Abstract
Soil drying is a limiting factor for crop production worldwide. Yet, it is not clear how soil drying impacts water uptake across different soils, species, and root phenotypes. Here we ask (1) what root phenotypes improve the water use from drying soils? and (2) what root hydraulic properties impact water flow across the soil–plant continuum? The main objective is to propose a hydraulic framework to investigate the interplay between soil and root hydraulic properties on water uptake. We collected highly resolved data on transpiration, leaf and soil water potential across 11 crops and 10 contrasting soil textures. In drying soils, the drop in water potential at the soil–root interface resulted in a rapid decrease in soil hydraulic conductance, especially at higher transpiration rates. The analysis reveals that water uptake was limited by soil within a wide range of soil water potential (−6 to −1000 kPa), depending on both soil textures and root hydraulic phenotypes. We propose that a root phenotype with low root hydraulic conductance, long roots and/or long and dense root hairs postpones soil limitation in drying soils. The consequence of these root phenotypes on crop water use is discussed.
Keywords: drought, leaf water potential, root hairs, root hydraulic conductance, root length, root water uptake, soil hydraulic conductivity, soil texture
Summary statement
During soil drying, the drop in soil–plant hydraulic conductance causes a decline in root water uptake, which is impacted by soil and root hydraulic phenotypes. Lower root conductance, longer root length and longer root hairs would allow plants to maintain water uptake at lower soil matric potential.
1. INTRODUCTION
Drought is an unavoidable natural hazard affects the entire ecosystem, especially agriculture (Boyer, 1982; Wilhite, 2000). Under drought conditions, plants reduce stomatal conductance to avoid an excessive decline in leaf water potential. Stomatal regulation determines the water flux across the soil–plant–atmosphere system. The mechanisms of stomatal response to water stress from the molecular and physiological aspects were intensively investigated (Buckley, 2005, 2019). Water use efficiency is positively affected by stomatal closure (Lawson & Vialet‐Chabrand, 2019; Yang et al., 2021), and phenotypes with high water use efficiency have been proposed for improving drought tolerance (Tracy et al., 2020). Although stomatal conductance is a key aboveground hydraulic variable regulating water use by crops, it does not mean that belowground hydraulics have no role in it. Indeed, changes in soil–plant hydraulic conductance have been shown to drive stomatal closure (Abdalla, Carminati, et al., 2021). It has been proposed that stomatal regulation is intimately linked to a decline in soil–plant hydraulics (Carminati & Javaux, 2020; Sperry & Love, 2015). This link allows one to predict the water use based on the decline in hydraulic conductivities of key components of soil and root hydraulics (Sperry & Love, 2015). For trees, much of the attention has been placed on xylem embolism (Cochard, 2002; Savi et al., 2015; Sperry et al., 2002; Venturas et al., 2017). However, recent studies have shown that, both in trees (Albuquerque et al., 2020; Anderegg et al., 2017) and crops (Corso et al., 2020), stomatal closure before xylem embolism takes place. For crops, Vadez (2014) proposed in an inspiring paper that root hydraulics is the key to improve drought tolerance. He proposed that rather than root length, root hydraulics might determine the water use of crops. However, experimental evidence linking root hydraulics to stomatal regulation is lacking. In this context, the following questions are still open: what root hydraulic properties impact the ability of roots to extract water from drying soil? And what is the hydraulic limit across the soil–plant–atmosphere continuum? Is it the soil (as proposed by Carminati & Javaux, 2020), the root–soil interface (as measured for olive trees by Rodriguez‐Dominguez & Brodribb, 2020), the root (Bourbia et al., 2021), an increase in soil–root resistance (Abdalla, Carminati, et al., 2021) and/or the outer xylem tissues (in roots [Cuneo et al., 2021, 2016] or leaves [Scoffoni & Sack, 2017])? In this review, we presented an analysis of soil–plant hydraulic measurements across species and soils and proposed a link between root hydraulic phenotypes and water use during drought.
Water flow along the soil–plant continuum is determined by a series of hydraulic conductivities and gradients in water potential (Figure 1a). In wet conditions, the water potential gradient at the soil–root interface is minimum, and root hydraulic conductance (K root, cm3 kPa−1 s−1) at a given transpiration rate determines the difference in water potential between soil and leaf (Nobel & Cui, 1992; Sperry et al., 1998). The root hydraulic conductance depends on different root traits, for example, root architecture (Doussan et al., 2006), root length (Kato & Okami, 2011), root anatomy (e.g., metaxylem vessel size, [Strock et al., 2021]), root hairs (Carminati et al., 2017) and aquaporin expression (Grondin et al., 2020; McLean et al., 2011). During soil drying, the root hydraulic conductivity (kroot, K root normalized by root surface area) might decrease due to lacunae formation in fine root cortical cells (Cuneo et al., 2016) and downregulation of aquaporin (AQP) activity (Caldeira et al., 2014; Rodríguez‐Gamir et al., 2019). Similarly, soil hydraulic conductivity decreases and the water potential gradient around the roots increases markedly, particularly at high transpiration rates (Figure 1a) (Gardner, 1960). Additionally, root shrinkage (Carminati et al., 2009; Nobel & Cui, 1992) further aggravates the drop in water potential in drying soil. As a result, root water uptake might be constrained at the soil–root interface due to poor soil–root contact, especially in well‐structured soils, as speculated by White and Kirkegaard (2010).
Figure 1.
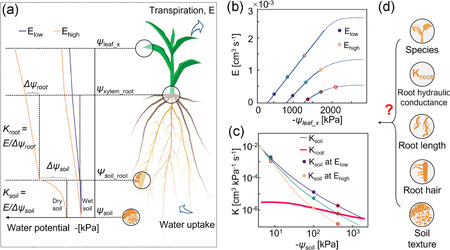
Schematic of water flow in the soil–plant continuum and corresponding variation of root and soil hydraulic properties during soil drying. (a) Water potential gradients from soil to leaf at low and high transpiration rates (E) in wet and dry conditions. ψ soil, ψ soil_root, ψ xylem_root, and ψ leaf_x are the water potentials in the soil, at the soil–root interface, root xylem and leaf xylem, respectively. Δψ soil and Δψ root are potential differences between soil and soil–root interface, and between soil–root interface and root xylem. K soil and K root are the soil and root hydraulic conductance. (b) Relation between E and ψ leaf_x in drying soil (for a hypothetical crop ca. 1‐month‐old). Dashed lines are from soil hydraulic nonlinearity. (c) Hypothetical relation between K soil and K root at different transpiration rates in drying soil. Declines in K soil and K root during soil drying cause nonlinearity in the relation between E and ψ leaf_x. Circle symbols with blue and red edges are for wet and dry conditions. (d) In this study, we explored how soil textures and root hydraulic phenotypes (e.g., root hydraulic conductance, root length, and root hairs from different species) impact root water uptake in drying soils [Color figure can be viewed at wileyonlinelibrary.com]
Direct measurements of the hydraulic conductance of the soil–plant continuum and its elements are challenging. In their literature review, Draye et al. (2010) compared root and soil conductivity across tree species and soils. Despite the importance of such information, their estimation is based on individual excised roots, including roots grown in hydroponics, and the assumption of soil water flow toward the root. In this way, it is not possible to investigate to what extent root hydraulic properties change with soil texture. Therefore, there is the need to collect information across species and soils on intact root systems growing in soils. Using the root pressure chamber (Passioura, 1980), root hydraulic conductance and the drop in water potential at the soil–root interface could be identified in intact plants during soil drying. The method provided accurate measurements of the relationship between transpiration rate (E) and leaf xylem water potential (ψ leaf_x) during soil drying (Abdalla, Carminati, et al., 2021; Cai, Ahmed, Reth, et al., 2020; Cai et al., 2021; Carminati et al., 2017). Root hydraulic conductance could be obtained from transpiration rate and water potential gradient along the pathway between soil and leaf. The E(ψ leaf_x) relation is typically linear in wet soil and becomes nonlinear as the soil dries (Figure 1b), especially at higher transpiration rates, mainly due to the increasing gradient in water potential at the soil–root interface (Carminati et al., 2017; Carminati & Javaux, 2020). The method was successfully used to investigate the response of various species to soil drying (Abdalla et al., 2021b; Cai, Ahmed, Dippold, et al., 2020; Cai et al., 2021; Carminati et al., 2017; Hayat et al., 2020; Passioura, 1980). However, a direct comparison across species and soils has never been done.
In this study, the main objective was to propose a hydraulic framework to investigate the interplay between soil and root hydraulic properties on water uptake. To this end, we investigated how soil and root hydraulic properties determine the decline in soil water availability to crops. We compared the decline in soil and root hydraulic conductances for varying species and soils to find out at what soil water potential, one of these elements started to limit root water uptake. Finally, we discussed how given root hydraulic phenotypes impacted water use by crops in different soil textures.
