Abstract
We aim to compare endometrial cancer survival in women with or without histological proven endometriosis or adenomyosis. We identified all women with endometrial cancer between 1990 and 2015 from the Netherlands Cancer Registry (NCR). Data were linked to the Dutch pathology database (PALGA) to select all women with histological proven endometriosis/adenomyosis. Overall survival was compared between women with endometrial cancer with or without endometriosis/adenomyosis. We used multivariable Cox proportional hazard analysis to estimate hazard ratios (HRs). We included 1701 women with endometrial cancer and endometriosis/adenomyosis, of whom 1236 (72.7%) women had adenomyosis, 320 (18.8%) had endometriosis and 145 (8.5%) had both. We compared these women to 39 139 women with endometrial cancer without endometriosis/adenomyosis. Women in the combined endometriosis/adenomyosis cohort were younger at endometrial cancer diagnosis, had earlier disease stage, more often had endometrioid endometrial cancer and low grade tumors. The 5‐year survival rate in the combined endometriosis/adenomyosis cohort was 84.8% (95% CI 84.6‐88.1) and 71.6% (95% CI 71.1‐72.0) in the nonendometriosis/adenomyosis cohort. Univariable analysis resulted in a crude HR of 0.63 (95% CI 0.59‐0.69). Significant confounding factors were age, stage, cancer subtype, histological grading, surgery and chemotherapy rate. Correction for these confounders resulted in a HR of 0.98 (95% CI 0.90‐1.06). Including endometriosis/adenomyosis status as a categorical factor resulted in similar HRs. In conclusion, women with endometrial cancer and histologically proven endometriosis/adenomyosis have a better overall survival when compared to women with endometrial cancer without endometriosis/adenomyosis. This better survival was correlated to stage, grade, age and histological subtype, but not to the presence of endometriosis/adenomyosis.
Keywords: adenomyosis, endometrial cancer, endometriosis, prognosis
What's new?
Whether women with adenomyosis and endometrial cancer have better overall survival rates compared to women with endometrial cancer without adenomyosis remains unclear. In this nationwide study involving more than 40,000 women with endometrial cancer, women with endometrial cancer and histologically proven endometriosis or adenomyosis were found to survive longer after cancer diagnosis than women without endometriosis/adenomyosis. Women with endometriosis/adenomyosis were diagnosed with endometrial cancer at a younger age and had tumor characteristics, including stage, grade, and histological subtype, that generally are associated with more favorable cancer outcome. Following correction for confounders, endometriosis/adenomyosis status was not independently prognostic for endometrial cancer.
Abbreviations
- 95% CI
95% confidence interval
- BSO
bilateral salpingo‐oophorectomy
- FIGO
International Federation of Gynecology and Obstetrics
- HR
hazard ratio
- ICD‐O
International Classification of Oncology Diseases
- IQR
inter quartile range
- LVSI
lymphovascular space invasion
- NA
not applicable
- NCR
Netherlands Cancer Registry
- NOS
not otherwise specified
- PALGA
Dutch nationwide registry of histopathology and cytopathology
- SRR
summarized relative risk
- TNM
tumor, node and metastasis
1. INTRODUCTION
Endometrial cancer is the most common gynecological cancer in developed countries with over 377 227 endometrial cancer cases diagnosed in these countries in 2020. 1 Endometrial cancer incidence is rising, which is associated with an increase in worldwide obesity, life expectancy and hormone replacement therapy. 2 , 3 Recently, Kvaskoff et al performed a meta‐analysis which showed a summarized relative risk (SRR) of 1.23 (0.97‐1.57) for endometrial cancer. 4 The association was nonsignificant but positive in case‐control studies. In prospective cohort studies that required temporality, the association was consistently null, with a SRR of 0.99 (95% CI 0.72‐1.37). 4 More recently, we published a study including around 130 000 women which showed that women with endometriosis or adenomyosis have an increased incidence of endometrial cancer which may indicate a possible association between endometriosis/adenomyosis and endometrial cancer. 5 Endometrial cancer is usually detected in an early stage due to postmenopausal bleeding and therefore has a high overall survival rate. 6 Factors resulting in a poorer prognosis are high tumor stage or grade, histological subtype other than endometrioid, cervical stromal involvement and lymphovascular space invasion (LVSI). 6 Endometriosis and adenomyosis are often associated with low grade tumors, early‐stage disease, with endometrioid histology, 5 , 7 , 8 possibly leading to a better endometrial cancer prognosis in endometriosis/adenomyosis patients. A recent meta‐analysis showed in crude analysis that women with adenomyosis and endometrial cancer have an increased overall survival when compared to women with endometrial cancer without adenomyosis. 7 Another study showed that adenomyosis was not an independent prognostic factor after correcting for confounders in 103 women with adenomyosis with endometrial cancer. 9 Additionally a recent case‐control study, did not find different survival for women with endometriosis or adenomyosis and endometrial cancer. 10 Due to small sample sizes and lack of correction for confounders, larger studies are warranted to study the role of endometriosis and adenomyosis in endometrial cancer survival. We therefore aimed to assess endometrial cancer survival in women with or without histological proven endometriosis or adenomyosis.
