Abstract
Objectives
Despite the high frequency of segmentation anomalies in the human sacrum, their evolutionary and clinical implications remain controversial. Specifically, inconsistencies involving the classification and counting methods obscure accurate assessment of lumbosacral transitional vertebrae. Therefore, we aim to establish more reliable morphological and morphometric methods for differentiating between sacralizations and lumbarizations in clinical and paleontological contexts.
Materials and Methods
Using clinical CT data from 145 individuals aged 14–47 years, vertebral counts and the spatial relationship between the sacrum and adjoining bony structures were assessed, while the morphological variation of the sacrum was assessed using geometric morphometrics based on varied landmark configurations.
Results
The prevalence of lumbosacral and sacrococcygeal segmentation anomalies was 40%. Lumbarizations and sacralizations were reliably distinguishable based on the spatial relationship between the iliac crest and the upward or downward trajectory of the linea terminalis on the sacrum. Different craniocaudal orientations of the alae relative to the corpus of the first sacral vertebra were also reflected in the geometric morphometric analyses. The fusion of the coccyx (32%) was frequently coupled with lumbarizations, suggesting that the six‐element sacra more often incorporate the coccyx rather than the fifth lumbar vertebra.
Conclusions
Our approach allowed the consistent identification of segmentation anomalies even in isolated sacra. Additionally, our outcomes either suggest that homeotic border shifts often affect multiple spinal regions in a unidirectional way, or that sacrum length is highly conserved perhaps due to functional constraints. Our results elucidate the potential clinical, biomechanical, and evolutionary significance of lumbosacral transitional vertebrae.
Keywords: geometric morphometrics, homeotic border shifts, lumbarization, lumbosacral transitional vertebrae, sacralization
Direct morphological comparison of lumbosacral segmentation anomalies, that is, sacralization (blue) and lumbarization (yellow).
1. INTRODUCTION
The adult human sacrum typically consists of a total of five fused sacral vertebrae. Developmentally, multiple primary and secondary ossification centers within each vertebra ossify from birth until age 25, while intersegmental fusion is completed at around age 18 (Broome et al., 1998; Cardoso et al., 2014). However, the sacrum shows a high frequency of numeric deviations, commonly attributed to segmentation anomalies (Bertolotti, 1917; Nastoulis et al., 2019; Paterson, 1892; Williams et al., 2019). The segmentation of the vertebral column occurs during embryonic development when the paraxial mesoderm starts to form transversal clefts around Day 21 that separate it into a total of 42–44 somites (Töndury & Theiler, 1990). This eventually results in five series of morphologically similar vertebrae (7 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 3–5 coccygeal vertebrae) that collectively form the human spine. A segmentation anomaly is present when one spinal region deviates from this typical number of vertebrae (Schmorl & Junghans, 1968).
Interestingly, segmentation anomalies are more likely to occur in the caudal part of the spine compared to the cranial spine. As such, the cervical count is highly conserved (Bronn, 1874; Le Double, 1912; Pilbeam & Young, 2004; Todd, 1922), with aberrations being often associated with detrimental effects, including late‐term miscarriages and stillbirths (Galis et al., 2006; Schmorl & Junghans, 1968; ten Broek et al., 2012). Variation is more frequently observed at the thoracolumbar border and particularly the lumbosacral border, although the number of presacral vertebrae remains remarkably stable, both within humans and within mammals (Haeusler et al., 2002; Pilbeam, 2004; Schultz & Straus, 1945; Todd, 1922; Williams & Russo, 2015). In general, meristic changes, that is, additional or missing segments (Baur, 1891; von Ihering, 1878; Williams et al., 2019; Zuckerman, 1938), are less common than homeotic changes, which manifest as the transformation of one segment into a different segment (Barnes, 1994; Keith, 1902; Rosenberg, 1875, 1899; ten Broek et al., 2012; Williams et al., 2019). Such homeotic transformations shift the boundaries between spinal regions. The vertebra that assumes characteristics of two adjacent spinal regions is often referred to as a transitional vertebra (or “Zwischenwirbel,” Dürr, 1860).
Hox genes offer a genetic explanation for these homeotic changes since they encode for proteins controlling axial patterning and play a major role in the specification of the morphological identity of the vertebrae (Carapuço et al., 2005; Mallo et al., 2010; Müller et al., 2010). Thus, Hox11 is primarily responsible for the correct genesis of the sacral and caudal vertebrae. A complete lack of Hox11 gene products results in sacral vertebrae that are morphologically identical to lumbar vertebrae. Hox10 gene products are also important because of their rib‐suppressing activity. Simultaneous inactivation of Hox11 and Hox10 leads to vertebrae bearing ribs in the lumbar and sacral spine (Mallo et al., 2010). Overexpression of Hox11, on the other hand, leads to signs of sacralization by fusion of adjacent vertebrae and a cranial shift of the first sacral vertebra (Carapuço et al., 2005).
Homeotic border shifts have also been implicated in the evolution of the spinal segmentation formulae of mammals and particularly of hominins (e.g., Haeusler et al., 2002, 2011; Machnicki & Reno, 2020; McCollum et al., 2010; Pilbeam, 2004; Williams et al., 2016, 2019; Williams & Pilbeam, 2021). However, the precise mechanisms responsible for the number and morphological identities of the vertebrae are still elusive (Kudlicki, 2019; Tague, 2018), and it is unknown whether these Hox genes are responsible for segmentation anomalies since Hox gene mutations also involve severe perturbation of limb and pelvic morphology (Wellik & Capecchi, 2003).
At the lumbosacral junction, border shifts can result in the last lumbar vertebra partly or completely fusing to the sacrum, resulting in a condition termed sacralization. In contrast, a partial or complete detachment of the first sacral element is referred to as lumbarization, in which case the corresponding vertebra morphologically resembles a last lumbar vertebra. The presence of transitional lumbar vertebrae has been linked to an increased risk for degenerative spine disorders, disc herniation, and low back pain (e.g., Bertolotti, 1917; Bron et al., 2007; Castellvi et al., 1984; Matson et al., 2020; Nardo et al., 2012; Vergauwen et al., 1997). Moreover, the patient's neurological symptoms may be at odds with the findings of Magnetic Resonance Imaging (MRI) examination, and spine surgery may consequently be performed at the incorrect segment if metameric variation is not considered.
Given their clinical relevance, segmentation anomalies have been extensively studied using X‐ray, CT, and MRI imaging modalities (e.g., Hahn et al., 1992; Hughes & Saifuddin, 2006; Tins & Balain, 2016). For instance, vascular structures such as the right renal and the superior mesenteric arteries, the aortic bifurcation, the inferior vena cava confluence, and the celiac trunk have been investigated in terms of their spatial relationship to specific vertebrae in order to evaluate the presence and identity of transitional vertebrae (Lee et al., 2007; Ralston et al., 1992). Further, the origin of the iliolumbar ligament, which usually attaches at the costal process of the fifth lumbar vertebra (L5) (Hughes & Saifuddin, 2004, 2006; Lee et al., 2007), as well as vertebra and disc shape (Hsieh et al., 2000; O'Driscoll et al., 1996) have been also used for classifying transitional vertebrae. Nevertheless, most authors agree that analyzing the entire spine and counting the vertebrae from the atlas (Hahn et al., 1992; Hughes & Saifuddin, 2004; Tins & Balain, 2016), or at least from T12 (Bron et al., 2007 and references therein), is necessary to establish a reliable diagnosis, while successfully distinguishing sacralizations from lumbarizations remains challenging.
Despite their clinical importance, few studies offer reliable classification systems for the different stages of sacralizations and lumbarizations (Castellvi et al., 1984; Mahato, 2013; Tini et al., 1977). In fact, most studies (see Table 1) focus exclusively on sacralization while ignoring lumbarization, or they fail to distinguish between the two, thereby propagating further confusion in the literature. Additionally, some studies do not clearly state how the distinction was made or fail to consider the complete spinal count. This accounts for the broad range reported for the prevalence of segmentation anomalies, which varies between 4% and 35% (see Table 1, adapted from Bron et al., 2007). Even less attention has been dedicated to segmentation anomalies at the sacrococcygeal border. In fact, sacralization, that is, the synostosis of the first coccygeal element to the last sacral vertebrae, is a frequent occurrence and also results in a change in sacral element number (Lee et al., 2016; Tague, 2011; Woon et al., 2013).
TABLE 1.
Previously published frequencies of transitional vertebrae (TV) from clinical studies and anatomical collections (studies based on archaeological material were omitted but see Drew & Kjellström, 2021 for an overview)
| Publication | Data | Sample origin | n | Lumbosacral transitional vertebrae | Sacralizations | Lumbarizations | |||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| Andrew (1954) | X‐ray | Great Britain | 300 | 31 | 10.3 | ||||
| Apazidis et al. (2011) | X‐ray | United States | 211 | 75 | 35.5 | ||||
| Benlidayi et al. (2015) | X‐ray | Turkey | 1588 | 96 | 6.0 | ||||
| Cadeddu et al. (1997) | X‐ray | United States | 299 | 16 | 5.4 | ||||
| Castellvi et al. (1984) | X‐ray | United States | 200 | 60 | 30.0 | ||||
| Chaijaroonkhanarak et al. (2006) | Osteological material | Thailand | 206 | 9 | 4.4 | 9 | 4.4 | ||
| Chithriki et al. (2002) | MRI | United States | 441 | 37 | 8.4 | 22 | 5.0 | 15 | 3.4 |
| Dar and Peled (2014) | CT | Israel | 436 | 57 | 13.1 | ||||
| Delport et al. (2006) | X‐ray | United States | 300 | 90 | 30.0 | ||||
| Elster (1973) | CT | United States | 2000 | 140 | 7.0 | ||||
| Erken et al. (2002) | X‐ray | Turkey | 729 | 262 | 35.9 | ||||
| French et al. (2014) | X‐ray | Australia | 5429 | 540 | 9.9 | 225 | 4.1 | 315 | 5.8 |
| Gopalan and Yerramshetty (2018) | X‐ray | India | 596 | 145 | 24.3 | 125 | 21.0 | 20 | 3.4 |
| Hahn et al. (1992) | MRI | United States | 200 | 24 | 12.0 | 15 | 7.5 | 9 | 4.5 |
| Hald et al. (1995) | X‐ray | Germany (males only) | 10,922 | 792 | 7.3 | 850 | 7.8 | 650 | 6.0 |
| Hsieh et al. (2000) | X‐ray | United States | 1668 | 67 | 4.0 | ||||
| Hughes and Saifuddin (2006) | MRI | Great Britain | 500 | 67 | 13.4 | 46 | 9.2 | 21 | 4.2 |
| Kim (1997) | MRI | Korea | 690 | 41 | 5.9 | 12 | 1.7 | 29 | 4.2 |
| Leboeuf et al. (1989) | X‐ray | Australia | 530 | 61 | 11.5 | 29 | 5.5 | 32 | 6.0 |
| Lee et al. (2007) | MRI | South Korea | 534 | 127 | 23.8 | 74 | 13.9 | 53 | 9.9 |
| Luoma et al. (2004) | MRI | Finland (males only) | 163 | 43 | 26.4 | ||||
| Mahato (2010) | Osteological material | India | 330 | 20 | 6.1 | ||||
| Nardo et al. (2012) | X‐ray | United States | 4636 | 841 | 18.1 | ||||
| O'Driscoll et al. (1996) | MRI | Great Britain | 100 | 15 | 15.0 | ||||
| Otani et al. (2001) | MRI/CT | Asia | 1009 | 119 | 11.8 | ||||
| Peh et al. (1999) | MRI | China | 129 | 17 | 13.2 | 8 | 6.2 | 9 | 7.0 |
| Peterson et al. (2005) | X‐ray | Canada | 353 | 43 | 12.2 | ||||
| Quinlan et al. (2006) | MRI | Ireland | 769 | 35 | 4.6 | ||||
| Santiago et al. (2001) | CT | Spain | 138 | 26 | 18.8 | 16 | 11.6 | 10 | 7.2 |
| Steinberg et al. (2003) | X‐ray | Israel | 464 | 85 | 18.3 | 65 | 14.0 | 20 | 4.3 |
| Tague (2009) | Osteological material | United States | 2086 | 131 | 6.3 | ||||
| Tang et al. (2014) | X‐ray | China | 5860 | 928 | 15.8 | 0.0 | |||
| Taskaynatan et al. (2005) | X‐ray | Turkey | 881 | 48 | 5.4 | 40 | 4.5 | 8 | 0.9 |
| Tini et al. (1977) | X‐ray | Switzerland | 4000 | 269 | 6.7 | ||||
| Tins and Balain (2016) | MRI | Great Britain | 418 | 14 | 3.3 | 8 | 1.9 | 4 | 1.0 |
| Uçar et al. (2013) | X‐ray | Turkey | 3607 | 683 | 18.9 | 17.2 | 1.7 | ||
| Vergauwen et al. (1997) | CT | Belgium | 350 | 53 | 15.1 | ||||
| Total | 53,192 | 6146 | 12 | ||||||
The correct recognition of the exact type of segmentation anomaly is crucial to understanding its relationship to disc herniation, degenerative joint disorders, and low back pain. Moreover, lumbarization cannot reliably be distinguished from sacralization in fragmentary skeletal remains, thereby obscuring any potential functional implications caused by such anomalies. Understanding the morphological variation, that these anomalies introduce, has important implications in both clinical and archaeological contexts.
