑ | ↑ | ↓ | ↑ | ↓ |
| LH | ↔↓ | ↔↓ | ↔ | ↔ | ↔ |
| FSH | ↔ | ↔ | ↔ | ↔ | ↔ |
| Testes/Ovaries/Adrenal cortex | |||||
| Oestradiol | ↑ | ↑ | ↓ | ↑ | ↓ |
| Testosterone | ↔↑↓ | ↔↑ | ↔ | ↑ | ↓ |
| Thyroid | |||||
| T3 | ↔↑ | ↔↑ | ↔↓ | ↔↑ | ↔↓ |
| T4 | ↔↑ | ↔↑ | ↔↓ | ↔↑ | ↔↓ |
Note: Hormonal responses to exercise differ based on specific exercise protocols, individual responses, and other factors (e.g., time of day and feeding status).
In nontraining columns:
↓ denotes lower plasma concentrations with increased exercise characteristic (column title).
↑ denotes higher plasma concentrations with increased exercise characteristic (column title).
↔ denotes no change in plasma concentrations with increased exercise characteristic (column title).
In training column (independent of changes in background concentrations):
↓ denotes lower plasma concentrations relative to concentrations at the same (absolute) workload before training.
↑ denotes higher plasma concentrations relative to concentrations at the same (absolute) workload before training.
↔ denotes no change in plasma concentrations relative to concentrations at the same (absolute) workload before training.
Abbreviations: ACTH, adrenocorticotropic hormone; GH, growth hormone; LH, luteinizing hormone; T3, triiodothyronine; T4, thyroxine.
This athletic hormonal milieu reflects a greater sensitivity of the target tissue to the hormonal stimulus and the degree of increase in neural, humoral, and hormonal factors that influence the responsiveness of various endocrine glands being lower. 25 This training adaptation has significant metabolic consequences such as lowered adrenaline‐mediated hepatic glycogen breakdown. The exception to this general rule is maximal or supramaximal exercise, for which trained athletes may present with augmented sympathoadrenal system responses compared with untrained subjects. This is due to the higher absolute workloads necessary to elicit a maximum response and/or possible training‐induced glandular adaptations (i.e., adrenal medulla hypertrophy) that increase its hormonal secretory capacity. 25
Trained sportspeople can present with decreased resting basal glucagon concentrations as well as lower fasted and stimulated insulin concentrations. 26 , 27 The influence of training status on resting levels of basal levels of hormones related to the hypothalamic–pituitary–gonadal axis in men and women is somewhat equivocal in literature and detailed explorations of the topic have been reviewed elsewhere. 28
Ultimately, endocrine adaptations to exercise training translate as an improved ability to maintain energy homoeostasis in the face of subsequent physiological or metabolic stressors. These hormonal changes are paralleled with metabolic and/or morphological adaptations in several organs with wider health benefits. This underscores the potential value of physical exercise as a therapeutic tool for the management of many NCDs. However, exposure to intense training regimes with inadequate rest and recovery can result in ‘Overtraining Syndrome’. 29 In such instances, imbalances within endocrine function become apparent, with possible downregulation of the hypothalamic–pituitary–gonadal axis.
4. RELATIVE ENERGY DEFICIENCY SYNDROME
Despite the name, RED‐S is not restricted to athletes participating in competitive sport. RED‐S can occur in exercisers of all levels, wherever an imbalance in exercise and nutritional behaviours occurs, resulting in low energy availability (LEA). 30
The clinical consequences of LEA were first described in the female athlete triad. 31 The triad covers a clinical spectrum from normal eating patterns, bone health, and menstrual function through to eating disorders, osteoporosis, and amenorrhoea. Furthermore, the clinical consequences of LEA are far reaching, reflecting widespread dysregulation of endocrine networks (Figure 1) negatively impacting health and exercise performance, as adaptive responses to exercise are driven by a fully functioning endocrine system.
Figure 1.
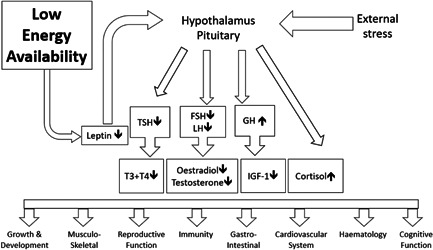
The impact of low energy availability on hormone networks. FSH, follicle‐stimulating hormone; GH, growth hormone; LH, luteinising hormone; IGF‐1, insulin‐like growth factor‐1; T3, triiodothyronine; T4, thyroxine; TSH, thyroid stimulating hormone
This multisystem clinical syndrome, RED‐S was first described in 2014 in the International Olympic Committee consensus statement 32 and subsequently updated in 2018. 33 The aetiology of RED‐S is LEA. Energy availability is the residual energy available from energy intake, once the energy demands of exercise have been covered and can be quantified in terms of kcal/kg lean body mass. LEA can arise intentionally, or unintentionally where there is a mismatch of energy intake and energy expenditure through exercise. 34 For this reason, RED‐S can occur in nonelite athletes, male, or female of any age.