2. METHODS
2.1. Study population and design
We performed a retrospective nationwide cohort study in which all women with epithelial endometrial cancer between 1990 and 2015 were selected from the Netherlands Cancer Registry (NCR). We identified all patients with histological proven endometriosis/adenomyosis from the Dutch nationwide registry of histopathology and cytopathology (PALGA) in the same period. We used a histological diagnosis of endometriosis or adenomyosis as this remains considered the gold standard for endometriosis or adenomyosis diagnoses. 11 We linked these two databases and selected all women with endometrial cancer and histological proven endometriosis and/or adenomyosis as cases. Women with endometrial cancer but without histological proven endometriosis/adenomyosis were included as controls. Women with an endometriosis/adenomyosis diagnosis more than a half year after endometrial cancer were excluded.
All newly diagnosed cancers are registered nationwide since 1989. Data on tumor characteristics, treatment and survival status were retrieved. Yearly the NCR is linked to the municipal basic administration, which provides vital status, date of death, migration status and date of emigration. All patients were censored after death, emigration or end of study (31 January 2020).
Morphology was coded according to the International Classification of Oncology Diseases (ICD‐O). The stage of the tumor is determined according to the tumor, node and metastasis (TNM) classification (1989‐1992 fourth edition; 1993‐1998 revision fourth edition; 1999‐2002 fifth edition; 2003‐2009 sixth edition; 2010‐2016 seventh edition, from 2017 eighth edition). The International Federation of Gynecology and Obstetrics (FIGO) staging was derived from the TNM classification (1989‐2013 FIGO staging 1989 edition, 2014‐2017 FIGO staging 2013 edition).
2.2. Statistical analyses
Overall survival rate and 5‐year survival with 95% CI were calculated. We plotted the overall survival data by using the Kaplan‐Meier method, and the endometriosis, the adenomyosis and control cohort were compared by using Log‐rank test. One‐Way ANOVA and χ 2 tests were used to compare the cohorts. Endometriosis/adenomyosis status was defined as a categorical factor (endometrioses; adenomyosis; endometriosis and adenomyosis; no histological endometriosis/adenomyosis). Endometrial cancer histologic subtypes were endometrioid, clear cell, serous, mucinous and adenocarcinoma not otherwise specified (NOS). Grading was defined as low, intermediate or high grade.
We estimated hazard ratios (HRs) with 95% CI for overall survival and corrected for confounders with univariable and multivariable Cox proportional hazard analysis. All significant confounders in univariable and multivariable analyses were included. Backward selection was used to remove factors that did not significantly contribute to the multivariable model. We assessed the interaction between the presence of endometriosis/adenomyosis and other possible confounding factors by including interaction terms in the model and testing for significance. Violation of the proportional hazards assumption was checked by plotting the log‐log plot of survival for all significant confounders, none were found. Additionally, we performed an analysis including endometriosis/adenomyosis status as a binomial factor (yes/no). All statistical tests were two‐sided, and a P‐value of less than .005 was considered significant to account for multiple comparisons. SPSS version 25.0.0.1 for Windows (SPSS, Chicago, IL) and STATA v16.0 (StataCorp LLC, College Station, TX) were used for statistical analyses.
3. RESULTS
3.1. Cohort characteristics
We assessed 41 001 women with endometrial cancer for eligibility between 1990 and 2015. Linking the NCR and PALGA resulted in 1755 women with both endometrial cancer and histological proven endometriosis/adenomyosis. A total of 161 women were excluded as 114 women did not have epithelial endometrial cancer and 47 women had their endometriosis or adenomyosis diagnosis more than half a year after the endometrial cancer diagnosis. This resulted in a total of 40 840 women with endometrial cancer of whom 1701 (4.2%) had histologically proven endometriosis or adenomyosis and 39 139 (95.8%) had no histologically proven endometriosis or adenomyosis (Figure 1).
