Abstract
Major depressive disorder is a leading cause of disability worldwide. Because conventional therapies are ineffective in many patients, novel strategies are needed to overcome treatment‐resistant depression (TRD). Limiting factors of successful drug development in the last decades were the lack of (1) knowledge of pathophysiology, (2) translational animal models and (3) objective diagnostic biomarkers. Here, we review novel drug targets and drug candidates currently investigated in Phase I–III clinical trials. The most promising approaches are inhibition of glutamatergic neurotransmission by NMDA and mGlu5 receptor antagonists, modulation of the opioidergic system by κ receptor antagonists, and hallucinogenic tryptamine derivates. The only registered drug for TRD is the NMDA receptor antagonist, S‐ketamine, but add‐on therapies with second‐generation antipsychotics, certain nutritive, anti‐inflammatory and neuroprotective agents seem to be effective. Currently, there is an intense research focus on large‐scale, high‐throughput omics and neuroimaging studies. These results might provide new insights into molecular mechanisms and potential novel therapeutic strategies.
Keywords: antidepressant, glutamate, monoamine, neuroimaging, neuroinflammation, neuroplasticity, opioid

Abbreviations
- BDNF
brain‐derived neurotrophic factor
- HAM‐D
Hamilton Depression Rating Scale
- HDAC
histone deacetylase
- MDD
Major depressive disorder
- SNRI
serotonin‐noradrenaline reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
- TRD
Treatment‐resistant depression
1. TREATMENT‐RESISTANT DEPRESSION IS AN UNMET MEDICAL NEED
Major depressive disorder (MDD) is one of the most common and debilitating public health problems with a cross‐national lifetime risk of 15%–18% (Bromet et al., 2011) and accounts for 4.4% of the disease burden worldwide (Murray et al., 2012). MDD is often recurrent; 50%–80% of patients have at least two episodes, typically with increasing severity and frequency. The crucial STAR*D study revealed that one third of MDD patients had not achieved remission after four consecutive antidepressant trials and developed a chronic treatment course (Rush et al., 2006). Whereas the symptoms of MDD can be severe and, at its worst, depression is a life‐threatening condition, pharmacological treatment resistance to antidepressant therapy is one of the most challenging situations in the clinical management of affective disorders. Treatment‐resistant depression (TRD) was traditionally defined as an MDD episode with failed or suboptimal response to an adequate course of antidepressant treatment; however, there has been a multitude of approaches yet.
According to the most recent and widely accepted definition, TRD is characterized by an inadequate response to at least two trials of antidepressant treatment at adequate dose and duration in monotherapy (Bartova et al., 2019; Brown et al., 2019; European Medicines Agency [EMA], 2013; Reutfors et al., 2018; Rush et al., 2006; Souery et al., 2007). Nevertheless, inconsistencies exist in the number of ineffective antidepressant trials and the way of choosing them (i.e., whether medications should be taken from the same or various pharmacological classes), across the TRD definitions (Cepeda et al., 2018; Dold & Kasper, 2017; Kautzky et al., 2019; Malhi & Mann, 2018; Reutfors et al., 2018, 2019; Souery & Pitchot, 2013). To classify the severity of treatment resistance in MDD, various clinical ‘staging’ models of TRD have been introduced. The initial model of TRD comprised a spectrum of treatment resistance ranging from one ineffective antidepressant to resistance to ECT (Fava, 2003; Thase & Rush, 1995). More recent staging models include the assessment of the number and type of unsuccessful antidepressant and augmentation pharmacotherapies, as well as the lack of response to other biological therapies (e.g., ECT or transcranial magnetic stimulation) (Voineskos et al., 2020). A new term, ‘difficult‐to‐treat depression’, has recently been proposed to destigmatize affected patients and to avoid ‘therapeutic nihilism’ (McAllister‐Williams et al., 2020); however, for uniformity and meaningful understanding, we use the term TRD in our review.
Most recently, the TRD‐III study found that symptom severity, presence of psychotic symptoms, suicidal risk, comorbid anxiety, inpatient status, the higher number of antidepressants administered previously and lifetime depressive episodes, as well as longer duration of the current episode were the strongest clinical predictors of treatment resistance in a large multinational sample (Kautzky et al., 2019). MDD has a sexually dimorphic character: Females show a twofold greater risk for MDD with a higher incidence during puberty, peripartum periods and menopause and differ in clinical presentation and course (Kornstein & Schneider, 2001). Despite the consistent data on sexual dimorphism of MDD, there is little evidence of sex differences in the rate of TRD compared with that of MDD. However, females respond better to serotonergic antidepressants than males, while postmenopausal females show a reduced antidepressant response to serotonergic agents compared with younger women (e.g., LeGates et al., 2019; Sramek et al., 2016). The reasons for this have not been fully clarified yet, but sex differences in pharmacokinetics and pharmacodynamics, neuronal circuitry, the effect of female reproductive hormone levels, pregnancy and menopause may all play a role (LeGates et al., 2019; Sramek et al., 2016). In addition, late‐life depression and depression with early onset have been frequently proved to be treatment resistant. Late‐life depression is often complicated by factors that can increase the risk of TRD, such as psychotic features and comorbid medical conditions (Kornstein & Schneider, 2001); and it can be difficult to separate it from incipient dementia. Moreover, onset at an early age is also associated with treatment resistance, as it is often coupled with personality disorders, substance use and family history of MDD (Kornstein & Schneider, 2001).
TRD has a negative impact on disease outcomes compared with MDD with adequate treatment response. TRD patients exhibit a higher rate of comorbid psychiatric and general medical conditions, and self‐harming behaviour not resulting in death compared with non‐TRD patients (Döme et al., 2021; Parker & Graham, 2015). Also, TRD patients have a significantly higher rate of mortality due to external causes, that is, suicide and accidents (Döme et al., 2021; Reutfors et al., 2018), and after acute coronary syndrome (Carney & Freedland, 2009; Scherrer et al., 2012).
A most recent European cohort study of patients with TRD with moderate to severe depression found that 69.2% of TRD patients had no treatment response within one year, and despite the low remission rate, 60% of them remained on unchanged treatment for a longer period (Heerlein et al., 2021). Thus, there is an urgent need to develop effective treatment strategies and an adequate medical approach to TRD. There are intensive ongoing efforts to find novel investigational drugs for further development and drug repurposing, to identify effective treatment strategies (Garay et al., 2017). However, the heterogeneity of MDD as well as the lack of biomarkers to create more homogenous clinical groups make it very difficult to find optimal treatment approaches in TRD.
2. CURRENT THERAPEUTIC STRATEGIES FOR TRD
According to the most recent guidelines, therapeutic strategies to improve inadequate antidepressant response in TRD patients usually start with the exclusion of pseudo‐resistance; that is, they optimize the dosage and duration of the antidepressant treatment—after ensuring TRD patients' drug compliance. Concerning combination therapies, combining monoamine reuptake inhibitors with presynaptic α2‐adrenoceptor antagonists can be regarded as having better efficacy in TRD. Presently, augmentation strategies are considered the most evidence‐based options for the management of TRD. Augmentation with second‐generation antipsychotics and lithium seem to be treatment options with the strongest augmentative evidence in TRD. Some evidence supports the efficacy and tolerability of thyroid hormone therapy in TRD. However, the augmentative potential of anticonvulsive drugs, nutraceuticals and glutamatergic as well as anti‐inflammatory agents is not fully established and needs further research. For other somatic therapies, stimulation therapies, particularly ECT and repetitive transcranial magnetic stimulation, have been found to be effective in TRD (most recently reviewed by Voineskos et al., 2020, and Goh et al., 2020).
Among the novel therapeutic agents, intravenous ketamine and intranasal S‐ketamine are most extensively investigated. Most recently, intranasal S‐ketamine was approved by the US Food and Drug Administration (FDA) and the EMA and has been introduced as a novel augmentation therapy with a restricted indication in TRD.
3. S‐KETAMINE IS A NOVEL ADD‐ON TREATMENT SPECIFICALLY APPROVED FOR TRD
Ketamine is an arylcyclohexylamine derivative, an old drug used for anaesthetic purposes for decades. The first data demonstrating the antidepressant effect of racemic ketamine were published by Berman et al. in 2000. Since then, several papers have described its efficacy in depressed patients including TRD. Its pharmacokinetic properties are well known; it has a rapid distribution mainly in the highly vascularized tissues, high volume of distribution, short half‐life time and renal elimination (Iqbal & Mathew, 2020). It is metabolized by the cytochrome P450 (CYP) system in the liver, which can lead to drug interactions. The obvious disadvantage of ketamine use is the widespread side effect profile. Besides cardiopulmonary, urinary tract and gastrointestinal symptoms, the most problematic adverse effects occur in the nervous system: dissociative symptoms, memory deterioration, hyperreflexia, clonus and vestibular complaints (Li & Vlisides, 2016).
Ketamine has a rapid onset followed by sustained effects mediated by a complex, multi‐target mechanism of action. It is basically considered to be a non‐competitive NMDA glutamate receptor antagonist, preferentially at the GluN2B subtype of NMDA receptors (Alexander, Christopoulos, et al., 2021) located on GABAergic interneurons, particularly in low, subanaesthetic doses. This results in disinhibition and a consequently increased glutamate and brain‐derived neurotrophic factor (BDNF) release, as well as up‐regulation of AMPA receptors (Alexander, Christopoulos, et al., 2021). These processes activate the mammalian target of rapamycin (mTOR) and BDNF in the hippocampus and prefrontal cortex. Other proposed mechanisms are the modulation of the intracellular signalling pathways: calcium/calmodulin‐dependent protein kinase II, the eukaryotic elongation factor 2 kinase pathway and the inhibition of brain glycogen synthase kinase‐3 (GSK3). Besides inhibiting the glutamatergic system, ketamine has been shown to inhibit adrenergic, dopaminergic and cholinergic receptors (Sleigh et al., 2014) as well as the 5‐HT2 serotonin receptor, which may be involved in the mechanism of action of psilocybin (Voineskos et al., 2020). Ketamine, similarly to other rapidly acting antidepressants, is suggested to stimulate synaptic plasticity via increasing BDNF production. The reactivated cortical plasticity leads to the readjustment of neuronal networks to better adapt to the environmental challenges. The pronounced recovery of synaptogenesis might mediate many of the prolonged effects (Li et al., 2010; Pryazhnikov et al., 2018), which is also supported by the fact that long‐lasting beneficial actions occur hours after ketamine is eliminated from the body (Fagerholm et al., 2021; for review, see Czéh & Simon, 2021). Recent data demonstrated that BDNF‐dependent methyl‐CpG‐binding protein 2 (MeCP2) phosphorylation seems to be essential in synaptogenesis and consequent sustained antidepressant actions of ketamine, similarly to scopolamine (Johnson & Liston, 2021; Kim et al., 2021) and imipramine (Hutchinson et al., 2012). It has been shown that ketamine treatment also results in a specific gene expression profile in the mouse brain (Bagot et al., 2017).
The routes of administration are diverse, such as intravenous (i.v.), intramuscular, intranasal, oral, rectal and even subcutaneous and epidural routes are available. Intranasal administration of the L‐stereoisomer of ketamine (S‐ketamine) has very recently been approved for TRD and MDD with suicidal ideations in both the United States and Europe. A meta‐analysis of eight randomized double‐blinded placebo‐controlled trials including 1488 patients provided evidence that intranasal S‐ketamine showed rapid antidepressant effects in these conditions already at 2 h and lasting for 28 days. However, the total adverse events including dissociation, blood pressure increment, nausea, vertigo, dysgeusia, dizziness and somnolence were more frequent in the S‐ketamine than in placebo group (Wang et al., 2021).
The majority of the clinical trials have been conducted with i.v. infusions of subanaesthetic doses of the racemic mixture (specific details are provided below). Despite not being officially registered for TRD, this is off‐label used in such patients.
4. NOVEL INVESTIGATIONAL DRUGS AND REGIMENS UNDER CLINICAL TRIALS
Clinical trials presented here were collected from www.clinicaltrials.gov and www.clinicaltrialsregister.eu, the official register of the US National Institutes of Health (NIH) and EMA, respectively. All Phase I–III pharmacological studies on TRD available till the end of 2020 are reviewed. The search term used was ‘treatment resistant depression’; all Phase I–III studies were filtered between the period 2010 and 2020. Clinical trials were identified by their database identification number, grouped by clinical phase in which they had been investigated, although the classification is not an easy task because there were several parallel studies with the same compound (Tables 1 and 2, Figures 1 and 2).
TABLE 1.
Summary of targets and drug candidates in Phase II studies
| Target | Drug candidate (mechanism of action) | Title, NCT/EudraCT number |
Study design, endpoints (1. = primary outcome, 2. = secondary outcome measures) |
Study arms N, age (years) | Current status (outcome, results) |
|---|---|---|---|---|---|
| NMDA receptor | Ketamine (antagonist) |
Ketamine in treatment resistant major depression (TRD) 2010‐023414‐31 |
Randomized, double‐blind, placebo‐controlled study 1. HAM‐D score (24 h) 2. Glutamine/glutamate ratio measured with MR spectroscopy (after 60 min and 24 h) |
Single arm: Single 50 mg·ml−1 ketamine i.v. infusion N = 80, age: 18–64 |
Completed (2012–2016): Change in glutamine/glutamate ratio is significantly larger 24 h after ketamine infusion compared with placebo (Li et al., 2016) |
|
Ketamine for depression and suicide risk |
Open‐label, single‐group assignment study 1. MADRS score (2 weeks) 2. MADRS percentage, several factor scores, CGI‐S |
Single arm: 0.3 mg·kg−1·h−1 infusion of ketamine for 100 min N = 12, age: 18–65 |
Completed (2014–2017): Response rate: 58%, remission rate: 42%, 4‐week‐long continuation‐phase administration of ketamine at weekly intervals extends remission duration for further 4 weeks (Vande Voort et al., 2016) | ||
|
A study of ketamine in patients with treatment‐resistant depression |
Randomized, double‐blind, placebo‐controlled, dose‐frequency, parallel assignment 1. Change in MADRS score (Day 15) 2. MADRS (Day 29), rate of responders, remitters and sustained responders; CGI‐S, ‐I, PGI‐S, ‐C, pharmacokinetics |
Single arm: 0.5 mg·kg−1 i.v. ketamine over 40 min 2–3 times a week N = 68, age: 18–64 |
Completed (2012–2020): Ketamine effectively induces rapid onset of antidepressant effects and induces improvement through 15 days (Johnson et al., 2016; Lewis et al., 2019) | ||
|
Ketamine in adolescents with treatment‐resistant depression |
Open‐label, single‐group assignment study 1. CGI (Week 2) 2. Depression scales, clinician‐administered dissociative scale, heart rate, BP, pulse oximetry |
Single arm: Infusions of 0.5 mg·kg−1 ketamine hydrochloride over 40 min (6 times for 2 weeks) N = 14, age: 12–18 |
Completed (2014–2020): Depression reduction correlated with increased nucleus accumbens entropy and insulin/mTOR/GSK3β signalling (Roy et al., 2020) | ||
|
Intravenous ketamine for treatment resistant depression: Exploring biomarkers of response and relapse A double‐blind, randomized controlled trial 2016‐001715‐21 |
Randomized, double‐blind, placebo‐controlled, parallel assignment 1. Relief of depressive symptoms (3 weeks) 2. Sampling method, biomarkers for response/relapse, psychophysiology of stress |
2 arms: Infusion of 0.5 mg·kg−1 ketamine over 40 min N = 54, age: 18–84 |
Completed (2016–2019): Results are not published | ||
|
Low dose intravenous ketamine in treatment‐resistant depression patients |
Open‐label, single‐group assignment 1. Cortical excitability in the dmPFC (TMS‐EEG; 4 h, 24 h, 7 days) 2. MADRS (4 h, 24 h, 7 days), adverse events |
Single arm: Slow ketamine hydrochloride infusion for 40 min N = 9, age: 18–60 |
Completed (2016–2020): Results are not published | ||
|
Efficacy of repeated ketamine infusions for treatment‐resistant depression |
Randomized, triple‐blind, parallel assignment, active comparator (midazolam) in combination 1. MADRS score (12 days) 2. Antidepressant response, remission rate, remission, post‐infusion relapse time |
2 arms: 6 ketamine (0.5 mg·kg−1) infusions over 2 weeks and the same ketamine treatment preceded by midazolam N = 62, age: 18–75 |
Completed (2015–2019): Results are not published | ||
|
A preliminary study of intravenous ketamine in selective serotonin reuptake inhibitor (SSRI)‐resistant depression 2011‐003654‐40 |
Open‐label, single‐group assignment study 1. Relief of depressive symptoms (5 weeks) 2. Biomarkers (cytokines, tryptophan metabolism markers), endocrine parameters |
Single‐arm: i.v. ketamine infusion weekly over 3 weeks N = 20, age: 18–84 |
Active (2011–) | ||
|
Biomarkers of response to ketamine in depression: MRI and blood assays before and after open‐label intranasal ketamine |
Open‐label, single‐group assignment study 1. Change in MADRS score, cortical thickness 2. Neural activity response to affective stimuli, white matter anisotropy, cognitive control network, functional brain connectivity |
Single‐arm: Intranasal 40‐mg ketamine hydrochloride (intranasal atomization device) N = 8, age: 18–65 |
Recruiting (2019–) | ||
|
Ketamine for treatment‐resistant depression: A multicentre clinical trial in Mexican population |
Randomized, triple‐blind, placebo‐controlled, parallel assignment 1. Change in HAM‐D scores (40 min, 1–7 days) 2. BP (every 5 min for 5 h) |
2 arms: 6 ketamine (0.5 mg·kg−1) infusion over 40 min and placebo (saline) N = 60, age: 18–65 |
Recruitment status unknown (2013–2016) | ||
| Dextromethorphan (antagonist) (+ quinidine) |
Nuedexta in treatment‐resistant major depression |
Open‐label, single‐group assignment study 1. Change in MADRS score 2. Enjoyment and satisfaction, functional impairment, illness‐related disability, adverse events, suicidality, overall treatment response, cognitive and executive dysfunction, anxiety |
Single arm: Up to 45/10‐mg test compound twice a day (7 days) + 45/10 mg once daily (7 days) N = 20, age: 18–65 |
Completed (2013–2018): Significant reduction in MADRS score and acceptable tolerability (Murrough et al., 2017) | |
|
AVP‐786/d6‐dextromethorphan hydrobromide (antagonist) (+ quinidine) |
Efficacy, safety and tolerability study of AVP‐786 as an adjunctive therapy in patients with major depressive disorder with an inadequate response to antidepressant treatment |
Randomized, placebo‐controlled, quadruple‐blind study 1. Change in MADRS score 2. Antidepressant treatment response, disability, illness severity, anxiety |
2 arms: Test compound and placebo (10 weeks) N = 206, age: >18 |
Completed (2014–2016): Results are not published | |
| Nitrous oxide |
Inhaled nitrous oxide for treatment‐resistant depression: Optimizing dosing strategies (NARSAD) |
Randomized, placebo‐controlled, double‐blind study 1. Change in HAM‐D score |
3 arms: 25%, 50% test compound or placebo inhalation (4 weeks) N = 34, age: 18–75 |
Completed (2016–2020): Results are not published | |
|
Cerebrovascular reaction to nitrous oxide in Resistant Depression: Pilot study (PROTO‐BRAIN) 2019‐002769‐37 |
Open‐label study 1. Cerebral pulsatility indices as measured by TPI ultrasonic imaging (2 months) 2. Change in MARDS, SSI, YMRS, CADSS, BPRS scores (6 months) |
Single arm: N2O diffusion N = 30, age: 25–50 (female) |
Active (2019–) | ||
|
Nitrous oxide for major depressive disorder |
Randomized, placebo‐controlled, double‐blind, study 1. Change in HAMD, QIDS‐SR, VAS‐D 2. EEG, VBM and functional connectivity of the brain, blood cytokines, feces bacterial flora, executive and cognitive performance, anhedonia, anxiety |
2 arms: Test compound and placebo (2 weeks) N = 44, age: 18–60 |
Recruiting (2019–; estimated completion 2020) | ||
| EVT 101 (antagonist) |
Safety and efficacy of EVT 101 in treatment‐resistant depression |
Randomized, placebo‐controlled, double‐blind 1. Safety and tolerability profile 2. MADRS, percentage of responders, remission rate |
2 arms: Test compound and placebo (4 weeks) N = 8, age: 18–55 |
Terminated (clinical hold issued by the FDA, 2010–2016) | |
| AZD6765/lanicemine (antagonist) |
AZD6765 for treatment‐resistant depression |
Randomized, multicentre, placebo‐controlled, triple‐blind, parallel assignment 1. MADRS total score change 2. Safety and tolerability |
2 arms: Test compound and placebo (3 weeks) N = 34, age: 21–65 |
Completed (2007–2011): Two‐compartment model with zero‐order input and first‐order elimination, significant efficacy without clinically appreciable dissociative and psychotomimetic adverse effects (Agbo et al., 2017; Sanacora et al., 2014) | |
|
An investigation of the antidepressant effects of an NMDA antagonist in treatment‐resistant major depression |
Randomized, placebo‐controlled, quadruple‐blind, crossover assignment 1. MADRS score change 2. MADRS remission, treatment response, suicidal ideation, anxiety, dissociative experiences, severity of depression, positive symptoms of schizophrenia |
2 arms: Single infusion of 150‐mg test compound and placebo (7 days) N = 22, age: 18–60 |
Completed (2009–2011): Onset of antidepressant effects: 110 min; duration of antidepressant effects: 2 days |
||
| MIJ821 (negative allosteric modulator) |
Proof of concept study evaluating the efficacy and safety of MIJ821 in patients with treatment‐resistant depression |
Randomized, placebo‐controlled, double‐blind study 1. Change in MADRS score 2. YMRS, efficacy in the melancholic subtype, safety and tolerability, most effective dose and dosing regimen, pharmacokinetics in plasma, impact on suicidality, efficacy on measures of response/remission, mixed mood symptoms, anxiety |
6 arms: Low and high doses of test compound, placebo and ketamine (6 + 1 weeks) N = 72, age: 18–65 |
Completed (2018–2020): Results are not published | |
|