Considering the endocrine effects of LEA, metabolic and external stressors are processed by the hypothalamic neuroendocrine gatekeeper. This results in downregulation of many hypothalamic–pituitary axes. For the reproductive axis, this functional suppression will be demonstrated by low end range luteinising hormone and oestradiol (females) or testosterone (males) in the presence of normal prolactin. This may manifest as functional hypothalamic amenorrhoea in females and symptoms of low testosterone such as reduced libido or erectile dysfunction in males. Hypothalamic–pituitary downregulation of the thyroid axis will be shown by low range thyroid stimulating hormone, thyroxine (T4), and triiodothyronine (T3) (and possible increase in reverse T3) which results in lowering of metabolic rate in an attempt to ‘conserve’ energy. Clinically this may explain why those in cumulative LEA will not necessarily be losing weight, or below normal body mass index. There is a concomitant increase in GH and decrease inIGF‐1, possibly due to an increase in binding proteins. The hypothalamic–pituitary–adrenal axis is activated, with cortisol levels consistently raised, lacking diurnal variation. This characteristic endocrine profile of RED‐S 35 is linked with clinical consequences, in particular poor bone health 36 and bone stress injuries. 37
In a patient presenting with suspected RED‐S on clinical history, checking baseline endocrine static function excludes most underlying medical conditions such as prolactinoma and so confirm functional endocrine downregulation due to LEA. Management can then be directed towards addressing behaviours around food and exercise. 38 This would include reduction in intensity of exercise and consistency of carbohydrate intake. In functional hypothalamic amenorrhoea, temporizing treatment with hormone replacement therapy to offer bone protection in the form of transdermal oestradiol and cyclic progesterone is indicated where Z score of the lumbar spine <1 or in presence of 2 or more stress fractures. Treatment with combined oral contraceptive pill is not advised by the Endocrine Society in functional hypothalamic amenorrhoea. 39 RED‐S is a functional endocrine dysregulation occurring in exercisers of all levels. Static hormone testing is essential in confirming RED‐S as a diagnosis of exclusion. Management of a patient with RED‐S will require a multidisciplinary team approach to provide medical, dietetic, and psychological input as clinically indicated. 40
5. THE FEMALE ATHLETE
Regular menstrual periods are the barometer of a healthy hormonal milieu for all women of reproductive age. This is normal physiology, regardless of how much physical activity is being taken. Given that a responsive, healthy endocrine network is essential for driving beneficial adaptative changes to exercise, then regular fluctuation in menstrual cycle hormones is essential not only for health but also for exercise performance (Figure 2). Note that the average age of menopause is 45–55 years.
Figure 2.
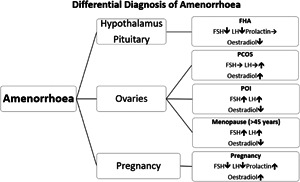
Differential diagnosis of amenorrhoea. FHA, functional hypothalamic amenorrhoea; FSH, follicle‐stimulating hormone; LH, luteinising hormone; PCOS, polycystic ovary syndrome; POI, premature ovarian insufficiency
Yet, there is surprisingly limited research assessing the impact of the menstrual cycle on exercise performance, and female athletes are sometimes uncertain of effective strategies to put in place to optimize performance throughout the menstrual cycle. 41 Although the ovarian hormones follow a well‐documented variation through the follicular and luteal phases, there are individual differences in timing, hormone concentrations, and tissue responses. This uncertainty in assessing the exact phase of the cycle for an individual was the underlying reason why a recent review concluded that no significant impact of menstrual cycle phase on performance was found. 2 Nevertheless, marginal gains in sport can be important for the podium. This has led to recommendations on standardizing assessment of menstrual cycle timing when conducting research in this field. 42 Machine learning in healthcare could also be of value in endocrine networks where feedback loops and biochronometers render mathematical modelling of these biological systems possible. 43 This approach could also be of value for nonathlete females where female hormones are usually measured on Day 3 of the cycle and so gives time‐limited information on menstrual cycle female hormone health.
Even though menstrual periods are a clinical indicator of female hormone health, there are some instances of an erroneous view that oligomenorrhoea and amenorrhoea is a ‘normal’ state. Periods are a very sensitive and personalized training metric for an athlete. Menstrual dysfunction can be a warning sign that athlete behaviours in terms of training load, nutrition, and recovery are not optimally periodized (See Section 4 on RED‐S). Any woman presenting with menstrual disruption requires investigation to exclude any underlying medical condition. Specifically, for secondary amenorrhoea, having excluded pregnancy, investigations in line with classification from the World Health Organisation will distinguish a hypothalamic–pituitary cause, from ovarian causes, based on follicle‐stimulating hormone and luteinising hormone. 44 Low range follicle‐stimulating hormone, luteinising hormone and oestradiol found in the presence of normal prolactin, would be indicative of functional hypothalamic amenorrhoea. Furthermore, the underling aetiology of LEA would be supported by low range thyroid function tests and clinical history.
It is a personal choice as to the form of contraception an athlete may wish to use. While hormonal contraception can help manage certain medical conditions associated with menstrual disturbance, such as dysmenorrhoea, menorrhagia, or polycystic ovary syndrome, there are some further specific considerations for female athletes. Prescribing hormonal contraception for young female athletes with menstrual disruption can adversely affect bone health 45 and mask any underlying functional hypothalamic amenorrhoea. 46 Hormonal contraception also switches off the personalized training metric of menses, which barrier methods of contraception do not do. Hormonal contraception use varies across sports disciplines.
Regular exercise is well‐established to be beneficial for female hormone health, when combined with other positive behaviours. Thus, research exploring female health in successful athletes can be beneficial for the wider female population.
6. ENDOCRINOLOGICAL ISSUES IN PAEDIATRIC ATHLETES
In many sports, the pursuit of elite status starts early, such that by the time a child reaches their teens they already have undertaken several years' worth of intense training and competition accompanied by the considerable bodily demands these events entail. Despite the consensus beneficial effects of exercise on a child's health overall, 47 , 48 there are inherent physical, psychological, dietetic, and physiological concerns associated with intense, long‐term training that should be considered when dealing with paediatric athletes.
The ‘female athlete triad’ is a common disorder among young female athletes 49 who often encounter disruption to normal menstrual function (i.e., delayed menarche, oligomenorrhea, and amenorrhoea). 50 Exercise‐related reproductive dysfunction may compromise growth velocity and peak bone mass acquisition with an accelerated risk of developing osteoporosis in later life. 51 LEA can detrimentally alter a variety of regulatory metabolic hormones known to support linear growth, for example, insulin, cortisol, GH, IGF‐1, ghrelin, and leptin. In clinical practice, screening for signs of the female athlete triad in adolescent athletes may allow early intervention that circumvents long‐term complications. Ensuring energy balance should be the primary point of call for those overseeing the care of young female athletes.
