Abstract
Chlorprothixene is commonly used off‐label in low doses for sedative‐hypnotic purposes although it might carry a risk of cardiometabolic adverse events due to its pharmacodynamic profile. We investigated the risk of diabetes and major adverse cardiovascular events (MACE) with use of low‐dose chlorprothixene, compared with use of low‐dose quetiapine in a nationwide cohort study, including all new users of low‐dose chlorprothixene (n = 81 328) and low‐dose quetiapine (n = 91 163) in Denmark 2000–2017. Main outcomes were diabetes and MACE (myocardial infarction, stroke and death from cardiovascular causes). The association between cumulative dose of chlorprothixene and the outcomes was tested in a case–control analysis. Low‐dose chlorprothixene use was associated with increased risk of diabetes (intention‐to‐treat [ITT]‐hazard ratio [HR]: 1.16; 95% CI: 1.08–1.25), compared with low‐dose quetiapine use. This association strengthened when follow‐up was restricted to time on treatment (as‐treated [AT]‐HR: 1.34; 95% CI: 1.14–1.56). Low‐dose chlorprothixene use was also associated with increased risk of MACE (ITT‐HR: 1.12; 95% CI: 1.04–1.21) and stroke (ITT‐HR: 1.21; 95% CI: 1.06–1.37) but not with myocardial infarction (ITT‐HR: 1.11; 95% CI: 0.95–1.30) nor death from cardiovascular causes (ITT‐HR: 1.07; 95% CI: 0.96–1.20). Cumulative dose of chlorprothixene ≥6000 mg was associated with increased risk of diabetes (OR: 1.15–1.63; test for trend: p < 0.001), whereas cumulative dose of chlorprothixene ≥1500 mg was associated with increased risk of MACE (OR: 1.10–1.85; test for trend: p < 0.001). In conclusion, low‐dose chlorprothixene use is associated with increased risk of cardiometabolic adverse events compared with low‐dose quetiapine use.
Keywords: antipsychotic agents, cardiovascular diseases, chlorprothixene, diabetes mellitus, pharmacoepidemiology, quetiapine fumarate
1. INTRODUCTION
Chlorprothixene is a low‐potency, first‐generation antipsychotic drug, which was first marketed in 1959 and is still marketed in 16 countries despite the growing number of second‐generation antipsychotics available for clinical use. 1 , 2
Licenced indications for chlorprothixene differ among countries. The licenced indications in Denmark are schizophrenia and other psychoses, 3 whereas in Norway, it also includes anxiety and withdrawal symptoms 4 and in Germany symptoms of mania. 5 Despite the limited indications, chlorprothixene remains a commonly used antipsychotic in Scandinavia and in a number of other countries 6 , 7 with evidence of substantial off‐label use. 8
Unlike most other first‐generation antipsychotics, chlorprothixene has high affinity for 5‐HT2C‐, H1‐ and M3‐receptors (Table 1). 9 Antagonistic action at these receptors is responsible for some of the anxiolytic and hypnotic properties of chlorprothixene but has also been connected to the development of metabolic disturbances (impaired glucose metabolism or dyslipidaemia). 10 , 11 Overall, the pharmacological profile of chlorprothixene is very similar to that of olanzapine and clozapine, which have been associated with high rates of metabolic disturbances. 12 , 13
TABLE 1.
Receptor binding profile of chlorprothixene with receptors involved in metabolic dysregulation in comparison with other antipsychotics associated with metabolic adverse events
| Antipsychotic | ||||
|---|---|---|---|---|
| Receptor | Chlorprothixene | Quetiapine | Olanzapine | Clozapine |
| D2 | 5.6 | 770 | 20 | 210 |
| 5‐HT1A | 138 | 300 | 610 | 160 |
| 5‐HT2C | 4.5 | 3500 | 4.1 | 4.8 |
| α2 | 186 | 80 | 280 | 158 |
| H1 | 3.8 | 19 | 0.1 | 3.1 |
| M3 | 22 | 1320 | 126 | 109 |
Chlorprothixene was first approved in the late 1950s, and clinical studies did not focus on metabolic adverse events at the time but rather on adverse effects like extrapyramidal symptoms. 1 , 14
1.1. Aims of the study
Given the potential for cardiometabolic adverse events with chlorprothixene due to its pharmacological properties, further studies on the cardiometabolic safety of chlorprothixene are warranted. Our aim was therefore to assess the risk of diabetes, major cardiovascular events and mortality with off‐label, low‐dose use of chlorprothixene, by using Danish nationwide registers and comparing with off‐label, low‐dose use of the commonly used second‐generation antipsychotic quetiapine.
2. METHODS
2.1. Study design
We conducted a new‐user, active‐comparator cohort study based on nationwide Danish health registers. To minimize confounding‐by‐indication, that is, outcomes that are related to the indication of the drug rather than an intrinsic biological effect, we used initiators of low‐dose quetiapine as comparator group, as this antipsychotic is also commonly used off‐label for anxiolytic and hypnotic purposes. 8 , 15
2.2. Study population
A cohort was created of new users of chlorprothixene and quetiapine, respectively, who were identified in the Danish National Prescription Register 16 between 1 January 2000 and 31 December 2017. The date of first chlorprothixene or quetiapine prescription was used as the index date. New use was defined as no records of prescriptions for the antipsychotic (chlorprothixene or quetiapine) between the Danish National Prescription Register's establishment in 1995 and the index date.
Exclusion criteria:
Prescriptions for both chlorprothixene and quetiapine on the index date
Prescription(s) for quetiapine, chlorprothixene or other antipsychotics within 365 days before the index date
Previously registered with a diagnosis of severe mental disorders, that is, schizophrenia, schizoaffective disorder and bipolar disorder
Less than 365 days of register coverage prior to the index date
Age above 85 years at the index date
Chlorprothixene or quetiapine prescriptions for tablet strengths >50 mg on the index date
We excluded individuals with a history of severe mental disorders and individuals who had filled chlorprothixene or quetiapine prescriptions for tablet strengths greater than 50 mg on the index date (‘high‐dose use’) to compare off‐label, low‐dose use of both drugs. Individuals that met any of these criteria after inclusion were censored. The data sources are described in further detail in Appendix S1, and the codes used for exposure assessment are presented in Appendix S2.
