Graphic abstract
Chemical compounds studied in this article: 5-Hydroxymethylfurfural (5-HMF, PubChem CID237332); β-Sitosterol (PubChem CID222284); β-Sitosterol-3-O-glucose (PubChem CID5742590); α-Linolenic (PubChem CID5280934); 1-Monopalmitin (PubChem CID14900); Chaenomic acid A (PubChem CID102339344)
Keywords: Pickled radish, Fermented food, Separated, Purified, Molecular docking
Highlights
-
•
β-Sitosterol, β-sitosterol-3-o-glucose glycosides, α-linolenic acid, 1-monopalmitin and chaenomic acid A were identified from 5-year-old pickled radish.
-
•
Production of the merad product 5-hydroxymethylfurfural in fresh white radish after salting and fermentation.
-
•
β-Sitosterol, β-sitosterol-3-O-glucose glycosides have good affinity with antioxidant enzymes.
Abstract
In this study, we aimed to isolate and identify the bioactive compounds from 5-year pickled radish. The pickled radish was extracted with methanol or ethyl acetate. Sephadex LH-20, normal phase and reverse phase silica gel column chromatography were used for separation and purification, combined with thin layer chromatography (TLC), high performance liquid chromatography (HPLC), electrospray mass spectrometry (ESI-MS), nuclear magnetic resonance spectroscopy (NMR) technology for structural identification. The results showed that 6 compounds were separated and purified from methanol and ethyl acetate extracts of 5-year-old pickled radish. The structures were identified as 5-hydroxymethylfurfural, β-sitosterol, β-sitosterol-3-O-glucose glycosides, α-linolenic acid, 1-monopalmitin and chaenomic acid A. Using molecular docking, it was determined that β-sitosterol and its derivative β-sitosterol-3-O-glucose glycosides have high affinity for five antioxidant enzymes, and there were multiple hydrogen bonds between them. These results indicated that pickled radishes might be used as an important source of natural chemical substances.
Introduction
Pickled radish is a kind of pickled food, which is favored by people because of its unique flavor, rich nutrition and long shelf life. Locally, pickled radish is made from high-quality fresh radish, salted with traditional sea salt and naturally fermented for many years. Pickled radishes can even be stored for decades in southern Fujian of China. Since this ancient, pickling is a traditional cooking technique that is popular all over the world (Chakraborty & Roy, 2018). The fresh food is treated by dehydration and salt, and then put in a closed container through anaerobic fermentation to get the pickled food (Sawada et al., 2021). During salting and fermentation, complex biochemical reactions, such as changes in nutrient content, microbial composition and product texture, will occur in radishes (Cheigh, & Park, 1994). According to related folk applications, the pickled radish in the region has a variety of potential dietary healing properties. However, the nutritional benefits of pickled radish are only known to be folklore. Previously we have published studies in which we analysed the chemical fractions of local pickled radishes in different years. The results of the experiment revealed that the content of specific chemical components of pickled radish varied from year to year and that there was a correlation between the content and the year. Also, after spectral peak identification and matching, the study revealed that the chemical fractions of 5 year old pickled radish were richer and more reproducible across batches compared to fresh white radish (Xiong et al., 2016). Therefore, the more researchful 5 year old pickled radish was chosen for the next analysis.
More and more evidences showed that pickled radish had a variety of health benefits and biological activities, including antibacterial, anti-atherosclerosis and lipid-lowering (Manivannan et al., 2019, Terras et al., 1992). Previous studies have shown that antihypertensive factors such as polyphenols, arginine, and α-linolenic acid contained in salted radish can reduce heart rate and systolic blood pressure of spontaneously hypertensive rats by inhibiting sympathetic nerve activity and ACE activity (Kumakura et al., 2017). In addition, another report showed that the consumption of radish leaves ethyl acetate extract may improve the hypertensive state of rats by increasing serum NO concentration, fecal Na+ concentration and antioxidant enzyme activity (Chung et al., 2012). In our previous research, it was determined that several phenolic compounds in pickled radish have antibacterial and antioxidant effects (Li et al., 2020). In another study, pickled radish phenolics could reduce lipid levels in high-fat mice by regulating the intestinal flora (Li et al., 2021). Therefore, as a high-quality source of natural active chemical substances, the biologically active factors contained in pickled radishes need to be further explored.
The oxidation reaction will inevitably lead to accelerated food spoilage and deterioration during storage. Some harmful by-products, such as ROS and so on, will be overproduced under oxidation conditions (He et al., 2017). Therefore, effective antioxidants have become the key to food preservation. Many evidences prove that the inhibition of some key enzymes can improve the adverse effects of oxidative stress. For example, inhibition of NADPH oxidase (Gao et al., 2019), Cytochrome p450 (He et al., 2017), Hematopoetic cell kinase (Kong et al., 2020), Xanthine oxidase (Bergamini et al., 2009), Myeloperoxidase (Aratani, 2018) and other enzyme proteins plays a positive role in improving oxidative stress. Molecular docking helps to screen potential enzyme inhibitors and has a strong advantage in the leading link of drug development. Studying the interaction between small molecules and target enzymes is helpful to the design and development of lead drugs.
