Abstract
Background
Cancer cachexia is a syndrome characterized by anorexia and decreased body weight. This study evaluated the efficacy and safety of anamorelin, an orally active, selective ghrelin receptor agonist, in patients with cancer cachexia and a low body mass index (BMI).
Methods
This multicenter, open‐label, single‐arm study enrolled Japanese patients with non–small cell lung cancer or gastrointestinal cancer with cancer cachexia (BMI < 20 kg/m2, involuntary weight loss > 2% in the last 6 months, and anorexia). Patients were administered 100 mg of anamorelin once daily for up to 24 weeks. The primary end point was a composite clinical response (CCR) at 9 weeks, which was defined as an increase in body weight of ≥5% from the baseline, an increase of ≥2 points in the score of the 5‐item Anorexia Symptom Scale of the Functional Assessment of Anorexia/Cachexia Therapy, and being alive.
Results
One hundred two patients were eligible and enrolled. The means and standard deviations for age and BMI were 71.0 ± 8.2 years and 17.47 ± 1.48 kg/m2, respectively. The CCR rate at 9 weeks was 25.9% (95% confidence interval [CI], 18.3%‐35.3%), which met the primary end point with a lower 95% CI exceeding the prespecified minimum of 8%. Improvements in body weight and anorexia were durable and were accompanied by improvements in patients' global impression of change for appetite/eating‐related symptoms and overall condition. Adverse drug reactions occurred in 37 of 101 treated patients (36.6%), with the most common being glycosylated hemoglobin increases, constipation, and peripheral edema.
Conclusions
Anamorelin improved body weight and anorexia‐related symptoms in patients with cancer cachexia and a low BMI with durable efficacy and favorable safety and tolerability.
Lay Summary
Anamorelin is a drug that stimulates appetite and promotes weight gain.
This clinical trial was aimed at determining its efficacy and safety in Japanese cancer patients with a low body mass index and cachexia, a syndrome associated with anorexia and weight loss.
Anamorelin was found to improve body weight and anorexia‐related symptoms in these patients, and these effects were durable for up to 24 weeks. Moreover, anamorelin was generally well tolerated.
These findings suggest that anamorelin is a valuable treatment option for patients with cancer cachexia and a low body mass index.
Keywords: anamorelin, anorexia, body weight, cancer cachexia, patient‐reported outcomes
Short abstract
This single‐arm trial is aimed at investigating the efficacy and safety of anamorelin in Japanese patients with cancer cachexia and a low body mass index. Anamorelin improves body weight and anorexia‐related symptoms with durable efficacy and favorable safety and tolerability.
Introduction
Cancer cachexia is defined as a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment. 1 Cancer cachexia diminishes the patient's physical function and reduces his or her quality of life and ability to tolerate chemotherapy, and this results in a poor prognosis. 2 , 3 , 4 , 5 , 6 , 7
Although several societies have developed guidelines for managing cancer cachexia that mainly encompass nutritional support and pharmacotherapies for addressing symptoms, 8 , 9 , 10 no medications are explicitly indicated for treating cancer cachexia. These guidelines generally suggest corticosteroids and progestins. 8 , 9 , 10 However, corticosteroids increase appetite for a short period of up to 2 to 3 weeks, and the effect usually is attenuated with more extended treatment. Progestins also improve appetite and body weight but not muscle mass or quality of life. Moreover, the risk of serious side effects, including thromboembolic events, must be considered. Therefore, there is a need to develop effective and safe pharmacotherapies for cancer cachexia.
Anamorelin (ONO‐7643) is an orally active, selective ghrelin receptor agonist with appetite‐enhancing and anabolic activity. 11 , 12 In Japan, the results of 3 clinical studies 13 , 14 , 15 consistently showed that anamorelin was associated with increases in lean body mass (LBM) and body weight as well as aspects of anorexia‐related symptoms. After these trials, anamorelin was approved in January 2021 for managing cachexia in 4 types of cancer: non–small cell lung cancer (NSCLC), gastric cancer, pancreatic cancer, and colorectal cancer. 16
According to an international consensus, 1 cancer cachexia is diagnosed as weight loss of >5% or of >2% in patients already showing depletion of body mass index (BMI; <20 kg/m²) or skeletal muscle mass (sarcopenia). The prognostic impact of weight loss differs according to the body size in patients with cancer. For example, the prognostic impact of a weight loss of 2.5% to 5.9% in patients with a BMI of <20 kg/m2 is equivalent to a weight loss of 6.0% to 10.9% in patients with a BMI of ≥20 kg/m2. 17 This points toward underdiagnosis of cachexia in small‐body‐size patients with a weight loss of <5%, especially in Asians, who constitutionally have low BMIs. 18 However, most trials of anamorelin have adopted a cutoff of >5% weight loss as an entry criterion, 13 , 14 , 15 , 19 , 20 , 21 and there are limited data on anamorelin for cachectic patients with a BMI of <20 kg/m2 and weight loss of <5%.
Clinical studies have repeatedly demonstrated the benefits of anamorelin for anorexia and increasing body weight, including LBM. 13 , 14 , 15 , 19 However, these studies did not evaluate the effects of anamorelin with appetite as the primary end point. Because anorexia and weight loss are the main symptoms of cancer cachexia, improvements in both of these end points are considered to be clinically important. 1 , 8 , 9 , 10 Regulatory authorities have also suggested that body mass should be combined with other clinically relevant outcomes when used as an end point in clinical trials of cancer cachexia. 22 Therefore, the primary objective of this study was to assess the efficacy of anamorelin with a new clinical end point (composite clinical response [CCR]) integrating clinically significant improvements in both anorexia‐related symptoms and body weight. Accordingly, we conducted this single‐arm, multicenter clinical study to evaluate the efficacy and safety of anamorelin in cancer patients with a BMI of <20 kg/m2 and a weight loss of >2%.
