Abstract
Background
Several pharmaceutical treatments for chronic pain caused by osteoarthritis (OA) and chronic low back pain (CLBP) are available or currently under development, each associated with different adverse events (AEs) and efficacy profiles. It is therefore important to understand what trade‐offs patients are willing to make when choosing between treatments.
Methods
A discrete‐choice experiment (DCE) was conducted with 437 adults with chronic pain caused by OA and/or CLBP. Respondents were presented with a series of scenarios and asked to choose between pairs of hypothetical treatments, each defined by six attributes: level of symptom control; risks of heart attack, rapidly progressive osteoarthritis and dependency; frequency and mode of administration and cost. Attributes were based on known profiles of oral nonsteroidal anti‐inflammatory drugs, opioids and injected nerve growth factor inhibitors, the last of which were under clinical development at the time of the study. Data were analysed using a latent class (LC) model to explore preference heterogeneity.
Results
Overall, respondents considered improving symptom control and reducing risk of physical dependency to be the most important attributes. The LC analysis identified four participant classes: an ‘efficacy‐focused’ class (33.7%), a ‘cost‐averse’ class (29.4%), a ‘physical‐dependence–averse’ class (19.6%) and a ‘needle‐averse’ class (17.3%). Subgroup membership was incompletely predicted by participant age and their responses to comprehension questions.
Conclusions
Preference heterogeneity across respondents indicates a need for a personalized approach to offering treatment options. Symptom improvement, cost, physical dependence and route of administration might be important to different patients.
Significance
Multiple treatment options that differ substantially in terms of efficacy and adverse events are available for the management of chronic pain. With a growing emphasis on a patient‐centred care model that incorporates patients’ priorities and values into treatment decisions, there is a need to understand how individuals with chronic musculoskeletal pain balance the benefits and risks of treatment and how treatment priorities vary among individuals. This study was designed to identify patient preferences for different characteristics of treatments for the management of chronic pain and to investigate how preferences differ among respondents.
1. INTRODUCTION
Osteoarthritis (OA) and chronic low back pain (CLBP) are common chronic pain conditions worldwide, resulting in substantial personal and socioeconomic burden (Bindawas et al., 2015; Farr et al., 2013; Gore et al., 2011, 2012; Hartvigsen et al., 2018; Ma et al., 2014). Nonsteroidal anti‐inflammatory drugs (NSAIDs), paracetamol (acetaminophen) and opioids are frequently used systemic pharmaceutical therapies for OA or CLBP (McAlindon et al., 2014; Qaseem et al., 2017). However, these therapies are limited by suboptimal efficacy and/or considerable risks. Some individuals are not appropriate candidates for NSAID treatment (Aronson, 2009; Kissin, 2010), and several guidelines have questioned the appropriateness of opioid use in these conditions (Bannuru et al., 2019; Chou et al., 2007). Injectable nerve growth factor inhibitors have emerged as potential nonopioid pain treatments (Ghouri & Conaghan, 2021; Patel et al., 2018). Types of associated adverse events (AEs) differ substantially between these three therapy classes. For example NSAIDs are associated with cardiovascular and gastrointestinal events, opioids carry risks for physical dependency (as well as somnolence, constipation, nausea, etc), and nerve growth factor inhibitors are associated with risks of rapidly progressive OA and abnormal peripheral sensations.
Although information asymmetry often means that health care demand is typically driven by doctors or other health care professionals, patient preference is a key driver of treatment choice within modern patient‐centred care (US Food & Drug Administration, 2021; Elwyn et al., 2010; Michel et al., 2020; National Health Service [NHS], 2021), alongside other factors such as treatment availability. The choice among diverse treatments requires an understanding of the importance to patients of different risks and benefits and the trade‐offs that patients are willing to make. Treatment preferences might differ among patients with different characteristics or for different chronic pain conditions. Understanding preferences, as well as how they vary across different people within the population, can inform policymakers’ decisions regarding whether a treatment should be made available, and it is important to identify subpopulations that might display different preferences to ensure that they are not disadvantaged by those decisions. Moreover, understanding how preferences differ across patient populations can inform discussions between the prescriber and the patient, helping them choose the treatment most appropriate to specific patients’ needs and values. Consideration of patients’ preferences can inform medical product development to ensure that research and development efforts are focused on treatments with characteristics that meet a genuine patient need.
Prior research has explored patient preferences for pharmaceutical treatments for chronic OA and CLBP (Hauber et al., 2013; Laba et al., 2013; Mühlbacher et al., 2015; Poder et al., 2019; Turk et al., 2020). Risks associated with NSAIDs (Hauber et al., 2013; Laba et al., 2013; Turk et al., 2020) and opioids (Mühlbacher et al., 2015; Turk et al., 2020) have been identified as key drivers of preferences. New treatments may bring new risks which may change perspectives on established therapies. Overall, the potential for pain relief from a treatment might be considered at least as important by people with OA or CLBP as are risks of adverse events (Turk et al., 2020). Risks associated with nerve growth factor blocking antibodies were considered to be of no greater importance than those of established analgesic treatments by people with OA and/or CLBP in the USA (Turk et al., 2020). Patient preferences might be expected to differ between countries with different cultures and healthcare systems. Furthermore, clinical experience indicates differences between individuals in how they weigh advantages and disadvantages of treatments. Little is known about preference heterogeneity for pharmaceutical treatments for chronic OA or CLBP. Latent class (LC) analysis is a statistical tool which can identify discrete subgroups (referred to as classes) within a population but has not previously been reported exploring unique preference segments among people with OA, CLBP or both.
With a growing emphasis on a patient‐centred care model that incorporates patients’ priorities and values into treatment decisions, a need exists to understand how individuals with chronic musculoskeletal pain balance the benefits and risks of treatment and how treatment priorities vary among individuals. This study was designed to identify patient preferences for different characteristics of treatments for the management of chronic pain and to investigate how preferences differ among respondents.
2. METHODS
2.1. Survey development
The study was developed following recommended good research practices (following the 10‐items checklist proposed by Bridges et al., 2011 covering: research question, attributes and levels, construction of tasks, experimental design, preference elicitation, instrument design, data collection plan, statistical analyses, results and conclusions and study presentation). There are multiple preference‐elicitation methods available to researchers (Soekhai, Whichello, et al., 2019). Each one has advantages and limitations, and the choice of approach may be determined by the research team to ensure the data collected can answer the primary research question. Considering the study objectives, the discrete‐choice experiment (DCE) methodology was selected from a number of stated‐preference methods, as it is very well suited to quantifying preferences for multiple attributes, quantifying the relative importance of those attributes, and simultaneously exploring trade‐offs among changes in multiple treatment attributes that survey respondents are willing to make (Soekhai, Whichello, et al., 2019). Discrete‐choice experiments have been widely employed in recent decades to quantify patients’ treatment preferences and explore the trade‐offs they are willing to accept in a range of therapeutic areas (Clark et al., 2014; de Bekker‐Grob et al., 2010; Marshall et al., 2010; Ryan et al., 2008; Soekhai, Whichello, et al., 2019). In the DCE, respondents were asked to assume that their level of symptom control had become very poor (i.e. very severe symptoms that are intolerable and that do not allow the respondent to engage in normal activities) and that their doctor suggested changing their treatment to one of two hypothetical medicines, Medicine A or Medicine B, each with a different level of six features, presented in each choice task (Figure 1).
FIGURE 1.

Example of choice question
The survey instrument was initially developed in a parallel study based in the United States (Turk et al., 2020) and was then adapted to the United Kingdom. Five categories of treatment attributes were selected to be included in the DCE survey instrument as follows: efficacy, tolerability (risk of heart attack and risk of rapidly progressive osteoarthritis), risk of addiction/dependency, frequency and mode of administration and out‐of‐pocket cost or insurance coverage. The descriptions of the attributes and levels included in the DCE were based on observations from the focus groups; empirical evidence for NSAIDs (Celecoxib, 2018; Meloxicam, 2016; Wongrakpanich et al., 2018), opioids (Hochberg et al., 2021; Oxycodone, 2015) and nerve growth factor inhibitors (Dakin et al., 2019; Hochberg, 2015) and guidance from clinical experts (Table 1).
TABLE 1.
Attributes and levels in the discrete‐choice experiment questions
| Attribute | Patient‐facing attribute label | Patient‐facing attribute levels | |
|---|---|---|---|
| Symptom control (patient global assessment) | Symptom control: Symptom control while you are taking the medicine | Very good (no symptoms; no limitations on normal activities) | |
| Good (mild symptoms; no limitations on normal activities) | |||
| Fair (moderate symptoms; limitations on some normal activities) | |||
| Poor (severe symptoms; unable to carry out most normal activities) | |||
| Incremental treatment‐related risk of severe rapidly progressive joint problems requiring total joint replacement a | Additional risk of severe joint problems: Additional risk each year of having joint problems that are severe enough that you would need a total joint replacement while you are taking the medicine or within 6 months of stopping the medicine | No additional risk (0%) | |
| Five people out of 1000 (0.5%) | |||
| 40 people out of 1000 (4%) | |||
| Risk of heart attack | Additional risk of heart attack: Additional risk each year of a heart attack while you are taking the medicine | No additional risk (0%) | |
| Two people out of 1000 (0.2%) | |||
| Five people out of 1000 (0.5%) | |||
| Risk of physical dependency | Risk of physical dependency: Risk each year of becoming physically dependent on the medicine | No risk (0%) | |
| 50 people out of 1000 (5%) | |||
| 250 people out of 1000 (25%) | |||
| Mode and frequency of administration | How you take the medicine | Oral pills two or more times a day | |
| Oral pills once a day | |||
| Injection every 4 weeks (about once a month) | |||
| Injection every 8 weeks (about once every 2 months) | |||
| Variable created for scope test (not an attribute visible to respondents) b | Narrow range | Wide range | |
| Personal (out‐of‐pocket) cost per month | Cost: Personal cost of the medicine to you every month | £0 every month | £0 every month |
| £30 every month | £30 every month | ||
| £55 every month | £75 every month | ||
| £85 every month | £110 every month | ||
The description of this attribute reflects what was known about rapidly progressive osteoarthritis type 2 (severe rapidly progressive joint problems requiring total joint replacement) at the time of the survey.
