Abstract
The effect of effluent irrigation on community composition and function of ammonia-oxidizing bacteria (AOB) in soil was evaluated, using techniques of molecular biology and analytical soil chemistry. Analyses were conducted on soil sampled from lysimeters and from a grapefruit orchard which had been irrigated with wastewater effluent or fertilizer-amended water (FAW). Specifically, comparisons of AOB community composition were conducted using denaturing gradient gel electrophoresis (DGGE) of PCR-amplified fragments of the gene encoding the α-subunit of the ammonia monooxygenase gene (amoA) recovered from soil samples and subsequent sequencing of relevant bands. A significant and consistent shift in the population composition of AOB was detected in soil irrigated with effluent. This shift was absent in soils irrigated with FAW, despite the fact that the ammonium concentration in the FAW was similar. At the end of the irrigation period, Nitrosospira-like populations were dominant in soils irrigated with FAW, while Nitrosomonas-like populations were dominant in effluent-irrigated soils. Furthermore, DGGE analysis of the amoA gene proved to be a powerful tool in evaluating the soil AOB community population and population shifts therein.
Agricultural irrigation with wastewater effluent is a common practice in arid and semiarid regions, and it is used as a readily available and inexpensive option to fresh water. However, irrigation with effluent has possible public health and environmental side effects, as effluents may contain pathogens and high levels of sodium, dissolved organic carbon, detergents, and toxic metals (22). Furthermore, the addition of such a “mixed bag” of compounds may cause significant shifts in structure and function of a microbial community, which in turn may influence the viability of the soil for agriculture. One important group of organisms that may be affected is the chemolithotrophic ammonia-oxidizing bacteria (AOB). The AOB, which are responsible for the first, rate-limiting step in nitrification in which ammonia (NH3) is transformed to nitrate (NO3−) via nitrite (NO2−), play a critical role in natural nitrogen cycling (9, 21).
The AOB have previously been demonstrated to be affected by a variety of chemical conditions including, but not limited to levels of ammonium, organic matter, O2, toxic metals, salt, and pH (6, 11, 17, 21, 24, 29–31). Traditional biological methods to study the AOB are extremely time-consuming and generally unrepresentative, as the AOB exhibit slow growth rates, low biomass yields, and a limited number of distinguishing phenotypic characteristics (26).
To address these concerns, molecular methods may be implemented. Application of molecular techniques targeting the 16S ribosomal DNA (rDNA) of the AOB has shown that this group is comprised of two monophyletic lineages within the class Proteobacteria. These lineages include the genus Nitrosococcus in the γ subgroup and the two genera Nitrosospira (containing the former genera Nitrosovibrio and Nitrosolobus) and Nitrosomonas in the β subgroup (7). One major drawback of using the 16S rRNA gene as a molecular marker for cultivation-independent study of AOB in environmental samples is that PCR primers targeting the 16S rDNA gene may cross-react with members of other phylogenetic and physiological groups (23, 28, 32). Furthermore, 16S rDNA sequence similarity among different AOB is so high that only limited phylogenetic information can be obtained using this gene as a molecular marker, and none of the published PCR primers and probes for detection of these organisms in the environment show both 100% sensitivity and 100% specificity (1, 27). Alternatively, the functional gene encoding the α-subunit ammonia monooxygenase (amoA), found only in AOB, has been shown to be useful as a specific molecular marker in environmental studies of AOB (28, 30). The successful use of a highly specific set of PCR primers to amplify a fragment of amoA from a variety of pure cultures of β-subgroup AOB and environmental samples was previously demonstrated (8, 14, 27, 28, 30). In addition, Purkhold et al. (27) have demonstrated that the phylogeny of the amoA gene corresponds with the phylogeny of the 16S rDNA of these bacteria. Denaturing gradient gel electrophoresis (DGGE) is a powerful tool for analyzing microbial communities and has been used to separate mixed PCR products (13, 18, 20, 35, 36), including those of AOB 16S rDNA fragments (5, 12, 14–16, 31). However, DGGE analysis of 16S rDNA fragments amplified with AOB-specific primers did not adequately resolve environmental populations of AOB (5, 12, 14–16, 31).
In this study we examined the impact of soil irrigation with effluent on the community composition of autotrophic AOB. Total DNA was extracted from lysimeter and orchard soils irrigated with effluent or fertilizer-amended water (FAW) and from the effluents used for irrigation. DGGE analyses of PCR-amplified amoA gene fragments from the respective soils were conducted in tandem with chemical analyses.
MATERIALS AND METHODS
Soil, irrigation, and experimental setup.
