Abstract
Background
There is substantial interest in immunotherapy and biologicals in IgE‐mediated food allergy.
Methods
We searched six databases for randomized controlled trials about immunotherapy alone or with biologicals (to April 2021) or biological monotherapy (to September 2021) in food allergy confirmed by oral food challenge. We pooled the data using random‐effects meta‐analysis.
Results
We included 36 trials about immunotherapy with 2126 mainly child participants. Oral immunotherapy increased tolerance whilst on therapy for peanut (RR 9.9, 95% CI 4.5.–21.4, high certainty); cow's milk (RR 5.7, 1.9–16.7, moderate certainty) and hen's egg allergy (RR 8.9, 4.4–18, moderate certainty). The number needed to treat to increase tolerance to a single dose of 300 mg or 1000 mg peanut protein was 2.
Oral immunotherapy did not increase adverse reactions (RR 1.1, 1.0–1.2, low certainty) or severe reactions in peanut allergy (RR 1,6, 0.7–3.5, low certainty), but may increase (mild) adverse reactions in cow's milk (RR 3.9, 2.1–7.5, low certainty) and hen's egg allergy (RR 7.0, 2.4–19.8, moderate certainty). Epicutaneous immunotherapy increased tolerance whilst on therapy for peanut (RR 2.6, 1.8–3.8, moderate certainty). Results were unclear for other allergies and administration routes.
There were too few trials of biologicals alone (3) or with immunotherapy (1) to draw conclusions.
Conclusions
Oral immunotherapy improves tolerance whilst on therapy and is probably safe in peanut, cow's milk and hen's egg allergy. More research is needed about quality of life, cost and biologicals.
Keywords: biological, food allergy, IgE‐mediated, immunotherapy, peanut
We systematically reviewed 39 randomized controlled trials about allergen‐specific immunotherapy and/or biologicals in 2244 people with IgE‐mediated food allergy, mostly children. We found that oral immunotherapy is probably safe and effective in peanut, milk and egg allergy. The number needed to treat (NNT) to increase tolerance to a single dose of 300 mg or 1000 mg peanut protein was 2.Abbreviation: NNT, number needed to treat

Abbreviation
- NNT
number needed to treat
1. INTRODUCTION
1.1. Background
Around 6% of people live with immunoglobulin E (IgE)‐mediated food allergy 1 which affects quality of life, social activities, anxiety and nutrition. 2 , 3 Patients are advised to avoid the allergen and use medications to relieve symptoms, but accidental exposure is common and can be life‐threatening. 4
There is growing interest in allergen‐specific immunotherapy (hereafter immunotherapy) and biological therapies. However, there are conflicting results, with some studies finding improved allergen tolerance and others emphasizing increased adverse reactions. 5
Past reviews sometimes combined studies of various designs and therapies, focused on only one type of food allergy or are outdated. 6 , 7 , 8 , 9 , 10 We compiled an up‐to‐date systematic review about the efficacy and safety of immunotherapy and/or biologicals to inform forthcoming Global Allergy and Asthma European Network (GA2LEN) guidelines and add to existing guidelines. 11 , 12
1.2. Objectives
We prioritized the following review questions after feedback from people with food allergy, patient advocates, healthcare professionals, teachers and policymakers. No industry representatives were involved.
What is the efficacy, safety and cost‐effectiveness of a) immunotherapy alone, b) immunotherapy with a biological or c) biologicals alone for children and adults with any IgE‐mediated food allergy compared to no active treatment agent?
What is the efficacy, safety and cost‐effectiveness of immunotherapy administered by different routes for children and adults with any IgE‐mediated food allergy?
2. METHODS
A task force of allergy specialists, patient representatives, primary care doctors, psychologists, other clinicians, teachers and methodologists from 19 countries undertook the review.
The full methods are available in the protocol (International Prospective Register of Systematic Reviews PROSPERO registration: CRD42021250940) so methods are only briefly summarized here.
2.1. Eligibility criteria
Studies meeting the following criteria were eligible:
Population: infants (<3 years), children (3–17 years) or adults (18+ years) with IgE‐mediated food allergy confirmed using oral food challenge.
Intervention: allergen‐specific immunotherapy alone, with a biological or biological alone.
Comparator: placebo, no intervention or routine management without active treatment. For review question 2, the compactor was immunotherapy using a different route.
Outcomes: quality of life, desensitization (ability to tolerate the allergen whilst being treated), sustained unresponsiveness (ability to tolerate the allergen after discontinuing therapy), adverse reactions, severe adverse reactions and cost‐effectiveness, all as defined by the original studies.
Study types: randomized controlled trials (hereafter trials) published from the beginning of databases (1946) to 30 April 2021 for immunotherapy and trials and controlled comparisons up to 30 September 2021 for biological monotherapy.
2.2. Study selection
Clinicians, patients and information specialists developed a search strategy (Supplement S1). Methodologists (CS, DdS) searched six databases (CINAHL, Cochrane Library, EMBASE, ISI Web of Science, MEDLINE, Scopus), reviewed bibliographies of reviews, guidelines and identified studies and contacted experts in the field for additional research.
Two methodologists independently screened the titles, abstracts and full text of potentially relevant studies (CS, DdS). Shortlisted studies were rescreened by the task force for consensus. Some studies included in previous reviews did not meet our stringent criteria (S2).
2.3. Data extraction
Pairs of methodologists (MG, DdS, CS) and task force members (all authors) extracted study characteristics and outcomes independently. Clinicians independently extracted additional data about adverse reactions and thresholds (PRdR, EK, GR, PB, AWN, ADG, SA, ME, TZ), including the different definitions used (S3). A senior clinician acted as an arbitrator if needed, but there was consensus (GR).