In this study, we selected 5-year-old pickled radishes as the research object, and extracted and analyzed the active compounds with the aim of providing a useful reference for the nutritional development of pickled radishes. Sephadex LH-20 column chromatography, positive phase silica gel column chromatography and reversed phase C18 (RP-C18) silica gel column chromatography was used to separate and purify methanol and ethyl acetate components. The structures of the compounds were further identified by thin layer chromatography (TLC), ultraviolet detection (UV), nuclear magnetic resonance spectroscopy (NMR) and electrospray ionization mass spectrometry (ESI-MS). In addition, the binding degree of six pickled radish compounds to some enzymes involved in oxidative stress was studied by molecular docking, and their interaction patterns were analyzed. Therefore, considering the importance of pickled radish to traditional Chinese pickled products, these findings help to provide further insight into the nutritional benefits of pickled radish.
Materials and methods
Materials
Five-year-old pickled radishes were purchased from Zhangpu market in 2015 in Fujian Province, and samples were prepared from fresh white radish root. Different batches of test samples from the region were purchased and tested for this trial to ensure that the chemical composition of each batch was consistent. Methanol, ethyl acetate and deuterated methanol are all chromatographic grade reagents (≥99 %), chloroform is a pure reagent for analysis (≥97 %), purchased from Guangdong Guanghua Technology Co., ltd. The high-precision analytical balance used in the test (accuracy: 0.0001 g) was purchased from Mettler Toledo Technology (China) Co., ltd. The metrological class of glassware used (500 ml rotary evaporation flask, 500 ml measuring cylinder, 10 ml pipette, 1L filtration flask, 1L Separatory funnel) were purchased from Fuzhou North Glass Experimental Instrument Co., ltd. Sephadex LH-20 was purchased from GE Healthcare (Uppsala, Sweden); Reverse phase Silica gel 60 RP-C18 was purchased from Biotage Company (Sweden); Normal-phase chromatography Silica gel P60 was purchased from SiliCycle (Canada); Precoated silica gel GF 254 plates was purchased from Qingdao Marine Chemical Factory (Qingdao, China).
Preparation of crude extract
The 5-year pickled radish were sliced and dried in an oven at 45 ℃ to constant weight. Samples were mixed with 10-fold volumes of methanol followed by crushing. The homogenate was extracted by stirring at 40 ℃ for 6 h, and the entire extraction procedures were repeated twice. After filtration, the supernatants were combined and evaporated to obtain the methanol crude extracts of pickled radish. Furthermore, the ethyl acetate extracts were prepared. Pickled radish powder was mixed with 10-fold volumes of ethyl acetate, and the other steps were the same as above.
Separation and purification of crude extract
Dissolve the crude extract sample with methanol, Sephadex LH-20 column and Silica gel 60 RP-C18 column were used for preliminary separation. The collected components were tracked by thin layer chromatography (TLC) and the same target point were combined. The compounds were further isolated and purified by normal-phase chromatography Silica gel P60. The purified compounds were stored at 4 ℃.
Analysis of the mass spectrometry
The separated and purified compound was dissolved in 0.1 % formic acid–methanol solution and the mass spectrum was measured. The ionization source is an electrospray ionization (ESI) source or an atmospheric pressure chemical ionization (APCI) source. Mass spectrometry was obtained by Xevo™ G2 Q Tof (Waters MS Technologies, Manchester, UK). It is a quadrupole and orthogonal acceleration time-of-flight tandem mass spectrometer.
Analysis of nuclear magnetic resonance analysis (NMR)
The compounds dissolved with deuterated reagents and moved into nuclear magnetic tube, the sample was measured on AVANCE III HD 400 MHz Nuclear magnetic resonance (NMR, Bruker Biospin GmbH, Karlsruhe, Germany) in deuterated reagents. All the samples were identified by 1H NMR, 13C NMR. Chemical shift values (δ) were given in parts per million (ppm) and coupling constant (J) in Hz. Signal multiplicities were described as follows: s (singlet), d (doublet), dd (doublet of doublets), t (triplet), m (multiplet), br s (broad singlet). The data were processed by phase correction and baseline correction using MestReNova software.
Analysis of high performance liquid chromatography (HPLC)
The purified compounds were dissolved into appropriate concentrations and analyzed by Ultimate 3000 Analytical high performance liquid chromatograph (HPLC, Thermo Company) under the same conditions. Chromatographic conditions: HPLC was used to perform gradient elution, methanol (A) 0.1 % formic acid water (B) system as the mobile phase. The flow rate was 1 ml/ min, the injection volume was 10 μL, keep the column temperature at 35 ℃ and use multiple wavelengths for detection. Elution gradient: 0–2 min, 10 % A and 90 % B; 2–10 min, 25 % A and 75 % B; 10–15 min, 25 % A and 75 % B; 15–20 min, 50 % A and 50 % B; 20–25 min, 75 % A and 25 % B; 25–35 min, 100 % A and 0 % B; 35–45 min, 100 % A and 0 % B; 45–50 min, 10 % A and 90 % B.