The studies were conducted in two types of experimental systems, lysimeters and orchard soil. The lysimeters consisted of asbestos containers that were 120 cm high with a surface area of 0.5 m2, yielding a total soil volume of 600 liters. The lysimeters contained a sandy loam soil (Hamra) which was obtained from the coastal plain in Israel. The chemical and physical properties of the soils are summarized in Table 1. The lysimeters received one of two water treatments: drip irrigation with secondary effluent of treated urban sewage from the Shomrat reservoir located near Ackre in Israel, or drip irrigation with FAW amended with nitrogen (in the form of ammonium sulfate), phosphorus (monopotassium phosphate), and potassium (potassium chloride and monopotassium phosphate) at concentrations similar to those in the effluent. The ammonium concentration in the FAW was designed to be similar to the sum of ammonium and organic nitrogen concentrations in the effluent. The properties of the irrigation waters are summarized in Table 2. Each treatment was conducted in triplicate in separate lysimeters, each growing maize. Two lysimeters with no plants, one irrigated with FAW and the other with effluent, were also maintained. Irrigation of the lysimeters began in July 1998, and soil was sampled for AOB analysis during the second season of maize growth, which began in June 1999. The lysimeters were sampled on days 25, 55, and 82 after maize seeding during the second growth season (referred to as the first, second, and third samplings, respectively) from different horizontal holes located at two sampling depths, 15 and 65 cm below the upper rim (three holes at each depth). At each sampling date, approximately 100 g of soil was removed per sampling depth. Molecular analysis was performed on the samples obtained on the last sampling date.
TABLE 1.
Properties of the soil used in the lysimeter experiment
| Component or property | Value |
|---|---|
| Clay (%a) | 10 |
| Silt (%a) | 6.9 |
| Sand (%a) | 83.1 |
| Organic matter (%a) | 0.3 |
| Calcium carbonate (%a) | 2.1 |
| CEC (meq/100 g of soil)b | 3 |
| EC (dS m−1)c | 0.12 |
| pHc | 6.6 |
Percent composition (wt/wt).
CEC, cation exchange capacity.
Measured in aqueous extract with 1:2 soil:water ratio.
TABLE 2.
Properties of irrigation water used in the experimentsa
| Source | EC (dS m−1) | pH | N-NH4 (mg liter−1) | N-NO3 (mg liter−1) | Total nitrogen (mg liter−1)b | Phosphate (mg liter−1) | Total P (mg liter−1) | Potassium (mg liter−1) | BOD (mg liter−1) |
|---|---|---|---|---|---|---|---|---|---|
| Lysimeter | |||||||||
| Effluent | 1.4 ± 0.1 | 7.6 ± 0.4 | 18.8 ± 8.6 | 2.8 ± 5.0 | 28.8 ± 12.0 | 6.2 ± 2.2 | 11.1 ± 4.4 | 29.8 ± 0.5 | 44.6 ± 22.4 |
| FAW | 1.1 ± 0.2 | 7.6 ± 0.4 | 31.0 | 5.5 ± 3.3 | 36.5 ± 3.3 | 6.0 | 6.0 | 30.0 | 0 |
| Orchard | |||||||||
| Effluent | 1.7 ± 0.1 | 7.6 ± 0.2 | 30.8 ± 5.2 | 0.28 ± 0.7 | 32.2 ± 5.0 | 6.2 ± 2.8 | NDc | 32.1 | NDc |
| FAW | 1.1 ± 0.1 | 7.4 ± 0.2 | 9.8 ± 3.6 | 23.8 ± 5.2 | 33.6 ± 8.7 | 4.65 ± 1.6 | 4.65 | 20.0 | 0 |
Average or mean (± standard deviation) values for 1999.
Calculated as the sum of the nitrate concentration (determined with a Lachat System IV flow injection autoanalyzer) and ammonium and organic nitrogen concentrations (measured using the rapid wet digestion method for N and P [33]).
ND, not determined.
Orchard sampling.
In addition to soil sampling from lysimeters, soil was also sampled (at a depth of 15 cm) from a sandy-loam grapefruit orchard located in Ramat-Hakovesh in northern Israel. Three samples of FAW-irrigated orchard soil and three samples of effluent-irrigated soil were taken for molecular analysis on January 2000, at the end of a 9-month irrigation period of four irrigations per week. The physical and chemical characteristics of the orchard soil were similar to those of the lysimeters (data not shown). The grapefruit orchard was first planted in 1980, and since 1995 the plots have been irrigated either with effluent or FAW with nitrogen, phosphorus, and potassium content similar to that of the effluent. The ammonium concentration in the FAW was designed to be similar to the sum of ammonium and organic nitrogen concentrations in effluent. The properties of the irrigation waters are summarized in Table 2.
Nitrogen compounds in waters and lysimeters soils.
Ammonium and nitrate concentrations were determined in the effluent, in the FAW, and in 1 N KCl extracts of soil with a Lachat System IV flow injection autoanalyzer (Lachat, Milwaukee, Wis.). The sum of organic nitrogen and ammonium concentrations in effluents used to irrigate the lysimeters was measured using a rapid wet digestion method for N and P, as described previously (33). Total nitrogen concentrations in effluents used to irrigate the orchard were measured using the Kjeldahl method (4).
BOD.
Biological oxygen demand (BOD) was measured using a HACH BOD apparatus (model 2173B; HACH Company, Loveland, Colo.) in accordance with the manufacturer's instructions.
pH and EC in soil.
pH and electroconductivity (EC) in soil were measured in water extracts of a 1:2 soil-to-water ratio. pH was measured with an El-Hama pH meter (El-Hama Instruments, Rosh Pina, Israel). EC was measured with an El-Hama conductometer TH 27.
DNA extraction and purification from soil and effluent.