Lastly, two sub‐cohorts were established based on the initial cohort: one for analysis of diabetes, where individuals with a diagnosis of diabetes or prescriptions for anti‐diabetic medications (Appendix S2) were excluded, and one for analyses of cardiovascular outcomes and death, where individuals with diagnoses of myocardial infarction, stroke or cancer (except non‐melanoma skin cancer) were excluded.
2.3. High‐dimensional propensity score
The propensity for receiving treatment with off‐label chlorprothixene was estimated separately in both cohorts using logistic regression. The regression model included age, sex, year of treatment initiation (forced covariates) and the 50 covariates with the highest potential for confounding (i.e., diagnoses or medications associated both with increased risk of the outcome and with uneven distribution between groups, see Appendices S4 and S5). These covariates were identified using a high‐dimensional propensity score algorithm, 17 assessing all filled prescriptions and registered hospital diagnoses within 365 days before the index date. Chlorprothixene users were then matched 1:1 with quetiapine users, using nearest‐neighbour matching allowing a calliper of 0.02 after trimming non‐overlapping regions of the propensity score distribution. Covariate balance in the matched cohort was assessed using standardized mean differences, with values ≤0.1 indicating adequate balance. 18
2.4. Outcomes
The two primary outcomes were diabetes and major adverse cardiovascular events (MACE). Diabetes onset was defined as the first occurrence of either (i) first prescription of an anti‐diabetic medication in the Danish National Prescription Register, (ii) first diagnosis of diabetes in the Danish National Patient Register or (iii) first measurement of glycated haemoglobin A1c (HbA1c) of ≥6.4%/48 mmol/mol recorded in the Danish National Laboratory Databank. 19 MACE was defined by first occurrence of either (i) myocardial infarction, (ii) stroke or (iii) death from a cardiovascular cause. Secondary outcomes were myocardial infarction, stroke, death from cardiovascular causes (the elements of MACE) as separate entities and death from any cause. In analyses of the secondary outcomes, their occurrence was assessed regardless of other events. Codes used for outcome assessment are listed in Appendix S2.
2.5. Follow‐up and censoring
In the intention‐to‐treat (ITT) analyses of the association between treatment initiation and outcomes, individuals were followed from 30 days after their first prescription of low‐dose chlorprothixene or quetiapine until they experienced the outcome of interest, died or were censored (see below). In the as‐treated (AT) analyses of the association between continuous treatment and outcomes, we followed individuals from 30 days after their first prescription until they experienced the outcome of interest, died, were censored (see below) or reached the end of their first treatment episode (see below and Appendix S3). The 30‐day lag time was used to avoid reverse causation, such that chlorprothixene would be prescribed for anxiety and insomnia related to imminent vascular events or unusual stressful conditions.
Reasons for censoring were (i) fulfilment of an exclusion criterion, (ii) emigration, (iii) 10 years of follow‐up, (iv) end of data availability (31 December 2018), (v) switching to the other study drug, (vi) filling of prescriptions for chlorprothixene/quetiapine in tablet strengths >50 mg, and (vii) filling of prescriptions for other antipsychotics or receiving a diagnosis of a severe mental disorder, whatever came first.
Treatment duration was calculated based on the number of tablets filled, assuming average use of one tablet per day. We added 90 days of additional follow‐up to prescriptions to account for irregular use and stockpiling. Any gap between prescription fills exceeding 90 days was considered treatment breaks and end of the current treatment episode. We also added 90 days of additional follow‐up to the duration of the last treatment episode to capture events occurring shortly after (but potentially associated with) the treatment episode and to avoid immortal time bias (see Appendix S3 for illustration). 20
2.6. Statistical analysis
2.6.1. Main analysis
In both ITT and AT analyses, the number of events during follow‐up was used to calculate incidence rates (IRs) expressed as events per 1000 person‐years of follow‐up. Furthermore, we used Cox proportional hazard regression models to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) for the association between low‐dose use of chlorprothixene and the outcomes, compared with low‐dose use of quetiapine. In analyses of diabetes, the models were additionally adjusted for history of dementia as this risk factor was not evenly distributed among individuals in the matched cohorts. In analyses of cardiovascular outcomes, the models were additionally adjusted for history of major depression. Lastly, we calculated the number of cases attributable to low‐dose chlorprothixene use in comparison with low‐dose use of quetiapine.
2.6.2. Cumulative incidence
Cumulative incidence of events was calculated using the Nelson–Aalen cumulative hazard function and depicted as the cumulative incidence function considering death as competing event (death from non‐cardiovascular causes in analyses of MACE/death from cardiovascular causes).
2.6.3. Subgroup analyses
In order to compare risk between different subgroups, we conducted a number of pre‐specified subgroup analyses, stratifying by (i) sex, (ii) age group (0–17, 18–64 and 65–85 years), (iii) prediabetes at baseline (in analyses of diabetes, [HbA1c 39–47 mmol/mol or 5.7–6.3%]), (iv) history of diabetes or ischaemic heart disease (in analyses of cardiovascular outcomes), (v) history of alcohol‐related conditions, (vi) history of substance abuse and (vii) history of dementia (in analyses of cardiovascular outcomes). Codes used for definition of subgroups are listed in Appendix S2.