To implement the scope test, respondents were assigned to one of the two cost ranges in the discrete‐choice experiment questions: a narrow or wide range of cost. The scope test tested whether respondents evaluated a given difference in cost similarly regardless of whether that change in cost occurred within a narrow range of costs or within a wide range of costs. If the respondents passed the scope test, the impact of, for example a difference in cost between £0 and £30 should be the same whether respondents evaluated other costs ranging from £0 to £85 or other costs ranging from £0 to £110.
Previous studies of patient and physician preferences for pain management (Arden et al., 2012; Hauber et al., 2013) have demonstrated that pain and function are confounded and cannot be treated effectively as independent attributes in a DCE. Therefore, respondents were presented with a scenario in which they were told to assume that they were experiencing a very poor symptoms control state (described to the patients following the patient global assessment). Changes in the patient global assessment from very poor were then experimentally included in the DCE to represent the benefit attribute. Because treatment side effects are important to patients and likely differ substantially among the three different classes of treatments of interest in this study, the risk of a major and concerning AE associated with each treatment type was included as an attribute. The primary risk related to opioids identified by patients in the US focus groups was the risk of physical dependency. The two key risks commonly associated with NSAIDs are cardiovascular risks and gastrointestinal risks. Finally, the primary risk of concern with nerve growth factor inhibitors was the risk of rapidly progressive osteoarthritis (RPOA, Pokrovnichka, 2012). The study team had to choose whether to include a cardiovascular risk or a gastrointestinal risk in the DCE. Because gastrointestinal risks can be reduced by coprescribing proton‐pump inhibitors with NSAIDs, the study team decided to include the risk of a major cardiovascular event (heart attack) in the DCE. In addition, mode and frequency of administration that differentiate treatments are important considerations to patients. Specifically, mode of administration was defined as being either oral medications (i.e. NSAIDs and opioids) or subcutaneous injections (i.e. nerve growth factor inhibitor). The frequency of oral medications was specified as either once daily or two or more times per day, while the frequency of subcutaneous injections was specified as either once every 4 weeks or once every 8 weeks. Finally, out‐of‐pocket cost was included as an attribute in this study to enable the research team to scale the preference estimates in monetary terms. The amount of out‐of‐pocket cost needed to reduce a patient's utility by the same amount that improving symptoms control or reducing the risk of a given adverse event increases utility is known as willingness to pay and can be interpreted as a measure of the true economic value that a patient would place on the utility gain, whether or not he or she had to pay for it. The benefits of quantifying the monetary benefits of health interventions in this way, even in ‘free at the point of use’ health care systems such as the UK’s NHS, have been explored previously (e.g. Boeri et al., 2019; Ikenwilo et al., 2018; Porteous et al., 2016; Scalone et al., 2011).
The survey also included written descriptions of each attribute and level in the DCE, questions designed to assess patients’ comprehension of each attribute description and the choice tasks, questions about respondents’ experiences using treatments for moderate‐to‐severe OA pain and CLBP, and questions regarding respondents’ demographic characteristics. Multiple comprehension questions were specifically included to assess the relationship between patient comprehension and preference estimates. Additionally, the survey contained an exploratory assessment of respondent health locus of control (HLOC) using the Multidimensional Health Locus of Control (MHLC) Scale—Form C (Wallston et al., 1994) to assess the relationship between patient preferences and HLOC—a psychological construct describing the extent to which individuals attribute their health to their own actions or to external agents. HLOC may influence patient preferences for their role in healthcare decision making, and treatment preferences might be expected to have greater relevance for people who believe they have choice, rather than treatment being beyond the patient's control. Including such a psychological construct in preference studies have been suggested as part of the research agenda promoted by the European public‐private partnership PREFER (‘Patient Preferences in Benefit and Risk Assessments during the Drug Lifecycle’) (Smith et al., 2021). Data from this study were leveraged to explore a research question from PREFER regarding whether HLOC provides insight into preference heterogeneity. The survey instruments differed between conditions (i.e. OA pain only, CLBP only and concurrent OA and CLBP) only where necessary in the questions developed to elicit disease and treatment experience.
Before the survey instrument was administered to the study sample, a draft was qualitatively pretested during in‐person interviews with eight adults with moderate‐to‐severe OA and/or CLBP in the United Kingdom (UK), in accordance with good research practices (Bridges et al., 2011). During the pretest interviews, participants were asked to read the survey instrument and think aloud as they completed it. The study team observed participants’ general reactions to the survey instrument (e.g. whether they understood the hypothetical choice questions and considered the treatment attributes relevant), iteratively revised the instrument and ensured that the survey instrument was performing as intended. This process confirmed that the survey questions were understandable and that the attributes and levels were relevant to people with OA and/or CLBP and were described appropriately.
After completing the pretest interviews, the survey instrument was finalized by developing a fractional‐factorial experimental design using a D‐optimal algorithm, to determine the set of choice questions shown to each respondent (Kuhfeld, 2010; Kuhfeld et al., 1994; Reed Johnson et al., 2013). The full experimental design included 48 choice questions to allow gathering a sufficient number of observations on trade‐offs to model preferences. The experimental design was split into six blocks of eight questions each to reduce respondent burden. Each respondent was presented with one randomly assigned block, and the questions within each block were randomly ordered to mitigate ordering effects.
The study was reviewed and determined to be exempt from ethics committee review by the RTI International institutional review board. In addition, the study was exempt from review by a UK research ethics committee according to guidelines of the UK Health Research Authority Guidelines (Health Research Authority, 2018) because respondents were not recruited through the National Health Service. All survey respondents provided electronic informed consent.
2.2. Study population
Potential respondents in the UK who were members of panels maintained by the health care market research company SSI, now Dynata, and its partners were invited by e‐mail to participate in the online survey and asked to complete a 12‐item screening questionnaire to determine study eligibility. Eligible respondents were residents of the UK and were aged 18 years or older with a self‐reported physician diagnosis of hip or knee OA and/or CLBP diagnosed at least 3 months prior to taking the survey. Eligible respondents also self‐reported moderate‐to‐severe pain in the hip, knee, and/or lower back, defined as a self‐assessed rating of 5 or greater, on average, in the past week on an 11‐point numeric rating scale (NRS) ranging from 0 (no pain) to 10 (worst possible pain). For respondents with concurrent OA pain and CLBP, a rating of 5 or greater was required for either OA pain or CLBP for the respondent to be eligible. Respondents were required to be taking or have taken (1) at least three classes of pain treatment in the past 2 years; (2) two prior classes of pain treatment, either excluding NSAIDs because of a contraindication or excluding opioids because the respondent could not take or was unwilling to take opioids or (3) one prior class of pain treatment excluding NSAIDs because of a contraindication and excluding opioids because the respondent could not or was unwilling to take opioids. Respondents were able to read and understand English and provided informed consent. To explore differences depending on the specific condition, we originally targeted a soft quota of 150 respondents with hip or knee OA, 150 with CLBP and 150 with both for a total of 450 respondents.
Individuals were excluded if they self‐reported a diagnosis of Alzheimer's disease, axial spondylarthritis, fibromyalgia, major depressive disorder, migraine headaches, myopathy, neuropathic pain, psoriatic arthritis, radiculopathy or sciatica, rheumatoid arthritis, spinal stenosis, spondyloarthropathy or pain as a result of having had surgery in the past 3 months.
Recruitment quotas were imposed to achieve a balanced composition of participants, such that (1) approximately one‐third had OA only, one‐third had CLBP only and one‐third had concurrent OA and CLBP; and (2) approximately half were taking or had taken an opioid to treat OA and/or CLBP within the past 2 years, and approximately half had not. The recruitment team sequentially filled as respondents completed the survey.
Respondents who completed the survey received modest compensation (equivalent to approximately £8) for their participation.
2.3. Statistical analyses
The analysis of the data from a DCE is grounded in the conventional random utility theory framework (Manski, 1977; Thurstone, 1927), which assumed that respondents maximize their utility when selecting the most preferred option. In our analysis, the utility specification assumed that all parameters but cost to be effects‐coded as categorical values. Cost was interacted with the natural log of income (calculated as the midpoint of the range of income selected by the respondent) to control for income effects. Based on the attribute list presented in Table 1, the following indirect utility function (V) was used in the analysis:
| (1) |
As a partial internal validity test, a scope test was conducted to assess whether respondents were attentive to the actual levels of the personal cost of the medicine each month when making treatment choices in the DCE (Ho et al., 2015; Özdemir et al., 2009; Poulos et al., 2018; Poulos, Standaert, et al., 2018; Poulos et al., 2018). The scope test hypothesized that respondents would evaluate a difference in cost similarly regardless of whether that change in cost occurred within a narrow range of costs or within a wide range of costs (see Table 1). A Wald test was used to jointly evaluate whether the two cost levels (£0 every month and £30 every month) that appeared in both the narrow and wide cost arms had the same preference weights (Greene, 2012).
DCE questions generate cross‐section/time‐series data that can be explored using mixed logit models (Hauber et al., 2016; Train, 2009; Train & Sonnier, 2005). Depending on the researcher's assumptions on the preference distribution, mixed logit models can be estimated as a random parameter logit (RPL) model or as a latent class (LC) model (Boeri et al., 2020; Greene & Hensher, 2003; Louviere, 2006; Zhou et al., 2018).
