Abstract
Introduction
We estimate societal value of a disease‐modifying Alzheimer's disease (AD) treatment that reduces progression by 30% in early stages.
Methods
Using the International Society for Pharmacoeconomics and Outcomes Research value flower as framework, we estimate gross societal value, that is, not including treatment cost, from avoided medical and social care costs, productivity and quality‐adjusted life‐years (QALY) gains for patients and caregivers, adjusting for severity of disease, value of financial insurance, and value of insurance for currently unafflicted adults with a Markov model.
Results
Predicted societal value from 2021 until 2041 is $2.62 trillion for the overall afflicted US population and $986 billion for the 2021 prevalent cohort or $134,418 per person, with valuation of patients’ QALY gains (63%) and avoided nursing‐home costs (20%) as largest components. Delays in access because of health system capacity constraints could reduce realized value between 52% and 69%. The value of insurance for the unafflicted is $4.52 trillion or $18,399 on average per person.
Discussion
With a total of $5.5 trillion, the projected gross societal value of a hypothetical AD treatment is substantial, which may help to put the cost of treatment into perspective.
Keywords: Alzheimer's disease, budget impact, disease‐modifying treatment, nursing home, societal value
1. INTRODUCTION
The advent of amyloid‐directed treatments for Alzheimer's disease (AD) gives hope to patients and their families, but the budget implications are likely to be substantial because of the large number of potentially eligible patients. A previous study has estimated that as many as 10 to 14 million patients live with mild cognitive impairment (MCI) of any etiology in the United States today, 1 and 55% have AD as the underlying pathology. 2 The exact number of patients with mild dementia is unknown but is likely in the millions.
This combination of a large patient pool and potentially high prices has triggered a debate about the value of AD modification. Addressing this question is unusually complex for two methodologic reasons. First, conventional cost‐effectiveness analysis, the most commonly used method to determine value for money, looks at the incremental net cost of a treatment, that is, cost of treatment less any reductions in spending on care, and the gain in quality‐adjusted life‐years (QALYs) relative to standard of care. 3 For the first disease‐modifying treatment (DMT), such a comparative analysis will have limitations because standard of care consists of relatively inexpensive symptom management and patient support, rendering the incremental cost of a treatment high. Second, cost‐effectiveness analysis often only considers the value to the patient, 4 whereas a substantial part of the value of a treatment might accrue to family caregivers. 5 Hurd et al. estimated that between 31% and 49% of the attributable cost of dementia stems from lost caregiver productivity. 6 Spillover effects on other sectors of society, such as decreased labor supply and diminished productivity and lower wages, are also not considered. 7 Moreover, there is the equity consideration that cost‐effectiveness analysis tends to assign a lower valuation to geriatric treatments, 8 because it uses gains in life‐years that are naturally limited in an aged population, 9 and the important consideration of unmet need is not captured. 10
To overcome limitations of conventional cost‐effectiveness analysis and provide guidance on valuation of innovative medical technologies, the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) recently published recommendations from an expert task force. 10 One of the products of this project was the ISPOR value flower, which outlines 12 potential elements of value including 8 that represent novel concepts not conventionally included in value assessment. 11 In this study, we use the ISPOR value flower as a framework for a comprehensive determination of the value of a hypothetical disease‐modifying AD treatment. We analyze which components are conceptually appropriate for such a treatment and are theoretically and empirically well enough understood to apply them. We use the published literature and secondary data to estimate the overall value of a hypothetical AD treatment from a societal perspective as well as the incidence of the generated value to individual stakeholders.
2. METHODS
2.1. Implementation of the ISPOR value flower
The ISPOR value flower is depicted in Figure 1. The green circles reflect the core elements of cost‐effectiveness analysis of net cost and gain in QALYs. As the actual price of a DMT is currently unknown, we are calculating gross societal value. The light blue circles are elements of value that are sometimes but not always considered in value assessments. Productivity refers to the ability of a treatment to reduce loss of days of work (absenteeism) and of performance at work (presenteeism), which is considered in analyses from the societal and employer‐payer perspectives.
FIGURE 1.
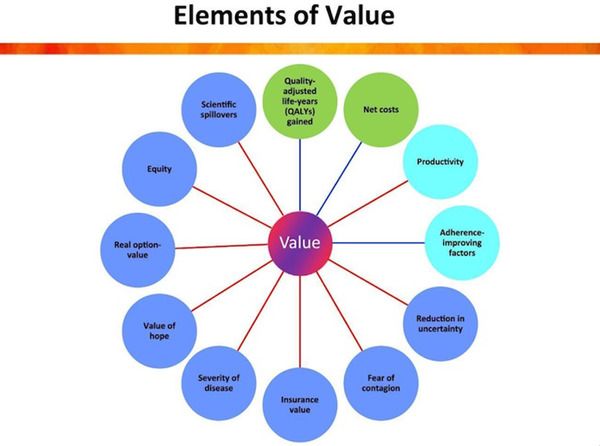
International Society for Pharmacoeconomics and Outcomes Research (ISPOR) value flower, ISPOR 2018, reproduced with permission
Adherence‐improving factors capture the ability of a new treatment to increase treatment adherence relative to standard of care under real‐world conditions, in which—unlike in clinical trials—adherence is not closely monitored or enforced. With all disease‐modifying AD treatments currently in late‐stage trials being injectables, 12 this component is irrelevant for our analysis.