Molecular docking
Autodock Vina software was used to conduct docking experiments on pickled radish compounds and enzymes related to oxidation reaction, and explain their interactions. Protein crystal structure was downloaded from RCSB Protein Data Bank (https://www.RCSB PDB). Namely NADPH oxidase (PDB ID: 2CDU), Cytochrome p450 (1OG5), Hematopoetic cell kinase (PDB ID: 2HCK), Xanthine oxidase (PDB ID: 3DBJ), Myeloperoxidase (PDB ID: 1DNU). Ten ligand 3D structures were mapped by Chem3D Ultra 8.0. The ligand and receptor were optimized by sybyl-X 2.0 Tripos field molecular mechanics program Mnimize, Loadeding the Gasteiger-Huckel charge, the Power energy gradient method was used for optimization, and a stable conformation was obtained after optimization. Delete the water molecules in the protein before docking, and Assign hydrogen atoms and Gasteiger charges to the PDB file. The Grid Box containing the active pocket was defined as: 60A˚* 60A˚* 60A˚. The grid spacing was 0.375 A˚. Keep the default values for other parameters and select the optimal conformation for bonding analysis.
Results
Determination of detection wavelength
An ultraviolet spectrophotometer was used to scan the whole wavelength of the 5-year pickled radish, and the scanning wavelength ranged from 190 nm to 600 nm. The scanning results showed that the sample has larger absorption peaks at 225 nm and 274 nm. Therefore, 225 nm and 274 nm were selected as the detection wavelengths for HPLC.
Chemical structure identification
Six compounds were obtained by separation of the crude extracts. The compounds were characterized by mass spectrometry, 1H NMR, and 13C NMR.
Purified components of methanol extract
Compound 1 was a light-yellow needle-like solid, which was easily soluble in water and more polar organic solvents. The mass spectrometry revealed that ESI (+)-MS: m/z = 127 [M + H + H2O] + and ESI-MS2: 109 [M + H] +, and the molecular weight was 126. To judge from its mass spectrum, compound 1 probably contained –OH. 1H NMR spectra showed 5 groups of subsidiar signals, included an aldehyde proton (δH 9.61) and two heterocyclic aforementers (δH 6.53 and δH 7.23. Moreover, the 13C NMR spectrum showed that 6 groups of carbon signals, which revealed an aldehyde carbon (δC 177.8) and four heterocyclic aromatic hydrocarbon (δC 160.6, δC 110.1, δC 122.7 and δC 152.6). In 1H NMR and 13C NMR spectrum, δH 6.53 (1H, d, J = 3.2 Hz) and δC 110.1 showed feature signals of furan (Table 1). After data analysis and comparison with the literature, it was identified as 5-hydroxymethylfurfural (5-HMF), the molecular formula is C6H6O2 (Dibenedetto et al., 2016).
Table 1.
1H NMR and 13C NMR Spectral Data of Compound 1 to 3 (1H 400 MHz, 13C 100 MHz, TMS, δ ppm).
| 5-HMF (1) |
β-sitosterol (2) |
β-sitosterol-3-O-glucose glycosides (3) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Solvent | CDCl3 |
CDCl3 |
DMSO‑d6 |
||||||
| Position | 13C | 1H | Multiplicity; J (Hz) |
13C | 1H | Multiplicity; J (Hz) |
13C | 1H | Multiplicity; J (Hz) |
| 1 | 57.8 | 4.73 | s | 37.3 | — | 37.3 | — | ||
| 2 | 160.6 | — | 31.6 | — | 29.8 | — | |||
| 3 | 110.1 | 6.53 | d; 3.2 | 71.8 | 3.55 | m | 77.4 | — | |
| 4 | 122.7 | 7.23 | d; 3.2 | 42.4 | — | overlapped | — | ||
| 5 | 152.6 | — | 140.9 | — | 140.9 | — | |||
| 6 | 177.8 | 9.61 | s | 121.7 | 5.38 | d; 5.0 | 121.7 | 5.27 | d; 4.0 |
| — | 3.71 | s | |||||||
| 7 | 31.6 | — | 31.9 | — | |||||
| 8 | 31.6 | — | 31.9 | — | |||||
| 9 | 50.1 | — | 50.1 | — | |||||
| 10 | 36.5 | — | 36.7 | — | |||||
| 11 | 21.1 | — | 21.1 | — | |||||
