Abstract
Background
The objective of this study was to investigate the role and molecular mechanism of cyclin‐dependent kinase 5 (CDK5) in regulating the growth of tongue squamous cell carcinoma (TSCC).
Methods
The authors used multiple methods to detect the levels of CDK5 expression in samples of TSCC and to explore the relation between CDK5 expression and various clinicopathologic factors. In vivo and in vitro cell experiments were performed to detect the proliferation, invasion, and migration of TSCC cells with CDK5 knockdown or overexpression. These studies verified that CDK5 regulates the occurrence and development of TSCC cells through the microRNA 513c‐5p/cell division cycle 25B pathway.
Results
An elevated level of CDK5 expression in TSCC tissues was identified as an independent risk factor affecting TSCC growth and patient prognosis. Patients who had TSCC with low levels of CDK5 expression had a higher survival rate than those with high levels. Knockdown of CDK5 reduced the proliferation, migration, and invasion of TSCC cells both in vitro and in vivo. In addition, the authors observed that CDK5 regulated the growth of TSCC through the microRNA 513c‐5p/cell division cycle C25B pathway.
Conclusions
CDK5 functions as an oncogene in TSCC and might serve as a molecular marker for use in the diagnosis and treatment of TSCC.
Lay Summary
Tongue squamous cell carcinoma (TSCC) is 1 of the most common malignant tumors of the head and neck, and the survival rate of patients with tongue cancer has been very low.
Therefore, it is important to study the molecular mechanism of TSCC progression to identify biomarkers that can be used to improve its clinical diagnosis and treatment.
Cyclin‐dependent kinase 5 (CDK5) is an atypical member of the cyclin‐dependent kinase family and is involved in regulating the cell cycle.
Changes in the cell cycle are of great significance for the occurrence and development of tumor cells; and, in recent years, increasing evidence has suggested that CDK5 exists in a disordered state in cancer cells.
In this study, the authors demonstrate that CDK5 functions as an oncogene in TSCC and might serve as a molecular marker for use in the diagnosis and treatment of TSCC.
Keywords: cell division cycle 25 B (CDC25B), cyclin‐dependent kinase 5 (CDK5), microRNA 513c‐5p (miR513c‐5p), tongue cancer
Short abstract
The role of cyclin‐dependent kinase 5 and its molecular mechanism in regulating the growth of tongue squamous cell carcinoma (TSCC) are investigated. The results indicate that cyclin‐dependent kinase 5 is involved in the occurrence and development of TSCC and could possibly serve as a new prognostic marker and molecular target for treating TSCC.
Introduction
Tongue squamous cell carcinoma (TSCC) is 1 of the most frequently diagnosed malignant tumors of the head and neck. 1 Although the technology used to diagnose and treat TSCC has continuously improved in recent years, the 5‐year survival rate of patients with TSCC has stagnated at 50%. 2 , 3 Therefore, it is important to identify biomarkers for TSCC and to find new therapeutic targets that might improve the effectiveness of clinical treatment. Cyclin‐dependent kinase 5 (CDK5) is an atypical member of the cyclin‐dependent kinase (CDK) family and is involved in regulating the cell cycle. The nucleoplasmic localization of CDK5 is very important for its many pathologic and physiologic functions. 4 , 5 , 6 , 7 Previous studies have demonstrated that an increased level of CDK5 target protein is a specific biomarker for cancer and is essential for regulating the growth and migration of tumor cells. 8 , 9 , 10 , 11 , 12 , 13 There are 3 members of the cell division cycle 25 (CDC25) protein family, namely, CDC25A, CDC25B, and CDC25C, all of which are directly or indirectly involved in the transition of cells in G1/S, S, and G2/M phases and in controlling mitosis. 14 , 15 , 16 MicroRNA 513c‐5p (miR513c‐5p) is a newly discovered microRNA (miRNA) that is associated with tumorigenesis and development. However, its clinical significance and mechanism of action in head and neck tumors remain unclear. 17
We examined the levels of CDK5 expression in TSCC tissues and cell lines and studied its effects on cell proliferation in vivo and in vitro; we also conducted a preliminary exploration of its possible pathways of action. Our results indicate that CDK5 is involved in the occurrence and development of TSCC and could possibly serve as a new prognostic marker and molecular target for treating TSCC.
Materials and Methods
Oncomine Database Analysis
Oncomine is currently the world's largest oncogene chip database and integrated data mining platform. We searched the Oncomine database for information regarding CDK5 expression patterns in TSCC.
Patient Data and Tissue Specimens
Fresh samples of TSCC and adjacent normal tissue (n = 8) were collected from patients undergoing tongue cancer surgery in Sun Yat‐sen University Cancer Center. In total, 136 surgical specimens were obtained. According to the International Union Against Cancer classification, all medical records obtained had a diagnosis of cancer of the anterior two‐thirds of the tongue (tongue body), which is oral cancer. Cancer staging was performed according to the American Joint Commission on Cancer seventh edition tumor, node, metastasis (TNM) staging system.
Immunohistochemistry
All staining results were independently evaluated by 2 pathologists under double‐blind conditions. The pathologists used a semiquantitative method that combined staining intensity and staining range to evaluate the results. The staining intensity and staining range scores of each tissue section were multiplied, and the final score was obtained. A total score ≤6 was considered to be low expression of CDK5, and a total score >6 was considered to be high expression of CDK5.
Cell Culture
A normal oral mucosal epithelial cell line (NOK) and 4 human TSCC cell lines (SCC9, SCC15, SCC25, and Cal27) were used in this study. The cancer cell culture medium was high‐sugar Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Normal epithelial cells were cultured in minimal essential medium supplemented with 15% fetal bovine serum. All cell lines were cultured in a humidified 5% CO2 incubator at 37 °C.
Western Blot Analysis
Each sample was separated by electrophoresis performed on gel, and the protein bands were transferred onto a polyvinylidene difluoride membrane. Next, the membrane was sequentially incubated with specific antibodies, and the immunostained protein bands were detected using an enhanced chemiluminescence method.
RNA Extraction and Quantitative Reverse Transcriptase‐Polymerase Chain Reaction Analysis
An RNA Quick Purification kit was used to extract the total RNA from cells and tissues, and a Fast Reverse Transcription kit was used to generate endogenous complementary DNA. Each quantitative reverse transcriptase‐polymerase chain reaction (PCR) analysis was performed in triplicate and using 2x Super SYBR Green qPCR Master Mix reagent. Gene expression was calculated using the 2−△△Ct method, and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) served as an internal standard.