DNA was extracted either from 0.4 g of soil or from a pellet of a 500-ml effluent sample centrifuged at 4,000 × g for 5 min. DNA was extracted using a FastPrep machine (Bio 101, Holbrook, N.Y.) with the FastSoil DNA purification kit (Bio 101, Vista, Calif.) in accordance with the manufacturer's instructions.
PCR and DGGE analysis of amoA fragments.
Fragments (491 bp) of the amoA gene were enzymatically amplified using a 1650 Air Thermo-Cycler (Idaho Technology, Idaho Falls, Idaho) with the primers amoA-1F and amoA-2R (28). A GC clamp (5′-CCGCCGCGCGGCGGGCGGGGCGGGGGCACGGGG-3′) was attached to the forward primer in order to increase DGGE gel separation (18). Reactions were performed in accordance with the manufacturer's recommendations in a total volume of 50 μl by using 40 mM MgCl2 reaction buffer (Idaho Technology), 1.5 U of Taq DNA polymerase (RedTaq; Sigma, St. Louis, Mo.), 1 μl of template DNA, 50 pmol of each primer, 20 nmol of each deoxynucleoside triphosphate, and 12.5 μg of bovine serum albumin. Thermal cycling was carried out with an initial denaturation step of 94°C for 30 s followed by 35 cycles of denaturation at 94°C for 15 s, annealing at 55°C for 20 s, and elongation at 72°C for 40 s; cycling was completed by a final elongation step of 72°C for 2 min. The presence and size of the amplification products were determined by agarose (2%) gel electrophoresis of the reaction product and ethidium bromide staining.
DGGE analysis was performed with a D-Gene system (Bio-Rad, Hercules, Calif.) using the following ingredients and conditions: 1× TAE (40 mM Tris, 20 mM acetic acid, 1 mM EDTA [pH 8.3]); 1-mm thick gels; a denaturant gradient of 20 to 50% urea-formamide at 60°C (19); 250 V for 3 h. Ethidium bromide-stained DGGE gels were photographed on a UV transillumination table (302 nm) with a Kodak digital camera, and inverse images were prepared with Photoshop version 4.0 (Adobe). Alternatively, gels were photographed using a Polaroid camera, and pictures were scanned and prepared with Photoshop.
Excision and reamplification of DGGE bands.
DGGE bands were carefully excised on an UV transilluminator with a scalpel and subsequently transferred to a 1.5-ml tube containing 500 μl of TE and 500 mg of glass beads (0.75- to 1.0-mm diameter). The acrylamide was disrupted by bead beating (FastPrep, Bio101) at maximum speed for 1 min. The samples were incubated for 30 min at 37°C, and 1 μl of the supernatant was subsequently used for reamplification of the DNA fragment. A second DGGE analysis was performed to confirm that only one band was reamplified and that it had the same position in the gel as the excised band.
Cloning of PCR products.
The pGEM-T Easy Vector System (Promega, Madison, Wis.) was used for cloning of excised and reamplified DGGE bands. Ligation and transformation reactions were performed according to the manufacturer's instructions. Clones were screened by suspending colony material in the PCR mixture and amplifying as described before. Plasmids from selected colonies were purified with the Wizard Plus Miniprep DNA Purification System (Promega) according to the manufacturer's instructions.
Sequencing.
Clones were sequenced using the Applied Biosystems PRISM Dye Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq DNA polymerase. The sequencing products were analyzed with an Applied Biosystems 377 DNA sequencer.
Phylogenetic analyses.
Nucleotide and deduced amoA amino acid sequences were analyzed using the program package ARB (http://www.biol.chemie.tu _munchen.de/Pub/ARB). Phylogenetic analyses were performed by applying protein parsimony (PHYLIP 3.55), neighbor-joining (using the PAM correction), and maximum likelihood programs implemented in the ARB software package. The topologies of the resulting trees were compared. Branching order was supported by all methods. Primer sequences were not included in the phylogenetic analyses.
Nucleotide sequence accession numbers.
The sequences discussed in this study are available from EMBL under accession numbers AF353246 to AF353264.
RESULTS
Ammonium input.
Ammonium concentration in the FAW used for irrigation was 31.0 mg liter−1 N-NH4+ (Table 2). Assuming a fast mineralization rate of organic nitrogen to ammonium in the effluents, the average ammonium concentration in the effluents used for irrigation was 26.0 mg liter−1 N-NH4+ (Table 2) (2).
Inorganic nitrogen compounds in soil.
Average nitrate concentrations in the soil samples obtained from the effluent- and FAW-irrigated lysimeters with plants were less than 1.5 μg/g of soil at both depths, on the first and second sampling dates (Fig. 1). However, during the last sampling, nitrate concentrations in the lysimeters increased at a depth of 15 cm in both the effluent- and the FAW-irrigated soils, to 17 and 16 μg/g of soil, respectively. A similar trend was observed in the lysimeters with no plants (Fig. 1). In these lysimeters, nitrate concentrations on the first and second sampling dates were similarly low (less than 5 μg/g of soil) at both depths. On the last sampling date, nitrate concentrations at the depth of 15 cm increased to 16 and 37 μg/g of soil in the effluent- and FAW-irrigated lysimeters, respectively. At a depth of 65 cm, nitrate concentrations remained low during the entire irrigation season, in all irrigation treatments.