2. OVERVIEW OF DATA COLLECTION AND PROCESS
The selected studies used the same method (plant pressurization) and included E(ψ leaf_x) measurements from eight cereal and three Solanaceae varieties, covering different root traits (e.g., root hairs, root length) and soil textures (from sand to loam). The corresponding plant and soil information is summarized in Table 1. The plants were 3‐ to 6‐week old with differences in shoot (leaf) and root growth. Soil hydraulic parameters were inversely fitted using the Brooks and Corey (1964) model. The water retention and hydraulic conductivity curves are shown in Figure 2. The soil hydraulic properties differed markedly even though some soils were in the same texture category, for example, sandy loam for maize showed a steeper slope than sandy loam for barley, millet and tomato. Taken together, this allows doing a systematic analysis of the interactive impact of soil and root characteristics on plant response to water stress.
Table 1.
Plants and soil textures used in this study
| Mono‐/dicot (M/D) | Family | C3/4 | Root hairs | Plant age (day) | Leaf area (cm2) | Mean root length (cm) | Mean root length density (cm−3) | r 0 (cm) | Soil texture (bulk density, g cm−3) | References a | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat | M | Poaceae | C3 | + | 21–30 | 30–190 |
1531 9294 6971 3776 |
5.2 8 6 3.25 |
0.015 0.014 0.016 0.015 |
Loam (1.27) Clay loam (Una, 1.6) Clay loam (Re b , 1.3) Sand (Re b , 1.6) |
|
| Barley | M | Poaceae | C3 | ± | 21–28 | / |
3660 3310 |
3.35 3.71 |
0.016 (WT) 0.013 (Mut) |
Sandy loam (0.93) Red Chromosolb (0.9) |
Carminati et al. (2017) |
| Maize | M | Poaceae | C4 |
+ ± ± |
40–50 26–36 26–36 |
/250–350 |
28 000 2461 (WT) 2865 (Mut) 5414 (WT) 3764 (Mut) |
21.1 2.91 (WT) 3.38 (Mut) 6.39 (WT) 4.44 (Mut) |
0.06 0.019 (WT, Mut) 0.012 (WT, Mut) |
Sandy loam (1.4) Sand (1.47) Loam (1.26) |
Hayat et al. (2020) Cai et al. (2021) |
| Millet | M | Poaceae | C4 | + | 30–45 | 250–380 | 27 617 | 13.5 | 0.04 | Sandy loam (1.3) | Cai, Ahmed, Dippold, et al. (2020) |
| Tomato | D | Solanaceae | C3 | + |
22–30 22–30 30–40 |
846–1116 943–1145 960–2016 |
3700 (long root) c 1700 (short root) 7540 (normal) |
1.81 0.83 3.68 |
0.05 | Sandy loam (1.3) |
Abdalla, Ahmed, et al. (2021) Abdalla, Carminati, et al. (2021) |
Abbreviations: Mut, mutant; WT, wild type.
The data of wheat were extracted from the literature using the software WebPlotDigitizer (Rohatgi, 2020). The other data were obtained from the authors directly.
Un, undisturbed soil; Re, repacked soil.
Red chromosol is from southeast Australia and is characterized by a strong contrasting texture (including aggregates and macropores) with low bulk density (ca. 0.9 g cm−3).
‘Long root’, ‘short root’ and ‘normal’ were different phenotypes. Tomato with a long and short root system was grafted with an identical shoot.
Figure 2.
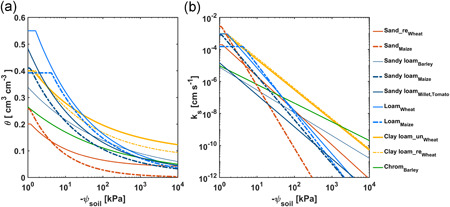
Soil hydraulic properties of the soils from the collected studies. (a) Soil water retention curves (θ, soil water content, ψ soil, soil matric potential). (b) Soil hydraulic conductivity curves (k s, soil hydraulic conductivity). The curves were reproduced using Brooks and Corey model. Chrom, red chromosol soil; re, repacked; un, undisturbed (see Table 1) [Color figure can be viewed at wileyonlinelibrary.com]
The E(ψ leaf_x) relations were reproduced for individual soil moisture using a soil–plant hydraulic model (Carminati & Javaux, 2020) by adjusting root hydraulic conductance and root length active in water uptake (Abdalla, Carminati, et al., 2021; Cai, Ahmed, Dippold, et al., 2020, 2021). In the model, water flow from soil towards root, xylem and leaf is driven by the gradients in water potential and regulated by soil hydraulic conductivity (k s, cm s−1) and plant hydraulic conductance (including root and aboveground xylem). The detailed description of this model is in Carminati and Javaux (2020) and the Methods S1. Briefly, a uniform water flux (q, cm s−1) is assumed to flow into a fraction of total root length from surrounding soil:
| (1) |
where r 0 (cm) is the root radius, and L act (cm) is a model parameter and is defined as the root length active in water uptake. No flow is assumed out of the casing soil, q(r b) = 0, where r b (cm) is the radius of the casing soil and calculated from soil volume (V, cm3), r b = (V/πL act)0.5. The water flux in soil q depends on k s and the gradient in soil matric potential (ψ soil, kPa). The nonlinear equations can be solved analytically (for simplified parameterization and boundary conditions) or numerically yield the gradient in soil matric potential around roots (Gardner, 1960; Passioura, 1980). The overall soil hydraulic conductance (K soil, cm3 kPa−1 s−1) is defined as:
| (2) |
where ψ soil_root (kPa) is the matric potential at the soil–root interface.
The water flow in the root system is described by:
| (3) |
where K root (cm3 kPa−1 s−1) is the root hydraulic conductance, ψ xylem_root (kPa) is the matric potential at the root collar. The water flow in the xylem is defined as:
| (4) |
where K x (cm3 kPa−1 s−1) is the aboveground xylem conductance, a function of K root and ψ soil. ψ leaf_x (kPa) is the water potential in leaf xylem. The detailed derivation of ψ soil_root, ψ xylem_root, K x, and ψ leaf_x is described in Methods S1. The measured and modelled parameters are described in the appendix.
3. ROOT HYDRAULIC PHENOTYPES AND WATER UPTAKE
Root phenotypes differed not only between species but also within the same species grown in different soil types (Table 1). In the following sections, we discuss how the interactions between root phenotypes and soil textures impact key soil and root hydraulic properties and thus the water uptake process.
3.1. Variation in root hydraulic conductance across species and soils
The maximum root hydraulic conductance, K root_max, is defined as the slope of E(ψ leaf_x) in wet soils. K root_max was soil texture independent and hence was positively related to root length across species and textures (Figure 3a), which was in line with Maurel et al. (2010) and Judd et al. (2016). For cereals, K root_max of maize in sand and loam, barley in sandy loam, and millet was similar. The value was around 10−6 cm3 kPa−1 s−1, which was four times higher than wheat and barley in red chromosol (green triangle in Figure 3c) but one‐fifth of the older maize with longer roots (Table 1). The magnitude of K root_max was in line with plants at similar ages using other methods (e.g., measuring exudate from immersed roots via pressurization), for example, wheat by Zhao et al. (2005), maize by Sunita et al. (2014) and barley by Kodama et al. (2021). Tomato with long and short root systems (grafted with an identical shoot) had surprisingly the highest K root_max (around 10−4 cm3 kPa−1 s−1), which was similar to the tomato at a similar age in commercial soil (Tsuda & Tyree, 2000), and ten times higher than the genotype without grafting (hexagram symbol).
Figure 3.