2.6.4. Sensitivity analyses
To test whether analytic choices or assumptions could have affected our results, we conducted the following sensitivity analyses: (i) estimation of associations between exposure and outcomes in the full cohorts using standardized mortality ratio (SMR) weights to control for baseline confounding as alternative to high‐dimensional propensity score matching 21 ; (ii) variation of the maximum follow‐up from 10 years to 0.5, 1, 3 and 5 years; (iii) extension of the exclusion period for prior use of quetiapine, chlorprothixene or other antipsychotics from 1 to 5 years to assess the potential impact from prior antipsychotic exposure on the results; (iv) excluding HbA1c measurements from the outcome definition; (v) stratifying on year of cohort entry (</≥2012 where HbA1c values were adapted in the diagnostic criteria for diabetes; and (vi) considering death as competing risk in analyses of diabetes and MACE using Fine and Gray's method. To supplement sensitivity analyses, we conducted a quantitative bias analysis by calculating E values for all outcomes showing an increased risk with use of chlorprothixene (see Appendix S22 for further description).
2.6.5. Analysis of relation with cumulative dose
To examine the relation between cumulative dose of chlorprothixene and the risk of adverse events, we conducted a case–control analysis nested in the overall cohort of chlorprothixene users. Each case was matched with up to 10 controls on age and sex using risk‐set sampling in this population. We used a sampling frame similar to the follow‐up used in ITT analyses. With the use of conditional logistic regression, we estimated odds ratios (ORs) with 95% CIs, for the relation between cumulative dose categories of chlorprothixene and the respective outcomes (1501–3000/3001–6000/6001–12 000/12 001–24 000/24 001–50 000/>50 000 mg). A cumulative dose of ≤1500 mg was used as reference, as this corresponds to the smallest package of chlorprothixene currently marketed in Denmark. Test for trend in the association between cumulative dose of chlorprothixene and the outcomes was tested using conditional logistic regression with dose strata as independent variable.
2.7. Other
Access to pseudonymized data was provided by the Danish Health Data Authority (FSEID‐4279). No ethical approval or informed consent is needed for purely register‐based studies in Denmark. All analyses were conducted using Stata MP, release 17.0 (StataCorp, College Station, TX, USA).
3. RESULTS
3.1. Characteristics of the study population
We identified 120 585 new users of chlorprothixene and 168 316 new users of quetiapine in the DNPR between 1 January 2000 and 31 December 2017. From this population, 81 328 low‐dose chlorprothixene users and 91 163 low‐dose quetiapine users adhered to the in‐ and exclusion criteria and were eligible to be included in the study (67% and 54% of all new users, respectively; Appendix S6). After high‐dimensional propensity score matching, the cohort without a history of diabetes consisted of 43 094 chlorprothixene users and 43 094 quetiapine users and the cohort without history of myocardial infarction, stroke or cancer consisted of 42 818 chlorprothixene users and 42 818 quetiapine users, representing 53% and 52% of all eligible chlorprothixene users, respectively. The cohorts were overall well‐balanced, including year of treatment initiation. Two exceptions, where history of dementia was more prevalent among quetiapine users than among chlorprothixene users in the cohort without history of diabetes, and similar difference was observed for history of major depression in the cohort without history of myocardial infarction, stroke or cancer (Table 2). Variables included in the propensity score models, the propensity score distributions and baseline characteristics for the full cohorts are shown in Appendices S4, S5, S7 and S8.
TABLE 2.
Baseline characteristics of the high‐dimensional propensity score‐matched cohorts
| Without history of diabetes | Without history of MI, stroke or cancer | |||||
|---|---|---|---|---|---|---|
| Chlorprothixene | Quetiapine | Chlorprothixene | Quetiapine | |||
| (N = 43 094) | (N = 43 094) | SMD | (N = 42 818) | (N = 42 818) | SMD | |
| Sex, N (%) | ||||||
| Female | 23 079 (54) | 22 657 (53) | <0.1 | 22 916 (54) | 22 768 (53) | <0.1 |
| Age, N (%) | ||||||
| Median (IQR) | 41 (27–55) | 39 (26–53) | 40 (26–53) | 39 (26–51) | ||
| 0–17 years | 2673 (6) | 2104 (5) | 0.1 | 3308 (8) | 2148 (5) | 0.1 |
| 18–44 years | 21 739 (50) | 23 816 (55) | 0.1 | 21 984 (51) | 24 406 (57) | 0.1 |
| 45–64 years | 13 177 (31) | 11 316 (26) | 0.1 | 12 717 (30) | 11 995 (28) | <0.1 |
| 65–85 years | 5505 (13) | 5858 (14) | <0.1 | 4809 (11) | 4269 (10) | <0.1 |
| Year of cohort entry, N (%) | ||||||
| 2000–2005 | 1432 (3) | 1727 (4) | <0.1 | 1139 (3) | 1465 (3) | <0.1 |
| 2006–2011 | 18 779 (44) | 18 621 (43) | <0.1 | 18 864 (44) | 18 843 (44) | <0.1 |
| 2012–2017 | 22 883 (53) | 22 746 (53) | <0.1 | 22 815 (53) | 22 510 (53) | <0.1 |
| Comorbidities, N (%) | ||||||
| Ischaemic heart disease | 2096 (5) | 2006 (5) | <0.1 | 1525 (4) | 1319 (3) | <0.1 |
| Heart failure | 510 (1) | 542 (1) | <0.1 | 380 (<1) | 350 (<1) | <0.1 |
| Peripheral vascular disease | 845 (2) | 844 (2) | <0.1 | 737 (2) | 625 (1) | <0.1 |
| Hypertension | 6546 (15) | 6101 (14) | <0.1 | 6514 (15) | 5808 (14) | <0.1 |
| COPD | 5886 (14) | 5196 (12) | <0.1 | 5725 (13) | 5070 (12) | <0.1 |
| Diabetes | ‐ | ‐ | ‐ | 2083 (5) | 2006 (5) | <0.1 |
| Obesity | 2544 (6) | 2521 (6) | <0.1 | 2955 (7) | 3072 (7) | <0.1 |
| Alcohol‐related disorders | 12 690 (29) | 13 100 (30) | <0.1 | 12 737 (30) | 13 210 (31) | <0.1 |
| Neurological disorders | 6766 (16) | 7211 (17) | <0.1 | 6460 (15) | 6885 (16) | <0.1 |
| Dementia | 564 (1) | 2046 (5) | 0.2 | 540 (1) | 1149 (3) | 0.1 |
| Major depression | 10 199 (24) | 12 764 (30) | 0.1 | 9518 (22) | 13 963 (33) | 0.2 |
| Anxiety disorders | 14 669 (34) | 15 185 (35) | <0.1 | 14 798 (35) | 15 388 (36) | <0.1 |
| Personality disorders | 5134 (12) | 5915 (14) | 0.1 | 5093 (12) | 6095 (14) | 0.1 |
| Drugs used in the past year, N (%) | ||||||
| Acetylsalicylic acid | ‐ | ‐ | ‐ | 2444 (6) | 2193 (5) | <0.1 |
| Statins | ‐ | ‐ | ‐ | 4016 (9) | 3547 (8) | <0.1 |
| Antidiabetic agents | ‐ | ‐ | ‐ | 1744 (4) | 1702 (4) | <0.1 |
| Oral glucocorticoids | 2500 (6) | 2370 (5) | <0.1 | ‐ | ‐ | ‐ |
| Mirtazapine | 7499 (17) | 6803 (16) | <0.1 | ‐ | ‐ | ‐ |
| Antihistamines | 4058 (9) | 3711 (9) | <0.1 | ‐ | ‐ | ‐ |
| Haemoglobin A1c at baseline, N (%) a | ||||||
| Normal | 2377 (6) | 2436 (6) | <0.1 | ‐ | ‐ | ‐ |
| Prediabetes | 751 (2) | 609 (1) | <0.1 | ‐ | ‐ | ‐ |
| Missing | 39 966 (93) | 40 049 (93) | <0.1 | ‐ | ‐ | ‐ |
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range; MI, myocardial infarction; N, number; SMD, standardized mean difference.