We had most data about peanut allergy so we compiled the proportion able to tolerate 300 mg (level at which there is a substantial reduction in risk from products labelled with ‘may contain’) 13 and 1000 mg peanut protein as both a single last tolerated and cumulative dose. Our approach was conservative, so when someone was reported to tolerate 300 mg to 600 mg peanut protein we classified them as tolerating a 300 mg dose. We used a range of 1000 mg to 2000 mg for the 1000 mg threshold. We used these ranges because an individual's threshold may vary by at least a factor of two when they are challenged twice within a short time. 14
2.4. Risk of bias in individual studies
Four methodologists and clinicians independently assessed the risk of bias in individual studies using the Cochrane Risk of Bias tool 2 (ROB2) 15 (pairs of all authors). Arbitration was available if needed from a senior clinician (GR) but there was agreement in the risk of bias assessments.
2.5. Synthesis of results
We used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to synthesize data about each outcome. 16
We pooled intention‐to‐treat data using random‐effects Mantel‐Haenszel meta‐analysis (Revman 5.4) because the studies included different populations, regimes and time periods and to avoid overweighting large but imprecise studies.
We divided studies based on the food allergy and therapy route. We undertook subgroup analysis based on risk of bias, age, allergy severity, comparator and threshold tolerated. In sensitivity analysis, we used a continuity correction (adding 0.05 to numerators and denominators) where there were no events in each study arm for severe or life‐threatening events, anaphylaxis and adrenaline use.
We used funnel plots to help assess publication bias. We quantified the heterogeneity of studies using the I2 statistic, with values <25% indicating low heterogeneity. 17
All task force members developed conclusions by consensus, recognizing any potential conflicts of interest, which were declared in advance. We used standardized GRADE statements to summarize the conclusions 18 (S4 lists our wording conventions). We used the word ‘may’ to represent low certainty evidence, ‘probably’ for moderate certainty and ‘resulted in’ for high certainty evidence.
3. RESULTS
3.1. Study characteristics
We included 36 trials of immunotherapy with 2126 participants, mainly children, and 3 trials about biological monotherapy with 118 participants, mainly teenagers and adults. (Table 1 and Figure 1).
TABLE 1.
Characteristics of included studies
| Allergy | Administration | Studies | Participants | Allergy severity | Region |
|---|---|---|---|---|---|
| Peanut (13 studies) | Oral 24 , 25 , 26 , 27 , 28 , 29 , 30 | 7 |
1031 children 55 mixed age |
1 study moderate 6 studies mixed |
4 studies Europe 2 studies USA 1 study multiple |
| Epicutaneous 31 , 32 , 33 | 3 |
356 children 295 mixed age |
2 studies mild/moderate 1 study mixed |
1 study USA 2 studies multiple |
|
| Sublingual 34 | 1 | 40 adults | Mild/moderate | 1 study USA | |
| Subcutaneous 35 | 1 | 8 mixed age | Severe | 1 study USA | |
| Oral vs. sublingual 36 | 1 | 21 children | Mild/moderate | 1 study USA | |
| Cow's milk (11 studies) | Oral 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 | 8 |
91 infants 144 children 42 mixed age |
3 studies mild/moderate 2 studies severe 3 studies mixed |
1 study Asia 6 studies Europe 1 study USA |
| Epicutaneous 45 | 1 | 19 mixed age | Mixed | 1 study Europe | |
| Oral vs. sublingual 46 | 1 | 30 children | Mixed | 1 study USA | |
| Oral + biological 47 | 1 | 16 children | Severe | 1 study Asia | |
| Hen's egg (7 trials) | Oral 48 , 49 , 50 , 51 , 52 , 53 , 54 | 7 | 327 children |
3 studies mild/moderate 1 study severe 1 study mixed |
2 studies Asia 5 studies Europe |
| Other (1 hazelnut. 1 peach, 1 wheat, 2 multiple foods) | Oral 55 , 56 , 57 | 3 |
69 children 49 mixed age |
2 studies mild/moderate 1 study mixed |
2 studies Europe 1 study USA |
| Sublingual 58 , 59 | 2 | 78 adults | Mixed | 1 study Europe | |
| Total immunotherapy |
25 studies oral 4 epicutaneous 1 subcutaneous 3 sublingual 2 oral vs. sublingual 1 oral +biological |
36 |
2126 2 studies infants 23 children 3 adults 8 mixed age |
12 studies mild/moderate 1 moderate 5 severe 18 mixed |
4 studies Asia 20 studies Europe 9 studies USA 3 studies multiple |
| Biological monotherapy |
1 etokimab 19 1 omalizumab 20 1 TNX−901 21 |
3 |
118 2 studies 13+ years 1 study half children and half 13+ years |
3 studies mixed | 3 studies USA |
Allergy severity was based on definitions in individual studies and descriptions of inclusion criteria.
FIGURE 1.
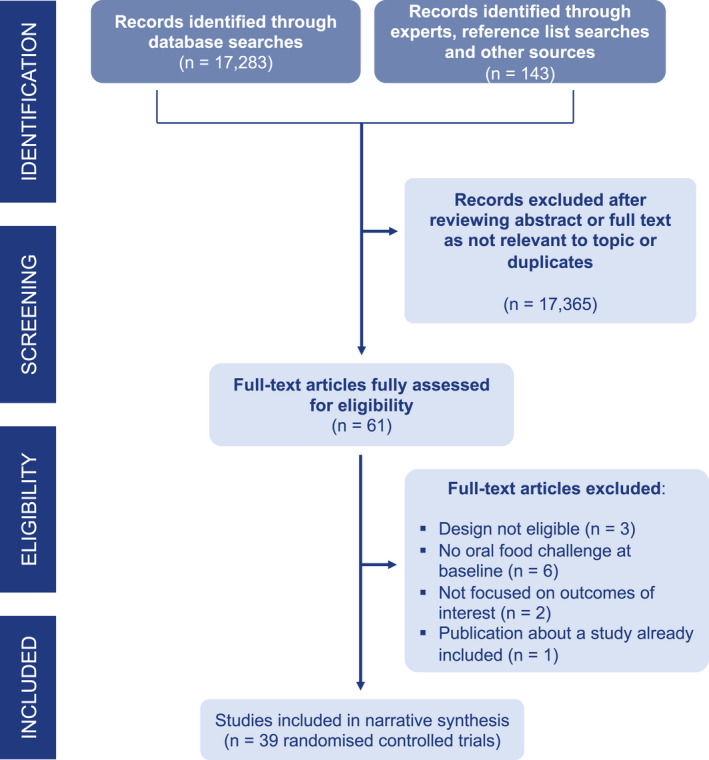
PRISMA diagram showing study selection
The GRADE certainty of evidence was generally low to moderate (S6). The certainty of evidence was mainly downgraded due to risk of bias (S5), indirectness and imprecision. About one quarter of studies were at low risk of bias (28%), half at moderate risk (47%) and quarter at high risk (25%).