| 12 | 39.8 | — | 39.8 | — | |||||
| 13 | 42.3 | — | 42.3 | — | |||||
| 14 | 56.7 | — | 56.6 | — | |||||
| 15 | 24.3 | — | 24.3 | — | |||||
| 16 | 28.3 | — | 28.2 | — | |||||
| 17 | 56.1 | — | 55.9 | — | |||||
| 18 | 11.9 | 0.71 | s | 12.1 | 0.67 | s | |||
| 19 | 19.4 | 1.03 | s | 19.5 | 0.97 | s | |||
| 20 | 36.1 | — | 36.0 | — | |||||
| 21 | 18.8 | 0.95 | d; 6.5 | 19.1 | 0.89 | d; 5.6 | |||
| 22 | 33.0 | — | 34.0 | — | |||||
| 23 | 26.2 | — | 26.2 | — | |||||
| 24 | 45.8 | — | 45.9 | — | |||||
| 25 | 29.2 | — | 29.3 | — | |||||
| 26 | 19.8 | 0.84 | d; 7.0 | 19.6 | 0.80 | m | |||
| 27 | 19.0 | 0.86 | d; 7.0 | 19.4 | 0.81 | m | |||
| 28 | 23.1 | — | 23.1 | — | |||||
| 29 | 12.0 | 0.87 | t; 7.4 | 12.2 | 0.83 | m | |||
| Hexose | |||||||||
| 1′ | 101.2 | 4.16 | d; 4.0 | ||||||
| 2′ | 73.9 | 2.95 | m | ||||||
| 3′ | 77.1 | 3.06 | m | ||||||
| 4′ | 70.6 | 3.00 | m | ||||||
| 5′ | 77.3 | 2.97 | m | ||||||
| 6′ | 61.6 | 3.40, 3.57 | m,m | ||||||
Purified components of ethyl acetate methanol extract
Compound 2 was a transparent needle-like compound, which was easily soluble in dimethyl sulfoxide (DMSO) and insoluble in water. The mass spectrometry revealed that ACPI (+)-MS: [M−H2O + H] + = 397. The results inferred that the molecular weight of compound 2 was 414. The 1H NMR spectra of compound 2 indicated a doublet of olefinic proton at δH 5.38 (1H, d, J = 5.0 Hz, H-6), a signal of oxymethine proton at δH 3.55 (1H, m, H-3). There were two methyl single chains at δH 0.71 (3H, s, H-18) and δH 1.03 (3H, s, H-19), which were characteristic of hydroxyl sterols of the mother nucleus for Δ5-3β-. Compound 2 had δH 0.95 (3H, d, J = 6.5 Hz, H-21), δH 0.84 (3H, d, J = 7.0 Hz, H-26) and δH 0.86 (3H, d, J = 7.0 Hz, H-27), appeared of three methyl doublets, and a methyl triplet at δH 0.87 (3H, t, J = 7.5 Hz, H-29). In the 13C NMR spectra of compound 2, three -C- groups were seen at δC 140.9 (C-5), δC 42.3 (C-13) and δC 36.5 (C-10); nine –CH- groups were observed at δC 121.7 (C-6), δC 71.8 (C-3), δC 56.1 (C-17), δC 56.7 (C-14), δC 50.1 (C-9), δC 45.8 (C-24), δC 36.1 (C-20), δC 31.6 (C-8) and δC 29.2 (C-25); eleven –CH2- groups were observed at δC 42.4 (C-4), δC 39.8 (C-12), δC 33.0 (C-22), δC 31.6 (C-2), δC 37.3 (C-1), δC 31.6 (C-7), δC 26.2 (C-23), δC 24.3 (C-15), δC 28.3 (C-16), δC 23.1 (C-28) and δC 21.1 (C-11); and six –CH3- groups were observed at δC 19.4 (C-19), δC 11.9 (C-18), δC 18.8 (C-21), δC 19.8 (C-26), δC 19.0 (C-27) and δC 12.0 (C-29) (Table 1). Based on the above information, after identification and comparison with the literature, the compound 2 was determined to be β-sitosterol, and the molecular formula was C29H5O (Li et al., 2008).
Compound 3 was obtained as a white powder with poor water solubility, readily dissolved in a mixture of dimethyl sulfoxide (DMSO) and chloroform. The ACPI (+)-MS result was [M−C6H11O6+] + = 397, and the molecular weight of compound 3 was 576. Compared 1H NMR and 13C NMR spectrum between compound 2 and compound 3, the results were similar except for the signal of more group of hexa-carbon in compound 3. The compound 3 might be inferred to be sugar derivatives of β-sitosterol. In the 13C NMR spectrum, δC 101.2 (C-1′), δC 73.9 (C-2′), δC 77.1 (C-3′), δC 70.6 (C-4′), δC 77.3 (C-5′), δC 61.6 (C-6′) were signals of glucose, it indicated that the sugar attached to the compound 3 was glucose (Table 1). According to the results of the above data analysis, it was finally determined that the compound was β-sitosterol-3-O-glucoside, and the molecular formula was C35H60O6 (Lee et al., 2002).