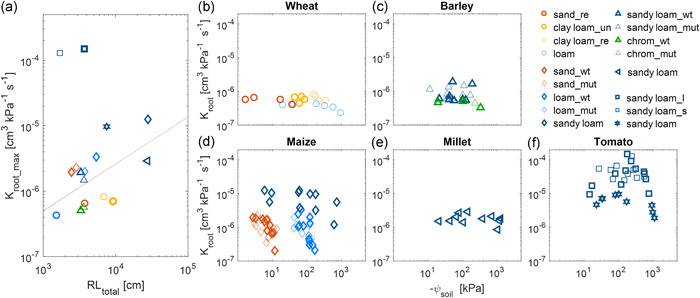
Variation of root hydraulic conductance (K root) of different species in drying soils. (a) Relation between root hydraulic conductance (K root_max) in wet conditions and measured total root length (RL total). The regression line excluded the grafted tomatoes (square) (r 2 = 0.35, p = 0.027). (b–f) Variation of K root in drying soils. In subplot (d), maize in sandy loam (dark cyan diamond) was around 2 weeks older than maize in sand and loam. In subplot (f), tomatoes with long (thick square) and short (thin square) root systems were grafted with an identical shoot whereas the one with hexagon symbol was not grafted. The grafted tomatoes were around 10 days older than the nongrafted ones. Millet (e) and tomato (f) were grown in the same sandy loam soil. The difference in plants and soils is shown in Table 1 and Figure 2. chrom, red chromosol soil; l, long root system; mut, mutant; re, repacked; s, short root system; un, undisturbed; wt, wild type. The same colour is used for similar soil textures [Color figure can be viewed at wileyonlinelibrary.com]
K root declined as the soil dried and the decline differed between species and textures (Figure 3b–f). Comparing textures, K root decreased at less negative soil water potentials in sand (ψ soil > −10 kPa) compared to loam (ψ soil > −60 kPa) (Figure 3d). This is expected considering the sharp drop in hydraulic conductivity in sand (Figure 2), which would cause a steeper gradient in soil water potential around roots. Similarly, a great root length reduces the decline in water potential gradient at the soil–root interface and softens the reduction in K root, for example, maize in loam (Figure 3d, dark blue diamond), and tomato with long roots (Figure 3f, thick square). Compared with other species, the decrease of K root was steeper in maize and tomato.
3.2. Water potential gradient at the soil–root interface
Gradients in matric potential occur between the bulk soil and the soil–root interface once plants start to transpire. During soil drying, how quickly and how far ψ soil_root deviates from ψ soil with increasing transpiration depends on soil textures and root hydraulic phenotypes.
3.2.1. Soil texture
ψ soil_root deviates at less negative ψ soil in sand than in loam because of the sharp decrease in hydraulic conductivity of sand. This is visible in Figure 4a,i,j, which shows that, in sand, ψ soil_root deviated remarkably from the 1:1 line. The comparison of ψ soil_root between sand and loam for different crops is shown in Figure 5a,b. These results are consistent with the findings of Dodd et al. (2010). Additionally, roots might shrink and form gaps between roots and the surrounding soils earlier in sand (Koebernick et al., 2018), resulting in a steeper decline in conductivity at the soil–root interface (North & Nobel, 1997) and thus more negative ψ soil_root.
Figure 4.
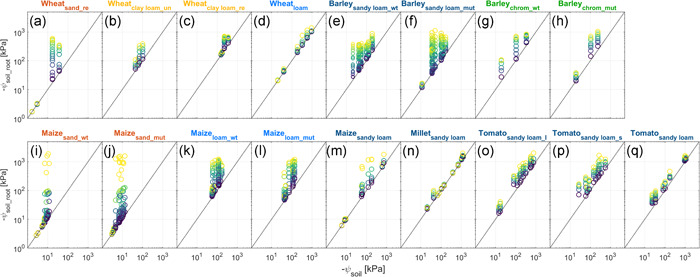
Variation of water potential at the soil–root interface (ψ soil_root) with increasing transpiration rates (from dark blue to yellow, note that same colour does not mean same transpiration rate) as a function of soil matric potential (ψ soil). (a,b,c,d) Wheat grown in repacked sand, undisturbed clay loam, repacked clay loam, and loam. (e,f) Wildtype and mutant (roothairless brb) barley grown in sandy loam. (g,h) Same barley genotypes as (e,f) grown in chromosol soil. (i,j) Wildtype and mutant (roothairless 3) grown in sand. (k,l) Same maize genotypes as (i,j) grown in loam. (m) Maize grown in sandy loam. (n) Millet grown in sandy loam. (o,p) Tomato with long and short root length grown in sandy loam, respectively. (q) Tomato grown in sandy loam. chrom, red chromosol soil; l, long root system; mut, mutant; re, repacked; s, short root system; un, undisturbed; wt, wild type. The same colour is used for similar soil textures [Color figure can be viewed at wileyonlinelibrary.com]
Figure 5.
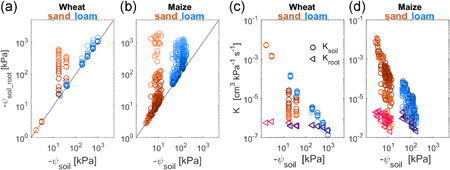
Comparison of water potential at the soil–root interface and of soil and root hydraulic conductance between sand and loam. (a,b) Water potential gradient at the soil–root interface (ψ soil_root) with increasing transpiration rate and soil (ψ soil) in sand and loam for both wheat and maize (from Figure 4a,d,i–l). (c,d) Difference between soil hydraulic conductance (K soil) with increasing transpiration rate and root hydraulic conductance (K root) in sand and loam for both wheat and maize [Color figure can be viewed at wileyonlinelibrary.com]
3.2.2. Root length
Concerning root length (and assuming a similar root diameter), longer roots (thus larger root surface) result in a lower water flow velocity in soil and allow for sustaining higher transpiration rates in drying soils (Faiz & Weatherley, 1982; Taylor & Willatt, 1983), which lead to a less negative ψ soil_root (Xu, 2001). This could explain the nearly 1:1 relation between ψ soil_root and ψ soil in millet (Figure 4n) and tomato (Figure 4q), since their root length was much longer than other varieties (Figure 4e,f,o,p). Note that these plants (Figure 4n–q) were grown in the same soil. It also explains the slightly less negative ψ soil_root of the tomato with long roots (Figure 4o) than with short roots (Figure 4p). However, it is neither the case for barley (Figure 4e,f) and tomato with contrasting root systems (Figure 4o,p) as these plants had a similar root length (Table 1), and nor the case for wheat (Figure 4d) and maize in loam (Figure 4i‐k) as maize even had longer roots. This apparent contradiction could be explained by the differences in transpiration rate and root hydraulic conductance.
3.2.3. Species
The transpiration rate of maize in loam was around two to four times higher than that of wheat at similar soil matric potential and leaf water potential (Cai et al., 2021; Passioura, 1980). Additionally, the difference in soil hydraulic conductivity curves of the two soils is very close (Figure 2). Therefore, maize in loam showed more negative ψ soil_root. For tomato and barley in sandy loam, it is not the case. Although K root and transpiration of tomato were both higher than that of barley (Figure 3; Abdalla, Carminati, et al., 2021, Carminati et al., 2017), the decline in soil hydraulic conductivity of the loamy soil for tomato was steeper compared to that for bayley (Figure 2). Lower ψ soil_root of barley in sandy loam indicates that the role of soil hydraulic conductivity is rather dominating the variations in ψ soil_root. The results also indicate that, during soil drying, variations in ψ soil_root with increasing transpiration are not determined by a single root hydraulic parameter but are impacted by both soil and root hydraulic properties.
3.2.4. Root hairs
Root hairs were hypothesized to soften the drop in soil matric potential around the roots (Ahmed, Passioura, et al., 2018; Cai et al., 2021; Carminati et al., 2017). Less negative ψ soil_root manifested in wildtype barley (Figure 4e) compared with mutant in sandy loam, which was, however, not observed in the wild type of maize either in sand or in loam (Figure 4i–l). This might be due to differences in root hair length between barley and maize (Cai et al., 2021). Indeed, recent studies showed that root hairs of barley were denser and around as two times long as that of maize both in lab and field measurements (Burak et al., 2021; Cai et al., 2021; Carminati et al., 2017; Marin et al., 2021). Furthermore, Burak et al. (2021) showed that, in barley, denser and longer root hairs formed thicker rhizosheath than that of maize, which probably amplified intimate contact between root and surrounding soils.
3.3. Comparison between root and soil hydraulic conductances
Figures 5c,d and 6 show the comparison between K soil and K root across species and textures during soil drying. In wet conditions, K soil was maintained at a high level regardless of the variations of transpiration rate. K soil was around four orders of magnitude higher than K root in sand (Figure 6a,i,j) and one order of magnitude higher than or even close K root in sandy loam for tomato (Figure 6o–q). As the soil dried, K soil decreased more rapidly than K root. The decline of K soil was sharper in sand than in other soils, and more marked at higher transpiration rates (see the comparison in Figure 5c,d). As transpiration rate increased, K soil decreased by one to two orders of magnitude at a specific ψ soil (e.g., Figure 6a,b,e,f,i,l) due to a remarkable decline in ψ soil_root (Figure 4). Though with different reduction patterns, decreasing K soil with increasing transpiration approached dropping K root in all drying soils. Afterwards, K soil became even much lower than K root, especially in tomato (Figure 6o,p). The effect of root length on ψ soil_root (Figure 4) was also reflected on K soil, for example, the drop in K soil with increasing transpiration was less abrupt in plants with longer roots (Figure 6k,l,n,q).