RESEARCH IN CONTEXT
Systematic review: We searched the peer‐reviewed and “gray” literature on estimation of value of a disease‐modifying Alzheimer's disease treatment and identified four publications using conventional cost‐effectiveness analysis. We used the International Society for Pharmacoeconomics and Outcomes Research value flower as a framework to expand to additional value components.
Interpretation: Our study operationalized severity adjustment of quality‐adjusted life‐years gains and value of insurance for afflicted and unafflicted persons as novel value components and show that those increase the value of a treatment substantially compared to value from easing burden on patients and caregivers. However, an empirical approach is lacking to estimate several components that could be particularly relevant for this treatment, such as the ability to stimulate future research and the opportunity to increase health equity.
Future directions: The results point to the need of further research into operationalization of additional value components to understand the holistic value of the treatment.
The remaining eight elements in the dark blue circles are not routinely considered in existing practice of value assessment and we identified two that could be quantified for an assessment of the societal value of an AD treatment, which are severity of disease and insurance value. The others were not included. Reduction in uncertainty refers to the added value of a companion diagnostic that allows identification of responders prior to treatment start. As coverage of the treatment will likely require biomarker confirmation of the AD pathology, this element is irrelevant for our analysis. Fear of contagion only matters for treatment or prevention of communicable diseases. Value of hope has been used in the literature for treatments for severe conditions, which have a skewed distribution of treatment effects, based on the logic that patients value the hope for a potentially strong effect over a certain weak effect. Real option value refers to the possibility that, by prolonging patients’ lives, they would be able to benefit from better treatments that are discovered in the future. It is an important consideration for an AD treatment, but we lack data to operationalize this. Equity would theoretically be an important element of value, given the disproportionate burden of the disease on disadvantaged populations, 13 as would be scientific spillovers, which is the ability of a novel treatment to stimulate future scientific advances. However, empirical approaches to quantify those concepts are lacking so far.
2.2. Markov model
Our model has three parts that were programmed in Visual Basic for Microsoft Excel and Stata 16. The first projects the annual number of individuals who will be formally diagnosed with MCI due to AD (the early disease stage at which treatment is expected to be effective) in the United States from 2021—the year in which we assumed the hypothetical treatment to become available—to 2041. The projection combines epidemiologic data on population trends, mortality, incidence, and prevalence with health systems data, as previous research has shown that capacity constraints in terms of availability of dementia specialists and biomarker testing will limit the number of patients diagnosed and treated each year. 14
The second part models disease progression from MCI through the three stages of dementia (mild, moderate, and severe) with and without treatment using published transition probabilities based on data from the National Alzheimer's Coordinating Center. 15 We modeled treatment effect as a 30% relative reduction of baseline progression rates from MCI to mild dementia and from mild to moderate dementia, with no effect on progression rates thereafter, based on published trial results. 16 , 17 , 18 The evolution of patients across stages, in turn, determines our outcomes, like mortality, medical cost, and caregiver burden. Details for these two parts have been previously published. 19 , 20
The third part computes the differences in outcomes between the treatment and no‐treatment groups and projects the effects of changing the disease progression on the included elements of the value flower, as detailed under the description of those elements. Sources, values, and distributions for all parameters can be found in the Technical Appendix in supporting information. We describe the estimation of the value components conceptually in the main document and mathematical details in the Technical Appendix.
All cost data are inflated to 2021 US dollars based on the medical care consumer price index (CPI) for medical spending and community and institutional costs, and overall CPI for the remaining components. 21 , 22 We discount future costs and benefits by the standard 3% annually. 23
2.3. Gain in QALYs
Mortality was modeled alongside disease progression based on age‐ and sex‐specific mortality rates of the US population 24 adjusted for increased mortality risks associated with disease progression. The treatment had no direct impact on mortality, but rather life‐year gains were achieved through slower progression to more severe disease. Stage‐specific health utility scores that serve as multipliers to transform those life‐years into QALYs were obtained from Mesterton et al. for patients 25 and from Bell et al. 26 and Neumann et al. 27 for caregivers. We assume for all caregiver‐related components that 75% of patients have a family caregiver based on prior publications 28 , 29 and that caregivers’ utility scores upon the death of a patient were equal to those caring for a severely demented patient.
2.4. Costs
Costs of formal medical and social care, that is, for professionally provided services to patients, by disease state were obtained from Gustavsson et al. 30 and GERAS data published by Robinson et al. 31 Both studies obtained costs with the Resource Utilization in Dementia (RUD) instrument 32 and multiplied by unit cost estimates. The GERAS study provides US‐specific estimates of the attributable social and medical costs for MCI and mild dementia, whereas Gustavsson et al. estimated values for mild, moderate, and severe dementia due to AD for the United States from a multinational study. For consistency, we scaled the GERAS estimate for mild dementia with the ratio of Gustavsson et al.’s values for moderate over mild and severe over mild dementia to compute moderate and severe AD costs. Details are noted in the Technical Appendix.