FIGURE 1.
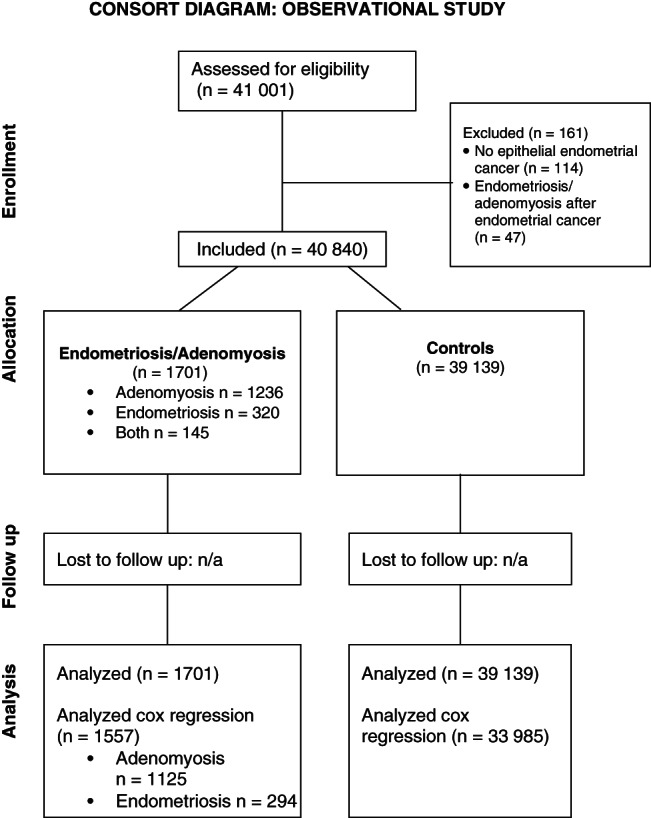
Consort diagram of included records
The endometriosis/adenomyosis group consisted of 1236 (72.7%) women with adenomyosis, 320 (18.8%) women with endometriosis and 145 (8.5%) with both histologically proven endometriosis and adenomyosis. Median age at endometriosis diagnosis was 57 years (IQR 51‐67) and 61 years (IQR 55‐69) at the time of adenomyosis diagnosis. Most endometriosis/adenomyosis diagnosis (93.7%) were concurrent with endometrial cancer diagnosis (endometriosis/adenomyosis diagnosis between a half year before or a year after endometrial cancer diagnosis). Of the 107 women with endometriosis/adenomyosis more than a year after endometrial cancer diagnosis 61 (57%) had adenomyosis, 35 (33%) had endometriosis and 11 (10%) had both endometriosis and adenomyosis.
Baseline characteristics for all included women with endometrial cancer are reported in Table 1. This table shows that women in the three endometriosis/adenomyosis cohorts were significantly younger at endometrial cancer diagnosis, more often had stage I endometrial cancer and more frequently had endometrioid endometrial cancer. Furthermore, the women in the endometriosis/adenomyosis cohorts more often had low‐grade endometrial cancer. In all cohorts between 40% and 44% of women had adenocarcinoma NOS. Exact details on these cancers are lacking but 63% in the endometriosis cohort, 57% in the adenomyosis cohort, 70% in the endometriosis and adenomyosis cohort and 52% in the control cohort of these cancers were low grade cancers (data not shown). Finally, the women in the endometriosis/adenomyosis cohort more often had surgery for their endometrial cancer and less often had chemotherapy. Women in the adenomyosis cohort more often had low disease stage when compared to the endometriosis cohort, more often had low grade tumors and less often had chemotherapy.
TABLE 1.