Materials and Methods
Ethics
This multicenter (29‐center), open‐label, single‐arm study (ONO‐7643‐06) was conducted according to the Declaration of Helsinki and all appropriate regulations and laws in Japan. The study was approved by the institutional review board at each participating center. The study was registered with the Japan Pharmaceutical Information Center Clinical Trials Database (JapicCTI‐194735).
Patients
Patients with unresectable advanced or recurrent cancer (NSCLC, colorectal cancer, gastric cancer, or pancreatic cancer) were eligible if they met the following criteria: unsuitability for radical resection/radical radiation therapy or postoperative recurrence, a BMI of <20 kg/m2 and involuntary weight loss of >2% in the last 6 months, cancer‐associated anorexia, and a performance status of 0 to 2 (0‐1 for pancreatic cancer). Cancer‐associated anorexia was defined as a Functional Assessment of Anorexia/Cachexia Therapy 5‐item Anorexia Symptom Scale (FAACT‐5IASS) 23 score of ≤17 points and FAACT anorexia/cachexia‐specific subscale score of ≤37 points. All patients provided written informed consent. The supporting information provides information on the exclusion criteria and prohibited therapies.
Intervention
After a screening/observation period of up to 4 weeks, eligible patients entered a 24‐week treatment period during which they were administered 100 mg of anamorelin once daily 1 hour before breakfast and a subsequent 2‐week follow‐up period. A comparator group was not set because body weight decreased consistently in the placebo groups in prior trials. 13 , 14 , 19
End Points and Assessments
The primary end point was the CCR rate at 9 weeks, which was defined as the proportion of living patients with an increase in body weight of ≥5% from the baseline 1 plus an increase in the FAACT‐5IASS score of ≥2. 24 The supporting information includes more information on the efficacy end points and patient‐reported outcomes. The safety end points included the following: adverse events (AEs), adverse drug reactions (ADRs), laboratory tests, 12‐lead electrocardiogram, vital signs, overall survival, and tumor status. ADRs were defined as any AEs for which a possible relationship with the study drug could not be denied.
Statistical Analyses
In a post hoc analysis of the ROMANA‐1 and ROMANA‐2 studies, 19 the CCR rate at 9 weeks in cachectic patients with a BMI of <20 kg/m2 was 21.2% and 5.9% in the anamorelin and placebo groups, respectively. The CCR was not a prespecified end point in those studies. Under the assumption of an expected CCR rate of 20.0%, with a threshold of 8% for the lower 95% confidence interval (CI), a sample of 100 patients would provide statistical power of 95.3%. Considering prior studies in Japan, we planned to enroll 100 patients to provide a sufficient sample size to evaluate the primary outcome and safety of anamorelin in this patient population.
The intention‐to‐treat analysis set was used as the denominator to calculate the CCR rate. The percentage of patients with a CCR at each assessment time was calculated with 100 sets of complete data after the imputation of missing data via the multiple imputation method as described in the supporting information. The Wilson method 25 was used to calculate 95% CIs for percentages with complete data after the imputation of missing data. All data were analyzed with descriptive statistics, including means and standard deviations for continuous variables and numbers and percentages of patients for categorical variables. Analyses were performed with SAS (version 9.3 or higher; SAS Institute, Cary, North Carolina).
Results
Patients
Between June 2019 and February 2020, a total of 107 patients provided consent to participate (Fig. 1); 102 of these patients were scheduled to start treatment with anamorelin and were included in the intention‐to‐treat analysis set. However, 1 patient met a criterion for study discontinuation (the development of poorly controlled pleural effusion or pericardial effusion) and did not start anamorelin treatment. This patient was excluded from the safety analysis set, which therefore comprised 101 patients. There were 19 deaths. AEs led to study discontinuation in 2 patients. Eight patients asked to withdraw from the study, and 7 were withdrawn at the physician's discretion. The means and standard deviations of age, weight, and BMI for the overall population were 71.0 ± 8.2 years, 44.62 ± 6.25 kg, and 17.47 ± 1.48 kg/m2, respectively. Two‐thirds (66.7%) of the patients had a performance status of 1 (Table 1). There were 81 patients with NSCLC and 21 patients with gastrointestinal (GI) cancer (10 had colorectal cancer, 5 had gastric cancer, and 6 had pancreatic cancer). The disease status was locally advanced and unresectable in 7.8%, metastatic in 66.7%, and recurrent after surgery in 25.5% of the patients. Approximately half of the patients had received 1 prior anticancer regimen, and most (87.3%) were receiving concomitant cancer therapies, including chemotherapy (59.8%), immunotherapy (27.5%), and a tyrosine kinase inhibitor (20.6%). Twenty‐four patients died because of disease progression during the study.
Figure 1.

Patient disposition. *The cancer type was unknown in all 5 patients. †Development of poorly controlled pleural effusion or pericardial effusion before treatment with anamorelin was started. This patient did not start anamorelin treatment and was excluded from the safety analysis set. GI indicates gastrointestinal; NSCLC, non–small cell lung cancer.
TABLE 1.