Compound 4 was a light-yellow oily compound, soluble in small polar organic solvents, but insoluble in water. The mass spectrometry revealed that ESI (+)–MS: m/z = 279 [M + H] +, M = 278; ESI-MS2: m/z = 261 [M−H2O + H] +, m/z = 243 [M−2H2O + H]+, m/z = 223 [M−55 + H]+. As indicated by the 1H NMR and 13C NMR results, the multiplet signal at δH 5.37 (6H, m, H-9, 10, 12, 13, 15, 16) represented olefinic unsaturated protons, and the δC 129.7 (C-9), δC 126.8 (C-10), δC 127.8 (C-12), δC 127.7 (C-13), δC 127.4 (C-15), δC 131.2 (C-16) appeared, which were correlated to carbons. A typical triplet signal at the δH 0.97 (3H, t, J = 7.3 Hz, CH3-18) demonstrated three protons, and the carbon at δC 13.3 represented a terminal methyl (omega-3, CH3-18) group. A multiplet signal at δH 2.09 (4H, m, CH2-8, 17) assigned to four protons correlated with the carbon at δC 26.7(CH2-8), and δC 20.1 (CH2-17) indicated two methylene groups. A triplet signal in δH 2.82 (4H, t, J = 6.2 Hz, CH2-11, 14) showed four protons correlated with the carbons at δC 25.1 and δC 25.0 in two individual methylene (CH2-11, 14) groups. An intense singlet at δH 1.32 (8H, s, H-4, 5, 6, 7) showed eight protons correlated to the carbons at δC 28.7–29.3 of four methylene (CH2-4, 5, 6, 7) groups. A multiplet at δH 1.61 (2H, m, H-3) attributed to protons correlated directly with a carbon at δC 24.6 (CH2-3). A triplet signal at δH 2.28 (2H, t, J = 7.0 Hz, H-2) was assigned to the protons correlated to a carbon at δC 33.4 in the methylene (CH2-2) group, respectively. The δC 174.6 (COOH-1) was a typical carboxyl carbon signal (Table 2). After data analysis, it was identified that compound 4 was α-linolenic acid, and the molecular formula was C18H30O2 (Li et al., 2010).
Table 2.
1H NMR and 13C NMR Spectral Data of Compound 4 to 6 (1H 400 MHz, 13C 100 MHz, TMS, δ ppm).
| α-linolenic acid (4) |
1-monopalmitin (5) |
chaenomic acid A (6) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Solvent | CD3OD |
CDCl3 |
CDCl3 |
||||||
| Position | 13C | 1H | Multiplicity; J (Hz) |
13C | 1H | Multiplicity; J (Hz) |
13C | 1H | Multiplicity; J (Hz) |
| 1 | 174.6 | — | 174.4 | — | 176.4 | — | |||
| 2 | 33.4 | 2.28 | t; 7.0 | 34.2 | 2.35 | t; 6.5 | 33.6 | 2.31 | t; 6.5 |
| 3 | 24.6 | 1.61 | m | 24.9 | 1.62 | m | 25.5 | 1.59 | m |
| 4 | 28.7 | 1.32 | br s | 29.1 | 1.28 | br s | 28.9 | 1.38 | br s |
| 5 | 28.8 | 1.32 | br s | 29.3 | 1.28 | br s | 29.2 | 1.38 | br s |
| 6 | 28.9 | 1.32 | br s | 29.4 | 1.28 | br s | 28.9 | 1.38 | br s |
| 7 | 29.3 | 1.32 | br s | 29.5 | 1.28 | br s | 24.7 | 1.38 | br s |
| 8 | 26.7 | 2.09 | m | 29.6 | 1.28 | br s | 36.9 | 1.55 | m |
| 9 | 129.7 | 5.37 | m | 29.7 | 1.28 | br s | 71.6 | 4.15 | q; 12 |
| 10 | 126.8 | 5.37 | m | 29.6 | 1.28 | br s | 134.6 | 5.73 | m |
| 11 | 25.1 | 2.82 | t; 6.2 | 29.5 | 1.28 | br s | 129.4 | 5.73 | m |
| 12 | 127.8 | 5.37 | m | 29.4 | 1.28 | br s | 74.6 | 3.95 | m |
| 13 | 127.7 | 5.37 | m | 29.3 | 1.28 | br s | 74.6 | 3.95 | m |
| 14 | 25.0 | 2.82 | t; 6.2 | 31.9 | 1.28 | br s | 129.6 | 5.73 | m |
| 15 | 127.4 | 5.37 | m | 22.7 | 1.28 | br s | 135.1 | 5.73 | m |
| 16 | 131.2 | 5.37 | m | 14.1 | 0.88 | t; 8.0 | 74.3 | 4.15 | m |
| 17 | 20.1 | 2.09 | m | 29.3 | 1.55 | m | |||
| 18 | 13.3 | 0.97 | t; 7.3 | 13.0 | 0.94 | t; 7.4 | |||
| — | 5.35 | – | |||||||
| 1′ | 65.2 | 4.18 | dd | ||||||
| 2′ | 70.3 | 5.36, 3.70 | m, dd; 4,12 | ||||||
| 3′ | 63.3 | 3.94, 3.60 | m, dd; 4,12 | ||||||
Compound 5 was a light buttery compound, and easily soluble in organic solvents. The mass spectrometry revealed that ESI (+)-MS: m/z = 331 [M + H] +, m/z = 312 [M−H2O] +, m/z = 353 [M + Na]+, and the molecular weight was 330. As indicated by the 1H NMR and 13C NMR data of compound 5, the typical triplet signal at the δH 0.88 (3H, t, J = 8.0 Hz, CH3-16) was three protons, and the carbon correlated to δC 14.1, which represented a terminal methyl (omega-3, CH3-16) group. An intense singlet at δH 1.28 (26H, s, H-4–15) showed eight protons correlated to the carbons at δC 22.7–31.9 of four methylene (CH2-4–15) groups. A multiplet at δH 1.62 (2H, m, H-3) attributed to protons correlated directly with a carbon at δC 24.9 (CH2-3). A triplet signal at δH 2.35 (2H, t, J = 6.5 Hz, H-2) was assigned to the protons correlated to a carbon at δC 34.2 in the methylene (CH2-2) group, respectively. The δC 174.4 (COOH-1) was a typical carboxyl carbon signal. The carbon values of δC 65.2 (CH2-1′), δC 70.3 (CH-2′), and δC 63.3 (CH2-3′) shift to the low field, which was guessed to be the effect of –OH group (Table 2). Combining the physical and chemical properties and data analysis, compound 5 was 1-monopalmitin and the molecular formula was C19H38O4.