FIG. 1.
Changes in nitrate concentrations (microgram per gram of soil) in soil of lysimeters with (A) and without (B) maize. Analysis was conducted on FAW-irrigated soil at depths of 15 (■) and 65 (□) cm and on effluent-irrigated soils at depths of 15 (●) and 65 (○) cm. Values in panel A are mean of three independent measurements, and values in panel B are from one measurement.
The soil ammonium concentrations in the lysimeters with and without plants increased from a range of 6 to 10 μg/g of soil during the first sampling to a range of 13 to 21 μg/g of soil during the second sampling, and then they decreased to a range of 0 to 10 μg/g of soil during the last sampling (Fig. 2). Ammonium concentrations in the lysimeters with plants at all depths approached near-identical values in the final analysis.
FIG. 2.
Changes in ammonium concentrations (microgram per gram of soil) in soil of lysimeters with (A) and without (B) maize. Analysis was conducted on FAW-irrigated soil at depths of 15 (■) and 65 (□) cm and on effluent-irrigated soils at depths of 15 (●) and 65 (○) cm. Values in panel A are means of three independent measurements, and values in panel B are from one measurement.
In brief summary, the highest ammonium concentrations at the depth of 15 cm were observed in all of the samples during the second sampling, and the highest nitrate concentrations were observed during the last sampling.
pH and EC in soil.
pH measurements of replicate lysimeter soils taken during the final sampling period indicated that the average pH values of the effluent- and FAW-irrigated lysimeters differed by no more than 0.1 pH units, being between 7.4 and 7.6 (data not shown).
A similar trend was observed in the orchard soil. Average pH values of the effluent-irrigated orchard soil were 0.1 pH units higher than the pH values of the FAW-irrigated orchard soil (data not shown).
EC measurements of replicate lysimeter soils taken during the last sampling period revealed that the EC of both effluent- and FAW-irrigated lysimeters with plants were 0.17 dS m−1. Similarly, average EC values for orchard soil at the end of the irrigation period were 0.19 and 0.18 dS m−1 in the effluent- and FAW-irrigated soils, respectively.
AOB community composition in lysimeter soil.
Analysis of the AOB community composition by PCR-DGGE was performed on all lysimeters, with and without plants. Figure 3 presents the DGGE banding pattern of amoA fragments amplified from total DNA extracted during the third sampling, from all the lysimeters and depths studied, with the exception of one sample from a depth of 65 cm which amplified poorly. Examination of the DGGE showed that banding patterns obtained from identically treated lysimeters were similar and that two main general distributions of dominant bands were present. Dominant DGGE bands detected in the FAW-irrigated soils (w2521, w627, and w2404) migrated to the lower third of the gel, while those detected in the effluent-irrigated soils migrated both to the lower (w2521 and w2404) and to the upper (w637, w614, w735, w716, w1203 and w537) third of the gel (Fig. 3). In only one of the FAW-irrigated lysimeters was a band detected in the upper third of the gel (w2406).
FIG. 3.
DGGE analysis of amoA fragments obtained from soils of lysimeters from the last sampling period. Samples were analyzed from depths of 15 and 65 cm. Five samples were from FAW-irrigated soil from lysimeters with plants (FeS+P); six samples were from effluent-irrigated soil from lysimeters with plants (EfS+P); two samples were from FAW-irrigated soil from lysimeters without plants (FeS); and two samples were from effluent-irrigated soils from lysimeters without plants (EfS).
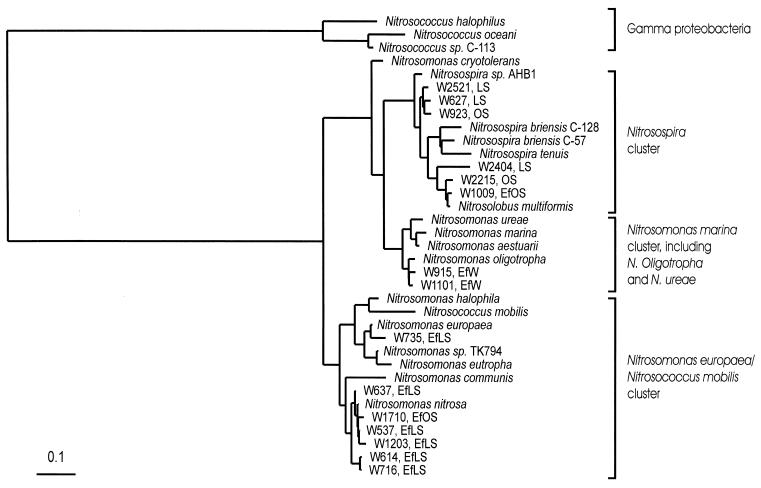
Ten bands were excised from the DGGE gel and sequenced. All excised fragments were confirmed as amoA by sequence analysis, with the exception of one fragment which was detected in the upper third of the gel in only one of the FAW-irrigated lysimeters (w2406). Sequence analysis also revealed that the bands in the lower third of the DGGE gel (w2521, w627, and w2404) were phylogenetically most closely related to Nitrosospira species, while the bands in the upper third of the gel (w637, w614, w735, w716, w1203, and w537) were phylogenetically most closely related to Nitrosomonas species (Fig. 3 and 4).