Patient Characteristics
| Characteristic | Overall Population (n = 102) | NSCLC (n = 81) | GI Cancers (n = 21) |
|---|---|---|---|
| Sex | |||
| Male | 59 (57.8) | 46 (56.8) | 13 (61.9) |
| Female | 43 (42.2) | 35 (43.2) | 8 (38.1) |
| Age, y | 71.0 ± 8.2 | 72.0 ± 7.7 | 67.0 ± 9.1 |
| Weight, kg | 44.62 ± 6.25 | 44.59 ± 6.36 | 44.74 ± 5.94 |
| BMI, kg/m2 | 17.47 ± 1.48 | 17.43 ± 1.54 | 17.60 ± 1.28 |
| ECOG PS | |||
| 0 | 20 (19.6) | 11 (13.6) | 9 (42.9) |
| 1 | 68 (66.7) | 56 (69.1) | 12 (57.1) |
| 2 | 14 (13.7) | 14 (17.3) | 0 |
| Cancer type | |||
| NSCLC | 81 (79.4) | 81 (100.0) | — |
| Colorectal cancer | 10 (9.8) | — | 10 (47.6) |
| Gastric cancer | 5 (4.9) | — | 5 (23.8) |
| Pancreatic cancer | 6 (5.9) | — | 6 (28.6) |
| Disease status | |||
| Locally advanced unresectable | 8 (7.8) | 5 (6.2) | 3 (14.3) |
| Metastatic | 68 (66.7) | 57 (70.4) | 11 (52.4) |
| Recurrent after surgery | 26 (25.5) | 19 (23.5) | 7 (33.3) |
| No. of prior anticancer regimens | |||
| 0 | 5 (4.9) | 5 (6.2) | — |
| 1 | 49 (48.0) | 37 (45.7) | 12 (57.1) |
| 2 | 24 (23.5) | 19 (23.5) | 5 (23.8) |
| ≥3 | 24 (23.5) | 20 (24.7) | 4 (19.0) |
| Concomitant cancer therapy | |||
| Yes | 89 (87.3) | 70 (86.4) | 19 (90.5) |
| No | 13 (12.7) | 11 (13.6) | 2 (9.5) |
| Type of anticancer therapy | |||
| Chemotherapy | 61 (59.8) | 41 (50.6) | 20 (95.2) |
| Immunotherapy | 28 (27.5) | 27 (33.3) | 1 (4.8) |
| Tyrosine kinase inhibitor | 21 (20.6) | 21 (25.9) | — |
| Time from diagnosis to start of study drug administration, d | 560.3 ± 711.4 | 525.3 ± 622.1 | 695.3 ± 991.8 |
| FAACT‐5IASS total score | 9.2 ± 4.1 | 9.1 ± 4.1 | 9.5 ± 4.3 |
| QOL‐ACD score | |||
| Item 8 | 2.8 ± 1.0 | 2.8 ± 1.1 | 2.7 ± 0.9 |
| Item 9 | 2.8 ± 1.0 | 2.8 ± 1.0 | 2.7 ± 1.1 |
| Item 11 | 3.2 ± 1.3 | 3.2 ± 1.3 | 3.2 ± 1.5 |
Abbreviations: BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status; FAACT‐5IASS, Functional Assessment of Anorexia/Cachexia Therapy questionnaire 5‐item Anorexia Symptom Scale; GI, gastrointestinal; NSCLC, non–small cell lung cancer; QOL‐ACD, Quality of Life Questionnaire for Cancer Patients Treated With Anticancer Drugs.
Values are presented as number of patients (%) or mean ± standard deviation.
CCR
At 9 weeks, 92 patients (90.2%) were alive and on trial, and 82 patients were continuing anamorelin treatment (80.4%). The 9‐week body weight or FAACT‐5IASS total score values were missing for 6 patients; these missing values were imputed via the multiple imputation method as described in the Materials and Methods section. The CCR rate at 9 weeks was 25.9% (95% CI, 18.3%‐35.3%; Fig. 2A and Supporting Table 1). The lower 95% CI exceeded the predetermined threshold efficacy rate of 8.0% and met the primary end point. The percentage of patients with CCR rates ranged from 20.6% to 33.6% during the 24‐week treatment period. By cancer type, 29.0% of the patients with NSCLC (95% CI, 20.1%‐39.7%) and 14.3% of the patients with GI cancers (95% CI, 5.0%‐34.6%) achieved a CCR at 9 weeks (Supporting Table 1). The percentage of patients with an increase in body weight of ≥5% was 43.2% at 9 weeks, with rates thereafter exceeding 36% throughout the treatment period (Fig. 2B). The percentage of patients with an increase in the FAACT‐5IASS score of ≥2 points was 61.0% at 9 weeks and remained >45% throughout the treatment period (Fig. 2C).
Figure 2.
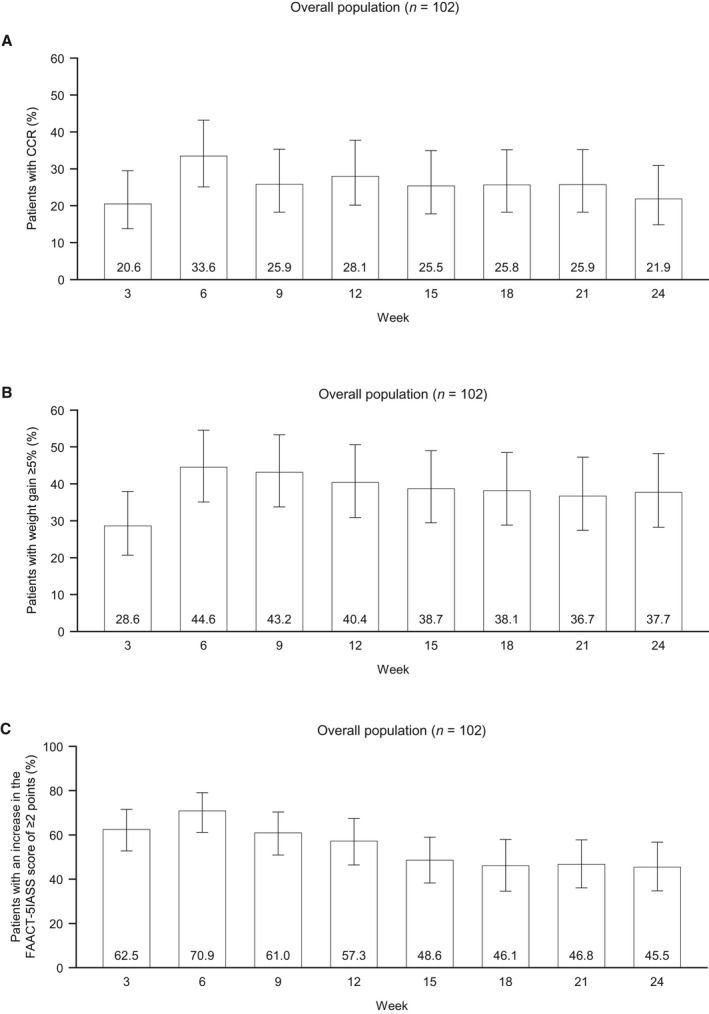
Percentage of patients with a CCR (an increase in body weight of ≥5% from the baseline, an increase in the FAACT‐5IASS score of ≥2, and survival) at each time point: (A) CCR, (B) body weight (increase of ≥5% from the baseline), and (C) FAACT‐5IASS (increase of ≥2 from the baseline). Values are shown as the percentage (95% confidence interval) with 102 as the denominator at each time point. CCR indicates composite clinical response; FAACT‐5IASS, Functional Assessment of Anorexia/Cachexia Therapy 5‐item Anorexia Symptom Scale; GI, gastrointestinal; NSCLC, non–small cell lung cancer.