Tables 2, 3, 4 list the key findings, with details in S6 and S7. Trends were broadly similar for people with mild/moderate or severe allergy and studies at lower and higher risk of bias (S7). There was insufficient information to be able to distinguish immunotherapy with raw vs. cooked foods or to draw conclusions about the most effective regimen or duration of treatment.
TABLE 2.
Summary of findings about immunotherapy for peanut allergy
| Outcome | % active | % control | Absolute difference | Relative risk (95% CI) | NNT | Certainty of evidence | Comparisons & participants |
|---|---|---|---|---|---|---|---|
| Oral immunotherapy | |||||||
| Desensitization | 68 | 6 | 62%, p < .05 | 9.9 (4.5–21.4) | 2 | High | 7 (n = 1023) |
| Tolerate single dose of 300 mg | 76 | 13 | 63%, p < .05 | 5.7 (4.0–7.9) | 2 | Moderate | 5 (n = 820) |
| Tolerate single dose of 1000 mg | 56 | 2 | 54%, p < .05 | 16.6 (8.0–34.4) | 2 | High | 5 (n = 906) |
| Sustained unresponsiveness | 35 | 4 | 31%, p < .05 | 8.8 (1.2–61.6) | 4 | Low | 1 (n = 85) |
| Adverse reactions | 98 | 89 | 9% p > .05 | 1.1 (1.0–1.2) | NA | Low | 7 (n = 953) |
| Severe adverse reactions | 4 | 2 | 2%, p > .05 | 1.6 (0.7–3.5) | NA | Low | 6 (n = 950) |
| Epicutaneous immunotherapy | |||||||
| Desensitization | 46 | 17 | 29%, p < .05 | 2.6 (1.8–3.8) | 3 | Moderate | 3 (n = 651) |
| Tolerate single dose of 300 mg | 30 | 13 | 18%, p < .05 | 2.4 (1.5–3.8) | 6 | Low | 3 (n = 333) |
| Adverse reactions | 78 | 61 | 17%, p > .05 | 1.2 (0.95–1.6) | NA | Low | 4 (n = 676) |
| Severe adverse reactions | 6 | 2 | 4%, p > .05 | 2.0 (0.8–5.1) | NA | Low | 4 (n = 676) |
| Sublingual immunotherapy | |||||||
| Desensitization | 70 | 15 | 55%, p < .05 | 4.7 (1.6–13.8) | 2 | Very low | 1 (n = 40) |
| Tolerate single dose of 300 mg | 25 | 15 | 10%, p > .05 | 1.7 (0.5–6.1) | NA | Very low | 1 (n = 40) |
| Severe adverse reactions | 0 | 0 | 0 | Not calculated | NA | Very low | 1 (n = 40) |
| Subcutaneous immunotherapy | |||||||
| Desensitization | 33 | 0 | 33%, p > .05 | 1.5 (0.1–22.6) | NA | Very low | 1 (n = 4) |
Rounded to nearest whole number. Absolute difference is % active minus control.
Adverse reactions and severe adverse reactions are all reactions, not necessarily linked to the therapy. Multiple definitions of severe reactions were used (see S3). In general these involved marked impact on participants (eg symptomatic bronchospasm, hypotension) and need for medical intervention. Online supplement S7 contains additional data such as the proportion of doses experiencing reactions and adverse events linked to therapy. ‘Comparisons’ means the number of comparisons between active and control groups. In most cases this is the total number of trials contributing the meta‐analysis but some studies compared different doses with placebo. Each comparison was included separately in the meta‐analysis. Data about threshold reactivity at 1000 mg peanut protein were only available for oral immunotherapy.
Abbreviations: CI, confidence interval; NA, not applicable as no statistically significant difference; NNT, number needed to treat.
TABLE 3.
Summary of findings about oral immunotherapy for cow's milk allergy
| Outcome | % active | % control | Absolute difference | Relative risk (95% CI) | NNT | Certainty of evidence | Trials and participants |
|---|---|---|---|---|---|---|---|
| Desensitization | 68 | 15 | 53%, p < .05 | 5.7 (1.9–16.7) | 2 | Moderate | 7 (n = 249) |
| Adverse reactions | 85 | 17 | 67%. p < .05 | 3.9 (2.1–7.5) | 2 | Low | 6 (n = 210) |
| Severe adverse reactions | 3 | 0 | 3%, p > .05 | 7.0 (0.4–124.8) | NA | Very low | 4 (n = 204) |
TABLE 4.
Summary of findings about oral immunotherapy for hen's egg allergy
| Outcome | % active | % control | Absolute difference | Relative risk (95% CI) | NNT | Certainty of evidence | Trials and participants |
|---|---|---|---|---|---|---|---|
| Desensitization | 84 | 5 | 79%, p < .05 | 8.9 (4.4–18.0) | 2 | Moderate | 6 (n = 259) |
| Sustained unresponsiveness | 35 | 4 | 30%, p < .05 | 7.1 (1.7–29.4) | 4 | Low | 2 (n = 91) |
| Adverse reactions | 79 | 8 | 71%, p < .05 | 7.0 (2.4–19.8) | 2 | Moderate | 6 (n = 291) |
| Severe adverse reactions | 6 | 0 | 6%, p > .05 | 3.4 (0.6–19.6) | NA | Very low | 4 (n = 211) |
The note under Table 2 also applies to this table.