Compound 6 was a light-yellow solid, and soluble in water. The mass spectrometry results showed that ESI (+)-MS: m/z = 367 [M + Na] +, 299 [M−COOH] +, and the molecular weight was 344. As indicated by the 1H NMR and 13C NMR data of compound 6, the multiplet signal at the δH 5.73 (4H, m, H-10,11,14,15) represented olefinic unsaturated protons, and carbons correlated to δC 134.6 (C-10), δC 129.4 (C-11), δC 129.6 (C-14) and δC 135.1 (C-15) were appeared. A typical triplet signal at the δH 0.94 (3H, t, J = 7.3 Hz, CH3-18) demonstrated three protons correlated to the carbon at δC 13.0 represented a terminal methyl (omega-3, CH3-18) group. A multiplet signal at δH 1.55 (4H, m, CH2-8, 17) assigned to four protons correlated with the carbon at δC 36.9(CH2-8) and δC 29.3 (CH2-17) indicated two methylene groups. The two a multiplet at δH 4.15 (2H, m, H-9, 16) and δH 3.95 (2H, m, H-12, 13) correlated to δC 71.6 (C-9), δC 74.3 (C-16) and δC 74.6 (C-12, 13), which connected to the –OH group, respectively. An intense singlet at δH 1.38 (8H, s, H-4, 5, 6, 7) showed eight protons correlated to the carbons at δC 24.7–29.2 of four methylene (CH2-4, 5, 6, 7) groups. A multiplet at δH 1.59 (2H, m, H-3) attributed to protons correlated directly with a carbon at δC 25.5 (CH2-3). A triplet signal at δH 2.31 (2H, t, J = 6.5 Hz, H-2) was assigned to the protons correlated to a carbon at δC 33.6 in the methylene (CH2-2) group, respectively. The δC 176.4 (COOH-1) was a typical carboxyl carbon signal (Table 2). After data analysis, it was identified that compound 6 was chaenomic acid A, and the molecular formula was C18H32O6 (Kim et al., 2014).
Comparison of HPLC results
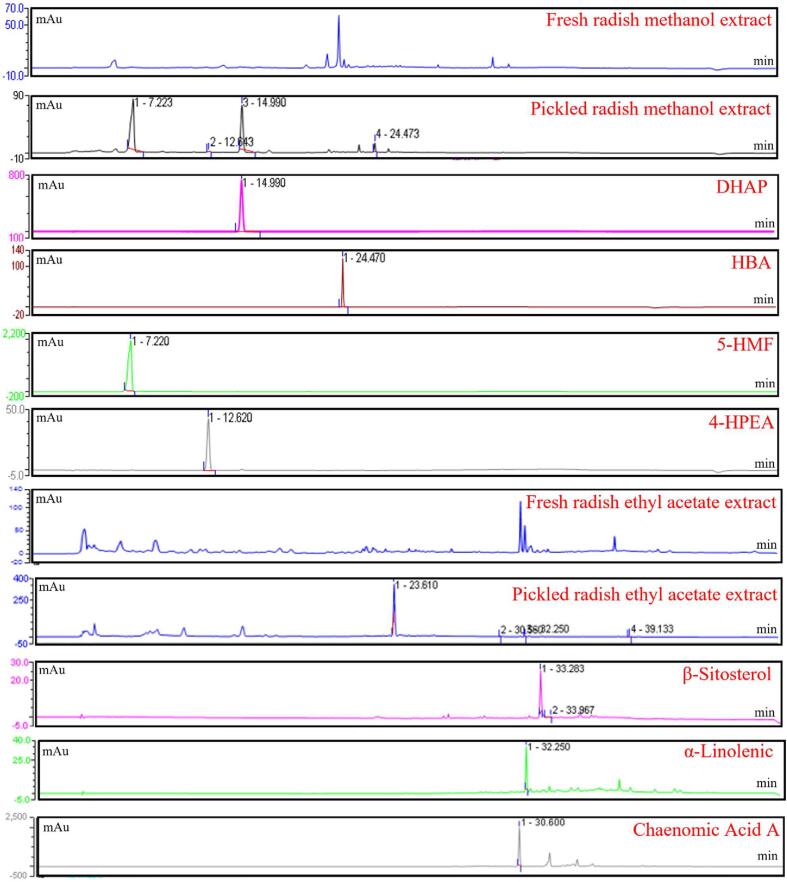
Whereas high performance liquid chromatography (HPLC) is an efficient separation and identification technology with high precision and good reproducibility. 6 compounds were obtained from 5-year of pickled radish through separation, purification and structural identification. The methanol component and ethyl acetate component of pickled radish were analyzed by HPLC (methanol extract was detected at 296 nm wavelength, ethyl acetate extract was detected at 256 nm wavelength). The comparative explanation of the methanol and ethyl acetate components was shown in Fig. 1. Except β-sitosterol-3-O-glucose glycosides and 1-monopalmitin, other compounds were detected. The methanol components of fresh radishes and pickled radishes were different, and 5-HMF was only detected in pickled radishes, but not in fresh white radish. It was speculated that 5-HMF was a new compound produced by pickling fresh white radish. In the ethyl acetate extraction of pickled radish, the content of β-sitosterol, α-linolenic acid and chaenomic acid A were lower than those of fresh radish (Fig. 1, Fig. 2).