The male gonadal axis is also vulnerable to energy deprivation, but may not be recognized by men who lack observable hypogonadal features such as amenorrhoea. 52 Calorie‐deficient diets and overload training programmes may result in hormonal abnormalities of IGF‐1, testosterone, and luteinizing hormone concentrations appearing below the reference ranges. 52 This can present adverse symptoms of depression, lowered libido, and low energy in some male adolescent athletes. 53
In weight category sports such as boxing, judo, and wrestling, deliberate under‐eating is an employed strategy to lose weight before competition, leading to reductions in IGF‐1 and GH binding protein. 54 , 55 In young male high‐level gymnasts, IGF‐1:cortisol ratio reduces during the strength and conditioning, and routine development, phases of the season. 56 Paradoxical to competing in a lower weight category, improper nutrition during the competitive season and its recovery phases can lead to adverse effects on subsequent performance. Collection of baseline and training/competition‐related hormonal changes may provide good markers of the athlete's general condition during the competitive season, though may not always be used to indicate performance. 54
Proper care of the paediatric athlete is essential in ensuring a child's normal growth, timely pubertal development, and psychological well‐being both within and outside of an exercising environment. Failure to do so may manifest in acute and chronic endocrine disruptions that implicate health status leading into and throughout adulthood. Considering the relative sparsity of literature examining endocrinological issues in male compared with female child/adolescent athletes, future research may benefit from longitudinal (more than one season) studies in this area with examination of nutritional intake and intensity and volume of the training.
7. LESSONS TO BE LEARNED FROM ATHLETES FOR POPULATION HEALTH
Irrespective of nationality or sport, Olympic‐level medal winners live on average 2.8 years longer than the general population. 57 What makes this possible? Though genetic potential plays a role, elite athletes undertake years of deliberate practice and adherence to rigorous training regimes in the pursuit of sporting success. International level athletes often train in excess of 500−1000 h per year, performed as 400−800 individual training sessions within a structured periodization protocol. 58 The bodily adaptations associated with such enduring efforts typify the physiology underpinning an athlete. Yet, beyond the pursuit of sporting success in athletic cohorts, many of these exercise‐induced adaptations harness powerful health‐related outcomes that can be gleaned through much smaller ‘doses’ of exercise regardless of training status. There are then lessons to be learned from the routines of elite athletes as we look to encourage physical activity in the wider population in clinical practice.
But how do we prescribe exercise to those unfamiliar or unable? This brief synopsis introduces the main factors but for a more thorough discussion the reader is directed to endurance 59 , 60 and strength 21 , 61 , 62 training references. Athletes understand the principle that adaptation will occur if the training load is frequently above their habitual level of activity. Figure 3 details the key factors for specific physical training adaptation through progressive overload.
Figure 3.
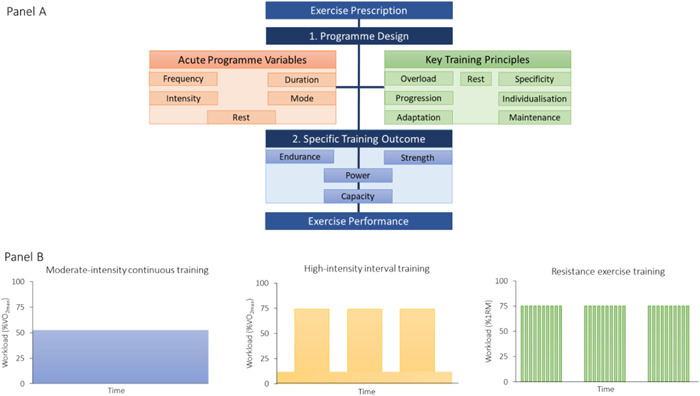
Panel A: Important characteristics of exercise prescription. Panel B: Example workload format of different exercise modalities. Note: figures in panel B are used for graphical purposes only. Definitions: Acute Programme Variables: (i) Frequency: How often the activity is performed. (ii) Intensity: How hard the individual is working. (iii) Duration: How long the activity is sustained for. (iv) Mode: The specific type of activity. Rest: Rest within and between different sessions. Physical adaptations occur during the recovery and nonactive period of training session. Key Training Principles: (i) Overload: Judicious application of work through acute programme variables to enhance metabolic and physiological capacity. (ii) Specificity: Training must be relevant to the individual and their activity to deliver adaptations in metabolic or physiological systems. (iii) Progression: Training should gradually become more difficult. Once the body has adapted, the performer should make further demands on physiological and metabolic systems. However, increases must be gradual so that the athlete avoids a plateau in performance, overtraining, or injury. (iv) Individualization: Recognition that a given stimulus does not affect all individuals equally. (v) Adaptation: The process of the body getting accustomed to a particular exercise or training programme through repeated exposure. All training is aimed at creating long‐term physical changes in the body systems. (vi) Maintenance/Reversibility: Physiological and metabolic systems will revert to pretrained state unless training is continued, and performance will decrease. Also known as ‘use it or lose it’. Specific Training Outcomes are usually directed to the development of either endurance or strength power and capacity. Optimizing programme design and identifying the specific training outcome can lead to improvement in exercise performance (e.g., power, speed, or time) and functional outcome, for example, ease of completion of daily tasks and improved quality of life [Color figure can be viewed at wileyonlinelibrary.com]
Endurance training leads to cardiovascular and musculoskeletal adaptations (e.g., mitochondrial biogenesis, respiratory capacity, and capillarization) that enhance the body's ability to deliver and utilize oxygen to generate energy. Conventional endurance training methods include: (1) long duration, moderate intensity; commonly referred to as ‘long, slow distance’ or ‘base’ training (2) moderate‐duration, high‐intensity; ‘pace/tempo’ training and (3) short‐duration, high‐intensity ‘interval’ training.