Figure 6.
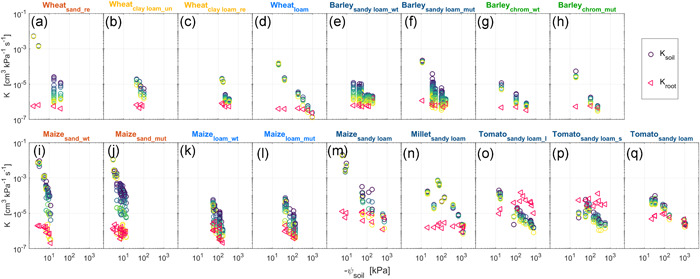
Variation of soil hydraulic conductance (K soil) and root hydraulic conductance (K root) for varying transpiration rates (from dark blue to yellow, note that same colour does not mean same transpiration rate) as a function of soil matric potential ψ soil: soil matric potential. (a,b,c,d) Wheat grown in repacked sand, undisturbed clay loam, repacked clay loam, and loam. (e,f) Wildtype and mutant (root‐hairless brb) barley grown in sandy loam. (g,h) Same barley genotypes as (e,f) grown in chromosol soil. (i,j) Wildtype and mutant (roothairless 3) grown in sand. (k,l) Same maize genotypes as (i,j) grown in loam. (m) Maize grown in sandy loam. (n) Millet grown in sandy loam. (o,p) Tomato with long and short root length grown in sandy loam, respectively. (q) Tomato grown in sandy loam.The same colour is used for the titles of similar soil textures [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Soil water limitation as a function of soil and plant hydraulic traits
We define ψ c soil as the point at which soil hydraulic conductance drops to lower values than the root hydraulic conductance. This is the point when the soil starts to limit the water fluxes in the soil–plant continuum relative to other plant tissues. This point corresponds to the onset of nonlinearity in the E(ψ leaf_x) relation (Figure 7), similarly to the definition given in Carminati and Javaux (2020). In the next sections, we explored how soil and root hydraulic traits impact ψ c soil.
Figure 7.
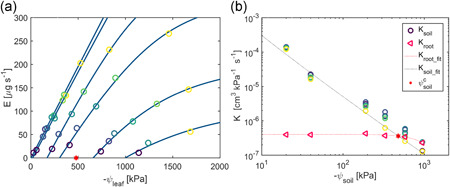
Definition of critical soil matric potential (ψ c soil). It is indicated as the pink star in both subplots. (a) Relation between transpiration rate (E) and leaf xylem water potential (ψ leaf_x) in wheat (Passioura, 1980). (b) Soil and root hydraulic conductance (K soil and K root) as a function of soil matric potential ψ soil. Colour from dark blue to yellow in (a) stands for increasing E. The critical soil matric potential ψ c soil is defined as the point where K root equals K soil was obtained by finding the intersection of fitted curves across K root (K root_fit) and K soil (K soil_fit) at the highest transpiration rate [Color figure can be viewed at wileyonlinelibrary.com]
We fitted the decreases of K root and K soil at the highest transpiration rate and obtained the soil matric potential at the intersection (ψ c soil) of the two fittings (Figure 7). There was no unique ψ c soil at which transpiration of different species was limited by soil. ψ c soil was affected not only by soil hydraulic but also by root hydraulic parameters (Figure 7b). Compared with other soils, transpiration in sand was constrained earlier, with ψ c soil around −10 to −20 kPa, when the soil hydraulic conductivity was still high (Figure 2). In loamy soils, ψ c soil was around −100 to −500 kPa.
3.4.1. Soil texture
To evaluate the impact of soil properties on ψ c soil, we plotted ψ c soil versus τ, which is the parameter that determines the steepness of the hydraulic conductivity. Lower τ meant a less steep decline in soil hydraulic conductivity with decreasing ψ soil, thereby the soil–root interface was more conductive and water flow towards root was less constrained. The larger is τ, the steeper is the decrease in conductivity (Figure 8a). It shows that the transpiration rate is constrained at more negative ψ soil by the soil with a lower τ. ψ c soil was round −10 kPa and τ was over 0.4 in sand, whereas these values were around −100 kPa and 0.2 and 0.3 in sandy loam and loam, respectively.
Figure 8.
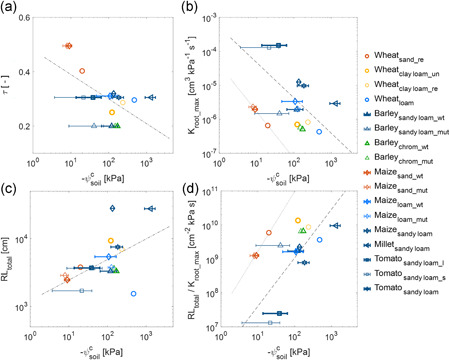
Relation between critical soil matric potential (ψ c soil) and soil and root hydraulic parameters. Relation between ψ c soil and (a) τ, which determines the slope of soil hydraulic conductivity, (b) root hydraulic conductance in wet conditions (K root_max), (c) root length (RL total) and (d) ratio of RL total to K root_max. Fittings were justified using analysis of covariance (ANCOVA) for sand and other soils, and only one regression line was justified in (c). r 2 of the regression is 0.35 in (a), 0.31 (without sand) and 0.999 (sand only) in (b), 0.25 in (c) and 0.50 (without sand) and 0.98 (sand only) in (d) [Color figure can be viewed at wileyonlinelibrary.com]
3.4.2. Root hydraulic conductance
The effect of root phenotype on ψ c soil is investigated by plotting ψ c soil with key root hydraulic properties. Figure 8b shows a general negative relation between K root_max and ‐ψ c soil. Plants with lower K root_max showed more negative ψ c soil. Although the relation in sandy soil seems off, the decreasing trend is similar (Figure 8b). Tomato had the highest K root_max and a high ψ c soil of ca. −30 kPa. Maize, barley, and wheat exhibited much lower K root_max and the corresponding ψ c soil was between −100 and −500 kPa. High K root_max helps plants to sustain high transpiration demand. However, it makes the E(ψ leaf_x) relation more sensitive to the declines in soil hydraulic conductance (Figure 6). The total soil–root hydraulic conductance is the harmonic mean of the root and soil hydraulic conductance. As the harmonic mean is controlled by the smallest values, plants with high K root_max would be more responsive to soil drying.
3.4.3. Root length
The effect of root length is shown in Figure 8c. It shows a positive relationship between root length and ‐ψ c soil in all soils. The effect of root length was distinct for millet and tomato grown in the same soil (sandy loam) and for maize grown in sand and loam. Maize in sandy loam and wheat in loam were off this relation but followed better the relation in Figure 8b. The mechanism by which root length impacts ψ c soil is that a longer root system reduces the local fluxes around roots and thus attenuates the gradient in soil water potential and delays the onset of nonlinearity of the E(ψ leaf_x) relation. Thus, plants with longer roots are expected to have a greater ability to extract water from drying soils. However, it has to be kept in mind that root length is not akin to root length active in water uptake (Ahmed, Zarebanadkouki, et al., 2018, 2016), which depends on the radial and axial hydraulic conductivities of roots. Indeed, in this review, the estimated fraction of roots active in water uptake across species and soils ranged from 4% to 30%.
3.4.4. Root hairs
The relation between root hairs and soil–plant hydraulics remains inconclusive. In barley, the wild type with similar K root_max showed −10 to −50 kPa lower ψ c soil than that of the mutant in sandy loam but not in red chromosol (probably due to fewer measurements) (Figure 8b). Maize with similar K root_max did not show a significant (p > 0.05) effect of root hairs on ψ c soil between wild type and mutant either in sand or in loam (Cai et al., 2021). Root hair length was two to three times longer in barley than in maize (Burak et al., 2021; Carminati et al., 2017; Marin et al., 2021). Longer root hairs were probably more effective in maintaining the hydraulic continuity across the rhizosphere than varieties with shorter hairs. The underlying mechanism is that root hairs facilitate water uptake by increasing root absorption radius (Segal et al., 2008) or buffering the drop in matric potential at the soil–root interface (Ahmed et al., 2020; Ahmed, Passioura, et al., 2018; Carminati et al., 2017). Up to now, studies of the role of root hairs in water uptake are still limited to a few species. Further investigations should be performed on more species under different soil and water conditions to explore their contributions to root hydraulic properties and water uptake, especially given that the development (length and density) of root hair may be a plastic trait under nutrient deficiency conditions (Bahmani et al., 2016; Zhu et al., 2010).