Considering the observation by Boeri et al. (2020) that the LC model has an advantage over the RPL approach, as it is a model that can simultaneously estimate marginal preference weights associated with different attribute levels characterizing alternative medical treatments for different segments in the sample (referred to as classes) and can assign each class a probability that can be dependent on the respondent's characteristics, the LC model approach was selected for the main analysis of this paper. To determine the optimal number of classes for the model we considered three information criteria based on maximizing the log‐likelihood of the model and minimizing the number of parameters to be estimated: the Bayesian information criteria (BIC, Hurvich & Tsai, 1989), the Akaike information criteria (AIC, McLachlan & Peel, 2000) and the AIC3, a variation of the AIC that penalizes more for the number of parameters used in estimation (Wedel & Kamakura, 2000). Nevertheless, it is worth noting that these criteria also fail some of the regularity conditions for a valid test under the null (Leroux, 1992). Following the various goodness‐of‐fit measures presented for the various LC model specifications, the LC model with four classes was considered the optimal model specification for this dataset because it generated the best BIC and convergence for the other AIC measures. The results are presented in the supplementary material, Figure A1.
Furthermore, preference heterogeneity was explored by modelling the class membership probability as a function of respondent condition (OA, CLBP or both); opioid experience; age; time living with chronic pain; baseline pain level; number of correct responses on the comprehension questions; ‘internal’, ‘powerful others’ and ‘chance’ dimensions of HLOC and history of joint replacement.
3. RESULTS
3.1. Respondents
SSI invited 381,382 potential respondents in the UK via e‐mail to participate in an online survey through SSI’s panels and partner panels. Of those who received the invitation, 17,627 respondents (4.6%) accessed the link to the online survey. Of those who accessed the survey, 1615 respondents (9.2%) completed the screening questions and met the eligibility criteria. Of those who were eligible, 1501 respondents (92.9%) consented to participate and were administered the survey. Of those who consented to participate and were administered the survey, 437 respondents (29.1%) completed the survey and were included in the final sample.
Of the 437 respondents included in the final sample, 171 had OA only, 188 had CLBP only and 78 had concurrent OA and CLBP. Because of difficulty identifying respondents for the survey who had no prior opioid experience, and those who had concurrent OA and CLBP, data collection was discontinued before the target of 450 completed surveys was reached. Four respondents did not answer the questions used to model the membership probability function and were excluded from the LC analysis sample.
Among all respondents, mean age was 54 years; 55% were female, 69% were married or living as married and 95% self‐identified as white or Caucasian (Table 2). Respondents’ mean (standard deviation [SD]) baseline pain NRS in the past week was 6.7 (1.2) among those with only OA and 7.0 (1.2) among those with only CLBP. Among those with both OA and CLBP, their mean (SD) rating for pain associated with OA was 6.5 (1.7) and for pain associated with CLBP was 6.7 (1.7). On average, 15%–31% of participants with each condition were somewhat dissatisfied or very dissatisfied with their current treatment to control their pain.
TABLE 2.
Respondent characteristics
| Question | Respondents with only OA (n = 171) | Respondents with only CLBP (n = 188) | Respondents with OA and CLBP (n = 78) | All respondents (N = 437) | ||
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Gender | ||||||
| Male | 65 (38.0) | 101 (53.7) | 29 (37.2) | 195 (44.6) | ||
| Female | 106 (62.0) | 87 (46.3) | 49 (62.8) | 242 (55.4) | ||
| Prefer not to answer | 0 | 0 | 0 | 0 | ||
| Age (in years) | ||||||
| Mean (SD) | 60.1 (11.5) | 46.6 (14.0) | 61.0 (11.2) | 54.4 (14.3) | ||
| Median | 62.0 | 45.0 | 63.0 | 56.0 | ||
| Min, max | 18, 79 | 18, 76 | 35, 91 | 18, 91 | ||
| Race/ethnicity a | ||||||
| White | 165 (96.5) | 174 (92.6) | 76 (97.4) | 415 (95.0) | ||
| Mixed/multiple ethnic groups | 2 (1.2) | 3 (1.6) | 0 | 5 (1.1) | ||
| Asian/Asian British | 2 (1.2) | 5 (2.7) | 0 | 7 (1.6) | ||
| Black/African/Caribbean/Black British | 1 (0.6) | 4 (2.1) | 0 | 5 (1.1) | ||
| Other ethnic groups | 0 | 1 (0.5) | 1 (1.3) | 2 (0.5) | ||
| Prefer not to say | 1 (0.6) | 1 (0.5) | 1 (1.3) | 3 (0.7) | ||
| Highest level of education | ||||||
| No formal education | 1 (0.6) | 0 | 1 (1.3) | 2 (0.5) | ||
| Primary school education or less | 0 | 0 | 0 | 0 | ||
| Secondary school education up to general, ordinary or standard level | 52 (30.4) | 38 (20.2) | 17 (21.8) | 107 (24.5) | ||
| Secondary school education up to intermediate, advanced or higher level | 24 (14.0) | 30 (16.0) | 13 (16.7) | 67 (15.3) | ||
| Professional or work‐related qualifications obtained from a college or university | 47 (27.5) | 40 (21.3) | 26 (33.3) | 113 (25.9) | ||
| Undergraduate university degree | 22 (12.9) | 60 (31.9) | 18 (23.1) | 100 (22.9) | ||
| Postgraduate university degree or equivalent qualification | 25 (14.6) | 20 (10.6) | 3 (3.8) | 48 (11.0) | ||
| Prefer not to say | 0 | 0 | 0 | 0 | ||
| What was your total household income before tax and other deductions for last year? | ||||||
| Less than £15,000 | 22 (12.9) | 22 (11.7) | 11 (14.1) | 55 (12.6) | ||
| £15,000 to £29,999 | 53 (31.0) | 37 (19.7) | 21 (26.9) | 111 (25.4) | ||
| £30,000 to £49,999 | 46 (26.9) | 49 (26.1) | 20 (25.6) | 115 (26.3) | ||
| £50,000 to £74,999 | 18 (10.5) | 39 (20.7) | 14 (17.9) | 71 (16.2) | ||
| £75,000 to £99,999 | 9 (5.3) | 18 (9.6) | 3 (3.8) | 30 (6.9) | ||
| £100,000 to £149,999 | 4 (2.3) | 4 (2.1) | 3 (3.8) | 11 (2.5) | ||
| £150,000 to £199,999 | 3 (1.8) | 2 (1.1) | 1 (1.3) | 6 (1.4) | ||
| £200,000 or more | 1 (0.6) | 2 (1.1) | 0 | 3 (0.7) | ||
| Don't know or not sure | 5 (2.9) | 3 (1.6) | 1 (1.3) | 9 (2.1) | ||
| Prefer not to say | 10 (5.8) | 12 (6.4) | 4 (5.1) | 26 (5.9) | ||
| Clinical characteristics | ||||||
| Use of opioids for OA pain or CLBP in the past 2 years | ||||||
| Weak opioids | 116 (67.8) | 110 (58.5) | 54 (69.2) | 280 (64.1) | ||
| Strong opioids | 42 (24.6) | 60 (31.9) | 17 (21.8) | 119 (27.2) | ||
| Respondent eligibility based on classes of pain treatment | ||||||
| Respondents who met the minimum of three current or prior medications | 132 (77.2) | 148 (78.7) | 63 (80.8) | 343 (78.5) | ||
| Respondents who selected two current or prior medications but could not take NSAIDs | 15 (8.8) | 5 (2.7) | 8 (10.3) | 28 (6.4) | ||
| Respondents who selected two current or prior medications but could not take opioids or would never consider taking opioids | 19 (11.1) | 29 (15.4) | 6 (7.7) | 54 (12.4) | ||
| Selected two current or prior medications, but could not take NSAIDs and could not take opioids or would never consider taking opioids | 1 (0.6) | 2 (1.1) | 0 | 3 (0.7) | ||
| Respondents who selected one current or prior medication but could not take NSAIDs and could not take opioids or would never consider taking opioids | 4 (2.3) | 4 (2.1) | 1 (1.3) | 9 (2.1) | ||
| How long has it been since you first had symptoms related to [OA] [CLBP] [OA and CLBP]? | ||||||
| Less than 1 year | 4 (2.3) | 14 (7.4) | 1 (1.3) | 19 (4.3) | ||
| At least 1 year, but less than 2 years | 14 (8.2) | 44 (23.4) | 6 (7.7) | 64 (14.6) | ||
| At least 2 years, but less than 5 years | 39 (22.8) | 46 (24.5) | 8 (10.3) | 93 (21.3) | ||
| 5 years or more | 112 (65.5) | 83 (44.1) | 63 (80.8) | 258 (59.0) | ||
| Don't know or not sure | 2 (1.2) | 1 (0.5) | 0 | 3 (0.7) | ||
| Have you ever had a joint replaced because of severe joint problems? | ||||||
| Yes | 40 (23.4) | 8 (4.3) | 20 (25.6) | 68 (15.6) | ||
| No | 131 (76.6) | 180 (95.7) | 58 (74.4) | 369 (84.4) | ||
| How satisfied are you with how well your current treatment works to control your [OA] [CLBP] [OA and CLBP] pain? (Please tick only one answer) | ||||||
| Very satisfied | 10 (5.8) | 23 (12.2) | 0 | 33 (7.6) | ||
| Somewhat satisfied | 73 (42.7) | 99 (52.7) | 30 (38.5) | 202 (46.2) | ||
| Neither satisfied nor dissatisfied | 42 (24.6) | 37 (19.7) | 24 (30.8) | 103 (23.6) | ||
| Somewhat dissatisfied | 41 (24.0) | 27 (14.4) | 21 (26.9) | 89 (20.4) | ||
| Very dissatisfied | 5 (2.9) | 2 (1.1) | 3 (3.8) | 10 (2.3) | ||
| Responses to locus of control questions (scoring divided by dimensions) | ||||||
| Internal (health is the result of an individual's effort and habits: items 1, 6, 8, 12, 13, 17) | ||||||
| Mean (SD) | 3.4 (0.9) | 3.7 (0.9) | 3.3 (0.9) | 3.5 (0.9) | ||
| Median | 3.3 | 3.8 | 3.3 | 3.5 | ||
| Min, max | 1.0, 5.8 | 1.3, 6.0 | 1.0, 5.3 | 1.0, 6.0 | ||
| Chance (health depends on fate and chance: items 2, 4, 9, 11, 15, 16) | ||||||
| N | 170 | 188 | 78 | 436 | ||
| Mean (SD) | 3.1 (1.0) | 3.5 (1.0) | 3.1 (1.0) | 3.3 (1.0) | ||