We distinguished social care costs for community‐dwelling and institutionalized patients and assumed that only patients with dementia but not MCI patients would be admitted to nursing home. As Gustavsson et al. did not provide an estimate for institutional care cost at the mild AD stage, we used their estimate for moderate AD. 30
For caregiver medical cost, we use the GERAS estimates 31 for MCI and mild dementia and applied the estimates for caregivers of patients with mild AD to those who cared for patients with moderate and severe AD, as we were unable to identify specific data for those stages. We set attributable medical cost to zero upon the death of the patient.
2.5. Productivity
2.5.1. Patients
We assume that labor earnings reflect workers’ productivity and follow guidance of the Second Panel on Cost‐Effectiveness in Health and Medicine that productivity gains are not well reflected in utility scores. 33 Improving patient outcomes not only helps them remain productive longer, but it also affects their likelihood of being recipients of public assistance. To estimate the detrimental effect of AD on labor earnings, we need to account for the patient's characteristics and estimate the (counterfactual) earnings they would make in absence of disease progression, if they were to remain in MCI. We used a matching algorithm and data from the Health and Retirement Study 34 to estimate the effect of each stage of AD on labor earnings and public program receipt from Supplemental Security Income (SSI) and Social Security Disability Insurance (SSDI). The Technical Appendix explains the details.
An important consideration in calculating the impact of reducing receipt of public assistance (SSI and SSDI) on societal value is that those assistance programs simply transfer public funds to individuals, which means that there is no creation or destruction of value. However, the taxes to fund these programs lead to distortion in the economy—called deadweight loss by economists. This loss rather than the transfer of funds leads to reduction of social welfare caused by public programs. We use the standard formula in the economics literature 35 and estimate the deadweight loss at 3% of social assistance payment. Details are documented in the Technical Appendix.
2.5.2. Caregivers
For caregivers, we estimate indirect cost based on losses in productivity and leisure time based on the GERAS data for community care costs 5 , 31 and Gustavsson et al. 30 for moderate and severe dementia patients in nursing homes. We follow Gustavsson et al., who calculate the value of lost productive hours by multiplying with the US average gross wage and of lost leisure time by multiplying with 35% of US average gross wage for as long as the patient is alive. Details are documented in the Technical Appendix.
2.6. Severity of disease
Conventional cost‐effectiveness analysis uses a linear valuation of health gain. 36 If a treatment cured a minor acute illness with a QALY multiplier of 0.84, it would get the same valuation as one that prevented progression from mild to moderate dementia. Lakdawalla and Phelps have argued that this approach is inconsistent with intuition, as most people would value the same effect in a severe illness more highly than in a minor illness, and proposed a framework and empirical approach to account for that fact. 37
2.7. Financial insurance value
Conceptually, a DMT resembles an insurance policy against the private cost of the disease, as it reduces an individual's expected out‐of‐pocket payments for medical and social care. 38 As insurance premiums typically exceed the expected cost, the so‐called premium load, they reflect how much individuals value the insurance beyond the amount covered. We approximate the financial insurance value of treatment by applying the premium load estimated by Brown and Finkelstein 39 to the out‐of‐pocket components of medical and long‐term care costs.
2.8. Insurance value to the unafflicted
The advent of a disease‐modifying AD treatment improves the outlook even for individuals who are not afflicted by the disease, because they have the reassurance of a therapeutic option should they need it in the future. An empirical analogy for this mechanism is the introduction of the COVID‐19 vaccine being associated with reduced mental distress even in individuals who were not yet fully vaccinated. 40 In another study, Shafrin et al. found the value of a future lung cancer treatment accruing to healthy individuals to be higher than the value for afflicted persons, and concluded the societal willingness to pay for generous insurance coverage to be above and beyond the traditional value to patients. 41
We evaluate this expected improvement in (gross) well‐being of the currently unafflicted before the realization of the uncertain health state, where a DMT would act as insurance against the physical and financial consequences of developing the disease. We calculate the combined future probability of survival and developing MCI due to AD for unafflicted individuals between the ages of 18 and 85. If they developed MCI, they would be able to benefit from the treatment, and we calculate this lifetime value as the actuarially fair expected private benefit conditional on surviving to that stage and developing the disease. We quantify this using our simulation results for forecasted benefits of treatment in terms of increased earnings, value of gains in QALYs adjusted for severity of disease, financial insurance value, and reduced out‐of‐pocket spending on medical and social care, assuming no costs for the treatment itself. We refrain from assuming risk aversion or behavioral aspects that would lead to increased valuation; therefore, this component is not the full valuation of risk reduction but instead reflects the expected benefits from a decrease in health risk due to DMT. Because of this limitation, we interpret it as a lower bound on the valuation of insurance. Details are in the Technical Appendix.
2.9. Calculation of societal value plus sensitivity analysis
We calculate the societal value of a disease‐modifying AD treatment from 2021 through 2041 for the cohort of all 2021 prevalent MCI cases, the 2021 cohort of unafflicted persons, and the full population with MCI due to AD. The cohort approach exposes all individuals to the same model duration, thus being more suitable for calculation of overall and by‐component per‐patient value and sensitivity analyses. The population approach adds the incident cases each year, which provides a complete picture of the overall societal value over the horizon of the model. As the actual price of the treatment is currently unknown, we calculate the gross societal value generated from which cost and disutility of the treatment will have to be deducted.