3.2. Peanut allergy (Table 2)
3.2.1. Desensitization
Most studies about peanut allergy enrolled children (Table 2). Oral immunotherapy resulted in a large increase in the proportion able to tolerate peanut during therapy (absolute difference 62%, relative risk (RR) 9.9, 95% confidence interval (CI) 4.5–21.4, high certainty). Those receiving oral immunotherapy were six times more likely than controls to tolerate a single dose of 300 mg peanut (roughly one peanut, number needed to treat (NNT) 2) and 17 times more likely to tolerate 1000 mg (NNT 2). Before immunotherapy, the mean maximum tolerated dose was only about 34 mg (S5).
Epicutaneous immunotherapy probably increased the proportion able to tolerate peanut during therapy (absolute difference 29%, RR 2.6, 95% CI 1.8–3.8, moderate certainty). Those receiving epicutaneous immunotherapy were twice as likely as controls to be able to tolerate a single dose of 300 mg peanut (NNT 6).
Sublingual immunotherapy may result in a large increase in the proportion able to tolerate peanut during therapy (absolute difference 55%, RR 4.7, 95% CI 1.6–13.8, low certainty). It is unclear whether subcutaneous immunotherapy had any impact because the certainty of evidence was very low.
3.2.2. Sustained unresponsiveness
Peanut oral immunotherapy may increase the proportion of children able to tolerate peanut after stopping therapy (absolute difference 31%, RR 8.8, 05% CI 1.2–61.6, low certainty). No data were available about sustained unresponsiveness using other administration routes.
3.2.3. Adverse reactions
There was no statistically significant increase in the proportion of people who had adverse reactions due to peanut immunotherapy, regardless of the administration route, though oral immunotherapy was on the borderline for increasing adverse reactions (RR 1.1, 95% CI 1.0–1.2, p = .06, low certainty).
The most common adverse reactions were gastrointestinal, including swelling and itching in the mouth, vomiting, abdominal pain and diarrhoea. Most symptoms (80%–90%) were mild in severity and <10% withdrew from treatment as a result. Some experienced respiratory and skin symptoms such as rhinitis, wheezing, dyspnoea, asthma, laryngeal oedema, urticaria, angioedema and erythema. These ranged in severity but were usually responsive to treatment. Immune system disorders, infections, eye symptoms, ear and labyrinth disorders and cardiovascular events were not frequently reported. Eosinophilic esophagitis was rare. The frequency of adverse reactions appeared to reduce with increasing duration of immunotherapy.
Skin reactions were the most common adverse reactions to peanut epicutaneous immunotherapy. Most cutaneous reactions were mild, limited to the patch site and generally occurred in the first month of treatment. Skin manifestations extending beyond the borders of the patch were uncommon, as were extra‐cutaneous reactions (respiratory, thoracic and mediastinal disorders, immune reactions or eye symptoms).
No deaths were reported due to immunotherapy, and there was no increase in anaphylaxis or life‐threatening reactions (S7).
3.3. Cow's milk allergy (Table 3)
All but one study about cow's milk allergy enrolled children (Table 3). Oral immunotherapy probably resulted in a higher proportion able to tolerate cow's milk protein during therapy (absolute difference 53%, RR 5.7, 95% CI 1.9–16.7, moderate certainty).
An increased proportion may have experienced adverse reactions (absolute difference 67%, RR 3.9, 95% CI 2.1–7.5, low certainty), but few studies focused solely on reactions caused by the therapy (S7) and there was no significant difference in the 3 studies comparing with placebo.
The most common adverse reactions were mild gastrointestinal symptoms. A small number experienced respiratory symptoms. It was unclear whether oral immunotherapy increased severe reactions because the certainty of evidence was very low. Reports of severe reactions, anaphylaxis, use of adrenaline and discontinuation were rare. No hospital admissions or deaths were reported due to treatment.
There was insufficient evidence to draw conclusions about other administration routes in cow's milk allergy.
3.4. Hen's egg allergy (Table 4)
All studies about hen's egg allergy were in children and all but one compared with an elimination diet (Table 4). Oral immunotherapy probably resulted in a large increase in the proportion of children able to tolerate hen's egg during therapy (absolute difference 79%, RR 8.9, 95% CI 4.4–18, moderate certainty). It may increase the proportion able to continue tolerating hen's egg after therapy ends (absolute difference in sustained unresponsiveness 30%, RR 7.1, 95% CI 1.7–29.4, low certainty).
Egg oral immunotherapy probably resulted in a large increase in the proportion of children experiencing adverse reactions (absolute difference 71%, RR 7.0, 95% CI 2.4–19.8, moderate certainty), but few studies focused only on reactions caused by the treatment. The one study that compared with placebo found no significant difference in adverse reactions. It was unclear whether there was any impact on severe reactions because the certainty of evidence was very low.
Around 95% of reactions were mild. They occurred in about 1 in 20 doses during the build‐up phase and decreased to 1 in 40 doses during the first months of maintenance. The most frequently reported symptoms were oral pruritus and gastrointestinal pain. Anaphylaxis or symptoms that were severe enough to discontinue immunotherapy were rare.
3.5. Other food allergies and outcomes
We identified few studies about other food allergies or directly comparing immunotherapy routes to present meaningful results (see S6 and S7). There was also little information about the impact on quality of life, despite being key to whether people are motivated to continue immunotherapy (S6). None of the included studies reported cost‐effectiveness. We did not find a clear relationship between the duration of treatment and desensitization, sustained responsiveness or safety (S7).