Prediabetes at baseline defined as haemoglobin A1c 39–47 mmol/mol or 5.7–6.3%.
Total follow‐up was 335 018 person‐years in the matched cohort without a history of diabetes and 342 409 person‐years in the matched cohort without history of myocardial infarction, stroke or cancer. In AT analyses, the median follow‐up was 0.5 years (interquartile range [IQR]: 0.5–0.6 years) for low‐dose chlorprothixene users and 0.5 years (IQR: 0.5–1.0 years) for low‐dose quetiapine users in both cohorts. However, 13% of low‐dose chlorprothixene users and 24% of low‐dose quetiapine users had more than 1 year of follow‐up. The median number of prescriptions was 1 (IQR: 1–2) for low‐dose chlorprothixene users and 2 (IQR: 1–5) for low‐dose quetiapine users. Further details on follow‐up and reasons for censoring can be found in Appendices S9 and 10.
3.2. Risk of diabetes
In ITT analyses, diabetes occurred in 1579 low‐dose chlorprothixene users and 1192 low‐dose quetiapine users, resulting in an increased risk of diabetes with initiation of low‐dose chlorprothixene, compared with initiation of low‐dose quetiapine (HR: 1.16; 95% CI: 1.08–1.25; Table 3). In AT analyses, the risk of diabetes was further increased with continuous use of low‐dose chlorprothixene compared with continuous use of low‐dose quetiapine (HR: 1.34, 95% CI: 1.14–1.56), and a clear difference from use of low‐dose quetiapine was evident with long‐term treatment (i.e., after 3 years of continuous treatment; Figure 1A,B). The cumulative incidence of diabetes with use of chlorprothixene was higher in AT analysis than in ITT analysis (~11 vs. 8%). The proportion of diabetes events attributable to continuous use over 10 years of low‐dose chlorprothixene (attributable proportion among exposed) was 16% (~48 cases).
TABLE 3.
Association between use of chlorprothixene, diabetes, major adverse cardiovascular events and all‐cause mortality, compared with use of low‐dose quetiapine in the hdPS‐matched cohorts
| Chlorprothixene | Quetiapine | ||||
|---|---|---|---|---|---|
| Events, N | Incidence rate, events per 1000 person‐years | Events, N | Incidence rate, events per 1000 person‐years | HR (95% CI) a | |
| Intention‐to‐treat analysis | |||||
| Diabetes | 1579 | 8.8 | 1192 | 7.7 | 1.16 (1.08–1.25) |
| Major adverse cardiovascular events | 1462 | 8.0 | 1125 | 7.1 | 1.12 (1.04–1.21) |
| Myocardial infarction | 370 | 2.0 | 291 | 1.8 | 1.11 (0.95–1.30) |
| Stroke | 585 | 3.2 | 419 | 2.6 | 1.21 (1.06–1.37) |
| Death from cardiovascular causes | 676 | 3.6 | 535 | 3.3 | 1.07 (0.96–1.20) |
| Death from any cause | 2349 | 12.6 | 2076 | 12.9 | 0.97 (0.91–1.02) |
| As‐treated analysis | |||||
| Diabetes | 310 | 10.6 | 349 | 8.9 | 1.34 (1.14–1.56) |
| Major adverse cardiovascular events | 323 | 11.0 | 439 | 11.2 | 1.07 (0.92–1.24) |
| Myocardial infarction | 56 | 1.9 | 83 | 2.1 | 0.95 (0.67–1.35) |
| Stroke | 125 | 4.3 | 136 | 3.5 | 1.36 (1.06–1.74) |
| Death from cardiovascular causes | 169 | 5.7 | 267 | 6.8 | 0.92 (0.75–1.12) |
| Death from any cause | 592 | 20.1 | 955 | 24.2 | 0.88 (0.80–0.98) |
Abbreviations: CI, confidence interval; hdPS, high‐dimensional propensity score; HR, hazard ratio.
Adjusted for age, sex and 50 covariates through matching on a high‐dimensional propensity score. Follow‐up began 30 days after the first prescription. Maximum follow‐up in all analyses was 10 years. Analyses with diabetes as outcome additionally adjusted for history of dementia. Analyses of cardiovascular outcomes and death from natural causes additionally adjusted for history of major depression.
FIGURE 1.