Development of Stable Cell Lines and Transfection Experiments With Small Interfering RNAs
The viral vectors used were pLVX‐C‐FLAG‐mCMV‐ZsGreen‐IRES‐Puro and pLVX‐shRNA2‐Puro, both of which carry ZsGreen fluorescence and puro (puromycin) resistance gene markers. A lentivirus with a final virus titer of 1 × 108 transducing units per milliliter was isolated and transfected into SCC25, Cal27, SCC15, and SCC9 cells. Stable cells were identified by screening with puromycin at a concentration of 2 μg/mL for 2 weeks. SCC25‐short hairpin CDK5 (shCDK5) and Cal27‐shCDK5 cells with stable, low expression of CDK5 were transfected with small interfering CDC25B (si‐CDC25B)/si‐negative control (si‐NC) and miR513c‐5p mimics/miR513c‐5p‐NC according to instructions.
Cholecystokinin‐8 Assay for the Detection of Cell Proliferation
Five 96‐well plates were seeded with suspended cells (100 μL per well), and 1 plate was removed every 12 hours for measuring cell proliferation. Cholecystokinin‐8 (CCK8) solution (10 μL) was added to each well and thoroughly mixed. Next, the absorbance of each well at 450 nm was read with a microplate reader. After recording the optical density measurements, 5 time points were used to create a cell growth curve.
Transwell Cell Migration Assay
Complete culture medium containing 20% fetal bovine serum was added to each lower chamber of a 24‐well Transwell plate, and 150 to 200 µL of prepared cell suspension (5 × 105 cells/mL) was added to each upper chamber. After 24 hours of incubation, the nonmigrated cells in the chamber were removed with a cotton swab, and the migrated cells on the bottom of the membrane were fixed with methanol and stained with crystal violet. The numbers of migrated cells in 5 randomly selected high‐power fields were counted under a microscope.
Clone‐Formation Studies
Cells were inoculated into the wells of a 6‐well plate (500 cells per well), which was then incubated for 3 weeks. Next, the cell colonies in the plate were washed with phosphate‐buffered saline (PBS) and then fixed with methanol for 60 minutes; after that, they were stained with crystal violet solution for 30 minutes and washed with PBS using the following formula: clone formation rate = cell inoculation survival rate = (the number of clones/the number of inoculated cells) × 100%.
Cell Cycle Analysis
Cells were digested, washed, and then resuspended in PBS at a concentration of 1 × 106 cells/mL. Next, 100 µL of the cell suspension was slowly added in a dropwise manner to −20 °C frozen alcohol, and the ruptured cells were frozen and fixed overnight. The ruptured cells were then centrifuged. After removing the supernatant, the cells were suspended in PBS and centrifuged. Finally, the cells were treated with propidium iodide/RNase, and the cell cycles were analyzed.
Subcutaneous Tumor Formation in Nude Mice
Female Balb/c‐nu nude mice (6‐7 weeks old; 18‐22 g) were housed in the Animal Experimental Center of Sun Yat‐sen University Cancer Center (specific pathogen free level). SCC25 cells (short hairpin [sh]NC and shCDK5 cells) and overexpressing (OE) SCC15 cells (OE‐NC and OE‐CDK5 cells) were subcutaneously injected into the lower abdomen of mice (1 × 106 cells per mouse). Four weeks later, the mice were killed, and their tumors were measured and analyzed. Tumor volume was calculated as follows: tumor volume = one‐half length × width squared.
Statistical Analysis
All statistical results were calculated using the software package IBM Statistics for Windows, version 23, and a P value < .05 was considered to be statistically significant.
Results
CDK5 Expression Was Up‐Regulated in TSCC Tissue
A bioinformatics analysis performed using the Oncomine database showed that the levels of CDK5 miRNA expression in TSCC tissues were significantly higher than those in normal tissues (Fig. 1A‐C). 18 , 19 , 20 The results of Western blot analysis (Fig. 1D) and PCR (Fig. 1E) studies indicated that the levels of CDK5 protein and miRNA in 8 pairs of TSCC tissues were significantly higher than those in normal tissues (P < .05). An immunohistochemical analysis of the TSCC sections confirmed that CDK5 expression increased in conjunction with the tumor's clinical stage (Fig. 1G).
Figure 1.
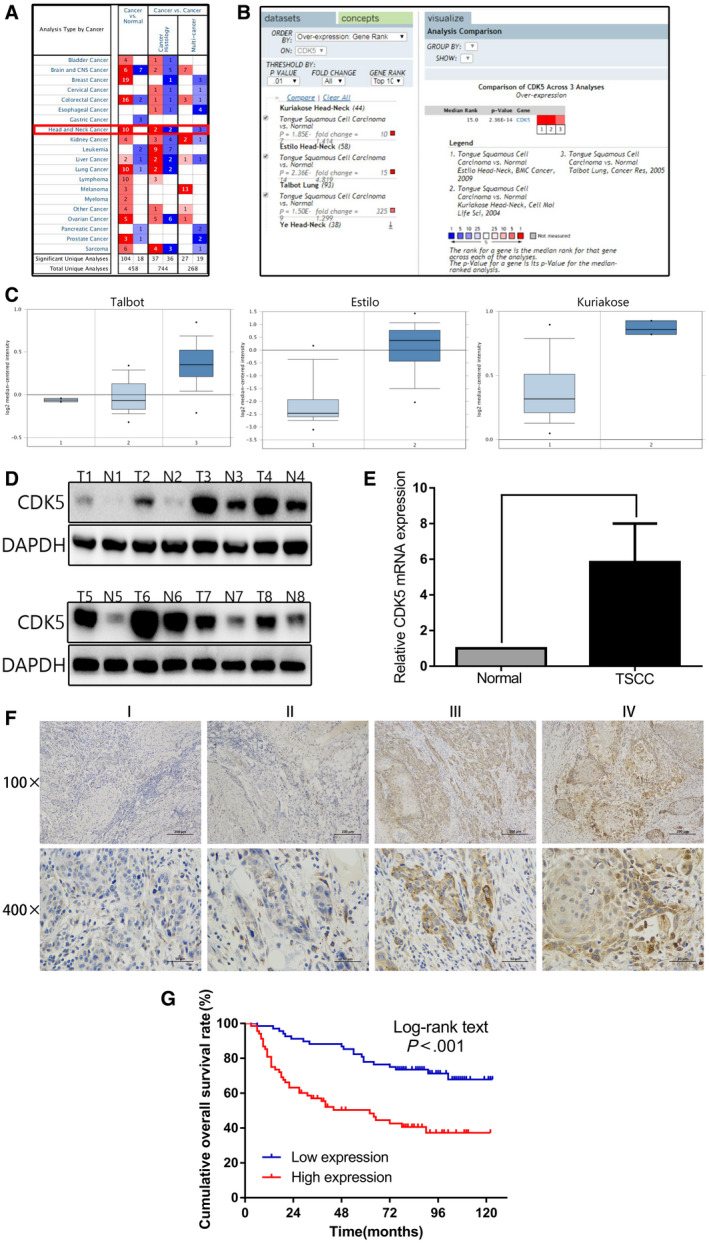
Cyclin‐dependent kinase 5 (CDK5) is overexpressed in tongue squamous cell carcinoma (TSCC) tissue and is associated with a poor prognosis. (A‐C) The levels of CDK5 in TSCC and adjacent normal tissue (ANT) were determined using the iOncomine database. CNS indicates central nervous system. (D) CDK5 protein expression in TSCC tissue and ANT was determined by Western blot analysis. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) served as a reference standard. (E) Polymerase chain reaction detection of CDK5 messenger RNA levels in 8 pairs of TSCC tissue and ANT is shown. (F) Representative images illustrate immunohistochemical results for 136 TSCC samples. (G) Kaplan‐Meier analysis of overall survival is illustrated in 136 patients with TSCC stratified according to CDK5 expression.