The effect of root hydraulic traits on ψ c soil shown in Figure 8b,c was integrated into Figure 8d. Figure 8d considered root water uptake ability (K root_max), root length (including root hairs), and uptake circumstances (soil texture). Due to the particular hydraulic property of sand (Figure 2), plants in sand did not follow the same relation between root hydraulic traits and ψ c soil as those in other soils but showed a similar trend. This relation indicates that plants with a lower K root_max, a longer root system and longer root hairs remain in the linear part of the relationship between transpiration rate and water potential for a broader range of soil water potentials.
3.5. Implications
Among root hydraulic traits that impacted soil water availability, we investigated the effects of K root, root length and root hairs. We introduced the concept of critical soil water potential, ψ c soil, as the point when the soil starts to limit root water uptake relative to root conductivity. Here, we discuss the meaning and importance of these traits as well as the critical soil water potential for water use.
3.5.1. Root hydraulic conductance
K root comprises the effects of root length, root architecture and the distribution of axial and radial conductivities (Doussan et al., 2006). The axial conductivity depends on the xylem vessel radius and number (Frensch & Steudle, 1989). The radial conductivity depends on the formation of Casparian band in exodermis (Zimmermann & Steudle, 1998), suberization (Kreszies et al., 2019) of the endodermis (Kreszies et al., 2020; Ranathunge et al., 2017), root cortical senescence (Schneider et al., 2017) and AQP expression (Martre et al., 2001). Variation of these elements impacts root hydraulic properties and thus root water uptake pattern (Ahmed, Passioura, et al., 2018; Ahmed, Zarebanadkouki, et al., 2018; Bramley et al., 2009; Javaux et al., 2008; Vadez, 2014).
Besides the absolute value of K root in wet conditions (K root_max), the decline of K root for decreasing soil water potentials is central to predict water use by crops. A loss of K root would induce nonlinearities in the E(ψ leaf_x) curve. The decline in K root during soil drying has several potential causes. Daily downregulation of AQP activity has been shown to occur particularly in water‐stressed plants and has been suggested as a mechanism to trigger stomatal regulation at the peak of transpiration demand (Caldeira et al., 2014). A passive mechanism leading to a decrease in K root is the formation of lacunae in the root cortex (Cuneo et al., 2016). Besides these mechanisms taking place at cellular and root cross‐section scales, a root‐system mechanism that affects K root is that during soil drying the drying front moves deeper in the soil, and fewer roots have access to soil water (Hayat et al., 2019). The relevance of this mechanism depends on the root architecture and its temporal changes (Ahmed, Passioura, et al., 2018).
Our analysis suggests that root water uptake of plants with higher K root_max was limited by the soil at less negative soil matric potential. The meaning of this result is not straightforward. According to the concept introduced by Carminati and Javaux (2020), the point at which the soil starts to limit water fluxes, ψ c soil, is reflected into a nonlinearity in the E(ψ leaf_x) relationship, and this triggers stomatal closure. The idea behind this model is that stomatal regulation prevents plants from nonlinearities in the E(ψ leaf_x) relationship. A similar concept was proposed by Sperry and Love (2015) and Sperry et al. (2016). A mechanism that allows stomata to accomplish this function has been recently proposed by Wankmüller and Carminati (2021). The idea is that stomatal conductance is regulated by the abscisic acid (ABA) level, which depends on the dynamic equilibrium between ABA production (assumed to increase with decreasing leaf water potential) and catabolism (assumed to increase with assimilation). The result of such a model is that stomatal conductance depends on the ratio between transpiration rate and leaf water potential. This concept still needs to be proven, but some preliminary evidence has been reported in Carminati and Javaux (2020) and Abdalla, Carminati, et al. (2021). If we apply the concept of Carminati and Javaux (2020) and Sperry and Love (2015), we would reach the conclusion that plants with high conductivity would show an earlier decrease in transpiration during soil drying. In other words, their stomata would start to downregulate transpiration at a less negative ψ c soil. This conclusion is counterintuitive and has to be taken cautiously. Indeed, if stomatal conductance was simply a function of leaf water potential, a high plant hydraulic conductance would have the opposite effect on transpiration response to soil drying: the plant would close the stomata at a more negative leaf water potential. The latter would fit with the results of a breeding programme initiated for wheat around 30 years ago (Richards & Passioura, 1989). The authors showed that smaller xylem vessels with lower hydraulic conductance would decelerate root water uptake and extend the plant water use. It is not necessarily that these two concepts are in contradiction, as the time scales of these observations are different, that is, daily regulation of transpiration in our concept compared to seasonal water use in the work of Richards and Passioura (1989). Possibly, a high plant hydraulic conductance would maintain transpiration in wet soils at a high vapour pressure deficit (VPD), while it would trigger stomatal closure during soil drying. The underlying mechanism of stomatal closure is still not fully explored (Brodribb & Holbrook, 2003; Buckley, 2005; Tardieu, 2016; Tombesi et al., 2015). Stomatal closure is proposed to be driven by ABA (Buckley, 2019), and ABA biosynthesis increases with decreasing leaf water potential (McAdam & Brodribb, 2016). But transport (Tardieu & Davies, 1993) and catabolism of ABA are also relevant for ABA levels. So, it is possible that stomatal conductance is both function (1) of leaf water potential, and (2) of a ratio between transpiration (or assimilation) and leaf water potential, with the latter component being important for the fine regulation of stomatal opening. In conclusion, the fact that a high plant conductance causes an earlier nonlinearity in the relationship between transpiration and leaf water potential does not translate in a straightforward way into an earlier decrease in transpiration rate.
3.5.2. Root length
We have shown that root length is an important root trait impacting the easiness of water uptake. First of all, K root increases with root length (Figure 3a). Additionally, a long root length means a slow rate of water flow in soils. Thus, it is beneficial to maintain water uptake under moderate drought. Opposite to the discussion on K root_max, independently from the stomatal model, a longer root length is expected to sustain transpiration during soil drying. Deeper rooting was shown to be important under dry conditions (Fan et al., 2017; Lynch, 2013; Wasson et al., 2012; White & Kirkegaard, 2010). However, the effects of a profuse root system on soil water availability and water use are not unique and their effects on water uptake are context‐dependent (see the discussion in Ahmed, Zarebanadkouki, et al., 2018, and Ahmed et al., 2020). Vadez (2014) showed that plants with a profuse root system (high root length densities) contributed positively to water uptake under drought in some studies whereas in others did not. A profuse root system may not be conducive to water use if most roots distribute at surface layers.
3.5.3. Root hairs
Another root hydraulic phenotype that needs more attention is root hairs. Root hairs have been shown to play an important role in nutrient absorption; however, their role in water uptake is still unclear. Root hairs are expected to increase the effective root radius and reduce the gradients in soil water potential needed to sustain high transpiration rates in drying soils (Segal et al., 2008). Experiments with barley with and without hairs supported this concept, both in the lab (Carminati et al., 2017) and in the field (Marin et al., 2021). However, there are measurements with similar genotypes that did not show significant differences (Dodd & Diatloff, 2016). For maize, Cai et al. (2021) found no difference between a wild type and its hairless mutant. The reason might be related to the shorter length of root hairs of maize compared to barley. Additionally, the effects of root hairs might be soil‐dependent. Therefore, it seems that there is no unique answer about the positive role of hairs on soil–plant hydraulics.
In summary, we proposed a concept to link root hydraulic phenotypes to water use by crops. Root hydraulic traits that attenuate declines in soil limitation (for instance, root hairs or long roots) would help to sustain the use of water during soil drying. Traits that cause an earlier drop in conductance, such as a high K root_max or a decline in K root with soil drying, could lead to a more conservative use of water. The ‘conservation’ here only links with plant water budget or plant hydraulics, not with drought tolerance (Ahmed, Passioura et al., 2018; Vadez, 2014).
3.6. Limitations
Besides the factors discussed above, root hydraulic conductance can be impacted by other factors, such as root architecture (Doussan et al., 2006; Javaux et al., 2008), root types (Ahmed, Zarebanadkouki, et al., 2016, 2018) and rhizosphere hydraulics changing over time (Ahmed et al., 2014; Ahmed, Kroener, et al., 2016), which further affect water uptake. In this study, K root was a ‘effective conductance’, and was an integration of root hydraulic properties of the whole root systemin during the drying process. Except in wet conditions, when E was not limited by soil, K root was an optimized variable based on the measurements of E, water potential in the soil and leaves. More complex models that consider dynamic root hydraulic properties, root architecture and nonuniform soil moisture distribution may be better in estimating root water uptake. However, estimation from those models depends on the accuracy of detailed root distribution and hydraulic properties of individual roots. These parameters are not available and cannot be easily measured, especially in roots growing in soils.