Baseline characteristics of all patients with endometrial cancer 1990 to 2015
| Endometriosis (n = 320) | Adenomyosis (n = 1236) | Endometriosis and adenomyosis (n = 145) | No endometriosis or adenomyosis (n = 39 139) | |
|---|---|---|---|---|
| Age at endometrial cancer diagnosis in years (IQR) a | 62 (IQR 52‐68) | 62 (IQR 55‐70) | 57 (IQR 52‐65) | 67 (IQR 59‐75) |
| Year of diagnosis (IQR) b | 2004 (IQR 1996‐2010) | 2003 (IQR 1996‐2009) | 2004 (IQR 1997‐2010) | 2004 (IQR 1997‐2010) |
| Endometrial cancer stage a | ||||
| Stage 1 | 258 (80.6%) | 1116 (90.3%) | 122 (84.1%) | 28 595 (73.1%) |
| Stage 2 | 27 (8.4%) | 47 (3.8%) | 10 (6.9%) | 3047 (7.8%) |
| Stage 3 | 22 (6.9%) | 45 (3.6%) | 10 (6.9%) | 3462 (8.8%) |
| Stage 4 | 9 (2.8%) | 11 (0.9%) | 2 (1.4%) | 2284 (5.8%) |
| Unknown | 4 (1.3%) | 17 (1.4%) | 1 (0.7%) | 1751 (4.5%) |
| Histological tumor type a | ||||
| Endometrioid | 175 (54.7%) | 643 (52.0%) | 80 (55.2%) | 19 763 (50.5%) |
| Clear cell | 4 (1.3%) | 14 (1.1%) | 1 (0.7%) | 778 (2.0%) |
| Serous | 7 (2.2%) | 23 (1.9%) | 0 (0.0%) | 1460 (3.7%) |
| Mucinous | 5 (1.6%) | 9 (0.7%) | 1 (0.7%) | 323 (0.8%) |
| Adenocarcinoma NOS | 129 (40.3%) | 547 (44.3%) | 63 (43.4%) | 16 815 (43.0%) |
| Histological grading a | ||||
| Low | 156 (48.8%) | 661 (53.5%) | 92 (63.4%) | 15 256 (39.0%) |
| Intermediate | 90 (28.1%) | 337 (27.3%) | 39 (26.9%) | 12 301 (31.4%) |
| High | 48 (15.0%) | 136 (11.0%) | 7 (4.8%) | 7123 (18.2%) |
| Unknown | 26 (8.1%) | 102 (8.3%) | 7 (4.8%) | 4459 (11.4%) |
| Surgery a | ||||
| Yes | 308 (96.2%) | 1228 (99.4%) | 144 (99.3%) | 35 949 (91.8%) |
| No | 12 (3.8%) | 8 (0.6%) | 1 (0.7%) | 3190 (8.2%) |
| Chemotherapy a | ||||
| Yes | 16 (5.0%) | 19 (1.5%) | 4 (2.8%) | 1641 (4.2%) |
| No | 304 (95.0%) | 1217 (98.5%) | 141 (97.2%) | 37 498 (95.8%) |
| Vital status as of 31 January 2020 a | ||||
| Alive | 190 (59.4%) | 719 (58.2%) | 102 (70.3%) | 17 637 (45.1%) |
| Passed away | 130 (40.6%) | 517 (41.8%) | 43 (29.7%) | 21 502 (54.9%) |
Note: Data are in numbers (percentage) unless otherwise specified.
Abbreviations: IQR, interquartile range; NOS, not otherwise specified.
P < .005 for comparison between patients with endometrial cancer with histological proven endometriosis and/or adenomyosis and those without.
P value not statistically significant for comparison between patients with endometrial cancer with histological proven endometriosis and/or adenomyosis and those without.
Endometrial cancer incidence increased gradually during the study period from 1188 endometrial cancer cases in 1990 to 1932 endometrial cancer cases in 2015 (data not shown). Median follow‐up from endometrial cancer diagnosis to death, emigration or end of study (31 January 2020) was 11 years (IQR 6‐18) in the endometriosis/adenomyosis cohort and 8 years (IQR 3‐14) in the control cohort. In total, 16 women emigrated in the endometriosis/adenomyosis cohort and 198 women in the control cohort.
3.2. Survival data
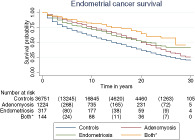
Figure 2 displays the Kaplan‐Meier curve of overall survival which shows that women with endometrial cancer in the endometriosis/adenomyosis cohorts had increased overall survival when compared to women with endometrial cancer without histological proven endometriosis/adenomyosis P < .005. Comparing the overall survival between the endometriosis/adenomyosis cohorts did not result in statistically significant differences. Median survival was 20 years (IQR 10‐Not reached) in the endometriosis/adenomyosis cohort and 13 years (IQR 4‐25) in the control cohort (P < .0005). Additionally, the 5‐year survival rate was 82.5% (95% CI 77.8‐86.2) in the endometriosis cohort; 87.2% (95% CI 85.2‐88.9) in the adenomyosis cohort; 89.6% (95% CI 83.4‐93.6) in the cohort with women with both endometriosis and adenomyosis; and 71.6% (95% CI 71.1‐72.0) in the control cohort. The 5‐year survival rate in the entire endometriosis/adenomyosis cohort combined was 84.8% (95% CI 84.6‐88.1). Figure S1 shows the Kaplan‐Meier curve of overall survival for all endometriosis/adenomyosis cases combined, compared to the control cohort. Additionally, Kaplan‐Meier curves stratified per histological subtype and early/late stage only resulted in significantly different survival for early and late‐stage endometrioid endometrial cancer and early and late‐stage adenocarcinoma NOS (Figure S2).