The Relation Between CDK5 Expression and Various Clinicopathologic Characteristics of Patients With TSCC
Immunohistochemical studies were performed on paraffin‐embedded tissue sections from 136 patients with TSCC to detect CDK5 expression. The results showed that TNM stage and clinical stage were related to CDK5 expression. There was no significant correlation between CDK5 expression and the age or sex of the patients (Table 1).
TABLE 1.
Relation Between Clinical Characteristics of the Patients and Cyclin‐Dependent Kinase 5 Protein Expression
| Variable | No. of Patients | CDK5 Expression, No. of Patients | χ2 Statistic | P | |
|---|---|---|---|---|---|
| Low | High | ||||
| Age, y | 1.882 | .170 | |||
| ≤55 | 68 | 38 | 30 | ||
| >55 | 68 | 30 | 38 | ||
| Sex | 2.504 | .114 | |||
| Men | 83 | 37 | 46 | ||
| Women | 53 | 31 | 22 | ||
| T classification | 5.647 | .017 a | |||
| T1 + T2 | 102 | 57 | 45 | ||
| T3 + T4 | 34 | 11 | 23 | ||
| N classification | 49.142 | <.001 a | |||
| N0 | 82 | 61 | 21 | ||
| N1 + N2 + N3 | 54 | 7 | 47 | ||
| M classification | 6.908 | .009 a | |||
| M0 | 126 | 67 | 59 | ||
| M1 | 10 | 1 | 9 | ||
| Clinical stage | 25.176 | <.001 a | |||
| I + II | 77 | 53 | 24 | ||
| III + IV | 59 | 15 | 44 | ||
Abbreviations: CDK5, cyclin‐dependent kinase 5; M, metastasis; N, lymph node; T, tumor.
These P values indicate a statistically significant difference.
CDK5 Was an Independent Predictor of Prognosis for Patients With TSCC
Kaplan‐Meier analysis demonstrated that the 3‐year and 5‐year survival rates of patients who had TSCC with a low level of CDK5 expression were 88.2% and 77.9%, respectively; the 3‐year and 5‐year survival rates of those who had TSCC with a high level of CDK5 expression were 57.1% and 50.3%, respectively; and the difference in survival rates between the 2 groups (low and high CDK5 expression) was statistically significant (Fig. 1F). Next, a single‐factor Cox regression analysis was performed on all the clinicopathologic parameters, and a multifactor Cox regression analysis was performed on the risk factors that had P values < .05. The univariate Cox regression model analysis showed that T, N, and M classification; clinical stage; and CDK5 expression were all risk factors affecting the growth of TSCC. The multivariate Cox regression analysis identified CDK5 expression and N stage as independent risk factors that affected the growth of TSCC (Table 2).
TABLE 2.
Cox Regression Analysis
| Variable | Single‐Factor Cox Regression Analysis | Multivariate Cox Regression Analysis | ||||
|---|---|---|---|---|---|---|
| 95% CI | HR | P | 95% CI | HR | P | |
| Age | 0.643‐1.788 | 1.072 | .79 | |||
| Sex | 0.378‐1.146 | 0.658 | 0.14 | |||
| T classification | 1.072‐3.156 | 1.839 | .027 a | 0.849‐2.905 | 1.570 | .151 |
| N classification | 1.660‐4.673 | 2.785 | <.001 a | 1.054‐5.886 | 2.491 | .037 a |
| M classification | 1.095‐5.385 | 2.429 | .029 a | 0.431‐2.454 | 1.029 | .949 |
| Clinical stage | 1.063‐2.968 | 1.777 | .028 a | 0.221‐1.141 | 0.502 | .100 |
| CDK5 expression | 1.779‐5.291 | 3.068 | <.001 a | 1.196‐4.601 | 2.346 | .013 a |
Abbreviations: CDK5, cyclin‐dependent kinase 5; CI, confidence interval; HR, hazard ratio; M, metastasis; N, lymph node; T, tumor.
These P values indicate a statistically significant difference.
CDK5 Promoted the Proliferation of TSCC Cells
Before constructing the stable strain, we tested for the expression of CDK5 in the 3 strains of TSCC cells used in our study. On the basis of those results, we selected the SCC25 and Cal27 cell lines for use in CDK5 knockout experiments because they showed relatively high levels of CDK5 expression. The SCC15 cell line, which had a relatively low level of CDK5 expression, was selected for use in CDK5 overexpression studies conducted in vivo and in vitro. Lentivirus was used to construct stable, CDK5 low‐expressing cell lines (SCC25‐shCDK5 and Cal27‐shCDK5) and a CDK5 overexpressing cell line (SCC15‐OE‐CDK5) (Fig. 2A). The results of CCK8 assays demonstrated that, compared with the control group, the proliferative ability CDK5 knockout cells was significantly decreased (Fig. 2B). The results of cell cloning experiments indicated that, compared with the control group, the number of colonies produced by the CDK5 knockout cells was significantly reduced (Fig. 2C).