Resistance exercise training leads to an increase in muscle strength and power because of neuromuscular adaptations, increases in muscle cross‐section area, and alterations in connective tissue stiffness. Programmes can be tailored to develop muscular endurance (high volume, low loads, and short rest), hypertrophy (moderate‐high volume, moderate loads, short‐moderate rest periods), strength (moderate volume, high loads, and moderate‐long rest periods), and dynamic power (explosive and/or ballistic movements, low volume, heavy loads, and long rest periods). 62 Deliberate manipulation of acute programme variables determines the specific training outcome by modifying the acute hormonal responses. Programmes that are higher in volume with shorter rest periods produce the greatest elevations in circulating concentrations of anabolic (testosterone, GH, and IGF‐1) and catabolic (cortisol) hormones and are therefore most likely to maximize hypertrophy. 21
It is essential that training programmes incorporate adequate periods of rest within each stage of the training phase (micro‐, meso‐, and macro‐cycles). Failure to do so may result in overreaching and/or overtraining, both of which compromise exercise performance and neuroendocrine health. 63 Thus, another lesson from athletes is to structure training appropriately and avoiding under‐ or over‐training.
The dedication required to attain the physical adaptations from chronic training is the product of an athlete's psychology. Strategies employed by athletes to maintain motivation and positive thinking during training include short and long‐term goal setting, close management of progress, and focusing on internal reasons of why they are competing in the sport. Practically speaking, athletes create attainable goals, set up methods to track progress, and continue to exercise on the basis of their own intrinsic reasons. 64 These principles can be applied to stimulate behavioural change in nonathletes and promote goal attainment for increasing physical activity levels. 65
8. IMPACT OF REGULAR PHYSICAL ACTIVITY ON ENDOCRINE DISORDERS
How can we take the lessons learned from elite athletes and use them in clinical practice? The point of this review is not to encourage the prescription of Olympic level exercise programming for individuals we routinely see in clinical practice. Rather it is to emphasize the potential value of exercise, even in minor amounts, in alleviating or averting the progression of numerous NCDs. 66 Beyond the local adaptations that occur within skeletal muscle, exercise induces positive adaptions in several other tissues. Though these adaptive processes undoubtedly serve to benefit the elite athlete from a sports performance perspective, they also lead to various health‐related outcomes that reduce the risk of disease onset or progression (Figure 4). 66
Figure 4.
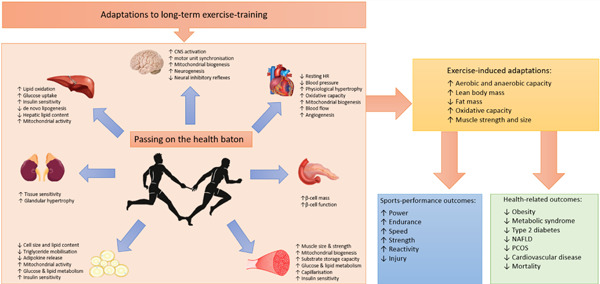
The effects of exercise regular exercise training on key endocrine tissues involved in the regulation of energy homoeostasis. The multisystemic effects of exercise training have direct relevance for the management of patients with energy imbalance, metabolic (glucose and lipid) dysregulation, insulin resistance, chronic inflammation, and hypertension; pathogenic features of many endocrine disorders. CNS, central nervous system; HR, heart rate; NAFLD, nonalcoholic fatty liver disease; PCOS, polycystic ovary syndrome [Color figure can be viewed at wileyonlinelibrary.com]
8.1. The evidence
Epidemiological data from large prospective cohort studies indicate that 150 min/week of moderate‐to‐vigorous intensity exercise can considerably reduce the incidence of type 2 diabetes (T2D) in high‐risk individuals. 67 , 68 When included as part of a lifestyle modification intervention, exercise training (moderate intensity for at least 150 min/week) is considered the most effective means of reducing the risk of T2D, out‐performing a drug only treatment approach. 67
The benefit of exercise for weight management goes beyond its immediate effects on increasing energy expenditure (and hence aiding the attainment of a caloric deficit). Indeed, there is unequivocal evidence to support the positive cardiometabolic effects (i.e., ↓ hyperlipidaemia, ↓hypertension, ↓body mass index, ↓insulin resistance, ↓fasting blood glucose, ↓HbA1c) of regular exercise in people with T2D, 69 , 70 polycystic ovary syndrome, 71 metabolic syndrome, 72 nonalcoholic fatty liver disease, 73 and/or those who are overweight or obese. 74 Given that cardiovascular disease (CVD) prevails as the leading cause of mortality in many NCDs, the benefits of exercise in mitigating its risk are noteworthy. 75
Physical inactivity is emerging as an independent risk for NCDs, causing an estimated 9% of premature all‐cause mortality, 6% of CVD, and 7% of T2D. 74 The associated economic costs are astronomical, equating to £39 billion/year worldwide (2013) 76 and £1 billion/year to the UK National Health Service (2006–7). 77 It is reasonable to suggest that physical activity promotion should be a public health priority.
8.2. Putting it into practice
Individuals with NCDs that are routinely seen in clinical practice may be among those most unlikely to exercise. Hence, primary health‐care providers are well placed to communicate the benefits of regular exercise to those who may stand to benefit most. 78 , 79 Advocation of regular exercise in clinical practice could be a simple, cost‐effective strategy that yields impactful results. 80 The ideal training regimen should include a variety of exercise activities (namely those the patient most enjoys, and is therefore most likely to sustain) that contribute to some form of daily movement in alignment with governmental guidelines (i.e., The UK's Chief Medical Officers Guidelines for Physical Activity 81 ). The ‘FITT’ (i.e., exercise Frequency, Intensity, Time, and Type) mnemonic is commonly used as a guidance source for exercise prescription guidelines and could be implemented alongside achievable goal setting (Figure 5).
Figure 5.