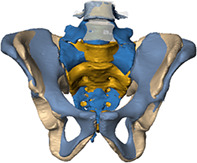
In this study, we evaluate morphological characteristics associated with segmentation anomalies of the modern human sacrum. Specifically, we aim to establish repeatable methods that reliably distinguish sacralizations from lumbarizations, while emphasizing informative morphological changes at the base of the sacrum, namely the diagnostic relationship between the iliac crest and the linea terminalis that co‐occur with segmentation anomalies.
2. MATERIALS AND METHODS
Our modern human sample consisted of 145 clinical CT scans, of which 127 included the entire vertebral column (n = 51) or a complete thoracolumbosacral spine (n = 76). Resolution ranged from 0.5 to 2.0 mm, with slice thicknesses being 0.8–3.0 mm. The sample comprised 88 patients aged 14.5–20.0 years from the Hôpital de la Timone, Marseille, and 58 adults aged 22–47 from the Department of Biomedical Imaging and Image‐guided Therapy, Medical University of Vienna, Vienna. The individuals were analyzed in two age cohorts (subadults and adults) based on the extent of closure at the primary ossification centers (about 20 years). The patients from both the Viennese and French datasets were randomly selected from larger data sets, provided they did not suffer any developmental or skeletal growth disorders. The French data were anonymized according to the personal privacy standards of the French National Ethical Committee (Corron, 2016). The Viennese data were anonymized by the Data Clearing House of the Medical University of Vienna, Austria, after clearance by the ethic commission of the Medical University of Vienna (votum 1196/2017).
The datasets were segmented using Amira (www.fei.com), and surface models were generated to represent the sacrum, the hip bones, the lumbar and the thoracic vertebrae, as well as the costae. The cranialmost rib‐bearing vertebra was classified as the first thoracic vertebra (T1). Vertebrae at the thoracolumbar border with rudimentary ribs were recorded as either thoracic (if the rib was mobile and oriented caudally) or as lumbar (if the rib was broad and blunt and shaped like a detached costal process directed laterally or cranially; see Haeusler et al., 2002). A laterally oriented costal process warranted a lumbar classification. Vertebrae with asymmetrical transitions were counted as half belonging to one spinal region and half belonging to the other spinal region. Thus, sacral vertebrae were counted according to the number of sacral foramina divided by two plus one (Schultz & Straus, 1945). Lumbosacral transitional vertebrae were classified according to Castellvi et al. (1984).
Additionally, we used the iliac crest (Andrew, 1954; Farshad‐Amacker et al., 2015) and the linea terminalis (Tague, 2009) as references to identify the type of segmentation anomaly (i.e., sacralization or lumbarization, Figure 1). For that purpose, we established four different categories for the relationship between the iliac crest and L4/L5, with the level of the iliac crest ranging between (1) the center of L4, (2) the inferior surface of L4, (3) the superior surface of L5, and (4) the center of L5. Levels were recorded from the 3D models generated from the clinical CTs in the supine position. Similarly, three different configurations were recognized for the trajectory of the linea terminalis. Specifically, the trajectory of the margin of the pelvic brim onto the sacral alae (the iliac portion of linea terminalis) could either be (1) upwards, (2) straight, or (3) downwards with respect to the arcuate line (Figure 1). Moreover, we recorded the location of the promontory, that is, that part of the spinal column that was most anteriorly protruding into the pelvic inlet, either sacral or lumbar, and the presence of a second promontory (Lierse, 1987). Associations between all variables, including sex, were investigated using χ2 and Cramérs V using SPSS (www.ibm.com).
FIGURE 1.
(a) Drawing of the lower spine and the pelvis in anterior view illustrating the spatial relationships between the iliac crest and the fourth and fifth lumbar vertebrae, the linea terminalis, which is composed of the arcuate line and the margin of the pelvic brim on the ala of the first sacral vertebra (marked as “ala margin”). (b) Visualization of the categorial relationship between the iliac crest and the fourth (L4) and fifth (L5) lumbar vertebrae, namely (1) center of L4, (2) inferior surface of L4, (3) superior surface of L5, and (4) center of L5. (c) The trajectories from the arcuate line on the hipbones to the margin of the pelvic brim of the sacral alae. The pelvic inlet thereby forms a flat circular plane (2) but can also be elongated, bent upwards (1), or curtailed and bent downwards (3)
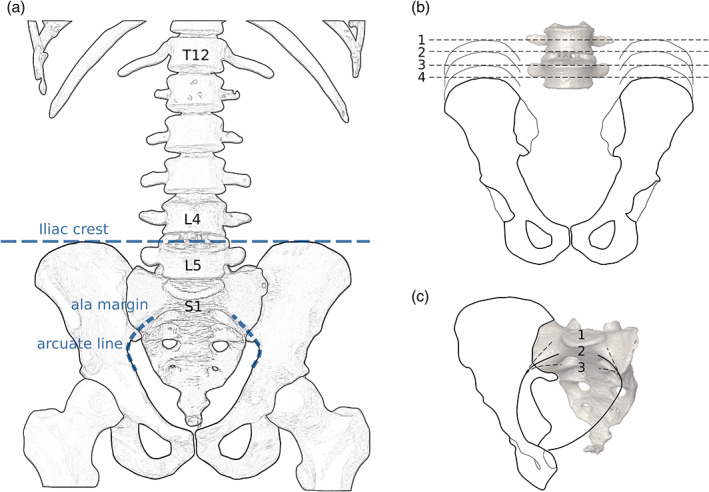
We also recorded aberrations at the sacrococcygeal level. The sacrococcygeal articulation is a cartilaginous joint reinforced by numerous sacrococcygeal ligaments. At the sacrococcygeal border, sacralizations of the coccyx or coccygealizations, that is, detachment of the fifth sacral vertebra, have rarely been investigated (Lee et al., 2016; Tague, 2011; Woon et al., 2013; Woon & Stringer, 2012). Nevertheless, sacralizations of the first coccygeal vertebra are quite common, either by synostosis or calcification of the lateral sacrococcygeal or the interarticular ligaments (sometimes also called ligamentum intercornuale, Woon et al., 2013). The caudalmost fused element of the sacrum was classified as sacral (S5) or coccygeal (Co1) according to Russo and Williams (2015). Thus, a vertebra was recognized as sacral when the sacral cornua were present and pointed caudally, and as coccygeal when caudally projecting cornua were absent and no ligaments attached inferiorly (Figure 2).
FIGURE 2.
Variation at the sacrococcygeal border based on CT generated 3D models of the human sacrum (depicted in gray) (a, b) ligaments of the sacrococcygeal joint, including the ligamentum sacrococcygeum laterale (dark blue), the lig. interarticulare (light blue), the lig. posterius superficiale (yellow) and the lig. posterius profundum (orange) in dorsal (a) and lateral (b) view. (c–j) Four exemplary specimens of the sacrococcygeal border in dorsal (c, e, g, i) and lateral (d, f, h, j) view. In (c–f), the cornua between the sacrum and the coccyx are not fused. In (g–j), the lateral and interarticular ligaments have calcified, leading to the sacralization of the first coccygeal vertebra
The shape of the sacrum was investigated with geometric morphometric techniques to identify morphological features associated with segmentation anomalies. Additionally, the relative position of the sacrum in relation to the iliac crests and the pelvic inlet was investigated to assess whether different types of segmentation anomalies were correlated with the relative craniocaudal position of the sacrum within the pelvic girdle. We tested eight different landmark sets to obtain the most suitable configuration to capture the morphological variation associated with the presence of transitional vertebrae. The landmark sets included 6–26 landmarks (LMs) on the sacrum and hip bones and up to 32 curve semilandmarks (sLMs) along the iliac crest and the linea terminalis that either represented the S1 (13 LMs), the entire sacrum (20 LMs, 4 sLMs), the pelvic inlet (6 LMs, 10 sLMs), or various combinations of these configurations representing the sacrum, the pelvic inlet, as well as the iliac crest (Figure 3, Table 2). To maintain homology within a sample containing both lumbarization and sacralizations for the 3D GM analyses, the first vertebra of the sacrum was landmarked, regardless of whether it was ontogenetically a “true” S1. However, we did not consider partially fused lumbar or detached sacral vertebrae (i.e., only Castellvi Typ IIIB lumbosacral transitional vertebrae were considered). We standardized the landmark sets with a generalized Procrustes analysis and analyzed the Procrustes shape coordinates via principal component analyses (PCA). The shape differences between the male and female group means as well as the mean shapes of different types of segmentation anomalies were tested employing a permutation test of the Procrustes distances with 10,000 random iterations.
FIGURE 3.
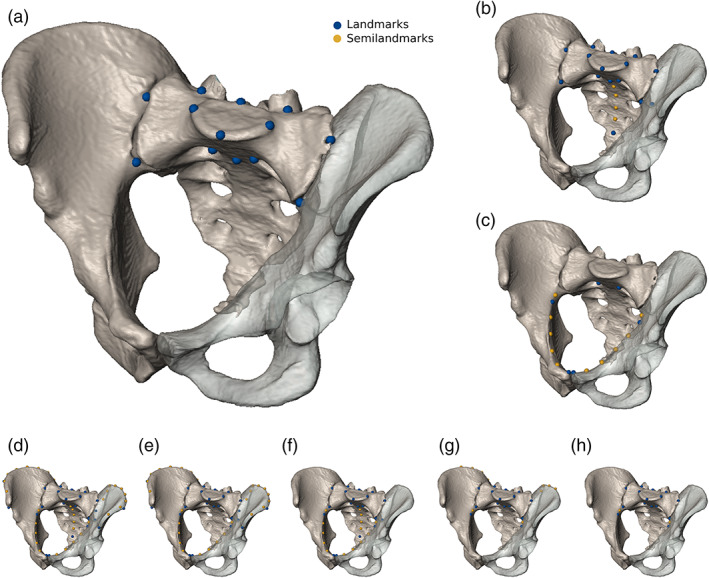
Landmarks and semilandmarks superimposed on a digital representation of the pelvis to show the configurations used in this study. Landmarks were placed on the sacrum and surrounding structures of the human hip bones, in eight different ways: (a) First sacral vertebra (S1). (b) Entire sacrum. (c) Pelvic inlet. (d) Sacrum, iliac crest, and pelvic inlet. (e) S1, iliac crest, and pelvic inlet. (f) Sacrum and pelvic inlet. (g) Proximate portion of the iliac crest and arcuate line in combination with the S1. (h) S1 and arcuate line. The size of the pelvic models correlates with the performance of the corresponding landmark configurations to detect segmentation anomalies at the lumbosacral border. Accordingly, a landmark configuration focusing on the first sacral vertebra was most informative, followed by a configuration representing the entire sacrum and a configuration covering the pelvic inlet. For a detailed description of landmarks see Table 2
TABLE 2.