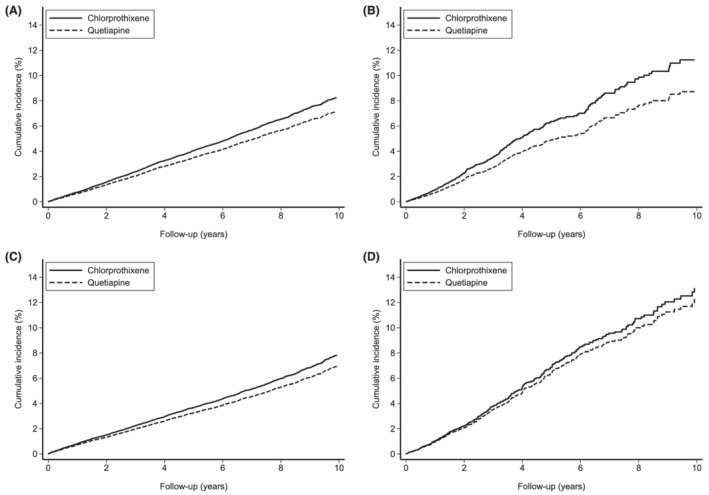
Cumulative incidence of diabetes and major adverse cardiovascular events in the high‐dimensional propensity score‐matched cohort. (A) Intention‐to‐treat analysis of diabetes, (B) as‐treated analysis of diabetes, (C) intention‐to‐treat analysis of major adverse cardiovascular events and (D) as‐treated analysis of major adverse cardiovascular events
IRs for diabetes with continuous low‐dose use of chlorprothixene did not differ between females and males (IR: 10.8 vs. 10.3/1000 person‐years; Figure 2) but increased with age (18–64 years: 9.9/1000 person‐years vs. 65–85 years: 16.7/1000 person‐years). The IRs of diabetes among females and 18‐ to 64‐year‐old individuals were considerably higher with continuous low‐dose treatment with chlorprothixene compared with quetiapine (AT analysis: HR [females]: 1.49; 95% CI: 1.20–1.84 and HR [18–64 years]: 1.28; 95% CI: 1.07–1.53). There were less than 5 cases of diabetes among those aged below 18 years at treatment initiation. Among those with prediabetes at treatment initiation, the IRs of diabetes were not considerably higher among low‐dose chlorprothixene users than among low‐dose quetiapine users (AT analysis: IR 32.8 vs. 15.9/1000 person‐years). However, the low number of individuals with blood glucose measurements at baseline (7–8% in both groups) did not allow for a meaningful formal comparison of users with prediabetes at treatment initiation.
FIGURE 2.
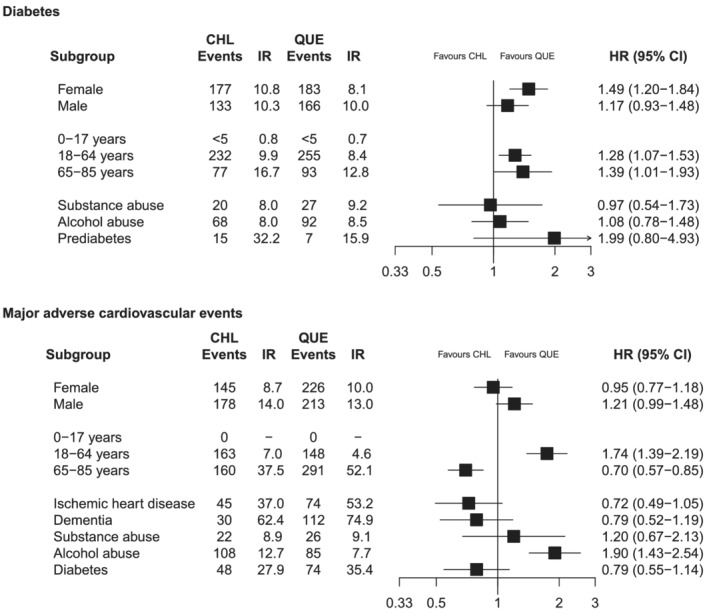
Association between use of chlorprothixene, diabetes and major adverse cardiovascular events in subgroups of the high‐dimensional propensity score‐matched cohort. Results from as‐treated analyses. Analyses with diabetes as outcome additionally adjusted for history of dementia. Analyses of major adverse cardiovascular events additionally adjusted for history of major depression. Abbreviations: CHL: chlorprothixene, CI: confidence interval, HR: hazard ratio, IR: incidence rate (events per 1000 person‐years), QUE: quetiapine
Cumulative dose of chlorprothixene >6000 mg as low‐dose treatment was associated with increased risk of diabetes compared with use of ≤1500 mg (OR: 1.16–1.63), with evidence of a dose–response relationship (test for trend p < 0.001; Figure 3A and Appendix S11).
FIGURE 3.