4. CONCLUSIONS
This review highlights the role of soil and key root hydraulic properties to predict water uptake by crops under limiting soil water conditions. We showed at what soil water potential the soil starts to limit the water fluxes through the soil–plant continuum across species and soils. Coarse textured soils exhibit an earlier limitation to root water uptake due to their sudden loss in conductivity for decreasing soil water potentials. We have introduced the concept of critical soil water potential ψ c soil and showed how it is affected by key hydraulic traits. The critical soil matric potential was not unique and varied with soil textures and root hydraulic phenotypes. Lower K root_max, longer root length and longer root hairs have a more negative ψ c soil.
According to the concept proposed by Carminati and Javaux (2020) and Sperry and Love (2015), ψ c soil corresponds to the point at which plants downregulate transpiration. This would imply that plants with a high root hydraulic conductivity would be more responsive in reducing transpiration during soil drying. However, a high hydraulic conductance would result in a less negative leaf water potential, which might have the opposite effect of maintaining transpiration. Despite this controversy on the effect of plant hydraulic conductance on stomatal regulation, this review of hydraulic traits and their impact on soil–plant conductance could help us to reveal the role of root hydraulics in crop water use. The effect of different root hydraulic phenotypes on drought tolerance will depend on environmental conditions and precipitation/irrigation patterns (Ahmed et al., 2020; Cai et al., 2021; Comas et al., 2013). Root hydraulic phenotypes that trigger an earlier stomatal closure would be convenient to save water transiently. On the other hand, root hydraulic phenotypes that maintain transpiration during soil drying would be favourable to maintain growth (at the cost of faster soil water depletion).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
The authors acknowledge the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) for funding of the priority program 2089, project numbers 403670197 ‘Emerging effects of root hairs and mucilage on plant scale soil water relations’ to M. A. A. and A. C. The position of G. Cai was founded by the BMBF, Project 02WIL1489 (Deutsch‐Israelische Wassertechnologie‐Kooperation). The authors thank the editor and two anonymous reviewers for their constructive comments and suggestions on the manuscript. Open access funding provided by Eidgenossische Technische Hochschule Zurich.
1.
Parameters and units used in the figures and table:
E: transpiration rate (measured), cm3 s−1
ψ soil: soil matric potential (measured), kPa
ψ soil_root: water potential at the soil–root interface (modelled), kPa
ψ c soil: critical soil matric potential (modelled), kPa
ψ leaf: leaf xylem water potential (measured), kPa
K root: root hydraulic conductance (modelled), cm3 kPa−1 s−1
K root_max: maximum root hydraulic conductance (modelled), cm3 kPa−1 s−1
kroot: root hydraulic conductivity (modelled), cm kPa−1 s−1
kroot max: maximum root hydraulic conductivity (modelled), cm kPa−1 s−1
K soil: soil hydraulic conductance (modelled), cm3 kPa−1 s−1
k s: soil hydraulic conductivity (measured), cm s−1
RL total: measured total root length (measured), cm
Cai, G. , Ahmed, M.A. , Abdalla, M. & Carminati, A. (2022) Root hydraulic phenotypes impacting water uptake in drying soils. Plant, Cell & Environment, 45, 650–663. 10.1111/pce.14259
DATA AVAILABILITY STATEMENT
The raw data are from published studies (described in Table 1). The analysed data that support the findings of this study are available from the first and corresponding authors upon reasonable request.
REFERENCES
- Abdalla, M. , Ahmed, M.A. , Cai, G. , Wankmüller, F. , Schwartz, N. & Litig, O. et al. (2021) Stomatal closure during water deficit is controlled by belowground hydraulics. Annals of Botany, mcab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdalla, M. , Carminati, A. , Cai, G. , Javaux, M. & Ahmed, M.A. (2021) Stomatal closure of tomato under drought is driven by an increase in soil–root hydraulic resistance. Plant, Cell & Environment, 44, 425–431. [DOI] [PubMed] [Google Scholar]
- Ahmed, M.A. , Vetterlein, D. & Carminati, A. (2020) Advances in understanding plant root water uptake. Cambridge, UK: Burleigh Dodds Science Publishing Limited. [Google Scholar]
- Ahmed, M.A. , Kroener, E. , Benard, P. , Zarebanadkouki, M. , Kaestner, A. & Carminati, A. (2016) Drying of mucilage causes water repellency in the rhizosphere of maize: measurements and modelling. Plant and Soil, 407, 161–171. [Google Scholar]
- Ahmed, M.A. , Kroener, E. , Holz, M. , Zarebanadkouki, M. & Carminati, A. (2014) Mucilage exudation facilitates root water uptake in dry soils. Functional Plant Biology, 41, 1129–1137. [DOI] [PubMed] [Google Scholar]
- Ahmed, M.A. , Passioura, J. & Carminati, A. (2018) Hydraulic processes in roots and the rhizosphere pertinent to increasing yield of water‐limited grain crops: a critical review. Journal of Experimental Botany, 69, 3255–3265. [DOI] [PubMed] [Google Scholar]
- Ahmed, M.A. , Zarebanadkouki, M. , Kaestner, A. & Carminati, A. (2016) Measurements of water uptake of maize roots: the key function of lateral roots. Plant and Soil, 398, 59–77. [Google Scholar]
- Ahmed, M.A. , Zarebanadkouki, M. , Meunier, F. , Javaux, M. , Kaestner, A. & Carminati, A. (2018) Root type matters: measurement of water uptake by seminal, crown, and lateral roots in maize. Journal of Experimental Botany, 69, 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque, C. , Scoffoni, C. , Brodersen, C.R. , Buckley, T.N. , Sack, L. & McElrone, A.J. (2020) Coordinated decline of leaf hydraulic and stomatal conductances under drought is not linked to leaf xylem embolism for different grapevine cultivars. Journal of Experimental Botany, 71, 7286–7300. [DOI] [PubMed] [Google Scholar]
- Anderegg, W.R.L. , Wolf, A. , Arango‐Velez, A. , Choat, B. , Chmura, D.J. , Jansen, S. et al. (2017) Plant water potential improves prediction of empirical stomatal models. PLoS ONE, 12, e0185481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani, R. , Kim, D.G. , Kim, J.A. & Hwang, S. (2016) The density and length of root hairs are enhanced in response to cadmium and arsenic by modulating gene expressions involved in fate determination and morphogenesis of root hairs in Arabidopsis. Frontiers in Plant Science, 7, 1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbia, I. , Pritzkow, C. & Brodribb, T.J. (2021) Herb and conifer roots show similar high sensitivity to water deficit. Plant Physiology, 186, 1908–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, J.S. (1982) Plant productivity and environment. Science, 218, 443–448. [DOI] [PubMed] [Google Scholar]
- Bramley, H. , Turner, N.C. , Turner, D.W. & Tyerman, S.D. (2009) Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiology, 150, 348–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb, T.J. & Holbrook, N.M. (2003) Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology, 132, 2166–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, R. & Corey, T. (1964) HYDRAU uc properties of porous media. Hydrology Papers, Colorado State University, 24, 37. [Google Scholar]
- Buckley, T.N. (2005) The control of stomata by water balance. New Phytologist, 168, 275–292. [DOI] [PubMed] [Google Scholar]
- Buckley, T.N. (2019) How do stomata respond to water status? New Phytologist, 224, 21–36. [DOI] [PubMed] [Google Scholar]
- Burak, E. , Quinton, J.N. & Dodd, I.C. (2021) Root hairs are the most important root trait for rhizosheath formation of barley (Hordeum vulgare L.), maize (Zea mays L.), and Lotus japonicus (Gifu). Annals of Botany, 128, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, G. , Ahmed, M.A. , Dippold, M.A. , Zarebanadkouki, M. & Carminati, A. (2020) Linear relation between leaf xylem water potential and transpiration in pearl millet during soil drying. Plant and Soil, 447, 565–578. [Google Scholar]
- Cai, G. , Ahmed, M.A. , Reth, S. , Reiche, M. , Kolb, A. & Carminati, A. (2020) Measurement of leaf xylem water potential and transpiration during soil drying using a root pressure chamber system. Acta Horticulturae, 1300, 131–138. [Google Scholar]
- Cai, G. , Carminati, A. , Abdalla, M. & Ahmed, M.A. (2021) Soil textures rather than root hairs dominate water uptake and soil–plant hydraulics under drought. Plant Physiology, 187, 858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, G. , Vanderborght, J. , Langensiepen, M. , Schnepf, A. , Hüging, H. & Vereecken, H. (2018) Root growth, water uptake, and sap flow of winter wheat in response to different soil water conditions. Hydrology and Earth System Sciences, 22, 2449–2470. [Google Scholar]
- Caldeira, C.F. , Jeanguenin, L. , Chaumont, F. & Tardieu, F. (2014) Circadian rhythms of hydraulic conductance and growth are enhanced by drought and improve plant performance. Nature Communications, 5, 5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati, A. & Javaux, M. (2020) Soil Rather than xylem vulnerability controls stomatal response to drought. Trends in Plant Science, 25, 868–880. [DOI] [PubMed] [Google Scholar]