2.10. Sensitivity and scenario analyses
We conducted two kinds of sensitivity analyses using the 2021 prevalent cohort. A univariate analysis varied each parameter in the model by ±10% individually to understand whether any parameter has a disproportionate effect on the prediction. A probabilistic analysis provides insights into the overall uncertainty in the prediction. We varied all parameters simultaneously by randomly drawing a value out of their underlying distributions (see Table A3 in the Technical Appendix), recomputed the projection, and replicated this process 1000 times. We added scenarios with an assumed treatment effect of 22% and 40% (Technical Appendix Table A5).
The main analysis includes all patients because the hypothetical societal value of a treatment does not depend on how many patients receive the treatment under real‐world conditions. As access to treatment is likely to be constrained by the existing capacity to diagnose, 1 we project what proportion of societal value will be realized with a previously published model. 19 We use two assumptions on how patients will be identified in primary care settings and referred to a dementia specialist for further evaluation. The first is that referrals are based on a positive Mini‐Mental State Examination (MMSE) test alone, the second that a blood test for the AD pathology is conducted in patients with a positive MMSE and patients are only referred if both tests are positive.
2.11. Incidence analysis
The total value of QALY gains, severity adjustment, increased patient earnings, and insurance value accrues to patients and those from increased QALYS for caregivers and reduced caregiver time loss to caregivers. Decreases in deadweight loss from social programs go to society and as does the value of insurance for the unafflicted. The allocation of medical and social care costs to different payers was derived using the Medical Expenditure Panel Survey (MEPS) as described in the Technical Appendix.
3. RESULTS
3.1. Overall societal value
The predicted value of a disease‐modifying AD treatment from a US societal perspective from 2021 until 2041 amounts to $2.62 trillion for all afflicted persons. The existence of this disease‐modifying AD treatment implies $4.52 trillion in lifetime value of insurance for unafflicted adults in 2021. The average value of insurance per unafflicted individual would be $18,399, with a range from $13,661 for 18‐year‐olds to $25,888 for 49‐year‐old persons.
The value corresponding to the 2021 cohort of prevalent cases with MCI due to AD is $986 billion, which represents a 13% difference between the treated and the untreated cohort. The year‐by‐year cumulative value by component for the cohort analysis is shown in Figure 2 and the underlying data can be found in the Technical Appendix (Table A5). Value accrual starts slowly, as expected for a slowly progressive disease, and enters a rapid growth phase around 2025 before leveling out toward the end of the simulation. With an estimated 7.3 million prevalent cases in 2021, the value per person is estimated to be $134,418.
FIGURE 2.
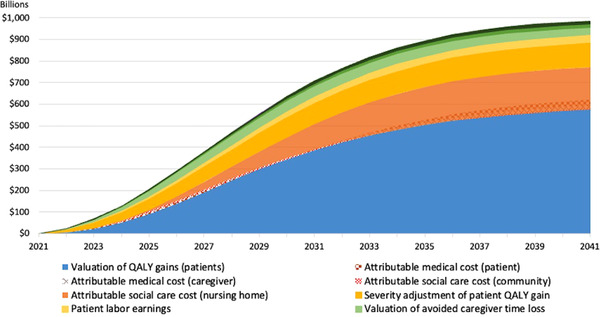
Cumulative societal value from disease‐modifying Alzheimer's disease treatment, cohort analysis. Shaded areas where two components overlap indicate components whose contribution turns negative and detracts from the positive components. QALY, quality‐adjusted life‐years
3.2. Scenario and sensitivity analyses using 2021 prevalent cohort
While the theoretical societal value of a treatment should not depend on a health system's ability to make it accessible, the large number of prevalent cases when the treatment will first become available will result in wait times for specialist appointments and biomarker testing, hence reducing the realized value under real‐world conditions. If patients were referred to diagnostic evaluation of treatment eligibility solely based on a positive MMSE, a large number of individuals without an eventual treatment indication would clog the pipeline for specialist appointments, thereby reducing realized value by 69% to $302 billion ($41,119 per person) as treatment‐eligible patients would progress on the wait list. If referrals were restricted to those who also had a positive blood biomarker test for the AD pathology, the more efficient triage process means that realized value would be reduced by 52% to $477 billion ($65,058 per person).
Figure 3 displays the results from the univariate sensitivity analysis that varied input parameters by ±10% from their baseline value. The results show that the changes of neither parameter led to a change of 10% or more in the predicted value and that changing patient QALYs, nursing home cost and transition to death had the largest impact on the predicted value, whereas changes to other parameters altered the prediction by ≈1% or less. The probabilistic sensitivity analysis results (Figure 4) show an interquartile range of $915 to $1057 billion and a 95% confidence interval of $797 to $1218 billion.
FIGURE 3.