FIGURE 2.
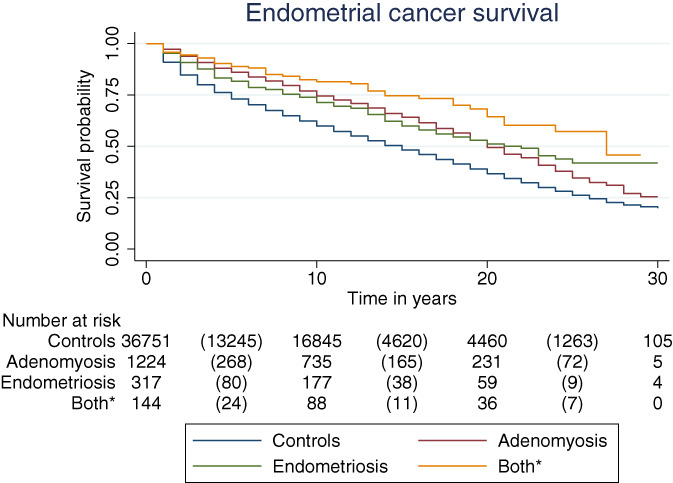
Kaplan‐Meier curve of overall survival, by endometriosis/adenomyosis status. *Both endometriosis and adenomyosis. Comparing all endometriosis/adenomyosis cohorts separately with the control cohorts resulted in a P‐value < .0005. Comparing the endometriosis/adenomyosis cohort did not result in P‐value smaller than .005 [Color figure can be viewed at wileyonlinelibrary.com]
Due to missing values, 5298 women were not included in the Cox proportional hazard analysis. This resulted in 35 542 women eligible for analysis. Baseline characteristics of these women are reported in Table S1. The crude HR for overall survival, when compared to the control cohort, was 0.68 (95% CI 0.57‐0.82) in the endometriosis cohort, 0.65 (95% CI 0.59‐0.71) in the adenomyosis cohort and 0.45 (95% CI 0.33‐0.61) in the endometriosis/adenomyosis cohort. After correction for confounders, none of the HRs for endometriosis/adenomyosis status were significant (Table 2). Included confounders were age at endometrial cancer diagnosis, stage, histological subtype, grading, surgery for endometrial cancer and chemotherapy. All included confounders had a significant effect in both univariable and multivariable analyses. We tested for interaction terms but none of the significant interaction terms significantly changed the analysis.
TABLE 2.
Hazard ratios of overall survival among women with endometrial cancer in univariable and multivariable analysis (n = 35 542)
| Hazard ratio (95% CI) | ||
|---|---|---|
| Univariable analysis | Multivariable analysis | |
| Endometriosis status | ||
| No endometriosis/adenomyosis | 1.00 (ref) | 1.00 (ref) |
| Endometriosis | 0.68 (0.57‐0.82) b | 1.01 (0.85‐1.21) c |
| Adenomyosis | 0.65 (0.59‐0.71) b | 0.98 (0.89‐1.08) c |
| Endometriosis and adenomyosis | 0.45 (0.33‐0.61) b | 0.90 (0.67‐1.22) c |
| Age | 1.09 (1.08‐1.09) b | 1.08 (1.08‐1.08) b |
| Endometrial cancer stage a | ||
| Stage 1 | 1.00 (ref) | 1.00 (ref) |
| Stage 2 | 1.64 (1.56‐1.72) b | 1.40 (1.33‐1.47) b |
| Stage 3 | 2.69 (2.57‐2.81) b | 2.31 (2.20‐2.42) b |
| Stage 4 | 7.38 (6.98‐7.80) b | 4.23 (3.95‐4.52) b |
| Histological tumor type a | ||
| Endometrioid | 1.00 (ref) | 1.00 (ref) |
| Clear cell | 2.12 (1.91‐2.37) b | 1.10 (0.98‐1.23) c |
| Serous | 3.13 (2.89‐3.38) b | 1.25 (1.14‐1.36) b |
| Mucinous | 1.28 (1.10‐1.48) b | 1.04 (0.90‐1.21) c |
| Adenocarcinoma NOS | 1.29 (1.25‐1.33) b | 1.20 (1.16‐1.24) b |
| Histological grading a | ||
| Low | 1.00 (ref) | 1.00 (ref) |
| Intermediate | 1.48 (1.43‐1.53) b | 1.21 (1.17‐1.25) b |
| High | 2.65 (2.56‐2.76) b | 1.71 (1.64‐1.78) b |
| Surgery | 0.14 (0.13‐0.15) b | 0.39 (0.36‐0.41) b |
| Chemotherapy | 2.56 (2.39‐2.75) b | 1.21 (1.12‐1.32) b |
Note: Only significant factors in univariable analysis are displayed.