Fig. 1.
Comparison of HPLC Chromatogram of Methanol Extract and Ethyl Acetate Extract.
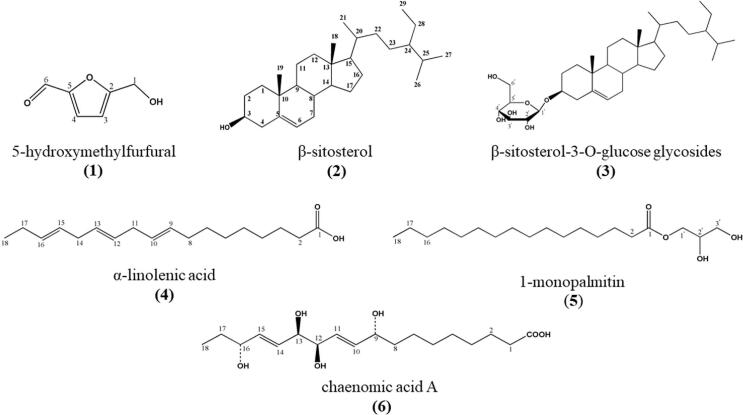
Fig. 2.
Structures of 6 Compounds Extracted from Pickled Radish.
Computational docking studies
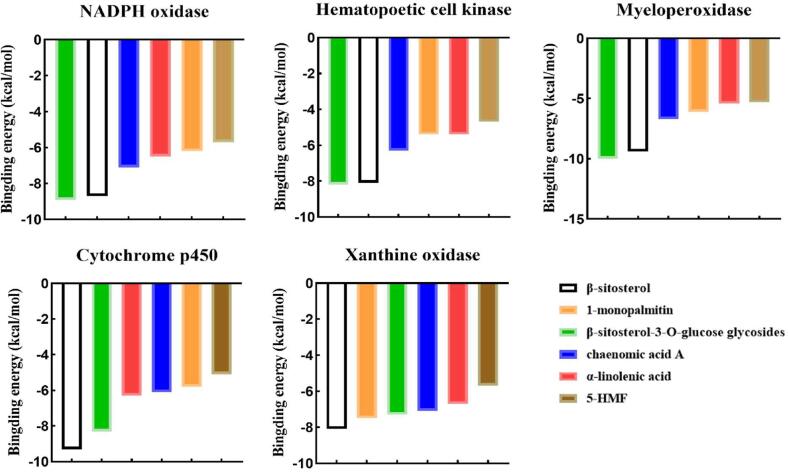
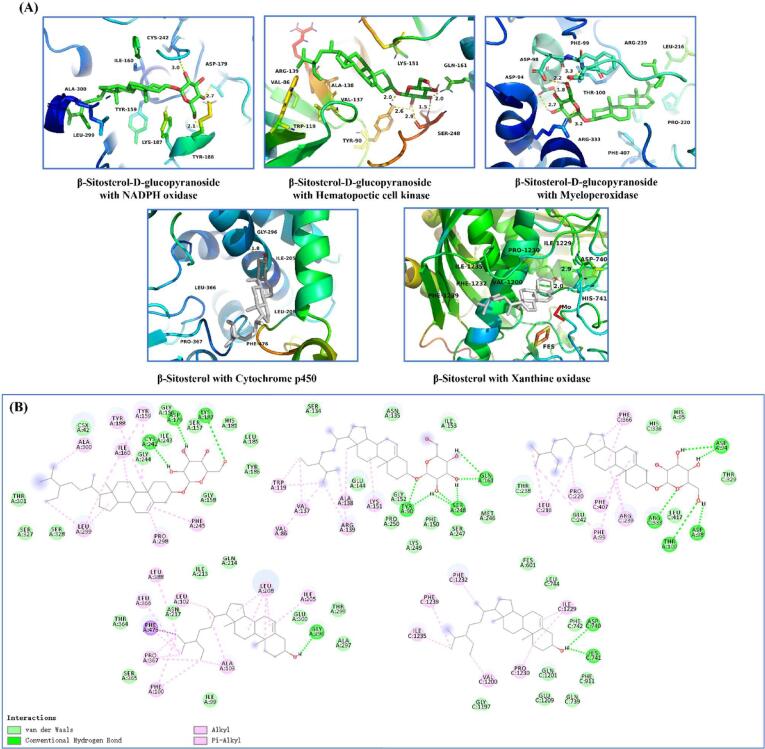
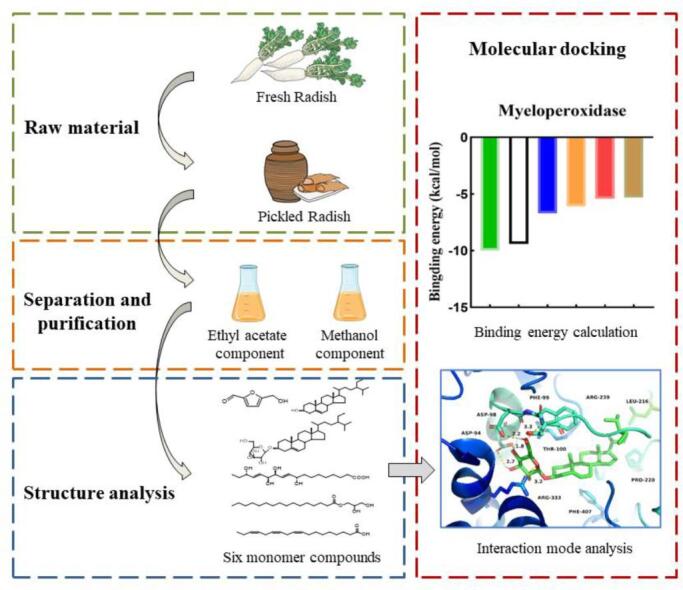
Through molecular docking, the compounds in pickled radish that bind most favorably to the enzyme proteins related to oxidation reaction were determined, and the conformation with the lowest binding energy was selected among the 9 results of docking output. As shown in Fig. 3, better combination of β-sitosterol-3-O-glucose glycosides with NADPH oxidase, Hematopoetic cell kinase and Myeloperoxidase. The binding energy was: −8.9, −8.2, −10 kcal/mol. β-sitosterol has a better combination with Cytochrome p450 and Xanthine oxidase. The binding energy was: −9.3, −8.1 kcal/mol. Further analyze the interaction between them, such as Fig. 4A. The glucoside structure of β-sitosterol-3-O-glucose glycosides has affinity for the inside of the pocket. The hydroxyl group on its glycoside forms hydrogen bonds with ASP-179, TYR-188 and CYS-242 of NADPH oxidase, with bond lengths of 2.7. Å, 2.1 Å, 3.0 Å. The hydroxyl groups on β-sitosterol-3-O-glucose glycosides form hydrogen bonds with TYR-90, GLN-161 and SER-248 of Hematopoetic cell kinase. The bond lengths were: 2.6 Å, 1.5 Å, and 2.0 Å, respectively. In addition, the hydroxyl group of the glycoside structure tends to form hydrogen bonds with ASP-94, ASP-98, and THR-100 located in the Myeloperoxidase pocket. The bond lengths were: 2.7 Å, 1.8 Å, 2.2 Å, and 3.3 Å, respectively. The hydroxyl end of β-Sitosterol was located