3.6. Biological therapies
There were too few studies to draw meaningful conclusions about biologicals alone or added to immunotherapy (S6). In those aged 13+ years, there was a trend towards improved tolerance of peanut during biological monotherapy, with few side effects. However the certainty of evidence was very low, with only three studies, each about different therapy. 19 , 20 , 21
4. DISCUSSION
4.1. Summary of evidence
Proactive treatments are needed to reduce the burden of food allergy. Our review suggests that oral immunotherapy may be safe and effective for increasing tolerance whilst on therapy in children allergic to peanut, cow's milk or hen's egg. Three‐quarters receiving peanut oral immunotherapy tolerated a single dose of 300 mg peanut protein at the end of therapy and a half a single dose of 1000 mg, which would protect against most accidental exposure. About one‐third may maintain tolerance after stopping therapy, at least in the short term (3 months). Oral immunotherapy was usually well tolerated. It is likely to result in more adverse reactions, but mainly mild. Clinicians and families will need to weigh up the benefits vs. harms when considering whether immunotherapy is appropriate for individuals.
Epicutaneous immunotherapy may also be safe and effective amongst children with peanut allergy, but less is known about this for other food allergies, or about sublingual or subcutaneous immunotherapy for any food allergy. We found insufficient evidence to draw conclusions about benefits or harms from biologicals.
4.2. Comparison with previous research
Many studies, reviews and opinion pieces have explored immunotherapy for people with food allergy, sometimes with inconsistent results. 22 Our review differs from others because it includes the highest quality and most up‐to‐date evidence, divided by type of food allergy and administration route. We excluded trials that did not use food challenges to confirm food allergy before treatment, as this may introduce bias into absolute event rates. We examined tolerated thresholds in detail, which we believe is the first analysis of its kind. Our review supports others suggesting that clinicians could consider immunotherapy for some groups of patients. 6 , 7
4.3. Implications for research
There is now a good pool of studies about oral immunotherapy for peanut allergy, but we can only draw limited conclusions about other food allergies, administration routes and biologicals due to sparse good quality evidence.
An important area not explored in our review is patient preferences and motivation to use immunotherapy and/or biologicals. Our findings suggest that (oral) immunotherapy may improve tolerance whilst people continue the therapy, but gains may not be sustained after stopping therapy. Therefore, people need to consider whether they are willing to continue therapy for an extended period. Future research using standardized threshold levels for each allergen may help patients make more informed decisions.
Future studies could create core outcome sets 23 and use standardized definitions and measures of quality of life, desensitization, sustained unresponsiveness and adverse reactions to allow robust comparisons across populations, regions and types of immunotherapy. Standardized immunotherapy regimens would also assist comparisons. More high quality trials are needed to directly compare different administration routes and to explore the impacts of adding biologicals.
4.4. Strengths and limitations
A strength of our review is that it was conducted by a large group of clinicians, allied health professionals, patient representatives, teachers and researchers from different parts of the world.
By analyzing detailed data about desensitization thresholds, we showed that only 2 children needed to be treated with peanut oral immunotherapy to increase 1 child's reactivity threshold up to 300 mg and 1000 mg. This would reduce the chance of reactions from accidental consumption of packaged food containing peanut. 11 Given variability in reactivity thresholds, 12 achieving tolerance to a single dose of 1000 mg is likely protective to real‐life exposures.
However, our conclusions are limited by methodological variations in the available studies and a lack of eligible studies about many topics. We found that adverse reactions tended to increase, but we do not know whether these reactions were due to the therapy, accidental exposure or unrelated causes. This limits conclusions about the safety profile. Some studies excluded patients with previous anaphylaxis, which may also affect generalizability.
A number of trials have pragmatic designs and do not confirm food allergy at the outset using oral food challenges. We excluded these studies to ensure that participants definitely had food allergy, but there is an argument for including such research in decision‐making if the selection criteria reflect real‐life clinical practice.
5. CONCLUSIONS
There may be merit in allergen‐specific immunotherapy and/or biologicals for a carefully selected group of patients, but there are also downsides including (mainly mild) reactions to manage, relatively long treatment periods and benefits potentially only lasting whilst people maintain the regimen.
Immunotherapy is also specific to the culprit food(s) and requires exposure to allergens, which may be off‐putting for some. We know little about how patient motivation to sustain the therapy may differ in various parts of the world or based on patient characteristics. When making patient‐centred shared decisions about whether to offer immunotherapy and/or biologicals to an individual, clinicians and patients need to weigh up factors such as patient preferences, motivation, accessibility and cost alongside the evidence about safety and effectiveness.
CONFLICT OF INTEREST
Debra de Silva, none declared. Organisation received GA2LEN funding to support review process. Pablo Rodríguez del Río, Personal fees: Aimmune, Nicolette W. de Jong, none declared. Ekaterina Khaleva, none declared. Chris Singh, none declared. Organisation received GA2LEN funding to support review process. Anna Nowak‐Wegrzyn, Personal fees: Nestle, Novartis, Sanofil. Antonella Muraro, Personal fees: Aimmune, DVB, Mylan, ALK, Nestle, Novartis, Nutricia Research. Grant: Aimmune, Sanofil. Philippe Begin, Personal fees: ALK, DBV, Novartis, Sanofil, Giovanni Pajno, none declared. Alessandro Fiocchi, Personal fees: Ferrero. Grant: Danone, HIPP. Angel Sanchez, none declared. Carla Jones, none declared. Organisation received funding to support projects. Caroline Nilsson, Grant: Aimmune, Thermofisher. Carsten Bindslev‐Jensen, Personal fees: Allakos, CEV SA. Grant: Novartis. Gary Wong, none declared. Hugh Sampson, Personal fees: DBV, N‐fold, Siolta. Kirsten Beyer, Personal fees: Aimmune, Bencard, Danone, DBV, HIPP, Hycor, Infectopharm, Jenapharma, Mylan/Meda, Nestle, Novartis, Nutricia Research, Thermofisher. Grants: Aimmune, Danone/Nutricia/Milipa, DBV, Hipp, Hycor, Infectopharm. Mary‐Jane Marchisotto, none declared. Montserrat Fernandez Rivas, Personal fees: Therapeutics, ALK, Allergy Therapeutics, DBV, Diater, GSK, Thermofisher Scientific, SPRIM. Rosan Meyer, Personal fees: Abbott, Danone, Nestle, Mead Johnson. Susanne Lau, none declared. Ulugbek Nurmatov, none declared. Graham Roberts, Grant: UK Food Standards Agency, DBV, European Union.