| Missing | 1 | 0 | 0 | 1 | ||
| Median | 3.2 | 3.5 | 3.2 | 3.3 | ||
| Min, max | 1.0, 5.5 | 1.2, 6.0 | 1.0, 6.0 | 1.0, 6.0 | ||
| Missing | 1 | 0 | 0 | 1 | ||
| Powerful others (health depends on others, such as doctors: items 3, 5, 7, 10, 14, 18) | ||||||
| Mean (SD) | 3.7 (0.8) | 3.9 (0.8) | 3.8 (0.9) | 3.8 (0.8) | ||
| Median | 3.7 | 4.0 | 3.9 | 3.8 | ||
| Min, max | 1.7, 5.7 | 1.5, 6.0 | 1.5, 5.3 | 1.5, 6.0 | ||
| Powerful others can be divided into the following: | ||||||
| Doctors (items 3, 5, 14) | ||||||
| Mean (SD) | 4.1 (0.9) | 4.1 (1.0) | 4.1 (1.1) | 4.1 (1.0) | ||
| Median | 4.0 | 4.0 | 4.0 | 4.0 | ||
| Min, max | 2.0, 6.0 | 1.7, 6.0 | 1.7, 6.0 | 1.7, 6.0 | ||
| Other people (items 7, 10, 18) | ||||||
| Mean (SD) | 3.4 (1.0) | 3.6 (1.0) | 3.6 (1.1) | 3.5 (1.0) | ||
| Median | 3.3 | 3.7 | 3.7 | 3.7 | ||
| Min, max | 1.0, 6.0 | 1.0, 6.0 | 1.0, 6.0 | 1.0, 6.0 | ||
| Comprehension question success frequencies | ||||||
| Respondents who answered all comprehension questions correctly | 14 (8.2) | 8 (4.3) | 4 (5.1) | 26 (5.9) | ||
| Respondents who answered six comprehension questions correctly | 33 (19.3) | 22 (11.7) | 15 (19.2) | 70 (16.0) | ||
| Respondents who answered five comprehension questions correctly | 37 (21.6) | 41 (21.8) | 20 (25.6) | 98 (22.4) | ||
| Respondents who answered four comprehension questions correctly | 45 (26.3) | 43 (22.9) | 18 (23.1) | 106 (24.3) | ||
| Respondents who answered three comprehension questions correctly | 24 (14.0) | 44 (23.4) | 14 (17.9) | 82 (18.8) | ||
| Respondents who answered two comprehension questions correctly | 15 (8.8) | 22 (11.7) | 6 (7.7) | 43 (9.8) | ||
| Respondents who answered one comprehension question correctly | 2 (1.2) | 6 (3.2) | 0 | 8 (1.8) | ||
| Respondents who did not answer any of the comprehension questions correctly | 1 (0.6) | 2 (1.1) | 1 (1.3) | 4 (0.9) | ||
Values are n (%) unless otherwise stated. The percentage totals may not sum exactly to 100% because of rounding.
Abbreviations: CLBP, chronic low back pain; NSAID, nonsteroidal anti‐inflammatory drug; OA, osteoarthritis; SD, standard deviation.
Respondents could provide multiple responses to these questions. Therefore, the totals may exceed the total number of respondents.
3.2. Discrete‐choice experiment
3.2.1. Preference analysis
The scope test analysis was conducted with the full sample (N = 437). The joint test for the overlapping cost levels indicated that the preferences for the two levels were not statistically significantly different from one another regardless of the range of costs shown (χ 2 = 1.92; p = 0.17). Therefore, the sample was determined to pass the scope test, and data from respondents seeing the different cost ranges were pooled in the analysis.
Overall, the estimated preference weights were ordered as expected for all attributes; that is more desirable levels of an attribute were preferred to worse levels of the attribute (Figure 2). On average, respondents placed more importance on better symptom control and avoiding treatment‐related risk of physical dependency over avoiding treatment‐related risk of heart attack and severe, rapidly progressive joint problems requiring arthroplasty (Figure 2).
FIGURE 2.

Mean preference weights from the latent class model output (N = 433). The change in utility associated with a change in the levels of each attribute is represented by the vertical distance between the preference weights for any two levels of that attribute. The mean preference weight for each attribute is set as 0.0. Larger differences between preference weights indicate that respondents viewed the change as having a relatively greater effect on overall utility. For example a change from poor to fair symptom control was considered more important by participants than from good to very good, and more important than a change in treatment‐related risk of heart attack from 0% to 0.5%. Differences in estimated preference weights between each level of symptoms control and each level of risk of treatment related risk of physical dependency were statistically significant (p < 0.05). Preference differences between the highest and the lowest incremental risks of severe joint problems and heart attack were also statistically significant, but, overall, respondents did not differentiate between modes or frequencies of administration. Attributes are presented in the order in which they appeared in the discrete‐choice experiment questions. The vertical bars surrounding each relative importance weight estimate denote the 95% confidence interval (computed by the delta method). Note. Four respondents did not answer the questions used to model the membership probability function and were excluded from this LC analysis
Figure 3 presents the results from the four classes LC model, which was selected as the optimal LC model for these data after comparing a range of models from two to six classes using the criteria described above. The largest identified class, with a membership probability of 34%, made choices driven by getting better efficacy (symptom control) in terms of larger improvement from ‘Poor’ on the patient global assessment scale. This class was therefore referred to as the ‘efficacy‐focused’ class. Respondents in this class were also averse to the risk of physical dependency, preferred to pay a lower personal out‐of‐pocket monthly cost, and preferred taking oral pills once a day or oral pills two or more times a day compared with having an injection every 4 or 8 weeks.
FIGURE 3.
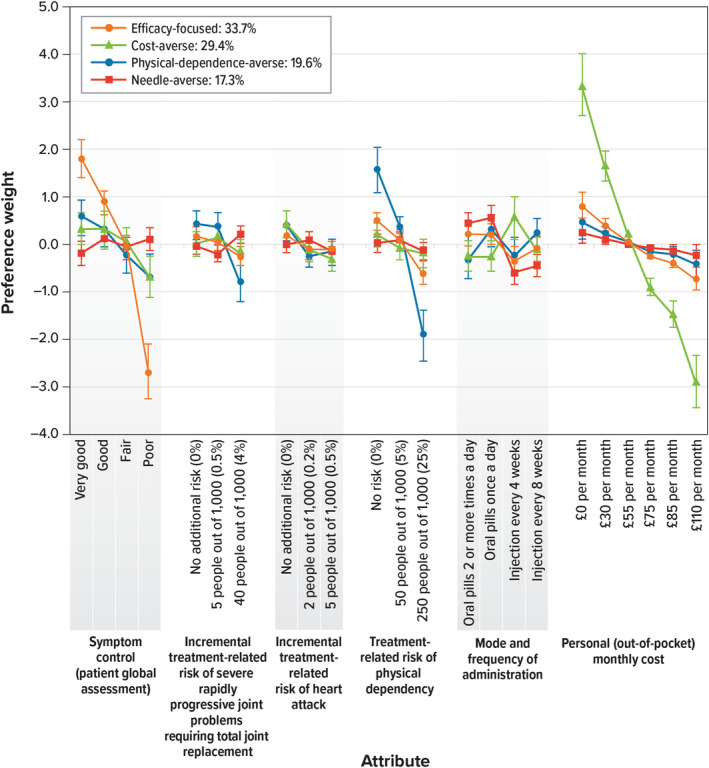
Latent Class Model: Preference Weights for Each Class (N = 433). Within each attribute, a higher preference weight indicates that a level is more preferred. The mean preference weight for each attribute is set as 0.0. Larger differences between preference weights indicate that respondents viewed the change as having a relatively greater effect on overall utility. As in any LC analysis, the order in which the classes are presented is only for convenience and does not have impact on the interpretation of the results. Attributes are presented in the order in which they appeared in the discrete‐choice experiment questions. The vertical bars around each mean preference weight represent the 95% confidence interval around the point estimate. Note: Four respondents did not answer the questions used to model the membership probability function and were not included in this LC analysis
Respondents associated with the second‐highest membership probability (approximately 29%) were strongly averse to paying a personal (out‐of‐pocket) cost for the treatment. They did not strongly differentiate between very good, good and fair symptom control, but they preferred these levels of symptom control to poor symptom control. Respondents associated with this class preferred to have an injection every 4 and 8 weeks compared with taking oral pills once a day or two or more times a day and were averse to the incremental treatment‐related risk of heart attack. This preference class is referred to as the ‘cost‐averse’ class.
A class with an average membership probability of approximately 20% was defined predominantly by a significant preference for a low risk of physical dependency and hence was deemed the ‘physical‐dependence–averse’ class. Respondents in this class were also averse to the risk of severe rapidly progressive joint problems requiring total joint replacement.
Finally, the smallest class, with a membership probability of approximately 17%, included respondents particularly focused on mode and frequency of administration, preferring taking oral pills once a day or two or more times a day rather than an injection every 4 or 8 weeks. Respondents associated with this class did not present strong preferences for any other attribute, although they did prefer to pay a lower personal out‐of‐pocket monthly cost. This class was named as the ‘needle‐averse’ class.
In an attempt to explain preference heterogeneity and better describe the classes highlighted in the LC model, we modelled the class membership probability function to include respondent characteristics (results are presented in Table 3). Respondents of median age (56 years) or older were more likely than younger respondents to be in the ‘efficacy‐focused’ class (hence, younger respondents were more likely to be in the ‘cost‐averse’ and ‘needle‐averse’ classes). Respondents who provided incorrect responses to three or more comprehension questions were more likely to be in any class (the ‘needle‐averse’ the ‘physical‐dependence–averse’ and the ‘cost‐averse’ classes) than in the ‘efficacy‐focused’ class. As presented in Table 3, other respondent characteristics such as whether respondents had OA, CLBP, or both; duration in which they lived with chronic pain; whether they self‐reported severe baseline pain (ranked 7–10); or their HLOC classifications were not significantly associated with the probability of membership in a specific class (i.e. covariates and the constant were not significantly different from zero).