Figure 2.
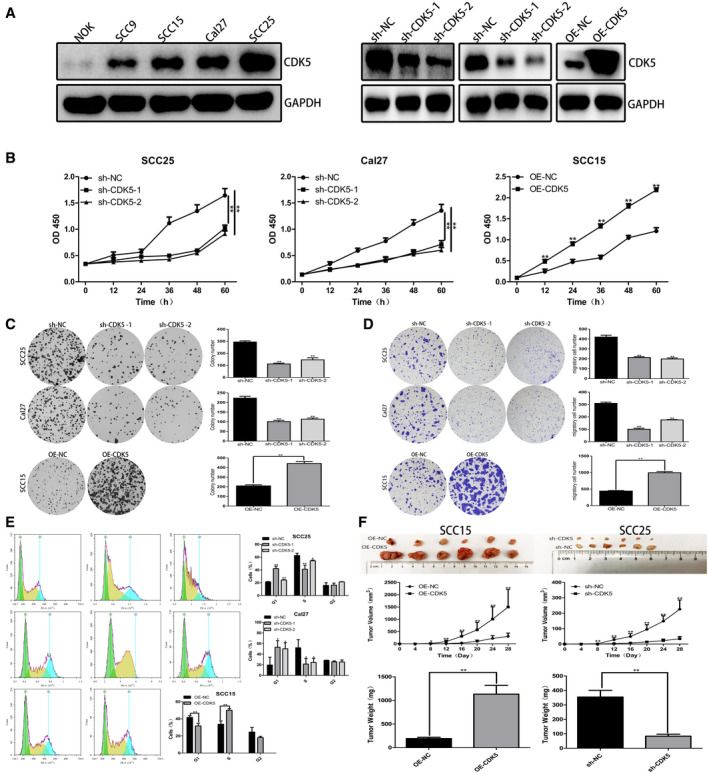
Cyclin‐dependent kinase 5 (CDK5) promotes tongue squamous cell carcinoma (TSCC) cell proliferation. (A) Right: Western blot analysis was used to detect CDK5 expression in TSCC cell lines and in a normal oral epithelial cell line. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) served as a control. Left: Western blot analysis revealed that CDK5 protein expression was downregulated in SCC25 and Cal27 cells and upregulated in SCC15 cells. GAPDH served as a control. sh‐NC indicates short hairpin negative control. (B) Cholecystokinin‐8 (CCK8) assays performed to detect TSCC cell proliferation revealed the absorbance at different time points at 450 nm (optical density [OD] 450). (C) Cell cloning experiments demonstrated that CDK5 overexpression promoted clone formation. (D) Cell migration experiments demonstrated that CDK5 overexpression promoted cell migration. (E) Flow cytometry analyses of the cell cycles of overexpressing CDK5 (OE‐CDK5)–treated and sh‐CDK5–treated cells and their respective negative control cells are illustrated. PE‐A indicates phycoerythrin assay; PI‐A, propidium iodide assay. (F) Xenograft tumors in nude mice after 28 days are shown. The mean volume and weight of xenograft tumors formed by OE‐CDK5 and sh‐CDK5 cells and their respective negative control cells are indicated. All data are expressed as mean ± standard deviation values. A single asterisk indicates P < .05; double asterisks, P < .01.
CDK5 Promoted the Migration of TSCC Cells
We used the Transwell migration assay to determine whether CDK5 affected the migration ability of TSCC cells. The results indicated that, compared with the control group, CDK5 knockdown caused a significant decrease in cell migration, whereas CDK5 overexpression caused an increase in cell migration (Fig. 2D).
CDK5 Regulated Cell Cycle Changes
Knockout of CDK5 resulted in an accumulation of cells in G1 phase: the percentage of G1 phase SCC25 cells transfected with CDK5 shRNA increased from 21.45% to 42.32% and 24.24%, and the percentage of G1 phase Cal27 cells increased from 19.53% to 53.16% and 50.23%. The proportions of cells in S phase decreased from 62.64% and 52.05% to 41.26%, 51.39%, 21.42%, and 24.51%, respectively. At the same time, overexpression of CDK5 promoted the transition of cells from G1 phase to S phase. After transfection, the number G2 phase cells in each group did not significantly change (Fig. 2E). These results showed that knockout of CDK5 could block the transition of cells from G1 phase to S phase.
Down‐Regulation of CDK5 Inhibited Tumor Growth in Vivo
The SCC25sh‐CDK5 cell line with low CDK5 expression and the SCC15OE‐CDK5 cell line with CDK5 overexpression were used for the in vivo studies. Our results indicated that shRNA‐CDK5 could significantly inhibit the growth of tumor cells. Compared with the control group, the xenograft tumors obtained from mice injected with CDK5 knockout cells were smaller and lighter, whereas the xenograft tumors obtained from mice injected with CDK5 overexpressing cells were larger and heavier (Fig. 2F).
CDC25B Was the Downstream Gene Affected by CDK5
We used the STRING, Pathwaycommon, HitPredict, and GeneMANIA databases to search for and identify key genes that interact with CDK5 and affect the cell cycle. Those genes included CDKN1A, CDC25A, CDC25B, and CDC25C, of which CDC25B and CDK5 had the highest correlation. The results indicated that knockout of CDK5 significantly increased CDC25B protein expression, whereas the levels of CDKN1A, CDC25A, and CDC25C expression did not significantly change (Fig. 3A). PCR results further confirmed that knockout of CDK5 led to an upregulation of CDC25B messenger RNA expression in TSCC cells (Fig. 3D). When CDK5 was overexpressed, the opposite result occurred.
Figure 3.