Body Weight
The percent changes in body weight over time in the overall population are illustrated in Figure 3A, with actual changes over time displayed in Supporting Figure 1A. Body weight increased by 3 weeks and exceeded the 5% threshold after 6 weeks. This improvement was sustained through 24 weeks. Similar trajectories for the changes in body weight were observed in patients with NSCLC and GI cancers (Fig. 3B,C and Supporting Fig. 1B,C).
Figure 3.

(A‐C) Percent changes in body weight over time in (A) the overall population, (B) patients with NSCLC, and (C) patients with GI cancers. (D‐F) Changes in FAACT‐5IASS in (D) the overall population, (E) patients with NSCLC, and (F) patients with GI cancers. The dashed lines represent a percent change in body weight of ≥5% or a change in FAACT‐5IASS of ≥2 points. Values are presented as the mean ± standard deviation for patients with available data at each time point. FAACT‐5IASS indicates Functional Assessment of Anorexia/Cachexia Therapy 5‐item Anorexia Symptom Scale; GI, gastrointestinal; NSCLC, non–small cell lung cancer.
Appetite
FAACT
The FAACT‐5IASS score increased by a mean ± SD of 4.0 ± 4.7 points at 3 weeks and 3.9 ± 4.8 points at 9 weeks from the baseline value (9.2 ± 4.1 points). This increase was sustained through 24 weeks (Fig. 3D). Similar trajectories for changes in FAACT‐5IASS scores were observed in patients with NSCLC and GI cancers (Fig. 3E,F).
Quality of Life Questionnaire for Cancer Patients Treated With Anticancer Drugs
As exploratory end points, we also determined the changes in the mean scores for Quality of Life Questionnaire for Cancer Patients Treated With Anticancer Drugs (QOL‐ACD) items 8, 9, and 11, which are related to appetite. For all 3 items, there were increases in the mean scores of approximately 1 point with respect to the baseline scores that were apparent at 3 weeks and remained broadly stable thereafter (Supporting Figs. 2‐4).
Patient global impression
The Patient Global Impression of Change (PGIC) for appetite/eating‐related symptoms and overall condition at 6 and 9 weeks is summarized in Supporting Figure 5. Overall, 76 patients (74.5%) reported some improvement (minimal, much, or very much) in their appetite/eating‐related symptoms at 6 and 9 weeks from the baseline. Four patients at 6 weeks and 3 patients at 9 weeks reported worsening appetite/eating‐related symptoms. For their overall condition, 67 patients (65.7%) reported an improvement at 6 weeks, and 66 (64.7%) did so at 9 weeks. The overall condition was reported as worse by 12 patients (11.8%) at 6 weeks and by 6 patients (5.9%) at 9 weeks. Data for both PGIC outcomes were missing for 9 and 15 patients at 6 and 9 weeks, respectively. The results were comparable between patients with NSCLC and those with GI cancers.
Safety
AEs occurred in 88 patients (87.1%) overall (Table 2) and included serious AEs in 23 patients (22.8%). AEs led to treatment discontinuation in 12 patients (11.9%). There were 3 deaths due to AEs (pneumonia, sepsis, and pneumonitis; 1 patient each); none were reported to be related to anamorelin treatment. ADRs occurred in 37 patients (36.6%) and were classified as grade 1, 2, 3, and 4 in 10.9%, 15.8%, 7.9%, and 2.0% of patients, respectively. The most common ADRs were glycosylated hemoglobin increases (5.9%), constipation (5.0%), and peripheral edema (5.0%; Table 2). The ADRs were classified as serious in 7 patients (6.9%); they included hyperglycemia and pneumonia in 1 patient and diabetes mellitus, supraventricular extrasystoles, gastric perforation, hepatic function abnormal, transient ischemic attack, and pneumonitis in 1 patient each. Seven patients (6.9%) discontinued anamorelin because of ADRs: hyperglycemia (grade 3) and pneumonia (grade 4) in 1 patient and sinus tachycardia (grade 2), supraventricular extrasystoles (grade 3), gastric perforation (grade 4), malaise (grade 3), transient ischemic attack (grade 2), and pneumonitis (grade 2) in 1 patient each.
TABLE 2.