TABLE 3.
Membership probability model for the latent class main‐effects model (N = 433 a )
| Covariate | ‘Cost‐averse’—class | ‘Physical‐dependence–averse’ class | ‘Needle‐averse’ class | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate | p value | Estimate | p value | Estimate | p value | |||
| Constant | −0.756 | 0.254 | −0.986 | 0.176 | −3.090 | 0.007 | ||
| Respondents with comorbid OA and CLBP | Reference | |||||||
| Respondents with OA only | 0.199 | 0.629 | 0.004 | 0.994 | 1.166 | 0.115 | ||
| Respondents with CLBP only | −0.065 | 0.878 | −0.170 | 0.727 | 1.094 | 0.144 | ||
| Respondents without opioid experience | Reference | |||||||
| Respondents with opioid experience | 0.151 | 0.634 | −0.447 | 0.247 | 0.744 | 0.113 | ||
| Age: Younger than median age | Reference | |||||||
| Age: Median age or older | −0.682 | 0.043 | −0.382 | 0.353 | −1.002 | 0.025 | ||
| Respondents living with chronic pain for less than 5 years | Reference | |||||||
| Respondents living with chronic pain for 5 years or more | 0.143 | 0.672 | 0.147 | 0.718 | −0.088 | 0.839 | ||
| Respondents with moderate baseline pain (ranked 5–6) | ||||||||
| Respondents with severe baseline pain (ranked 7–10) | 0.084 | 0.781 | 0.585 | 0.141 | 0.342 | 0.396 | ||
| Respondents who are classified as low ‘internal’ locus of control (the median score or less) | Reference | |||||||
| Respondents who are classified as high ‘internal’ locus of control (above the median score) | 0.221 | 0.473 | 0.343 | 0.367 | 0.479 | 0.239 | ||
| Respondents who are classified as low ‘chance’ locus of control (the median score or less) | Reference | |||||||
| Respondents who are classified as high ‘chance’ locus of control (above the median score) | 0.243 | 0.424 | −0.702 | 0.081 | 0.263 | 0.510 | ||
| Respondents who are classified as low ‘powerful others’ locus of control (the median score or less) | Reference | |||||||
| Respondents who are classified as high ‘powerful others’ locus of control (above the median score) | 0.179 | 0.549 | −0.180 | 0.634 | 0.220 | 0.573 | ||
| Respondents who provided incorrect responses to fewer than three comprehension questions | Reference | |||||||
| Respondents who provided incorrect responses to three or more comprehension questions | 0.872 | 0.003 | 1.364 | 0.000 | 1.365 | 0.001 | ||
For the model to be identified, one class (the ‘efficacy‐focused’ class) was omitted and was the reference for the model estimates. Therefore, a positive and significant estimate for the constant and each covariate in the class membership probability model was interpreted as increasing the probability that a respondent with this characteristic would be in a specific class rather than in the ‘efficacy‐focused’ class compared with the baseline respondents (for which each covariate was equal to zero). Furthermore, dummy coded variables were used for the respondent characteristics, and the baseline for each effect is defined by the constant and all variables at the reference levels. Bold values indicate significant association with the probability of membership in a specific class (i.e. covariates and the constant were significantly different from zero).
Abbreviations: CLBP, chronic low back pain; LC, latent class; OA, osteoarthritis.
Four respondents did not answer the questions used to model the membership probability function and were excluded from this LC analysis.
4. DISCUSSION
This study explored patient preferences for current and emergent pharmaceutical treatments for OA pain and/or CLBP. Overall, participants put at least equal weight on analgesic benefit as on treatment‐associated risks, but marked heterogeneity was identified between participants. Four discrete participant subgroups were identified. The largest class put the greatest weight on analgesic efficacy, but smaller subgroups focused on out‐of‐pocket costs, risk of physical dependency and mode and frequency of administration. Subgroup membership was incompletely predicted by participant age and their responses to comprehension questions.
The growing availability of pharmaceutical treatments for the management of chronic pain and the variety of efficacy and AEs related to each of these treatments (Ghouri & Conaghan, 2021) emphasizes the importance of understanding the trade‐offs that patients are willing to make among benefits and risks linked to specific treatments. This study addressed this topic by administering a DCE survey instrument to 437 respondents with chronic pain related to OA and/or CLBP. Respondents were asked to assume that their level of symptom control had become very poor and they were presented with a series of scenarios and asked to choose between pairs of hypothetical treatments, each defined by six attributes, including symptom control, safety, drug administration and cost. Attributes were based on known profiles of oral NSAIDs, opioids and injected nerve growth factor inhibitors, the latter of which were under clinical development at the time of the study.
The results presented in Figure 2 indicate that, on average, respondents placed more importance on better symptom control and avoiding treatment‐related risk of physical dependency over avoiding treatment‐related risk of heart attack and severe, rapidly progressive joint problems requiring arthroplasty. Comparison with our previous findings using the identical methodology in population with OA and/or CLBP in the USA (Turk et al., 2020) indicates a remarkable similarity in treatment preferences between people in countries with different cultures and healthcare systems. However, in our LC analysis, we found evidence of preference heterogeneity across four classes: the ‘efficacy‐focused’ class (with a membership probability of 33.7%), the ‘cost‐averse’ class (with a membership probability of 29.4%), the ‘physical‐dependence–averse’ class (with a membership probability of 19.6%) and the ‘needle‐averse’ class (with a membership probability of 17.3%). Therefore, we suggest that preferences vary across the sample as different respondents are focused on different characteristics of pharmaceutical treatments to control their pain (subgroup analysis).
Participant characteristics only weakly predicted subgroup allocation through LC analysis, emphasizing the need to address patient preferences in individual consultations. However, we found that respondents of median age (56 years) or older and those who provided incorrect answers to fewer than three comprehension questions were more likely to be in the ‘efficacy‐focused’ class, whereas younger respondents were more likely to be in the ‘needle‐averse’ and ‘cost‐averse’ classes. Association of treatment preference class with age might reflect different life experiences, including previous treatment experience, or cultural attitudes and beliefs that might differ between generations.
Although this was only marginally explored in our analysis, we did not find any significant associations between HLOC classification and patient preferences. This finding was not completely surprising, but research on the predictive utility of the HLOC construct has been mixed. Some analyses have found HLOC to be a weak to moderate predictor of health behaviour (e.g. Grisolía et al., 2015; Norman & Bennett, 1996). Others have found that individuals with higher external HLOC prefer less involvement in health care decision making (Schneider et al., 2006), and those with lower external HLOC prefer an active and collaborative role in health care decisions (Marton et al., 2021). The role of HLOC in health preferences is an empirical matter, and thus further investigation would seem warranted to better understand how it impacts preferences.
Comprehension questions are, as here, frequently included in stated‐preference studies as an indicator of whether respondents understand the material with which they are being presented (e.g. Marshall et al., 2017). Our finding that respondents’ answers to the comprehension questions differentiated their preferences might indicate suboptimal comprehension of the survey. However, we used gold‐standard methodology to pretest the survey instrument (Bridges et al., 2011), and if respondents failed a comprehension question, the information was repeated to them. Therefore, a failed comprehension question need not mean that the respondent did not understand all the information about the hypothetical treatments when they answered the treatment choice questions. Association with incorrect answering of comprehension questions might alternatively reflect cultural attitudes (e.g. associated with educational level or social class). Recognizing heterogeneity in comprehension among patients is important in helping physicians make fully informed treatment choices in clinical practice, and our findings suggest that comprehension may particularly influence how patients balance benefits against risks of treatments.
Our study confirms previous findings that OA and CLBP treatment preferences are influenced by efficacy, side effects, out‐of‐pocket costs and treatment schedule (Laba et al., 2013). We found, overall, that pain relief was considered more important than adverse events such as cardiovascular events (specific to NSAIDs) or risks of rapidly progressive OA (specific to nerve growth factor inhibitors). Mühlbacher and colleagues (2015) similarly found in German patients with chronic pain (44% with neuropathic pain) that pain reduction was approximately twice as important as reduction in risk of addiction. We furthermore show that different adverse events might contribute differently to preferences in different patient subgroups. Patients were willing to accept a wide range of adverse events in exchange for reductions in pain, extending previous findings that patients in the UK were willing to accept significant cardiovascular and gastrointestinal risks in exchange for reductions in pain from nonopioid OA treatments (Arden et al., 2012; Hauber et al., 2013). We show here and in a related study from the USA (Turk et al., 2020), that physical dependency, as associated with opioid treatment, may be considered more important by patients than are cardiovascular and gastrointestinal risks, particularly by a subgroup of the UK OA and CLBP populations.
In addition, the monetary value of achieving very good symptom control was estimated to be £69 per month, which is not out of line with previous research on the value of OA treatment that estimated an economic value for viscosupplement injections of up to £69 per treatment (Posnett et al., 2015). The cost attribute was included in the study as a benchmark to scale the preference estimates in monetary terms and was not itself anticipated to be a motivating factor driving patient preferences. Influence by cost on treatment preferences might seem surprising in the UK, whose NHS is based on the principle that treatments should be free at the point of delivery. However, NHS treatments may present a financial burden to patients through prescription charges and accessing sites in which treatments are delivered. Furthermore, not all treatments may be readily accessible through the NHS, and many patients self‐fund treatments in the UK. Our findings suggest that, even when health care is nominally free at the point of delivery, cost is an important driver of preferences to a group of individuals in the UK.