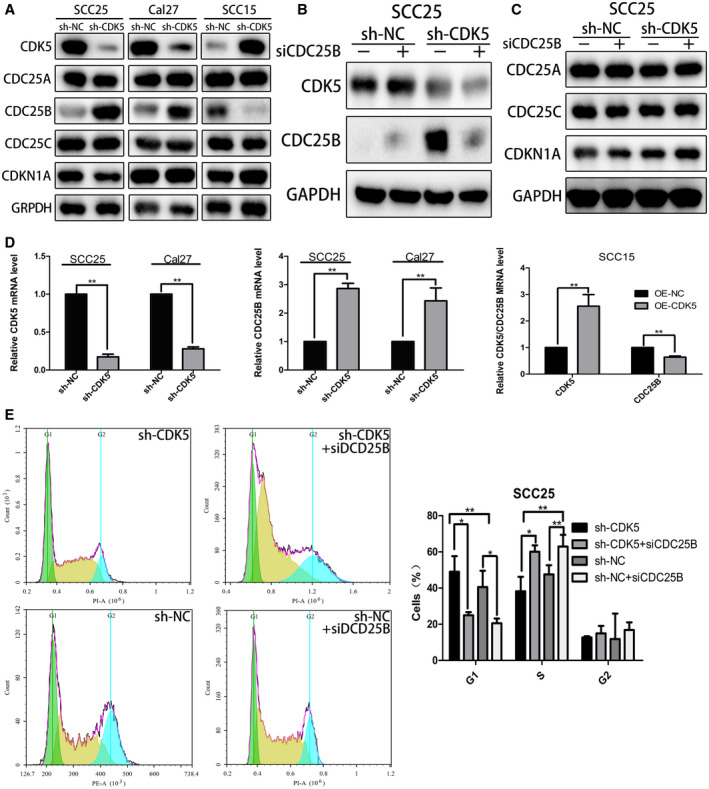
Cell division cycle 25B (CDC25B) is a downstream gene affected by cyclin‐dependent kinase 5 (CDK5). (A) Western blot analysis of key genes related to the cell cycle is illustrated. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) served as a control. sh‐NC indicates short hairpin negative control. (B) Western blot analysis was used to detect CDK5 and CDC25B protein expression in SCC25‐shCDK5 cells after knock‐down of CDC25B. GAPDH served as a control. si‐CDC25B indicates small interfering CDC25B; TSCC, tongue squamous cell carcinoma. (C) Western blot analysis was used to detect the expression of proteins related to the cell cycle in SCC25‐shCDK5 cells. GAPDH served as a control. Mut indicates mutant; WT, wild type. (D) Polymerase chain reaction analysis of CDK5 and CDC25B messenger RNA expression is illustrated in overexpressing CDK5 (OE‐CDK5) and sh‐CDK5 cells and their respective negative control cells. (E) After transfection with si‐CDC25B or negative control (si‐NC), the cell cycle of SCC25‐shCDK5 cells was analyzed by flow cytometry. PE‐A indicates phycoerythrin assay; PI‐A, propidium iodide assay. All data points represent mean ± standard deviation values. A single asterisk indicates P < .05; double asterisks, P < .01.
To verify the effect of CDC25B on cell cycle arrest, we used siRNA to inhibit CDC25B expression in SCC25‐shCDK5 cells (Fig. 3B). Flow cytometry results showed, that after transfection with CDC25B siRNA, the percentage of G1 phase CDK5 knockdown cells decreased from 49.03% to 24.97%, indicating that CDK5 knockdown could change the cell cycle process and depended on CDC25B (Fig. 3E). At the same time, the levels of CDKN1A, CDC25A, and CDC25C proteins were not affected by transfection with CDC25B siRNA (Fig. 3C). These results indicated that knockout of CDK5 could block the cell cycle at the G1/S phase through the CDC25B pathway.
Overexpression of CDK5 Reversed the Inhibitory Effect of miR‐513c‐5p on the Growth of TSCC
We first used TargetscanHuman and MiRanda software to predict the miRNAs that might regulate CDK5 expression. Then, we used the Targetscan and Miranda databases to screen all of the above miRNAs that were differentially expressed at the same time (differential screening criteria: P value < .05 and fold‐change ≥2). Those results indicated that the miRNA that was simultaneously differentially downregulated was miR513c‐5p. After screening the miRNAs, we checked the expression of miR513c‐5p in TSCC tissues using the graph engine service data set. The results indicated that, compared with normal tissues, miR513c‐5p expression was significantly downregulated in TSCC tissues (Fig. 4A). Next, we examined the expression of miR513c‐5p in 8 pairs of TSCC tissue and adjacent normal tissue samples collected at our center. The results showed that, compared with normal tissues adjacent to the cancer, the levels of miR513c‐5p in TSCC tissues were significantly reduced (Fig. 4B). Furthermore, we performed a dual luciferase reporter assay, which verified that CDK5 was a downstream target gene of miR513c‐5p (Fig. 4C). Our data also indicated that, when miR513c‐5p expression increased, the levels of CDK5 messenger RNA and protein significantly decreased, and those changes were accompanied by an increase in CDC25B protein levels (Fig. 4D‐F).
Figure 4.