Frequency of AEs and ADRs: Safety Population (n = 101)
| Classification | No. (%) | ||||
|---|---|---|---|---|---|
| AEs | 88 (87.1) | ||||
| Serious AEs | 23 (22.8) | ||||
| AEs leading to treatment discontinuation | 12 (11.9) | ||||
| AEs leading to death | 3 (3.0) | ||||
| ADRs | 37 (36.6) | ||||
| Serious ADRs | 7 (6.9) | ||||
| ADRs leading to treatment discontinuation | 7 (6.9) | ||||
| ADRs in ≥2 Patients or All Grade ≥3 ADRs | All | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Any ADR | 37 (36.6) | 11 (10.9) | 16 (15.8) | 8 (7.9) | 2 (2.0) |
| Glycosylated hemoglobin increased | 6 (5.9) | 4 (4.0) | 2 (2.0) | — | — |
| Constipation | 5 (5.0) | 3 (3.0) | 2 (2.0) | — | — |
| Peripheral edema | 5 (5.0) | 4 (4.0) | 1 (1.0) | — | — |
| γ‐Glutamyltransferase increased | 4 (4.0) | 2 (2.0) | — | 2 (2.0) | — |
| Hyperglycemia | 4 (4.0) | 2 (2.0) | 1 (1.0) | 1 (1.0) | — |
| Blood alkaline phosphatase increased | 3 (3.0) | 2 (2.0) | 1 (1.0) | — | — |
| Diabetes mellitus | 3 (3.0) | — | 2 (2.0) | 1 (1.0) | — |
| Hypertension | 3 (3.0) | 1 (1.0) | 1 (1.0) | 1 (1.0) | — |
| Atrioventricular block first degree | 2 (2.0) | 2 (2.0) | — | — | — |
| Supraventricular extrasystoles | 2 (2.0) | 1 (1.0) | — | 1 (1.0) | — |
| Nausea | 2 (2.0) | 1 (1.0) | 1 (1.0) | — | — |
| Malaise | 2 (2.0) | — | 1 (1.0) | 1 (1.0) | — |
| Hepatic function abnormal | 2 (2.0) | — | 1 (1.0) | 1 (1.0) | — |
| Alanine aminotransferase increased | 2 (2.0) | — | 2 (2.0) | — | — |
| Aspartate aminotransferase increased | 2 (2.0) | 2 (2.0) | — | — | — |
| Electrocardiogram QT prolonged | 2 (2.0) | 2 (2.0) | — | — | — |
| Proteinuria | 2 (2.0) | 1 (1.0) | 1 (1.0) | — | — |
| Anemia | 1 (1.0) | — | — | 1 (1.0) | — |
| Gastric perforation | 1 (1.0) | — | — | — | 1 (1.0) |
| Pneumonia | 1 (1.0) | — | — | — | 1 (1.0) |
Abbreviations: ADR, adverse drug reaction; AE, adverse event.
Discussion
This multicenter, open‐label, single‐arm study is the first to evaluate the efficacy of anamorelin with a new clinical end point (ie, CCR) that integrates a patient‐reported outcome related to anorexia and body weight in patients with cancer cachexia and a low BMI (<20 kg/m2). There are 2 main findings. First, anamorelin achieved clinically significant improvements in both anorexia and body weight. These effects were durable throughout the 24‐week treatment period. Second, anamorelin showed favorable safety in cachectic patients with a low BMI that was consistent with the results of previous studies, which adopted a 5% weight loss threshold without restriction to the patient's BMI category. 13 , 14 , 15 , 20 , 21
This study met the primary end point because the lower 95% CI of the CCR rate at 9 weeks (25.9%; 95% CI, 18.3%‐35.3%) exceeded the planned threshold of 8%. The CCR rate was generally maintained above 20% throughout the treatment period, and this demonstrated sustained efficacy on anorexia and body weight. The durable efficacy through 24 weeks was consistent with the results of the ROMANA‐3 trial. 26 Anamorelin quickly improved anorexia, which was followed by body weight, and it reached the CCR threshold. At 9 weeks, 43.2% and 61.0% of the subjects met the CCR criteria for body weight and appetite, respectively, and this suggests that patients may be more likely to achieve the appetite criterion than the body weight criterion (ie, weight gain of ≥5%). Because patients with cancer cachexia typically suffer from progressive weight loss, the improvement in body weight (≥5%) in approximately 40% of the patients is a clinically significant effect of anamorelin. It is also notable that the administration of anamorelin led to improvements in patient‐reported outcomes, including not only appetite‐related measures such as the FAACT‐5IASS subscale scores, the QOL‐ACD items related to anorexia (items 8, 9, and 11), and the PGIC assessment of appetite/eating‐related symptoms but also the PGIC assessment of overall condition. More than 60% of the patients reported an increase in the FAACT‐5IASS score of ≥2 points at 9 weeks, and this remained above 45% through 24 weeks. These results demonstrate a robust effect of anamorelin on anorexia and a potential impact on the patient's general condition. Body weight gain and anorexia improvement were observed in patients with NSCLC and GI cancers, and this was consistent with the results of the previous clinical studies. Although the improvement in the anorexia score was slightly greater in patients with NSCLC than in those with GI cancer, the overall trends were similar. Taken together, these results demonstrate beneficial effects of anamorelin on anorexia symptoms, including appetite and the resultant body weight gain, in this study of cachectic patients with cancer and a low BMI.
The most common ADRs were glycosylated hemoglobin increases, constipation, and peripheral edema. Several of the ADRs were related to metabolism and nutrition disorders; they were similar to those reported in prior Japanese studies. 13 , 14 , 15 The frequency of grade 4 ADRs was low (2.0%), and serious ADRs or ADRs that led to treatment discontinuation occurred in 6.9% of patients each. Although 3 patients died of AEs, none of these AEs were related to anamorelin. Hyperglycemia and cardiac conduction disorder have been reported as notable categories of ADRs among patients treated with anamorelin. In this study, grade 3 hyperglycemia, grade 3 diabetes mellitus, and grade 3 supraventricular extrasystoles occurred in 1 patient each. Among these ADRs, anamorelin was discontinued in the patients with hyperglycemia and supraventricular extrasystoles. None of these categories of ADRs were grade 4; the remainder were grade 1 or 2. Overall, these safety findings demonstrate the tolerability of anamorelin in low‐BMI patients. However, we should consider that this cohort of patients may be susceptible to ADRs because of their clinical situation, which encompasses cachexia, sarcopenia, and recent/ongoing anticancer treatment. Thus, clinicians should be aware of the risk of ADRs and the appropriate interventions.