Landmark definitions and configurations used
| Landmark definitions | Right | Midsagittal | Left | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Sacrum | Pelvic inlet | Inlet+ sacrum+crest | inlet+S1+ crest | Inlet+ sacrum | S1+ curve segments | S1+inlet segment | ||||
| Sacral promontorium | LM01 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | |||
| Posterior point of the superior sacral articular surface on the midsagittal plane | LM02 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | |||
| Most lateral point of the sacral articular surface | LM03 | LM04 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ||
| Midpoint at the root of the articular process, on the posterior aspect of the sacral base | LM05 | LM06 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ||
| Point at the corner of the first sacral foramen obtained at the intersection between the line passing for its highest point and that passing from its medial aspect | LM07 | LM08 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | |
| Point (on the linea transversa) between S1 and S2 on the midsagittal plane | LM09 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | |||
| The most caudal point of the ventral aspect of the sacrum on the midsagittal plane | LM10 | ☑ | ☑ | ☑ | |||||||
| Most cranial point of the auriculum (on the ilium) | LM11 | LM19 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ||
| The anteriormost point of the auriculum on the linea terminalis | LM12 | LM20 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ||
| The most lateral point of the pelvic inlet along the linea terminalis | LM13 | LM21 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | |||
| The most anterior superior point of the internal aspect of the pelvic inlet at the pubic symphysis (end of the linea terminalis) | LM14 | LM22 | ☑ | ☑ | ☑ | ☑ | |||||
| Posterior superior iliac spine | LM15 | LM23 | ☑ | ☑ | |||||||
| Posterior inferior iliac spine | LM16 | LM24 | ☑ | ☑ | ☑ | ☑ | |||||
| Anterior superior iliac spine | LM17 | LM25 | ☑ | ☑ | |||||||
| The most lateral point on the outer aspect of the iliac crest | LM18 | LM26 | ☑ | ☑ | |||||||
| Linea terminalis, curve semilandmarks | CV1 SLM1 | CV4 SLM1 | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | |||
| CV1 SLM2 | CV4 SLM2 | ☑ | ☑ | ☑ | ☑ | ||||||
| CV1 SLM3 | CV4 SLM3 | ☑ | ☑ | ☑ | ☑ | ||||||
| CV1 SLM4 | CV4 SLM4 | ☑ | ☑ | ☑ | ☑ | ||||||
| CV1 SLM5 | CV4 SLM5 | ☑ | ☑ | ☑ | ☑ | ||||||
| Midline of the iliac crest, curve semilandmarks | CV2 SLM1 | CV5 SLM1 | ☑ | ☑ | |||||||
| CV2 SLM2 | CV5 SLM2 | ☑ | ☑ | ☑ | |||||||
| CV2 SLM3 | CV5 SLM3 | ☑ | ☑ | ☑ | |||||||
| CV2 SLM4 | CV5 SLM4 | ☑ | ☑ | ☑ | |||||||
| CV2 SLM5 | CV5 SLM5 | ☑ | ☑ | ☑ | |||||||
| CV2 SLM6 | CV5 SLM6 | ☑ | ☑ | ||||||||
| CV2 SLM7 | CV5 SLM7 | ☑ | ☑ | ||||||||
| CV2 SLM8 | CV5 SLM8 | ☑ | ☑ | ||||||||
| CV2 SLM9 | CV5 SLM9 | ☑ | ☑ | ||||||||
| Midsagittal line of the sacrum, curve semilandmarks | CV3 SLM1 | ☑ | ☑ | ☑ | |||||||
| CV3 SLM2 | ☑ | ☑ | ☑ | ||||||||
| CV3 SLM3 | ☑ | ☑ | ☑ | ||||||||
| CV3 SLM4 | ☑ | ☑ | ☑ |
Note: A, B, C, E, F, G, and H refer to the configurations presented in Figure 3.
Abbreviations: CV SLM, curve semilandmark; LM, landmark; S1, first sacral vertebra.
3. RESULTS
Segmentation anomalies were present in 60 of the 145 individuals (41%, Figure 4). Eleven patients expressed changes at the thoracolumbar (TL) border, 21 at the lumbosacral (LS) border, and 46 at the sacrococcygeal (SC) junction (Figure 4). Sixteen of these individuals showed variations at multiple levels. In 18 individuals, rudimentary ribs were recorded, but only two individuals exhibited a unilateral thoracic and a contralateral lumbar rib. True numerical (meristic) aberrations due to additional or missing vertebrae were observed in 10 individuals (six additions, four subtractions), half of which in addition possessed transitional vertebrae. All other individuals showed border shifts that were compensated in other spinal regions. Of the 50 CTs that represented the whole spine, all had seven cervical vertebrae, and no transitional anomalies or cervical ribs were encountered at the cervicothoracic border. We found a higher frequency of segmentation anomalies within our male sample (50%) than in the female sample (33%), which was just above the level of significance. Males were more likely to express numeral aberration and multilevel border shifts. However, there was no difference in prevalence between the sexes for sacralizations and lumbarizations (p = 0.436).
FIGURE 4.
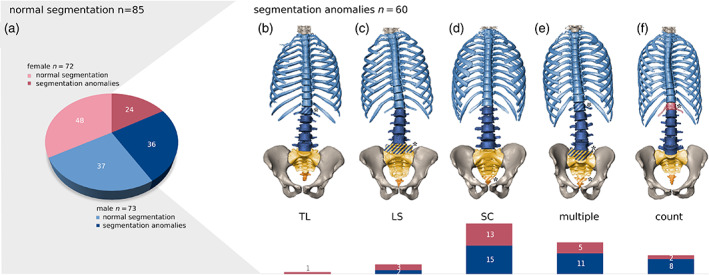
Typical segmentation anomalies. (a) Frequencies of various types of segmentation anomalies at different levels of the spine are shown as pie charts differentiated by sexes (blue, male; red, female). Typical configurations of the spine associated with segmentation anomalies at the (b) thoracolumbar (TL), (c) lumbosacral (LS), (d) sacrococcygeal (SC) level, (e) Multiple border shifts, and (f) true numerical (i.e., meristic) aberrations (count). Corresponding frequency bar charts are shown at the bottom. Out of 145 individuals, 85 possessed the typical spinal count of 12 thoracic (T), 5 lumbar (L), and 5 sacral (S) vertebrae and 60 expressed some type of segmentation anomaly (11–13 T, 4–6 L, and 4–6 S). Transitional vertebrae are marked by a diagonal patterning and an asterisk
Regarding the spatial relationship with the hip bones, for the majority of the individuals the iliac crest level varied from the inferior endplate of L4 to the superior endplate of L5, that is, they ranged between categories two and three (Figure 1, see also Figure 5). There was a significant association between the level of the iliac crest and the presence of segmentation anomalies (χ2 p < 0.05, Cramérs V = 0.302, see Table 3), as well as the level at which the border shifts occurred (χ2 p < 0.01, Cramérs V = 0.269), and particularly the presence of sacralizations and lumbarizations (χ2 p < 0.01, Cramérs V = 0.374). In individuals with coccyx sacralization and S1 lumbarizations, which are often coupled, the iliac crest was lower and corresponded with the center of L5. Contrastingly, L5 sacralizations were associated with a high position of the iliac crest at the center of L4. The level of the iliac crest also differed significantly between the sexes (p < 0.01). In most males, the iliac crest was in line with the L4, whereas in females, the iliac crest was level with L5. The age cohorts showed no significant difference.
FIGURE 5.
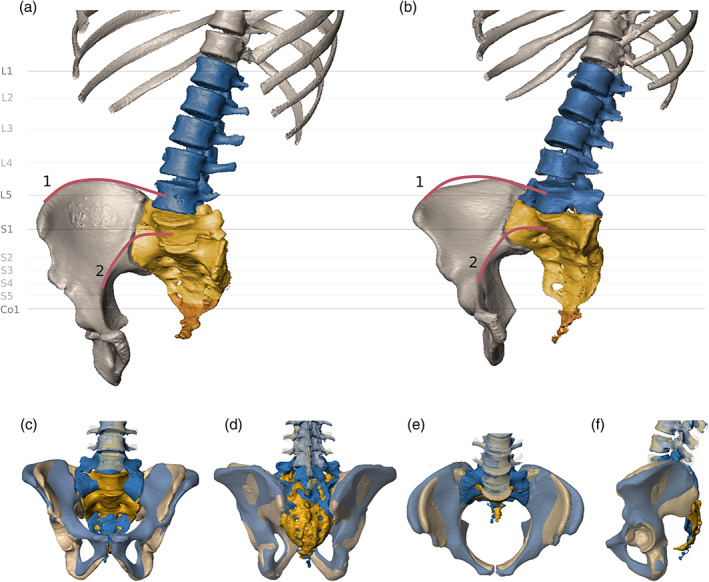
Direct comparison of segmentation anomalies. (a) Lumbarization of the first sacral vertebra and associated sacralization of the first coccygeal element. (b) Sacralization of the fifth lumbar vertebra. Blue, lumbar spine, yellow, sacral vertebrae, and orange, coccyx. The red lines indicate the trajectories of the iliac crest (1) and the linea terminalis (2). (c–f) Superimposition of the two specimens presented in (a) and (b), showing the morphological difference associated with the segmentation anomalies (yellow, lumbarization, blue, sacralization) in anterior (c), posterior (d), superior (e), and lateral (f) view
TABLE 3.
Cross tables and effect sizes for the association between the level of the iliac crest and the trajectory of the linea terminalis with respect to the presence of anomalies, anomaly type, and type of the lumbosacral border shift (numbers represent absolute numbers of specimens)
| Anomaly presence | Iliac crest level | Linea terminalis trajectory | ||||||
|---|---|---|---|---|---|---|---|---|
| L4 center | L4 lower surface | L5 upper surface | L5 center | Upwards | Linear | Downwards | Total | |
| Normal spine | 13 | 34 | 33 | 5 | 4 | 70 | 11 | 85 |
| Segmentation anomaly | 12 | 14 | 19 | 15 | 14 | 30 | 16 | 60 |
| Total | 25 | 48 | 52 | 20 | 18 | 100 | 27 | 145 |
| χ2 statistics and effect size | χ2 significance | Cramérs V | φ | χ2 significance | Cramérs V | φ | ||
| 0.004 | 0.302 | 0.302 | 0.000 | 0.359 | 0.359 | |||
| Anomaly type | Iliac crest level | Linea terminalis trajectory | ||||||
|---|---|---|---|---|---|---|---|---|
| L4 center | L4 lower surface | L5 upper surface | L5 center | Upwards | Linear | Downwards | Total | |
| Normal | 13 | 34 | 33 | 5 | 4 | 70 | 11 | 85 |
| TL | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 |
| LS | 2 | 0 | 1 | 2 | 1 | 0 | 4 | 5 |
| SC | 3 | 11 | 8 | 6 | 3 | 21 | 4 | 28 |
| Count | 4 | 2 | 3 | 1 | 2 | 5 | 3 | 10 |
| Multiple | 3 | 1 | 7 | 5 | 8 | 3 | 5 | 16 |
| Total | 25 | 48 | 52 | 20 | 18 | 100 | 27 | 145 |
| χ2 statistics and effect size | χ2 significance | Cramérs V | φ | χ2 significance | Cramérs V | φ | ||
| 0.008 | 0.269 | 0.465 | 0.000 | 0.414 | 0.585 | |||
| Type of lumbosacral border shift | Iliac crest level | Linea terminalis trajectory | ||||||
|---|---|---|---|---|---|---|---|---|
| L4 center | L4 lower surface | L5 upper surface | L5 center | Upwards | Linear | Downwards | Total | |
| Normal | 20 | 46 | 44 | 13 | 8 | 97 | 18 | 123 |
| Sacralization | 6 | 0 | 1 | 0 | 0 | 1 | 6 | 7 |
| Lumbarization | 0 | 2 | 7 | 7 | 10 | 2 | 4 | 16 |
| Total | 25 | 48 | 52 | 20 | 18 | 100 | 27 | 145 |
|
χ2 statistics and effect size |
χ2 significance | Cramérs V | φ | χ2 significance | Cramérs V | φ | ||
| 0.000 | 0.374 | 0.529 | 0.000 | 0.483 | 0.683 | |||
Abbreviations: L4, fourth lumbar vertebra; L5, fifth lumbar vertebra; LS, lumbosacral; SC, sacrococcygeal; TL, thoracolumbar (see Figure 4 for explanation).