AUTHOR CONTRIBUTION
All authors conceptualized the work, extracted or checked data, undertook risk of bias assessments, provided substantive comments and approved the review for submission. In addition, CS and DdS undertook searches and meta‐analysis and constructed summary of findings tables. PRdR, NdJ, GR, EK, ANW, PB and ADG extracted additional data and contributed to analysis. DdS, GR and EK managed the process.
CONTRIBUTORS
Susanne Halken, Hans Christian Andersen Children's Hospital, Odense University Hospital, Denmark. Margitta Worm, Charité ‐ Universitätsmedizin Berlin, Germany. Audrey Dunn‐Galvin, University College Cork, Ireland. Liz Angier, University of Southampton, England. Amena Warner, Allergy UK, England. Antoine Deschildre, University Lille, France. Antonella Cianferoni, University of Florence, Italy. Barbara Ballmer, UniversitätsSpital Zürich, Switzerland. Berber Vlieg‐Boerstra, OLVG Hospital, the Netherlands. Bertine Flokstra‐de Blok, University of Groningen, the Netherlands. Bright Nwaru, University of Gothenburg, Sweden. Carina Venter, Children's Hospital Colorado, USA. Celine Demoulin, Association Française pour la Prévention des Allergies Siège social, France. Clare Mills, University of Manchester, England. Gary Wong, Chinese University of Hong Kong, Hong Kong. Giovanni Pajno, Policlinico Hospital‐University of Messina, Italy. Hania Szajewska, Medical University of Warsaw, Poland. Hasan Arshad, University of Southampton, England. Jennifer Gerdts, Food Allergy Canada, Canada. Josefine Gradman, Hans Christian Andersen Children's Hospital, Odense University Hospital, Denmark. Kate Grimshaw, Salford Care Organisation, England. Lars K. Poulsen, Copenhagen University Hospital, Denmark. Lynne Regent, Anaphylaxis Campaign, England. Marcia Podestà, Food Allergy Italia, Italy. Matthew Geromi, The Evidence Centre, USA. Mika Makela, Helsinki University Hospital, Finland. Montserrat Alvaro Lozano, Hospital Sant Joan de Déu, Esplugues, Spain. Montserrat Fernandez Rivas, Hospital Clínico San Carlos, Spain. Motohiro Ebisawa, Sagamihari National Hospital, Japan. Pete Smith, Griffith University School of Medicine, Australia. Richard Loh, Perth Children's Hospital, Australia. Robert Wood, John Hopkins Bloomberg School of Public Health, USA. Ronald Van Ree, Amsterdam UMC, the Netherlands. Sabine Schnadt, DAAB, Deutscher Allergie‐ und Asthmabund e.V, Germany. Stefania Arasi, Bambino Gesù Children's Research Hospital, Italy. Torsten Zuberbier, Charité ‐ Universitätsmedizin Berlin, Germany.
Supporting information
App S1
App S2
App S3
App S4
App S5
App S6
App S7
ACKNOWLEDGEMENTS
The Global Allergy and Asthma European Network (GA2LEN) contributed funding towards the systematic review process. Authors and contributors volunteered their time.
de Silva D, Rodríguez del Río P, de Jong NW, et al; the GA2LEN Food Allergy Guidelines Group . Allergen immunotherapy and/or biologicals for IgE‐mediated food allergy: A systematic review and meta‐analysis. Allergy. 2022;77:1852–1862. doi: 10.1111/all.15211
REFERENCES
- 1. Nwaru BI, Hickstein L, Panesar SS, et al. The epidemiology of food allergy in Europe: a systematic review and meta‐analysis. Allergy. 2014;69(1):62‐75. [DOI] [PubMed] [Google Scholar]
- 2. Warren C, Dyer A, Lombard L, Dunn‐Galvin A, Gupta R. The psychosocial burden of food allergy among adults: a US population‐based study. J Allergy Clin Immunol Pract. 2021;9(6):2452‐2460.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polloni L, Muraro A. Anxiety and food allergy: a review of the last two decades. Clin Exp Allergy. 2020;50(4):420‐441. [DOI] [PubMed] [Google Scholar]
- 4. Pouessel G, Turner PJ, Worm M, et al. Food‐induced fatal anaphylaxis: from epidemiological data to general prevention strategies. Clin Exp Allergy. 2018;48(12):1584‐1593. [DOI] [PubMed] [Google Scholar]
- 5. Eiwegger T, Anagnostou K, Arasi S, et al. Conflicting verdicts on peanut oral immunotherapy from the institute for clinical and economic review and US food and drug administration advisory committee: where do we go from here? J Allergy Clin Immunol. 2020;145:1153‐1156. [DOI] [PubMed] [Google Scholar]
- 6. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE‐mediated food allergy: a systematic review and meta‐analysis. Allergy. 2017;72:1133‐1147. [DOI] [PubMed] [Google Scholar]
- 7. Romantsik O, Tosca MA, Zappettini S, Calevo MG. Oral and sublingual immunotherapy for egg allergy. Cochrane Database Syst Rev. 2018;4(4):CD010638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiong L, Lin J, Luo Y, Chen W, Dai J. The efficacy and safety of epicutaneous immunotherapy for allergic diseases: a systematic review and meta‐analysis. Int Arch Allergy Immunol. 2020;181(3):170‐182. [DOI] [PubMed] [Google Scholar]
- 9. Brożek JL, Terracciano L, Hsu J, et al. Oral immunotherapy for IgE‐mediated cow's milk allergy: a systematic review and meta‐analysis. Clin Exp Allergy. 2012;42(3):363‐374. [DOI] [PubMed] [Google Scholar]
- 10. Fisher HR, du Toit G, Lack G. Specific oral tolerance induction in food allergic children: is oral desensitisation more effective than allergen avoidance? A meta‐analysis of published RCTs. Arch Dis Child. 2011;96(3):259‐264. [DOI] [PubMed] [Google Scholar]