Two international, phase 3, randomized, placebo‐controlled, quadruple‐masked studies (the SCALA studies) are now underway to further evaluate the efficacy and safety of anamorelin for the treatment of cancer cachexia in patients with NSCLC (NCT03743064 and NCT03743051). 27 , 28 Both studies have adopted the same entry criteria: a BMI of <20 kg/m2 and involuntary weight loss of >2% within 6 months of screening. The coprimary outcomes are the durations of clinically meaningful improvements in body weight gain and the FAACT‐5IASS until 12 weeks. Furthermore, the studies will evaluate the response rate with the CCR. Thus, it will be possible to compare the results of those studies with our results.
The previous and current clinical studies were not designed to examine the survival benefits of anamorelin. However, we believe that anamorelin may have the potential to improve overall survival in cachectic patients with cancer because of its consistent, positive effects on body weight/LBM in the prior studies. 13 , 14 , 15 , 19 , 20 , 21 In addition, there is abundant evidence regarding a relationship between body weight/LBM loss and worse survival. 29 , 30 , 31 , 32 Therefore, we should investigate the prognostic effect of anamorelin in future clinical studies.
Limitations
Some limitations of this study warrant mention. In particular, it was designed as an uncontrolled, open‐label study without a placebo group. This design was selected because a decrease in body weight was consistently observed in the placebo groups in previous studies of anamorelin. 17 , 18 , 19 However, the study designs and eligible patients were not identical, so we should take care when extrapolating the results of such studies. It is also important to consider that there were no specific treatment options for cancer cachexia. In addition, unlike prior studies, we did not measure LBM or handgrip strength. Instead, we focused on patient‐reported outcomes to evaluate their perceptions of appetite and anorexia symptoms.
Despite these limitations, important strengths of this study include the use of multiple patient‐reported outcomes of appetite and anorexia and the enrollment of a broader range of patients with cancer cachexia and a low BMI (ie, BMI of <20 kg/m2 and body weight loss of >2% rather than the 5% of other studies).
In conclusion, this study provides further evidence showing the clinical efficacy and safety of anamorelin for managing cancer cachexia. The study met the primary end point with a CCR of 25.9%, and this was accompanied by improvements in body weight and patient‐reported outcomes, including the FAACT‐5IASS, QOL‐ACD items, and PGIC. Anamorelin was also generally well tolerated in this population. Overall, these findings support the clinical use of anamorelin for managing cancer cachexia in low‐BMI patients.
Funding Support
This work was funded by Ono Pharmaceutical Co, Ltd.
Conflict of Interest Disclosures
Tateaki Naito reports lecture fees and travel expenses from Ono Pharmaceutical, institutional research funds from Ono Pharmaceutical in relation to this work, and institutional research funds from Otsuka Pharmaceutical outside the submitted work. Junji Uchino, Toru Kojima, Yutaka Matano, Koichi Minato, Kentaro Tanaka, Takuro Mizukami, and Shinji Atagi report institutional research funds from Ono Pharmaceutical in relation to this work. Kentaro Tanaka reports institutional research funds from Ono Pharmaceutical in relation to this work and honoraria from AstraZeneca and Chugai outside the submitted work. Takuro Mizukami reports institutional research funds from Ono Pharmaceutical in relation to this work; institutional research funds from Taiho Pharmaceutical and Eli Lilly outside the submitted work; and honoraria from Taiho Pharmaceutical, Chugai, Bayer, Merck Serono, Takeda, Eli Lilly, Asahi Kasei, Otsuka Pharmaceutical, Bristol‐Myers Squibb, and Ono Pharmaceutical outside the submitted work. Shinji Atagi reports institutional research funds from Ono Pharmaceutical in relation to this work; research grants from AstraZeneca, Eli Lilly, Ono Pharmaceutical, Taiho Pharmaceutical, Boehringer Ingelheim, Pfizer, Bristol‐Myers Squibb, MSD, Chugai, Merck, and F. Hoffmann‐La Roche outside the submitted work; honoraria from AstraZeneca, Eli Lilly, Ono Pharmaceutical, Taiho Pharmaceutical, Boehringer Ingelheim, Pfizer, Bristol‐Myers Squibb, Hisamitsu, MSD, Chugai, Kyowa Hakko Kirin, Merck, Novartis Pharma, and Thermo Fisher Scientific outside the submitted work; and other financial or nonfinancial interests in F. Hoffmann‐La Roche outside the submitted work. Takashi Higashiguchi reports consultancy fees and travel expenses from Ono Pharmaceutical outside the submitted work. Kei Muro reports consultancy fees, lecture fees, and travel expenses from Ono Pharmaceutical in relation to this work; institutional research funds from Solasia Pharma, Merck Serono, Daiichi Sankyo, Parexel International, Pfizer, MSD, Amgen, Sanofi, and Taiho Pharmaceutical outside the submitted work; consultancy fees from Amgen, Ono Pharmaceutical, and AstraZeneca outside the submitted work; and honoraria from Ono Pharmaceutical, Sanofi, Taiho Pharmaceutical, Chugai Pharma, Takeda, Eli Lily, Bristol‐Myers Squibb, and Bayer outside the submitted work. Koichi Takayama reports consultancy fees, lecture fees, travel expenses, and institutional research funds from Ono Pharmaceutical in relation to this work; institutional research funds from Ono Pharmaceutical, Taiho Pharmaceutical Co, Eli Lilly, and Fukuda Lifetech Co outside the submitted work; lectures fees from Ono Pharmaceutical, AstraZeneca, MSD, Chugai‐Roche, Eli Lilly, and Boehringer Ingelheim outside the submitted work; and travel expenses from Ono Pharmaceutical outside the submitted work. Junji Furuse reports consultancy fees, lecture fees, travel expenses, and institutional research funds from Ono Pharmaceutical in relation to this work; institutional research funds from Ono Pharmaceutical, MSD, Merck Bio, J‐Pharma, Taiho Pharmaceutical, Takeda, Chugai Pharma, Astra Zeneca, Yakult Honsha, Eisai, Daiichi Sankyo, Mochida, Sanofi, Sumitomo Dainippon Bayer, Astellas, and Incyte Japan outside the submitted work; and honoraria or personal fees from Ono Pharmaceutical, Bayer, Eisai, Eli Lilly Japan, MSD, Yakult Honsha, Chugai Pharma, Novartis Pharma, Astra Zeneca, Pfizer, Takeda, Taiho Pharmaceutical, Sanofi, Mylan EPD, EA Pharma, Kyowa Hakko Kirin, Daiichi Sankyo, Teijin Pharma, Servier Japan, and Incyte Japan outside the submitted work. Eiichiro Morishima and Toru Takiguchi are employees of Ono Pharmaceutical. Kazuo Tamura reports consultancy fees, lecture fees, and travel expenses from Ono Pharmaceutical in relation to this work; consultancy fees from AC Medical outside the submitted work; and participation on advisory boards for Symbio and Eisai outside the submitted work.