A multi‐center, randomized, subject and investigator‐blinded, placebo‐controlled, active comparator, parallel‐group proof of concept study to evaluate the efficacy, safety, tolerability and pharmacokinetics of MIJ821 in patients with treatment‐resistant depression 2018‐003002‐12 |
Randomized, double‐blinded, placebo‐controlled study 1. MADRS 2. YMRS, CADSS, Bech‐Rafaelsen Melancholia Scale, Dissociative Experiences Scale |
6 arms: 0.16 and 0.32 mg·kg−1 MIJ821 weekly and biweekly, ketamine and placebo weekly (36 days) N = 70, age: 18–84 |
Completed (2019–2020): Results are not published | ||
| Riluzole (antagonist) |
Efficacy and tolerability of riluzole in treatment‐resistant depression |
Randomized, placebo‐controlled, triple‐blind study 1. Change in MADRS 2. Responders having at least a 50% improvement in MADRS, Assessment for Treatment Emergent Events scale |
3 arms: 100‐mg test compound and placebo added to ongoing SSRI or SNRI antidepressant (8 weeks); 100‐mg test compound added to ongoing SSRI or SNRI antidepressant (4 weeks), and placebo (4 weeks) N = 104, age: 18–65 |
Completed (2010–2018): Results are not published | |
| REL‐1017/dextromethadone (antagonist) |
Safety, tolerability, PK profile and symptom response of a 7‐day dosing with 25 mg daily and 50 mg daily of REL‐1017 in major depressive disorder |
Randomized, placebo‐controlled, double‐blind study 1. Treatment‐emergent adverse events 2. ECG and laboratory parameters, MADRS score, pharmacokinetic parameters |
3 arms: 25‐, 50‐mg test compound and placebo (7 days) N = 62, age: 18–65 |
Completed (2017–2019): Results are not published | |
| GLYX‐13 (partial agonist) |
Single IV dose of GLYX‐13 in patients with treatment‐resistant depression |
Randomized, placebo‐controlled, quadruple‐blind study 1. Change in depression scores 2. Change in BPRS+ scores |
4 arms: 1, 5, 10 mg·kg−1 single i.v. dose test compound and placebo (14 days) N = 115, age: 18–60 |
Completed (2011–2012): U‐shaped dose–response curve, 5 and 10 mg·kg−1 of test compound induced dose‐dependent reductions in depression score from Day 1 until Day 7, no serious adverse events (Preskorn et al., 2015) | |
| d‐Cycloserine (partial agonist) (broad‐spectrum antibiotic) |
d‐Cycloserine for major depressive disorder |
Randomized, placebo‐controlled, triple‐blind study 1. Change in HAM‐D scores, safety measures, change in anxiety scores |
2 arms: 1 g·day−1 test compound and placebo (6 weeks) N = 26, age: 18–75 |
Completed (2007–2010): Results are not published | |
| mGlu 5 receptor | RO4917523/basimglurant (negative allosteric modulator) |
A study of RO4917523 versus placebo as adjunctive therapy in patients with major depressive disorder and an inadequate response to ongoing antidepressant therapy |
Randomized, double‐blind, placebo‐controlled, parallel assignment study 1. MADRS 2. CGI‐S, CGI‐I (6 weeks), adverse effects, remission rate, MADRS score (2 years) |
2 arms: Oral 0.5–1.5 mg of test compound or placebo once daily added to ongoing antidepressant therapy (6 weeks) N = 333, age: 18–70 |
Completed (2011–2013): Adjunctive 1.5‐mg basimglurant modified‐release had a significant antidepressant effect at Day 42 (Quiroz et al., 2016) |
|
A study of RO4917523 in patients with treatment‐resistant depression |
Randomized, placebo‐controlled, double‐blind study 1. Safety and tolerability 2. MADRS score, TRD symptoms |
2 arms: Up to 5 different doses of test compound and placebo (10 days) N = 46, age: 18–65 |
Completed (2009–2011): Results are not published | ||
| mGlu 2 /mGlu 3 receptors | RO4995819/decoglurant (negative allosteric modulator) |
Efficacy and safety of RO4995819 versus placebo, as adjunctive therapy in patients with major depressive disorder having inadequate response to ongoing antidepressant treatment 2011‐002160‐24 |
Randomized, placebo‐controlled, double‐blind, parallel assignment study 1. MADRS 2. Safety, pharmacokinetics, pharmacodynamics, efficacy |
4 arms: Oral 5‐, 15‐, 30‐mg RO4995819 and placebo (6 weeks) N = 353, age: 18–64 |
Completed (2012–2014): Results are not published |
| AMPA receptor | TAK‐653 (positive allosteric modulator) |
Evaluation of the efficacy and safety of TAK‐653 in the treatment of subjects with TRD 2017‐002232‐16 |
Randomized, double‐blind, placebo‐controlled study 1. MADRS (57 days) 2. CGI‐S, QIDS‐SR16, ECG, adverse effects |
2 arms: TAK‐653 and placebo N = 0, age: >18 |
Terminated (no participants enrolled, 2017–2018) |
|
Efficacy and safety in TRD (ketamine non‐responders) |
Randomized, quadruple‐blind, parallel assignment, placebo‐controlled, sequential study 1. MADRS scores (up to 57 days) |
4 arms, 2 cohorts N = 0, age: 18–65 |
Withdrawn (business decision; 2017–2018) | ||
| GABA A receptor | Propofol (positive modulator) |
Neural and antidepressant effects of propofol |
Open‐label single group study (Phase 2/3) 1. HAM‐D score (3 weeks) |
Single arm N = 0, age: 18–55 |
Withdrawn (change in study design based on Phase 1 data) |
|
Neural and antidepressant effects of propofol |
Randomized, quadruple‐blind crossover design study (Phase 2/3) 1. HAM‐D scores (17 days) 2. HAM‐D scores (36 days) |
2 arms: (low and high dose) propofol i.v.; 6–12 series) N = 24, age: 18–55 |
Recruiting (2018–; proposed completion 2024) | ||
| Opioid receptors | Buprenorphine (partial μ receptor agonist + κ opioid receptor antagonist) |
Buprenorphine for Late‐Life TRD (BUILD) safety and clinical effect of low‐dose buprenorphine |
Open‐label study Efficacy outcomes : Clinical change in depression, anxiety, sleep, positive and negative effects and quality of life. Tolerability outcomes : Change in vital signs, weight, cognitive functions and side effects |
Single arm (pretreatment and post‐treatment self‐control): Buprenorphine dose from 0.2 to 1.6 mg·day−1 N = 15, age: >50 |
Completed (2010–2012): Decline in depression severity during the first 3 weeks, pessimism and sadness, executive function and learning improved. Side effects (nausea, constipation) lasted 1–2 weeks (Karp et al., 2014) |
| Tramadol (μ, κ, δ receptor agonist) |
Antidepressant activity of Viotra™ compared with amitriptyline in the treatment of major depressive disorder (MDD) in patients who have an unsatisfactory response/are resistant to SSRIs 2013‐000719‐26 |
Randomized, double‐blind, non‐inferiority study 1. Antidepressant activity 2. Safety and tolerability |
3 arms: Low and high dose of ETS6103 and amitriptyline (8 weeks) N = 367, age: 18–84 |
Completed (2013–2015): Results are not published | |
| CERC‐501 (κ receptor antagonist) |
Proof‐of‐concept trial of CERC‐501 augmentation of antidepressant therapy in TRD (RAPID KOR) |
Double‐blind, placebo‐controlled, proof‐of‐concept study with parallel sequential design 1. HAM‐D6 score change (72 h) 2. HAM‐D6 (20 days), MADRS, CGI‐S, CGI‐I, self‐rated depression, stress, positive affect and social health; suicidal ideation and behaviour (72, 20 days), ECG, laboratory parameters |
5 arms: Low and high doses N = 8, age: >18 |
Terminated (slow enrolment; 2013–2016) | |
| Monoaminergic system | Psilocybin (5‐HT1A, 5‐HT2A and 5‐HT2C activator) |
Assessing the subjective intensity of oral psilocybin in patients with TRD: A pilot study 2013‐003196‐35 |
Open‐label study 1. Efficacy (QIDS) (1 week to 3 months) 2. Changes in brain activity (fMRI) |
Single arm: Oral 10‐ and 25‐mg psilocybin (7 days apart, all patient) N = 20, age: 18–64 |
Completed (2015–2016): Psilocybin reduces depressive symptoms and improves anxiety and anhedonia (Carhart‐Harris et al., 2016, 2018) |
|
A phase II randomized, double‐blind, active placebo‐controlled parallel group trial to examine the efficacy and safety of psilocybin in TRD 2019‐003984‐24 |
Randomized, double‐blind, active placebo‐controlled, parallel group study 1. HAM‐D (6 weeks) 2. Efficacy and safety (ECG, C‐SSRS, DSS‐4, adverse effects) |
3 arms: Oral 5‐, 25‐mg, psilocybin and 100‐mg niacin N = 144, age: 18–64 |
Active (2020–) | ||
|
A multicentre study to assess safety and efficacy of psilocybin in patients with TRD following completion of COMP 001 and COMP 003 trials (P‐TRD LTFU) 2020‐001348‐25 |
Long‐term follow‐up study 1. MADRS, suicidality, hospitalization (52 weeks) 2. QIDS‐SR‐16, WSAS, SDS |
Single arm: 52‐week‐long follow‐up of the groups (oral 1‐, 10‐ and 25‐mg psilocybin) N = 150, age: >18 |
Active (2020–) | ||
|
The safety and efficacy of psilocybin in participants with TRD (COMP001) 2017‐003288‐36 |
Randomized, double‐blind study 1. MADRS 2. Safety and tolerability (C SSRS) |
3 arms: 25‐, 10‐ and 1‐mg psilocybin (3–12 weeks) N = 398, age: >18 |
Active (2018–) | ||
|
The safety and efficacy of psilocybin as an adjunctive therapy in participants with TRD (COMP003) 2018‐002577‐22 |
Open‐label study 1. Efficacy 2. Safety and tolerability (C‐SSRS, ECG) |
Single arm: Oral 25‐mg psilocybin (adjunct therapy, 3 weeks) N = 20, age: >18 |
Restarted (2019–) | ||
|
Safety and efficacy of psilocybin in participants with TRD |
Multicentre (23 locations), randomized, quadruple‐blind, parallel, noncontrolled study 1. Change in MADRS‐10 score |
3 arms: Low–medium–high dose of psilocybin (up to 12 weeks) N = 216, age: >18 |
Recruiting (2018–) | ||
|
Safety and efficacy of psilocybin in participants with TRD |
Open‐label, single‐group assignment study Change in MADRS score |
Single arm: 25‐mg psilocybin·day−1 (3 weeks) N = 15, age: 18–65 |
Not yet recruiting (estimated completion January 2021) | ||
|
A randomised, placebo controlled trial of psilocybin in TRD: A feasibility study 2018‐003573‐97 |
Randomized, double‐blind, parallel, placebo‐controlled study 1. MADRS 2. Efficacy, safety and tolerability |
2 arms: Oral 25‐mg psilocybin versus placebo (6 weeks) N = 120, age: 25–80 |
Terminated (no longer in EU/EEA; 2020) | ||
| PF‐04995274 (partial agonist of serotonin 5‐HT4 receptor) |
PF‐04995274 and emotional processing in TRD |
Randomized, placebo‐controlled, quadruple‐blind, parallel assignment study (Phase 1/2) 1. Facial emotion recognition task 2. Emotional memory task, auditory verbal learning task, probabilistic instrumental learning task |
2 arms: 3 × 5‐mg test compound and placebo (7–9 days) N = 50, age: 16–65 |
Active (estimated completion July 2020) | |
| GH001 (5‐methoxy‐N,N‐dimethyl‐tryptamine) |
A phase 1/2 study of GH001 in patients with treatment‐resistant depression 2018‐004208‐20 |
Open‐label study MARDS, safety and tolerability (7 days) |
Single arm: Single dose inhalation of 5‐methoxy‐N,N‐dimethyltryptamine N = 16, age: 18–64 |
Active (2019–) | |
| Ayahuasca |
Antidepressant effects of ayahuasca: A randomized placebo controlled trial in TRD |
Randomized, triple‐blind, parallel assignment, placebo‐controlled study (Phase 1/2) 1. Change in HAM‐D (7 days) 2. Changes of MADRS |
2 arms: Test compound and placebo (follow‐up at 7–21 days) N = 29, age: 18–60 |
Completed (2014–2017): In ayahuasca‐treated patients significant decrease in suicidality and negative correlation between serum BDNF levels and depressive symptoms (de Almeida et al., 2019, Galvão et al., 2018, Zeifman et al., 2019) | |
| BMS‐820836 (SNDRI) |
Evaluation of the efficacy BMS‐820836 as compared with continued duloxetine/escitalopram |
Multicentre (93 locations), randomized, triple‐blind (outcomes assessor), active‐controlled study 1. MADRS score change (Week 13) 2. Change in disability score (Week 13) |
5 arms: p.o. 0.25, 0.5, 1, 2 mg·day−1 BMS‐820836 + placebo versus duloxetine + escitalopram + placebo N = 976, age: 18–65 |
Completed (2011–2013): BMS‐820836 was well tolerated, but not superior to continuation of duloxetine/escitalopram (Bhagwagar et al., 2015) | |
|
Evaluation of the efficacy of BMS‐820836 as compared with continued duloxetine |
Multicentre (84 locations), randomized, double‐blind, active‐controlled study 1. MADRS score change 2. Change in disability and anhedonia |
5 arms: Duloxetine (6/8 weeks), placebo matching with BMS‐820836 (14 weeks), BMS‐820836 (1.5–2 mg·day−1; 6 weeks), placebo matching with duloxetine (8 weeks) N = 889, age: 18–65 |
Completed (2011–2013): BMS‐820836 was well tolerated, but not superior to continuation of duloxetine/escitalopram (Bhagwagar et al., 2015) | ||
|
Efficacy and safety of flexibly‐dosed BMS‐820836 in patients with TRD 2010‐022841‐93 |
Multicentre, randomized, double‐blind, active‐controlled study 1. MADRS 2. CGI‐I, SDS, safety and tolerability |
2 arms: Flexibly dosed (0.5–2 mg·day−1) BMS‐820836 versus duloxetine (6 weeks) N = 346, age: 18–65 |
Completed (2011–2013): Flexibly dosed BMS‐820836 was well tolerated, but not superior to continuation of duloxetine | ||
|
Comparative, fixed‐dose, dose response study of the efficacy and safety of BMS‐820836 in patients with TRD 2011‐000778‐71 |
Multicentre, randomized, double‐blind, active‐controlled study 1. MARDS (13 weeks) 2. SDS |
5 arms: 0.25‐, 0.5‐, 1‐, 2‐mg BMS‐820836 versus duloxetine/escitalopram (6 weeks) N = 502, age: 19–65 |
Completed (2011–2013): Fixed doses (≥1 mg·day−1) of BMS‐820836 were not superior to continuation of duloxetine or escitalopram and dose‐related increases were observed in heart rate and systolic BP | ||
|
A 58 week rollover study to assess the safety and tolerability of BMS‐820836 in TRD 2010‐024371‐12 |
Multicentre, double‐blind, placebo‐controlled study 1. Long‐term safety and tolerability 2. Frequency and severity of adverse effects |
4 arms: 0.5‐, 1‐, 2‐mg BMS‐820836 versus placebo (54 weeks) N = 788, age: 18–84 |
Completed (2011–2013): Results are not published | ||
|
Long‐term safety and tolerability of BMS‐820836 in patients with TRD |
Multicentre (144 locations), randomized, triple‐blind, placebo‐controlled, 58‐week rollover study 1. Long‐term effects on BP 2. Adverse effects |
3 arms: Test compound 0.5, 1, 2 mg·day−1 (54 weeks) N = 789, age: 18–65 |
Terminated (failure to meet primary endpoint; 2011–2013) | ||
| CX157/TriRima (reversible MAO inhibitor) |
Evaluation of the efficacy, safety and tolerability of CX157 modified‐release tablet in TRD patients |
Multicentre (30 locations), randomized, double‐blind, placebo‐controlled, parallel assignment 1. MADRS score change 2. CGI‐S, CGI‐I, HADS |
2 arms: CX157/TriRima (125 mg 2 times a day) and placebo (6 weeks) N = 360, age 20–65 |
Completed (2010–2012): Results are not published | |
| Tranylcypromine (irreversible MAO inhibitor) |
Efficacy of tranylcypromine (TCP) in daily doses up to 60 mg and lithium augmentation (Li.‐Aug.) of antidepressants in the acute treatment of TRD 2012‐001209‐26 |
Randomized, open‐label, Simon‐phase‐II‐design study 1. MADRS 2. HAMD‐17, CGI, BDI‐II, IDS, ASEC, TSQ, QLESQ, ASEX |
2 arms: 60‐mg tranylcypromine and lithium augmentation of antidepressants N = 71, age: >18 |
Restarted (2013–) | |
| Cholinergic system | Botulinum toxin A |
Glabellar botulinum toxin injections for the treatment of geriatric depression (BOTDEP) |
Randomized, placebo‐controlled, quadruple‐blind, crossover study (Phase 1/2) 1. MADRS 2. Remission, quality of life assessment, safety/tolerability |
2 arms: Test compound and placebo (16 weeks) N = 50, age: 65–99 |
Not yet recruiting |
| TC‐5214/ S‐mecamylamine (non‐selective, non‐competitive antagonist of the nAChRs) |
Assessment of the safety and efficacy of 2 fixed dose groups of TC‐5214 as monotherapy treatment in patients with TRD 2010‐023816‐15 |
Multicentre, randomized, double‐blind, placebo‐ and active‐controlled, parallel group study 1. MADRS (16 weeks) 2. HAMD, CGI‐S, CGI‐I, HAM‐A, SDS, EQ‐5D, pharmacokinetic properties |
4 arms: 1, 4 mg of TC5214, duloxetine and placebo N = 145, age: 18–64 |
Terminated (2011–2012) | |
| Others | NV‐5138 (leucine analogue, mTORC1 activator, binding to Sestrin2) |
Safety, tolerability, PK and efficacy of single doses of NV‐5138 in healthy volunteers and subjects with TRD |
Randomized, two‐step, double‐blind, placebo‐controlled, single‐dose study (Phase 1/2: Part A single ascending dosage levels in healthy subjects orally, Part B: TRD patients: Single dose) |
2 arms: Single dose of 150, 300, 600, 1000, 1600 or 2400 mg single dose of NV‐5138 or placebo orally in healthy subjects, and one dose of NV‐5138 or placebo in patients (28 days) N = 88, age: 18–55 |
Completed (2018–2019): Results are not published |
| S‐adenosyl methionine (SAMe) |
Optimizing the effectiveness of selective serotonin reuptake inhibitors (SSRIs) in treatment‐resistant depression |
Randomized, double‐blind, placebo‐controlled, parallel assignment 1. HAM‐D remission rate (Week 6) 2. HAM‐D remitters (Week 6) |
2 arms: SAMe (oral SAMe tosylate, up to 1600 mg·day−1) and placebo (6 weeks) N = 73, age: 18–80 |
Completed (2004–2014): Results are not published | |
| Erythropoietin |
Effects of erythropoietin on depressive symptoms and neurocognitive deficits in depression and bipolar disorder |
Randomized, double‐blind, placebo‐controlled, parallel assignment 1. HAM‐D (Week 14) 2. HAM‐D remission rate (Weeks 9 and 14) |
2 arms: Epoetin alfa (40,000 IU·ml−1 i.v. infusions over 15 min) and placebo weekly for 8 weeks N = 83, age: 18–65 |
Completed (2009–2012): Results are not published | |
| l‐Leucine |
Rapid antidepressant effects of leucine |
Randomized, double‐blind, placebo‐controlled, parallel assignment 1. QIDS‐SR 2. QIDS‐SR, adverse effects, fatigue, psychosocial functions, anhedonia |
2 arms: Leucine and placebo (maltodextrin) (2 weeks) N = 40, age: 18–64 |
Suspended (2017–; due to the Covid‐19 pandemic) | |
| Tofacitinib (JAK/STAT inhibitor) |
Tofacitinib in depression |
Randomized, quadruple‐blind, parallel assignment, placebo‐controlled study (Phase 1/2) 1. Facial emotional recognition task 2. EMEM, ECAT, EREC, brain neuronal activity (BOLD fMRI, FDOT, PILT, AVLT, cerebral perfusion) |
2 arms: Test compound (5 mg oral 2 times a day for 7–10 days) and placebo N = 50, age: 18–60 |
Suspended (2019–; due to the Covid‐19 pandemic) | |
| Isoflurane |
Efficacy of deep anaesthesia with isoflurane as a fast‐response antidepressant agent |
Open‐label, randomized, parallel assignment study 1. MADRS and HAM‐D score change (Week 6) 2. Adverse effects, MADRS and HAM‐D (Week 24), maintenance and duration of action |
2 arms: Isoflurane (for 6 weeks, one deep anaesthesia per week) + oral antidepressants and isoflurane without oral antidepressants (no placebo, no control; responders followed for 24 weeks) N = 30, age: 18–60 |
Not yet recruiting (2019–) | |
| L‐DOPA |
Dopaminergic dysfunction in late‐life depression |
Randomized, quadruple‐blind, placebo‐controlled, crossover assignment (7‐day washout period); 1. Intelligence and executive functions, gait, cost/reward fMRI task (Week 6) 2. Overall cognitive index, reward anticipation and receipt fMRI task, MADRS and QIDS scores |
2 arms: 3 times oral 25/150–450 mg·day−1 carbidopa/levodopa and placebo (3 weeks) N = 60, age: >60 |
Not yet recruiting (2020–2026) | |
| Masitinib (TK c‐Kit inhibitor) |
Safety and efficacy of masitinib in the treatment of mood disorders in patients with antidepressant‐resistant major depression or patients with dysthymic disorder 2010‐022744‐21 |
Randomized, double blind, placebo‐controlled study 1. HAMD‐17, CGI‐I, safety (12 weeks) 2. CGI‐S, LESQ, C‐SSRS, MADRS |
2 arms: 4.5 mg·kg−1·day−1 test compound and placebo N = 120, age: >18 |
Terminated (2013–2018) | |
| JNJ26489112 (voltage‐gated Na+ channel and N‐type Ca2+ channel inhibitor, K+ channel opener) |
A safety and efficacy study of JNJ26489112 in patients with treatment‐resistant major depressive disorder |
Randomized, triple‐blind, parallel assignment 1. MADRS (6 weeks) 2. Adverse effects, changes in depressive symptoms, CGI, ophthalmological symptoms (Weeks 2–9) |
3 arms: JNJ26489112 (oral 500 to 1000 mg·day−1 by Week 4), venlafaxine (75 mg·day−1) and placebo (6 weeks) N = 12, age: 18–65 |
Terminated (sponsor portfolio decision, 2011–2012) | |
| Combination approaches (Phase 1/2 studies) | MDL100.907 + citalopram |
An investigation of the antidepressant efficacy of the 5‐HT2A antagonist, M100907, in combination with citalopram in treatment‐resistant depression |
Randomized (double‐blind), open treatment, placebo‐controlled, add‐on study 1. HAM‐D score 2. Sleeping pattern (polysomnography) |
2 arms, 2 steps: Escitalopram + M100907 or continued escitalopram + placebo (4 weeks) then all escitalopram + M100907 (4 weeks). Patients showing remission received escitalopram + M100907 for additional 6 months N = 96, age: 18–65 |
Completed (2003–2004): Results are not published |
| Antidepressant + minocycline |
Adjunct minocycline in treatment‐resistant depression |
Randomized, multicentre, double‐blind, placebo‐controlled, add‐on, parallel assignment 1. MADRS response 2. Remission (MADRS), HAM‐D, CGI, SCL‐90‐R, transcriptomic changes in the PBMC, inflammatory markers (blood) |
2 arms: Oral 200 mg·day−1 minocycline or placebo (add‐on, 6 weeks) N = 168, age: 18–75 |
Completed (2015–2020): Results are not published | |
|
Multicentre proof‐of‐principle trial of adjunctive minocycline for patients with treatment resistant unipolar major depressive disorder (MDD) 2015‐001456‐29 |
Randomized, double‐blind, placebo‐controlled study 1. MADRS (7 weeks) 2. CGI‐S, BDI, HAMD‐17, SCL‐90‐R, serum concentration of cytokines, cell‐specifically markers, neurotrophic and inflammatory factors |
2 arms: Oral 50 mg·day−1 minocycline or placebo N = 160, age: >18 |
Completed (2015–2020): Results are not published | ||
| Venlafaxine XR + buprenorphine |
Buprenorphine used with treatment‐resistant depression in older adults |
Randomized, triple‐blind, placebo‐controlled, parallel assignment 1. MADRS, side effect checklist (8 weeks) and rating (1, 8 weeks) 2. Suicide ideation, anxiety and pain assessment |
2 arms: Venlafaxine (oral up to 300 mg·day−1) + buprenorphine (oral 0.2–2 mg·day−1) and venlafaxine + placebo (up to 32 weeks) N = 18, age: >50 |
Completed (2014–2019): Results are not published | |
| Antidepressant + aspirin |
Salicylic augmentation in depression |
Randomized, double‐blind, placebo‐controlled, augmentation, parallel assignment 1. HAM‐D score change 2. Inflammatory biomarkers in the blood and their correlations with the antidepressant effect |
2 arms: 325 mg·day−1 oral aspirin and placebo along with the antidepressant treatment for 8 weeks N = 74, age: 18–65 |
Recruiting (2017–2021) | |
| Ethosuximide + escitalopram |
Investigate the clinical responses of ethosuximide in patients with treatment‐resistant depression |
Randomized, quadruple‐blind, placebo‐controlled, add‐on, parallel assignment 1. MADRS score change (43 days) 2 . QIDS, anxiety and mania scores |
2 arms: Oral ethosuximide (2 weeks) + escitalopram (4 weeks) and placebo (2 weeks) + escitalopram (4 weeks) N = 40, age: 18–65 |
Recruiting (2019–) | |
| Oxytocin + tibolone |
Oxytocin and tibolone adjuncts in treatment‐resistant depression—A pilot study |
Randomized, double‐blind, placebo‐controlled, parallel assignment 1. MADRS score change (1, 2, 4, 8 weeks) 2. HAM‐D, BDI‐II, state and trait anxiety, adverse symptom checklist, perceived stress scale, sleep quality, quality of life test |
3 arms: Oxytocin (20 IU intranasal, 2 times a day, for 8 weeks) and oxytocin + tibolone (2.5 mg oral) and placebo N = 15, age: 18–46 (females) |
Unknown (2010–2012) |
Note: Inclusion criteria: Phase I/II or Phase II studies available on https://clinicaltrials.gov or https://www.clinicaltrialsregister.eu/, patients with treatment‐resistant (major) depression.