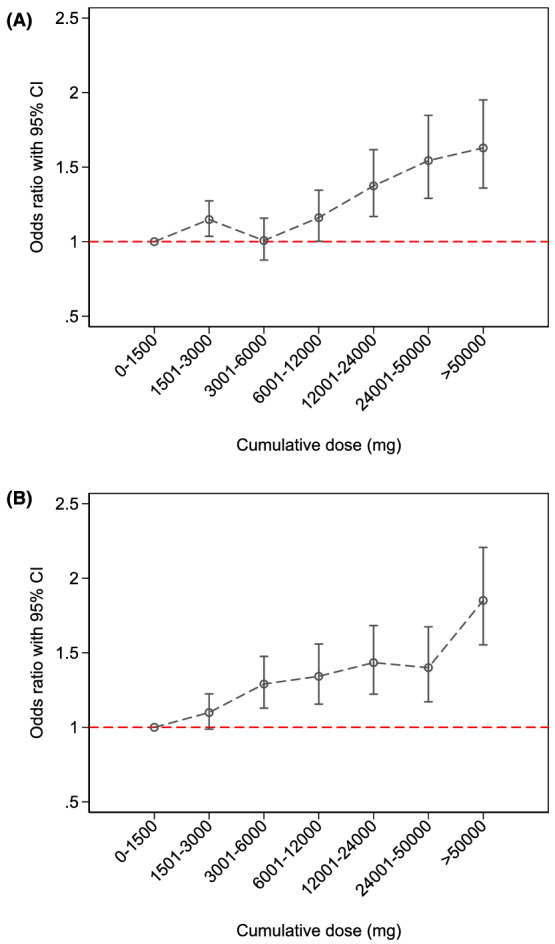
Association between cumulative dose of chlorprothixene and diabetes or major adverse cardiovascular events. (A) Diabetes and (B) major adverse cardiovascular events
3.3. Risk of major cardiovascular events
MACE occurred in 1462 users of low‐dose chlorprothixene and 1125 users of low‐dose quetiapine during follow‐up. Initiation of low‐dose chlorprothixene was associated with increased risk of MACE, compared with initiation of low‐dose quetiapine (ITT‐adjusted hazard ratio [aHR] 1.12; 95% CI: 1.04–1.21; Table 2). However, the risk of continuous use of chlorprothixene did not differ significantly from the risk of continuous use of low‐dose quetiapine (AT‐aHR: 1.07; 95% CI: 0.92–1.24). While the overall HR did not differ between treatment groups, the cumulative incidence of MACE was higher among individuals who used chlorprothixene continuously compared with continuous use of low‐dose quetiapine (~13% vs. ~8%; Figure 1C,D).
The IR of MACE among low‐dose chlorprothixene users was higher among males compared with females (IR: 14.0 vs. 8.7/1000 person‐years) with a similar pattern observed among low‐dose quetiapine users (Figure 2). The IR of MACE was significantly higher among low‐dose chlorprothixene users aged 18–64 years, compared with low‐dose quetiapine users of the same age (7.0 vs. 4.6/1000 person‐years; HR: 1.74; 95% CI: 1.39–2.19). There were no events of MACE among those aged below 18 years at treatment start. History of alcohol‐related conditions was associated with increased rates of MACE among low‐dose chlorprothixene users in comparison with low‐dose quetiapine users (IR: 12.7 vs. 7.7/1000 person‐years; HR: 1.90; 95% CI: 1.43–2.54), whereas a lower, although still high, risk was observed among those with ischaemic heart disease (IR: 37.0 vs. 53.2/1000 person‐years).
Any cumulative dose of chlorprothixene above >3000 mg was associated with increased risk of MACE compared with use of ≤1500 mg (OR: 1.29–1.85), and increasing cumulative dose was associated with increased risk of MACE (test for trend p < 0.001; Figure 3B and Appendix S11).
3.4. Risk of myocardial infarction, stroke, death from cardiovascular causes and death from any cause
The risk of myocardial infarction did not differ significantly between low‐dose chlorprothixene users and low‐dose quetiapine users (ITT‐aHR: 1.11; 95% CI: 0.95–1.30 and AT‐aHR: 0.95; 95% CI: 0.67–1.35; Table 3 and Appendices S12 and S13) and showed no relation with cumulative dose (OR: 0.77–1.08; test for trend p = 0.81; Appendix S11). The risk of stroke was significantly increased with initiation of low‐dose chlorprothixene, compared with initiation of low‐dose quetiapine (ITT‐aHR: 1.21; 95% CI: 1.06–1.37 and AT‐aHR: 1.36; 95% CI: 1.06–1.74; Table 3), and the cumulative incidence of stroke was higher during long‐term low‐dose treatment with chlorprothixene compared with quetiapine (~6 vs. ~4%; Appendix S12). The IR of stroke was higher among those aged 18–64 years when treated with chlorprothixene (IR: 2.8 vs. 1.8; HR: 1.80; 95% CI: 1.25–2.58; Appendix S13). Overall, the risk of death from cardiovascular causes and death from any cause did not differ between low‐dose chlorprothixene‐ and low‐dose quetiapine users, although subgroup analyses found increased risk for both outcomes with use of low‐dose chlorprothixene among those aged 18–64 years and those with a history of alcohol abuse (Appendices S14 and S15).
3.5. Sensitivity analyses
Using SMR weights to account for baseline confounding in the full cohort or extending the exclusion period for prior antipsychotic use from 1 to 5 years did not alter the observed associations considerably (Appendices S16 and S17). HRs were generally smaller with shorter maximum follow‐up durations, and the increased risk of diabetes was present even at a maximum follow‐up length of 3 years or longer (Appendix S18). Excluding individuals with a history of substance abuse did not negate the observed risk of diabetes (Appendix S19). Exclusion of HbA1c measurement from the outcome definition did not negate the observed increase in risk related to continuous use of chlorprothixene, compared with continuous use of quetiapine (Appendix S20). The increased risk of diabetes seen with use of chlorprothixene was mainly driven by individuals initiating treatment before 2012 (Appendix S21). For an unmeasured confounder to negate the observed increases in risk of diabetes, MACE and stroke, the association would have to result in a HR of at least 1.49 (Appendix S22). Considering death as a competing risk did not attenuate the observed associations between chlorprothixene, diabetes, and MACE (Appendix S23).
4. DISCUSSION
In this nationwide cohort study of cardiometabolic outcomes to off‐label, low‐dose use of chlorprothixene, we found increased risk of diabetes with long‐term use of low‐dose chlorprothixene, compared with quetiapine. The risk of MACE did not clearly differ between low‐dose chlorprothixene and quetiapine users, although results suggested an increased risk of MACE and stroke, especially with long‐term chlorprothixene treatment and among those aged 18–64 years, compared with use of quetiapine.