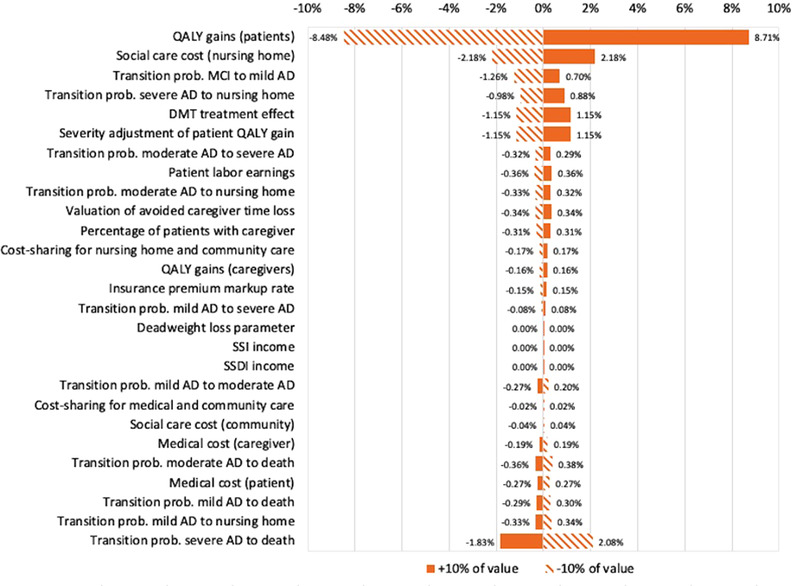
Results from univariate sensitivity analysis, cohort analysis. Results show relative change in overall societal value if the respective parameter is changed by ±10%. AD, Alzheimer's disease; DMT, disease‐modifying treatment; MCI, mild cognitive impairment; QALY, quality‐adjusted life‐years; SSDI, Social Security Disability Insurance; SSI, Supplemental Security Income; prob: probability
FIGURE 4.
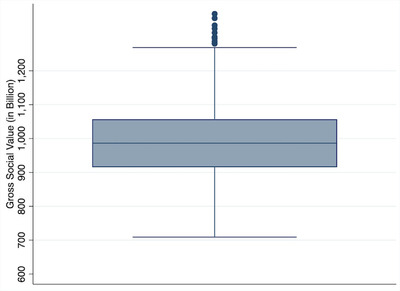
Distribution of predicted societal value based on probabilistic sensitivity analysis, cohort analysis
FIGURE 5.
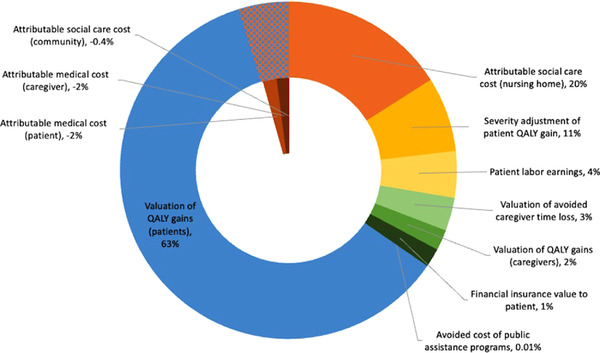
Relative contribution of individual components to value for the 2021 prevalent cohort from disease‐modifying Alzheimer's disease treatment. The patterned area represents negative contributions to overall value that is deducted from the total value. This includes the components of the value flower derived from treating the 2021 prevalent cohort, therefore it excludes the $4.52 trillion insurance value attributed to unafflicted individuals. QALY, quality‐adjusted life‐years
Technical Appendix Table A6 illustrates the effect of the assumed treatment effect size. Assuming an effect of 22% rather than 30% reduces societal value by $293 billion (30%) and of 40% increases it by $400 billion (40%).
3.3. Breakdown by components
Figure 5 illustrates the relative contribution of each component to overall societal value for the 2021 prevalent cohort. More than half of the cumulative value (63%) stems from the valuation of the average gain of 0.75 QALYs (reflecting an average gain of 0.58 life‐years) per patient at the standard amount of $150,000 with severity adjustment of those gains adding another 11%. Avoided nursing home costs contribute 20%, whereas the role of all other components is small with 4% or less each. Medical and community social care costs reduce value, as the longer lifespan of treated individuals results in a net increase of spending.
3.4. Incidence of value
Incidence of each value component for the 2021 patient cohort is described in Table 1. Almost all the value generated for afflicted individuals accrues to patients (82%), mainly from QALY gains, and to Medicaid programs as the main payer of nursing home care (14%). However, the expected benefits to the unafflicted adults may be interpreted as accruing to society at large.
TABLE 1.
Incidence of value to different stakeholders (million US$)
| Overall value | Patients | Caregivers | Payers | Rest of society | |||
|---|---|---|---|---|---|---|---|
| Medicare | Medicaid & other public insurance | Private insurance | |||||
| Valuation of QALY gains (patients) | $621 | $621 | |||||
| Severity adjustment of patient QALY gain | $113 | $113 | |||||
| Valuation of QALY gains (caregivers) | $16 | $16 | |||||
| Attributable medical cost (patient) | –$25 | –$5 | –$13 | –$3 | –$4 | ||
| Attributable medical cost (caregiver) | –$19 | –$2 | –$4 | –$5 | –$8 | ||
| Attributable social care cost (community) | –$3 | –$1 | –$1 | –$1 | –$0.2 | ||
| Attributable social care cost (nursing home) | $199 | $52 | $24 | $123 | |||
| Patient labor earnings | $36 | $36 | |||||
| Avoided cost of public assistance programs | $0.1 | $0.1 | |||||
| Valuation of avoided caregiver time loss | $33 | $33 | |||||
| Financial insurance value to patient | $15 | $15 | |||||
| Overall | $986 | $830 (84%) | $47 (5%) | $6 (1%) | $114 (12%) | –$12 (–1%) | $0.1 (0.01%) |
Abbreviation: QALY, quality‐adjusted life‐years.