Abbreviations: ASEC, Antidepressant Side Effect Scale; ASEX, Arizona Sexual Functioning Questionnaire; AVLT, Auditory Verbal Learning Task; BDI‐II, Beck Depression Inventory‐II; BPRS+, Brief Psychiatric Rating Scale Positive Symptoms Subscale; CADSS, Clinician Administered Dissociative States; CGI, Clinical Global Impressions scale; CGI‐I, Clinical Global Impression—Improvement; CGI‐S, Clinical Global Impression—Severity; C‐SSRS, Columbia‐Suicide Severity Rating Scale; dmPFC, dorsomedial prefrontal cortex; DSS‐4, Dissociation Scale; ECAT, Emotional Categorization Task; EMEM, Emotional Memory Task; EQ‐5D, European Quality of Life; EREC, Emotional Recall Task; FDOT, Faces Dot Probe Task; fMRI, functional MRI; HADS, Hospital Anxiety and Depression Scale; HAM‐A, Hamilton Anxiety Assessment; HAM‐D (6/17), Hamilton Depression Rating Scale (6‐ or 17‐item version); IDS, Inventory of Depressive Symptoms; LESQ, Leeds Evaluation Sleeping Questionnaire; MADRS (10), Montgomery–Åsberg Depression Rating Scale (10‐item version); PBMC, patient‐specific peripheral blood‐derived monocytic cells; PGI‐C, Patient Global Impression‐Change; PGI‐S, Patient Global Impression‐Severity; PILT, Probabilistic Instrumental Learning Task; QIDS(‐SR), Quick Inventory of Depressive Symptomatology (self‐reported); QLESQ, Quality of Life Questionnaire; SCL‐90‐R, Symptom Checklist‐90‐Revised; SDS, Sheehan Disability Scale; SSI, Scale for Suicidal Ideation; TMS, transcranial magnetic stimulation; TRD, treatment‐resistant depression; TSQ, Treatment Satisfaction Questionnaire; VAS‐D, Visual Analogue Scale‐Depression; VBM, voxel‐based morphometry; WSAS, Work and Social Adjustment Score; YMRS, Young Mania Rating Scale.
TABLE 2.
Summary of targets and drug candidates in Phase III studies
| Target | Drug candidate (mechanism of action) | Title, aim, NCT number | Study design, endpoints (1. = primary outcome, 2. = secondary outcome measures) | Study arms N, age (years) | Current status (outcome) |
|---|---|---|---|---|---|
| NMDA receptor | Ketamine (antagonist) |
Action of ketamine in treatment‐resistant depression |
Randomized, double‐blind, crossover assignment, 2‐step study 1. Change in MADRS score, BDNF gene VAL/MET polymorphism 2. Plasma and salivary concentrations of cortisol, melatonin and inflammatory mediators; CGI‐S, and self‐reported depression, and suicidality in comparison with midazolam |
2 arms in 2 steps Step 1: Single i.v. 0.5 mg·kg−1 bolus infusion ketamine or 1 mg·kg−1 midazolam over 40 min Step 2: 6 ketamine or midazolam infusions N = 46, age: 18–65 |
Completed (2013–2017): Cumulative and sustained antidepressant effects |
|
Evaluation of schemes of administration of intravenous ketamine in depression |
R andomized, initially double‐blind then open‐label, placebo‐controlled, parallel assignment study 1. Changes of HAM‐D and MADRS scores, brain glutamate and GABA concentrations (up to 12 weeks) |
2 arms: Ketamine i.v. 0.5 mg·kg−1 over 40 min, twice weekly for 8 weeks or placebo (saline) N = 30, age: 18–65 |
Recruiting (2018–2020) | ||
|
Ketamine for treatment‐resistant late‐life depression |
Randomized, quadruple‐blind, parallel assignment study 1. MADRS score (4‐week follow‐up) 2. Clinician‐administered dissociative state scale, cognitive test batteries, dementia screening test, general side effect rating scale, BDNF, resting‐state EEG, blood inflammatory biomarkers |
4 arms: Single i.v. 0, 1, 0.25, 0.5 mg·kg−1 bolus infusion ketamine or 1, 0.03 mg·kg−1 midazolam over 40 min N = 46, age: >55 |
Active, not recruiting (2015–) | ||
| AXS‐05 (dextromethorphan + bupropion) |
A study to assess the efficacy and safety of AXS‐05 in subjects with treatment‐resistant major depressive disorder |
Randomized, active‐controlled, double‐blind study 1. Changes of MADRS score 2. Clinical Global Impressions‐Severity, HAM‐D |
2 arms: Oral test compound or bupropion (6 weeks) N = 312, age: 18–65 |
Completed (2016–2020): Results are not published | |
|
Open‐label safety study of AXS‐05 in subjects with depression |
Open‐label, multicentre, long‐term, single group assignment study 1. Safety (types and rates of adverse events) 2. Change in MADRS score |
2 arms: Oral test compound (2 times a day) for up to 12 months N = 300, age: 18–65 |
Recruiting (2019–, estimated completion 2020) | ||
| Opioid receptors | Buprenorphine (partial μ receptor agonist + κ opioid receptor antagonist) |
Buprenorphine for TRD (BUP‐TRD) |
Randomized, triple‐blind, placebo‐controlled, parallel design study 1. Changes in MADRS score, BP, side effect rating scale, heart rate, weight 2. Brief Symptom Inventory—Anxiety Scale, Positive/Negative Affect Scale |
2 arms: Buprenorphine (0.2–1.6 mg sublingual; 8 weeks) or placebo N = 13, age: >21 |
Completed (2011–2018): Results are not published |
| Combination approaches | Antidepressant + lamotrigine |
Efficacy and safety of antidepressant augmentation with lamotrigine |
Randomized, triple‐blind, placebo‐controlled, parallel add‐on assignment pilot study 1. MADRS score changes 2. CGI score |
2 arms: Oral 200 mg·day−1 lamotrigine or placebo added to the ongoing antidepressant (8 weeks) N = 34, age: 18–60 |
Completed (2004–2006): No differences compared with placebo; side effects: dyspepsia, nausea, rashes |
| Antidepressant + olanzapine |
The study of olanzapine plus fluoxetine in combination for treatment of TRD |
Randomized, double‐blind, active compound‐controlled, parallel assignment study 1. MADRS score changes 2. Side effects, laboratory parameters, HAM‐A, HAM‐D, CGI and BCRS scores |
3 arms: Olanzapine + fluoxetine or olanzapine or fluoxetine (8 weeks) N = 605, age: 18–65 |
Completed (2002–2006): Significantly greater antidepressant effect of the combination | |
|
Olanzapine augmentation therapy in treatment‐resistant depression: A double‐blind placebo‐controlled trial |
Randomized, quadruple‐blind, placebo‐controlled add‐on parallel assignment study, 2 steps 1. HAM‐D score changes 2. MADRS, HAM‐D subscales, CGI |
2 arms: Oral 10 mg·day−1 olanzapine or placebo added to the ongoing antidepressant (2‐week treatment, responder selection, further 2‐month treatment, 2‐week follow‐up) N = 60, age: 18–65 |
Terminated (2006–2016) | ||
| Antidepressant + risperidone |
Risperidone vs. bupropion ER augmentation of SSRIs in TRD |
Randomized, open‐label, comparative crossover, add‐on assignment study 1. MADRS score changes 2. HAM‐D. BDI, HAM‐A, CGI scores |
2 arms: Oral risperidone or bupropion ER added to the ongoing SSRI (6 weeks) N = 30, age: >18 |
Completed (2005–2015): Results are not published | |
|
A study of the effectiveness and safety of risperidone to augment SSRI therapy in patients with TRD |
Randomized, double‐blind, placebo‐controlled, parallel add‐on assignment study 1. MADRS score changes 2. HAM‐D and CGI scores |
2 arms: Oral risperidone (0.25, 0.5, 0, 2 mg·day−1) or placebo (30 weeks) added to citalopram (20–40 mg, 36 weeks) N = 258, age: 18–85 |
Completed (2002–2004): Results are not published | ||
| Antidepressant + simvastatin |
Simvastatin as an augmentation treatment for treatment‐resistant depression: Randomized controlled trial |
Randomized, double‐blind, placebo‐controlled add‐on parallel assignment study 1. MADRS score changes |
2 arms: Oral 20 mg·day−1 simvastatin or placebo added to the ongoing antidepressant (3 months) N = 150, age: 18–75 |
Recruiting (2019–) | |
| Antidepressant + minocycline |
Minocycline as adjunctive treatment for treatment‐resistant depression |
Randomized, quadruple‐blind, placebo‐controlled add‐on parallel assignment study 1. HAM‐D score changes 2. CGI, quality of life scale, GAD‐7 |
2 arms: Oral 100–200 mg·day−1 minocycline or placebo added to the ongoing antidepressant (6 weeks) N = 100, age: 18–80 |
Recruiting (2020–2021): Interim report‐minocycline is well tolerated and effective in reducing depressive symptoms | |
| Antidepressant + aripiprazole |
Aripiprazole augmentation therapy in treatment‐resistant depression |
Open‐label pilot study 1. HAM‐D score changes 2. HAM‐D, MADRS, CGI, BDI |
Single arm: Oral 10 mg·day−1 aripiprazole to concurrent antidepressant (pre–post comparison, 3 weeks) N = 20, age: 18–70 |
Terminated (2006–2016) |
Note: Inclusion criteria: Phase III studies available on https://clinicaltrials.gov or https://www.clinicaltrialsregister.eu/, patients with treatment‐resistant (major) depression.
Abbreviations: BCRS, Brief Cognitive Rating Scale; BDI, Beck Depression Inventory; BDNF, brain‐derived neurotrophic factor; CGI, Clinical Global Impression; CGI‐S, CGI‐Severity; GAD‐7, Generalized Anxiety Disorder 7‐item scale; HAM‐A, Hamilton Anxiety Rating Scale; HAM‐D, Hamilton Depression Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; VAL/MET, valine/methionine.
FIGURE 1.
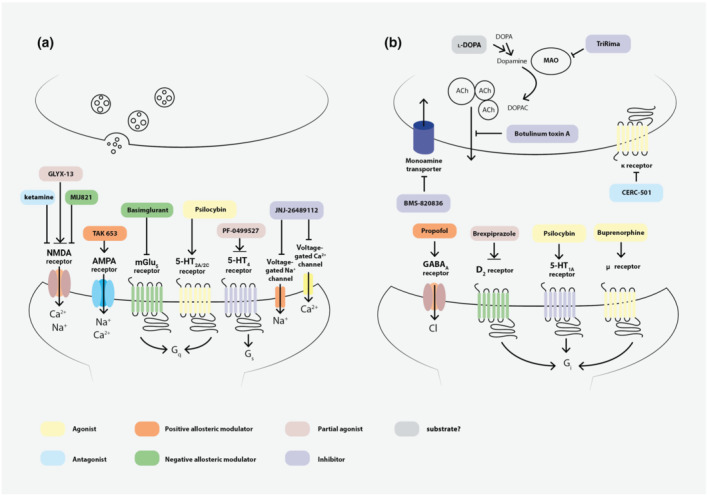
Synaptic targets for the drug candidates investigated in Phase I–III studies. Panel (a) represents the excitatory ionotropic and G protein coupled receptors (GPCRs), as well as the ion channels serving as targets for novel compounds for TRD treatment. Panel (b) represents the postsynaptic inhibitory receptors and presynaptic targets on which the new drug candidates exert their effects. Colour boxes indicate the mechanisms of action for the representative members of the different drug groups. ↓ denotes activation, ┴ denotes inhibition, double arrowhead denotes partial agonism. D2 receptor, D2 dopamine receptor; mGlu, metabotropic glutamate receptor; κ receptor, κ opioid receptor; μ receptor, μ opioid receptor
FIGURE 2.
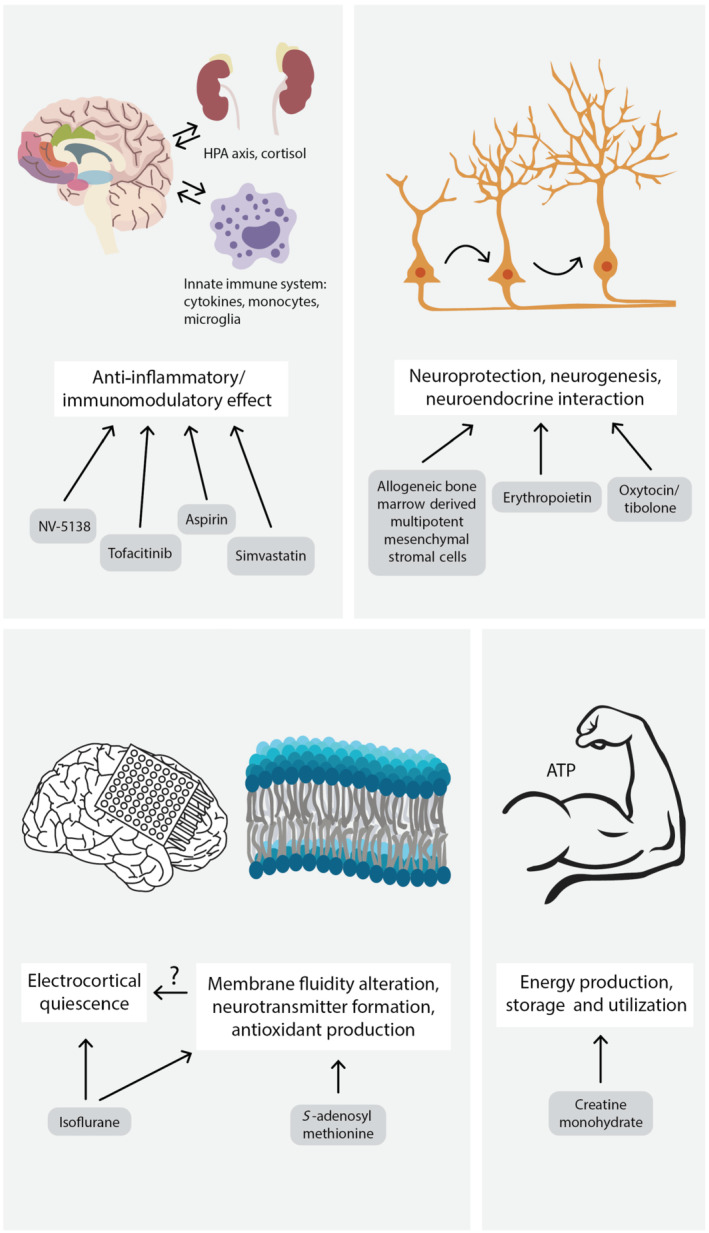
Other mechanisms leading to antidepressant effects in TRD patients. Anti‐inflammatory and immunomodulatory effects can be achieved by several different mechanisms. Activation of rapamycin complex 1 (mTORC1) by NV‐5138, inhibition of JAK by tofacitinib, COX by aspirin and HMG‐CoA‐reductase by simvastatin lead to beneficial effects. Drugs promoting neuroprotection, neurogenesis and neuroendocrine interactions can also improve the patient's symptoms, although the precise mechanism of action is unknown in most cases. Inducing electrocortical quiescence by isoflurane, influencing the membrane fluidity or neurotransmitter formation by S‐adenosyl‐l‐methionine and affecting the energy production, storage and utilization, by creatine monohydrate, can also be effective mechanisms. HPA axis, hypothalamic–pituitary–adrenal axis, ATP, adenosine triphosphate
Phase I studies are performed to determine the tolerability and pharmacokinetic parameters of the candidates. They involve small groups of participants, who are healthy volunteers for originally developed drugs, but trials using nontherapeutic doses of an already registered drug for repositioning purposes might also include patients with chronic, stable diseases. Phase II exploratory studies provide the proof of concept for the efficacy and safety of the drug (therapeutic dose) in a small group of patients. Finally, Phase III, multi‐centric, often multi‐national, studies are conducted to confirm the efficacy in large groups of patients (https://clinicaltrials.gov/ct2/about-studies/glossary; https://www.abpi.org.uk/media/4992/guidelines-for-phase-i-clinical-trials-2018-edition-20180626.pdf).
4.1. Drug candidates acting on the glutamatergic system
Undisputedly, the major group of drug candidates acts on the glutamatergic system. Glutamate (Glu) is the most important amino acid excitatory neurotransmitter of the CNS and present in half of the synapses. It plays a role in several neurological and psychiatric disorders (Li et al., 2019). There is increasing evidence that impairing glutamate effects is a promising target mechanism for fast‐acting antidepressant agents. Both ionotropic and metabotropic glutamate receptors are involved in the regulation of mood. Most of the drug candidates under development act antagonistic on NMDA receptors (Jaso et al., 2017).
4.1.1. Non‐selective NMDA receptor antagonists
Ketamine
Several Phase I, II and III clinical trials (NCT04101474, 2010‐023414‐31, 2016‐001715‐21, 2011‐003654‐40) are currently running with i.v. racemic ketamine in order to understand its exact mechanism of action as well as to determine the most optimal dose, administration route and treatment regimen in patients with TRD (and other concomitant disorders). Fourteen trials are available altogether in the registries, from these six are ongoing and eight completed studies. Only five completed studies have published results, demonstrating the significant improvement of depressive symptoms after ketamine infusions. Three of them are randomized, controlled, double blind and two open‐label (Figure 3). These data obviously support the rationale of ketamine use in this indication. Significantly reduced depressive symptoms were detected in a time frame of 1 day to 6 weeks, after 1–6 infusions of 0.3–0.5 mg·kg−1 racemic ketamine in the different studies, in which repeated administrations led to cumulative and long‐lasting antidepressant effect. Suggested mechanisms of the effect of ketamine include glutamine/glutamate ratio changes in the pregenual anterior cingulate cortex, nucleus accumbens entropy and insulin/mTOR/GSK3β signalling (Johnson et al., 2016; Lewis et al., 2019; Li et al., 2016; Phillips et al., 2019; Roy et al., 2020; Vande Voort et al., 2016; see details in Tables 1 and 2).
FIGURE 3.
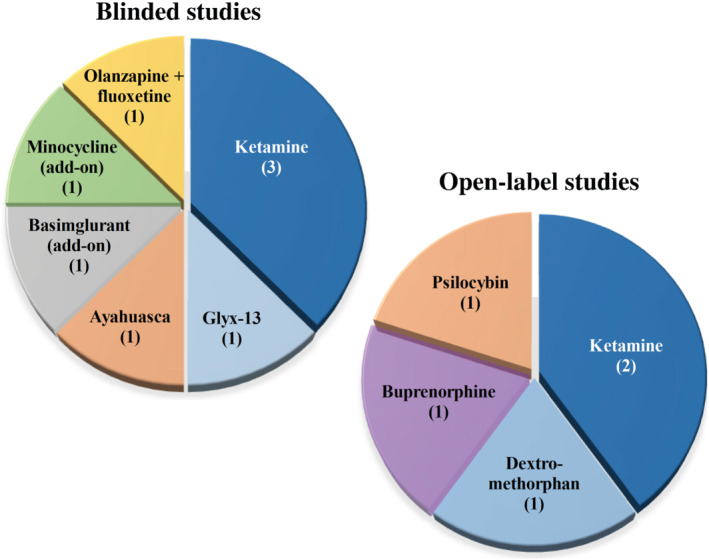
Summary of the published clinical studies with positive outcomes. Pie charts demonstrate compounds listed on https://clinicaltrials.gov or https://www.clinicaltrialsregister.eu/, which were studied in randomized, controlled, blinded or open‐label trials and published with positive results in TRD patients (the number of trials is shown in brackets)
Dextromethorphan, AVP‐786 and AXS‐05
Dextromethorphan is a synthetic, nonopioid derivate of morphine, which is commonly used as a centrally acting antitussive drug by suppressing the cough reflex. On the other hand, it can bind to NMDA receptors and act as a non‐competitive antagonist.
Furthermore, the fixed combination of dextromethorphan with quinidine is also approved for Pseudobulbar Affect (Nuedexta). Quinidine is an inhibitor of CYP2D6 enzyme and a beneficial combination partner to reduce the first‐pass metabolism (O‐demethylation) of dextromethorphan (Pope et al., 2004). A Phase II study (NCT01882829) evaluated the efficacy and tolerability of the drug combination dextromethorphan/quinidine in a 45/10‐mg dosage. The combination treatment significantly decreased the Montgomery‐Åsberg Depression Rating Scale (MADRS) scores and was well tolerated—the most common side effect was constipation (Murrough et al., 2017).
Another Phase II investigation (NCT02153502) analysed the effects of AVP‐786, which is a deuterated (d6)‐dextromethorphan/quinidine combination. The modulation with deuterium, an isotope of hydrogen, can lead to further attenuation of the first‐pass effect and the quinidine dose can also be reduced (Garay et al., 2017; Wilkinson & Sanacora, 2019). Although the study was finished in 2016, the results have not yet been published.
AXS‐05 is a newer combination of dextromethorphan with bupropion. Bupropion has been used for decades albeit its mechanism of action is not completely understood (inhibition of noradrenaline and dopamine transporters, antagonism on nicotinic ACh receptor) (Sadock et al., 2014). Antidepressant actions of the two compounds are additive while bupropion acts also as an enzyme inhibitor on CYP2D6 increasing the bioavailability of dextromethorphan (Wilkinson & Sanacora, 2019). Two Phase III studies (NCT04039022 and NCT02741791) are running presently (Tables 1 and 2).
Memantine
Memantine is a well‐known compound used in Alzheimer's disease for the symptomatic treatment of cognitive dysfunction (Vaz & Silvestre, 2020). Based on this, a special patient population—older adults over 55—was investigated in a Phase I study (NCT01392287). The results have not been published.
Nitrous oxide
Nitrous oxide (NO), known also as laughing gas, is a common anaesthetic drug with unique pharmacology. Its minimal alveolar concentration (MAC) value is 105%, meaning that hyperbaric pressure is for anaesthesia. Solubility in blood is low, which leads to a prompt onset and offset of action. The molecular mechanism of action is also very complex: NMDA antagonism is the most important factor, but AMPA, kainate and nicotinic ACh receptors as well as calcium and potassium channels are involved in the effect (Nagele et al., 2018). Several clinical Phase I/II trials (NCT02994433, NCT03283670, NCT03932825 and 2019‐002769‐37) are currently investigating NO as a potential antidepressant drug including treatment‐resistant cases. The treatment regimens are variable: Inhalation may last 1 h every day or three times a week; the whole treatment can be 2 or 4 weeks long. Some trials are in a very early stage, but some have been completed; however, the results are still unavailable (Table 1).