Abbreviation: NOS, not otherwise specified.
P‐value < .0005 for categorical factor in univariable and multivariable analyses.
P‐value < .0005.
P‐value not statistically significant.
Including endometriosis/adenomyosis status as a binary factor resulted in a crude HR for overall survival of 0.63 (95% CI 0.59‐0.69) for women with endometrial cancer and endometriosis/adenomyosis vs women with only endometrial cancer (Table S2). After correction for confounders, the HR was 0.98 (95% CI 0.90‐1.06), P = .867.
4. DISCUSSION
4.1. Principal findings
Our study in a large nationwide cohort shows that women with histological proven endometriosis/adenomyosis and endometrial cancer have a longer overall survival when compared to women without endometriosis/adenomyosis. Women in the endometriosis/adenomyosis cohort were younger at endometrial cancer diagnosis and had more beneficial tumor characteristics and more often had surgical treatment. After correction for these confounders, no increased survival was found. This shows that women with endometriosis/adenomyosis and endometrial cancer have a better prognosis, but this is due to younger age at cancer diagnosis, earlier stage disease, low grade tumors with more beneficial histological subtype, more surgery for endometrial cancer and a lower chemotherapy rate. In our study, endometriosis/adenomyosis status itself was not an independent prognostic factor for endometrial cancer.
4.2. Results of the study in the context of other observations
A recent meta‐analysis found an increased overall survival with a crude HR of 0.51 (95% CI 0.38‐0.69) in 855 women with endometrial cancer and histologically proven adenomyosis, which was similar to our crude HR of 0.63 (95% CI 0.59‐0.69). 7 They concluded that women with endometrial cancer and adenomyosis had a decreased ratio of deep myometrial invasion, less often had LVSI, more often had low‐grade cancer and early cancer stage. In our study, no correction for confounding was performed, so it is unknown whether this would have resulted in a nonsignificant HRs due to the same pattern of more beneficial confounders in the adenomyosis cohort. Unfortunately, we do not have any specific information on LVSI or myometrial invasion and therefore could not correct for these confounders. However myometrial invasion is an important factor for determining stage. Furthermore, the study by Aslan et al showed similar overall survival between women with endometrial cancer with or without adenomyosis and did find similar factors associated with prolonged survival. 9 In our study, only women with endometrioid endometrial cancer were included, possibly resulting in a different outcome.
One small study assessed endometrial cancer prognosis in women with endometriosis and found that they were younger at endometrial cancer diagnosis and more often had endometrioid endometrial cancer subtype. 12 They found that women with adenomyosis had the best prognosis, but women with endometriosis also had longer overall survival when compared to controls without endometriosis/adenomyosis. These were all crude analyses and no correction for confounders was performed. Additionally, the study by Johnatty et al assessed the co‐existence of adenomyosis and endometriosis in patients with endometrial cancer and evaluated survival in these subgroups. 10 They observed more co‐existence of leiomyomas, adenomyosis and endometriosis than expected, but did not find any differences in survival for the group of women with adenomyosis or endometriosis after correcting for covariates like age, stage, grade or histological subtype. 10 In our study we also compared endometrial cancer survival between women with endometriosis, adenomyosis, endometriosis and adenomyosis combined and women without either. In crude analyses, this resulted in similar HRs for women with endometriosis or adenomyosis or a combination of both. However, in adjusted analyses, no significant HRs were found.