The trajectory of the arcuate line with respect to the margin of the pelvic brim on the sacral alae was in most instances linearly pointing to the center of S1, thereby forming a flat pelvic inlet (Figure 1, see also Figure 5). A strong up‐ or downwards deviation from the linear trajectory of the arcuate line to the alar margin was often indicative of a border shift. The association between the trajectory and the presence of segmentation anomalies was significant and moderate (χ2 p < 0.01, Cramérs V = 0.359, see also Table 3), but became stronger when the level of the border shift (i.e., TL, LS, SC, count, multiple) was taken into account (χ2 p < 0.01, Cramérs V = 0.414) and strongest when distinguishing only between sacralizations and lumbarizations (χ2 p < 0.01, Cramérs V = 0.483). Sacralizations were associated with a downward deviation while lumbarizations had an upward trajectory. There was no significant difference between the sexes or age cohorts regarding the aforementioned linea terminalis features.
As expected, we also found a strong association between the level of the iliac crest and the trajectory from the arcuate line to the sacral margin (χ2 p < 0.01, Cramérs V = 0.433). A downward deviation from the arcuate line was more likely present when the iliac crest was at the level of L4, and an upward deviation occurred more frequently when it was level with L5.
In 46 individuals (32%), a sacralization was present at the sacrococcygeal border. In most instances, either the lateral processes of the last sacrum element and the coccyx or the interarticular cornua (or both) were fused. In 14 individuals (30% of the coccyx sacralizations), we observed only a one‐sided fusion of the transverse coccygeal process to the sacrum (resulting in an additional half sacral vertebra, e.g., 5.5 sacral vertebrae in total). In only one individual did we encounter a coccygealization, that is, a detachment of the last sacral element, which occurred in combination with a sacralization of the last lumbar vertebra (Figure 4e). Coccyx anomalies were moderately associated with both the level of the iliac crest and arcuate line trajectory (χ2 p < 0.05, Cramérs V > 0.3). They occurred more often with an iliac crest level at the L5 and an upward trajectory from the arcuate line. We also found a significant and moderate association between the presence of a coccyx sacralization and a lumbosacral transitional vertebra (χ2 p < 0.01, Cramérs V = 0.389).
Based on the spinal counts and the above‐mentioned spatial relationship between L4, L5, S1, Co1, the iliac crest, and the linea terminalis, 23 individuals (15.8%) showed signs of sacralization or lumbarization. Seven sacralizations (4.8%) and 16 lumbarizations (11.0%), with Castellvi classifications ranging between IA and IV (Table 4) were identified in the study. Two exemplary specimens are shown in Figure 5.
TABLE 4.
Frequencies of sacralizations and lumbarizations based on the categories according to Castellvi et al. (1984)
| Castellvi categories | Frequency |
|---|---|
| IA | 2 |
| IB | 1 |
| IIA | 3 |
| IIB | 3 |
| IIIA | 2 |
| IIIB | 11 |
| IV | 1 |
| Total | 23 |
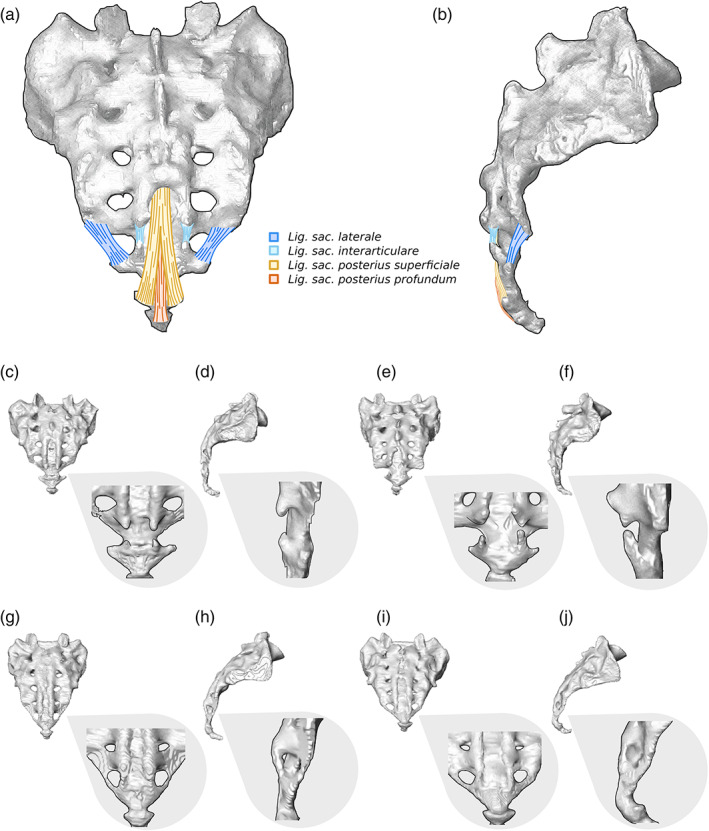
In the isolated sacrum, the presence of a lumbosacral transitional vertebra was morphologically reflected in two different ways (Figure 6; the corresponding 3D surface models can be found at this Zenodo link https://doi.org/10.5281/zenodo.5069789) (Krenn et al., 2021). In specimens where a lumbosacral transitional vertebra was detached or not fully incorporated into the sacrum, we observed a distinct morphology of the upper lateral angle of the cranial most fused sacral vertebra. It was characterized by the lateral expansion of the tip of the transverse process (the transverse process ontogenetically derives from the bone center of the neural arch, see Gray & Lewis, 1918), which results in smooth and elongated sacral alae without apparent indentation from the costal process. Thereby, the sacral base sits deeply between the sacral alae (Figures 6a and 7a–d). This morphological characteristic was similar in specimens with either sacralizations or lumbarizations. Nevertheless, sacralizations and lumbarizations were always distinguishable based on the number of fused sacral elements that followed the lumbosacral transitional vertebra. In specimens with sacralizations, five fused sacral elements followed, while in lumbarizations, either only four fused sacral elements followed, or if a fifth fused element was present, this last element was always a sacralized coccyx. Therefore, significant attention must be paid to the sacrococcygeal border as it has important implications for the distinction of sacralizations and lumbarizations. In two individuals that showed this morphology, the presacral spinal count did not support the presence of a complete lumbarization, and the ontogenetic identity of the last presacral vertebra remained indeterminate. Consequently, they were classified as numeric, that is, meristic aberrations missing one sacral element, although a coccygeal element was attached (Figure 7g).
FIGURE 6.
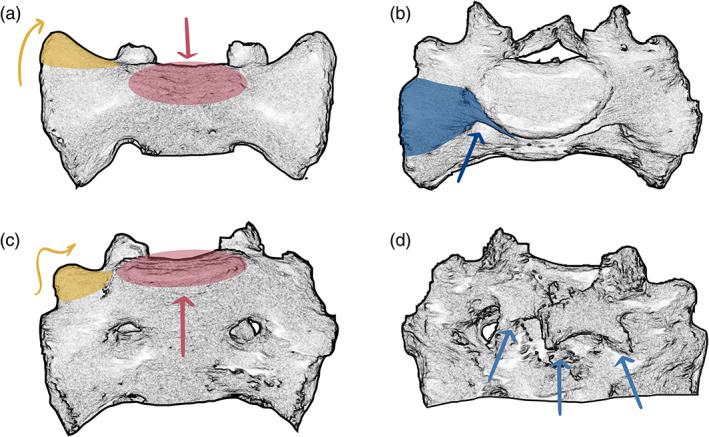
Morphological features in sacra with transitional vertebrae. (a) Typical morphology of the cranial most sacral element in frontal view, whose supra‐adjacent segment shows complete lumbarization. Note that this segmentation anomaly leads to smooth and elongated sacral alae without apparent indentation between the prominent tip of the transverse (yellow) and the costal process, while the sacral base (red) is set deep between the sacral alae. Extra joint facets (nearthroses) on the alae articulating with the costal process of the supra‐adjacent vertebra might be present. (b–d) Sacrum with partial lumbarization of S1. (b) Cranial view showing dorsal displacement of the first sacral costal process (blue) with respect to S2. (c) Frontal view showing the highly positioned sacral base with respect to the sacral alae and a well‐developed transverse process (yellow) that is set apart from the costal process (marked blue in b). (d) Dorsal view of the incomplete fusion of the first segment of the median crest
FIGURE 7.
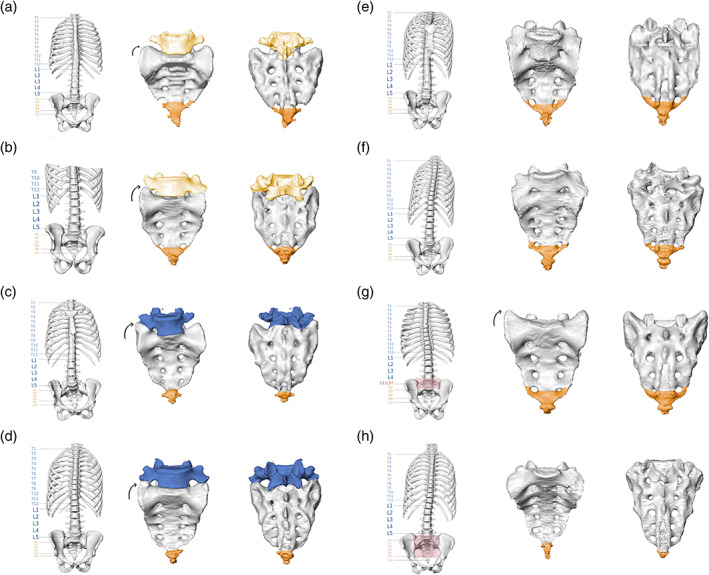
Typical morphological variants of lumbosacral and sacrococcygeal transitions in isolated sacra. Entire spine (left panels), isolated sacrum in ventral view (mid panels), and dorsal view (right panels). (a). Complete lumbarization with detachment of the first sacral element (S1; Castellvi Type IA) and partial sacralization of the coccyx. (b). Partial lumbarization of S1 with complete coccyx sacralization (Castellvi Type IV). (c) Partial sacralization of L5 (Castellvi Type IIIA). (d) Complete sacralization of L5 (Castellvi Type IIB). (e, f) Different stages of incomplete lumbarizations, which were always paired with coccyx sacralizations (Castellvi Type IIIB). Note that we did not find a morphologically corresponding case of lumbar sacralization. (g, h) Sacra without morphological evidence for a lumbosacral transitional vertebra, classified as numeric (meristic) aberrations. (g) Sacrum with four sacral elements and coccyx sacralization. (h) Sacrum with six sacral vertebrae and no indication of coccyx sacralization
The second type of morphological variation was a dorsal displacement of the costal process of the first sacral vertebra (Figure 6b). This was always coupled with a well‐developed transverse process whose tip was set apart from the tip of the costal process and a sacral base that was set high relative to the sacral alae (Figure 6c and 7e,f). In all individuals, this morphology was associated with a partial or complete coccyx sacralization. Therefore, these sacra included 5.5 or 6 elements. The spinal counts suggested that all these transitional vertebrae were ontogenetically derived from an S1. Accordingly, they represent partial lumbarizations rather than almost complete sacralizations. It should be noted that this expands on the traditional meaning of lumbarization because S1 does not morphologically resemble L5. The situation is better described as a partial or incomplete stage of lumbarization. Specifically, the first sacral element (ontogenetically corresponding to S1) is fully incorporated but has not entirely fused, which can also be recognized in the dorsal view (Figure 6d). In two individuals with an S6, no coccyx sacralization and no aberration of spinal counts in other regions could be detected, which were classified as additional sacral elements (Figure 7h).
Another morphological aspect that usually indicates a lumbosacral transitional vertebra is a second promontory within the sacrum. Thus, the longitudinal sacral curvature shows an angle between the first and second sacral elements. Such a second promontory was found in 13 specimens with lumbosacral transitional vertebra (60%). There was, however, no correlation with the type of the segmentation anomaly.