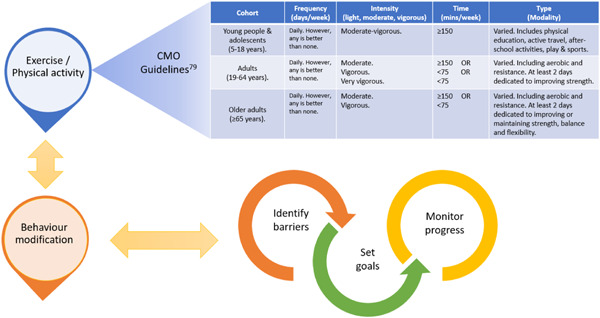
Exercise prescription model in alignment with Chief Medical Officers (CMO) physical activity guidelines using the ‘FITT’ principals alongside positive behaviour modification [Color figure can be viewed at wileyonlinelibrary.com]
Clearly not everyone is able to exercise intensely or indeed has the resources available to undertake bespoke exercise regimes with qualified professionals. However, many community‐based projects and online guidance material are free. Not to forget, walking is a practical, free, and user‐friendly means of contributing to physical activity guidelines. Indeed, just 30 min a day, 5 days per week can significantly reduce CVD risk 82 ; Taking small steps can have big impact.
Undoubtedly primary health‐care providers have a valuable role to play in exercise promotion at the population level. However, many report time constraints, inadequate resources, and a lack of confidence/knowledge as leading barriers to exercise prescription. 79 Unfortunately, not all countries offer referral schemes to a sports and exercise medicine specialist. Ongoing efforts are needed to address these concerns to optimize patient adherence and outcomes.
8.3. Next steps
Recent texts have given appraisals of exercise prescription in primary health care (See Khan and Seth 78 , 79 ) with resource direction and practical implementation points. Some prudent next steps could be:
Administer a physical activity questionnaire (i.e., the UK general practice physical activity questionnaire 83 ) to establish baseline activity levels.
Prescribe a periodized exercise plan according to acute programme variables and training principles for the patient (Figure 3). Align these with governmental guidelines if appropriate.
Establish a plan that is both feasible and effective for the patient. Set small, achievable goals to build confidence.
Provide a recorded exercise prescription plan that states the agreed upon goals. Free resource material can be found in the ‘exercise is medicine’ initiate co‐created by the American College of Sports Medicine and the American Medical Association (www.exerciseismedicine.com).
Know your local resources for physical activity and communicate these to the patient.
Follow‐up with the patient to assess progress, identify problems, fine tune the ‘dose’ and reset the goals.
Remember ‘no size fits all’ and potential health risk's need consideration. If uncertain about the appropriate advice to give, reach out to exercise professionals for help.
9. CONCLUSIONS
Many positive adaptations occur in athletes in a training‐dependent manner. Structuring training in a periodized fashion helps avoid maladaptation to physical training, an especially important factor for consideration in paediatric athletes. Great feats of exercise performance begin with small amounts of physical activity that are progressively increased and many of the principles of fitness can be employed to improve several endocrine disorders. Taken collectively, it is clear to see the reason behind the ‘exercise is medicine’ mantra with recognition of its value as a nonpharmacological therapy option for the treatment of many NCDs. Though not everyone can become an Olympian or professional athlete, adopting a healthy lifestyle can bring great health benefits to many, including people with endocrine disorders.
McCarthy O, Pitt JP, Keay N, et al. Passing on the exercise baton: what can endocrine patients learn from elite athletes? Clin Endocrinol (Oxf). 2022;96:781‐792. 10.1111/cen.14683
Olivia McCarthy and Jason P. Pitt are joint first authorship.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Sönksen PH, Holt RIG, Böhning W, et al. Why do endocrine profiles in elite athletes differ between sports? Clin Diabetes Endocrinol. 2018;4(1):1‐16. 10.1186/s40842-017-0050-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McNulty KL, Elliott‐Sale KJ, Dolan E, et al. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: a systematic review and meta‐analysis. Sport Med. 2020;50(10):1813‐1827. 10.1007/s40279-020-01319-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borer K. Hormones and fuel use in exercise. Advanced exercise endocrinology. Human Kinetics; 2013:97‐126. [Google Scholar]
- 4. Ball D. Metabolic and endocrine response to exercise: sympathoadrenal integration with skeletal muscle. J Endocrinol. 2015;224(2):R79‐R95. 10.1530/JOE-14-0408 [DOI] [PubMed] [Google Scholar]
- 5. Hearris MA, Hammond KM, Fell JM, Morton JP. Regulation of muscle glycogen metabolism during exercise: implications for endurance performance and training adaptations. Nutrients. 2018;10(3):1‐21. 10.3390/nu10030298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nielsen J, Holmberg HC, Schrøder HD, Saltin B, Ørtenblad N. Human skeletal muscle glycogen utilization in exhaustive exercise: Role of subcellular localization and fibre type. J Physiol. 2011;589(11):2871‐2885. 10.1113/jphysiol.2010.204487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen J, Farup J, Rahbek SK, De Paoli FV, Vissing K. Enhanced glycogen storage of a subcellular hot spot in human skeletal muscle during early recovery from eccentric contractions. PLOS One. 2015;10(5):0127808. 10.1371/journal.pone.0127808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gemmink A, Schrauwen P, Hesselink MKC. Exercising your fat (metabolism) into shape: a muscle‐centred view. Diabetologia. 2020;63(8):1453‐1463. 10.1007/s00125-020-05170-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meinild Lundby AK, Jacobs RA, Gehrig S, et al. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiol. 2018;222(1):1-14. 10.1111/apha.12905 [DOI] [PubMed] [Google Scholar]