Exact mechanisms behind the more beneficial endometrial cancer characteristics in women with endometriosis/adenomyosis are unknown. A possible explanation could be that women with endometriosis/adenomyosis are more aware of abnormal symptoms and get more opportunities for earlier detection of endometrial cancer and therefore possibly have an earlier diagnosis with earlier cancer disease stage and are younger age at diagnosis. Additionally, endometriosis and adenomyosis are associated with changes in genes like ARID1A, PTEN, KRAS and PIK3CA. 13 , 14 These genes also have known cancer driving mutations involved in endometrial cancer carcinogenesis, especially endometrioid endometrial cancer. 15 This could explain the higher incidence of endometrioid endometrial cancer in women with endometriosis/adenomyosis. Endometrioid endometrial cancers generally have better overall survival when compared to other endometrial cancer subtypes. 16
4.3. Strengths and limitations
In this large nationwide study, we combined two national registries with over 95% national coverage. This is, to the best of our knowledge, the largest study assessing endometrial cancer prognosis in both endometriosis and adenomyosis. Additionally, we only included histological proven endometriosis/adenomyosis which remains considered the gold standard for endometriosis/adenomyosis diagnosis. Due to using anonymous retrospective databases, we could not collect clinical data, and therefore could not correct for possible other confounders like hormonal treatment, obesity, parity or previous surgical treatment. Moreover, we could not re‐examine tissue samples and therefore do not know whether unreported endometriosis/adenomyosis existed in the control cohort or whether women had an earlier diagnosis of endometriosis or adenomyosis. Furthermore, most endometriosis/adenomyosis diagnosis were synchronous with endometrial cancer diagnosis. It is not known if both diagnoses were known before surgery or if either was an incidental finding at the time of surgery. We also found a high age at endometriosis or adenomyosis diagnosis which might be due to using a histological diagnosis as inclusion but could also be due to selecting women with endometrial cancer, as endometrial cancer is disease more frequent in older women. Finally, a large proportion of patients in both cohorts had adenocarcinoma NOS. Unfortunately, we do not have detailed information on these cases and could not specify the histological subtype in this group. However, as reported 57% to 70% in the endometriosis/adenomyosis and 52% in the control cohort had low‐grade adenocarcinoma NOS. We therefore hypothesize that these cases were endometrioid endometrial cancer, as serous and clear cell endometrial cancer are mostly high grade tumors. 17
5. CONCLUSION
In conclusion, we found an increased survival in women with endometrial cancer and histological proven endometriosis or adenomyosis. The increased prognosis seems large due to a younger age at endometrial cancer diagnosis, an earlier disease stage, a more favorable histological subtype and more low grade tumors with higher surgery rate and lower chemotherapy rate in the group of women with endometrial cancer and endometriosis/adenomyosis. Future studies should study the exact mechanism for these more favorable tumor characteristics.
CONFLICT OF INTEREST
All other authors declare no competing interests.
ETHICS STATEMENT
Our study is exempt from medical ethical approval because there is no physical involvement of the women in the study and data were not traceable to individual participants. The study was approved by the scientific and ethical committee of PALGA (lzv2017‐104) and the ethical committee of the NCR (K20.032). Data were supplied anonymously via a trusted third‐party agreement.
Supporting information
Figure S1 Kaplan‐Meier curve of overall survival, by endometriosis/adenomyosis status (binary).
Figure S2 Kaplan‐Meier curve of overall survival, by endometriosis/adenomyosis status (binary) per low/high stage disease and histological subtype.
Table S1 Baseline characteristics for patients included in the Cox proportional hazard analysis.
Table S2 Hazard ratios of overall survival among women with endometrial cancer in univariable and multivariable analysis (n = 35 542), including endometriosis/adenomyosis status as binary variable.
ACKNOWLEDGEMENTS
The authors thank the team of the Netherlands Cancer Registry (NCR) and the team of the Dutch nationwide registry of histopathology and cytopathology (PALGA), for providing the study data from the Dutch Pathology Registry.