AZD6765/lanicemine
AZD6765 is a low‐trapping NMDA channel blocker with pharmacological properties very similar to ketamine. Two Phase II studies (NCT00491686 and NCT00986479) were performed approximately 10 years ago, but despite favourable pharmacokinetics, they did not show significant effect (Agbo et al., 2017; Sanacora et al., 2014, 2017; Zarate, Duman, et al., 2013) (Table 1). As a continuation of this line of development, the prodrug of lanicemine, BHV‐5000, was synthesized, which is an orally active compound of Biohaven Pharmaceuticals (https://www.biohavenpharma.com/science-pipeline/glutamate/bhv-5000).
Riluzole
Riluzole was the first drug developed for the treatment of amyotrophic lateral sclerosis (ALS), approved in 1995. It is a benzothiazole derivative that has diverse actions on the glutamatergic system. Thus, it can inhibit glutamate release and promote reuptake by different cell types and interact with NMDA receptors and various inhibitory neurotransmitters (de Boer et al., 2019). Based on this, it could be a promising compound for the treatment of TRD. The results of the only ongoing Phase II study (NCT01204918) have not yet been published (Table 1).
REL‐1017/dextromethadone
REL‐1017 is the d‐stereoisomer of methadone, acting as a non‐competitive receptor antagonist on the NMDA receptor. Recent preclinical data demonstrated that it can influence BDNF release, stimulate mTORC1 signalling and enhance synaptic connectivity through NMDA antagonism, leading to a rapid antidepressant effect in rats (Fogaça et al., 2019). Hence, the pharmacokinetic profile of dextromethadone and its effect as adjunctive therapy in TRD were investigated in clinical trials (NCT03051256). Results are submitted to ClinicalTrials.gov but have not yet been published (Table 1).
4.1.2. GluN2B subtype selective NMDA receptor antagonists
EVT‐101
EVT‐101 is a potent and orally active antagonist on the NMDA receptors, selectively binding to the GluN2B subtype. There was only one trial (NCT01128452) investigating its safety and efficacy, but it was terminated in 2016 due to a clinical hold issued by the FDA (Table 1).
MIJ821
MIJ821 is a 2B subtype‐selective and a potent negative allosteric modulator of the NMDA receptors. Little is known about this compound. A ketamine‐like, rapid antidepressant effect without typical ketamine side effects is proposed by the sponsors (http://www.cadenttx.com/pipeline/). Two Phase II studies (NCT03756129 and 2018‐003002‐12) were finished early this year investigating the efficacy and safety of MIJ821, but the results are not yet published (Table 1).
4.1.3. Partial NMDA receptor agonists
Glyx‐13/rapastinel
Glyx‐13 is a tetrapeptide, acting as a weak partial agonist on the glycine site of NMDA receptors (Moskal et al., 2017). It is a promising drug candidate, because not only the effect on NMDA receptors can be identified, but several other mechanisms (AMPA, mTOR activation, BDNF release etc.) lead to rapid‐acting antidepressant actions (Kato & Duman, 2020; Witkin et al., 2019). A Phase II clinical trial (NCT01234558) ended in 2012. The published data demonstrated a U‐shaped dose–response curve, where 5 and 10 mg·kg−1 of Glyx‐13 induced a rapid drop in depression scores, lasting for 1 week. Furthermore, no serious adverse effect was observed (Preskorn et al., 2015). A more potent analogue of rapastinel, apimostinel (NRX‐1074), was developed and found safe in healthy volunteers (NCT01856556 and NCT02366364). Its effect in MDD patients has not been published (Wilkinson & Sanacora, 2019) and has not yet been investigated in TRD (Table 1).
d‐Cycloserine
d‐Cycloserine is a well‐known, broad‐spectrum antibiotic agent used for treating tuberculosis. However, increasing evidence supports the fact that it can be useful in the treatment of neuropsychiatric disorders (Schade & Paulus, 2016). A Phase II study (NCT00408031) was finished in 2010 with a smaller population of TRD patients, but the results have not been published since then (Table 1).
4.1.4. Drugs acting at the AMPA receptor
There is increasing evidence that the rapid antidepressant action of ketamine is partly mediated by AMPA receptors (Aleksandrova et al., 2017). Therefore, drugs potentiating this pathway (AMPAkines) including farmampator (CX‐691/ORG 2448) were developed and are tested in major depression (NCT00113022 and NCT00610649; Jaso et al., 2017). Furthermore, the ketamine metabolite (2R,6R)‐hydroxynorketamine is also involved in a Phase I study (NCT04711005), because it can evoke antidepressant effects through the activation of AMPA receptors (Henter et al., 2021).
The positive allosteric modulator, TAK‐653, was developed as a potential rapid‐acting antidepressant (Witkin et al., 2019). Phase II trials (NCT03312894 and 2017‐002232‐16) were designed to test the efficacy and safety of TAK‐653 in TRD patients, but both of them were withdrawn/terminated in 2018 (Table 1).
4.1.5. Metabotropic glutamate receptor ligands
Approximately 10 years ago, preclinical data indicated that mGlu2/3 antagonism can be a potential antidepressant mechanism (Witkin et al., 2019). Consequently, several metabotropic glutamate receptor (mGlu receptor) 2/3 antagonists were tested in clinical trials by BrainCells Inc. The original compound, BCI‐838, its metabolite BCI‐632 and pro‐drugs, like BCI‐1038, BCI‐1206 and BCI‐1283, were investigated in Phase I clinical trials (NCT01548703 and NCT01546051), but the results are not published. Similarly, RO4995819/decoglurant was a promising compound acting on mGlu2/3 receptors, tested in Phase II clinical trial (2011‐002160‐24), but the results remained likewise unpublished.
RO4917523/basimglurant ((2‐chloro‐4‐[1‐(4‐fluoro‐phenyl)‐2,5‐dimethyl‐1H‐imidazol‐4‐ylethynyl]‐pyridine) is a potent, selective, negative allosteric modulator of mGlu5 receptors. It has favourable pharmacokinetic parameters (high oral bioavailability and long half‐life time), and promising preclinical data support its efficacy in mood disorders (Lindemann et al., 2015). A Phase II study (NCT00809562) investigated basimglurant in TRD patients between 2009 and 2011. Although the results are not officially published, another publication mentioned this trial, in which basimglurant was well tolerated and showed a tendency of positive effect (Quiroz et al., 2016) (Table 1).
4.2. Drug candidates acting on the opioid system
The endogenous opioid system of the brain plays an important role in mood regulation, and disturbances may lead to depressive disorders. Based on this, compounds influencing the opioid cascade can be potential drug candidates for TRD. Furthermore, data suggested that mainly the post‐synaptic μ receptor and pre‐synaptic κ receptors (Alexander, Mathie, et al., 2021) are responsible for most of the symptoms experienced by the patients. The μ receptor agonism alone does not cause antidepressant effects, while antagonizing the κ receptor is likely to induce an antidepressant effect and reduce the severity of anhedonia. Therefore, drug development has mainly been focusing on κ receptors (Browne et al., 2020; Jacobson et al., 2020).
4.2.1. ETS6103
ETS6103 is a controlled‐release formulated form of tramadol. Tramadol is a synthetic μ receptor agonist and seems to be effective in the treatment of anxiety and depression recently (Rougemont‐Bücking et al., 2017). Its efficacy was also investigated compared with amitriptyline in TRD patients (2013‐000719‐26), but the results are not yet published (Table 1).
4.2.2. Aticaprant/CERC‐501/LY2456302
CERC‐501 is a pyrrolidine‐methylene‐phenoxy‐benzamide and a relative selective κ opioid receptor antagonist. Its effect is short (half‐life time: 2–4 h), but it has a high selectivity for κ receptor (compared with μ and δ receptors). It was efficacious in several preclinical studies (stress/depressive‐like behaviour). Former human studies revealed no serious side effects and found that the compound has a positive effect particularly on anhedonic symptoms (Krystal et al., 2020; Pizzagalli et al., 2020; Reed et al., 2020). Therefore, a Phase II study (NCT01913535) was carried out to confirm its efficacy as add‐on therapy in TRD patients. Although the study was terminated in 2016, because of slow enrolment, the results are published in a very recent paper (Table 1). The low sample size is an obvious limitation of the trial, but the results are positive, but not significant (Fava et al., 2020). Based on these, further investigations are proposed in a larger population of TRD patients.
4.2.3. Buprenorphine
Buprenorphine (Alexander, Mathie, et al., 2021) is a semi‐synthetic derivative of thebaine (isoquinoline derivative alkaloid of opium). It exerts its effect as a partial μ receptor agonist and κ receptor antagonist. This effect is relatively long lasting, due to the slow dissociation from the receptor. High plasma protein binding, relatively low oral bioavailability and excessive metabolism in the liver are characteristics of its pharmacokinetic profile. Phase II (NCT01071538) and Phase III trials (NCT01407575) are investigating its efficacy in younger and older TRD patients (Tables 1 and 2). Buprenorphine treatment results in increased emotional reactivity (Lin et al., 2019), and an impressive drop in depression score was detected. After refinement of the study design and after recruitment of more patients, buprenorphine may prove to be a promising drug candidate in TRD treatment (Karp et al., 2014).
4.3. Drug candidates acting on the serotonergic/monoaminergic system
4.3.1. PF‐04995274
PF‐04995274 (4‐[[4‐[[4‐[(3R)‐oxolan‐3‐yl]oxy‐1,2‐benzoxazol‐3‐yl]oxymethyl]piperidin‐1‐yl]methyl]oxan‐4‐ol) is a partial agonist of the serotonin 5‐HT4 receptor. PF‐04995274 was effective in an animal model of stress (Chen et al., 2020). In healthy people, PF‐04995274 was well tolerated, but a high impact on plasma aldosterone is an issue of concern. The compound has a long half‐life time (31–42 h) and shows no accumulation and food does not influence its plasma level (Nicholas et al., 2011). A presently ongoing Phase 1/2 (NCT03515733) study investigates the therapeutic effect on emotional processing in TRD (Table 1).
4.3.2. GH001
GH001 contains 5‐methoxy‐N,N‐dimethyl‐tryptamine (5‐MeO‐DMT), which can be applied by inhalation to the patients. Phase 1/2 study is initiated in the Netherlands (2018‐004208‐20), after investigating healthy volunteers (completed in 2020, NCT04640831), but the results are not yet published (Table 1).
4.3.3. Psychedelics
Classical psychedelics, such as psilocybin, ayahuasca, mescaline and the semisynthetic lysergic acid diethylamine (LSD), act at 5‐HT2A receptors. Psychedelic‐assisted therapies may be promising alternatives for MDD patients who do not benefit sufficiently when managed by conventional methods. Nevertheless, randomized controlled clinical trials in patients with TRD have been published only on psilocybin and ayahuasca so far (Table 1).
Psilocybin is a naturally occurring alkaloid (tryptamine) known for its psychedelic properties. It inhibits the serotonin transporter and is a partial agonist for 5‐HT2A, 5‐HT2C , 5‐HT1A and 5‐HT1B receptors. Despite its well‐known dose‐dependent adverse effects (sensory illusions, hallucinations, nausea, vomiting and headache), psilocybin has been proven to be effective in mood disorders (Reiff et al., 2020). Psilocybin was found to be effective in reducing depressive and anxiety symptoms, and therefore, it is considered to be a promising and safe agent, even relevant for first‐line treatment (2013‐003196‐35, Carhart‐Harris et al., 2016, 2018; Vargas et al., 2020), where a psychological support therapy comprising three parts (preparation, acute and periacute support, and integration session) was used together with the drug administration. Its short‐ and long‐term safety and efficacy in TRD will be tested in further Phase II studies (NCT03775200, NCT04433858, 2020‐001348‐25, 2019‐003984‐24, 2018‐002577‐22 and 2017‐003288‐36).
Ayahuasca, also known as ‘the liana of the soul’, is a ritual psychedelic that is traditionally administered as a plant decoction. It was originally used in South America for centuries, but in the last 25 years has been used all over the world. The active biological components are N,N‐dimethyltryptamine (DMT), acting mainly as a non‐selective serotonin (5‐HT) receptor agonist, and β‐carboline alkaloids, which are strong and short‐acting MAO type A (MAOI‐A) inhibitors. Ayahuasca has been reported to have anti‐anxiety and antidepressant effects, and its effectiveness in TRD seems to be promising, even comparable with ketamine. Some human studies report a rapid antidepressant effect even after a single exposure, lasting for 21 days (Osório et al., 2015). In 2018, a randomized, double‐blind trial involving 29 TRD patients showed a rapid antidepressant effect persisting up to 7 days after a single administration (Palhano‐Fontes et al., 2019). In another study of 17 TRD patients, increased SPECT blood flow was detected 8 h after a single administration in brain areas related to mood regulation, accompanied by significantly reduced depression severity for 21 days after administration (Sanches et al., 2016). In a randomized trial of TRD patients with increased salivary cortisol levels, a single ayahuasca administration (containing on average 0.36 ± 0.01 mg·ml−1 of N,N‐dimethyltryptamine, 1.86 ± 0.11 mg·ml−1 of harmine, 0.24 ± 0.03 mg·ml−1 of harmaline and 1.20 ± 0.05 mg·ml−1 of tetrahydroharmine) significantly improved the clinical symptoms and reduced the salivary cortisol (NCT02914769, de Almeida et al., 2019; Galvão et al., 2018; Zeifman et al., 2019). Although the clinical results are promising, there are risks of interaction and adverse effects, which are important due to the high variability of the herbal mixture compositions (Barabasz‐Gembczyk & Kucia, 2020).
4.3.4. BMS‐820836
BMS‐820836 is a safe, long‐acting novel triple monoamine reuptake inhibitor (SNDRI). It shows very high occupancy on the serotonin transporter, but it also inhibits the dopamine and noradrenaline transporters. The results of some Phase II studies (NCT01361555, NCT01309945, NCT01369095, 2010‐024371‐12, 2010‐022841‐93 and 2011‐000778‐71) show that its side effect profile is promising, but it does not prove superior to the resumption of an existing antidepressant (Bhagwagar et al., 2015) (Table 1).
4.3.5. MAO inhibitors
CX157/TriRima belongs to a group of reversible inhibitors of MAO‐A (RIMAs). After a successful Phase I study, a Phase II trial (NCT01246908) was conducted between 2010 and 2012, but the results have not been published.
Tranylcypromine is an irreversible, non‐selective MAO inhibitor. Recently, it was proved to be effective in combination with amitriptyline in ECT‐resistant depression (Ferreira‐Garcia et al., 2018). One Phase II study (2012‐001209‐26) is ongoing to investigate the efficacy of the drug in TRD (Table 1).
4.4. Drug candidates acting on the cholinergic system
The cholinergic system can also regulate mood. High central levels of ACh can lead to depression (Dulawa & Janowsky, 2019). Scopolamine was intensively investigated in different forms of depression (2017‐003112‐39, NCT03386448, NCT04211961, NCT03874130 and NCT03131050), but only one trial was designed to investigate its effects in TRD patients (NCT01613820), which was withdrawn due to the lack of funding.
4.4.1. Botulinum toxin A
Facial expressions influence emotional experience and local botulinum toxin A (BTA) treatment of muscles involved in facial expression of sadness, anger or anxiety induced quick, strong and sustained improvement of depressive symptoms. The first results demonstrating potent antidepressant action of BTA 2 months after its injection into the glabellar frown lines of 10 TRD patients were obtained by Finzi and Wasserman (2006). Later, a series of studies including three randomized controlled trials showed beneficial antidepressant effect as a sole or adjunctive therapy predominantly in female TRD patients. More evidence in this field might result in a widespread application of BTA in depression therapy, based on the assumption that superficial paralysis of facial muscles probably via proprioceptive feedback mechanisms could have profound effects on the emotional brain (Kruger & Wollmer, 2015). Recently, in a real‐world setting, with TRD patients, BTA injections significantly reduced depression scores both in males (n = 23) and in females (n = 19), and therefore, BTA has been suggested to be effective for TRD treatment and promoted for further longer lasting clinical trials (Chugh et al., 2018). Elderly TRD patients are currently planned to be involved in a crossover, placebo‐controlled study with BTA, which is considered as a very safe treatment without lasting side effects (NCT03833063; Table 1).
4.4.2. TC‐5214/S‐mecamylamine
TC‐5214/S‐mecamylamine is a non‐selective, non‐competitive antagonist of the nicotinic ACh receptors (nAChRs). There are several preclinical data about the beneficial effects of mecamylamine, but the clinical relevance has not been realized; the only Phase II study in TRD was terminated 9 years ago (2010‐023816‐15) (Table 1). Probably the high coincidence with smoking in patients with psychiatric diseases (depression and attention deficit hyperactivity disorder [ADHD]) strongly influences the evoked effect (Nickell et al., 2013).
4.5. Other tools and targets
4.5.1. Allogenic mesenchymal stem cells
Multipotent mesenchymal stem cells (MSCs) are considered to be promising novel candidates for cell transplantation therapy in various CNS disorders. MSCs are known to secrete soluble factors that stimulate the surrounding micro‐environment. Although MSC transplantation has the potential to elicit antidepressant effect, only a few studies have been conducted to investigate the underlying mechanisms. Intra‐hippocampal transplantation of MSCs in rats enhanced neurogenesis despite a short‐term graft survival; however, MSC transplantation did not exert an antidepressant‐like behavioural effect in this animal model (Coquery et al., 2012). Wistar‐Kyoto rats exhibiting TRD‐like behaviours were used to compare the effect of encapsulated MSCs with conventional antidepressant treatment (Kin et al., 2020). Encapsulated MSCs attenuated spontaneous locomotor and anxiety behaviour accompanied by activation of associated vascular endothelial growth factor (VEGF), BDNF, fibroblast growth factor (FGF)‐2 and ciliary neurotrophic factor (CNTF) pathways suggesting MSC therapy to be a novel tool for treating depression (Kin et al., 2020). A randomized, double‐blind, placebo‐controlled study was started to evaluate the safety and potential efficacy of the treatment, but it was terminated due to difficulties in recruitment and funding (NCT02675556).
4.5.2. Tyrosine kinase (TK) inhibitors
The JAK/STAT signalling pathway is a pleiotropic cascade leading to cytokine and growth factor release. The JAK/STAT pathway is involved in the regulation of several cellular mechanisms, such as neurogenesis, synaptic plasticity, gliogenesis and microglial activation, which all have been implicated in depression. JAK/STAT inhibitors restored stress‐induced reduction in adult neurogenesis, as well as an associated anxious‐depressive behaviour in mice (Gulbins et al., 2016). Furthermore, the mechanism of actions of some current antidepressants has been shown to be mediated by JAK/STAT‐dependent mechanisms. Although JAK inhibitors have been approved primarily for inflammatory and autoimmune conditions such as rheumatoid arthritis (RA), observational data obtained from these patients suggest that they significantly improve mood. Because JAK is mainly coupled to IL‐6 signalling, it is not surprising that biologicals against the interleukin (IL)‐6‐related pathway (e.g., tocilizumab) also have a favourable impact on depressive symptoms of RA patients (Tiosano et al., 2020). It is difficult to clearly differentiate between the potential direct antidepressive effect of JAK inhibitors from their secondary beneficial actions as a consequence of their anti‐inflammatory and analgesic effects in arthritis patients. Because not all studies showing the antidepressant effect of these drugs were performed in arthritic patients, but also in patients without inflammatory diseases, it is very likely that the advantageous effect on depression does not result only from pain control but also from the decrease of central neuroinflammatory mechanisms. Furthermore, results of some omics analyses of depressed patients also support the involvement of the JAK/STAT pathways in the pathophysiological mechanisms of the disease. Therefore, JAK/STAT inhibitors have been proposed as novel therapeutic options (Shariq et al., 2018); a Phase I/II study (NCT04141904) has already been started to evaluate its efficacy in TRD patients.
Masitinib, a TK c‐Kit inhibitor, was also tested in TRD (2010‐022744‐21), but the study was terminated in 2018 (Table 1).
4.5.3. Statins
The 3‐hydroxy‐3‐methylglutaryl co‐enzyme A (HMG‐CoA) reductase inhibitors, denoted statins, are lipid‐lowering drugs that have been shown to reduce depression‐like behaviours in animals. The lipophilic simvastatin was recently demonstrated to dose‐dependently (20–40 mg·kg−1) reduce the immobility time in FST in stress‐naïve mice, possibly by stimulating opioidergic pathways without inducing tolerance or withdrawal signs (Dolatshahi et al., 2020). NO‐cGMP‐KATP channels and the PPARγ receptor might also be involved in the simvastatin‐induced antidepressant effect, which also potentiated the action of fluoxetine (Naserzadeh et al., 2019).
The first clinical trial investigating the use of simvastatin as an augmentation strategy in TRD patients is currently going on as a randomized, double‐blind, placebo‐controlled study with two parallel arms involving 75 patients as add‐on therapy (NCT03435744; Table 2). If the results indicate that adjuvant simvastatin is effective for depressive symptoms, it will deliver immediate clinical benefit (Husain et al., 2019).
4.5.4. Leucine and the NV‐5138 analogue
Preclinical studies demonstrate that rapid‐acting antidepressants, including ketamine, require rapamycin complex 1 (mTORC1) signalling stimulation (Hasegawa et al., 2019; Kato et al., 2019). This pathway is regulated by neuronal activity, endocrine and metabolic signals, notably the amino acid leucine, which activates mTORC1 signalling via binding to the upstream regulator sestrin. The synthetic leucine analogue NV‐5138 is a novel highly selective modulator of sestrin that penetrates the brain. NV‐5138 has a rapid and sustained antidepressant action in rat and mouse models via mTORC1 signalling activation, up‐regulation of BDNF and the induction of rapid synaptic responses in the medial prefrontal cortex. These findings suggest that direct activation of mTORC1 signalling might be effective for depression therapy (Hasegawa et al., 2019; Kato et al., 2019), but Phase I/II study (NCT03606395) results have not yet confirmed the beneficial effect in humans (Table 1).
4.5.5. Tibolone and oxytocin
Specific guidelines for the treatment of perimenopausal depression were released in 2018 (Maki et al., 2019). These guidelines recommend selective serotonin (SSRIs) and serotonin‐noradrenaline reuptake inhibitors (SNRIs) as front‐line medications, but they are commonly ineffective. The efficacy of oestrogen in such conditions is well documented, but oestrogen is not approved to treat mood disturbances in perimenopausal women due to potentially severe side effects (Garay et al., 2019). However, novel oestrogenic compounds with improved benefit/risk ratios, which directly target hormonal fluctuations, such as tibolone, have potential in perimenopausal depression. Tibolone is metabolized to oestrogen‐like, gestagen‐like and androgen‐like products and therefore balances the hormonal status. In a randomized, double‐blind placebo‐controlled add‐on study involving 44 women, 12‐week long adjunctive tibolone (2.5 mg oral·day−1) was demonstrated to exert a significant improvement in depression scores, as compared with the placebo group without any significant side effects (Kulkarni et al., 2018).
Oxytocin plays a role in depressive disorders by interacting with various neuroendocrine and neuroinflammatory processes mediated via stressor‐provoked psychosocial responses (McQuaid et al., 2014). Local injection of oxytocin into the rat paraventricular nucleus reversed depressive‐like behaviours and high plasma corticosterone levels, as well as increased tropomyosin receptor kinase B (TrkB) expression, suggesting that the antidepressant effect of oxytocin is mediated by hypothalamic–pituitary–adrenal (HPA) axis modulation via TrkB (Wang et al., 2018). Currently, only one Phase I study is ongoing with oxytocin and tibolone in TRD (NCT01239888).