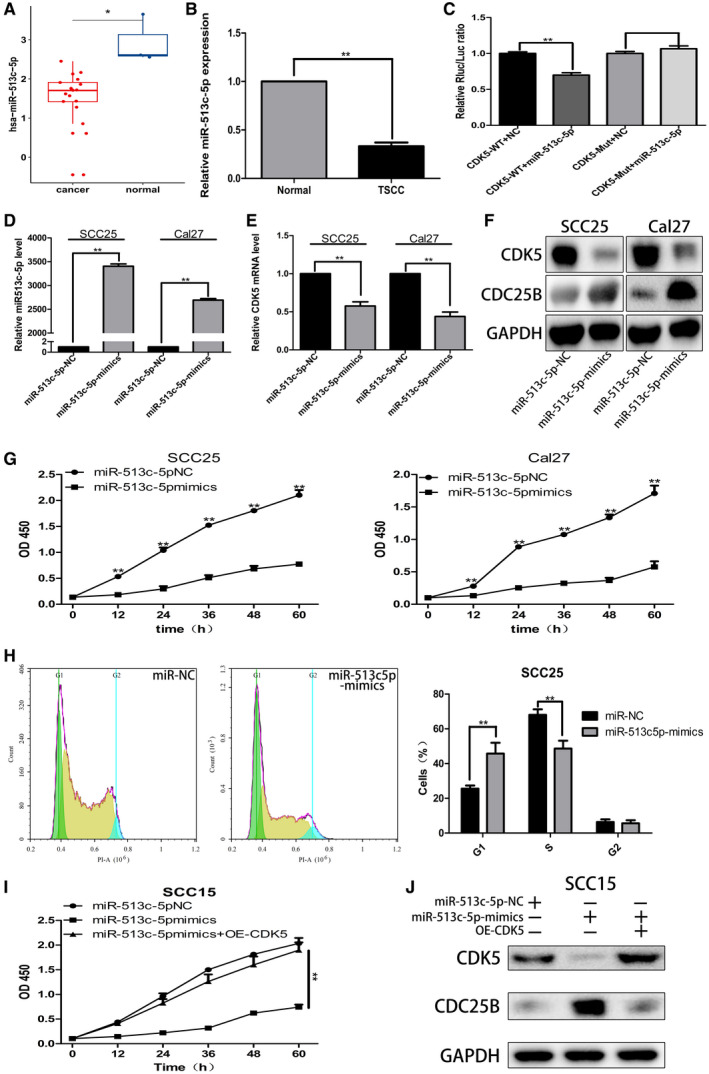
Cyclin‐dependent kinase 5 (CDK5) was identified as the downstream target gene of microRNA 513c‐5p (miR‐513c‐5p). (A) A bioinformatics query of miR513c‐5p expression in tongue squamous cell carcinoma (TSCC) tissues is illustrated. (B) Polymerase chain reaction (PCR) analysis was used to verify the expression of miR513c‐5p in TSCC tissues. (C) Results of a dual luciferase (Luc) reporter experiment are illustrated. Mut indicates mutation; NC, negative control; Rluc, Renilla luciferin; WT, wild type. (D,E) PCR was used to detect miR513c‐5p and CDK5 expression in TSCC cells transfected with miR513c‐5p mimics. (F) Western blot analysis was used to detect CDK5 and cell division cycle 25B (CDC25B) protein expression in TSCC cells transfected with miR513c‐5p mimics. (G) Cholecystokinin‐8 (CCK8) assays were performed to detect the effect of miR513c‐5p on the growth of TSCC cells. OD 450 indicates an optical density of 450 nm. (H) Flow cytometry was used to detect the effect of miR513c‐5p on the cell cycle of SCC25 cells. PI‐A indicates propidium iodide assay. (I) The miR513c‐5p mimics and a CDK5 overexpression lentivirus were simultaneously transfected into SCC15 cells that were used for CCK8 assays. (J) Western blot analysis was performed to detect the levels of CDK5 and CDC25B proteins in SCC15 cells that had been transfected with miR513c‐5p mimics and the CDK5 overexpression lentivirus separately or at the same time. All data points represent mean ± standard deviation values. A single asterisk indicates P < .05; double asterisks, P < .01.
Next, we conducted CCK8 and flow‐cytometry analyses. The CCK8 assays demonstrated that, compared with the control group, in the experimental group of SCC25 and Cal27 cells treated with miR513c‐5p‐mimics, the proliferative ability of cells was significantly reduced (Fig. 4G). The flow cytometry studies showed that the proportion of SCC25 cells in G1 phase treated with miR513c‐59 mimics in the experimental group increased from 25.57% to 45.75%, whereas the proportion of cells in S phase decreased from 68.04% to 48.63%, and there was no significant change in the proportion of cells in G2 phase (Fig. 4H). Subsequently, we conducted a reversion experiment in which SCC15 cells were simultaneously transfected with miR513c‐5p mimics and the CDK5 overexpressing lentivirus. Our results showed that, when CDK5 was overexpressed, the proliferative activity of SCC15 cells was significantly increased (Fig. 4I), and the inhibitory effect of miR513c‐5p on the growth of TSCC cells was reversed. Moreover, Western blot analysis confirmed that, when miR513c‐5p overexpression in SCC15 cells became increased, CDK5 protein levels decreased, CDC25B protein levels increased, and overexpression of CDK5 could reverse the expression of CDC25B protein when miR513c‐5p levels were increased (Fig. 4J). The above results indicated that CDK5 was the downstream target gene of miR513c‐5p.
Discussion
Our experimental results demonstrate that the levels of CDK5 in TSCC cells were higher than those in normal cells, and a high level of CDK5 expression was related to a poor prognosis for patients with TSCC. Previous studies indicated that, in patients with breast cancer, nonsmall cell lung cancer, liver cancer, or nasopharyngeal carcinoma, CDK5 overexpression was significantly associated with a poor prognosis and a low 5‐year survival rate, whereas knockdown of CDK5 produced the opposite effects. 18 , 19 , 20 , 21 Those findings support our current results and confirm that CDK5 functions as an oncogene.
We knocked out CDK5 expression in a TSCC cell line and observed that the viability, clonality, migration, and invasiveness of the cells in vitro were significantly reduced, and the transition of the cells from G1 to S phase was slowed, causing a large number of cells to remain in G1 phase. The opposite results were obtained in the same cell line when CDK5 was overexpressed. Subsequently, the regulatory effect of CDK5 on tumor growth was further verified in a mouse tumor transplantation model. Studies conducted by Zhang et al 21 and Wang et al 22 showed that, when CDK5 expression was knocked down, the proliferation and migration abilities of nonsmall cell lung cancer cells in vitro were significantly reduced, and the tumor formation ability of the cells in vivo was also significantly reduced. Those results confirmed that CDK5 plays an important role in the occurrence and development of tumors.