FIG. 4.
Phylogenetic tree constructed for partial amoA gene sequences, based upon 151 deduced amino acid sites. The tree was produced by using a neighbor-joining algorithm. The tree shows the relationship between cultured AOB and environmental sequences obtained from effluent-irrigated soil in lysimeters (EfLS) or orchard (EfOS), exclusively; effluent- or FAW-irrigated soil from lysimeters (LS) or orchard (OS); and effluents used for irrigation (EfW). The bar indicates an estimated 10% sequence divergence. The cluster nomenclature is that of Purkhold et al. (27).
AOB community composition in orchard soils.
Figure 5 presents the DGGE banding pattern of amoA fragments amplified from orchard soil samples. Since banding patterns obtained from similar irrigation treatments were similar, only one representative sample of each treatment is represented.
FIG. 5.
DGGE analysis of amoA fragments obtained from two representative samples of the last soil sampling from lysimeters without plants, irrigated with FAW (FeLS) or effluent (EfLS); two representative samples of the orchard soil irrigated with FAW (FeOS) or effluent (EfOS); and two representative samples of the effluents used for irrigation (EfW). Soil was sampled at a depth of 15 cm. The band designated by the ∗ is a PCR artifact and is not an amoA fragment.
In FAW-irrigated orchard soil samples, a single DGGE band was detected in the lower third of the DGGE gel (w923), while in the effluent-irrigated soil bands were detected both in the upper (w1710) and the lower (w923, w1009, and w2215) third (Fig. 5). Sequence analyses of these bands confirmed their phylogenetic affiliation with the sequences obtained from the lysimeter experiment (Fig. 4). A single Nitrosospira-like sequence was detected in the FAW-irrigated orchard (w923), while both Nitrosomonas-like (w1710) and Nitrosospira-like (w1009, w923, and w2215) sequences were found in the effluent-irrigated orchard.
Six of the seven Nitrosomonas-like sequences obtained from the effluent-irrigated soils of the lysimeter and the orchard experiment were grouped into one cluster with Nitrosomonas nitrosa. The only Nitrosomonas-like sequence which did not cluster within this group was w735, obtained from the 65-cm depth of an effluent-irrigated lysimeter, which grouped with Nitrosomonas europaea (Fig. 4). All seven sequences related to Nitrosomonas obtained from the effluent-irrigated soils belonged to the N. europaea-Nitrosococcus mobilis cluster (27) (Fig. 4). Three of the six Nitrosospira-like sequences obtained from the lysimeters and orchard soils grouped into one cluster together with Nitrosospira (formerly Nitrosolobus) multiformis. The other three bands (w627, w2521, and w923) clustered with the soil isolate Nitrosospira sp. AHB1.
AOB community composition in the effluent.
DGGE analysis of PCR products obtained from two samples of effluents used to irrigate the lysimeters (taken from different dates) indicated that only one strong band in the middle third of the gel was detected for each effluent sample (w915 and w1101) (Fig. 5). Both sequences migrated differently from all of the bands in soil samples. The two bands, although migrating similarly on the DGGE gel, were determined to be different organisms, based on amoA sequence analysis. This analysis revealed that both were closely related to Nitrosomonas oligotropha of the Nitrosomonas marina cluster and were distinctly different from the Nitrosomonas-like sequences obtained from the effluent-irrigated soils (27) (Fig. 4).
DISCUSSION
New developments in the application of molecular methods to the study of AOB have increased our ability to characterize microbial diversity and population shifts in environmental systems. These developments include the design of a new set of PCR primers that specifically amplify a fragment of amoA, a functional molecular marker for the β-subgroup of AOB (27, 28, 30), and also the use of DGGE to separate the PCR-amplified fragments on the basis of sequence composition. In this study, we present the first application of amoA DGGE to the study of AOB in complex environmental systems. The predominant AOB populations identified in this study, Nitrosospira-like species and Nitrosomonas-like species, were readily separated by DGGE analyses. Specifically, all six analyzed amoA bands which migrated to the lower third of the DGGE gel were determined to be phylogenetically related to Nitrosospira species, while all amoA bands migrating to the upper third of the gel were phylogenetically associated with Nitrosomonas species. Furthermore, bands consisting of highly similar sequences (i.e., bands w614 and w716, differing by 2 bp and identical in amino acid sequence) were clearly separated on the DGGE gel. The only band which migrated to the upper third of the gel and was not phylogenetically associated with Nitrosomonas species was determined not to be an amoA sequence (w2406). It is important to note that the amoA primers have two degenerate nucleotide positions (28); thus, generally, one PCR-amplified amoA sequence might be represented in two separate DGGE bands. However, no such case was observed in this research.
The findings of this study suggest that irrigation with wastewater effluent altered the predominant AOB population in the soil, as compared to soil irrigated with FAW. Towards the end of the irrigation period, Nitrosospira-like sequences were dominant in FAW-irrigated soil (Fig. 3, 4, and 5) and Nitrosomonas populations were absent, while in the effluent-irrigated soil both Nitrosospira-like and Nitrosomonas-like sequences were present (Fig. 3, 4, and 5). This community change manifested itself in a consistent manner in an established orchard and in lysimeters with and without plants.