- Carminati, A. , Passioura, J.B. , Zarebanadkouki, M. , Ahmed, M.A. , Ryan, P.R. , Watt, M. et al. (2017) Root hairs enable high transpiration rates in drying soils. New Phytologist, 216, 771–781. [DOI] [PubMed] [Google Scholar]
- Carminati, A. , Vetterlein, D. , Weller, U. , Vogel, H.‐J. & Oswald, S.E. (2009) When roots lose contact. Vadose Zone Journal, 8, 805–809. [Google Scholar]
- Cochard, H. (2002) Xylem embolism and drought‐induced stomatal closure in maize. Planta, 215, 466–471. [DOI] [PubMed] [Google Scholar]
- Comas, L. , Becker, S. , Cruz, V.M.V. , Byrne, P.F. & Dierig, D.A. (2013) Root traits contributing to plant productivity under drought. Frontiers in Plant Science, 4, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso, D. , Delzon, S. , Lamarque, L.J. , Cochard, H. , Torres‐Ruiz, J.M. , King, A. et al. (2020) Neither xylem collapse, cavitation, or changing leaf conductance drive stomatal closure in wheat. Plant, Cell & Environment, 43, 854–865. [DOI] [PubMed] [Google Scholar]
- Cuneo, I.F. , Barrios‐Masias, F. , Knipfer, T. , Uretsky, J. , Reyes, C. , Lenain, P. et al. (2021) Differences in grapevine rootstock sensitivity and recovery from drought are linked to fine root cortical lacunae and root tip function. New Phytologist, 229, 272–283. [DOI] [PubMed] [Google Scholar]
- Cuneo, I.F. , Knipfer, T. , Brodersen, C.R. & McElrone, A.J. (2016) Mechanical failure of fine root cortical cells initiates plant hydraulic decline during drought. Plant Physiology, 172, 1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deery, D.M. (2008). Water uptake by a single plant: analysis using experimentation and modeling. [Doctoral dissertation]. University of Melbourne. Retrieved from https://www.irrigationaustralia.com.au/documents/item/263
- Deery, D.M. , Passioura, J.B. , Condon, J.R. & Katupitiya, A. (2013) Uptake of water from a Kandosol subsoil. II. Control of water uptake by roots. Plant and Soil, 368, 649–667. [Google Scholar]
- Dodd, I.C. & Diatloff, E. (2016) Enhanced root growth of the brb (bald root barley) mutant in drying soil allows similar shoot physiological responses to soil water deficit as wild‐type plants. Functional Plant Biology, 43, 199–206. [DOI] [PubMed] [Google Scholar]
- Dodd, I.C. , Egea, G. , Watts, C.W. & Whalley, W.R. (2010) Root water potential integrates discrete soil physical properties to influence ABA signalling during partial rootzone drying. Journal of Experimental Botany, 61, 3543–3551. [DOI] [PubMed] [Google Scholar]
- Doussan, C. , Pierret, A. , Garrigues, E. & Pagès, L. (2006) Water uptake by plant roots: ii—modelling of water transfer in the soil root‐system with explicit account of flow within the root system—comparison with experiments. Plant and Soil, 283, 99–117. [Google Scholar]
- Draye, X. , Kim, Y. , Lobet, G. & Javaux, M. (2010) Model‐assisted integration of physiological and environmental constraints affecting the dynamic and spatial patterns of root water uptake from soils. Journal of Experimental Botany, 61, 2145–2155. [DOI] [PubMed] [Google Scholar]
- Faiz, S.M.A. & Weatherley, P.E. (1982) Root contraction in transpiring plants. New Phytologist, 92, 333–343. [Google Scholar]
- Fan, Y. , Miguez‐Macho, G. , Jobbágy, E.G. , Jackson, R.B. & Otero‐Casal, C. (2017) Hydrologic regulation of plant rooting depth. Proceedings of the National Academy of Sciences of the United States of America, 114, 10572–10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frensch, J. & Steudle, E. (1989) Axial and radial hydraulic resistance to roots of maize (Zea mays L.) 1. Plant Physiology, 91, 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, W.R. (1960) Dynamic aspects of water availability to plants. Soil Science, 89, 63–73. [Google Scholar]
- Grondin, A. , Affortit, P. , Tranchant‐Dubreuil, C. , de la Fuente‐Cantó, C. , Mariac, C. , Gantet, P. , et al. (2020) Aquaporins are main contributors to root hydraulic conductivity in pearl millet [Pennisetum glaucum (L.) R. Br.]. PLoS ONE, 15, e0233481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat, F. , Ahmed, M.A. , Zarebanadkouki, M. , Cai, G. & Carminati, A. (2019) Measurements and simulation of leaf xylem water potential and root water uptake in heterogeneous soil water contents. Advances in Water Resources, 124, 96–105. [Google Scholar]
- Hayat, F. , Ahmed, M.A. , Zarebanadkouki, M. , Javaux, M. , Cai, G. & Carminati, A. (2020) Transpiration reduction in maize (Zea mays L.) in response to soil drying. Frontiers in Plant Science, 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaux, M. , Schröder, T. , Vanderborght, J. & Vereecken, H. (2008) Use of a three‐dimensional detailed modeling approach for predicting root water uptake. Vadose Zone Journal, 7, 1079–1088. [Google Scholar]
- Judd, L.A. , Jackson, B.E. , Fonteno, W.C. & Domec, J.‐C. (2016) Measuring root hydraulic parameters of container‐grown herbaceous and woody plants using the hydraulic conductance flow meter. HortScience, 51, 192–196. [Google Scholar]
- Kato, Y. & Okami, M. (2011) Root morphology, hydraulic conductivity and plant water relations of high‐yielding rice grown under aerobic conditions. Annals of Botany, 108, 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama, A. , Watanabe, T. , Yamaguchi, M. , Narita, R. , Katsuhara, M. , Sato, K. et al. (2021) Accession difference in leaf photosynthesis, root hydraulic conductance and gene expression of root aquaporins under salt stress in barley seedlings. Plant Production Science, 24, 73–82. [Google Scholar]
- Koebernick, N. , Schlüter, S. , Blaser, S.R.G.A. & Vetterlein, D. (2018) Root‐soil contact dynamics of Vicia faba in sand. Plant and Soil, 431, 417–431. [Google Scholar]
- Kreszies, T. , Eggels, S. , Kreszies, V. , Osthoff, A. , Shellakkutti, N. , Baldauf, J.A. et al. (2020) Seminal roots of wild and cultivated barley differentially respond to osmotic stress in gene expression, suberization, and hydraulic conductivity. Plant, Cell & Environment, 43, 344–357. [DOI] [PubMed] [Google Scholar]
- Kreszies, T. , Shellakkutti, N. , Osthoff, A. , Yu, P. , Baldauf, J.A. , Zeisler‐Diehl, V.V. et al. (2019) Osmotic stress enhances suberization of apoplastic barriers in barley seminal roots: analysis of chemical, transcriptomic and physiological responses. New Phytologist, 221, 180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, T. & Vialet‐Chabrand, S. (2019) Speedy stomata, photosynthesis and plant water use efficiency. New Phytologist, 221, 93–98. [DOI] [PubMed] [Google Scholar]
- Lynch, J.P. (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany, 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin, M. , Feeney, D.S. , Brown, L.K. , Naveed, M. , Ruiz, S. , Koebernick, N. et al. (2021) Significance of root hairs for plant performance under contrasting field conditions and water deficit. Annals of Botany, 128, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martre, P. , North, G.B. & Nobel, P.S. (2001) Hydraulic conductance and mercury‐sensitive water transport for roots of opuntia acanthocarpa in relation to soil drying and rewetting. Plant Physiology, 126, 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel, C. , Simonneau, T. & Sutka, M. (2010) The significance of roots as hydraulic rheostats. Journal of Experimental Botany, 61, 3191–3198. [DOI] [PubMed] [Google Scholar]
- McAdam, S.A.M. & Brodribb, T.J. (2016) Linking turgor with ABA biosynthesis: implications for stomatal responses to vapor pressure deficit across land plants. Plant Physiology, 171, 2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean, E.H. , Ludwig, M. & Grierson, P.F. (2011) Root hydraulic conductance and aquaporin abundance respond rapidly to partial root‐zone drying events in a riparian Melaleuca species. New Phytologist, 192, 664–675. [DOI] [PubMed] [Google Scholar]
- Nobel, P.S. & Cui, M. (1992) Hydraulic conductances of the soil, the root—soil air gap, and the root: changes for desert succulents in drying soil. Journal of Experimental Botany, 43, 319–326. [Google Scholar]
- North, G.B. & Nobel, P.S. (1997) Root‐soil contact for the desert succulent Agave deserti in wet and drying soil. New Phytologist, 135, 21–29. [DOI] [PubMed] [Google Scholar]
- Passioura, J.B. (1980) The transport of water from soil to shoot in wheat seedlings. Journal of Experimental Botany, 31, 333–345. [Google Scholar]
- Ranathunge, K. , Kim, Y.X. , Wassmann, F. , Kreszies, T. , Zeisler, V. & Schreiber, L. (2017) The composite water and solute transport of barley (Hordeum vulgare) roots: effect of suberized barriers. Annals of Botany, 119, 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, R.A. & Passioura, J.B. (1989) A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain‐fed environments. Australian Journal of Agricultural Research, 40, 943–950. [Google Scholar]
- Rodriguez‐Dominguez, C.M. & Brodribb, T.J. (2020) Declining root water transport drives stomatal closure in olive under moderate water stress. New Phytol, 225, 126–134. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Gamir, J. , Xue, J. , Clearwater, M.J. , Meason, D.F. , Clinton, P.W. & Domec, J.‐C. (2019) Aquaporin regulation in roots controls plant hydraulic conductance, stomatal conductance, and leaf water potential in Pinus radiata under water stress: aquaporin activity regulates stomatal conductace. Plant, Cell & Environment, 42, 717–729. [DOI] [PubMed] [Google Scholar]
- Rohatgi, A. (2020) WebPlotDigitizer, Version 4.3.