First, CDK5 is closely related to drug resistance. In patients with cervical cancer, the CDK5/cyclin I complex is associated with cisplatin resistance; and, when CDK5 is inhibited, the cancer cells can regain their sensitivity to chemotherapy drugs. 23 , 24 Previous studies confirmed that the combined use of a CDK5 inhibitor and paclitaxel can help prevent the development of paclitaxel resistance and improve the efficacy of paclitaxel. 25 Second, studies have demonstrated that CDK5 is involved in initiating the DNA damage response. When cells are exposed to ultraviolet rays and other radiation, CDK5 upregulates p35 and p25 expression through the Egr1 promoter and thus activates the DNA damage pathway. 26 At the same time, CDK5 also plays an important role in the angiogenesis of tumor cells. CDK5 expression in endothelial cells is regulated by the proliferation‐promoting angiogenic factor (basic fibroblast growth factor) secreted by tumor cells, and angiostatin can reduce CDK5 expression and cause cell apoptosis. 27 In addition, CDK5 is also involved in tumor cell proliferation, 28 epithelial‐mesenchymal transition, 13 cytoskeleton regulation, 14 and other processes that are of great significance for cell growth. Finally, CDK5 also plays an important role in the phosphorylation process. Zhou et al 29 observed that, in nonsmall cell lung cancer, CDK5 activates the FAK/AKT signaling pathway to promote tumor angiogenesis. In medullary thyroid carcinoma, the retinoblastoma protein controls the transition of cells from G0/G1 phase to S phase by isolating transcription factor E2F, thereby altering the cell cycle. 30 In prostate cancer, CDK5 interacts with Ser‐727 in transcription activator 3 and phosphorylates the transcription factor androgen receptor, which is a prerequisite for cell proliferation. 31 , 32 Some literature suggests that an investigation of specific processes performed by CDK5 phosphorylation dual‐specific phosphatase CDC25 family members (including CDC25A‐CDC25C) is crucial for identifying factors that lead to the occurrence and development of TSCC, and we plan to investigate those processes in future studies.
CDC25B is an important cell cycle regulator needed for the activation of CDKs. 33 Previous studies demonstrated that CDC25B is involved in regulating cell cycle changes in nonmelanoma skin cancer, triple‐negative breast cancer, colorectal cancer, and other tumors 34 , 35 , 36 , 37 , 38 ; however, there have been no literature reports concerning the role played by CDC25B in head and neck tumors. Our results suggest that regulation of the TSCC cell cycle by CDK5 is CDC25B‐dependent, and CDC25B is a downstream gene affected by CDK5. However, follow‐up studies are needed to identify specific phosphorylation pathways and sites.
Few studies have investigated the miR513c‐5p gene, which is upstream of CDK5. However, studies of liver cancer and cervical cancer have indicated that a low level of miR513c‐5p expression is related to higher rates of local tissue invasion, lymphatic metastasis, and distant metastasis. 14 , 39 , 40
In summary, the miR513c‐5p/CDK5/CDC25B axis in TSCC cells may be highly regulated. As an oncogene, CDK5 drives the occurrence and development of TSCC tumors and might serve as a biomarker for diagnosing TSCC and a molecular target for treating the disease.
Funding Support
This study was supported by the National Natural Science Foundation of China (Fund 82072981).
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Yixuan Li: Collected patient samples, performed statistical analysis, performed the experiments, and made a major contribution to writing the article. Fan Yao: Collected patient samples, performed statistical analysis, performed the experiments, and made a major contribution to writing the article. Zan Jio: Performed the experiments. Xuan Su: Collected patient samples, performed statistical analysis, performed the experiments, and made a major contribution to writing the article. Tong Wu: Performed the experiments. Jin Peng: Collected patient samples and performed statistical analysis. Zhongyuan Yang: Made a major contribution to writing the article. Weichao Chen: Made a major contribution to writing the article. Ankui Yang: Designed the study and made a major contribution to writing the article. All authors read and approved the final version.
Li Y, Yao F, Jiao Z, Su X, Wu T, Peng J, Yang Z, Chen W, Yang A. Cyclin‐dependent kinase 5 promotes the growth of tongue squamous cell carcinoma through the microRNA 513c‐5p/cell division cycle 25B pathway and is associated with a poor prognosis. Cancer. 2022. 10.1002/cncr.34136
The first and the second authors contributed equally to this work.
References
- 1. Tang M, Dai W, Wu H, et al. Transcriptome analysis of tongue cancer based on high‐throughput sequencing. Oncol Rep. 2020;43:2004‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shetty SS, Kudpaje A, Jayaraj R, Rao V, Shah PK. Tongue cancer: a discrete oral cavity subsite. Oral Oncol. 2019;99:104348. [DOI] [PubMed] [Google Scholar]
- 3. Ansarin M, Bruschini R, Navach V, et al. Classification of GLOSSECTOMIES: proposal for tongue cancer resections. Head Neck. 2019;41:821‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Do PA, Lee CH. The role of CDK5 in tumours and tumour microenvironments. Cancers (Basel). 2020;13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sang Y, Li Y, Zhang Y, et al. CDK5‐dependent phosphorylation and nuclear translocation of TRIM59 promotes macroH2A1 ubiquitination and tumorigenicity. Nat Commun. 2019;10:4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cortes N, Guzman‐Martinez L, Andrade V, Gonzalez A, Macciono RB. CDK5: a unique CDK and its multiple roles in the nervous system. J Alzheimers Dis. 2019;68:843‐855. [DOI] [PubMed] [Google Scholar]
- 7. Roufayel R, Murshid N. CDK5: key regulator of apoptosis and cell survival. Biomedicines. 2019;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Navaneetha Krishnan S, Rosales JL, Lee KY. Targeting Cdk5 for killing of breast cancer cells via perturbation of redox homeostasis. Oncoscience. 2018;5(5‐6):152‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Masek V, Starha P, Harvanova M, et al. Interaction of selected platinum(II) complexes containing roscovitine‐based CDK inhibitors as ligands with human liver microsomal cytochrome P450. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:382‐387. [DOI] [PubMed] [Google Scholar]