The observed increased risk of diabetes and MACE related to low‐dose chlorprothixene use compared with low‐dose quetiapine use did not manifest before three years of continuous treatment. This temporal relationship is consistent with the underlying pathophysiology—that hyperglycaemia or dyslipidaemia might develop quickly after treatment initiation but will most likely not cause diabetes, coronary artery disease or cerebrovascular disease until years later. The observed difference in risk between low‐dose chlorprothixene‐ and low‐dose quetiapine users matched on a high number of potential confounders suggests that chlorprothixene carries a higher risk of cardiometabolic adverse events and is not solely attributable to differences between users. This could be explained by similarities of the pharmacodynamic profile between chlorprothixene and antipsychotic drugs like olanzapine and clozapine 9 that have been associated with high risk of metabolic disturbances 22 , 23 and development of cardiovascular disease. 24 All three drugs have a relatively high affinity for 5‐HT2C‐, H1‐ and M3‐receptors, 10 which are involved in the development of antipsychotic‐induced weight gain, hyperglycaemia and potentially dyslipidaemia. 11 , 25 , 26 The dose–response relationship seen in case–control analyses of the relation between cumulative dose and risk of outcomes is in accordance with the increased risk of diabetes seen in AT analyses.
Analyses of subgroups showed a higher risk of cardiovascular events with low‐dose chlorprothixene use compared with low‐dose quetiapine use among individuals below 65 years and with a history of alcohol abuse, which supports the contribution of chlorprothixene to development of cardiovascular disease. Although the highest IRs of MACE were generally observed among individuals with other risk factors for diabetes and cardiovascular events, for example, high age, ischaemic heart disease or alcohol abuse‐related disorders, the rates were not different from those observed among low‐dose quetiapine users.
To our knowledge, this is the first study to investigate the cardiometabolic safety of chlorprothixene in its most predominant role today—as anxiolytic or hypnotic used off‐label and at low doses. 6 , 8 Only one prior study has investigated the cardiovascular safety of chlorprothixene and found similar rates of MACE with use beyond one year. 27 However, that study was restricted to an elderly population (≥70 years) and did not investigate low‐dose use specifically and therefore did not generalize to the majority of chlorprothixene users, who are off‐label users and younger than 70 years. 27 , 28 No studies on the risk of diabetes with off‐label or low‐dose use of chlorprothixene were identified.
4.1. Strengths and limitations
There are several strengths of the present analysis and its design: Firstly, the use of nationwide data sources allowed us to include and follow a high number of subjects for extended periods of time and capture a substantial number of events for each outcome. Secondly, the application of strict eligibility and censoring criteria allowed us to conduct a cohort study emulating a hypothetical ‘trial’ of chlorprothixene against a clinically relevant comparator drug, isolating the effect of chlorprothixene and quetiapine and thus reducing the contributions to cardiometabolic risk from other risk factors such as recent or current use of other antipsychotics and severe mental illness. Thirdly, the application of a high‐dimensional propensity score allowed us to utilize the rich register data on prescriptions and diagnoses to control for a wide range of potential confounders. Lastly, the additional case–control analysis, with evidence of increasing risk with increasing cumulative dose, and a number of sensitivity analyses enforced the confidence in the main findings.
There are, however, also limitations associated with both the data sources and the analytical strategy: Firstly, a considerable proportion of chlorprothixene users were excluded from the analyses because of eligibility criteria and the use of propensity score matching. This was necessary to isolate the effect of chlorprothixene from other risk factors for cardiovascular disease and thus to ensure internal validity. Nonetheless, estimating the effect in a subpopulation might limit the generalizability of our results to the entire population of chlorprothixene users in Denmark. However, baseline characteristics for the full cohort were very similar to those of the matched cohorts (Table 1 and Appendix S6), and SMR‐weighted estimates using the entire eligible cohort were very similar, suggesting that this is a minor limitation. Secondly, the majority of chlorprothixene and quetiapine users had only one prescription within the follow‐up period, which means that results from the ITT analyses should be interpreted cautiously as the observed IRs are likely to reflect other exposures during the average follow‐up of 4–5 years after the first prescription. Therefore, we believe that the results from the AT analysis are of most importance. Thirdly, HbA1c measurements, used to substantiate the outcome‐definition for diabetes, were only available for the last part of the study period. Lastly, it is currently unknown if the comparator quetiapine, used in low doses, is associated with an increased risk of cardiovascular adverse events, even though a recent Danish cohort study rejected a substantial risk of diabetes with use of off‐label, low‐dose use of quetiapine. 29 Still, even if low‐dose quetiapine was associated with an increased risk of cardiovascular events, the study design would mimic the clinical dilemma: Should chlorprothixene or quetiapine be the chosen antipsychotic for anxiolytic and hypnotic purposes?
4.2. Conclusions and implications
In conclusion, long‐term use of low‐dose chlorprothixene is associated with increased risk of diabetes, compared with long‐term treatment with low‐dose quetiapine in off‐label situations. Furthermore, long‐term low‐dose chlorprothixene use is associated with increased risk of major cardiovascular events, including stroke and death from cardiovascular cause, compared with long‐term low‐dose quetiapine use. We recommend that clinicians consider the increased risk of cardiometabolic adverse events when using chlorprothixene off‐label by taking appropriate actions to monitoring and modification of risk factors for cardiometabolic disease. Although any dose of chlorprothixene or duration of treatment might increase the risk of cardiometabolic adverse events and should include appropriate monitoring, this is of special importance with long‐term use of chlorprothixene.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGEMENT
This study was supported by Mental Health Services in the Region of Southern Denmark (grant number A2957).
Højlund M, Wagner CB, Wesselhoeft R, Andersen K, Fink‐Jensen A, Hallas J. Use of chlorprothixene and the risk of diabetes and major adverse cardiovascular events: A nationwide cohort study. Basic Clin Pharmacol Toxicol. 2022;130(4):501-512. doi: 10.1111/bcpt.13711
Funding information Mental Health Services in the Region of Southern Denmark, Grant/Award Number: A2957
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available due to confidentiality issues but can be obtained through application to the Danish Health Data Authority.