This study shares similar limitations with any other stated‐preference studies. Respondents were asked to make hypothetical choices, which may differ from actual decisions made in a clinical setting. Diagnoses and participant characteristics were self‐reported and could not be independently confirmed by the investigators. The DCE choice task could allow the inclusion of only a limited number of adverse events, forcing the authors to select from the literature the most important attribute for each type of drug. Incomplete comprehension might compromise interpretation of reported treatment preferences in some participant groups, although respondents in the current study were provided with the correct answer to each comprehension question if answered incorrectly. Physician preferences were not explored in the current study, although physicians and patients may make similar choices with regard to OA treatments (Arden et al., 2012).
While the sample was considered sufficient to achieve the study objectives, a somewhat limited number of respondents with each pain condition of interest were included; thus, the possibility that a larger sample size may have revealed additional preference classes cannot be ruled out. Our sample included diversity in terms of gender, education, age, condition, chronic pain duration and prior treatment experience; however, there was a lack of diversity with regard to race and ethnicity. Owing to sampling limitations, the study sample may not be entirely representative of the target population, although our sample's characteristics were broadly similar to those of the populations of two recent clinical trials (Berenbaum et al., 2020; Schnitzer et al., 2019).
Our recruitment method and use of online survey might have introduced information and selection bias and limited the generalizability of our findings. Our respondents may be younger and more educated than actual patient populations (Deshpande et al., 2016; Smith et al., 2016), and we show that age and comprehension may influence treatment preferences. A high proportion of the UK population can access online surveys, but important minorities, households with lower incomes and senior citizens may be less likely to adopt Internet (InternetLiveStats.com, 2016). However, carefully administered online research can efficiently reach samples that are representative of the targeted population (Boyle et al., 2012), and can generate results not statistically different from face‐to‐face interviews (Marta‐Pedroso et al., 2007; Nielsen, 2011). In addition, there is evidence to suggest internet panels generate comparable preference data to mail‐based surveys (Determann et al., 2017), yet they are superior in terms of response rate (Ryan et al., 2020).
5. CONCLUSION
Physicians, regulators and drug developers endeavour to understand patients’ treatment priorities. Our findings provide insights into how patients balance symptom relief against other aspects of health that can inform shared decision making in clinical practice. We show that respondents, on average, were willing to accept degrees of risk associated with NSAIDs, opioids and nerve growth factor blocking antibodies in order to improve symptom control. Future research should explore to what extent these factors drive patients’ actual treatment choices.
Participants in the current sample displayed discrete patterns of preference, with the largest subgroup placing particular importance on symptom control. However, three smaller but important subgroups were identified who put greater weight on out‐of‐pocket costs, treatment‐related risk of physical dependency or mode and route of treatment administration. The investigated participant characteristics only weakly predicted preference class allocation, and unexplained heterogeneity in preferences highlights the need for a personalized approach to discussing treatment options with patients. Heterogeneity in treatment preferences between groups of people with chronic musculoskeletal pain emphasizes the need for a patient‐centred care model through which physicians consider an individual patient's preferences when making treatment recommendations.
CONFLICT OF INTEREST
Lucy Abraham, Jo Atkinson, Andrew Bushmakin, Joseph Cappelleri, Brett Hauber and Leo Russo are employees of and hold stock and/or stock options in Pfizer Inc. Lars Viktrup is an employee of Eli Lilly and Company and holds stock. Marco Boeri and Kathleen Klein are employees of RTI Health Solutions, who were paid consultants to Pfizer in connection with the development of this manuscript. In the past 36 months, Dennis C. Turk has received research grants and contracts from the US Food and Drug Administration and US National Institutes of Health and has received compensation for consulting on clinical trials and patient preferences from AccelRx, Eli Lilly and Company, Flexion, GlaxoSmithKline, Pfizer and Swing Therapeutics. Professor Walsh declares a personal financial interest in his employment by the University of Nottingham who received funding for his salary from the UK Government, Sherwood Forest Hospitals NHS Foundation Trust and UKRI. In the past 36 months David Walsh declares the following non‐personal financial interests: consultancy through his employment with the University of Nottingham to Pfizer Ltd, Eli Lilly, AbbVie Ltd, Galapagos and Reckitt Benckiser Health Ltd and GlaxoSmithKline Plc, and responsibilities for investigator‐led grants outside the work in this manuscript held by the University of Nottingham from Pfizer Ltd and Eli Lilly and Company.
AUTHORS’ CONTRIBUTIONS
DCT and DAW contributed to design the study, interpret the data and revised the article critically for important intellectual content. MB and BH designed the study, provided study oversight, conducted the analyses, interpreted the data and revised the article critically for important intellectual content. KK conducted the analyses, interpreted the data and revised the article critically for important intellectual content. LA, JA, AB, JCC, LR and LV contributed to the study design, participated in securing study funding, contributed to the analyses, interpreted the data and revised the article critically for important intellectual content. All authors approved the article for publication and agree to take responsibility for the content.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
Kimberly Moon of RTI Health Solutions provided overall project management for this study. Medical writing support was provided by Kate Lothman of RTI Health Solutions and was funded by Pfizer and Eli Lilly and Company
Walsh, D. A. , Boeri, M. , Abraham, L. , Atkinson, J. , Bushmakin, A. G. , Cappelleri, J. C. , Hauber, B. , Klein, K. , Russo, L. , Viktrup, L. , & Turk, D. (2022). Exploring patient preference heterogeneity for pharmacological treatments for chronic pain: A latent class analysis. European Journal of Pain, 26, 648–667. 10.1002/ejp.1892
Funding information
This study was sponsored by Pfizer Inc. and Eli Lilly and Company. The authors affiliated with Pfizer Inc. and Eli Lilly and Company participated in designing the study, interpreting the data and writing the manuscript and approved the final version for publication.
REFERENCES
- Arden, N. K. , Hauber, A. B. , Mohamed, A. F. , Johnson, F. R. , Peloso, P. M. , Watson, D. J. , Mavros, P. , Gammaitoni, A. , Sen, S. S. , & Taylor, S. D. (2012). How do physicians weigh benefits and risks associated with treatments in patients with osteoarthritis in the United Kingdom? Journal of Rheumatology, 39, 1056–1063. 10.3899/jrheum.111066 [DOI] [PubMed] [Google Scholar]
- Aronson, J. K. (2009). Meyler’s side effects of analgesics and anti‐inflammatory drugs. Elsevier. [Google Scholar]
- Bannuru, R. R. , Osani, M. C. , Vaysbrot, E. E. , Arden, N. K. , Bennell, K. , Bierma‐Zeinstra, S. , Kraus, V. B. , Lohmander, L. S. , Abbott, J. H. , Bhandari, M. , Blanco, F. J. , Espinosa, R. , Haugen, I. K. , Lin, J. , Mandl, L. A. , Moilanen, E. , Nakamura, N. , Snyder‐Mackler, L. , Trojian, T. , … McAlindon, T. E. (2019). OARSI guidelines for the non‐surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis and Cartilage, 27, 1578–1589. 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- Berenbaum, F. , Blanco, F. J. , Guermazi, A. , Miki, K. , Yamabe, T. , & Verburg, K. M. (2020). Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24‐week randomised phase III study with a 24‐week follow‐up period. Annals of the Rheumatic Diseases, 79, 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindawas, S. M. , Vennu, V. , & Auais, M. (2015). Health‐related quality of life in older adults with bilateral knee pain and back pain: Data from the Osteoarthritis Initiative. Rheumatology International, 2015, 2095–2101. 10.1007/s00296-015-3309-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeri, M. , Saure, D. , Schacht, A. , Riedl, E. , & Hauber, B. (2020). Modeling heterogeneity in patients’ preferences for psoriasis treatments in a multicountry study: A comparison between random‐parameters logit and latent class approaches. Pharmacoeconomics, 38, 593–606. 10.1007/s40273-020-00894-7 [DOI] [PubMed] [Google Scholar]
- Boeri, M. , Szegvari, B. , Hauber, B. , Mange, B. , Mountian, I. , Schiff, M. , & Maniadakis, N. (2019). From drug‐delivery device to disease management tool: A study of preferences for enhanced features in next‐generation self‐injection devices. Patient Prefer Adherence, 13, 1093–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle, J. , Ball, S. , Ding, H. , Srinath, K. P. , & Euler, G. (2012, May). Using online panels for national surveys of low incidence populations: findings from the CDC influenza vaccination monitoring survey of pregnant women. Abstract presented at the American Association for Public Opinion Research (AAPOR) 67th Annual Conference, Orlando, FL