Author Contributions
Tateaki Naito: Study design, data collection, and data interpretation. Junji Uchino: Data collection. Toru Kojima: Data collection. Yutaka Matano: Data collection. Koichi Minato: Data collection. Kentaro Tanaka: Data collection. Takuro Mizukami: Data collection. Shinji Atagi: Data collection. Takashi Higashiguchi: Conceptualization. Kei Muro: Study design and data interpretation. Koichi Takayama: Study conception, study design, and data interpretation. Junji Furuse: Study design, data collection, and data interpretation. Eiichiro Morishima: Study design, data collection, data analysis, and data interpretation. Toru Takiguchi: Study conception, study design, and data interpretation. Kazuo Tamura: Study design and data interpretation. All authors contributed to the drafting, critical review, and final approval of the manuscript and take accountability for the integrity of the work.
Supporting information
Supplementary Material
Naito T, Uchino J, Kojima T, Matano Y, Minato K, Tanaka K, Mizukami T, Atagi S, Higashiguchi T, Muro K, Takayama K, Furuse J, Morishima E, Takiguchi T, Tamura K. A multicenter, open‐label, single‐arm study of anamorelin (ONO‐7643) in patients with cancer cachexia and low body mass index. Cancer. 2022. 10.1002/cncr.34154
The results of this study were presented as an abstract and oral presentation at the 59th Annual Meeting of the Japan Society of Clinical Oncology; October 21 to 23, 2021; Yokohama, Japan.
We thank all the patients and their families, investigators, and site staff who participated in the current study. We also thank Nicholas D. Smith (EMC KK) for medical writing support, which was funded by Ono Pharmaceutical Co, Ltd.
Data Availability
Qualified researchers may ask Ono Pharmaceutical Co, Ltd, to disclose individual patient‐level data from clinical studies through the following website: https://www.clinicalstudydatarequest.com/. For more information on Ono Pharmaceutical's policy for the disclosure of clinical study data, please see the following website: https://www.ono.co.jp/eng/rd/policy.html.
References
- 1. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489‐495. doi: 10.1016/s1470-2045(10)70218-7 [DOI] [PubMed] [Google Scholar]
- 2. Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology‐epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2018;9:1200‐1208. doi: 10.1002/jcsm.12379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med. 1988;148:1586‐1591. [PubMed] [Google Scholar]
- 4. Robinson DW Jr, Eisenberg DF, Cella D, Zhao N, de Boer C, DeWitte M. The prognostic significance of patient‐reported outcomes in pancreatic cancer cachexia. J Support Oncol. 2008;6:283‐290. [PubMed] [Google Scholar]
- 5. Ross PJ, Ashley S, Norton A, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90:1905‐1911. doi: 10.1038/sj.bjc.6601781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491‐497. doi: 10.1016/s0149-2918(05)80001-3 [DOI] [PubMed] [Google Scholar]
- 7. Le‐Rademacher J, Lopez C, Wolfe E, et al. Weight loss over time and survival: a landmark analysis of 1000+ prospectively treated and monitored lung cancer patients. J Cachexia Sarcopenia Muscle. 2020;11:1501‐1508. doi: 10.1002/jcsm.12625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arends J, Strasser F, Gonella S, et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open. 2021;6:100092. doi: 10.1016/j.esmoop.2021.100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muscaritoli M, Arends J, Bachmann P, et al. ESPEN practical guideline: clinical nutrition in cancer. Clin Nutr. 2021;40:2898‐2913. doi: 10.1016/j.clnu.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 10. Roeland EJ, Bohlke K, Baracos VE, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol. 2020;38:2438‐2453. doi: 10.1200/jco.20.00611 [DOI] [PubMed] [Google Scholar]
- 11. Pietra C, Takeda Y, Tazawa‐Ogata N, et al. Anamorelin HCl (ONO‐7643), a novel ghrelin receptor agonist, for the treatment of cancer anorexia‐cachexia syndrome: preclinical profile. J Cachexia Sarcopenia Muscle. 2014;5:329‐337. doi: 10.1007/s13539-014-0159-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Currow DC, Skipworth RJ. The emerging role of anamorelin hydrochloride in the management of patients with cancer anorexia‐cachexia. Future Oncol. 2017;13:1767‐1783. doi: 10.2217/fon-2017-0141 [DOI] [PubMed] [Google Scholar]