- 11. Pajno GB, Fernandez‐Rivas M, Arasi S, et al. EAACI allergen immunotherapy guidelines group. EAACI guidelines on allergen immunotherapy: IgE‐mediated food allergy. Allergy. 2018;73(4):799‐815. [DOI] [PubMed] [Google Scholar]
- 12. Bégin P, Chan ES, Kim H, et al. CSACI guidelines for the ethical, evidence‐based and patient‐oriented clinical practice of oral immunotherapy in IgE‐mediated food allergy. Allergy Asthma Clin Immunol. 2020;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Remington BC, Krone T, Koppelman S. Quantitative risk reduction through peanut immunotherapy: safety benefits of an increased threshold in Europe. Pediatr Allergy Immunol. 2018;29(7):762‐772. [DOI] [PubMed] [Google Scholar]
- 14. Dua S, Ruiz‐Garcia M, Bond S, et al. Effect of sleep deprivation and exercise on reaction threshold in adults with peanut allergy: a randomized controlled study. J Allergy Clin Immunol. 2019;144(6):1584‐1594.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2, February 2021. Cochrane; 2021. [Google Scholar]
- 16. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383‐394. [DOI] [PubMed] [Google Scholar]
- 17. Melsen WG, Bootsma MCJ, Rovers MM, Bonten MJM. The effects of clinical and statistical heterogeneity on the predictive values of results from meta‐analyses. Clin Microbiol Infect. 2014;20(2):123‐129. [DOI] [PubMed] [Google Scholar]
- 18. Santesso N, Glenton C, Dahm P, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126‐135. [DOI] [PubMed] [Google Scholar]
- 19. Chinthrajah S, Cao S, Liu C, et al. Phase 2a randomized, placebo‐controlled study of anti‐IL‐33 in peanut allergy. JCI Insight. 2019;4(22):e131347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sampson HA, Leung DY, Burks AW, et al. A phase II, randomized, double‐blind, parallel‐group, placebo‐controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127(5):1309‐1310.e1. [DOI] [PubMed] [Google Scholar]
- 21. Leung DYM, Sampson HA, Yunginger JW, et al. Effect of anti‐IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986‐993. [DOI] [PubMed] [Google Scholar]
- 22. Chu DK, Wood RA, French S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta‐analysis of efficacy and safety. Lancet. 2019;393(10187):2222‐2232. [DOI] [PubMed] [Google Scholar]
- 23. Sim K, Mijakoski D, Stoleski S, et al. Outcomes for clinical trials of food allergy treatments. Ann Allergy Asthma Immunol. 2020;125(5):535‐542. [DOI] [PubMed] [Google Scholar]
- 24. Anagnostou K, Islam S, King Y, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383(9925):1297‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bird JA, Spergel JM, Jones SM, et al. Efficacy and safety of AR101 in oral immunotherapy for peanut allergy: results of arc001, a randomized, double‐blind, placebo‐controlled phase 2 clinical trial. J Allergy Clin Immunol Pract. 2018;6(2):476‐485.e3. [DOI] [PubMed] [Google Scholar]
- 26. Blumchen K, Trendelenburg V, Ahrens F, et al. Efficacy, safety, and quality of life in a multicenter, randomized, placebo‐controlled trial of low‐dose peanut oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol Pract. 2019;7(2):479‐491.e10. [DOI] [PubMed] [Google Scholar]
- 27. Chinthrajah RS, Purington N, Andorf S, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double‐blind, placebo‐controlled, phase 2 study. Lancet. 2019;394(10207):1437‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fauquert JL, Michaud E, Pereira B, et al. Peanut gastrointestinal delivery oral immunotherapy in adolescents: results of the build‐up phase of a randomized, double‐blind, placebo‐controlled trial (PITA study). Clin Exp Allergy. 2018;48(7):862‐874. [DOI] [PubMed] [Google Scholar]
- 29. Hourihane J, Beyer K, Abbas A, et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double‐blind, randomised, placebo‐controlled phase 3 trial. Lancet Child Adolesc Health. 2020;4(10):728‐739. [DOI] [PubMed] [Google Scholar]
- 30. Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379(21):1991‐2001. [DOI] [PubMed] [Google Scholar]
- 31. Fleischer DM, Greenhawt M, Sussman G, et al. Effect of epicutaneous immunotherapy vs placebo on reaction to peanut protein ingestion among children with peanut allergy: the PEPITES randomized clinical trial. JAMA. 2019;321(10):946‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones SM, Sicherer SH, Burks AW, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol. 2017;139(4):1242‐1252.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sampson HA, Shreffler WG, Yang WH, et al. Effect of varying doses of epicutaneous immunotherapy vs placebo on reaction to peanut protein exposure among patients with peanut sensitivity: a randomized clinical trial. JAMA. 2017;318(18):1798‐1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fleischer DM, Burks AW, Vickery BP, et al. Sublingual immunotherapy for peanut allergy: a randomized, double‐blind, placebo‐controlled multicenter trial. J Allergy Clin Immunol. 2013;131(1):119‐127.e1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992;90(2):256‐262. [DOI] [PubMed] [Google Scholar]
- 36. Narisety SD, Frischmeyer‐Guerrerio PA, Keet CA, et al. A randomized, double‐blind, placebo‐controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. 2015;135(5):1275‐1282.e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caminiti L, Passalacqua G, Barberi S, et al. A new protocol for specific oral tolerance induction in children with IgE‐mediated cow's milk allergy. Allergy Asthma Proc. 2009;30(4):443‐448. [DOI] [PubMed] [Google Scholar]