- Bridges, J. F. P. , Hauber, A. B. , Marshall, D. , Lloyd, A. , Prosser, L. A. , Regier, D. A. , Johnson, F. R. , & Mauskopf, J. (2011). Conjoint analysis applications in health—A checklist: A report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value in Health, 14, 403–413. 10.1016/j.jval.2010.11.013 [DOI] [PubMed] [Google Scholar]
- Celecoxib . (2018, July). Celecoxib prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020998s050lbl.pdf
- Chou, R. , Qaseem, A. , Snow, V. , Casey, D. , Cross, J. T. , Shekelle, P. , Owens, D. K. , & American Pain Society Low Back Pain Guidelines Panel . (2007). Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Annals of Internal Medicine, 147, 478–491. 10.7326/0003-4819-147-7-200710020-00006 [DOI] [PubMed] [Google Scholar]
- Clark, M. , Determann, D. , Petrou, S. , Moro, D. , & de Bekker‐Grob, E. W. (2014). Discrete choice experiments in health economics: A review of the literature. Pharmacoeconomics, 32, 883–902. 10.1007/s40273-014-0170-x [DOI] [PubMed] [Google Scholar]
- Dakin, P. , DiMartino, S. J. , Gao, H. , Maloney, J. , Kivitz, A. J. , Schnitzer, T. J. , Stahl, N. , Yancopoulos, G. D. , & Geba, G. P. (2019). The efficacy, tolerability, and joint safety of fasinumab in osteoarthritis pain: A phase IIb/III double‐blind, placebo‐controlled, randomized clinical trial. Arthritis & Rheumatology, 71, 1824–1834. 10.1002/art.41012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bekker‐Grob, E. W. , Ryan, M. , & Gerard, K. (2010). Discrete choice experiments in health economics: A review of the literature. Health Economics, 21, 145–172. 10.1002/hec.1697 [DOI] [PubMed] [Google Scholar]
- Deshpande, B. R. , Katz, J. N. , Solomon, D. H. , Yelin, E. H. , Hunter, D. J. , Messier, S. P. , Suter, L. G. , & Losina, E. (2016). Number of persons with symptomatic knee osteoarthritis in the US: Impact of race and ethnicity, age, sex, and obesity. Arthritis Care and Research, 68, 1743–1750. 10.1002/acr.22897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Determann, D. , Lambooij, M. S. , Steyerberg, E. W. , de Bekker‐Grob, E. W. , & de Wit, G. A. (2017). Impact of survey administration mode on the results of a health‐related discrete choice experiment: Online and paper comparison. Value Health, 20, 953–960. 10.1016/j.jval.2017.02.007 [DOI] [PubMed] [Google Scholar]
- Elwyn, G. , Laitner, S. , Coulter, A. , Walker, E. , Watson, P. , & Thomson, R. (2010). Implementing shared decision making in the NHS. BMJ, 341, c5146. 10.1136/bmj.c5146 [DOI] [PubMed] [Google Scholar]
- Farr, J., II , Miller, L. E. , & Block, J. E. (2013). Quality of life in patients with knee osteoarthritis: A commentary on nonsurgical and surgical treatments. Open Orthopaedics Journal, 7, 619–623. 10.2174/1874325001307010619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghouri, A. , & Conaghan, P. G. (2021). Prospects for therapies in osteoarthritis. Calcified Tissue International, 109, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore, M. , Sadosky, A. , Stacey, B. R. , Tai, K. S. , & Leslie, D. (2012). The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976), 37, E668–E677. [DOI] [PubMed] [Google Scholar]
- Gore, M. , Tai, K. S. , Sadosky, A. , Leslie, D. , & Stacey, B. R. (2011). Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. Journal of Medical Economics, 14, 497–507. 10.3111/13696998.2011.594347 [DOI] [PubMed] [Google Scholar]
- Greene, W. H. (2012). Econometric analysis (7th international ed.). Pearson. [Google Scholar]
- Greene, W. H. , & Hensher, D. A. (2003). A latent class model for discrete choice analysis: Contrasts with mixed logit. Transportation Research Part B: Methodological, 37(8), 681–698. [Google Scholar]
- Grisolía, J. M. , Longo, A. , Hutchinson, G. , & Kee, F. (2015). Applying health locus of control and latent class modelling to food and physical activity choices affecting CVD risk. Social Science and Medicine, 132, 1–10. 10.1016/j.socscimed.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Hartvigsen, J. , Hancock, M. J. , Kongsted, A. , Louw, Q. , Ferreira, M. L. , Genevay, S. , Hoy, D. , Karppinen, J. , Pransky, G. , Sieper, J. , Smeets, R. J. , Underwood, M. , Buchbinder, R. , Hartvigsen, J. , Cherkin, D. , Foster, N. E. , Maher, C. G. , Underwood, M. , van Tulder, M. , … Woolf, A. (2018). What low back pain is and why we need to pay attention. The Lancet, 391(10137), 2356–2367. 10.1016/S0140-6736(18)30480-X [DOI] [PubMed] [Google Scholar]
- Hauber, A. B. , Arden, N. K. , Mohamed, A. F. , Johnson, F. R. , Peloso, P. M. , Watson, D. J. , Mavros, P. , Gammaitoni, A. , Sen, S. S. , & Taylor, S. D. (2013). A discrete choice experiment of United Kingdom patients’ willingness to risk adverse events for improved function and pain control in osteoarthritis. Osteoarthritis Cartilage, 221, 289–297. 10.1016/j.joca.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Hauber, A. B. , González, J. M. , Groothuis‐Oudshoorn, C. G. M. , Prior, T. , Marshall, D. A. , Cunningham, C. , IJzerman, M. J. , & Bridges, J. F. P. (2016). Statistical methods for the analysis of discrete choice experiments: A report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value in Health, 19, 300–315. 10.1016/j.jval.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Health Research Authority . (2018) Do I need NHS REC review? http://www.hra‐decisiontools.org.uk/ethics/
- Ho, M. P. , Gonzalez, J. M. , Lerner, H. P. , Neuland, C. Y. , Whang, J. M. , McMurry‐Heath, M. , Brett Hauber, A. , & Irony, T. (2015). Incorporating patient‐preference evidence into regulatory decision making. Surgical Endoscopy, 29, 2984–2993. 10.1007/s00464-014-4044-2 [DOI] [PubMed] [Google Scholar]
- Hochberg, M. C. (2015). Serious joint‐related adverse events in randomized controlled trials of anti‐nerve growth factor monoclonal antibodies. Osteoarthritis and Cartilage, 23, S18–S21. 10.1016/j.joca.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Hochberg, M. C. , Carrino, J. , Schnitzer, T. , Guermazi, A. , Walsh, D. , White, A. , Nakajo, S. , Fountaine, R. J. , Hickman, A. , Pixton, G. , Viktrup, L. , Brown, M. T. , West, C. R. , & Viktrup, L. (2021). Long‐term safety and efficacy of subcutaneous tanezumab versus nonsteroidal antiinflammatory drugs for hip or knee osteoarthritis: A randomized trial. Arthritis & Rheumatology, 73, 1167–1177. [DOI] [PubMed] [Google Scholar]
- Hurvich, M. , & Tsai, C. (1989). Regression and time series model selection in small samples. Biometrika, 76(2), 297–307. 10.1093/biomet/76.2.297 [DOI] [Google Scholar]
- Ikenwilo, D. , Heidenreich, S. , Ryan, M. , Mankowski, C. , Nazir, J. , & Watson, V. (2018). The best of both worlds: An example mixed methods approach to understand men's preferences for the treatment of lower urinary tract symptoms. Patient, 11, 55–67. 10.1007/s40271-017-0263-7 [DOI] [PubMed] [Google Scholar]
- InternetLiveStats.com . (2016). Internet users by country. http://www.InternetLiveStats.com/internet‐users‐by‐country
- Kissin, I. (2010). The development of new analgesics over the past 50 years: A lack of real breakthrough drugs. Anesthesia & Analgesia, 110, 780–789. [DOI] [PubMed] [Google Scholar]
- Klojgaard, M. E. , Manniche, C. , Pedersen, L. B. , Beth, M. , & Sogaard, R. (2014). Patient preferences for treatment of low back pain—A discrete choice experiment. Value in Health, 17, 390–396. 10.1016/j.jval.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Kuhfeld, W. (2010). Marketing research methods in SAS: Experimental design, choice, conjoint, and graphical techniques. SAS Institute Inc. [Google Scholar]
- Kuhfeld, W. , Tobias, F. , & Garratt, M. (1994). Efficient experimental design with marketing research applications. Journal of Marketing Research, 31, 545–557. 10.1177/002224379403100408 [DOI] [Google Scholar]
- Laba, T. L. , Brien, J. A. , Fransen, M. , & Jan, S. (2013). Patient preferences for adherence to treatment for osteoarthritis: The MEducation Decisions in Osteoarthritis Study (MEDOS). BMC Musculoskeletal Disorders, 14, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux, B. G. (1992). Consistent estimation of mixing distributions. Annals of Statistics, 20, 1350–1360. [Google Scholar]
- Louviere, J. J. (2006). What you don’t know might hurt you: Some unresolved issues in the design and analysis of discrete choice experiments. Environmental and Resource Economics, 34, 173–188. 10.1007/s10640-005-4817-0 [DOI] [Google Scholar]
- Ma, V. Y. , Chan, L. , & Carruthers, K. J. (2014). Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Archives of Physical Medicine and Rehabilitation, 95, 986–995. 10.1016/j.apmr.2013.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manski, C. F. (1977). The structure of random utility models. Theory and Decision, 8, 229–254. 10.1007/BF00133443 [DOI] [Google Scholar]
- Marshall, D. , Bridges, J. F. P. , Hauber, A. B. , Cameron, R. , Donnalley, L. , Fyie, K. , & Johnson, F. R. (2010). Discrete‐choice experiment applications in health—How are studies being designed and reported? An update on current practice in the published literature between 2005 and 2008. Patient, 3, 249–256. [DOI] [PubMed] [Google Scholar]
- Marshall, T. , Pugh, A. , Fairchild, A. , & Hass, S. (2017). Patient preferences for device‐aided treatments indicated for advanced Parkinson disease. Value in Health, 20, 1383–1393. 10.1016/j.jval.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Marta‐Pedroso, C. , Freitas, H. , & Domingos, T. (2007). Testing for the survey mode effect on contingent valuation data quality: A case study of web based versus in‐person interviews. Ecological Economics, 62, 388–398. 10.1016/j.ecolecon.2007.02.005 [DOI] [Google Scholar]
- Marton, G. , Pizzoli, S. F. M. , Vergani, L. , Mazzocco, K. , Monzani, D. , Bailo, L. , Pancani, L. , & Pravettoni, G. (2021). Patients’ health locus of control and preferences about the role that they want to play in the medical decision‐making process. Psychology, Health & Medicine, 26, 260–266. 10.1080/13548506.2020.1748211 [DOI] [PubMed] [Google Scholar]