REFERENCES
- 1. Ravn J. Chlorprothixen (Truxal og Taractan). Nord Psykiatr Tidsskr. 1963;17(1):69‐82. doi: 10.3109/08039486309132147 [DOI] [Google Scholar]
- 2. H. Lundbeck A/S . Products. Accessed January 2, 2022. https://www.lundbeck.com/global/our-science/products
- 3. Danish Medicines Agency Summary of Product Characteristics: Truxal (Chlorprothixene). Published online February 24, 2021.
- 4. Truxal . «Lundbeck» ‐ Felleskatalogen. Accessed March 19, 2021. https://www.felleskatalogen.no/medisin/truxal-lundbeck-564882
- 5. Pharmindex . Chlorprothixen. Gelbe Liste Online. Accessed June 9, 2021. https://www.gelbe-liste.de/wirkstoffe/Chlorprothixen_2626
- 6. Højlund M, Pottegård A, Johnsen E, et al. Trends in utilization and dosing of antipsychotic drugs in Scandinavia: comparison of 2006 and 2016. Br J Clin Pharmacol. 2019;85(7):1598‐1606. doi: 10.1111/bcp.13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hálfdánarson Ó, Zoëga H, Aagaard L, et al. International trends in antipsychotic use: a study in 16 countries, 2005‐2014. Eur Neuropsychopharmacol. 2017;27(10):1064‐1076. doi: 10.1016/j.euroneuro.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 8. Højlund M, Andersen JH, Andersen K, Correll CU, Hallas J. Use of antipsychotics in Denmark 1997–2018: a nation‐wide drug utilisation study with focus on off‐label use and associated diagnoses. Epidemiol Psychiatr Sci. 2021;30:e28. doi: 10.1017/S2045796021000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. University of North Carolina at Chapel Hill . The National Institute of Mental Health's Psychoactive Drug Screening Program (PDSP) Database. Accessed June 9, 2021. https://pdsp.unc.edu/databases/kidb.php
- 10. Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry. 2010;25(S2):S12‐S21. doi: 10.1016/S0924-9338(10)71701-6 [DOI] [PubMed] [Google Scholar]
- 11. Holt RIG. Association between antipsychotic medication use and diabetes. Curr Diab Rep. 2019;19(10):96. doi: 10.1007/s11892-019-1220-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8(2):114‐126. doi: 10.1038/nrendo.2011.156 [DOI] [PubMed] [Google Scholar]
- 13. Correll CU, Detraux J, de Lepeleire J, de Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14(2):119‐136. doi: 10.1002/wps.20204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Remvig J, Sonne LM. Chlorprothixene ("Truxal") compared to chlorpromazine. Psychopharmacologia. 1961;2(3):203‐208. doi: 10.1007/bf00407980 [DOI] [PubMed] [Google Scholar]
- 15. Gjerden P, Bramness JG, Tvete IF, Slørdal L. The antipsychotic agent quetiapine is increasingly not used as such: dispensed prescriptions in Norway 2004‐2015. Eur J Clin Pharmacol. 2017;73(9):1173‐1179. doi: 10.1007/s00228-017-2281-8 [DOI] [PubMed] [Google Scholar]
- 16. Pottegård A, Schmidt SAJ, Wallach‐Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46(3):798‐798f. doi: 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High‐dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20(4):512‐522. doi: 10.1097/EDE.0b013e3181a663cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Webster‐Clark M, Stürmer T, Wang T, et al. Using propensity scores to estimate effects of treatment initiation decisions: state of the science. Stat Med. 2021;40(7):1718‐1735. doi: 10.1002/sim.8866 [DOI] [PubMed] [Google Scholar]
- 19. Danish Health Data Authority . Danish National Laboratory Databank [Den Nationale Labdatabank]. Accessed June 15, 2018. https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/doedsaarsager-og-biologisk-materiale/laboratoriedatabasen
- 20. Suissa S. Immortal time bias in pharmaco‐epidemiology. Am J Epidemiol. 2008;167(4):492‐499. doi: 10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]
- 21. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367:l5657. doi: 10.1136/bmj.l5657 [DOI] [PubMed] [Google Scholar]
- 22. Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta‐analysis. Lancet Psychiatry. 2020;7(1):64‐77. doi: 10.1016/S2215-0366(19)30416-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stahl SM, Sy S, Maguire GA. How and when to treat the most common adverse effects of antipsychotics: expert review from research to clinical practice. Acta Psychiatr Scand. 2021;143(2):172‐180. doi: 10.1111/acps.13266 [DOI] [PubMed] [Google Scholar]
- 24. Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first‐ and second‐generation antipsychotics: a state‐of‐the‐art clinical review. Ther Clin Risk Manag. 2017;13:757‐777. doi: 10.2147/TCRM.S117321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carnovale C, Lucenteforte E, Battini V, et al. Association between the glyco‐metabolic adverse effects of antipsychotic drugs and their chemical and pharmacological profile: a network meta‐analysis and regression. Psychol Med. 2021;30:1‐13. doi: 10.1017/S0033291721000180 [DOI] [PubMed] [Google Scholar]
- 26. Vantaggiato C, Panzeri E, Citterio A, Orso G, Pozzi M. Antipsychotics promote metabolic disorders disrupting cellular lipid metabolism and trafficking. Trends Endocrinol Metab. 2019;30(3):189‐210. doi: 10.1016/j.tem.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 27. Sahlberg M, Holm E, Gislason GH, Køber L, Torp‐Pedersen C, Andersson C. Association of selected antipsychotic agents with major adverse cardiovascular events and noncardiovascular mortality in elderly persons. J Am Heart Assoc. 2015;4(9):e001666. doi: 10.1161/JAHA.114.001666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Danish Health Data Authority . Danish Register of Medicinal Product Statistics. Accessed January 12, 2022. https://medstat.dk/en
- 29. Højlund M, Lund LC, Andersen K, Correll CU, Hallas J. Association of low‐dose quetiapine and diabetes. JAMA Netw Open. 2021;4(5):e213209. doi: 10.1001/jamanetworkopen.2021.3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Data Availability Statement
The data that support the findings of this study are not publicly available due to confidentiality issues but can be obtained through application to the Danish Health Data Authority.