4.5.6. Creatine
There is growing evidence from human neuroimaging, genetic, epidemiological and animal studies that disruptions in brain energy production, storage and utilization are implicated in the development and maintenance of depression. Creatine, a widely available nutritional supplement, has the potential to improve these disruptions in some patients, and early clinical trials indicate that it is a potential adjuvant antidepressant agent (Kious et al., 2019). There is evidence for its effectiveness in the secondary prevention of TRD in women. Creatine supplementation was proven to be effective in neuropsychological performance in vegetarians or vegans and heavy‐duty labourers, people who undergo intensive mental effort or in the elderly (Balestrino & Adriano, 2019). There are only mild side effects: nausea, diarrhoea, muscle cramps, hypomania/mania, peripheral oedema and increase of serum creatinine level induced by creatine (Hellem et al., 2015; Kious et al., 2019). A randomized, quadruple‐blind, parallel assignment, placebo‐controlled clinical study was carried out in 32 female patients between 2012 and 2016, but the results have not been published yet (NCT01601210).
4.5.7. General anaesthetics: Propofol, isoflurane and ethosuximide
There are converging preclinical and clinical data that the general anaesthetics, isoflurane (NCT04171193), propofol (NCT03923361 and NCT03684447) and ethosuximide (NCT03887624), acting mainly by activating the GABAergic system (Table 1), exert relatively rapid, robust and durable antidepressant effects and lack serious adverse effects (Tadler & Mickey, 2018).
4.5.8. Anticonvulsants and voltage‐gated ion channel blockers
Previous results of randomized controlled clinical trials investigating the effect of antiepileptic drugs in MDD were inconclusive for lamotrigine and carbamazepine; although, overall, lamotrigine may have a beneficial, but modest effect, negative results were found in TRD patients (NCT00652171). JNJ26489112 with complex mechanism of action (voltage‐gated Na+ channel and N‐type Ca2+ channel inhibitor, K+ channel opener) was investigated in a Phase II study (NCT01114698) but was terminated due to sponsor portfolio decision (Table 1).
4.5.9. l‐DOPA
Late‐life depression often presents with motivational deficits, and cognitive deficits including executive dysfunctions, slow information processing and mobility impairments. This triad of findings implicates dopaminergic dysfunction as a core pathophysiologic feature in depression and may contribute to cognitive decline and motor disability. Normal aging results in brain‐wide dopamine declines, decreased D1/D2 dopamine receptor density and loss of dopamine transporters. Although brain changes associated with depression and aging converge on dopamine circuits, the specific disturbances in depression and how responsive the system is to modulation remain unclear (Taylor, 2014). The integrative model in which aging, in concert with proinflammatory shifts, decreases dopamine signalling, as well as the effect of l‐DOPA + carbidopa in elderly depression are currently tested in a randomized, double‐blind placebo‐controlled clinical trial (NCT04469959; Table 1).
4.6. Augmentation and combination therapy
Besides switching to a different antidepressant, combining antidepressants or augmenting an antidepressant with another non‐antidepressant drug (add‐on therapy) are the most important approaches in TRD.
A large spectrum of various agents has been tried as an augmentation strategy. These include second‐generation antipsychotics, lithium, dopamine agonists, celecoxib, modafinil, benzodiazepines, l‐methylfolate, lamotrigine, pindolol, riluzole, ω‐3 fatty acids, topiramate, amphetamines, S‐adenosyl‐l‐methionine, herbal supplements, thyroid hormone and sex steroids that have been used in patients with TRD with various outcomes (Ionescu et al., 2015; Shelton et al., 2010; Tundo et al., 2015). Minocycline, a well‐known member of the second‐generation tetracycline antibiotics, is also a potentially beneficial agent for augmentation treatment (Pae et al., 2008), although the results of the Phase I/II studies (NCT02456948 and 2015‐001456‐29) are not yet published (Tables 1 and 2).
Augmentation of conventional SSRIs or SNRIs with atypical antipsychotics, such as quetiapine, risperidone, aripiprazole or brexpiprazole, is effective and more beneficial than switching to another antidepressant monotherapy, especially when comorbid anxiety or sleeping disorders are present. The combination of olanzapine and fluoxetine was one of the first pharmacotherapies approved for TRD, but its use is limited by the common metabolic side effects. Other effective strategies include augmentation with lithium, liothyronine (a synthetic T3), lamotrigine or a combination of antidepressants including bupropion, tricyclic antidepressants or mirtazapine. The efficacy of ziprasidone or levothyroxine is well established. A shared decision‐making approach is recommended to guide treatment selection to address each patient's individual needs (Cantù et al., 2020; Ruberto et al., 2020).
Based on the findings of the published trials, the most widely studied drugs with significant antidepressant activity in TRD are the inhibitors of the glutamatergic system (racemic ketamine, dextromethorphan, AZD6765 and GLYX‐13). Although the NMDA receptor was pointed out as the main target for novel drug development according to the clinical effectiveness of (S)‐ketamine, some of the selective antagonists were not effective in human studies. Because (S)‐ketamine has divergent mechanisms of action besides being an NMDA receptor antagonist, expectation regarding the potential therapeutic value of other selective NMDA receptor antagonists should be cautious. Further compelling therapeutics are buprenorphine, psilocybin and ayahuasca as well as the add‐on therapy of olanzapine, basimglurant or minocycline (Figure 3).
5. NEW TREATMENT STRATEGIES WITH NOVEL MECHANISMS OF ACTION
The purpose of this section is to briefly highlight a few pathophysiological theories that have been implicated in TRD and came into focus in recent drug development.
Since the unexpected discovery that ketamine infusion can rapidly improve core symptoms of depression (Berman et al., 2000), there has been intense research focusing on the glutamatergic system as a potential target for similar rapid‐acting compounds (Zarate, Mathews, et al., 2013). Clinical studies reported altered glutamate levels in the brains of depressed patients using in vivo proton magnetic resonance spectroscopy (Moriguchi et al., 2019), and post‐mortem studies found expressional changes of NMDA receptors (Duman et al., 2019; Feyissa et al., 2009). Altered functioning of astrocytes, as the third element of the tripartite synapse, is likely to contribute to disturbed glutamatergic signalling (Choudary et al., 2005). Based on these findings, numerous compounds have been developed to act on the glutamatergic system as candidates for TRD treatment. Therefore, at this moment, targeting the glutamatergic system appears to be the most promising approach to develop novel treatments for TRD.
Neuroinflammatory processes, the circulating cytokines and chemokines, as well as the altered functioning of microglial cells have also been implicated in the pathophysiology of MDD and TRD (Bhattacharya & Drevets, 2017). Chronic stress and the consequent release of proinflammatory cytokines contribute to the development of neuroinflammation, which is likely to play a significant role in depressive symptomatology (Yuan et al., 2019). A recent study reported that TRD patients had higher levels of numerous inflammatory proteins compared with controls and that the elevated levels of IL‐6, IL‐8, TNF, C‐reactive protein and macrophage inflammatory protein‐1 were associated with poorer treatment outcomes (Strawbridge et al., 2019). Another study found elevated C‐reactive protein in patients with TRD (Chamberlain et al., 2019). So far, only a few studies investigated the antidepressant effectiveness of anti‐inflammatory compounds. A double‐blind, placebo‐controlled, randomized clinical trial tested the TNF antagonist infliximab to treat TRD (Raison et al., 2013). This proof‐of‐concept study could not find a clear beneficial effect on the change of Hamilton Depression Rating Scale (HAM‐D) scores but reported that infliximab treatment could improve depressive symptoms in a subset of patients with high baseline inflammatory biomarkers (Raison et al., 2013). More recently, the efficacy of antidepressant augmentation with minocycline was tested in TRD patients with a 4‐week, placebo‐controlled, randomized clinical trial, but changes in HAM‐D scores and the proportion of partial responders did not differ between the study arms (Nettis et al., 2021). However, the same study reported that patients with elevated levels of serum C‐reactive protein showed the largest changes in HAM‐D scores and that responders had higher baseline IL‐6 concentrations than non‐responders (Nettis et al., 2021). Finally, there is a recent report on an ongoing double blind, placebo‐controlled, randomized trial of minocycline as adjunctive treatment for TRD (Husain et al., 2020).
Most recently, the gut microbiota and the gut–brain axis came into focus as a major contributing factor for the development of MDD (Sanada et al., 2020; Valles‐Colomer et al., 2019), and it has been implicated also in TRD (Fontana et al., 2020). The initial finding was that faecal microbiota transplantation from depressed patients to microbiota‐depleted rats can induce depressive‐like behaviour in the recipient animals (Kelly et al., 2016). Rapidly accumulating evidence documents that psychobiotic therapy, that is, nurturing gut microbiome with prebiotic treatment, can have an antidepressant‐like effect (Wallace & Milev, 2017), but to the best of our knowledge, probiotic therapy has not yet been tested in TRD patients.
6. NOVEL APPROACHES AND CONCEPTS FOR ANTIDEPRESSANT DRUG DEVELOPMENT
Despite significant efforts, there has not been a real breakthrough in the therapy of MDD during the past decades. The possible reasons for this are the inappropriate, hypothesis‐driven concepts and approaches to identify novel drug targets, as well as the lack of translationally relevant, predictive preclinical models and investigational techniques as experimental tools. Moreover, the heterogeneity of the symptoms and the broad range of comorbidities have not been adequately addressed during drug development. Recently, due to a paradigm shift in drug target identification, new strategies have been employed to understand the underlying neurobiological mechanisms. Such approaches include multi‐omics (transcriptomics, metabolomics and proteomics), complex neuroimaging techniques as well as bioinformatic network pathway analysis. Genetic and epigenetic factors, as well as neuroinflammatory and metabolic pathophysiological mechanisms, are thoroughly investigated by these methods (Maes et al., 2016).
Genetic and genomic factors influence treatment responsiveness in MDD; however, the underlying genes and specific gene networks are still unknown. Similarly, little is known about the interaction of genetic and environmental factors. However, some single nucleotide polymorphisms are associated with the responsiveness to certain antidepressants; for example, effectiveness of bupropion was implicated in pathways associated with circadian rhythm and growth factor‐associated neuroplasticity (Li et al., 2016). In an extended meta‐analysis that pooled data from five GWASs (Genome‐Wide Association Study), Li et al. (2020) analysed the largest cohort ever on antidepressant efficacy in MDD and identified loci associated with treatment response to SSRI, SNRI as well as with TRD. Particularly, genes and genetic sites associated with the immune regulation, such as the LTB (lymphotoxin β) gene on chromosome 6, an inducer of the inflammatory response, which underlines the role of the inflammatory response system in TRD. The dysregulation of this in the antidepressant response seems to be relevant. Moreover, in the antidepressant response to SSRI, the role of complement component (3b/4b) receptor 1‐like (CR1L) polymorphism was found to be significant. Gammie (2021) examined gene expression portraits using large‐scale neural gene expression depression datasets to identify potential treatments for depression. For known antidepressants, top‐scoring agents were fluoxetine, desipramine, imipramine, venlafaxine and ketamine. When examining potential novel antidepressants, exercise has been proved as highest ranking, beside other nontraditional treatments such as curcumin, elevated temperature, d‐serine, albiflorin, creatine, alfa‐ and γ‐tocopherol with vitamin E and nicotinamide ribonide. Although the Gammie (2021) study was not restricted to TRD, these results can be considered when searching for novel treatment targets for TRD as well. Some of them (e.g., curcumin and creatine) were tested in studies reviewed by us here.
Transcriptomic studies using functional pathway analyses in post‐mortem brain samples of patients with MDD also revealed disruptions of neuronal and glial plasticity, growth factors and circadian rhythm (Bunney et al., 2015; Duman & Duman, 2015), as well as regulatory changes of microRNAs (Dwivedi, 2014) and epigenetic signatures (Sun et al., 2013). Gene expression regulation by post‐translational histone transcription factors and chromatin regulatory protein modifications, cytosine base methylation of the DNA, translational regulation of RNAs by microRNAs, splicing and editing all play important roles in the emergence, progression of and therapy responsiveness of MDD patients (Bagot et al., 2014). Examining these mechanisms is particularly useful to explore the complexity of the pathophysiology and therapy responsiveness in MDD. However, a broad range of confounding factors can limit the post‐mortem sample analysis, such as age, comorbidities, multiple drugs including psychoactive medications as well as technical factors (post‐mortem interval, processing and evaluation methods). Regarding that MDD is a particularly heterogeneous nosological entity, much larger samples are required to uncover the relevant genetic factors (Tubbs et al., 2020). Results from multiple brain regions from a large number of patients are needed to be compared with those of healthy controls to draw convincing conclusions.
Neuroimaging techniques appear to be promising methods to detect structural and functional brain alterations in TRD. Neuroimaging can examine factors underlying the heterogeneity of treatment responsiveness, and the trajectory and outcome of MDD. Treatment responsiveness has an immediate impact on the clinical and functional outcome; it could be directly related to genetic risk load and manifested as a specific pattern of functional and structural brain abnormalities. Therefore, brain imaging can identify neuronal correlates that serve as predictive biomarkers of treatment resistance. Neuroimaging in combination with genetic studies could determine predictors of clinical outcome and therapeutic responsiveness (Fu et al., 2019). Furthermore, evaluating the occupancy of serotonin and norepinephrine transporters by specific PET radioligands assesses the in vivo target engagement and can predict the therapeutic effectivity of the antidepressant medication. Also, dopamine regulates functions that are often impaired in MDD and lead to hedonic deficits. Mesolimbic dopamine receptor sensitivity can predict the antidepressant response (Gershon et al., 2007). PET studies using dopamine D2 /3 receptor‐specific radiotracers can further characterize the D2/3 receptor binding in TRD and may yield informative results in the future. Nevertheless, to evaluate novel drug targets, specific PET radioligands need to be developed parallel with drug development (Arakawa et al., 2020).
Large‐scale neuroimaging consortia such as the ENIGMA project examined GWAS variation concerning brain structure of MDD patients and healthy subjects (Thompson et al., 2013). Such an approach may help to unravel associations of specific genetic variants with MRI‐based brain structural and functional alterations. Integrating data of pharmaco‐neuroimaging and imaging genetics is critical for reverse translation, that is, to determine to which extent animal models can mimic the pathophysiology of MDD and test their relation to specific clinical features including treatment responsiveness.
As more recent findings suggest, epigenetic regulation of gene expression plays a key role in disease development and therapeutic responsiveness of depressed patients (Akil et al., 2018). Epigenetic regulatory mechanisms include a broad range of molecular machineries, such as post‐translational histone modifications, methylation of cytosine bases on DNA, modulation of transcription factors and numerous chromatin‐regulatory proteins, and translational regulation of RNAs by microRNAs and RNA splicing; psychosocial stressors during development could reprogram the epigenome; and in consequence influence several functions during the whole lifespan by being incorporated in the germ cells. From the pathophysiological point of view, aberrant epigenomic modulation of the glucocorticoid receptor gene, as a result of early life stress, seems to link adverse childhood experiences with adult psychopathologies like MDD (Holmes et al., 2019; Juruena et al., 2021). Epigenetic mechanisms underlie the reduced expression of BDNF in an animal model of chronic stress, and hypermethylation of the BDNF promoter IV was observed in the Wernicke's area of suicide victims (Caraci et al., 2018). While epigenetic changes that are observed in peripheral blood samples are often in harmony with the alterations found in the brain (Han et al., 2018), the question still remains as to how well the findings from peripheral blood samples mirror the neurobiological underpinnings of MDD.
From the therapeutic point of view, targeting epigenetic mechanisms may yield a completely novel class of antidepressants. For example, it is well documented that chromatin remodelling mediated by histone deacetylase (HDAC) influences gene transcription. Furthermore, the experimental data documenting that HDAC inhibitors have antidepressant‐like efficacy in rodent‐models of depression led to the concept that HDAC inhibitors might be useful in TRD patients (Fuchikami et al., 2016). Recently, the FDA approved two HDAC inhibitors, vorinostat and romidepsin, for clinical use to treat malignancies, but to date, no clinical trials have been conducted to evaluate HDAC inhibitors for the treatment of depression (Fuchikami et al., 2016). Interestingly, an augmentation treatment with the antiepileptic valproic acid—which is also considered to be an HDAC inhibitor—yielded substantial clinical improvement and maintenance in a small cohort of severe TRD patients (Ghabrash et al., 2016). Another potentially beneficial approach is the use of various dietary DNA methylation modifying agents such folic acid, S‐adenosylmethionin or l‐acetylcarnitine (Hoepner et al., 2021; Nasca et al., 2018). Similarly, therapeutic application of probiotics to reduce depressive symptoms is another promising tactic because metabolic activity of gut microbiota seems to regulate epigenetic mechanisms of host tissues (Bambling et al., 2017; Kazemi et al., 2019; Miro‐Blanch & Yanes, 2019). Overall, targeting epigenetic regulation may represent a fruitful approach to develop novel drugs with potentially higher and persistent efficacy (Akil et al., 2018; Caraci et al., 2018).
7. TRANSLATIONAL ANIMAL MODELS FOR TESTING NOVEL ANTIDEPRESSANT CANDIDATES IN TRD
Animal models play a vital role in drug development, but to model complex mental disorders in animals is an extremely challenging task (Bale et al., 2019; Berton et al., 2012). Decades of research have been invested in developing various types of clinically relevant models for MDD (Czéh et al., 2016; Nestler & Hyman, 2010; O'Leary & Cryan, 2013; Pryce & Fuchs, 2016; Willner, 1997). Typically, these models are based either on genetic or pharmacological manipulations or on the application of social or environmental stressors. Until now, most studies focused on animals that were susceptible to the applied stressors and responded well to the antidepressant medication. It is known, however, that in the existing models, only a fraction of animals responds to the applied antidepressant therapy (for an overview, see Caldarone et al., 2015). In a rat model of chronic mild stress (Willner, 2016), approximately 50% of the animals normalize their disturbed behaviour in response to escitalopram or sertraline treatment (Christensen et al., 2011; Jayatissa et al., 2006). Comparable data have been shown in rats treated with fluoxetine or desipramine in a chronic social defeat stress model (Der‐Avakian et al., 2014). Another interesting finding is that chronic pretreatment with adrenocorticotropic hormone (ACTH) can block the antidepressant‐like effect of imipramine and desimipramine in the forced swim test (Kitamura et al., 2002; Walker et al., 2013).
So far, only a few research groups have made extra efforts to develop specific animal models for TRD. For example, the Wistar‐Kyoto rat model has been shown to be less responsive to chronic antidepressant treatment with imipramine (Lahmame et al., 1997; Willner & Belzung, 2015). More recently, Willner and co‐workers (2019) used this genetic model and subjected Wistar‐Kyoto rats to the chronic mild stress protocol and reported that these animals were nonresponsive to drug treatment with imipramine, citalopram or venlafaxine, but responded well to deep brain stimulation as well as to ketamine treatment. Another approach was to apply a high‐fat diet (45% fat) to mice in combination with the chronic mild stress protocol, and this high‐fat diet protocol could prevent the antidepressant effect of fluoxetine (Isingrini et al., 2010). Yet another study reported that centrally administered IL‐6 or endogenous overexpression of IL‐6 in the brain could prevent the antidepressant‐like response to systemic fluoxetine treatment (Sukoff Rizzo et al., 2012). Overall, these findings imply that a TRD‐like condition can be generated also in experimental animals.
In our view, a valid TRD model should include some kind of genetic susceptibility and an early life stressor because childhood adversity seem to contribute to the development of TRD (Tunnard et al., 2014; Williams et al., 2016). Afterwards, the adolescent or adult animals should be subjected to repeated periods of stress and antidepressant treatment, which would then lead to a condition where a subset of the animals is resistant to the drug treatment. This model would require a large number of animals that are subjected to various stressors and afterwards treated with different types of established antidepressant drugs to prove their resistance to the conventional therapy. Obviously, such a complex and work‐intensive, long‐term protocol would not be suitable for everyday drug screening, but it would be helpful for studying the underlying pathophysiology and to test selected, highly promising, novel drug candidates.
For screening the effectiveness of novel compounds, investigators can employ the widely available, simple behavioural assays. Typically, these tests assess the animal's response to acute stressors, for example, the forced swim or tail suspension tests. One should add that these tests have been criticized for the anthropomorphic interpretation of the ‘despair’ behaviour that the animals display in these tests. Other tests assess the reward‐seeking (hedonic) or anxiety‐like behaviour, grooming and social interaction between the animals. Clearly, none of these screening assays can mimic the depressed phenotype, but they can give a hint whether the tested compound can influence hedonic, or fearful behaviour, or engagement in motorically demanding tasks. Importantly, all these simple behavioural tests were optimized to screen for the potential efficacy of drugs under development. Physiological parameters, such as neuroendocrine responses (e.g., HPA‐axis activity and sex hormone levels), changes in core body temperature, ultradian sleep cycle or body weight gain, have also been used to assess the response of the animals. Regrettably, none of these measures yield unambiguous results. In conclusion, if these simple behavioural tests are employed as a battery of tests following a model of stress induction, then they can yield a fairly reliable assessment of the drug response of the animals and enable the researchers to identify certain subtypes of the behavioural responses that can be altered by novel compounds.
8. SUMMARY, CONCLUSIONS AND FUTURE PERSPECTIVES
For decades, traditional antidepressants have targeted the monoamine pathways, but one third of MDD patients still remain therapy resistant. Hence, there is an urgent need to identify novel targets with different mechanisms of action.
Here, we systematically reviewed American and European registries of clinical studies in the past 10 years. We aimed to outline promising trends in drug development that can be potentially useful in the treatment of TRD. We identified numerous novel targets; however, the majority of them are still in early developmental phases. In Figure 3, we present agents that were found to be clinically effective in TRD in published trials.
So far, intranasal S‐ketamine treatment, approved in 2019 by the FDA and EMA, is the sole treatment option specifically indicated in TRD, as add‐on therapy to antidepressants acting on monoaminergic system. S‐ketamine is considered to be relatively safe, but several psychiatric, cardiovascular and neurological side effects have been reported; therefore, its long‐term safety is heavily debated (Ramadan & Mansour, 2020). Because of the established clinical effectiveness of (S)‐ketamine, the NMDA receptor has been singled out as the main novel target for drug development. Disappointingly, some of the selective NMDA receptor antagonists have failed in clinical studies. Importantly, (S)‐ketamine acts not only on NMDA receptors, and the exact molecular and cellular mechanisms responsible for the antidepressant efficacy of ketamine are not completely understood; therefore, the therapeutic value of selective NMDA receptor antagonists should not be taken for granted. However, based on our present evaluation of the published trials, the non‐selective inhibitors and partial agonists of the NMDA receptors (i.e., racemic ketamine, GLYX‐13 and dextromethorphan) are the most promising candidates in TRD. Furthermore, basimglurant, a selective, negative allosteric modulator of a metabotropic glutamate receptor, mGlu5, yielded encouraging results in TRD.
Considering the opioid system, κ opioid receptor antagonists seem to have the most favourable effect in TRD, among which buprenorphine was the most effective in clinical trials.
Currently, there is a revival in the use of psychedelic drugs containing tryptamine derivates (e.g., psilocybin and ayahuasca) for therapeutic purposes. Results with psilocybin are especially convincing when it is applied together with operationalized psychotherapy. These compounds are believed to act on the serotonergic/monoaminergic system, and the optimal dosage and treatment regimens need to be determined. These drugs are likely to be valuable alternative therapeutic tools in TRD treatment.
Finally, it should be emphasized that currently, combination strategies are the most evidence‐based and convincing treatment options for TRD. Among the augmentative agents on top of conventional SSRIs or monoamine reuptake inhibitors, atypical (second‐ and third‐generation antipsychotics, i.e., aripiprazole, brexpiprazole, quetiapine and olanzapine) and lithium have the strongest evidence for effectiveness. Further studies are needed to evaluate the augmentative efficacy of anticonvulsants, thyroid hormones, novel glutamatergic agents, anti‐inflammatory agents and nutraceuticals.