Three of the six Nitrosospira-like sequences obtained in this study were phylogenetically related to N. multiformis (Fig. 4), which is known to be among the most common soil AOB (25). The other three bands clustered with a Nitrosospira species isolated from fertilized heath soil (Nitrosospira sp. AHB1) (3). Six of the seven Nitrosomonas-like sequences obtained from the effluent-irrigated soils in the lysimeter and orchard experiments clustered with N. nitrosa (Fig. 4), an AOB commonly found in eutrophic environments (10). The only Nitrosomonas-like sequence which did not cluster within this group was obtained from a depth of 65 cm of an effluent-irrigated lysimeter (w735) and grouped with N. europaea (Fig. 4). This finding may reflect the different conditions at the 65-cm depth.
In addition to population shifts, we also noted that effluent-irrigated systems supported more complex communities of AOB. In the FAW-irrigated systems, only a few Nitrosospira-like sequences were detected from the last sampling event (Fig. 3, 4, and 5), while in the effluent-irrigated systems additional Nitrosospira-like and Nitrosomonas-like sequences were detected.
The Nitrosomonas-like sequences identified in the effluent-irrigated soil in this study were clearly different than the Nitrosomonas-like sequences identified in the effluent used for irrigation. The sequences obtained from the effluents from different dates were closely related to each other and to N. oligotropha, which is commonly detected in industrial sewage disposal systems (10), but they were divergent from the effluent-irrigated soil clones (Fig. 4). Two possible mechanisms for this scenario may be considered. First, the Nitrosomonas-like species present in the effluent-irrigated soil originated from the soil and were selected for by the environmental conditions, which changed after irrigation with the effluent. The second possibility is that these species originated from the effluent itself but were either below the PCR-DGGE detection limit (approximately 1% of the target community [19]) or originated from the effluent used for irrigation during the first year of irrigation (this period was not analyzed). Currently, there is no information to resolve this issue. Regardless of the origin of the Nitrosomonas species, it is clear that the dominant Nitrosomonas species in the wastewater were unable to dominate in the effluent-irrigated soil.
As was demonstrated, the addition of effluent to the soil systems consistently favored the development of alternate Nitrosomonas populations in these soil communities, in addition to the already-present Nitrosospira populations. Several factors are considered in examining this shift in the microbial population from that found in the soil irrigated with FAW and that from the original effluent. Previous work has suggested that ammonium concentration determines which AOB species will dominate an environmental niche (29). However, ammonium concentration does not appear to explain solely the community shift in the systems examined in this study. Assuming a fast mineralization rate of organic nitrogen to ammonium in the effluents (2), ammonium inputs in the FAW- and effluent-irrigated soils were similar (Table 2). One of the differences in the constitution of the effluent and FAW was the BOD. The effluent water contained high organic levels (BOD, 44.6 mg liter−1) compared to the FAW (BOD, 0 mg liter−1). Previous studies have indicated that the growth of Nitrosomonas populations are encouraged in soil plots amended with swine manure (6), and in other organic-rich environments, such as activated sludge and biofilm reactors, Nitrosomonas species are readily detected (28, 34). It is possible that in effluent-irrigated soil, as in other organic-rich environments, environmental conditions favor Nitrosomonas species.
Additional determinants of the AOB population structure in environmental systems may be soil pH and salt concentration. Specifically, different Nitrosospira species have been shown to develop in neutral and acid soils (31, 32). However, no significant differences in pH and salt concentration were detected in the different irrigation systems (data not shown).
Surprisingly, the salient shifts in microbial community between the effluent- and FAW-irrigated soils seem not to have been accompanied by significant shifts in community function. In the lysimeters with plants, measurements of nitrate and ammonium were similar, particularly in the final sampling period. Significant nitrate development in the lysimeters with or without plants was seen only at the depth of 15 cm on the third sampling date (82 days), although it may have begun to accrete anytime after the second sampling period (55 days). Likewise, a decrease in the soil ammonium concentration was not seen until the third sampling period (Fig. 1 and 2). These results may suggest higher nitrification rates only toward the end of the 3-month irrigation period and are consistent with the slow growth rates of AOB. The chemical analysis has also demonstrated that nitrate concentrations were significantly lower at the depth of 65 cm. Several factors may result in this difference, including a smaller AOB population. In general, it should be noted, however, that both ammonium and nitrate concentrations may also be affected by other processes, such as denitrification and leaching.
Similar trends in population shifts were noted between lysimeters with or without plants, suggesting that the plants were not critical factors in the determination of AOB community. The observation is remarkable since plant roots are generally known to affect soil microflora (21). This study has demonstrated that DGGE of the amoA fragment is a powerful tool in evaluating AOB populations in natural samples. Such studies may provide not only insight into the distribution and diversity of AOB but may also help in assessing their response to changing environmental conditions.
ACKNOWLEDGMENTS
We thank Larissa Kautsky and Ori Haras for technical assistance. We greatly appreciate the help of Stefan Green and Edouard Jurkevitch in reviewing the manuscript and discussion.