- Savi, T. , Bertuzzi, S. , Branca, S. , Tretiach, M. & Nardini, A. (2015) Drought‐induced xylem cavitation and hydraulic deterioration: risk factors for urban trees under climate change? New Phytologist, 205, 1106–1116. [DOI] [PubMed] [Google Scholar]
- Schneider, H.M. , Wojciechowski, T. , Postma, J.A. , Brown, K.M. , Lücke, A. , Zeisler, V. et al. (2017) Root cortical senescence decreases root respiration, nutrient content and radial water and nutrient transport in barley. Plant, Cell & Environment, 40, 1392–1408. [DOI] [PubMed] [Google Scholar]
- Scoffoni, C. & Sack, L. (2017) The causes and consequences of leaf hydraulic decline with dehydration. Journal of Experimental Botany, 68, 4479–4496. [DOI] [PubMed] [Google Scholar]
- Segal, E. , Kushnir, T. , Mualem, Y. & Shani, U. (2008) Water uptake and hydraulics of the root hair rhizosphere. Vadose Zone Journal, 7, 1027–1034. [Google Scholar]
- Sperry, J.S. , Adler, F.R. , Campbell, G.S. & Comstock, J.P. (1998) Limitation of plant water use by rhizosphere and xylem conductance: results from a model. Plant, Cell & Environment, 21, 347–359. [Google Scholar]
- Sperry, J.S. , Hacke, U.G. , Oren, R. & Comstock, J.P. (2002) Water deficits and hydraulic limits to leaf water supply. Plant, Cell & Environment, 25, 251–263. [DOI] [PubMed] [Google Scholar]
- Sperry, J.S. & Love, D.M. (2015) What plant hydraulics can tell us about responses to climate‐change droughts. New Phytologist, 207, 14–27. [DOI] [PubMed] [Google Scholar]
- Sperry, J.S. , Wang, Y. , Wolfe, B.T. , Mackay, D.S. , Anderegg, W.R.L. , McDowell, N.G. et al. (2016) Pragmatic hydraulic theory predicts stomatal responses to climatic water deficits. New Phytologist, 212, 577–589. [DOI] [PubMed] [Google Scholar]
- Strock, C.F. , Burridge, J.D. , Niemiec, M.D. , Brown, K.M. & Lynch, J.P. (2021) Root metaxylem and architecture phenotypes integrate to regulate water use under drought stress. Plant, Cell & Environment, 44, 49–67. [DOI] [PubMed] [Google Scholar]
- Sunita, C. , Sinclair, T.R. , Messina, C.D. & Cooper, M. (2014) Hydraulic conductance of maize hybrids differing in transpiration response to vapor pressure deficit. Crop Science, 54, 1147–1152. [Google Scholar]
- Tardieu, F. (2016) Too many partners in root‐shoot signals. Does hydraulics qualify as the only signal that feeds back over time for reliable stomatal control? New Phytologist, 212, 802–804. [DOI] [PubMed] [Google Scholar]
- Tardieu, F. & Davies, W.J. (1993) Integration of hydraulic and chemical signalling in the control of stomatal conductance and water status of droughted plants. Plant, Cell & Environment, 16, 341–349. [Google Scholar]
- Taylor, H.M. & Willatt, S.T. (1983) Shrinkage of soybean roots 1. Agronomy Journal, 75, 818–820. [Google Scholar]
- Tombesi, S. , Nardini, A. , Frioni, T. , Soccolini, M. , Zadra, C. , Farinelli, D. et al. (2015) Stomatal closure is induced by hydraulic signals and maintained by ABA in drought‐stressed grapevine. Scientific Reports, 5, 12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy, S.R. , Nagel, K.A. , Postma, J.A. , Fassbender, H. , Wasson, A. & Watt, M. (2020) Crop improvement from phenotyping roots: highlights reveal expanding opportunities. Trends in Plant Science, 25, 105–118. [DOI] [PubMed] [Google Scholar]
- Tsuda, M. & Tyree, M.T. (2000) Plant hydraulic conductance measured by the high pressure flow meter in crop plants. Journal of Experimental Botany, 51, 823–828. [PubMed] [Google Scholar]
- Vadez, V. (2014) Root hydraulics: the forgotten side of roots in drought adaptation. Field Crops Research, 165, 15–24. [Google Scholar]
- Venturas, M.D. , Sperry, J.S. & Hacke, U.G. (2017) Plant xylem hydraulics: what we understand, current research, and future challenges. Journal of Integrative Plant Biology, 59, 356–389. [DOI] [PubMed] [Google Scholar]
- Wankmüller, F. & Carminati, A. (2021) Stomatal regulation prevents plants from critical water potentials during drought: result of a model linking soil‐plant hydraulics to ABA dynamics. Ecohydrology. e2386. [Google Scholar]
- Wasson, A.P. , Richards, R.A. , Chatrath, R. , Misra, S.C. , Prasad, S.V. , Rebetzke, G.J. et al. (2012) Traits and selection strategies to improve root systems and water uptake in water‐limited wheat crops. Journal of Experimental Botany, 63, 3485–3498. [DOI] [PubMed] [Google Scholar]
- White, R.G. & Kirkegaard, J.A. (2010) The distribution and abundance of wheat roots in a dense, structured subsoil–implications for water uptake. Plant, Cell & Environment, 33, 133–148. [DOI] [PubMed] [Google Scholar]
- Wilhite, D.A. (2000). Drought as a natural hazard: concepts and definitions. London, UK: Routledge.
- Xu, H.‐L. (2001) Soil‐root interface water potential in sweet corn as affected by organic fertilizer and a microbial inoculant. Journal of Crop Production, 3, 139–156. [Google Scholar]
- Yang, Y.‐J. , Bi, M.‐H. , Nie, Z.‐F. , Jiang, H. , Liu, X.‐D. , Fang, X.‐W. et al. (2021) Evolution of stomatal closure to optimize water‐use efficiency in response to dehydration in ferns and seed plants. New Phytologist, 230, 2001–2010. [DOI] [PubMed] [Google Scholar]
- Zhao, C.‐X. , Deng, X.‐P. , Shan, L. , Steudle, E. , Zhang, S.‐Q. & Ye, Q. (2005) Changes in root hydraulic conductivity during wheat evolution. Journal of Integrative Plant Biology, 47, 302–310. [Google Scholar]
- Zhu, J. , Zhang, C. & Lynch, J.P. (2010) The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Functional Plant Biology, 37, 313–322. [Google Scholar]
- Zimmermann, H.M. & Steudle, E. (1998) Apoplastic transport across young maize roots: effect of the exodermis. Planta, 206, 7–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The raw data are from published studies (described in Table 1). The analysed data that support the findings of this study are available from the first and corresponding authors upon reasonable request.