- 10. Ørtenblad N, Westerblad H, Nielsen J. Muscle glycogen stores and fatigue. J Physiol. 2013;591(18):4405‐4413. 10.1113/jphysiol.2013.251629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hackney AC. Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev Endocrinol Metab. 2006;1(6):783‐792. 10.1586/17446651.1.6.783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zouhal H, Rannou F, Gratas‐Delamarche A, Monnier M, Bentué‐Ferrer D, Delamarche P. Adrenal medulla responsiveness to the sympathetic nervous activity in sprinters and untrained subjects during a supramaximal Exercise. Int J Sports Med. 1998;19(3):172‐176. [DOI] [PubMed] [Google Scholar]
- 13. Gaitanos GC, Williams C, Boobis LH, Brooks S. Human muscle metabolism during intermittent maximal exercise. J Appl Physiol. 1993;75(2):712‐719. 10.1152/jappl.1993.75.2.712 [DOI] [PubMed] [Google Scholar]
- 14. Schroeder ET, Villanueva M, West DDW, Phillips SM. Are acute post‐resistance exercise increases in testosterone, growth hormone, and IGF‐1 necessary to stimulate skeletal muscle anabolism and hypertrophy? Med Sci Sports Exerc. 2013;45(11):2044‐2051. 10.1249/MSS.0000000000000147 [DOI] [PubMed] [Google Scholar]
- 15. Mulligan SE, Fleck SJ, Gordon SE, Koziris LP, Triplett‐McBride NT, Kraemer WJ. Influence of resistance exercise volume on serum growth hormone and cortisol concentrations in women. J Strength Cond Res. 1996;10(4):256‐262. 10.1519/00124278-199611000-00009 [DOI] [Google Scholar]
- 16. Stokes KA, Gilbert KL, Hall GM, Andrews RC, Thompson D. Different responses of selected hormones to three types of exercise in young men. Eur J Appl Physiol. 2013;113(3):775‐783. 10.1007/s00421-012-2487-5 [DOI] [PubMed] [Google Scholar]
- 17. Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sport Med. 2005;35(4):339‐361. 10.2165/00007256-200535040-00004 [DOI] [PubMed] [Google Scholar]
- 18. Kjaer M, Mikines KJ, Linstow MV, Nicolaisen T, Galbo H. Effect of 5 wk of detraining on epinephrine response to insulin‐induced hypoglycemia in athletes. J Appl Physiol. 1992;72(3):1201‐1204. 10.1152/jappl.1992.72.3.1201 [DOI] [PubMed] [Google Scholar]
- 19. Galbo H. Hormonal and metabolic adaptation to exercise. 1983:1‐11.
- 20. Bunt JC. Hormonal alterations due to exercise. Sport Med. 1986;3(5):331‐345. 10.2165/00007256-198603050-00003 [DOI] [PubMed] [Google Scholar]
- 21. Crewther B, Keogh J, Cronin J, Cook C. Possible stimuli for strength and power adaptation: acute hormonal responses. Sports Med. 2006;36(3):215‐238. 10.2165/00007256-200636030-00004 [DOI] [PubMed] [Google Scholar]
- 22. Melin B, Eclache JP, Geelen G, et al. Plasma AVP, neurophysin, renin activity, and aldosterone during submaximal exercise performed until exhaustion in trained and untrained men. Eur J Appl Physiol Occup Physiol. 1980;44(2):141‐151. 10.1007/BF00421092 [DOI] [PubMed] [Google Scholar]
- 23. Fellmann N. Hormonal and plasma volume alterations following endurance exercise: a brief review. Sport Med An Int J Appl Med Sci Sport Exerc. 1992;13(1):37‐49. 10.2165/00007256-199213010-00004 [DOI] [PubMed] [Google Scholar]
- 24. Deuster PA, Chrousos GP, Luger A, et al. Hormonal and metabolic responses of untrained, moderately trained, and highly trained men to three exercise intensities. Metabolism. 1989;38(2):141‐148. 10.1016/0026-0495(89)90253-9 [DOI] [PubMed] [Google Scholar]
- 25. Kjaer M, Galbo H. Effect of physical training on the capacity to secrete epinephrine. J Appl Physiol. 1988;64(1):11‐16. 10.1152/jappl.1988.64.1.11 [DOI] [PubMed] [Google Scholar]
- 26. Bloom SR, Johnson RH, Park DM, Rennie MJ, Sulaiman WR. Differences in the metabolic and hormonal response to exercise between racing cyclists and untrained individuals. J Physiol. 1976;258(1):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahrén B, Thorsson O. Increased insulin sensitivity is associated with reduced insulin and glucagon secretion and increased insulin clearance in man. J Clin Endocrinol Metab. 2003;88(3):1264‐1270. 10.1210/jc.2002-021547 [DOI] [PubMed] [Google Scholar]
- 28. Cano Sokoloff N, Misra M, Ackerman KE. Exercise, training, and the hypothalamic‐pituitary‐gonadal axis in men and women. Front Horm Res. 2016;47:27‐43. 10.1159/000445154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hackney AC, Battaglini C. The overtraining syndrome: neuroendocrine imbalances in athletes. Braz J Biomotricity. 2007;1(2):34‐44. [Google Scholar]
- 30. Keay N, Overseas AD, Francis G. Indicators and correlates of low energy availability in male and female dancers. BMJ Open Sport Exerc Med. 2020;6(1):906. 10.1136/bmjsem-2020-000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand: the female athlete triad. Med Sci Sports Exerc. 1997;29(5):i‐ix. 10.1097/00005768-199705000-00037 [DOI] [PubMed] [Google Scholar]
- 32. Mountjoy M, Sundgot‐Borgen J, Burke L, et al. The IOC consensus statement: beyond the female athlete triad‐relative energy deficiency in sport (RED‐S). Br J Sports Med. 2014;48(7):491‐497. 10.1136/bjsports-2014-093502 [DOI] [PubMed] [Google Scholar]
- 33. Mountjoy M, Sundgot‐Borgen JK, Burke LM, et al. IOC consensus statement on relative energy deficiency in sport (RED‐S): 2018 update. Br J Sports Med. 2018;52(11):687‐697. 10.1136/bjsports-2018-099193 [DOI] [PubMed] [Google Scholar]