The study was supported by the Israeli Ministry of Science (grant 8868) and by the Chief Scientist of the Israeli Ministry of Agriculture and Rural Development.
REFERENCE
- 1.Aakra A, Utaker J B, Nes I F. RFLP of rRNA genes and sequencing of the 16S–23S rDNA intergenic spacer region of ammonia-oxidizing bacteria: a phylogenetic approach. Int J Syst Bacteriol. 1999;49:123–130. doi: 10.1099/00207713-49-1-123. [DOI] [PubMed] [Google Scholar]
- 2.Avnimelech Y, Wallach R. Irrigation with treated effluent. Special report supported by the Ministries of Environment and Agriculture (Hebrew). Haifa, Israel: Technion; 1991. [Google Scholar]
- 3.Boer W D, Duyts H, Laanbroek H J. Urea stimulated autotrophic nitrification in suspensions of fertilized acid heath soil. Soil Biol Biochem. 1989;21:349–354. [Google Scholar]
- 4.Bremner J M, Mulvaney C S. Nitrogen—total. In: Page A L, Miller R H, Keeney D R, editors. Methods of soil analysis, pt. 2. 2nd ed. Madison, Wis: American Society of Agronomy and Soil Science Society of America; 1982. pp. 595–622. [Google Scholar]
- 5.Bruns M A, Stephen J R, Kowalchuk G A, Prosser J I, Paul E A. Comparative diversity of ammonia oxidizer 16S rRNA gene sequences in native, tilled and successional soils. Appl Environ Microbiol. 1999;65:2994–3000. doi: 10.1128/aem.65.7.2994-3000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hastings R C, Ceccherini M T, Micalus N, Saunders J R, Bazzicalupo M, McCarthy A J. Direct molecular biological analysis of ammonia oxidizing bacteria population in cultivated soil plots treated with swine manure. FEMS Microbiol Ecol. 1997;23:45–54. [Google Scholar]
- 7.Head I M, Hiorns W D, Embley T M, McCarthy A J, Saunders J R. The phylogeny of autotrophic ammonia-oxidizing bacteria as determined by analysis of 16S ribosomal RNA gene sequences. J Gen Microbiol. 1993;139:1147–1153. doi: 10.1099/00221287-139-6-1147. [DOI] [PubMed] [Google Scholar]
- 8.Horz H P, Rotthauwe J H, Lukow T, Liesack W. Identification of major subgroups of ammonia-oxidizing bacteria in environmental samples by T-RFLP analysis of amoA PCR products. J Microbiol Methods. 2000;39:197–204. doi: 10.1016/s0167-7012(99)00119-0. [DOI] [PubMed] [Google Scholar]
- 9.Jetten M S, Logemann S, Muyzer G, Robertson L A, de Vries S, van Loosdrecht M C, Kuenen J G. Novel principles in the microbial conversion of nitrogen compounds. Antonie Leeuwenhoek. 1997;71:75–93. doi: 10.1023/a:1000150219937. [DOI] [PubMed] [Google Scholar]
- 10.Koops H P, Boettcher B, Moeller U C, Pommerening R A, Stehr G. Classification of eight new species of ammonia-oxidizing bacteria: Nitrosomonas communis, new species, Nitrosomonas ureae, new species, Nitrosomonas aestuarii, new species, Nitrosomonas marina, new species, Nitrosomonas nitrosa, new species, Nitrosomonas eutropha, new species, Nitrosomonas oligotropha, new species and Nitrosomonas halophila, new species. J Gen Microbiol. 1991;137:1689–1700. [Google Scholar]
- 11.Koops H P, Moller U C. The lithotrophic ammonia-oxidizing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 2625–2637. [Google Scholar]
- 12.Kowalchuk G A, Bodelier P L E, Heilig G H J, Stephen J R, Laanbroek H J. Community analysis of ammonia-oxidizing bacteria, in relation to oxygen availability in soil and root-oxygenated sediments, using PCR, DGGE and oligonucleotide probe hybridization. FEMS Microbiol Ecol. 1998;27:339–350. [Google Scholar]
- 13.Kowalchuk G A, Gerards S, Woldendorp J W. Detection and characterization of fungal infections of Ammophila arenaria (marram grass) roots by denaturing gradient gel electrophoresis of specifically amplified 18S rDNA. Appl Environ Microbiol. 1997;63:3858–3865. doi: 10.1128/aem.63.10.3858-3865.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowalchuk G A, Naoumenko Z S, Derikx P J L, Felske A, Stephen J R, Arkhipchenko I A. Molecular analysis of ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in compost and composted materials. Appl Environ Microbiol. 1999;65:396–403. doi: 10.1128/aem.65.2.396-403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowalchuk G A, Stephen J R, De Boer W, Prosser J I, Embley T M, Woldendorp J W. Analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl Environ Microbiol. 1997;63:1489–1497. doi: 10.1128/aem.63.4.1489-1497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCaig A E, Phillips C J, Stephen J R, Kowalchuk G A, Harvey S M, Herbert R A, Embley T M, Prosser J I. Nitrogen cycling and community structure of proteobacterial beta-subgroup ammonia-oxidizing bacteria within polluted marine fish farm sediments. Appl Environ Microbiol. 