Our geometric morphometric analysis based on a landmark configuration using 13 fixed LMs on the first sacral vertebra (Figure 2a) was best suited to identify segmentation anomalies, forming discrete clusters encompassing the segmentation anomalies along PC1 in our PCA analyses (Figure 8). PC1 explained 47.8% of the total shape variation and was associated with a strong up‐ or downward inclination of the sacral alae relative to the sacral base. These features were discriminative of different types of border shifts. Individuals with initial stages of lumbarization or with an additional sacral element showed a strong downward trajectory of the alae (exemplary specimens are shown in Figure 7e,f,h), whereas individuals with complete lumbarization or a sacralized L5 clustered at the opposite sides of the axes, characterized by a low sacral base and smooth upward flaring of sacral alae (exemplary cases are depicted in Figure 7a–d,g). These shape changes corresponded to the qualitatively described morphological features in the section above. The group mean shapes of these two distinct morphologies, both indicative of segmentation anomalies, were significantly different from each other as well as from typical sacra without segmentation anomalies (p < 0.01). PC2 accounted for 17.1% of the total shape variation and reflected patterns traditionally attributed to sexual dimorphism, such as the ala to corpus ratio and coronal curvature. There was considerable overlap between the sexes as well as the age cohorts, although a combined sexual and ontogenetic trend was present from adult males at one end of the axis to subadult females on the opposing side, and the group mean shapes differed significantly (p < 0.01).
FIGURE 8.
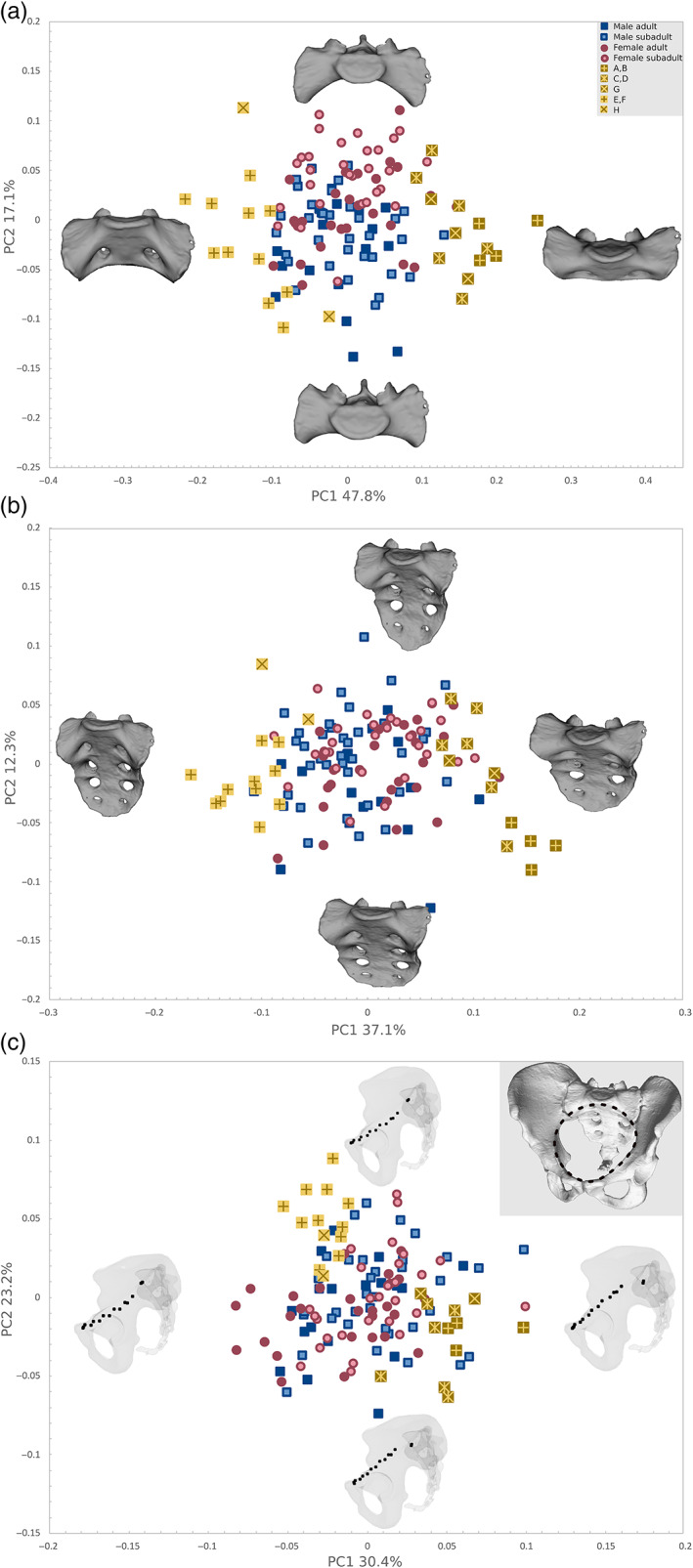
PCA plots of the Procrustes shape coordinates of the landmark configurations representing (a) the first sacral vertebra (S1), (b) the entire sacrum, and (c) the pelvic inlet. The thin‐plate‐spline warps represent the real shape variation at the extremes of the range of distribution along with the first two PCs. Note that the S1 configuration is best suited to distinguish segmentation anomalies along PC1, additionally showing a slight separation of males and females along PC2. (adult males, blue filled squares; subadult males, blue empty squares; adult females, red filled circles; subadult female, red empty circles; different shades of yellow squares correspond to sacral segmentation anomalies as represented in Figure 7: Dark yellow with cross, complete and partial lumbarization (a, b); dark yellow with star, partial and complete sacralization (c, d); dark yellow x, sacrum with four sacral elements (g); light yellow cross, different stages of incomplete lumbarizations (e, f); light yellow x, sacrum with six sacral elements (h). PCA, principal component analyses
The landmark configuration covering the entire sacrum also proved useful in distinguishing individuals with transitional vertebrae, reflecting similar shape changes in the first sacral vertebra but also changes in relative height, which was obviously influenced by the number of vertebrae composing the sacrum. This configuration was more susceptible to errors regarding the identification of coccyx sacralizations. In addition, the overlap between the sexes was more extensive. The pelvic inlet configuration was diagnostic as well, although it yielded a less clear separation, based on the relative position of the sacral body to the pelvic inlet. This reflected the deflection of the sacral alae with respect to the arcuate line. More comprehensive landmark configurations, especially the ones including the iliac crest, were the least informative (Figure S1).
4. DISCUSSION
The human spine can express a variety of segmentation anomalies that are particularly common in the caudal region of the vertebral column including the sacrum (e.g., Farshad‐Amacker et al., 2015; Konin & Walz, 2010; Le Double, 1912; Lian et al., 2018; Schmorl & Junghans, 1968; Wigh, 1980). These conditions have been extensively discussed with respect to potential clinical implications (e.g., Bron et al., 2007; Matson et al., 2020; Nardo et al., 2012; Peterson et al., 2005; Tini et al., 1977) or for their importance in hominin comparative morphology (Fornai et al., 2021; Haeusler, 2019; Haeusler et al., 2002, 2011, 2012; Latimer & Ward, 1993; Ogilvie et al., 1998; Robinson, 1972; Russo & Williams, 2015; Williams, 2012). Moreover, variation in spinal segmentation has been examined in the context of the evolution of the vertebral column in mammals and particularly primates (Haeusler et al., 2002; Machnicki & Reno, 2020; McCollum et al., 2010; Pilbeam, 2004; Schultz & Straus, 1945; Todd, 1922; Williams et al., 2016, 2019; Williams & Pilbeam, 2021). However, while consideration of the complete vertebral column is usually needed to differentiate sacralizations from lumbarizations and meristic changes, a reliable system for the assessment and classification of the morphological variation is still needed. This would aid the assessment of sacral elements found in isolation in archaeological settings, the morphological and taxonomic interpretation of fossil remains, as well as the possible exploration of their functional significance.
Our investigation focused primarily, but not exclusively, on the lumbosacral and sacrococcygeal borders. It confirms that aberrations and border shifts are more likely to occur in the lower spinal regions, mainly the lumbosacral and sacrococcygeal borders. When the whole spine is considered, the precaudal vertebrae number of 29 was remarkably stable. Based on the most parsimonious explanation, meristic changes, that is, additions or subtractions of individual vertebrae, were rarer than homeotic shifts, which were observed in 10 out of 145 specimens (7%; see also Kudlicki, 2019; Williams et al., 2019). Sacralizations and lumbarizations at the LS border were present in 23 out of 145 specimens (15.8%), which is within the published range of 4%–35% (Bron et al., 2007; Konin & Walz, 2010; Lian et al., 2018; Matson et al., 2020; Nastoulis et al., 2019). In this study, lumbarizations were more frequent than sacralizations (11% and 4.8%, respectively), contradicting previous works (e.g., Hughes & Saifuddin, 2006; Lee et al., 2007; Tins & Balain, 2016; see also Table 1). However, French et al. (2014) found a similar ratio in an extensive Australian sample (n = 5429).
A crucial aspect for the assessment of segmentation anomalies is the correct numbering of the vertebrae. In their reviews, Lian et al. (2018) and Konin and Walz (2010) emphasized the difficulties associated with the numbering of lumbar vertebrae. Our results showed that thoracolumbar transitional vertebrae are more frequent than previously recognized (10% in our sample, predominantly coupled with shifts in other spinal regions; see also Wigh, 1980; Farshad‐Amacker et al., 2015; Yun et al., 2018). Therefore, the use of lumbar radiographs only, or simple assumptions that every individual possesses 12 thoracic vertebrae, will impact the reported prevalence of sacralizations versus lumbarizations. Further, the high variability in the degree of expression of the segmentation anomalies often leads to difficulties in the interpretation of the anatomy, thereby affecting the accuracy of the classification. Castellvi et al. (1984) distinguished between true transitional characteristics (Castellvi Types II–IV) and transformed costal processes (Castellvi Type I). Some studies consider all Castellvi types (e.g., present study and Apazidis et al., 2011), others only Types II–IV (e.g., Benlidayi et al., 2015), still others include only specimens with complete bony fusion (Type III–IV; e.g., Dar & Peled, 2014). Thus, reported frequencies are highly dependent on the classification criteria and imaging technique utilized.
Our study of the sacrum and spine revealed that accurate characterization of the sacrum requires more than what can be inferred via basic vertebral element counts. The vertebral count alone cannot always reliably predict the presence of segmentation anomalies or explain the underlying etiology. Thus, sacra with six vertebral elements often result from a border shift between the sacrum and the coccyx rather than between the sacrum and the lumbar spine. In those specimens, the first coccygeal element is incorporated within the sacrum by complete fusion. In our sample, sacralizations of the coccyx were relatively common (32%) and frequently occurred in conjunction with lumbarizations, which is in accordance with previous findings (Derry, 1912; Mitchell, 1936; Tague, 2018). Therefore, attention must be paid not only to the first but also to the last sacral element, specifically the interarticular cornua, since such situations are otherwise not recognizable in isolated sacra. Alternatively, one could interpret such a morphology as a sacralization coupled with meristic changes. An additional sixth lumbar vertebra could be completely sacralized, and concomitantly one sacral element would either be missing or morphologically coccygealized. Yet, the morphological variation of the coccyx, the sacrococcygeal border, and the post‐sacral numeric variability have not yet been sufficiently explored to resolve this issue (Lee et al., 2016; Tague, 2011; Woon et al., 2013; Woon & Stringer, 2012). O'Connell (1951) and Andrew (1954) also described a similar morphological state as “occult sacralization,” which corresponds to a sacrum with six elements that are cranially placed relative to the inlet of the pelvis. As the number of thoracic vertebrae could not be assessed in their studies, they speculated that an additional border shift at the thoracolumbar level could have taken place. Thus, L1 would ontogenetically be a T12, and the transitional vertebra ontogenetically a L5, which does not correspond to what we observed based on our sample. This again highlights the importance of investigating the whole spine to verify the specific type of the segmentation anomaly. The occurrence of multiple meristic changes, in addition to homeotic shifts, cannot be ruled out but appears to be less likely based on inferences from complete spinal counts. In sum, the inconsistency and limitations of the classification approach easily account for the heterogeneity encountered in the literature regarding the frequency of sacralizations and lumbarizations.