In our opinion, the most promising leads for the treatment of TRD are non‐selective NMDA and mGlu5 receptor antagonists, κ opioid receptor antagonists and hallucinogenic tryptamine derivates.
8.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22 (Alexander, Christopoulos, et al., 2021; Alexander, Cidlowski, et al., 2021; Alexander, Fabbro, et al., 2021; Alexander, Kelly, et al., 2021; Alexander, Mathie, et al., 2021)
AUTHOR CONTRIBUTIONS
É.B. (novel investigational drugs and regimens under clinical trials), M.S. (treatment‐resistant depression is an unmet medical need), B.C. (new treatment strategies with novel mechanisms of action, translational animal models for testing novel antidepressant candidates in TRD) and Z.H. (S‐ketamine is a novel add‐on treatment specifically approved for TRD, novel approaches and concepts for antidepressant drug development) wrote the manuscript. E.F. and O.W. also helped to write and revise the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ACKNOWLEDGEMENTS
The authors were financially supported by the following grant agencies: Hungarian Brain Research Program (2017‐1.2.1‐NKP‐2017‐00002) and EU Social Funds (EFOP‐3.6.2‐16‐2017‐00008, ‘The role of neuro‐inflammation in neurodegeneration: from molecules to clinics’, and EFOP‐3.6.1‐16‐2016‐00004, ‘Comprehensive Development for Implementing Smart Specialization Strategies at the University of Pécs’), and the Project No. TKP2020‐IKA‐08 has been implemented with the support provided by the National Research, Development and Innovation Fund of Hungary, financed under the 2020‐4.1.1‐TKP2020 funding scheme. This paper was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences, by the ÚNKP‐21‐5 new National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund and Richer Gedeon NyRt. ZSH and ÉB was supported by the Hungarian National Research, Development and Innovation Office (Nemzeti Kutatási, Fejlesztési és Innovációs Hivatal) (OTKA K138046 and FK137951). MS was supported by the ÁOK‐KA Szolcsányi János research fund (Pécsi Tudományegyetem). These grant agencies had no influence in the writing of the report and in the decision to submit the article for publication.
Borbély, É. , Simon, M. , Fuchs, E. , Wiborg, O. , Czéh, B. , & Helyes, Z. (2022). Novel drug developmental strategies for treatment‐resistant depression. British Journal of Pharmacology, 179(6), 1146–1186. 10.1111/bph.15753
Funding information János Bolyai Research Scholarship of the Hungarian Academy of Sciences, Grant/Award Number: BO/00592/19/5; EU Social Fund, Grant/Award Number: EFOP‐3.6.1‐16‐2016‐00004 EFOP‐3.6.2‐16‐2017‐00008; Hungarian Brain Research Program, Grant/Award Number: 2017‐1.2.1‐NKP‐2017‐00002; Nemzeti Kutatási Fejlesztési és Innovációs Hivatal, Grant/Award Numbers: TKP2020‐IKA‐08, 2020‐4.1.1‐TKP2020, OTKA FK137951, OTKA K138046, ÚNKP‐21‐5‐PTE‐998; Pécsi Tudományegyetem (Szolcsányi János Research Fund)
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article because no new data were created or analysed in this study.
REFERENCES
- Agbo, F. , Bui, K. H. , & Zhou, D. (2017). Population pharmacokinetic analysis of lanicemine (AZD6765), an NMDA channel blocker, in healthy subjects and patients with major depressive disorder. Journal of Clinical Pharmacy and Therapeutics, 42(5), 539–546. 10.1111/jcpt.12541 [DOI] [PubMed] [Google Scholar]
- Akil, H. , Gordon, J. , Hen, R. , Javitch, J. , Mayberg, H. , McEwen, B. , Meaney, M. J. , & Nestler, E. J. (2018). Treatment resistant depression: A multi‐scale, systems biology approach. Neuroscience and Biobehavioral Reviews, 84, 272–288. 10.1016/j.neubiorev.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrova, L. R. , Phillips, A. G. , & Wang, Y. T. (2017). Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. Journal of Psychiatry & Neuroscience: JPN, 42(4), 222–229. 10.1503/jpn.160175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐Hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(Suppl 1), S27–S156. 10.1111/bph.15538 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Coons, L. , Fuller, P. J. , Korach, K. S. , & Young, M. J. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Nuclear hormone receptors. British Journal of Pharmacology, 178(S1), S246–S263. 10.1111/bph.15540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Boison, D. , Burns, K. E. , Dessauer, C. , Gertsch, J. , Helsby, N. A. , Izzo, A. A. , Koesling, D. , … Wong, S. S. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes. British Journal of Pharmacology, 178(S1), S313–S411. 10.1111/bph.15542 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Amarosi, L. , Anderson, C. M. H. , Beart, P. M. , Broer, S. , Dawson, P. A. , Hagenbuch, B. , Hammond, J. R. , Inui, K.‐i. , … Verri, T. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Transporters. British Journal of Pharmacology, 178(S1), S412–S513. 10.1111/bph.15543 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Aldrich, R. W. , Attali, B. , Baggetta, A. M. , Becirovic, E. , Biel, M. , Bill, R. M. , Catterall, W. A. , … Zhu, M. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Ion channels. British Journal of Pharmacology, 178(Suppl 1), S157–S245. 10.1111/bph.15539 [DOI] [PubMed] [Google Scholar]
- Arakawa, R. , Takano, A. , & Halldin, C. (2020). PET technology for drug development in psychiatry. Neuropsychopharmacology Reports, 40(2), 114–121. 10.1002/npr2.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot, R. C. , Cates, H. M. , Purushothaman, I. , Vialou, V. , Heller, E. A. , Yieh, L. , LaBonté, B. , Peña, C. J. , Shen, L. , Wittenberg, G. M. , & Nestler, E. J. (2017). Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience‐specific gene expression profiles. Biological Psychiatry, 81(4), 285–295. 10.1016/j.biopsych.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot, R. C. , Labonté, B. , Peña, C. J. , & Nestler, E. J. (2014). Epigenetic signaling in psychiatric disorders: Stress and depression. Dialogues in Clinical Neuroscience, 16(3), 281–295. 10.31887/DCNS.2014.16.3/rbagot [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale, T. L. , Abel, T. , Akil, H. , Carlezon, W. A. Jr. , Moghaddam, B. , Nestler, E. J. , Ressler, K. J. , & Thompson, S. M. (2019). The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 44(8), 1349–1353. 10.1038/s41386-019-0405-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrino, M. , & Adriano, E. (2019). Beyond sports: Efficacy and safety of creatine supplementation in pathological or paraphysiological conditions of brain and muscle. Medicinal Research Reviews, 39(6), 2427–2459. 10.1002/med.21590 [DOI] [PubMed] [Google Scholar]
- Bambling, M. , Edwards, S. C. , Hall, S. , & Vitetta, L. (2017). A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: An intestinal anti‐inflammatory response is suggested. Inflammopharmacology, 25(2), 271–274. 10.1007/s10787-017-0311-x [DOI] [PubMed] [Google Scholar]
- Barabasz‐Gembczyk, A. , & Kucia, K. (2020). Ayahuasca—Potential therapeutic properties in psychiatry. Research review. Ayahuaska—Potencjalne właściwości terapeutyczne w psychiatrii. Przegląd badań. Psychiatria Polska, 54(2), 381–389. 10.12740/PP/103364 [DOI] [PubMed] [Google Scholar]
- Bartova, L. , Dold, M. , Kautzky, A. , Fabbri, C. , Spies, M. , Serretti, A. , Souery, D. , Mendlewicz, J. , Zohar, J. , Montgomery, S. , Schosser, A. , & Kasper, S. (2019). Results of the European Group for the Study of Resistant Depression (GSRD)—Basis for further research and clinical practice. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, 20(6), 427–448. 10.1080/15622975.2019.1635270 [DOI] [PubMed] [Google Scholar]
- Berman, R. M. , Cappiello, A. , Anand, A. , Oren, D. A. , Heninger, G. R. , Charney, D. S. , & Krystal, J. H. (2000). Antidepressant effects of ketamine in depressed patients. Biological Psychiatry, 47(4), 351–354. 10.1016/s0006-3223(99)00230-9 [DOI] [PubMed] [Google Scholar]
- Berton, O. , Hahn, C. G. , & Thase, M. E. (2012). Are we getting closer to valid translational models for major depression? Science (New York, N.Y.), 338(6103), 75–79. 10.1126/science.1222940 [DOI] [PubMed] [Google Scholar]
- Bhagwagar, Z. , Torbeyns, A. , Hennicken, D. , Zheng, M. , Dunlop, B. W. , Mathew, S. J. , Khan, A. , Weisler, R. , Nelson, C. , Shelton, R. , Thase, M. E. , & Lane, R. (2015). Assessment of the efficacy and safety of BMS‐820836 in patients with treatment‐resistant major depression: Results from 2 randomized, double‐blind studies. Journal of Clinical Psychopharmacology, 35(4), 454–459. 10.1097/JCP.0000000000000335 [DOI] [PubMed] [Google Scholar]
- Bhattacharya, A. , & Drevets, W. C. (2017). Role of neuro‐immunological factors in the pathophysiology of mood disorders: Implications for novel therapeutics for treatment resistant depression. Current Topics in Behavioral Neurosciences, 31, 339–356. 10.1007/7854_2016_43 [DOI] [PubMed] [Google Scholar]
- Bromet, E. , Andrade, L. H. , Hwang, I. , Sampson, N. A. , Alonso, J. , de Girolamo, G. , de Graaf, R. , Demyttenaere, K. , Hu, C. , Iwata, N. , Karam, A. N. , Kaur, J. , Kostyuchenko, S. , Lépine, J. P. , Levinson, D. , Matschinger, H. , Mora, M. E. , Browne, M. O. , Posada‐Villa, J. , … Kessler, R. C. (2011). Cross‐national epidemiology of DSM‐IV major depressive episode. BMC Medicine, 9, 90. 10.1186/1741-7015-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, S. , Rittenbach, K. , Cheung, S. , McKean, G. , MacMaster, F. P. , & Clement, F. (2019). Current and common definitions of treatment‐resistant depression: Findings from a systematic review and qualitative interviews. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie, 64(6), 380–387. 10.1177/0706743719828965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne, C. A. , Jacobson, M. L. , & Lucki, I. (2020). Novel targets to treat depression: Opioid‐based therapeutics. Harvard Review of Psychiatry, 28(1), 40–59. 10.1097/HRP.0000000000000242 [DOI] [PubMed] [Google Scholar]
- Bunney, B. G. , Li, J. Z. , Walsh, D. M. , Stein, R. , Vawter, M. P. , Cartagena, P. , Barchas, J. D. , Schatzberg, A. F. , Myers, R. M. , Watson, S. J. , Akil, H. , & Bunney, W. E. (2015). Circadian dysregulation of clock genes: Clues to rapid treatments in major depressive disorder. Molecular Psychiatry, 20(1), 48–55. 10.1038/mp.2014.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarone, B. J. , Zachariou, V. , & King, S. L. (2015). Rodent models of treatment‐resistant depression. European Journal of Pharmacology, 753, 51–65. 10.1016/j.ejphar.2014.10.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantù, F. , Ciappolino, V. , Enrico, P. , Moltrasio, C. , Delvecchio, G. , & Brambilla, P. (2020). Augmentation with atypical antipsychotics for treatment‐resistant depression. Journal of Affective Disorders, 280(Pt A), 45–53. 10.1016/j.jad.2020.11.006 [DOI] [PubMed] [Google Scholar]
- Caraci, F. , Calabrese, F. , Molteni, R. , Bartova, L. , Dold, M. , Leggio, G. M. , Fabbri, C. , Mendlewicz, J. , Racagni, G. , Kasper, S. , Riva, M. A. , & Drago, F. (2018). International Union of Basic and Clinical Pharmacology CIV: The neurobiology of treatment‐resistant depression: From antidepressant classifications to novel pharmacological targets. Pharmacological Reviews, 70(3), 475–504. 10.1124/pr.117.014977 [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , Bolstridge, M. , Day, C. , Rucker, J. , Watts, R. , Erritzoe, D. E. , Kaelen, M. , Giribaldi, B. , Bloomfield, M. , Pilling, S. , Rickard, J. A. , Forbes, B. , Feilding, A. , Taylor, D. , Curran, H. V. , & Nutt, D. J. (2018). Psilocybin with psychological support for treatment‐resistant depression: Six‐month follow‐up. Psychopharmacology, 235(2), 399–408. 10.1007/s00213-017-4771-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , Bolstridge, M. , Rucker, J. , Day, C. M. , Erritzoe, D. , Kaelen, M. , Bloomfield, M. , Rickard, J. A. , Forbes, B. , Feilding, A. , Taylor, D. , Pilling, S. , Curran, V. H. , & Nutt, D. J. (2016). Psilocybin with psychological support for treatment‐resistant depression: An open‐label feasibility study. The Lancet. Psychiatry, 3(7), 619–627. 10.1016/S2215-0366(16)30065-7 [DOI] [PubMed] [Google Scholar]
- Carney, R. M. , & Freedland, K. E. (2009). Treatment‐resistant depression and mortality after acute coronary syndrome. The American Journal of Psychiatry, 166(4), 410–417. 10.1176/appi.ajp.2008.08081239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda, M. S. , Reps, J. , Fife, D. , Blacketer, C. , Stang, P. , & Ryan, P. (2018). Finding treatment‐resistant depression in real‐world data: How a data‐driven approach compares with expert‐based heuristics. Depression and Anxiety, 35(3), 220–228. 10.1002/da.22705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain, S. R. , Cavanagh, J. , de Boer, P. , Mondelli, V. , Jones, D. , Drevets, W. C. , Cowen, P. J. , Harrison, N. A. , Pointon, L. , Pariante, C. M. , & Bullmore, E. T. (2019). Treatment‐resistant depression and peripheral C‐reactive protein. The British Journal of Psychiatry: The Journal of Mental Science, 214(1), 11–19. 10.1192/bjp.2018.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B. K. , Mendez‐David, I. , Luna, V. M. , Faye, C. , Gardier, A. M. , David, D. J. , & Denny, C. A. (2020). Prophylactic efficacy of 5‐HT4R agonists against stress. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 45(3), 542–552. 10.1038/s41386-019-0540-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudary, P. V. , Molnar, M. , Evans, S. J. , Tomita, H. , Li, J. Z. , Vawter, M. P. , Myers, R. M. , Bunney, W. E. Jr. , Akil, H. , Watson, S. J. , & Jones, E. G. (2005). Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proceedings of the National Academy of Sciences of the United States of America, 102(43), 15653–15658. 10.1073/pnas.0507901102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, T. , Bisgaard, C. F. , & Wiborg, O. (2011). Biomarkers of anhedonic‐like behavior, antidepressant drug refraction, and stress resilience in a rat model of depression. Neuroscience, 196, 66–79. 10.1016/j.neuroscience.2011.08.024 [DOI] [PubMed] [Google Scholar]
- Chugh, S. , Chhabria, A. , Jung, S. , Kruger, T. , & Wollmer, M. A. (2018). Botulinum toxin as a treatment for depression in a real‐world setting. Journal of Psychiatric Practice, 24(1), 15–20. 10.1097/PRA.0000000000000277 [DOI] [PubMed] [Google Scholar]
- Coquery, N. , Blesch, A. , Stroh, A. , Fernández‐Klett, F. , Klein, J. , Winter, C. , & Priller, J. (2012). Intrahippocampal transplantation of mesenchymal stromal cells promotes neuroplasticity. Cytotherapy, 14(9), 1041–1053. 10.3109/14653249.2012.694418 [DOI] [PubMed] [Google Scholar]
- Czéh, B. , Fuchs, E. , Wiborg, O. , & Simon, M. (2016). Animal models of major depression and their clinical implications. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 64, 293–310. 10.1016/j.pnpbp.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Czéh, B. , & Simon, M. (2021). Benefits of animal models to understand the pathophysiology of depressive disorders. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 106, 110049. 10.1016/j.pnpbp.2020.110049 [DOI] [PubMed] [Google Scholar]
- de Almeida, R. N. , Galvão, A. , da Silva, F. S. , Silva, E. , Palhano‐Fontes, F. , Maia‐de‐Oliveira, J. P. , de Araújo, L. B. , Lobão‐Soares, B. , & Galvão‐Coelho, N. L. (2019). Modulation of serum brain‐derived neurotrophic factor by a single dose of ayahuasca: Observation from a randomized controlled trial. Frontiers in Psychology, 10, 1234. 10.3389/fpsyg.2019.01234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer, J. N. , Vingerhoets, C. , Hirdes, M. , McAlonan, G. M. , Amelsvoort, T. V. , & Zinkstok, J. R. (2019). Efficacy and tolerability of riluzole in psychiatric disorders: A systematic review and preliminary meta‐analysis. Psychiatry Research, 278, 294–302. 10.1016/j.psychres.2019.06.020 [DOI] [PubMed] [Google Scholar]
- Der‐Avakian, A. , Mazei‐Robison, M. S. , Kesby, J. P. , Nestler, E. J. , & Markou, A. (2014). Enduring deficits in brain reward function after chronic social defeat in rats: Susceptibility, resilience, and antidepressant response. Biological Psychiatry, 76(7), 542–549. 10.1016/j.biopsych.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolatshahi, M. , Davoudi, S. , Paridar, Y. , Naserzadeh, R. , & Ghorbanzadeh, B. (2020). Pharmacological evidence for the involvement of the opioid system in the antidepressant‐like effect of simvastatin in mice: Without tolerance and withdrawal syndrome. Neuroscience Letters, 714, 134578. 10.1016/j.neulet.2019.134578 [DOI] [PubMed] [Google Scholar]
- Dold, M. , & Kasper, S. (2017). Evidence‐based pharmacotherapy of treatment‐resistant unipolar depression. International Journal of Psychiatry in Clinical Practice, 21(1), 13–23. 10.1080/13651501.2016.1248852 [DOI] [PubMed] [Google Scholar]
- Döme, P. , Kunovszki, P. , Takács, P. , Fehér, L. , Balázs, T. , Dede, K. , Mulhern‐Haughey, S. , Barbreau, S. , & Rihmer, Z. (2021). Clinical characteristics of treatment‐resistant depression in adults in Hungary: Real‐world evidence from a 7‐year‐long retrospective data analysis. PLoS ONE, 16(1), e0245510. 10.1371/journal.pone.0245510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa, S. C. , & Janowsky, D. S. (2019). Cholinergic regulation of mood: From basic and clinical studies to emerging therapeutics. Molecular Psychiatry, 24(5), 694–709. 10.1038/s41380-018-0219-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman, C. H. , & Duman, R. S. (2015). Spine synapse remodeling in the pathophysiology and treatment of depression. Neuroscience Letters, 601, 20–29. 10.1016/j.neulet.2015.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman, R. S. , Sanacora, G. , & Krystal, J. H. (2019). Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron, 102(1), 75–90. 10.1016/j.neuron.2019.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi, Y. (2014). Emerging role of microRNAs in major depressive disorder: Diagnosis and therapeutic implications. Dialogues in Clinical Neuroscience, 16(1), 43–61. 10.31887/DCNS.2014.16.1/ydwivedi [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency . (2013). Guideline on clinical investigation of medicinal products in the treatment of depression. EMA/CHMP/185423/2010 Rev 2.