Hermens M, van Altena AM, van der Aa M, et al. Endometrial cancer prognosis in women with endometriosis and adenomyosis: A retrospective nationwide cohort study of 40 840 women. Int. J. Cancer. 2022;150(9):1439-1446. doi: 10.1002/ijc.33907
Funding information Stichting Catharina Onderzoeksfonds; Stichting Ruby & Rose
DATA AVAILABILITY STATEMENT
Data can be requested from the Netherlands Cancer Registry (NCR) by filling in their data application form on their website (https://iknl.nl/forms/dataapplication and/or the nationwide network and registry of histo‐ and cytopathology in the Netherlands (PALGA) by starting a request procedure in the PALGA Request System (https://aanvraag.palga.nl/). For questions about a NCR request, please contact gegevensaanvraag@iknl.nl. If you have any questions about a PALGA Request System, please contact aanvraag@palga.nl. Further information is available from the corresponding author upon request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491‐505. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Dos Santos Silva I, Moller H, Weiderpass E. Endometrial cancer incidence trends in Europe: underlying determinants and prospects for prevention. Cancer Epidemiol Biomarkers Prev. 2005;14:1132‐1142. [DOI] [PubMed] [Google Scholar]
- 4. Kvaskoff M, Mahamat‐Saleh Y, Farland LV, et al. Endometriosis and cancer: a systematic review and meta‐analysis. Hum Reprod Update. 2021;27:393‐420. [DOI] [PubMed] [Google Scholar]
- 5. Hermens M, van Altena AM, Velthuis I, et al. Endometrial cancer incidence in endometriosis and adenomyosis. Cancers (Basel). 2021;13:4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amant F, Mirza MR, Koskas M, Creutzberg CL. Cancer of the corpus uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):37‐50. [DOI] [PubMed] [Google Scholar]
- 7. An M, Duan H, Zhang Y. Prognostic significance of co‐existent adenomyosis on outcomes and tumor characteristics of endometrial cancer: a meta‐analysis. J Obstet Gynaecol Res. 2020;46:1851‐1863. [DOI] [PubMed] [Google Scholar]
- 8. Mogensen JB, Kjær SK, Mellemkjær L, Jensen A. Endometriosis and risks for ovarian, endometrial and breast cancers: a nationwide cohort study. Gynecol Oncol. 2016;143:87‐92. [DOI] [PubMed] [Google Scholar]
- 9. Aslan K, Sarı ME, Yalçın HR, Yalçın İ, Cüylan ZF, Özdal B. Coexistence of uterine adenomyosis is not associated with a better prognosis in endometrioid‐type endometrial cancer. Ir J Med Sci. 2020;189:835‐842. [DOI] [PubMed] [Google Scholar]
- 10. Johnatty SE, Stewart CJR, Smith D, et al. Co‐existence of leiomyomas, adenomyosis and endometriosis in women with endometrial cancer. Sci Rep. 2020;10:3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400‐412. [DOI] [PubMed] [Google Scholar]
- 12. Koshiyama M, Okamoto T, Ueta M. The relationship between endometrial carcinoma and coexistent adenomyosis uteri, endometriosis externa and myoma uteri. Cancer Detect Prev. 2004;28:94‐98. [DOI] [PubMed] [Google Scholar]
- 13. Hever A, Roth RB, Hevezi PA, et al. Molecular characterization of human adenomyosis. Mol Hum Reprod. 2006;12:737‐748. [DOI] [PubMed] [Google Scholar]
- 14. Kyo S, Sato S, Nakayama K. Cancer‐associated mutations in normal human endometrium: surprise or expected? Cancer Sci. 2020;111:3458‐3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toumpeki C, Liberis A, Tsirkas I, et al. The role of ARID1A in endometrial cancer and the molecular pathways associated with pathogenesis and cancer progression. In Vivo. 2019;33:659‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morice P, Leary A, Creutzberg C, Abu‐Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094‐1108. [DOI] [PubMed] [Google Scholar]
- 17. Clement PB, Young RH. Non‐endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2004;11:117‐142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Kaplan‐Meier curve of overall survival, by endometriosis/adenomyosis status (binary).
Figure S2 Kaplan‐Meier curve of overall survival, by endometriosis/adenomyosis status (binary) per low/high stage disease and histological subtype.
Table S1 Baseline characteristics for patients included in the Cox proportional hazard analysis.
Table S2 Hazard ratios of overall survival among women with endometrial cancer in univariable and multivariable analysis (n = 35 542), including endometriosis/adenomyosis status as binary variable.
Data Availability Statement
Data can be requested from the Netherlands Cancer Registry (NCR) by filling in their data application form on their website (https://iknl.nl/forms/dataapplication and/or the nationwide network and registry of histo‐ and cytopathology in the Netherlands (PALGA) by starting a request procedure in the PALGA Request System (https://aanvraag.palga.nl/). For questions about a NCR request, please contact gegevensaanvraag@iknl.nl. If you have any questions about a PALGA Request System, please contact aanvraag@palga.nl. Further information is available from the corresponding author upon request.