Besides changes in the vertebral count, we found that the spatial relationship of the sacrum, and specifically of S1, to the iliac crest and the linea terminalis is crucial in identifying segmentation anomalies and for distinguishing between lumbarizations and sacralizations. Andrew (1954) already noted the significance of the iliac crest relative to the level of the lumbar vertebrae, which Farshad‐Amacker et al. (2015) termed the “iliac crest tangent sign.” Both studies found the same general patterns of the association between the lumbar vertebrae and the iliac crest to distinguish sacralizations from lumbarizations, and they suggested that this relationship can be used if assessing the whole spinal count is not possible. This spatial relationship might also reflect the position of the iliolumbar ligament, which mostly originates at L5 and can consequently aid the classification of segmentation anomalies in clinical contexts (Hughes & Saifuddin, 2004, 2006; Lee et al., 2007). In addition, statistical evaluation of the relationship between the arcuate line and S1 proved useful to distinguish between sacralizations and lumbarizations by observing whether its trajectory deviates upwards or downwards. Our GM analysis of the inlet reflected the relative height of the sacral base to the arcuate line, supporting our univariate statistical approach.
Further, two distinct morphological features were indicative of segmentation anomalies, that is, the shape of the lateral mass of S1 and the position of the sacral base relative to the sacral alae (Figure 6). The associated characteristic shapes could be captured already by PC1 of our geometric morphometric analysis of S1 (Figure 8), confirming that this approach can be used to macroscopically assess sacrum morphology and identify segmentation anomalies even in isolated sacra.
In our GM analysis of the S1, male and female mean shapes differed significantly, although the sexes overlapped extensively in the ordination plots. The sexes also had significantly different spatial relationships between iliac crest and L4/L5, which can probably be explained by the generally longer iliac blades in males so that the iliac crests are at the level of L4 rather than L5. Further, males were more likely to express segmentation anomalies, a result achieved also by Nardo et al. (2012). However, we found no significant difference in the prevalence of sacralizations and lumbarizations between males and females.
Many authors suggested that obstetric disadvantages might result from segmentation anomalies (Breus & Kolisko, 1904; Buttenberg, 1962; Diehl & Holmberg, 1968; Kirchhoff, 1949; Kirchhoff, 1958; Mahato, 2018; Müller, 1932; Nastoulis et al., 2019; Tague, 2018; Winter, 1953). The pelvic inlet dimensions, for example, the conjugate, the inlet circumference, and the transverse diameter, may therefore vary with changes in the relative position of the promontory, sacral breadth, height, and sagittal curvature. Moreover, sacrum orientation within the pelvis was said to be affected by the presence of lumbosacral transitional vertebrae (Benlidayi et al., 2015; Diehl & Holmberg, 1968; Mahato, 2018), whereas pelvic outlet dimensions are potentially reduced by sacrococcygeal fusion (Tague, 2011). Obstetric radiographic studies particularly suggested that lumbosacral transitional vertebrae impact pelvic dimensions due to a more cranially positioned promontorium, a double promontorium, or an associated flatter ventral curvature, thus increasing the risk of anomalous fetal head presentations at the inlet, hindering fetal rotations and leading to dystocia with the arrest of labor at the inlet or midplane (Breus & Kolisko, 1904; Buttenberg, 1962; Kirchhoff, 1949; Kirchhoff, 1958; Maurer & Post‐Amon, 1961; Winter, 1953). Contrastingly, Mahato (2018) hypothesized based on osteological considerations that metric changes associated with lumbarizations could interfere with the descent of the fetal head, whereas sacralizations may inhibit the dynamics of sacral nutation. Nonetheless, in the most recent obstetric‐radiological study of 430 births, Diehl and Holmberg (1968) found that assimilated pelves, that is, pelves that show additional sacral elements, do not represent risk factors for obstetric complications. In addition, the lack of a different prevalence of lumbosacral transitional vertebrae between the sexes and the low sexual dimorphism of the sacrum compared to the hip bones both support the notion that no significant obstetrical implications are associated with segmentation anomalies. This implies that biomechanical constraints may outweigh the obstetric needs due to the high sacroiliac joint mobility and the backwards nutation of the apex of the sacrum during the second stage of labor (Krenn et al., n.d.; Krenn, Fornai, Webb, & Haeusler, 2021).
The relative position of the sacrum has biomechanical implications for the stability of the sacroiliac joint (Andrew, 1954; Illeez et al., 2018; Vleeming et al., 2012). It is thus plausible that a certain configuration of the sacrum is needed for the attachment of the ligaments and muscles leading to the spine, the hipbone, and the lower limb. This might explain why sacra with less than five elements are extremely rare and why lumbarizations of the first sacral vertebra are often coupled with coccyx sacralizations. Impaired biomechanics associated with lumbosacral transitional vertebrae have also been implicated with disc degeneration, osteoarthritic changes, and low back pain (see, e.g., Bertolotti, 1917; Castellvi et al., 1984; Vergauwen et al., 1997; Peterson et al., 2005; Matson et al., 2020). However, the often inconclusive and ambiguous results of these studies might be related to inconsistent classification methods. Similarly, a detailed evaluation and accurate classification of lumbosacral and sacrococcygeal transitional vertebrae is imperative for studying the evolution of vertebral column segmentation (see, e.g., Haeusler et al., 2002; Russo & Williams, 2015). Our results will therefore potentially help to understand the clinical, biomechanical, and evolutionary significance of lumbosacral transitional vertebrae.
5. CONCLUSION
This study recommends assessing the entire human spine and the hip bones in anatomical articulation when analyzing the segmentation of the vertebral column. We found that the evaluation of the trajectory of the linea terminalis and the shape of the first sacral segment is correlated with segmentation anomalies. In combination with the spinal count, this allows to reliably differentiate between sacralizations and lumbarizations. Importantly, these classification methods are repeatable and also work for isolated sacra. This approach should therefore not only be used in a clinical context but it has also implications for archaeological and paleontological settings.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Viktoria A. Krenn: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); project administration (lead); resources (equal); software (lead); visualization (lead); writing – original draft (lead). Cinzia Fornai: Data curation (supporting); supervision (equal); validation (equal); writing – review and editing (equal). Nicole M. Webb: Supervision (equal); writing – review and editing (equal). Mirella A. Woodert: Data curation (equal). Helmut Prosch: Resources (lead). Martin Hausler: Conceptualization (supporting); funding acquisition (lead); project administration (supporting); resources (equal); supervision (lead); validation (equal); writing – review and editing (equal).
Supporting information
Figure S1. PCA plots of the Procrustes shape coordinates of the landmark configurations representing A. sacrum, iliac crest, and pelvic inlet. B. first sacral vertebra (S1), iliac crest, and pelvic inlet. C. sacrum and pelvic inlet. D. proximate portion of the iliac crest and arcuate line in combination with the S1. E. S1 and arcuate line. (Adult males = blue filled squares, subadult males = blue empty squares, adult females = red filled circles, subadult female = red empty circles, different shades of yellow squares correspond to sacral segmentation anomalies as represented in Figure 7: Dark yellow with cross, complete and partial lumbarization (A, B); dark yellow with star, partial and complete sacralization (C, D); dark yellow x, sacrum with four sacral elements (G); light yellow cross, different stages of incomplete lumbarizations (E, F); light yellow x, sacrum with six sacral elements (H))
ACKNOWLEDGMENT
We are very grateful to Louise Corron (Department of Anthropology, University of Nevada, Reno, USA) for providing access to the CT scans from the Hôpital de la Timone, Marseille. Open access funding provided by Universitat Zurich.
Krenn, V. A. , Fornai, C. , Webb, N. M. , Woodert, M. A. , Prosch, H. , & Haeusler, M. (2022). The morphological consequences of segmentation anomalies in the human sacrum. American Journal of Biological Anthropology, 177(4), 690–707. 10.1002/ajpa.24466
Funding information Swiss National Science Foundation, Grant/Award Number: 31003A_176319
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Andrew, J. (1954). Sacralization: An aetiological factor in lumbar intervertebral disk lesions, and a cause of misleading focal signs. The British Journal of Surgery, 42, 304–311. [DOI] [PubMed] [Google Scholar]
- Apazidis, A. , Ricart, P. A. , Diefenbach, C. M. , & Spivak, J. M. (2011). The prevalence of transitional vertebrae in the lumbar spine. The Spine Journal, 11, 858–862. [DOI] [PubMed] [Google Scholar]
- Barnes, E. (1994). Developmental defects of the axial skeleton in paleopathology. University Press of Colorado. [Google Scholar]
- Baur, G. (1891). On intercalation of vertebrae. Journal of Morphology, 4, 331–336. [Google Scholar]
- Benlidayi, I. C. , Coskun, N. C. , & Basaran, S. (2015). Does lumbosacral transitional vertebra have any influence on sacral tilt. Spine, 40, E1176–E1179. [DOI] [PubMed] [Google Scholar]
- Bertolotti, M. (1917). Contributo alla conoscenza dei vizi di differenziazione regionale del rachide con speciale riguardo all assimilazione sacrale della v. lombare. Radiologique Medica, 4, 113–144. [Google Scholar]
- Breus, C. , & Kolisko, A. (1904). Die Pathologischen Beckenformen. Deuticke. [Google Scholar]
- Bron, J. L. , van Royen, B. J. , & Wuisman, P. I. (2007). The clinical significance of lumbosacral transitional anomalies. Acta Orthopaedica Belgica, 73, 687–695. [PubMed] [Google Scholar]
- Bronn, H. G. (1874). Klassen und Ordnungen des Thier‐Reichs. Bd. 6: Säugethiere, bearb. von C.G. Giebel und W. Leche. Winter. [Google Scholar]
- Broome, D. , Hayman, L. , Herrick, R. , Braverman, R. M. , Glass, R. B. , & Fahr, L. M. (1998). Postnatal maturation of the sacrum and coccyx: MR imaging, helical CT, and conventional radiography. AJR American Journal of Roentgenology, 170, 1061–1066. [DOI] [PubMed] [Google Scholar]
- Buttenberg, D. (1962). Geburtsmechanische Betrachtungen am Kanalbecken. Archiv für Gynäkologie, 197, 172–207. [DOI] [PubMed] [Google Scholar]
- Cadeddu, J. A., Benson, J. E., Silver, R. I., Lakshmanan, Y., Jeffs, R. D., & Gearhart, J. P. (1997). Spinal abnormalities in classic bladder exstrophy. BJU International, 79(6), 975–978. 10.1046/j.1464-410x.1997.00190.x [DOI] [PubMed] [Google Scholar]