1999;65:213–220. doi: 10.1128/aem.65.1.213-220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendum T A, Sockett R E, Hirsch P R. Use of molecular and isotopic techniques to monitor the response of autotrophic ammonia-oxidizing populations of the beta subdivision of the class Proteobacteria in arable soils to nitrogen fertilizer. Appl Environ Microbiol. 1999;65:4155–4162. doi: 10.1128/aem.65.9.4155-4162.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muyzer G, de Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muyzer G, Hottentrager S, Teske A, Wawer C. Denaturing gradient gel electrophoresis of PCR-amplified 16S-rDNA—a new molecular approach to analyze the genetic diversity of mixed microbial communities. In: Akkermans A D L, Van-Elsas J D, De-Bruijn F J, editors. Molecular microbial ecology manual. 1st ed. London, United Kingdom: Kluwer Academic Publishers; 1996. pp. 1–23. [Google Scholar]
- 20.Muyzer G, Smalla K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek. 1998;73:127–141. doi: 10.1023/a:1000669317571. [DOI] [PubMed] [Google Scholar]
- 21.Paul E A, Clark F E. Soil microbiology and biochemistry. San Diego, Calif: Academic Press, Inc.; 1989. [Google Scholar]
- 22.Pettygrove G S, Davenport D C, Asano T. Introduction: California's reclaimed municipal wastewater resource. In: Pettygrove G S, Asano T, editors. Irrigation with reclaimed municipal wastewater—a guidance manual. 2nd ed. Chelsea, Mich: Lewis Publishers; 1985. pp. 1–14. [Google Scholar]
- 23.Pommerening R A, Rath G, Koops H P. Phylogenetic diversity within the genus Nitrosomonas. Syst Appl Microbiol. 1996;19:344–351. [Google Scholar]
- 24.Princic A, Mahne I, Megusar F, Paul E A, Tiedje J M. Effects of pH and oxygen and ammonium concentrations on the community structure of nitrifying bacteria from wastewater. Appl Environ Microbiol. 1998;64:3584–3590. doi: 10.1128/aem.64.10.3584-3590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prosser J I. Nitrification. Oxford, United Kingdom: IRL Press; 1986. [Google Scholar]
- 26.Prosser J I. Autotrophic nitrification in bacteria. Adv Microb Physiol. 1989;30:125–181. doi: 10.1016/s0065-2911(08)60112-5. [DOI] [PubMed] [Google Scholar]
- 27.Purhkold U, Pommerening-Roser A, Juretschko S, Schmid M C, Koops H P, Wagner M. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implication for molecular diversity surveys. Appl Environ Microbiol. 2000;66:5368–5382. doi: 10.1128/aem.66.12.5368-5382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotthauwe J H, Witzel K P, Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol. 1997;63:4704–4712. doi: 10.1128/aem.63.12.4704-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schramm A, De Beer D, Wagner M, Amann R. Identification and activities in situ of Nitrosospira and Nitrospira spp. as dominant populations in a nitrifying fluidized bed reactor. Appl Environ Microbiol. 1998;64:3480–3485. doi: 10.1128/aem.64.9.3480-3485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephen J R, Chang Y J, Macnaughton S J, Kowalchuk G A, Leung K T, Flemming C A, White D C. Effect of toxic metals on indigenous soil beta-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl Environ Microbiol. 1999;65:95–101. doi: 10.1128/aem.65.1.95-101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephen J R, Kowalchuk G A, Bruns M A, McCaig A E, Phillips C J, Embley T M, Prosser J I. Analysis of beta-subgroup proteobacterial ammonia oxidizer populations in soil by denaturing gradient gel electrophoresis analysis and hierarchical phylogenetic probing. Appl Environ Microbiol. 1998;64:2958–2965. doi: 10.1128/aem.64.8.2958-2965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephen J R, McCaig A E, Smith Z, Prosser J I, Embley T M. Molecular diversity of soil and marine 16S rRNA gene sequences related to beta-subgroup ammonia-oxidizing bacteria. Appl Environ Microbiol. 1996;62:4147–4154. doi: 10.1128/aem.62.11.4147-4154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas T L, Sheard R W, Moyer J R. Comparison of conventional and automated procedures for nitrogen, phosphorus and potassium analysis of plant material using a single digestion. Agron J. 1967;59:240–250. [Google Scholar]
- 34.Wagner M, Rath G, Amann R, Koops H P, Schleifer K H. In situ identification of ammonia oxidizing bacteria. Syst Appl Microbiol. 1995;18:251–264. [Google Scholar]
- 35.Wawer C, Jetten M S, Muyzer G. Genetic diversity and expression of the [NiFe] hydrogenase large-subunit gene of Desulfovibrio spp. in environmental samples. Appl Environ Microbiol. 1997;63:4360–4369. doi: 10.1128/aem.63.11.4360-4369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wawer C, Muyzer G. Genetic diversity of Desulfovibrio spp. in environmental samples analyzed by denaturing gradient gel electrophoresis of [NiFe] hydrogenase gene fragments. Appl Environ Microbiol. 1995;61:2203–2210. doi: 10.1128/aem.61.6.2203-2210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]