4. DISCUSSION
4.1. Overall value
We have quantified the value of a disease‐modifying AD treatment from a US societal perspective from 2021 until 2041 as $2.62 trillion for all afflicted persons and as a lifetime value for the 2021 cohort of prevalent patients with MCI due to AD disease of $986 billion. The estimate appears not to be overly sensitive to any one single parameter and reasonably well bounded. We calculate $4.52 trillion in lifetime value of insurance for unafflicted adults (alive in 2021). Thus, overall gross societal value is estimated to be $5.5 trillion, the sum of value accrued to the afflicted and unafflicted cohorts in 2021, as the insurance value to the 2021 unafflicted cohort already captures the value to those who become symptomatic later.
The result that the overall aggregate value to unafflicted individuals is considerably larger than that to patients arises because there are 245 million unafflicted individuals, and roughly 7.3 million MCI patients in 2021. The average lifetime value to those unafflicted individuals and to the prevalent MCI cases is $18,399 and $134,418 per person, respectively.
4.2. Contribution of value components
The largest contribution to the value to afflicted persons was the valuation of an average of 0.75 per patient gain in QALYs, which is in line with recently published estimates of QALY gains of 0.73, 42 0.65, 43 and 0.225 5 assuming treatment effects of 25%, 31%, and 25%, respectively. The recently published Institute for Clinical and Economic Review report, which estimated a gain of 0.154 QALYs, did not disclose assumptions for treatment effect. 44
Like other researchers, we find that nursing home costs are by far the largest component of care purchased in the marketplace. Hurd et al., for example, estimated nursing home cost exceeded community‐based societal care cost and medical cost by a factor of about 2.4 and 5.0, respectively. 6 Based on a systematic review of 27 studies, Schaller et al. found the predominant determinant of costs to be long term care expenditures. 45
Conversely, caregiver burden, which others have identified as an important contributor to the cost of the disease, 8 only contributed marginally, potentially because of the particular nature of the treatment, which does not prevent disease onset but merely slows down progression to more severe stages. As caregivers’ loss of quality of life and productivity as well as their medical cost become substantial once a patient is symptomatic and do not change markedly with disease progression, the consequences for value generation are limited.
4.3. Incidence of value
It follows that most of the value generated by the treatment of the prevalent patients accrues to patients and to a lesser degree to the Medicaid program via reduced nursing home use, and only 4% to other stakeholder whereas the entire value to the unafflicted accrues to society.
4.4. Limitations
Important limitations must be kept in mind. First and foremost, modeling studies are fraught with uncertainty especially over long prediction horizons. We report on gross societal value, that is, exclude the monetary and physical cost of the treatment itself and added cost because of the treatment, such as monitoring for and managing of side effects. At the same time, important components, like scientific spillovers, real‐option value, and equity are not valued for lack of established empirical strategies. In particular, the value of scientific spillovers would be important to quantify, as experience from other therapeutic areas, such as oncology, has shown how initial treatments spur innovation. Our disease progression model oversimplifies the trajectory as it does not reflect differential changes in neuropsychiatric symptoms and may therefore misestimate caregiver impact. 46 Model parameters by disease stage were derived from a variety of sources that used different definitions and instruments, which may have introduced error.
4.5. Conclusions
Overall, our findings point to substantial societal value from an AD treatment that can help putting the cost of treatment into perspective. Value largely stems from components that are reflected in conventional cost‐effectiveness analysis—at least for afflicted persons, whereas the aggregate insurance value to all the unafflicted is more than twice the total value for the afflicted. As we were unable to operationalize several components of the value flower that are of particular salience in AD, equity, scientific spillovers, and real option value, further work is needed to arrive at a comprehensive estimate of the societal value of an AD treatment.
CONFLICTS OF INTEREST
The work was funded by a contract from Biogen to the University of Southern California. The sponsor had no role in the design of the study, interpretation of the findings, and decision to submit for publication. Outside of this work, USC has received funding for research projects on which Dr. Mattke is PI from Biogen, Eisai, and Roche. Dr. Prados is supported by the following grants: NIMHD #P50MD015705, NICHD 3R01HD093382‐02S1, NIDDK R01DK111169, MRDRC: RDR UM21‐Q2, MRDRC RDR18000002‐02 UM20‐08, MRDRC RDR18000002‐01 UM19‐06, MRDRC RDR UM19‐Q1, MRDRC RRC08098401, MRDRC RRC08098401. Dr. Liu's work was supported by grants and supports from National Institutes of Health, National Science Foundation, George Lucas Educational Foundation, and Union Bank. Dr. Lam was supported by a Bristol Myers Squibb Fellowship with funds made directly to USC from June 2019 to December 2020 and is currently employed by Bristol Myers Squibb. Dr. Mattke serves on the board of directors of Senscio Systems, Inc., and the scientific advisory board of AiCure Technologies, and Boston Millennia Partners. He has received consulting fees from AARP, Biogen, Biotronik, Bristol‐Myers Squibb, C2N, Eisai, Roche, and Defined Health. Dr. Prados has received honoraria for presentations at Mount Saint Mary's University, Los Angeles, and the Women Lawyers Association of Los Angeles. Ms. Jun reports no potential conflicts.