- Fagerholm, E. D. , Leech, R. , Williams, S. , Zarate, C. A. Jr. , Moran, R. J. , & Gilbert, J. R. (2021). Fine‐tuning neural excitation/inhibition for tailored ketamine use in treatment‐resistant depression. Translational Psychiatry, 11(1), 335. 10.1038/s41398-021-01442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava, M. (2003). Diagnosis and definition of treatment‐resistant depression. Biological Psychiatry, 53(8), 649–659. 10.1016/s0006-3223(03)00231-2 [DOI] [PubMed] [Google Scholar]
- Fava, M. , Mazzone, E. , Freeman, M. , Flynn, M. , Judge, H. , Hoeppner, B. , Hock, R. S. , Shui, A. , Macaluso, M. , Morrison, M. F. , Carpenter, L. L. , Shelton, R. , Zajecka, J. , & Papakostas, G. I. (2020). Double‐blind, placebo‐controlled, proof‐of‐concept trial of a kappa‐selective opioid receptor antagonist augmentation in treatment‐resistant depression. Annals of Clinical Psychiatry: Official Journal of the American Academy of Clinical Psychiatrists, 32(4), e16–e24. 10.12788/acp.0003 [DOI] [PubMed] [Google Scholar]
- Ferreira‐Garcia, R. , da Rocha Freire, R. C. , Appolinário, J. C. , Levitan, M. N. , Halkjær‐Lassen, R. D. , Bueno, J. R. , & Nardi, A. E. (2018). Tranylcypromine plus amitriptyline for electroconvulsive therapy‐resistant depression: A long‐term study. Journal of Clinical Psychopharmacology, 38(5), 502–504. 10.1097/JCP.0000000000000945 [DOI] [PubMed] [Google Scholar]
- Feyissa, A. M. , Chandran, A. , Stockmeier, C. A. , & Karolewicz, B. (2009). Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD‐95 in the prefrontal cortex in major depression. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 33(1), 70–75. 10.1016/j.pnpbp.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi, E. , & Wasserman, E. (2006). Treatment of depression with botulinum toxin A: A case series. Dermatologic Surgery: Official Publication for American Society for Dermatologic Surgery, 32(5), 645–650. 10.1111/j.1524-4725.2006.32136 [DOI] [PubMed] [Google Scholar]
- Fogaça, M. V. , Fukumoto, K. , Franklin, T. , Liu, R. J. , Duman, C. H. , Vitolo, O. V. , & Duman, R. S. (2019). N‐Methyl‐d‐aspartate receptor antagonist d‐methadone produces rapid, mTORC1‐dependent antidepressant effects. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 44(13), 2230–2238. 10.1038/s41386-019-0501-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana, A. , Manchia, M. , Panebianco, C. , Paribello, P. , Arzedi, C. , Cossu, E. , Garzilli, M. , Montis, M. A. , Mura, A. , Pisanu, C. , Congiu, D. , Copetti, M. , Pinna, F. , Carpiniello, B. , Squassina, A. , & Pazienza, V. (2020). Exploring the role of gut microbiota in major depressive disorder and in treatment resistance to antidepressants. Biomedicine, 8(9), 311. 10.3390/biomedicines8090311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, C. , Fan, Y. , & Davatzikos, C. (2019). Addressing heterogeneity (and homogeneity) in treatment mechanisms in depression and the potential to develop diagnostic and predictive biomarkers. NeuroImage. Clinical, 24, 101997. 10.1016/j.nicl.2019.101997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami, M. , Yamamoto, S. , Morinobu, S. , Okada, S. , Yamawaki, Y. , & Yamawaki, S. (2016). The potential use of histone deacetylase inhibitors in the treatment of depression. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 64, 320–324. 10.1016/j.pnpbp.2015.03.010 [DOI] [PubMed] [Google Scholar]
- Galvão, A. , de Almeida, R. N. , Silva, E. , Freire, F. , Palhano‐Fontes, F. , Onias, H. , Arcoverde, E. , Maia‐de‐Oliveira, J. P. , de Araújo, D. B. , Lobão‐Soares, B. , & Galvão‐Coelho, N. L. (2018). Cortisol modulation by ayahuasca in patients with treatment resistant depression and healthy controls. Frontiers in Psychiatry, 9, 185. 10.3389/fpsyt.2018.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie, S. C. (2021). Creation of a gene expression portrait of depression and its application for identifying potential treatments. Scientific Reports, 11(1), 3829. 10.1038/s41598-021-83348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay, R. P. , Charpeaud, T. , Logan, S. , Hannaert, P. , Garay, R. G. , Llorca, P. M. , & Shorey, S. (2019). Pharmacotherapeutic approaches to treating depression during the perimenopause. Expert Opinion on Pharmacotherapy, 20(15), 1837–1845. 10.1080/14656566.2019.1645122 [DOI] [PubMed] [Google Scholar]
- Garay, R. P. , Zarate, C. A. Jr. , Charpeaud, T. , Citrome, L. , Correll, C. U. , Hameg, A. , & Llorca, P. M. (2017). Investigational drugs in recent clinical trials for treatment‐resistant depression. Expert Review of Neurotherapeutics, 17(6), 593–609. 10.1080/14737175.2017.1283217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon, A. A. , Vishne, T. , & Grunhaus, L. (2007). Dopamine D2‐like receptors and the antidepressant response. Biological Psychiatry, 61(2), 145–153. 10.1016/j.biopsych.2006.05.031 [DOI] [PubMed] [Google Scholar]
- Ghabrash, M. F. , Comai, S. , Tabaka, J. , Saint‐Laurent, M. , Booij, L. , & Gobbi, G. (2016). Valproate augmentation in a subgroup of patients with treatment‐resistant unipolar depression. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, 17(2), 165–170. 10.3109/15622975.2015.1073856 [DOI] [PubMed] [Google Scholar]
- Goh, K. K. , Chang, S. C. , Chen, C. H. , & Lu, M. L. (2020). Therapeutic strategies for treatment‐resistant depression: State of the art and future perspectives. Current Pharmaceutical Design, 26(2), 244–252. 10.2174/1381612826666200110101604 [DOI] [PubMed] [Google Scholar]
- Gulbins, A. , Grassmé, H. , Hoehn, R. , Kohnen, M. , Edwards, M. J. , Kornhuber, J. , & Gulbins, E. (2016). Role of Janus‐kinases in major depressive disorder. Neuro‐Signals, 24(1), 71–80. 10.1159/000442613 [DOI] [PubMed] [Google Scholar]
- Han, L. , Aghajani, M. , Clark, S. L. , Chan, R. F. , Hattab, M. W. , Shabalin, A. A. , Zhao, M. , Kumar, G. , Xie, L. Y. , Jansen, R. , Milaneschi, Y. , Dean, B. , Aberg, K. A. , van den Oord, E. , & Penninx, B. (2018). Epigenetic aging in major depressive disorder. The American Journal of Psychiatry, 175(8), 774–782. 10.1176/appi.ajp.2018.17060595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa, Y. , Zhu, X. , & Kamiya, A. (2019). NV‐5138 as a fast‐acting antidepressant via direct activation of mTORC1 signaling. The Journal of Clinical Investigation, 129(6), 2207–2209. 10.1172/JCI129702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerlein, K. , Perugi, G. , Otte, C. , Frodl, T. , Degraeve, G. , Hagedoorn, W. , Oliveira‐Maia, A. J. , Perez Sola, V. , Rathod, S. , Rosso, G. , Sierra, P. , Malynn, S. , Morrens, J. , Verrijcken, C. , Gonzalez, B. , & Young, A. H. (2021). Real‐world evidence from a European cohort study of patients with treatment resistant depression: Treatment patterns and clinical outcomes. Journal of Affective Disorders, 290, 334–344. 10.1016/j.jad.2021.03.073 [DOI] [PubMed] [Google Scholar]
- Hellem, T. L. , Sung, Y. H. , Shi, X. F. , Pett, M. A. , Latendresse, G. , Morgan, J. , Huber, R. S. , Kuykendall, D. , Lundberg, K. J. , & Renshaw, P. F. (2015). Creatine as a novel treatment for depression in females using methamphetamine: A pilot study. Journal of Dual Diagnosis, 11(3–4), 189–202. 10.1080/15504263.2015.1100471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henter, I. D. , Park, L. T. , & Zarate, C. A. Jr. (2021). Novel glutamatergic modulators for the treatment of mood disorders: Current status. CNS Drugs, 35(5), 527–543. 10.1007/s40263-021-00816-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepner, C. T. , McIntyre, R. S. , & Papakostas, G. I. (2021). Impact of supplementation and nutritional interventions on pathogenic processes of mood disorders: A review of the evidence. Nutrients, 13(3), 767. 10.3390/nu13030767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, L. Jr. , Shutman, E. , Chinaka, C. , Deepika, K. , Pelaez, L. , & Dabney, K. W. (2019). Aberrant epigenomic modulation of glucocorticoid receptor gene (NR3C1) in early life stress and major depressive disorder correlation: Systematic review and quantitative evidence synthesis. International Journal of Environmental Research and Public Health, 16(21), 4280. 10.3390/ijerph16214280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain, M. I. , Chaudhry, I. B. , Khoso, A. B. , Husain, M. O. , Rahman, R. R. , Hamirani, M. M. , Hodsoll, J. , Carvalho, A. F. , Husain, N. , & Young, A. H. (2019). Adjunctive simvastatin for treatment‐resistant depression: Study protocol of a 12‐week randomised controlled trial. BJPsych Open, 5(1), e13. 10.1192/bjo.2018.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain, M. I. , Cullen, C. , Umer, M. , Carvalho, A. F. , Kloiber, S. , Meyer, J. H. , Ortiz, A. , Knyahnytska, Y. , Husain, M. O. , Giddens, J. , Diniz, B. S. , Wang, W. , Young, A. H. , Mulsant, B. H. , & Daskalakis, Z. J. (2020). Minocycline as adjunctive treatment for treatment‐resistant depression: Study protocol for a double blind, placebo‐controlled, randomized trial (MINDEP2). BMC Psychiatry, 20(1), 173. 10.1186/s12888-020-02553-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson, A. N. , Deng, J. V. , Cohen, S. , & West, A. E. (2012). Phosphorylation of MeCP2 at Ser421 contributes to chronic antidepressant action. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 32(41), 14355–14363. 10.1523/JNEUROSCI.2156-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu, D. F. , Rosenbaum, J. F. , & Alpert, J. E. (2015). Pharmacological approaches to the challenge of treatment‐resistant depression. Dialogues in Clinical Neuroscience, 17(2), 111–126. 10.31887/DCNS.2015.17.2/dionescu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal, S. Z. , & Mathew, S. J. (2020). Ketamine for depression clinical issues. Advances in Pharmacology (San Diego, Calif.), 89, 131–162. 10.1016/bs.apha.2020.02.005 [DOI] [PubMed] [Google Scholar]
- Isingrini, E. , Camus, V. , Le Guisquet, A. M. , Pingaud, M. , Devers, S. , & Belzung, C. (2010). Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: A model of fluoxetine resistance in mice. PLoS ONE, 5(4), e10404. 10.1371/journal.pone.0010404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, M. L. , Browne, C. A. , & Lucki, I. (2020). Kappa opioid receptor antagonists as potential therapeutics for stress‐related disorders. Annual Review of Pharmacology and Toxicology, 60, 615–636. 10.1146/annurev-pharmtox-010919-023317 [DOI] [PubMed] [Google Scholar]
- Jaso, B. A. , Niciu, M. J. , Iadarola, N. D. , Lally, N. , Richards, E. M. , Park, M. , Ballard, E. D. , Nugent, A. C. , Machado‐Vieira, R. , & Zarate, C. A. (2017). Therapeutic modulation of glutamate receptors in major depressive disorder. Current Neuropharmacology, 15(1), 57–70. 10.2174/1570159x14666160321123221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatissa, M. N. , Bisgaard, C. , Tingström, A. , Papp, M. , & Wiborg, O. (2006). Hippocampal cytogenesis correlates to escitalopram‐mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 31(11), 2395–2404. 10.1038/sj.npp.1301041 [DOI] [PubMed] [Google Scholar]
- Johnson, K. M. , Devine, J. M. , Ho, K. F. , Howard, K. A. , Saretsky, T. L. , & Jamieson, C. A. (2016). Evidence to support Montgomery‐Asberg Depression Rating Scale administration every 24 hours to assess rapid onset of treatment response. The Journal of Clinical Psychiatry, 77(12), 1681–1686. 10.4088/JCP.15m10253 [DOI] [PubMed] [Google Scholar]
- Johnson, S. , & Liston, C. (2021). MeCP2 for sustained antidepressant effects. Nature Neuroscience, 24(8), 1047–1048. 10.1038/s41593-021-00881-x [DOI] [PubMed] [Google Scholar]
- Juruena, M. F. , Gadelrab, R. , Cleare, A. J. , & Young, A. H. (2021). Epigenetics: A missing link between early life stress and depression. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 109, 110231. 10.1016/j.pnpbp.2020.110231 [DOI] [PubMed] [Google Scholar]
- Karp, J. F. , Butters, M. A. , Begley, A. E. , Miller, M. D. , Lenze, E. J. , Blumberger, D. M. , Mulsant, B. H. , & Reynolds, C. F. 3rd (2014). Safety, tolerability, and clinical effect of low‐dose buprenorphine for treatment‐resistant depression in midlife and older adults. The Journal of Clinical Psychiatry, 75(8), e785–e793. 10.4088/JCP.13m08725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, T. , & Duman, R. S. (2020). Rapastinel, a novel glutamatergic agent with ketamine‐like antidepressant actions: Convergent mechanisms. Pharmacology, Biochemistry, and Behavior, 188, 172827. 10.1016/j.pbb.2019.172827 [DOI] [PubMed] [Google Scholar]
- Kato, T. , Pothula, S. , Liu, R. J. , Duman, C. H. , Terwilliger, R. , Vlasuk, G. P. , Saiah, E. , Hahm, S. , & Duman, R. S. (2019). Sestrin modulator NV‐5138 produces rapid antidepressant effects via direct mTORC1 activation. The Journal of Clinical Investigation, 129(6), 2542–2554. 10.1172/JCI126859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautzky, A. , Dold, M. , Bartova, L. , Spies, M. , Kranz, G. S. , Souery, D. , Montgomery, S. , Mendlewicz, J. , Zohar, J. , Fabbri, C. , Serretti, A. , Lanzenberger, R. , Dikeos, D. , Rujescu, D. , & Kasper, S. (2019). Clinical factors predicting treatment resistant depression: Affirmative results from the European multicenter study. Acta Psychiatrica Scandinavica, 139(1), 78–88. 10.1111/acps.12959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi, A. , Noorbala, A. A. , Azam, K. , Eskandari, M. H. , & Djafarian, K. (2019). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clinical Nutrition (Edinburgh, Scotland), 38(2), 522–528. 10.1016/j.clnu.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Kelly, J. R. , Borre, Y. , O'Brien, C. , Patterson, E. , El Aidy, S. , Deane, J. , Kennedy, P. J. , Beers, S. , Scott, K. , Moloney, G. , Hoban, A. E. , Scott, L. , Fitzgerald, P. , Ross, P. , Stanton, C. , Clarke, G. , Cryan, J. F. , & Dinan, T. G. (2016). Transferring the blues: Depression‐associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research, 82, 109–118. 10.1016/j.jpsychires.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Kim, J. W. , Autry, A. E. , Na, E. S. , Adachi, M. , Björkholm, C. , Kavalali, E. T. , & Monteggia, L. M. (2021). Sustained effects of rapidly acting antidepressants require BDNF‐dependent MeCP2 phosphorylation. Nature Neuroscience, 24(8), 1100–1109. 10.1038/s41593-021-00868-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kin, K. , Yasuhara, T. , & Date, I. (2020). Encapsulation of mesenchymal stem cells: Dissecting the underlying mechanism of mesenchymal stem cell transplantation therapy. Neuroscience Insights, 15, 2633105520959064. 10.1177/2633105520959064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kious, B. M. , Kondo, D. G. , & Renshaw, P. F. (2019). Creatine for the treatment of depression. Biomolecules, 9(9), 406. 10.3390/biom9090406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura, Y. , Araki, H. , & Gomita, Y. (2002). Influence of ACTH on the effects of imipramine, desipramine and lithium on duration of immobility of rats in the forced swim test. Pharmacology, Biochemistry, and Behavior, 71(1–2), 63–69. 10.1016/s0091-3057(01)00625-6 [DOI] [PubMed] [Google Scholar]
- Kornstein, S. G. , & Schneider, R. K. (2001). Clinical features of treatment‐resistant depression. The Journal of Clinical Psychiatry, 62(Suppl 16), 18–25. [PubMed] [Google Scholar]
- Kruger, T. H. , & Wollmer, M. A. (2015). Depression—An emerging indication for botulinum toxin treatment. Toxicon: Official Journal of the International Society on Toxinology, 107(Pt A), 154–157. 10.1016/j.toxicon.2015.09.035 [DOI] [PubMed] [Google Scholar]
- Krystal, A. D. , Pizzagalli, D. A. , Smoski, M. , Mathew, S. J. , Nurnberger, J. Jr. , Lisanby, S. H. , Iosifescu, D. , Murrough, J. W. , Yang, H. , Weiner, R. D. , Calabrese, J. R. , Sanacora, G. , Hermes, G. , Keefe, R. , Song, A. , Goodman, W. , Szabo, S. T. , Whitton, A. E. , Gao, K. , & Potter, W. Z. (2020). A randomized proof‐of‐mechanism trial applying the ‘fast‐fail’ approach to evaluating κ‐opioid antagonism as a treatment for anhedonia. Nature Medicine, 26(5), 760–768. 10.1038/s41591-020-0806-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, J. , Gavrilidis, E. , Thomas, N. , Hudaib, A. R. , Worsley, R. , Thew, C. , Bleeker, C. , & Gurvich, C. (2018). Tibolone improves depression in women through the menopause transition: A double‐blind randomized controlled trial of adjunctive tibolone. Journal of Affective Disorders, 236, 88–92. 10.1016/j.jad.2018.04.103 [DOI] [PubMed] [Google Scholar]
- Lahmame, A. , del Arco, C. , Pazos, A. , Yritia, M. , & Armario, A. (1997). Are Wistar‐Kyoto rats a genetic animal model of depression resistant to antidepressants? European Journal of Pharmacology, 337(2–3), 115–123. 10.1016/s0014-2999(97)01276-4 [DOI] [PubMed] [Google Scholar]
- LeGates, T. A. , Kvarta, M. D. , & Thompson, S. M. (2019). Sex differences in antidepressant efficacy. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 44(1), 140–154. 10.1038/s41386-018-0156-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepow, L. , Luckenbaugh, D. A. , Park, L. , Henter, I. D. , & Zarate, C. A. Jr. (2017). Case series: Antidepressant effects of low‐affinity and low‐trapping NMDA receptor antagonists did not predict response to ketamine in seven subjects. Journal of Psychiatric Research, 86, 55–57. 10.1016/j.jpsychires.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S. , Romano, C. , De Bruecker, G. , Murrough, J. W. , Shelton, R. , Singh, J. B. , & Jamieson, C. (2019). Analysis of clinical trial exit interview data in patients with treatment‐resistant depression. The Patient, 12(5), 527–537. 10.1007/s40271-019-00369-8 [DOI] [PubMed] [Google Scholar]
- Li, C. T. , Yang, K. C. , & Lin, W. C. (2019). Glutamatergic dysfunction and glutamatergic compounds for major psychiatric disorders: Evidence from clinical neuroimaging studies. Frontiers in Psychiatry, 9, 767. 10.3389/fpsyt.2018.00767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , & Vlisides, P. E. (2016). Ketamine: 50 years of modulating the mind. Frontiers in Human Neuroscience, 10, 612. 10.3389/fnhum.2016.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Lee, B. , Liu, R. J. , Banasr, M. , Dwyer, J. M. , Iwata, M. , Li, X. Y. , Aghajanian, G. , & Duman, R. S. (2010). mTOR‐dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science (New York, N.Y.), 329(5994), 959–964. 10.1126/science.1190287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. S. , Tian, C. , Hinds, D. , & 23andMe Research Team . (2020). Genome‐wide association studies of antidepressant class response and treatment‐resistant depression. Translational Psychiatry, 10(1), 360. 10.1038/s41398-020-01035-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. S. , Tian, C. , Seabrook, G. R. , Drevets, W. C. , & Narayan, V. A. (2016). Analysis of 23andMe antidepressant efficacy survey data: Implication of circadian rhythm and neuroplasticity in bupropion response. Translational Psychiatry, 6(9), e889. 10.1038/tp.2016.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. , Karim, H. T. , Pecina, M. , Aizenstein, H. J. , Lenze, E. J. , Blumberger, D. M. , Mulsant, B. H. , Kharasch, E. D. , Reynolds Iii, C. F. , & Karp, J. F. (2019). Low‐dose augmentation with buprenorphine increases emotional reactivity but not reward activity in treatment resistant mid‐ and late‐life depression. NeuroImage. Clinical, 21, 101679. 10.1016/j.nicl.2019.101679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann, L. , Porter, R. H. , Scharf, S. H. , Kuennecke, B. , Bruns, A. , von Kienlin, M. , Harrison, A. C. , Paehler, A. , Funk, C. , Gloge, A. , Schneider, M. , Parrott, N. J. , Polonchuk, L. , Niederhauser, U. , Morairty, S. R. , Kilduff, T. S. , Vieira, E. , Kolczewski, S. , Wichmann, J. , … Jaeschke, G. (2015). Pharmacology of basimglurant (RO4917523, RG7090), a unique metabotropic glutamate receptor 5 negative allosteric modulator in clinical development for depression. The Journal of Pharmacology and Experimental Therapeutics, 353(1), 213–233. 10.1124/jpet.114.222463 [DOI] [PubMed] [Google Scholar]
- Maes, M. , Nowak, G. , Caso, J. R. , Leza, J. C. , Song, C. , Kubera, M. , Klein, H. , Galecki, P. , Noto, C. , Glaab, E. , Balling, R. , & Berk, M. (2016). Toward omics‐based, systems biomedicine, and path and drug discovery methodologies for depression‐inflammation research. Molecular Neurobiology, 53(5), 2927–2935. 10.1007/s12035-015-9183-5 [DOI] [PubMed] [Google Scholar]
- Maki, P. M. , Kornstein, S. G. , Joffe, H. , Bromberger, J. T. , Freeman, E. W. , Athappilly, G. , Bobo, W. V. , Rubin, L. H. , Koleva, H. K. , Cohen, L. S. , & Soares, C. N. (2019). Guidelines for the evaluation and treatment of perimenopausal depression: Summary and recommendations. Journal of Women's Health (2002), 28(2), 117–134. 10.1089/jwh.2018.27099.mensocrec [DOI] [PubMed] [Google Scholar]
- Malhi, G. S. , & Mann, J. J. (2018). Depression. Lancet (London, England), 392(10161), 2299–2312. 10.1016/S0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- McAllister‐Williams, R. H. , Arango, C. , Blier, P. , Demyttenaere, K. , Falkai, P. , Gorwood, P. , Hopwood, M. , Javed, A. , Kasper, S. , Malhi, G. S. , Soares, J. C. , Vieta, E. , Young, A. H. , Papadopoulos, A. , & Rush, A. J. (2020). The identification, assessment and management of difficult‐to‐treat depression: An international consensus statement. Journal of Affective Disorders, 267, 264–282. 10.1016/j.jad.2020.02.023 [DOI] [PubMed] [Google Scholar]
- McQuaid, R. J. , McInnis, O. A. , Abizaid, A. , & Anisman, H. (2014). Making room for oxytocin in understanding depression. Neuroscience and Biobehavioral Reviews, 45, 305–322. 10.1016/j.neubiorev.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Miro‐Blanch, J. , & Yanes, O. (2019). Epigenetic regulation at the interplay between gut microbiota and host metabolism. Frontiers in Genetics, 10, 638. 10.3389/fgene.2019.00638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi, S. , Takamiya, A. , Noda, Y. , Horita, N. , Wada, M. , Tsugawa, S. , Plitman, E. , Sano, Y. , Tarumi, R. , ElSalhy, M. , Katayama, N. , Ogyu, K. , Miyazaki, T. , Kishimoto, T. , Graff‐Guerrero, A. , Meyer, J. H. , Blumberger, D. M. , Daskalakis, Z. J. , Mimura, M. , & Nakajima, S. (2019). Glutamatergic neurometabolite levels in major depressive disorder: A systematic review and meta‐analysis of proton magnetic resonance spectroscopy studies. Molecular Psychiatry, 24(7), 952–964. 10.1038/s41380-018-0252-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskal, J. R. , Burgdorf, J. S. , Stanton, P. K. , Kroes, R. A. , Disterhoft, J. F. , Burch, R. M. , & Khan, M. A. (2017). The development of rapastinel (formerly GLYX‐13); a rapid acting and long lasting antidepressant. Current Neuropharmacology, 15(1), 47–56. 10.2174/1570159x14666160321122703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, C. J. , Vos, T. , Lozano, R. , Naghavi, M. , Flaxman, A. D. , Michaud, C. , Ezzati, M. , Shibuya, K. , Salomon, J. A. , Abdalla, S. , Aboyans, V. , Abraham, J. , Ackerman, I. , Aggarwal, R. , Ahn, S. Y. , Ali, M. K. , Alvarado, M. , Anderson, H. R. , Anderson, L. M. , … Memish, Z. A. (2012). Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England), 380(9859), 2197–2223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- Murrough, J. W. , Wade, E. , Sayed, S. , Ahle, G. , Kiraly, D. D. , Welch, A. , Collins, K. A. , Soleimani, L. , Iosifescu, D. V. , & Charney, D. S. (2017). Dextromethorphan/quinidine pharmacotherapy in patients with treatment resistant depression: A proof of concept clinical trial. Journal of Affective Disorders, 218, 277–283. 10.1016/j.jad.2017.04.072 [DOI] [PubMed] [Google Scholar]