- 13. Katakami N, Uchino J, Yokoyama T, et al. Anamorelin (ONO‐7643) for the treatment of patients with non–small cell lung cancer and cachexia: results from a randomized, double‐blind, placebo‐controlled, multicenter study of Japanese patients (ONO‐7643‐04). Cancer. 2018;124:606‐616. doi: 10.1002/cncr.31128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takayama K, Katakami N, Yokoyama T, et al. Anamorelin (ONO‐7643) in Japanese patients with non–small cell lung cancer and cachexia: results of a randomized phase 2 trial. Support Care Cancer. 2016;24:3495‐3505. doi: 10.1007/s00520-016-3144-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamauchi S, Furuse J, Takano T, et al. A multicenter, open‐label, single‐arm study of anamorelin (ONO‐7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer. 2019;125:4294‐4302. doi: 10.1002/cncr.32406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ONO receives a manufacturing and marketing approval of Adlumiz® (anamorelin), a ghrelin receptor agonist for the treatment of cancer cachexia in Japan. ONO Pharmaceutical Co., Ltd. Published January 22, 2021. Accessed August 5, 2021. (Japanese version at https://www.ono‐pharma.com/news/20210122.htmlhttps://www.ono.co.jp/news/20210122.html
- 17. Martin L, Senesse P, Gioulbasanis I, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol. 2015;33:90‐99. doi: 10.1200/jco.2014.56.1894 [DOI] [PubMed] [Google Scholar]
- 18. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300‐307.e2. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 19. Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non–small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol. 2016;17:519‐531. doi: 10.1016/s1470-2045(15)00558-6 [DOI] [PubMed] [Google Scholar]
- 20. Garcia JM, Boccia RV, Graham CD, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo‐controlled, double‐blind trials. Lancet Oncol. 2015;16:108‐116. doi: 10.1016/s1470-2045(14)71154-4 [DOI] [PubMed] [Google Scholar]
- 21. Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer‐related cachexia: a multicenter, randomized, double‐blind, crossover, pilot study. Support Care Cancer. 2013;21:129‐137. doi: 10.1007/s00520-012-1500-1 [DOI] [PubMed] [Google Scholar]
- 22. Fearon K, Argiles JM, Baracos VE, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle. 2015;6:272‐274. doi: 10.1002/jcsm.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. LeBlanc TW, Samsa GP, Wolf SP, Locke SC, Cella DF, Abernethy AP. Validation and real‐world assessment of the Functional Assessment of Anorexia‐Cachexia Therapy (FAACT) scale in patients with advanced non–small cell lung cancer and the cancer anorexia‐cachexia syndrome (CACS). Support Care Cancer. 2015;23:2341‐2347. doi: 10.1007/s00520-015-2606-z [DOI] [PubMed] [Google Scholar]
- 24. Gelhorn HL, Gries KS, Speck RM, et al. Comprehensive validation of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) Anorexia/Cachexia Subscale (A/CS) in lung cancer patients with involuntary weight loss. Qual Life Res. 2019;28:1641‐1653. doi: 10.1007/s11136-019-02135-7 [DOI] [PubMed] [Google Scholar]
- 25. Lott A, Reiter JP. Wilson confidence intervals for binomial proportions with multiple imputation for missing data. Am Stat. 2020;74:109‐115. doi: 10.1080/00031305.2018.1473796 [DOI] [Google Scholar]
- 26. Currow D, Temel JS, Abernethy A, Milanowski J, Friend J, Fearon KC. ROMANA 3: a phase 3 safety extension study of anamorelin in advanced non–small‐cell lung cancer (NSCLC) patients with cachexia. Ann Oncol. 2017;28:1949‐1956. doi: 10.1093/annonc/mdx192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A study to evaluate the efficacy and safety of anamorelin HCl for the treatment of malignancy associated weight loss and anorexia in adult patients with advanced non–small cell lung cancer (NSCLC) (NCT03743064). ClinicalTrials.gov. Accessed August 5, 2021. https://clinicaltrials.gov/ct2/show/NCT03743064
- 28.A study to evaluate the efficacy and safety of anamorelin HCl for the treatment of malignancy associated weight loss and anorexia in adult patients with advanced non–small cell lung cancer (NSCLC) (NCT03743051). ClinicalTrials.gov. Accessed August 6, 2021. https://clinicaltrials.gov/ct2/show/NCT03743051
- 29. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long‐term improved survival in definitively treated locally advanced non–small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52‐57. doi: 10.1016/j.lungcan.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 30. Martin L, Birdsell L, MacDonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539‐1547. doi: 10.1200/jco.2012.45.2722 [DOI] [PubMed] [Google Scholar]
- 31. Mytelka DS, Li L, Benoit K. Post‐diagnosis weight loss as a prognostic factor in non–small cell lung cancer. J Cachexia Sarcopenia Muscle. 2018;9:86‐92. doi: 10.1002/jcsm.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Topkan E, Parlak C, Selek U. Impact of weight change during the course of concurrent chemoradiation therapy on outcomes in stage IIIB non–small cell lung cancer patients: retrospective analysis of 425 patients. Int J Radiat Oncol Biol Phys. 2013;87:697‐704. doi: 10.1016/j.ijrobp.2013.07.033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Qualified researchers may ask Ono Pharmaceutical Co, Ltd, to disclose individual patient‐level data from clinical studies through the following website: https://www.clinicalstudydatarequest.com/. For more information on Ono Pharmaceutical's policy for the disclosure of clinical study data, please see the following website: https://www.ono.co.jp/eng/rd/policy.html.