- 34. Keay N, Francis G. Infographic. Energy availability: concept, control and consequences in relative energy deficiency in sport (RED‐S). Br J Sports Med. 2019;53(20):1310‐1311. 10.1136/bjsports-2019-100611 [DOI] [PubMed] [Google Scholar]
- 35. Keay N, Rankin A. Infographic. Relative energy deficiency in sport: an infographic guide. Br J Sports Med. 2019;53(20):1307‐1309. 10.1136/bjsports-2018-100354 [DOI] [PubMed] [Google Scholar]
- 36. Keay N, Francis G, Hind K. Low energy availability assessed by a sport‐specific questionnaire and clinical interview indicative of bone health, endocrine profile and cycling performance in competitive male cyclists. BMJ Open Sport Exerc Med. 2018;4(1):424. 10.1136/bmjsem-2018-000424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heikura IA, Uusitalo ALT, Stellingwerff T, Bergland D, Mero AA, Burke LM. Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int J Sport Nutr Exerc Metab. 2018;28(4):403‐411. 10.1123/ijsnem.2017-0313 [DOI] [PubMed] [Google Scholar]
- 38. Keay N, Francis G, Entwistle I, Hind K. Clinical evaluation of education relating to nutrition and skeletal loading in competitive male road cyclists at risk of relative energy deficiency in sports (RED‐S): 6‐month randomised controlled trial. BMJ Open Sport Exerc Med. 2019;5(1):523. 10.1136/bmjsem-2019-000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ackerman KE, Singhal V, Baskaran C, et al. Oestrogen replacement improves bone mineral density in oligo‐amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med. 2019;53(4):229‐236. 10.1136/bjsports-2018-099723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keay N. Raising Awareness of RED‐S in Male and Female Athletes and Dancers BJSM ‐ Health for Performance. 2018, Available online at: https://blogs.bmj.com/bjsm/2018/10/30/raising-awareness-of-red-s-in-male-and-female-athletes-and-dancers/
- 41. Bruinvels G, Burden RJ, McGregor AJ, et al. Sport, exercise and the menstrual cycle: where is the research? Br J Sports Med. 2017;51(6):487‐488. 10.1136/bjsports-2016-096279 [DOI] [PubMed] [Google Scholar]
- 42. Janse DE, Jonge X, Thompson B, Han A. Methodological recommendations for menstrual cycle research in sports and exercise. Med Sci Sports Exerc. 2019;51(12):2610‐2617. 10.1249/MSS.0000000000002073 [DOI] [PubMed] [Google Scholar]
- 43. van der Schaar M Annual report of the chief medical officer: Machine learning for individualised medicine.
- 44. Wass J, Owen K, Turner H. Oxford Textbook of Endocrinology and Diabetes. 3rd ed. Oxford University Press; 2014. [Google Scholar]
- 45. Cheng J, Santiago KA, Abutalib Z, et al. Menstrual irregularity, hormonal contraceptive use, and bone stress injuries in collegiate female athletes in the United States. P M R. 2020;13:1207‐1215. 10.1002/pmrj.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gordon CM, Ackerman KE, Berga SL, et al. Functional hypothalamic amenorrhea: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(5):1413‐1439. 10.1210/jc.2017-00131 [DOI] [PubMed] [Google Scholar]
- 47. Rasmussen M, Laumann K. The academic and psychological benefits of exercise in healthy children and adolescents. Eur J Psychol Educ. 2013;28(3):945‐962. 10.1007/s10212-012-0148-z [DOI] [Google Scholar]
- 48. Poitras VJ, Gray CE, Borghese MM, et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school‐aged children and youth. Appl Physiol Nutr Metab. 2016;41(6):S197‐S239. 10.1139/apnm-2015-0663 [DOI] [PubMed] [Google Scholar]
- 49. Brown KA, Dewoolkar AV, Baker N, Dodich C. The female athlete triad: special considerations for adolescent female athletes. Transl Pediatr. 2017;6(3):144‐149. 10.21037/tp.2017.04.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maïmoun L, Georgopoulos NA, Sultan C. Endocrine disorders in adolescent and young female athletes: impact on growth, menstrual cycles, and bone mass acquisition. J Clin Endocrinol Metab. 2014;99(11):4037‐4050. 10.1210/jc.2013-3030 [DOI] [PubMed] [Google Scholar]
- 51. Drinkwater BL, Bruemner B, Chesnut CH. Menstrual history as a determinant of current bone density in young athletes. JAMA J Am Med Assoc. 1990;263(4):545‐548. 10.1001/jama.1990.03440040084033 [DOI] [PubMed] [Google Scholar]
- 52. Wong HK, Hoermann R, Grossmann M. Reversible male hypogonadotropic hypogonadism due to energy deficit. Clin Endocrinol. 2019;91(1):3‐9. 10.1111/cen.13973 [DOI] [PubMed] [Google Scholar]
- 53. Cohen J, Nassau DE, Patel P, Ramasamy R. Low testosterone in adolescents & young adults. Front Endocrinol. 2020;10(January):1‐6. 10.3389/fendo.2019.00916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eliakim A, Nemet D. The endocrine response to exercise and training in young athletes. Pediatr Exerc Sci. 2013;25(4):605‐615. 10.1123/pes.25.4.605 [DOI] [PubMed] [Google Scholar]
- 55. Roemmich JN, Sinning WE. Weight loss and wrestling training: effects on growth‐related hormones. J Appl Physiol. 1997;82(6):1760‐1764. 10.1152/jappl.1997.82.6.1760 [DOI] [PubMed] [Google Scholar]
- 56. Daly RM, Rich PA, Klein R. Hormonal responses to physical training in high‐level peripubertal male gymnasts. Eur J Appl Physiol Occup Physiol. 1998;79(1):74‐81. 10.1007/s004210050476 [DOI] [PubMed] [Google Scholar]
- 57. Clarke PM, Walter SJ, Hayen A, Mallon WJ, Heijmans J, Studdert DM. Survival of the fittest: retrospective cohort study of the longevity of Olympic medallists in the modern era. BMJ. 2012;345(7888):8308. 10.1136/bmj.e8308 [DOI] [PubMed] [Google Scholar]