- 38. Lee JH, Kim WS, Kim H, Hahn YS. Increased cow's milk protein‐specific IgG4 levels after oral desensitization in 7‐ to 12‐month‐old infants. Ann Allergy Asthma Immunol. 2013;111(6):523‐528. [DOI] [PubMed] [Google Scholar]
- 39. Longo G, Barbi E, Berti I, et al. Specific oral tolerance induction in children with very severe cow's milk‐induced reactions. J Allergy Clin Immunol. 2008;121(2):343‐347. [DOI] [PubMed] [Google Scholar]
- 40. Martorell A, De la Hoz B, Ibáñez MD, et al. Oral desensitization as a useful treatment in 2‐year‐old children with cow's milk allergy. Clin Exp Allergy. 2011;41(9):1297‐1304. [DOI] [PubMed] [Google Scholar]
- 41. Morisset M, Moneret‐Vautrin DA, Guenard L, et al. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow's milk allergy and 90 children with egg allergy. Eur Ann Allergy Clin Immunol. 2007;39(1):12‐19. [PubMed] [Google Scholar]
- 42. Pajno GB, Caminiti L, Ruggeri P, et al. Oral immunotherapy for cow’s milk allergy with a weekly up‐dosing regimen: a randomized single‐blind controlled study. Ann Allergy Asthma Immunol. 2010;105(5):376‐381. [DOI] [PubMed] [Google Scholar]
- 43. Salmivesi S, Korppi M, Mäkelä MJ, Paassilta M. Milk oral immunotherapy is effective in school‐aged children. Acta Paediatr. 2013;102(2):172‐176. [DOI] [PubMed] [Google Scholar]
- 44. Skripak JM, Nash SD, Rowley H, et al. A randomized, double‐blind, placebo‐controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. 2008;122(6):1154‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dupont C, Kalach N, Soulaines P, Legoué‐Morillon S, Piloquet H, Benhamou PH. Cow's milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. 2010;125(5):1165‐1167. [DOI] [PubMed] [Google Scholar]
- 46. Keet CA, Frischmeyer‐Guerrerio PA, Thyagarajan A, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129(2):448‐455.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takahashi M, Soejima K, Taniuchi S, et al. Oral immunotherapy combined with omalizumab for high‐risk cow's milk allergy: a randomized controlled trial. Sci Rep. 2017;7(1):17453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Akashi M, Yasudo H, Narita M, et al. Randomized controlled trial of oral immunotherapy for egg allergy in Japanese patients. Pediatr Int. 2017;59(5):534‐539. [DOI] [PubMed] [Google Scholar]
- 49. Caminiti L, Pajno GB, Crisafulli G, et al. Oral immunotherapy for egg allergy: a double‐blind placebo‐controlled study, with postdesensitization follow‐up. J Allergy Clin Immunol Pract. 2015;3(4):532‐539. [DOI] [PubMed] [Google Scholar]
- 50. Dello Iacono I, Tripodi S, Calvani M, Panetta V, Verga MC, Miceli SS. Specific oral tolerance induction with raw hen's egg in children with very severe egg allergy: a randomized controlled trial. Pediatr Allergy Immunol. 2013;24(1):66‐74. [DOI] [PubMed] [Google Scholar]
- 51. Escudero C, Rodríguez Del Río P, Sánchez‐García S, et al. Early sustained unresponsiveness after short‐course egg oral immunotherapy: a randomized controlled study in egg‐allergic children. Clin Exp Allergy. 2015;45(12):1833‐1843. [DOI] [PubMed] [Google Scholar]
- 52. Itoh‐Nagato N, Inoue Y, Nagao M, Fujisawa T, Shimojo N, Iwata T. Desensitization to a whole egg by rush oral immunotherapy improves the quality of life of guardians: a multicenter, randomized, parallel‐group, delayed‐start design study. Allergol Int. 2018;67(2):209‐216. [DOI] [PubMed] [Google Scholar]
- 53. Martín‐Muñoz MF, Belver MT, Alonso Lebrero E, et al. Egg oral immunotherapy in children (SEICAP I): daily or weekly desensitization pattern. Pediatr Allergy Immunol. 2019;30(1):81‐92. [DOI] [PubMed] [Google Scholar]
- 54. Pérez‐Rangel I, Rodríguez Del Río P, Escudero C, Sánchez‐García S, Sánchez‐Hernández JJ, Ibáñez MD. Efficacy and safety of high‐dose rush oral immunotherapy in persistent egg allergic children: a randomized clinical trial. Ann Allergy Asthma Immunol. 2017;118(3):356‐364.e3. [DOI] [PubMed] [Google Scholar]
- 55. Patriarca G, Schiavino D, Nucera E, Schinco G, Milani A, Gasbarrini GB. Food allergy in children: results of a standardized protocol for oral desensitization. Hepatogastroenterology. 1998;45(19):52‐58. [PubMed] [Google Scholar]
- 56. Staden U, Rolinck‐Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62(11):1261‐1269. [DOI] [PubMed] [Google Scholar]
- 57. Nowak‐Węgrzyn A, Wood RA, Nadeau KC, et al. Multicenter, randomized, double‐blind, placebo‐controlled clinical trial of vital wheat gluten oral immunotherapy. J Allergy Clin Immunol. 2019;143(2):651‐661.e9. [DOI] [PubMed] [Google Scholar]
- 58. Enrique E, Pineda F, Malek T, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double‐blind, placebo‐controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005;116(5):1073‐1079. [DOI] [PubMed] [Google Scholar]
- 59. Fernández‐Rivas M, Garrido Fernández S, Nadal JA, et al. Randomized double‐blind, placebo‐controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy. 2009;64(6):876‐883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1
App S2
App S3
App S4
App S5
App S6
App S7