- Carapuço, M. , Nóvoa, A. , Bobola, N. , & Mallo, M. (2005). Hox genes specify vertebral types in the presomitic mesoderm. Genes & Development, 19, 2116–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, H. F. V. , Pereira, V. , & Rios, L. (2014). Chronology of fusion of the primary and secondary ossification centers in the human sacrum and age estimation in child and adolescent skeletons. American Journal of Physical Anthropology, 153, 214–225. [DOI] [PubMed] [Google Scholar]
- Castellvi, A. E. , Goldstein, L. A. , & Chan, D. P. (1984). Lumbosacral transitional vertebrae and their relationship with lumbar extradural defects. Spine, 9, 493–495. [DOI] [PubMed] [Google Scholar]
- Chaijaroonkhanarak, W. , Buranarugsa, M. , Umka, J. , & Namking, M. (2006). Sacralization of the 5th lumbar vertebra in Thais. Srinagarind Medical Journal, 21(3), 194–199. [Google Scholar]
- Corron, L. (2016). Juvenile age estimation in physical anthropology: A critical review of existing methods and the application of two standardised methodological approaches. Aix‐ Marseille Université. [Google Scholar]
- Dar, G. , & Peled, N. (2014). The association between sacralization and spondylolisthesis. Anatomical Science International, 89, 156–160. [DOI] [PubMed] [Google Scholar]
- Delport, E. G. , Cucuzzella, T. R. , Kim, N. , Marley, J. , Pruitt, C. , & Delport, A. G. (2006). Lumbosacral transitional vertebrae: incidence in a consecutive patient series. Pain Physician, 9(1), 53–56. [PubMed] [Google Scholar]
- Derry, D. E. (1912). The influence of sex on the position and composition of the human sacrum. Journal of Anatomy and Physiology, 46, 184. [PMC free article] [PubMed] [Google Scholar]
- Diehl, J. , & Holmberg, N. G. (1968). The assimilation pelvis—A radiological and obstetrical study: I. radiological part; II. Obsterical part. Acta Obstetricia et Gynecologica Scandinavica, 47, 5–91. [PubMed] [Google Scholar]
- Drew, R., & Kjellström, A. (2021). Sacralization in the Mary Rose and Kronan assemblages: An inconsistently recorded anomaly. International Journal of Osteoarchaeology, 31(5), 683–700. 10.1002/oa.2982 [DOI] [Google Scholar]
- Dürr, E. (1860). Über die Assimilation des letzten Bauchwirbels an das Kreuzbein. Zeitschrift für rationelle Medicin, 8, 185–200. [Google Scholar]
- Elster, A. D. (1973). Bertolotti's syndrome revisited. Transitional vertebrae of the lumbar spine. Spine, 14, 1373–1377. [PubMed] [Google Scholar]
- Erken, E. , Ozer, H. T. , Gulek, B. , & Durgun, B. (2002). The association between cervical rib and sacralization. Spine, 27(15), 1659–1664. [DOI] [PubMed] [Google Scholar]
- Farshad‐Amacker, N. A. , Aichmair, A. , Herzog, R. J. , & Farshad, M. (2015). Merits of different anatomical landmarks for correct numbering of the lumbar vertebrae in lumbosacral transitional anomalies. European Spine Journal, 24, 600–608. [DOI] [PubMed] [Google Scholar]
- Fornai, C. , Krenn, V. A. , Mitteroecker, P. , Webb, N. M. , & Haeusler, M. (2021). Sacrum morphology supports taxonomic heterogeneity of Australopithecus africanus at Sterkfontein member 4. Communications Biology, 4, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, H. D. , Somasundaram, A. J. , Schaefer, N. R. , & Laherty, R. W. (2014). Lumbosacral transitional vertebrae and its prevalence in the Australian population. Global Spine Journal, 4, 229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis, F. , van Dooren, T. , Feuth, J. D. , Metz, J. A. , Witkam, A. , Ruinard, S. , Steigenga, M. J. , & Wijnaendts, L. C. (2006). Extreme selection in humans against homeotic transformations of cervical vertebrae. Evolution, 60, 2643–2654. [PubMed] [Google Scholar]
- Gopalan, B., & Yerramshetty, J. S. (2018). Lumbosacral transitional vertebra‐related low back pain: Resolving the controversy. Asian Spine Journal, 12(3), 407–415. 10.4184/asj.2018.12.3.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, H. , & Lewis, W. H. (1918). Anatomy of the human body. Lea and Febiger. [Google Scholar]
- Haeusler, M. (2019). Spinal pathologies in fossil hominins. In Been E., Gómez‐Olivencia A., & Kramer P. A. (Eds.), Spinal evolution: Morphology, function, and pathology of the spine in hominoid evolution (pp. 213–245). Springer. [Google Scholar]
- Haeusler, M. , Martelli, S. , & Boeni, T. (2002). Vertebrae numbers of the early hominid lumbar spine. Journal of Human Evolution, 43, 621–643. [DOI] [PubMed] [Google Scholar]
- Haeusler, M. , Schiess, R. , & Boeni, T. (2011). New vertebral and rib material point to modern bauplan of the Nariokotome Homo erectus skeleton. Journal of Human Evolution, 61, 575–582. [DOI] [PubMed] [Google Scholar]
- Haeusler, M. , Schiess, R. , & Boeni, T. (2012). Modern or distinct axial bauplan in early hominins? A reply to Williams (2012). Journal of Human Evolution, 63, 557–559. [DOI] [PubMed] [Google Scholar]
- Hahn, P. Y. , Strobel, J. J. , & Hahn, F. J. (1992). Verification of lumbosacral segments on MR images: Identification of transitional vertebrae. Radiology, 182, 580–581. [DOI] [PubMed] [Google Scholar]
- Hald, H., Danz, B., Schwab, R., Burmeister, K., & Bähren, W. (1995). Röntgenologisch nachweisbare Wirbelsäulenveränderungen asymptomatischer junger Männer. RöFo ‐ Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren, 163(7), 4–9. 10.1055/s-2007-1015936 [DOI] [PubMed] [Google Scholar]
- Hsieh, C. Y. , Vanderford, J. D. , Moreau, S. R. , & Prong, T. (2000). Lumbosacral transitional segments: Classification, prevalence, and effect on disk height. Journal of Manipulative and Physiological Therapeutics, 23, 483–489. [DOI] [PubMed] [Google Scholar]
- Hughes, R. J. , & Saifuddin, A. (2004). Imaging of lumbosacral transitional vertebrae. Clinical Radiology, 59, 984–991. [DOI] [PubMed] [Google Scholar]
- Hughes, R. J. , & Saifuddin, A. (2006). Numbering of lumbosacral transitional vertebrae on MRI: Role of the iliolumbar ligaments. AJR. American Journal of Roentgenology, 187, W59–W65. [DOI] [PubMed] [Google Scholar]
- Illeez, O. G. , Atıcı, A. , Ulger, E. B. , Kulcu, D. G. , Ozkan, F. U. , & Aktas, I. (2018). The transitional vertebra and sacroiliac joint dysfunction association. European Spine Journal, 27, 187–193. [DOI] [PubMed] [Google Scholar]
- Keith, A. (1902). The extent to which the posterior segments of the body have been transmuted and suppressed in the evolution of man and allied primates. Journal of Anatomy and Physiology, 37, 18–40. [PMC free article] [PubMed] [Google Scholar]
- Kim, N. H. S. K. S. (1997). Kim, (1997). The role of transitional vertebrae in spondylolysis and spondylolytic spondylolisthesis. Bulletin Hospital for Joint Diseases, 56(3), 161–166. [PubMed] [Google Scholar]
- Kirchhoff, H. (1949). Das Lange Becken. Thieme. [Google Scholar]
- Kirchhoff, H. (1958). Zur Ätiologie und Diagnostik des “Hohen Gradstandes” [Aetiological and diagnostic aspects of “high longitudinal presentation”]. Deutsche Medizinische Wochenschrift, 83, 1651–1654. [DOI] [PubMed] [Google Scholar]
- Konin, G. P. , & Walz, D. M. (2010). Lumbosacral transitional vertebrae: Classification, imaging findings, and clinical relevance. AJNR. American Journal of Neuroradiology, 31, 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenn, V. A. , Fornai, C. , Webb, N. M. , & Haeusler, M. (2021). Sex determination accuracy in a Central European sample using the human sacrum. Anthropologischer Anzeiger, 79(2), 10.1127/anthranz/2021/1415 [DOI] [PubMed] [Google Scholar]
- Krenn, V. A. , Fornai, C. , Webb, N. M. , Woodert Mirella, A. , Helmut, P. , & Haeusler, M. (2021). Data from: Segmenation anomalies of the human sacrum. Zenodo. 10.5281/zenodo.5069789 [DOI]
- Krenn, V. A. , Fornai, C. , Webb, N. M. , & Haeusler, M. (n.d.). Sex classification using the human sacrum: Geometric morphometrics vs. conventional approaches. PLoS One. (in revision). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudlicki, A. (2019). Why a constant number of vertebrae? Digital control of segmental identity during vertebrate development. BioEssays, 42, 1900133. [DOI] [PubMed] [Google Scholar]
- Latimer, B. , & Ward, C. V. (1993). The thoracic and lumbar vertebrae. In Walker A. & Leakey R. (Eds.), The Nariokotome Homo erectus skeleton (pp. 266–293). Springer. [Google Scholar]
- Leboeuf, C. , Kimber, D. , & White, K. (1989). Prevalence of spondylolisthesis, transitional anomalies and low intercrestal line in a chiropractic patient population. Journal of Manipulative and Physiological Therapeutics, 12(3), 200–204. [PubMed] [Google Scholar]
- Le Double, A. F. (1912). Traité des variations de la colonne vertébrale de l'homme. Vigot Frères. [Google Scholar]
- Lee, C. H. , Park, C. M. , Kim, K. A. , Hong, S. J. , Seol, H. Y. , Kim, B. H. , & Kim, J. H. (2007). Identification and prediction of transitional vertebrae on imaging studies: Anatomical significance of paraspinal structures. Clinical Anatomy, 20, 905–914. [DOI] [PubMed] [Google Scholar]
- Lee, J. Y. , Gil, Y. C. , Shin, K. J. , Kim, J. N. , Joo, S. H. , Koh, K. S. , & Song, W. C. (2016). An anatomical and morphometric study of the coccyx using three‐dimensional reconstruction. Anat Rec, 299, 307–312. [DOI] [PubMed] [Google Scholar]
- Lian, J. , Levine, N. , & Cho, W. (2018). A review of lumbosacral transitional vertebrae and associated vertebral numeration. European Spine Journal, 27, 995–1004. [DOI] [PubMed] [Google Scholar]
- Lierse, W. (1987). Structural and functional anatomy of the pelvis. In Lierse W. (Ed.), Applied anatomy of the pelvis (pp. 3–45). Springer. [Google Scholar]
- Luoma, K., Vehmas, T., Raininko, R., Luukkonen, R., & Riihimäki, H. (2004). Lumbosacral Transitional Vertebra relation to disc degeneration and low back pain. Spine (Phila Pa 1976), 29(2), 200–205. 10.1097/01.brs.0000107223.02346.a8 [DOI] [PubMed] [Google Scholar]
- Machnicki, A. L. , & Reno, P. L. (2020). Great apes and humans evolved from a long‐backed ancestor. Journal of Human Evolution, 144, 102791. [DOI] [PubMed] [Google Scholar]
- Mahato, N. K. (2013). Redefining lumbosacral transitional vertebrae (LSTV) classification: Integrating the full spectrum of morphological alterations in a biomechanical continuum. Medical Hypotheses, 81, 76–81. [DOI] [PubMed] [Google Scholar]
- Mahato, N. K. (2010). Complete sacralization of L5 vertebrae: traits, dimensions, and load bearing in the involved sacra. The Spine Journal, 10(7), 610–615. 10.1016/j.spinee.2010.04.012 [DOI] [PubMed] [Google Scholar]
- Mahato, N. K. (2018). Transitional female sacrum: Dimensions, alterations in dorsal pelvic structure, and potential obstetric implications. Oman Medical Journal, 33, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo, M. , Wellik, D. M. , & Deschamps, J. (2010). Hox genes and regional patterning of the vertebrate body plan. Developmental Biology, 344, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson, D. M. , MacCormick, L. M. , Sembrano, J. N. , & Polly, D. W. (2020). Sacral dysmorphism and lumbosacral transitional vertebrae (LSTV) review. International Journal of Spine Surgery, 14, S14–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer, H.‐J. , & Post‐Amon, B. (1961). Zur Bedeutung des Assimilationsbeckens in der Geburtshilfe ‐ Radiologisch‐klinische Studie. Zeitschrift für Geburtshilfe und Gynäkologie, 157, 153–171. [PubMed] [Google Scholar]
- McCollum, M. A. , Rosenman, B. A. , Suwa, G. , Meindl, R. S. , & Lovejoy, C. O. (2010). The vertebral formula of the last common ancestor of African apes and humans. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 314, 123–134. [DOI] [PubMed] [Google Scholar]
- Mitchell, G. (1936). The significance of lumbosacral transitional vertebrae. British Journal of Surgery, 24, 147–158. [Google Scholar]