- 58. Tnønessen E, Sylta Ø, Haugen TA, Hem E, Svendsen IS, Seiler S. The road to gold: training and peaking characteristics in the year prior to a gold medal endurance performance. PLoS One. 2014;9(7):e101796. 10.1371/journal.pone.0101796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Seiler S. What is best practice for training intensity and duration distribution in endurance athletes? Int J Sports Physiol Perform. 2010;5(3):276‐291. 10.1123/ijspp.5.3.276 [DOI] [PubMed] [Google Scholar]
- 60. Cisternas NS. Deborah R, Jonathan KE, Liguori G, Magal M, eds. ACSM Guidelines for Exercise Testing and Prescription. 10 ed. Wolters Kluwer Health; 2018. [Google Scholar]
- 61. American College of Sports Medicine . American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687‐708. 10.1249/MSS.0b013e3181915670 [DOI] [PubMed] [Google Scholar]
- 62. Bird SP, Tarpenning KM, Marino FE. Designing resistance training programmes to enhance muscular fitness. Sport Med. 2005;35(10):841‐851. 10.2165/00007256-200535100-00002 [DOI] [PubMed] [Google Scholar]
- 63. Fry AC, Kraemer WJ. Resistance exercise overtraining and overreaching: neuroendocrine responses. Sport Med. 1997;23(2):106‐129. 10.2165/00007256-199723020-00004 [DOI] [PubMed] [Google Scholar]
- 64. Weinberg RS. Goal setting in sport and exercise: research and practical applications. Rev da Educ Fis. 2013;24(2):171‐179. 10.4025/reveducfis.v24.2.17524 [DOI] [Google Scholar]
- 65. Lachman ME, Lipsitz L, Lubben J, Castaneda‐Sceppa C, Jette AM. When adults don't exercise: behavioral strategies to increase physical activity in sedentary middle‐aged and older adults. Innov Aging. 2018;2(1):1‐12. 10.1093/geroni/igy007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pedersen BK, Saltin B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25:1‐72. 10.1111/sms.12581 [DOI] [PubMed] [Google Scholar]
- 67. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‐403. 10.1056/NEJMOA012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2009;344(18):1343‐1350. 10.1056/NEJM200105033441801 [DOI] [PubMed] [Google Scholar]
- 69. Grace A, Chan E, Giallauria F, Graham PL, Smart NA. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta‐analysis. Cardiovasc Diabetol. 2017;16:37. 10.1186/s12933-017-0518-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sampath Kumar A, Maiya AG, Shastry BA, et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta‐analysis. Ann Phys Rehabil Med. 2019;62:98‐103. 10.1016/j.rehab.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 71. Santos IK, Nunes FASS, Queiros VS, et al. Effect of high‐intensity interval training on metabolic parameters in women with polycystic ovary syndrome: a systematic review and meta‐analysis of randomized controlled trials. PLOS One. 2021;16(1 January):e0245023. 10.1371/journal.pone.0245023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ostman C, Smart NA, Morcos D, Duller A, Ridley W, Jewiss D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: a systematic review and meta‐analysis. Cardiovasc Diabetol. 2017;16(1):110. 10.1186/s12933-017-0590-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Golabi P, Locklear CT, Austin P, et al. Effectiveness of exercise in hepatic fat mobilization in nonalcoholic fatty liver disease: systematic review. World J Gastroenterol. 2016;22(27):6318‐6327. 10.3748/wjg.v22.i27.6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lee I‐M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Impact of physical inactivity on the World's major non‐communicable diseases. Lancet. 2012;380(9838):219‐229. 10.1016/S0140-6736(12)61031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Thompson PD, Buchner D, Piña IL, et al. Exercise and Physical Activity in the Prevention and Treatment of Atherosclerotic Cardiovascular Disease A Statement From the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcomittee on Physical Activity). Circulation. 2003;107(24):3109-3116. 10.1161/01.CIR.0000075572.40158.77 [DOI] [PubMed]
- 76. Ding D, Lawson KD, Kolbe‐Alexander TL, et al. The economic burden of physical inactivity: a global analysis of major non‐communicable diseases. Lancet. 2016;388(10051):1311‐1324. 10.1016/S0140-6736(16)30383-X [DOI] [PubMed] [Google Scholar]
- 77. Scarborough P, Bhatnagar PB, Wickramasinghe K, Allender S, Foster C, Rayner M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: an update to 2006‐07 NHS costs. J Public Health. 2011;33(4):527‐535. 10.1093/PUBMED/FDR033 [DOI] [PubMed] [Google Scholar]
- 78. Khan KM, Weiler R, Blair SN. Prescribing exercise in primary care. BMJ. 2011;343(7828):4141. 10.1136/BMJ.D4141 [DOI] [PubMed] [Google Scholar]
- 79. Seth A. Exercise prescription: what does it mean for primary care? Br J Gen Pract. 2014;64(618):12‐13. 10.3399/BJGP14X676294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Grandes G, Sanchez A, Sanchez‐Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169(7):694‐701. 10.1001/ARCHINTERNMED.2009.23 [DOI] [PubMed] [Google Scholar]
- 81. UK Chief Medical Officers' Physical Activity Guidelines . 2019, Available online at: https://www.gov.uk/government/publications/physical-activity-guidelines-uk-chief-medical-officers-report
- 82. Murtagh EM, Murphy MH, Boone‐Heinonen J. Walking – the first steps in cardiovascular disease prevention. Curr Opin Cardiol. 2010;25(5):490‐496. 10.1097/HCO.0B013E32833CE972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Department of Health and Social Care . General practice physical activity questionnaire (GPPAQ). 2013. Available online at: https://www.gov.uk/government/publications/general-practice-physical-activity-questionnaire-gppaq
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