Supporting information
Supporting Information
Prados MJ, Liu Y, Jun H, Lam J, Mattke S. Projecting the long‐term societal value of a disease‐modifying treatment for Alzheimer's disease in the United States. Alzheimer's Dement. 2022;18:142–151. 10.1002/alz.12578
REFERENCES
- 1. Li J, Hlávka JP, Hillestad R, Mattke S. Assessing the Preparedness of the U.S. Health Care System Infrastructure for an Alzheimer's Treatment. RAND Corporation; 2017. [PMC free article] [PubMed] [Google Scholar]
- 2. Rabinovici GD, Gatsonis C, Apgar C, et al. Association of Amyloid Positron Emission Tomography With Subsequent Change in Clinical Management Among Medicare Beneficiaries With Mild Cognitive Impairment or Dementia. JAMA. 2019; 321(13): 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drummon M, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford University Press; 2015. [Google Scholar]
- 4. Nguyen K‐H, Comans TA, Green C. Where are we at with model‐based economic evaluations of interventions for dementia? a systematic review and quality assessment. International Psychogeriatrics. 2018; 30(11): 1593‐1605. [DOI] [PubMed] [Google Scholar]
- 5. Ito K, Chapman R, Pearson SD, Tafazzoli A, Yaffe K, Gurwitz JH. Evaluation of the Cost‐effectiveness of Drug Treatment for Alzheimer Disease in a Simulation Model That Includes Caregiver and Societal Factors. JAMA Netw Open. 2021; 4(10): e2129392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary Costs of Dementia in the United States. N Engl J Med. 2013; 368(14): 1326‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gustavsson A, Pemberton‐Ross P, Montero MG, et al. Challenges in demonstrating the value of disease‐modifying therapies for Alzheimer's disease. Expert Rev Pharmacoecon Outcomes Res. 2020; 20(6): 563‐570. [DOI] [PubMed] [Google Scholar]
- 8. Pyenson BS, Pelizzari PM, Smith R, Latimer H. Assessing the value of therapies in Alzheimer's disease: Considerations to create a practical approach to value. Milliman Report, May 12, 2021. Available at: https://www.milliman.com/en/insight/assessing-thevalue-of-therapies-in-alzheimers-disease-considerations-to-create-a-practical. Accessed 4 January 2022.
- 9. Huter K, Kocot E, Kissimova‐Skarbek K, Dubas‐Jakóbczyk K, Rothgang H. Economic evaluation of health promotion for older people‐methodological problems and challenges. BMC Health Serv Res. 2016; 16(S5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neumann PJ, Willke RJ, Garrison LP. A Health Economics Approach to US Value Assessment Frameworks—Introduction: an ISPOR Special Task Force Report [1]. Value in Health. 2018; 21(2): 119‐123. [DOI] [PubMed] [Google Scholar]
- 11. Lakdawalla DN, Doshi JA, Garrison LP, Phelps CE, Basu A, Danzon PM. Defining Elements of Value in Health Care—A Health Economics Approach: an ISPOR Special Task Force Report [3]. Value Health. 2018; 21(2): 131‐139. [DOI] [PubMed] [Google Scholar]
- 12. Cumming J. Alzheimer's disease drug development pipeline: 2020. Alzheimer Dement. 2020; 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lines L. Racial and Ethnic Disparities Among Individuals with Alzheimer's Disease in the United States: A Literature Review. RTI Press; 2014. [Google Scholar]
- 14. Peterse R. Mild Cognitive Impairment. CONTINUUM: Lifelong Learn Neurol. 2016; 22(2, Dementia): 404‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Potashman M, Buessing M, Levitchi Benea M, et al. Estimating Progression Rates Across the Spectrum of Alzheimer's Disease for Amyloid‐Positive Individuals Using National Alzheimer's Coordinating Center Data. Neurol Ther. 2021: 941‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cumming J. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimer Res Ther. 2021; 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021; 384(18): 1691‐1704. [DOI] [PubMed] [Google Scholar]
- 18. Swanson C, Zhang Y, Dhadda S, et al. A randomized, double‐blind, phase 2b proof‐of‐concept clinical trial in early Alzheimer's disease with lecanemab, an anti‐Aβ protofibril antibody. Alzheimer Res Ther. 2021; 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lam J, Jun H, Cho SK, et al. Projection of budgetary savings to US state Medicaid programs from reduced nursing home use due to an Alzheimer's disease treatment. Alzheimers Dement (Amst). 2021; 13(1): e12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mattke S, Cho SK, Bittner T, et al. Blood‐based biomarkers for Alzheimer's pathology and the diagnostic process for a disease‐modifying treatment: projecting the impact on the cost and wait times. Alzheimer Dement. 2020; 12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Federal Reserve . December 11, 2019: FOMC Projections materials, accessible version. Jan 20, 2021]; https://www.federalreserve.gov/monetarypolicy/fomcprojtabl20191211.htm