- 10. Riccio O, van Gijn ME, Bezdek AC, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;94:377‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng Y, Liu Q, Tian C, et al. CDK5 activates Hippo signaling to confer resistance to radiation therapy via upregulating TAZ in lung cancer. Int J Radiat Oncol Biol Phys. 2020;108:758‐769. [DOI] [PubMed] [Google Scholar]
- 12. Teng M, Jiang J, He Z, et al. Development of CDK2 and CDK5 dual degrader TMX‐2172. Angew Chem Int Ed Engl. 2020;59:13865‐13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pozo K, Bibb JA. The emerging role of Cdk5 in cancer. Trends Cancer. 2016;2:606‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tao Y, Hao X, Ding X, et al. Medicinal chemistry insights into novel CDC25 inhibitors. Eur J Med Chem. 2020;201:112374. [DOI] [PubMed] [Google Scholar]
- 15. Alao JP, Sunnerhagen P. Caffeine as a tool for investigating the integration of Cdc25 phosphorylation, activity and ubiquitin‐dependent degradation in Schizosaccharomyces pombe. Cell Div. 2020;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu JC, Granieri L, Shrestha M, et al. Identification of CDC25 as a common therapeutic target for triple‐negative breast cancer. Cell Rep. 2018;23:112‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xia HL, Lv Y, Xu CW, et al. MiR‐513c suppresses neuroblastoma cell migration, invasion, and proliferation through direct targeting glutaminase (GLS). Cancer Biomark. 2017;20:589‐596. [DOI] [PubMed] [Google Scholar]
- 18. Saidy B, Rakha EA, Green AR, Ellis IO, Martin SG, Storr SJ. Retrospective assessment of cyclin‐dependent kinase 5 mRNA and protein expression and its association with patient survival in breast cancer. J Cell Mol Med. 2020;24:6263‐6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prince GMSH, Yang TY, Chen MC. Mechanistic insight of cyclin‐dependent kinase 5 in modulating lung cancer growth. Chin J Physiol. 2019;62:231‐240. [DOI] [PubMed] [Google Scholar]
- 20. Peng H, Zhang J, Zhang PP, et al. ARNTL hypermethylation promotes tumorigenesis and inhibits cisplatin sensitivity by activating CDK5 transcription in nasopharyngeal carcinoma. J Exp Clin Cancer Res. 2019;38:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Z, Wang J, Jia Y, et al. CDK5 neutralizes the tumor suppressing effect of BIN1 via mediating phosphorylation of c‐MYC at Ser‐62 site in NSCLC. Cancer Cell Int. 2019;19:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang F, Zhao W, Gao Y, et al. CDK5‐mediated phosphorylation and stabilization of TPX2 promotes hepatocellular tumorigenesis. J Exp Clin Cancer Res. 2019;38:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li R, Liu GZ, Luo SY, Chen R, Zhang JX. Cyclin I promotes cisplatin resistance via Cdk5 activation in cervical cancer. Eur Rev Med Pharmacol Sci. 2015;19:4533‐4541. [PubMed] [Google Scholar]
- 24. Lu JW, Chang JG, Yeh KT, Chen RM, Tsai JJP, Hu RM. Decreased expression of p39 is associated with a poor prognosis in human hepatocellular carcinoma. Med Oncol. 2011;28(suppl 1):S239‐S245. [DOI] [PubMed] [Google Scholar]
- 25. Zhang S, Lu Z, Mao W, et al. CDK5 regulates paclitaxel sensitivity in ovarian cancer cells by modulating AKT activation, p21Cip1‐ and p27Kip1‐mediated G1 cell cycle arrest and apoptosis. PLoS One. 2015;10:e0131833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ehrlich SM, Liebl J, Ardelt MA, et al. Targeting cyclin dependent kinase 5 in hepatocellular carcinoma—a novel therapeutic approach. J Hepatol. 2015;63:102‐113. [DOI] [PubMed] [Google Scholar]
- 27. Sharma MR, Tuszynski GP, Sharma MC. Angiostatin‐induced inhibition of endothelial cell proliferation/apoptosis is associated with the down‐regulation of cell cycle regulatory protein cdk5. J Cell Biochem. 2004;91:398‐409. [DOI] [PubMed] [Google Scholar]
- 28. Strock CJ, Park JI, Nakakura EK, et al. Cyclin‐dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res. 2006;66:7509‐7515. [DOI] [PubMed] [Google Scholar]
- 29. Zhou T, Wang H, Shen J, et al. The p35/CDK5 signaling is regulated by p75NTR in neuronal apoptosis after intracerebral hemorrhage. J Cell Physiol. 2019;234:15856‐15871. [DOI] [PubMed] [Google Scholar]
- 30. Yue CH, Oner M, Chiu CY, et al. RET regulates human medullary thyroid cancer cell proliferation through CDK5 and STAT3 activation. Biomolecules. 2021;11:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin H, Juang JL, Wang PS. Involvement of Cdk5/p25 in digoxin‐triggered prostate cancer cell apoptosis. J Biol Chem. 2004;279:29302‐29307. [DOI] [PubMed] [Google Scholar]
- 32. Lindqvist J, Imanishi SY, Torvaldson E, et al. Cyclin‐dependent kinase 5 acts as a critical determinant of AKT‐dependent proliferation and regulates differential gene expression by the androgen receptor in prostate cancer cells. Mol Biol Cell. 2015;26:1971‐1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cairns J, Ly RC, Niu N, Kalari KR, Carlson EE, Wang L. CDC25B partners with PP2A to induce AMPK activation and tumor suppression in triple negative breast cancer. NAR Cancer. 2020;2:zcaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al‐Matouq J, Holmes TR, Hansen LA. CDC25B and CDC25C overexpression in nonmelanoma skin cancer suppresses cell death. Mol Carcinog. 2019;58:1691‐1700. [DOI] [PubMed] [Google Scholar]
- 35. Xiao Y, Yu Y, Gao D, et al. Inhibition of CDC25B with WG‐391D impedes the tumorigenesis of ovarian cancer. Front Oncol. 2019;9:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu Y, Deng X, Wu D, Jin M, Yu B. PKCδ promotes fertilization of mouse embryos in early development via the Cdc25B signaling pathway. Exp Ther Med. 2019;18:3281‐3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cui L, Mahesutihan M, Zheng W, et al. CDC25B promotes influenza A virus replication by regulating the phosphorylation of nucleoprotein. Virology. 2018;525:40‐47. [DOI] [PubMed] [Google Scholar]
- 38. Wang M, Zhu XY, Wang L, Lin Y. Expression and significance of CDC25B, PED/PEA‐15 in esophageal carcinoma. Cancer Biother Radiopharm. 2015;30:139‐145. [DOI] [PubMed] [Google Scholar]
- 39. Delgir S, Ilkhani K, Safi A, et al. The expression of miR‐513c and miR‐3163 was downregulated in tumor tissues compared with normal adjacent tissue of patients with breast cancer. BMC Med Genomics. 2021;14:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang K, Zhao Z, Yu J, Chen W, Xu Q, Chen L. LncRNA FLVCR1‐AS1 acts as miR‐513c sponge to modulate cancer cell proliferation, migration, and invasion in hepatocellular carcinoma. J Cell Biochem. 2018;119:6045‐6056. [DOI] [PubMed] [Google Scholar]