- Nagele, P. , Zorumski, C. F. , & Conway, C. (2018). Exploring nitrous oxide as treatment of mood disorders: Basic concepts. Journal of Clinical Psychopharmacology, 38(2), 144–148. 10.1097/JCP.0000000000000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca, C. , Bigio, B. , Lee, F. S. , Young, S. P. , Kautz, M. M. , Albright, A. , Beasley, J. , Millington, D. S. , Mathé, A. A. , Kocsis, J. H. , Murrough, J. W. , McEwen, B. S. , & Rasgon, N. (2018). Acetyl‐l‐carnitine deficiency in patients with major depressive disorder. Proceedings of the National Academy of Sciences of the United States of America, 115(34), 8627–8632. 10.1073/pnas.1801609115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naserzadeh, R. , Abad, N. , Ghorbanzadeh, B. , Dolatshahi, M. , & Mansouri, M. T. (2019). Simvastatin exerts antidepressant‐like activity in mouse forced swimming test: Role of NO‐cGMP‐KATP channels pathway and PPAR‐gamma receptors. Pharmacology, Biochemistry, and Behavior, 180, 92–100. 10.1016/j.pbb.2019.03.002 [DOI] [PubMed] [Google Scholar]
- Nestler, E. J. , & Hyman, S. E. (2010). Animal models of neuropsychiatric disorders. Nature Neuroscience, 13(10), 1161–1169. 10.1038/nn.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettis, M. A. , Lombardo, G. , Hastings, C. , Zajkowska, Z. , Mariani, N. , Nikkheslat, N. , Worrell, C. , Enache, D. , McLaughlin, A. , Kose, M. , Sforzini, L. , Bogdanova, A. , Cleare, A. , Young, A. H. , Pariante, C. M. , & Mondelli, V. (2021). Augmentation therapy with minocycline in treatment‐resistant depression patients with low‐grade peripheral inflammation: Results from a double‐blind randomised clinical trial. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 46(5), 939–948. 10.1038/s41386-020-00948-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas, T. , Raunig, D. , Duvvuri, S. , Plotka, A. , Schwam, E. , Grimwood, S. , Park, Y. , Rowinski, C. , Rapp, T. L. , Bergeron, M. , & Leurent, C. (2011). Pharmacokinetics, safety and tolerability of PF‐04995274: A 5HT4 partial agonist being developed for the treatment of Alzheimer's disease. Alzheimer's & Dementia, 7(4), S786–S787. 10.1016/j.jalz.2011.05.2259 [DOI] [Google Scholar]
- Nickell, J. R. , Grinevich, V. P. , Siripurapu, K. B. , Smith, A. M. , & Dwoskin, L. P. (2013). Potential therapeutic uses of mecamylamine and its stereoisomers. Pharmacology, Biochemistry, and Behavior, 108, 28–43. 10.1016/j.pbb.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary, O. F. , & Cryan, J. F. (2013). Towards translational rodent models of depression. Cell and Tissue Research, 354(1), 141–153. 10.1007/s00441-013-1587-9 [DOI] [PubMed] [Google Scholar]
- Osório, F. , Sanches, R. F. , Macedo, L. R. , Santos, R. G. , Maia‐de‐Oliveira, J. P. , Wichert‐Ana, L. , Araujo, D. B. , Riba, J. , Crippa, J. A. , & Hallak, J. E. (2015). Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A preliminary report. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999), 37(1), 13–20. 10.1590/1516-4446-2014-1496 [DOI] [PubMed] [Google Scholar]
- Pae, C. U. , Marks, D. M. , Han, C. , & Patkar, A. A. (2008). Does minocycline have antidepressant effect? Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 62(5), 308–311. 10.1016/j.biopha.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Palhano‐Fontes, F. , Barreto, D. , Onias, H. , Andrade, K. C. , Novaes, M. M. , Pessoa, J. A. , Mota‐Rolim, S. A. , Osório, F. L. , Sanches, R. , Dos Santos, R. G. , Tófoli, L. F. , de Oliveira Silveira, G. , Yonamine, M. , Riba, J. , Santos, F. R. , Silva‐Junior, A. A. , Alchieri, J. C. , Galvão‐Coelho, N. L. , Lobão‐Soares, B. , … Araújo, D. B. (2019). Rapid antidepressant effects of the psychedelic ayahuasca in treatment‐resistant depression: A randomized placebo‐controlled trial. Psychological Medicine, 49(4), 655–663. 10.1017/S0033291718001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, G. B. , & Graham, R. K. (2015). Determinants of treatment‐resistant depression: The salience of benzodiazepines. The Journal of Nervous and Mental Disease, 203(9), 659–663. 10.1097/NMD.0000000000000348 [DOI] [PubMed] [Google Scholar]
- Phillips, J. L. , Norris, S. , Talbot, J. , Birmingham, M. , Hatchard, T. , Ortiz, A. , Owoeye, O. , Batten, L. A. , & Blier, P. (2019). Single, repeated, and maintenance ketamine infusions for treatment‐resistant depression: A randomized controlled trial. The American Journal of Psychiatry, 176(5), 401–409. 10.1176/appi.ajp.2018.18070834 [DOI] [PubMed] [Google Scholar]
- Pizzagalli, D. A. , Smoski, M. , Ang, Y. S. , Whitton, A. E. , Sanacora, G. , Mathew, S. J. , Nurnberger, J. Jr. , Lisanby, S. H. , Iosifescu, D. V. , Murrough, J. W. , Yang, H. , Weiner, R. D. , Calabrese, J. R. , Goodman, W. , Potter, W. Z. , & Krystal, A. D. (2020). Selective kappa‐opioid antagonism ameliorates anhedonic behavior: Evidence from the Fast‐fail Trial in Mood and Anxiety Spectrum Disorders (FAST‐MAS). Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 45(10), 1656–1663. 10.1038/s41386-020-0738-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope, L. E. , Khalil, M. H. , Berg, J. E. , Stiles, M. , Yakatan, G. J. , & Sellers, E. M. (2004). Pharmacokinetics of dextromethorphan after single or multiple dosing in combination with quinidine in extensive and poor metabolizers. Journal of Clinical Pharmacology, 44(10), 1132–1142. 10.1177/0091270004269521 [DOI] [PubMed] [Google Scholar]
- Preskorn, S. , Macaluso, M. , Mehra, D. O. , Zammit, G. , Moskal, J. R. , Burch, R. M. , & GLYX‐13 Clinical Study Group . (2015). Randomized proof of concept trial of GLYX‐13, an N‐methyl‐d‐aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. Journal of Psychiatric Practice, 21(2), 140–149. 10.1097/01.pra.0000462606.17725.93 [DOI] [PubMed] [Google Scholar]
- Pryazhnikov, E. , Mugantseva, E. , Casarotto, P. , Kolikova, J. , Fred, S. M. , Toptunov, D. , Afzalov, R. , Hotulainen, P. , Voikar, V. , Terry‐Lorenzo, R. , Engel, S. , Kirov, S. , Castren, E. , & Khiroug, L. (2018). Longitudinal two‐photon imaging in somatosensory cortex of behaving mice reveals dendritic spine formation enhancement by subchronic administration of low‐dose ketamine. Scientific Reports, 8(1), 6464. 10.1038/s41598-018-24933-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce, C. R. , & Fuchs, E. (2016). Chronic psychosocial stressors in adulthood: Studies in mice, rats and tree shrews. Neurobiology of Stress, 6, 94–103. 10.1016/j.ynstr.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz, J. A. , Tamburri, P. , Deptula, D. , Banken, L. , Beyer, U. , Rabbia, M. , Parkar, N. , Fontoura, P. , & Santarelli, L. (2016). Efficacy and safety of basimglurant as adjunctive therapy for major depression: A randomized clinical trial. JAMA Psychiatry, 73(7), 675–684. 10.1001/jamapsychiatry.2016.0838 [DOI] [PubMed] [Google Scholar]
- Raison, C. L. , Rutherford, R. E. , Woolwine, B. J. , Shuo, C. , Schettler, P. , Drake, D. F. , Haroon, E. , & Miller, A. H. (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment‐resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry, 70(1), 31–41. 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan, A. M. , & Mansour, I. A. (2020). Could ketamine be the answer to treating treatment‐resistant major depressive disorder? General Psychiatry, 33(5), e100227. 10.1136/gpsych-2020-100227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, B. , Butelman, E. R. , & Kreek, M. J. (2020). Kappa opioid receptor antagonists as potential therapeutics for mood and substance use disorders. Handbook of Experimental Pharmacology, 473–491. 10.1007/164_2020_401 [DOI] [PubMed] [Google Scholar]
- Reiff, C. M. , Richman, E. E. , Nemeroff, C. B. , Carpenter, L. L. , Widge, A. S. , Rodriguez, C. I. , Kalin, N. H. , McDonald, W. M. , & the Work Group on Biomarkers and Novel Treatments, a Division of the American Psychiatric Association Council of Research . (2020). Psychedelics and psychedelic‐assisted psychotherapy. The American Journal of Psychiatry, 177(5), 391–410. 10.1176/appi.ajp.2019.19010035 [DOI] [PubMed] [Google Scholar]
- Reutfors, J. , Andersson, T. M. , Brenner, P. , Brandt, L. , DiBernardo, A. , Li, G. , Hägg, D. , Wingård, L. , & Bodén, R. (2018). Mortality in treatment‐resistant unipolar depression: A register‐based cohort study in Sweden. Journal of Affective Disorders, 238, 674–679. 10.1016/j.jad.2018.06.030 [DOI] [PubMed] [Google Scholar]
- Reutfors, J. , Andersson, T. M. , Tanskanen, A. , DiBernardo, A. , Li, G. , Brandt, L. , & Brenner, P. (2019). Risk factors for suicide and suicide attempts among patients with treatment‐resistant depression: Nested case‐control study. Archives of Suicide Research: Official Journal of the International Academy for Suicide Research, 25, 424–438. 10.1080/13811118.2019.1691692 [DOI] [PubMed] [Google Scholar]
- Rougemont‐Bücking, A. , Gamma, F. , & Panksepp, J. (2017). Use of tramadol in psychiatric care: A comprehensive review and report of two cases. Swiss Medical Weekly, 147, w14428. 10.4414/smw.2017.14428 [DOI] [PubMed] [Google Scholar]
- Roy, A. V. , Thai, M. , Klimes‐Dougan, B. , Westlund Schreiner, M. , Mueller, B. A. , Albott, C. S. , Lim, K. O. , Fiecas, M. , Tye, S. J. , & Cullen, K. R. (2020). Brain entropy and neurotrophic molecular markers accompanying clinical improvement after ketamine: Preliminary evidence in adolescents with treatment‐resistant depression. Journal of Psychopharmacology (Oxford, England), 35, 168–177. 10.1177/0269881120928203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberto, V. L. , Jha, M. K. , & Murrough, J. W. (2020). Pharmacological treatments for patients with treatment‐resistant depression. Pharmaceuticals (Basel, Switzerland), 13(6), 116. 10.3390/ph13060116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush, A. J. , Trivedi, M. H. , Wisniewski, S. R. , Nierenberg, A. A. , Stewart, J. W. , Warden, D. , Niederehe, G. , Thase, M. E. , Lavori, P. W. , Lebowitz, B. D. , McGrath, P. J. , Rosenbaum, J. F. , Sackeim, H. A. , Kupfer, D. J. , Luther, J. , & Fava, M. (2006). Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. The American Journal of Psychiatry, 163(11), 1905–1917. 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- Sadock, B. J. , Sadock, V. A. , & Ruiz, P. (2014). Kaplan and Sadock's synopsis of psychiatry: Behavioral sciences/clinical psychiatry (Eleventh ed.). Wolters Kluwer Health. [Google Scholar]
- Sanacora, G. , Johnson, M. R. , Khan, A. , Atkinson, S. D. , Riesenberg, R. R. , Schronen, J. P. , Burke, M. A. , Zajecka, J. M. , Barra, L. , Su, H. L. , Posener, J. A. , Bui, K. H. , Quirk, M. C. , Piser, T. M. , Mathew, S. J. , & Pathak, S. (2017). Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: A randomized, placebo‐controlled study. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 42(4), 844–853. 10.1038/npp.2016.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora, G. , Smith, M. A. , Pathak, S. , Su, H. L. , Boeijinga, P. H. , McCarthy, D. J. , & Quirk, M. C. (2014). Lanicemine: A low‐trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Molecular Psychiatry, 19(9), 978–985. 10.1038/mp.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada, K. , Nakajima, S. , Kurokawa, S. , Barceló‐Soler, A. , Ikuse, D. , Hirata, A. , Yoshizawa, A. , Tomizawa, Y. , Salas‐Valero, M. , Noda, Y. , Mimura, M. , Iwanami, A. , & Kishimoto, T. (2020). Gut microbiota and major depressive disorder: A systematic review and meta‐analysis. Journal of Affective Disorders, 266, 1–13. 10.1016/j.jad.2020.01.102 [DOI] [PubMed] [Google Scholar]
- Sanches, R. F. , de Lima Osório, F. , Dos Santos, R. G. , Macedo, L. R. , Maia‐de‐Oliveira, J. P. , Wichert‐Ana, L. , de Araujo, D. B. , Riba, J. , Crippa, J. A. , & Hallak, J. E. (2016). Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A SPECT study. Journal of Clinical Psychopharmacology, 36(1), 77–81. 10.1097/JCP.0000000000000436 [DOI] [PubMed] [Google Scholar]
- Schade, S. , & Paulus, W. (2016). d‐Cycloserine in neuropsychiatric diseases: A systematic review. The International Journal of Neuropsychopharmacology, 19(4), pyv102. 10.1093/ijnp/pyv102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer, J. F. , Chrusciel, T. , Garfield, L. D. , Freedland, K. E. , Carney, R. M. , Hauptman, P. J. , Bucholz, K. K. , Owen, R. , & Lustman, P. J. (2012). Treatment‐resistant and insufficiently treated depression and all‐cause mortality following myocardial infarction. The British Journal of Psychiatry: The Journal of Mental Science, 200(2), 137–142. 10.1192/bjp.bp.111.096479 [DOI] [PubMed] [Google Scholar]
- Shariq, A. S. , Brietzke, E. , Rosenblat, J. D. , Pan, Z. , Rong, C. , Ragguett, R. M. , Park, C. , & McIntyre, R. S. (2018). Therapeutic potential of JAK/STAT pathway modulation in mood disorders. Reviews in the Neurosciences, 30(1), 1–7. 10.1515/revneuro-2018-0027 [DOI] [PubMed] [Google Scholar]
- Shelton, R. C. , Osuntokun, O. , Heinloth, A. N. , & Corya, S. A. (2010). Therapeutic options for treatment‐resistant depression. CNS Drugs, 24(2), 131–161. 10.2165/11530280-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Sleigh, J. , Harvey, M. , Voss, L. , & Denny, B. (2014). Ketamine—More mechanisms of action than just NMDA blockade. Trends in Anaesthesia and Critical Care, 4(2–3), 76–81. 10.1016/j.tacc.2014.03.002 [DOI] [Google Scholar]
- Souery, D. , Oswald, P. , Massat, I. , Bailer, U. , Bollen, J. , Demyttenaere, K. , Kasper, S. , Lecrubier, Y. , Montgomery, S. , Serretti, A. , Zohar, J. , Mendlewicz, J. , & Group for the Study of Resistant Depression . (2007). Clinical factors associated with treatment resistance in major depressive disorder: Results from a European multicenter study. The Journal of Clinical Psychiatry, 68(7), 1062–1070. 10.4088/jcp.v68n0713 [DOI] [PubMed] [Google Scholar]
- Souery, D. , & Pitchot, W. (2013). Definitions and predictors of treatment‐resistant depression. In Kasper S. & Montgomery S. A. (Eds.), Treatment‐resistant depression (pp. 1–20). John Wiley & Sons Ltd. [Google Scholar]
- Sramek, J. J. , Murphy, M. F. , & Cutler, N. R. (2016). Sex differences in the psychopharmacological treatment of depression. Dialogues in Clinical Neuroscience, 18(4), 447–457. 10.31887/DCNS.2016.18.4/ncutler [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge, R. , Hodsoll, J. , Powell, T. R. , Hotopf, M. , Hatch, S. L. , Breen, G. , & Cleare, A. J. (2019). Inflammatory profiles of severe treatment‐resistant depression. Journal of Affective Disorders, 246, 42–51. 10.1016/j.jad.2018.12.037 [DOI] [PubMed] [Google Scholar]
- Sukoff Rizzo, S. J. , Neal, S. J. , Hughes, Z. A. , Beyna, M. , Rosenzweig‐Lipson, S. , Moss, S. J. , & Brandon, N. J. (2012). Evidence for sustained elevation of IL‐6 in the CNS as a key contributor of depressive‐like phenotypes. Translational Psychiatry, 2(12), e199. 10.1038/tp.2012.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H. , Kennedy, P. J. , & Nestler, E. J. (2013). Epigenetics of the depressed brain: Role of histone acetylation and methylation. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38(1), 124–137. 10.1038/npp.2012.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadler, S. C. , & Mickey, B. J. (2018). Emerging evidence for antidepressant actions of anesthetic agents. Current Opinion in Anaesthesiology, 31(4), 439–445. 10.1097/ACO.0000000000000617 [DOI] [PubMed] [Google Scholar]
- Taylor, W. D. (2014). Clinical practice. Depression in the elderly. The New England Journal of Medicine, 371(13), 1228–1236. 10.1056/NEJMcp1402180 [DOI] [PubMed] [Google Scholar]
- Thase, M. E. , & Rush, A. J. (1995). Treatment resistant depression. In Bloom F. E. & Kupfer D. J. (Eds.), Psychopharmacology: The fourth generation of progress (pp. 1081–1097). Raven Press Ltd. [Google Scholar]
- Thompson, P. M. , Ge, T. , Glahn, D. C. , Jahanshad, N. , & Nichols, T. E. (2013). Genetics of the connectome. NeuroImage, 80, 475–488. 10.1016/j.neuroimage.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiosano, S. , Yavne, Y. , Watad, A. , Langevitz, P. , Lidar, M. , Feld, J. , Tishler, M. , Aamar, S. , Elkayam, O. , Balbir‐Gurman, A. , Molad, Y. , Ehrlich, S. , Abu‐Shakra, M. , Amital, D. , & Amital, H. (2020). The impact of tocilizumab on anxiety and depression in patients with rheumatoid arthritis. European Journal of Clinical Investigation, 50(9), e13268. 10.1111/eci.13268 [DOI] [PubMed] [Google Scholar]
- Tubbs, J. D. , Ding, J. , Baum, L. , & Sham, P. C. (2020). Systemic neuro‐dysregulation in depression: Evidence from genome‐wide association. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 39, 1–18. 10.1016/j.euroneuro.2020.08.007 [DOI] [PubMed] [Google Scholar]
- Tundo, A. , de Filippis, R. , & Proietti, L. (2015). Pharmacologic approaches to treatment resistant depression: Evidences and personal experience. World Journal of Psychiatry, 5(3), 330–341. 10.5498/wjp.v5.i3.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnard, C. , Rane, L. J. , Wooderson, S. C. , Markopoulou, K. , Poon, L. , Fekadu, A. , Juruena, M. , & Cleare, A. J. (2014). The impact of childhood adversity on suicidality and clinical course in treatment‐resistant depression. Journal of Affective Disorders, 152‐154, 122–130. 10.1016/j.jad.2013.06.037 [DOI] [PubMed] [Google Scholar]
- Valles‐Colomer, M. , Falony, G. , Darzi, Y. , Tigchelaar, E. F. , Wang, J. , Tito, R. Y. , Schiweck, C. , Kurilshikov, A. , Joossens, M. , Wijmenga, C. , Claes, S. , Van Oudenhove, L. , Zhernakova, A. , Vieira‐Silva, S. , & Raes, J. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology, 4(4), 623–632. 10.1038/s41564-018-0337-x [DOI] [PubMed] [Google Scholar]
- Vande Voort, J. L. , Morgan, R. J. , Kung, S. , Rasmussen, K. G. , Rico, J. , Palmer, B. A. , Schak, K. M. , Tye, S. J. , Ritter, M. J. , Frye, M. A. , & Bobo, W. V. (2016). Continuation phase intravenous ketamine in adults with treatment‐resistant depression. Journal of Affective Disorders, 206, 300–304. 10.1016/j.jad.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Vargas, A. S. , Luís, Â. , Barroso, M. , Gallardo, E. , & Pereira, L. (2020). Psilocybin as a new approach to treat depression and anxiety in the context of life‐threatening diseases—A systematic review and meta‐analysis of clinical trials. Biomedicine, 8(9), 331. 10.3390/biomedicines8090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz, M. , & Silvestre, S. (2020). Alzheimer's disease: Recent treatment strategies. European Journal of Pharmacology, 887, 173554. 10.1016/j.ejphar.2020.173554 [DOI] [PubMed] [Google Scholar]
- Voineskos, D. , Daskalakis, Z. J. , & Blumberger, D. M. (2020). Management of treatment‐resistant depression: Challenges and strategies. Neuropsychiatric Disease and Treatment, 16, 221–234. 10.2147/NDT.S198774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, A. J. , Burnett, S. A. , Hasebe, K. , McGillivray, J. A. , Gray, L. J. , McGee, S. L. , Walder, K. , Berk, M. , & Tye, S. J. (2013). Chronic adrenocorticotrophic hormone treatment alters tricyclic antidepressant efficacy and prefrontal monoamine tissue levels. Behavioural Brain Research, 242, 76–83. 10.1016/j.bbr.2012.12.033 [DOI] [PubMed] [Google Scholar]
- Wallace, C. , & Milev, R. (2017). The effects of probiotics on depressive symptoms in humans: A systematic review. Annals of General Psychiatry, 16, 14. 10.1186/s12991-017-0138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. M. , Kim, N. Y. , Na, H. R. , Lim, H. K. , Woo, Y. S. , Pae, C. U. , & Bahk, W. M. (2021). Rapid onset of intranasal esketamine in patients with treatment resistant depression and major depression with suicide ideation: A meta‐analysis. Clinical Psychopharmacology and Neuroscience: The Official Scientific Journal of the Korean College of Neuropsychopharmacology, 19(2), 341–354. 10.9758/cpn.2021.19.2.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T. , Shi, C. , Li, X. , Zhang, P. , Liu, B. , Wang, H. , Wang, Y. , Yang, Y. , Wu, Y. , Li, H. , & Xu, Z. D. (2018). Injection of oxytocin into paraventricular nucleus reverses depressive‐like behaviors in the postpartum depression rat model. Behavioural Brain Research, 336, 236–243. 10.1016/j.bbr.2017.09.012 [DOI] [PubMed] [Google Scholar]
- Wilkinson, S. T. , & Sanacora, G. (2019). A new generation of antidepressants: An update on the pharmaceutical pipeline for novel and rapid‐acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discovery Today, 24(2), 606–615. 10.1016/j.drudis.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, L. M. , Debattista, C. , Duchemin, A. M. , Schatzberg, A. F. , & Nemeroff, C. B. (2016). Childhood trauma predicts antidepressant response in adults with major depression: Data from the randomized international study to predict optimized treatment for depression. Translational Psychiatry, 6(5), e799. 10.1038/tp.2016.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner, P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: A 10‐year review and evaluation. Psychopharmacology, 134(4), 319–329. 10.1007/s002130050456 [DOI] [PubMed] [Google Scholar]
- Willner, P. (2016). The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiology of Stress, 6, 78–93. 10.1016/j.ynstr.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner, P. , & Belzung, C. (2015). Treatment‐resistant depression: Are animal models of depression fit for purpose? Psychopharmacology, 232(19), 3473–3495. 10.1007/s00213-015-4034-7 [DOI] [PubMed] [Google Scholar]
- Willner, P. , Gruca, P. , Lason, M. , Tota‐Glowczyk, K. , Litwa, E. , Niemczyk, M. , & Papp, M. (2019). Validation of chronic mild stress in the Wistar‐Kyoto rat as an animal model of treatment‐resistant depression. Behavioural Pharmacology, 30(2), 239–250. 10.1097/FBP.0000000000000431 [DOI] [PubMed] [Google Scholar]
- Witkin, J. M. , Martin, A. E. , Golani, L. K. , Xu, N. Z. , & Smith, J. L. (2019). Rapid‐acting antidepressants. Advances in Pharmacology (San Diego, Calif.), 86, 47–96. 10.1016/bs.apha.2019.03.002 [DOI] [PubMed] [Google Scholar]
- Yuan, N. , Chen, Y. , Xia, Y. , Dai, J. , & Liu, C. (2019). Inflammation‐related biomarkers in major psychiatric disorders: A cross‐disorder assessment of reproducibility and specificity in 43 meta‐analyses. Translational Psychiatry, 9(1), 233. 10.1038/s41398-019-0570-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate, C. , Duman, R. S. , Liu, G. , Sartori, S. , Quiroz, J. , & Murck, H. (2013). New paradigms for treatment‐resistant depression. Annals of the New York Academy of Sciences, 1292, 21–31. 10.1111/nyas.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate, C. A. Jr. , Mathews, D. , Ibrahim, L. , Chaves, J. F. , Marquardt, C. , Ukoh, I. , Jolkovsky, L. , Brutsche, N. E. , Smith, M. A. , & Luckenbaugh, D. A. (2013). A randomized trial of a low‐trapping nonselective N‐methyl‐d‐aspartate channel blocker in major depression. Biological Psychiatry, 74(4), 257–264. 10.1016/j.biopsych.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeifman, R. J. , Palhano‐Fontes, F. , Hallak, J. , Arcoverde, E. , Maia‐Oliveira, J. P. , & Araujo, D. B. (2019). The impact of ayahuasca on suicidality: Results from a randomized controlled trial. Frontiers in Pharmacology, 10, 1325. 10.3389/fphar.2019.01325 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no new data were created or analysed in this study.