- 22. Statistics USBoL . Consumer Price Index for All Urban Consumers. 2021 Jan 20, 2021]; https://www.bls.gov/
- 23. Neuman P, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost‐effectiveness in health and medicine. Oxford University Press; 2016. [Google Scholar]
- 24. Kochanek KD, Xu J, Arias E. Mortality in the United States. NCHS Data Brief. 2019; 2020(395): 1‐8. [PubMed] [Google Scholar]
- 25. Mesterton J, Wimo A, By A, Langworth S, Winblad B, Jonsson L. Cross Sectional Observational Study on the Societal Costs of Alzheimers Disease. Curr Alzheimer Res. 2010; 7(4): 358‐367. [DOI] [PubMed] [Google Scholar]
- 26. Bell CM, Araki SS, Neumann PJ. The association between caregiver burden and caregiver health‐related quality of life in Alzheimer disease. Alzheimer Dis Assoc Disord. 2001; 15(3): 129‐136. [DOI] [PubMed] [Google Scholar]
- 27. Neumann PJ, Sandberg EA, Araki SS, Kuntz KM, Feeny D, Weinstein MC. A comparison of HUI2 and HUI3 utility scores in Alzheimer's disease. Med Decis Making. 2000; 20(4): 413‐422. [DOI] [PubMed] [Google Scholar]
- 28. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009; 11(2): 217‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schulz R, Martire LM. Family Caregiving of Persons With Dementia: prevalence, Health Effects, and Support Strategies. Am J Geriatr Psychiatry. 2004; 12(3): 240‐249. [PubMed] [Google Scholar]
- 30. Gustavsso AN, Brinck P, Bergvall N, et al. Predictors of costs of care in Alzheimer's disease: a multinational sample of 1222 patients. Alzheimers Dement. 2011; 7(3): 318‐327. [DOI] [PubMed] [Google Scholar]
- 31. Robinso RL, Rentz DM, Andrews JS, et al. Costs of Early Stage Alzheimer's Disease in the United States: cross‐Sectional Analysis of a Prospective Cohort Study (GERAS‐US)1. J Alzheimers Dis. 2020; 75(2): 437‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wimo A. Application of Resource Utilization in Dementia (RUD) instrument in a global setting. Alzheimer's & Dementia. 2013; 9(4): 429‐435. [DOI] [PubMed] [Google Scholar]
- 33. Sander GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost‐effectiveness Analyses. JAMA. 2016; 316(10): 1093. [DOI] [PubMed] [Google Scholar]
- 34. Health and Retirement Study. (RAND HRS Longitudinal File 2016, V2). University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740). [Google Scholar]
- 35. Feldstein M. Tax Avoidance and the Deadweight Loss of the Income Tax. Rev Econ Stat. 1999; 81(4): 674‐680. [Google Scholar]
- 36. Rowe D, Brazier J, Mukuria C, et al. Eliciting Societal Preferences for Weighting QALYs for Burden of Illness and End of Life. Med Decis Making. 2016; 36(2): 210‐222. [DOI] [PubMed] [Google Scholar]
- 37. Lakdawalla D, Phelps CE. Health technology assessment with risk aversion in health. J Health Econ. 2020; 72: 102346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lakdawalla D, Malani A, Reif J. The insurance value of medical innovation. J Public Econ. 2017; 145: 94‐102. [Google Scholar]
- 39. Brown J, Finkelstein A. Insuring Long‐Term Care in the United States. J Econ Perspect. 2011; 25(4): 119‐142. [Google Scholar]
- 40. Perez‐Arc FE, Angrisani M, Bennett D, Darling J, Kapteyn A, Thomas K. COVID‐19 vaccines and mental distress. PLOS One. 2021; 16(9): e0256406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shafrin J, May SG, Zhao LM, et al. Measuring the Value Healthy Individuals Place on Generous Insurance Coverage of Severe Diseases: a Stated Preference Survey of Adults Diagnosed With and Without Lung Cancer. Value Health. 2021: 855‐861. [DOI] [PubMed] [Google Scholar]
- 42. Wimo A, Handels R, Winblad B, et al. Quantifying and Describing the Natural History and Costs of Alzheimer's Disease and Effects of Hypothetical Interventions. J Alzheimers Dis. 2020; 75(3): 891‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herring WL, Gould IG, Fillit H, et al. Predicted Lifetime Health Outcomes for Aducanumab in Patients with Early Alzheimer's Disease. Neurol Ther. 2021: 919‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin GAW, Melanie D, Synnott PG, et al. Aducanumab for Alzheimer's Disease: Effectiveness and Value; Draft Evidence Report. Institute for Clinical and Economic Review; 2021. ICER. [Google Scholar]
- 45. Schaller S, Mauskopf J, Kriza C, Wahlster P, Kolominsky‐Rabas PL. The main cost drivers in dementia: a systematic review. Int J Geriatr Psychiatry. 2015; 30(2): 111‐129. [DOI] [PubMed] [Google Scholar]
- 46. Gustavsson A, Green C, Jones RW, et al., Current issues and future research priorities for health economic modelling across the full continuum of Alzheimer's disease. Alzheimer Dement. 2017;13(3): 312‐321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