- Müller, J. , Scheyer, T. M. , Head, J. J. , Barrett, P. M. , Werneburg, I. , Ericson, P. G. P. , Pol, D. , & Sánchez‐Villagra, M. R. (2010). Homeotic effects, somitogenesis and the evolution of vertebral numbers in recent and fossil amniotes. Proceedings of the National Academy of Sciences of the United States of America, 107, 2118–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, W. (1932). Pathologische Physiologie der Wirbelsäule. Barth. [Google Scholar]
- Nardo, L. , Alizai, H. , Virayavanich, W. , Liu, F. , Hernandez, A. , Lynch, J. A. , Nevitt, M. C. , McCulloch, C. E. , Lane, N. E. , & Link, T. M. (2012). Lumbosacral transitional vertebrae: Association with low back pain. Radiology, 265, 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastoulis, E. , Karakasi, M. V. , Pavlidis, P. , Thomaidis, V. , & Fiska, A. (2019). Anatomy and clinical significance of sacral variations: A systematic review. Folia Morphologica, 78, 651–667. [DOI] [PubMed] [Google Scholar]
- O'Connell, J. E. (1951). Protrusions of the lumbar intervertebral discs. The Journal of Bone and Joint Surgery British, 33, 8–30. [DOI] [PubMed] [Google Scholar]
- O'Driscoll, C. , Irwin, A. , & Saifuddin, A. (1996). Variations in morphology of the lumbosacral junction on sagittal MRI: Correlation with plain radiography. Skeletal Radiology, 25, 225–230. [DOI] [PubMed] [Google Scholar]
- Ogilvie, M. D. , Hilton, C. E. , & Ogilvie, C. D. (1998). Lumbar anomalies in the Shanidar 3 Neandertal. Journal of Human Evolution, 35, 597–610. [DOI] [PubMed] [Google Scholar]
- Otani, K. , Konno, S. , & Kikuchi, S. (2001). Lumbosacral transitional vertebrae and nerve‐root symptoms. The Journal of Bone and Joint Surgery. British volume, 83(8), 1137–1140. [DOI] [PubMed] [Google Scholar]
- Paterson, A. M. (1892). The human sacrum. Proceedings of the Royal Society of London, 51, 520–524. [Google Scholar]
- Peterson, C. K. , Bolton, J. , Hsu, W. , & Wood, A. (2005). A cross‐sectional study comparing pain and disability levels in patients with low back pain with and without transitional lumbosacral vertebrae. Journal of Manipulative and Physiological Therapeutics, 28, 570–574. [DOI] [PubMed] [Google Scholar]
- Peh, W. C. , Siu, T. , & Chan, J. H. (1999). Determining the lumbar vertebral segments on magnetic resonance imaging. Spine, 24(17), 1852. [DOI] [PubMed] [Google Scholar]
- Pilbeam, D. (2004). The anthropoid postcranial axial skeleton: Comments on development, variation, and evolution. Journal of Experimental Zoology, 302B, 241–267. [DOI] [PubMed] [Google Scholar]
- Pilbeam, D. , & Young, N. (2004). Hominoid evolution: Synthesizing disparate data. Comptes Rendus Palevol, 3, 305–321. [Google Scholar]
- Quinlan, J. , Duke, D. , & Eustace, S. (2006). Bertolotti’s syndrome: a cause of back pain in young people. The Journal of Bone and Joint Surgery. British volume, 88(9), 1183–1186. [DOI] [PubMed] [Google Scholar]
- Ralston, M. , Dykes, T. , & Applebaum, B. (1992). Verification of lumbar vertebral bodies. Radiology, 185, 615–616. [DOI] [PubMed] [Google Scholar]
- Robinson, J. T. (1972). Early hominid posture and locomotion. University of Chicago Press. [Google Scholar]
- Rosenberg, E. (1875). Über die Entwicklung der Wirbelsäule und das Centrale carpi des Menschen. Morphologisches Jahrbuch, 1, 83–198. [Google Scholar]
- Rosenberg, E. (1899). Über eine primitive Form der Wirbelsäule des Menschen. Morphologisches Jahrbuch, 27, 1–118. [Google Scholar]
- Russo, G. A. , & Williams, S. A. (2015). “Lucy” (A.L. 288‐1) had five sacral vertebrae. American Journal of Physical Anthropology, 156, 295–303. [DOI] [PubMed] [Google Scholar]
- Santiago, F. , Milena, G. , Herrera, R. , Romero, P. , & Plazas, P. (2001). Morphometry of the lower lumbar vertebrae in patients with and without low back pain. European Spine Journal, 10(3), 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmorl, G. , & Junghans, H. (1968). Die gesunde und die kranke Wirbelsäule in Röntgenbild und Klinik. Thieme. (English translation: Schmorl G, Junghans H. (1971). The human spine in health and disease. Grune & Stratton). [Google Scholar]
- Schultz, A. H. , & Straus, W. L. (1945). The numbers of vertebrae in primates. Proceedings of the American Philosophical Society, 89, 601–626. [PubMed] [Google Scholar]
- Steinberg, E. L, Luger, E., Arbel, R., Menachem, A., & Dekel, S. (2003). A comparative roentgenographic analysis of the lumbar spine in male army recruits with and without lower back pain. Clinical Radiology, 58(12), 985–989. 10.1016/s0009-9260(03)00296-4 [DOI] [PubMed] [Google Scholar]
- Tague, R. G. (2009). High assimilation of the sacrum in a sample of American skeletons: Prevalence, pelvic size, and obstetrical and evolutionary implications. American Journal of Physical Anthropology, 138, 429–438. [DOI] [PubMed] [Google Scholar]
- Tague, R. G. (2011). Fusion of coccyx to sacrum in humans: Prevalence, correlates, and effect on pelvic size, with obstetrical and evolutionary implications. American Journal of Physical Anthropology, 145, 426–437. [DOI] [PubMed] [Google Scholar]
- Tague, R. G. (2018). Proximate cause, anatomical correlates, and obstetrical implication of a supernumerary lumbar vertebra in humans. American Journal of Physical Anthropology, 165, 444–456. [DOI] [PubMed] [Google Scholar]
- Tang, M., Yang, X.‐f., Yang, S.‐w., Han, P., Ma, Y.‐m., Yu, H., & Zhu, B. (2014). Lumbosacral transitional vertebra in a population‐based study of 5860 individuals: Prevalence and relationship to low back pain. European Journal of Radiology, 83(9), 1679–1682. 10.1016/j.ejrad.2014.05.036 [DOI] [PubMed] [Google Scholar]
- Taskaynatan, M. A., Izci, Y., Ozgul, A., Hazneci, B., Dursun, H., & Kalyon, T. A. (2005). Clinical significance of congenital lumbosacral malformations in young male population with prolonged low back pain. Spine, 30(8), E210–E213. 10.1097/01.brs.0000158950.84470.2a [DOI] [PubMed] [Google Scholar]
- ten Broek, C. M. , Bakker, A. J. , Varela‐Lasheras, I. , Bugiani, M. , van Dongen, S. , & Galis, F. (2012). Evo‐devo of the human vertebral column: On homeotic transformations, pathologies and prenatal selection. Evolutionary Biology, 39, 456–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tini, P. G. , Wieser, C. , & Zinn, W. M. (1977). The transitional vertebra of the lumbosacral spine: Its radiological classification, incidence, prevalence, and clinical significance. Rheumatology & Rehabilitation, 16, 180–185. [DOI] [PubMed] [Google Scholar]
- Tins, B. J. , & Balain, B. (2016). Incidence of numerical variants and transitional lumbosacral vertebrae on whole‐spine MRI. Insights Into Imaging, 7, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, T. W. (1922). Numerical significance in the thoracicolumbar vertebrae of the mammalia. Anatomical Records, 24, 261–286. [Google Scholar]
- Töndury, G. , & Theiler, K. (1990). Entwicklungsgeschichte und Fehlbildungen der Wirbelsäule. Hippokrates‐Verlag. [Google Scholar]
- Uçar, D., Uçar, B. Y., Coşar, Y., Emrem, K., Gümüşsuyu, G., Mutlu, S., Mutlu, B., Çaçan, M. A., Mertsoy, Y., & Gümüş, H. (2013). Retrospective cohort study of the prevalence of lumbosacral transitional vertebra in a wide and well‐represented population. Arthritis, 2013, 1–5. 10.1155/2013/461425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergauwen, S. , Parizel, P. M. , van Breusegem, L. , van Goethem, J. W. , Nackaerts, Y. , van den Hauwe, L. , & de Schepper, A. M. (1997). Distribution and incidence of degenerative spine changes in patients with a lumbo‐sacral transitional vertebra. European Spine Journal, 6, 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleeming, A. , Schuenke, M. D. , Masi, A. T. , Carreiro, J. E. , Danneels, L. , & Willard, F. H. (2012). The sacroiliac joint: An overview of its anatomy, function and potential clinical implications. Journal of Anatomy, 221, 537–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ihering, H. (1878). Das peripherische Nervensystem der Wirbelthiere als Grundlage für die Kenntnis der Regionenbildung der Wirbelsäule. Vogel. [Google Scholar]
- Wellik, D. M. , & Capecchi, M. R. (2003). Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science, 301, 363–367. [DOI] [PubMed] [Google Scholar]
- Wigh, R. E. (1980). The thoracolumbar and lumbosacral transitional junctions. Spine, 5, 215–222. [DOI] [PubMed] [Google Scholar]
- Williams, S. A. (2012). Modern or distinct axial bauplan in early hominins? Comments on Haeusler et al. (2011). Journal of Human Evolution, 63, 552–556. [DOI] [PubMed] [Google Scholar]
- Williams, S. A. , Gómez‐Olivencia, A. , & Pilbeam, D. R. (2019). Numbers of vertebrae in hominoid eolution. In Been E., Gómez‐Olivencia A., & Ann Kramer P. (Eds.), Spinal evolution: Morphology, function, and pathology of the spine in hominoid evolution (pp. 97–124). Springer. [Google Scholar]
- Williams, S. A. , Middleton, E. R. , Villamil, C. I. , & Shattuck, M. R. (2016). Vertebral numbers and human evolution. American Journal of Physical Anthropology, 159, S19–S36. [DOI] [PubMed] [Google Scholar]
- Williams, S. A. , & Pilbeam, D. (2021). Homeotic change in segment identity derives the human vertebral formula from a chimpanzee‐like one. American Journal of Physical Anthropology, 176, 283–294. [DOI] [PubMed] [Google Scholar]
- Williams, S. A. , & Russo, G. A. (2015). Evolution of the hominoid vertebral column: The long and the short of it. Evolutionary Anthropology: Issues, News, and Reviews, 24, 15–32. [DOI] [PubMed] [Google Scholar]
- Winter, H. (1953). Über das “Lange Becken” und seine geburtshilfliche Bedeutung. Deutsche Medizinische Wochenschrift, 78, 68–71. [DOI] [PubMed] [Google Scholar]
- Woon, J. T. , Perumal, V. , Maigne, J. Y. , & Stringer, M. D. (2013). CT morphology and morphometry of the normal adult coccyx. European Spine Journal, 22, 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon, J. T. K. , & Stringer, M. D. (2012). Clinical anatomy of the coccyx: A systematic review. Clinical Anatomy, 25, 158–167. [DOI] [PubMed] [Google Scholar]
- Yun, S. , Park, S. , Park, J. G. , Huh, J. D. , Shin, Y. G. , & Yun, J. H. (2018). Spinal enumeration by morphologic analysis of spinal variants: Comparison to counting in a cranial‐to‐caudal manner. Korean Journal of Radiology, 19, 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman, S. (1938). Observations on the autonomic nervous system and on vertebral and neural segmentation in monkeys. Transactions of the Zoological Society of London, 23, 315–378. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PCA plots of the Procrustes shape coordinates of the landmark configurations representing A. sacrum, iliac crest, and pelvic inlet. B. first sacral vertebra (S1), iliac crest, and pelvic inlet. C. sacrum and pelvic inlet. D. proximate portion of the iliac crest and arcuate line in combination with the S1. E. S1 and arcuate line. (Adult males = blue filled squares, subadult males = blue empty squares, adult females = red filled circles, subadult female = red empty circles, different shades of yellow squares correspond to sacral segmentation anomalies as represented in Figure 7: Dark yellow with cross, complete and partial lumbarization (A, B); dark yellow with star, partial and complete sacralization (C, D); dark yellow x, sacrum with four sacral elements (G); light yellow cross, different stages of incomplete lumbarizations (E, F); light yellow x, sacrum with six sacral elements (H))
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