- McAlindon, T. E. , Bannuru, R. R. , Sullivan, M. C. , Arden, N. K. , Berenbaum, F. , Bierma‐Zeinstra, S. M. , Hawker, G. A. , Henrotin, Y. , Hunter, D. J. , Kawaguchi, H. , Kwoh, K. , Lohmander, S. , Rannou, F. , Roos, E. M. , & Underwood, M. (2014). OARSI guidelines for the non‐surgical management of knee osteoarthritis. Osteoarthritis and Cartilage, 22, 363–388. 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- McLachlan, G. , & Peel, D. (2000). Finite mixture models. John Wiley & Sons. [Google Scholar]
- Meloxicam . (2016). Meloxicam prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020938s024s025,021530s014s015lbl.pdf
- Michel, P. , Brudon, A. , Pomey, M. P. , Durieu, I. , Baille, N. , Schott, A.‐M. , Dadon, I. , Saout, C. , Kouevi, A. , Blanchardon, F. , Volta‐Paulet, B. , Reynau, Q. , & Haesebaert, J. (2020). Approche terminologique de l’engagement des patients: Point de vue d’un établissement de santé français. Revue D'epidemiologie Et De Sante Publique, 68, 51–56. [DOI] [PubMed] [Google Scholar]
- Mühlbacher, A. C. , Junker, U. , Juhnke, C. , Stemmler, E. , Kohlmann, T. , Leverkus, F. , & Nübling, M. (2015). Chronic pain patients’ treatment preferences: A discrete‐choice experiment. European Journal of Health Economics, 16, 613–628. 10.1007/s10198-014-0614-4 [DOI] [PubMed] [Google Scholar]
- National Health Service . (2021). Involving people in their own care. https://www.england.nhs.uk/ourwork/patient‐participation/
- Nielsen, J. S. (2011). Use of the internet for willingness‐to‐pay survey: A comparison of face‐to‐face and web‐based interviews. Resource and Energy Economics, 33, 119–129. [Google Scholar]
- Norman, P. , & Bennett, P. (1996). Health locus of control. In Conner M. & Norman P. (Eds.), Predicting health behaviour (pp. 62–94). Open University Press. [Google Scholar]
- Oxycodone . (2015). Oxycodone hydrochloride prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022272s027lbl.pdf
- Özdemir, S. , Johnson, F. R. , & Hauber, A. B. (2009). Hypothetical bias, cheap talk, and stated willingness to pay for health care. Journal of Health Economics, 28, 894–901. 10.1016/j.jhealeco.2009.04.004 [DOI] [PubMed] [Google Scholar]
- Patel, M. K. , Kaye, A. D. , & Urman, R. D. (2018). Tanezumab: Therapy targeting nerve growth factor in pain pathogenesis. Journal of Anaesthesiology and Clinical Pharmacology, 34, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poder, T. G. , Beffarat, M. , Benkhalti, M. , Ladouceur, G. , & Dagenais, P. (2019). A discrete choice experiment on preferences of patients with low back pain about non‐surgical treatments: Identification, refinement and selection of attributes and levels. Patient Preference and Adherence, 13, 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrovnichka, A. (2012). Anti‐NGF drug class efficacy and safety: Regulator history (presentation), 12, 42–43. https://wayback.archive‐it.org/7993/20170405210041/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM301302.pdf
- Porteous, T. , Ryan, M. , Bond, C. , Watson, M. , & Watson, V. (2016). Managing minor ailments; the public's preferences for attributes of community pharmacies. A discrete choice experiment. PLoS One, 11, e0152257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnett, J. , Dixit, S. , Oppenheimer, B. , Kili, S. , & Mehin, N. (2015). Patient preference and willingness to pay for knee osteoarthritis treatments. Patient Prefer Adherence, 9, 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos, C. , Curran, D. , Anastassopoulou, A. , & De Moerlooze, L. (2018). German travelers’ preferences for travel vaccines assessed by a discrete choice experiment. Vaccine, 36, 969–978. 10.1016/j.vaccine.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Poulos, C. , Johnson, F. R. , Krishnarajah, F. , Anonychuk, A. , & Misurski, D. (2015). Pediatricians’ preferences for infant meningococcal vaccination. Value in Health, 18, 67–77. 10.1016/j.jval.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Poulos, C. , Standaert, B. , Sloesen, B. , Stryjewska, I. , Janitsary, A. , & Hauber, B. (2018). Preferences for vaccines against children’s diarrheal illness among mothers in Poland and Hungary. Vaccine, 36, 6022–6029. 10.1016/j.vaccine.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Qaseem, A. , Wilt, T. J. , McLean, R. M. , Forciea, M. A. , & Clinical Guidelines Committee of the American College of Physicians . (2017). Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine, 166, 514–530. 10.7326/M16-2367 [DOI] [PubMed] [Google Scholar]
- Ratcliffe, J. , Buxton, M. , McGarry, T. , Sheldon, R. , & Chancellor, J. (2004). Patients’ preferences for characteristics associated with treatments for osteoarthritis. Rheumatology, 43, 337–345. 10.1093/rheumatology/keh038 [DOI] [PubMed] [Google Scholar]
- Reed Johnson, F. , Lancsar, E. , Marshall, D. , Kilambi, V. , Mühlbacher, A. , Regier, D. A. , Bresnahan, B. W. , Kanninen, B. , & Bridges, J. F. P. (2013). Constructing experimental designs for discrete‐choice experiments: Report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value in Health, 16, 3–13. 10.1016/j.jval.2012.08.2223 [DOI] [PubMed] [Google Scholar]
- Ryan, M. , Gerard, K. , & Amaya‐Amaya, M. (2008). Using discrete choice experiments to value health and health care. Springer. [Google Scholar]
- Ryan, M. , Mentzakis, E. , Matheson, C. , & Bond, C. (2020). Survey modes comparison in contingent valuation: Internet panels and mail surveys. Health Economics (United Kingdom), 29, 234–242. 10.1002/hec.3983 [DOI] [PubMed] [Google Scholar]
- Scalone, L. , Watson, V. , Ryan, M. , Kotsopoulos, N. , & Patel, R. (2011). Evaluation of patients’ preferences for genital herpes treatment. Sexually Transmitted Diseases, 38, 802–807. 10.1097/OLQ.0b013e318218702c [DOI] [PubMed] [Google Scholar]
- Schneider, A. , Körner, T. , Mehring, M. , Wensing, M. , Elwyn, G. , & Szecsenyi, J. (2006). Impact of age, health locus of control and psychological co‐morbidity on patients’ preferences for shared decision making in general practice. Patient Education and Counseling, 61, 292–298. 10.1016/j.pec.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Schnitzer, T. J. , Easton, R. , Pang, S. , Levinson, D. J. , Pixton, G. , Viktrup, L. , Davignon, I. , Brown, M. T. , West, C. R. , & Verburg, K. M. (2019). Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: A randomized clinical trial. JAMA, 322, 37–48. 10.1001/jama.2019.8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, I. P. , DiSantostefano, R. L. , de Bekker‐Grob, E. W. , Levitan, B. , Berlin, C. , Veldwijk, J. , & de Wit, G. A. (2021). Methodological priorities for patient preferences research: Stakeholder input to the PREFER public–private project. Patient, 14(5), 449–453. 10.1007/s40271-021-00502-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. R. , Deshpande, B. R. , Collins, J. E. , Katz, J. N. , & Losina, E. (2016). Comparative pain reduction of oral non‐steroidal anti‐inflammatory drugs and opioids for knee osteoarthritis: Systematic analytic review. Osteoarthritis and Cartilage, 24, 962–972. 10.1016/j.joca.2016.01.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soekhai, V. , de Bekker‐Grob, E. W. , Ellis, A. R. , & Vass, C. M. (2019). Discrete choice experiments in health economics: Past, present and future. Pharmacoeconomics, 37, 201–226. 10.1007/s40273-018-0734-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soekhai, V. , Whichello, C. , Levitan, B. , Veldwijk, J. , Pinto, C. A. , Donkers, B. , Huys, I. , van Overbeeke, E. , Juhaeri, J. , & de Bekker‐Grob, E. W. (2019). Methods for exploring and eliciting patient preferences in the medical product lifecycle: A literature review. Drug Discovery Today, 24, 1324–1331. 10.1016/j.drudis.2019.05.001 [DOI] [PubMed] [Google Scholar]
- Thurstone, L. (1927). A law of comparative judgement. Psychological Review, 34, 273–286. [Google Scholar]
- Train, K. (2009). Discrete choice methods with simulation. Cambridge University Press. [Google Scholar]
- Train, K. , & Sonnier, G. (2005). Mixed logit with bounded distributions of correlated partworths. In Scarpa R. & Alberini A. (Eds.), Applications of simulation methods in environmental and resource economics (pp. 117–134). Springer. [Google Scholar]
- Turk, D. , Boeri, M. , Abraham, L. , Atkinson, J. , Bushmakin, A. G. , Cappelleri, J. C. , Hauber, B. , Klein, K. , Russo, L. , Viktrup, L. , & Walsh, D. (2020). Patient preferences for osteoarthritis pain and chronic low back pain treatments in the United States: A discrete‐choice experiment. Osteoarthritis and Cartilage, 28, 1202–1213. 10.1016/j.joca.2020.06.006 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration . (2021). CDER patient‐focused drug development. https://www.fda.gov/drugs/development‐approval‐process‐drugs/cder‐patient‐focused‐drug‐development
- Wallston, K. A. , Stein, M. J. , & Smith, C. A. (1994). Form C of the MHLC scales: A condition‐specific measure of locus of control. Journal of Personality Assessment, 63, 534–553. 10.1207/s15327752jpa6303_10 [DOI] [PubMed] [Google Scholar]
- Wedel, M. , & Kamakura, W. (2000). Market segmentation: Conceptual and methodological foundations (2nd ed.). Kluwer Academic Publishers. [Google Scholar]
- Wongrakpanich, S. , Wongrakpanich, A. , Melhado, K. , & Rangaswami, J. (2018). A comprehensive review of non‐steroidal anti‐inflammatory drug use in the elderly. Aging and Disease, 9(1), 143–150. 10.14336/AD.2017.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M. , Thayer, M. W. , & Bridges, J. F. P. (2018). Using latent class analysis to model preference heterogeneity in health: A systematic review. Pharmacoeconomics, 36(2), 175–187. 10.1007/s40273-017-0575-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