inside the hydrophobic pocket of Cytochrome p450 and Xanthine oxidase. A hydrogen bond with a bond length of 1.8 Å was formed between the H atom on the hydroxyl group and the GLY-296 of Cytochrome p450. The hydroxyl of β-sitosterol and ASP-740 and HIS-741 of Xanthine oxidase form hydrogen bonds with bond lengths of 2.9 Å and 2.0 Å, respectively. In addition, the pockets of the five enzyme proteins have multiple amino acid residues and π-π conjugates between the compounds (Fig. 4B).
Fig. 3.
Calculation of binding energy between pickled radish compound and protein.
Fig. 4.
Interaction patterns between pickled radish compounds and proteins.
Discussion
The emergence of fermented food not only provides people with a new diet choice, but also brings a lot of nutritional benefits. More and more people realize that fermented food has many benefits, such as improving food safety, providing nutritional factors, and unique flavor and taste (Licandro et al., 2020). For example, soy sauce and vinegar, as traditional fermented foods with a long history, have produced rich nutritional factors that are beneficial to the human body during the production process (Budak et al., 2014, Li et al., 2016). Pickled radish is a kind of fermented food popular all over the world. As a potential source of bioactive substances, their nutritional value has been widely concerned. However, reports on its nutritional content are scarce. In our previous research, we have stated that the three polyphenol compounds identified from pickled radish are the key to lasting freshness (Li et al., 2020). In this study, we used column chromatography to separate and purify 6 compounds from methanol extracts and ethyl acetate extracts of 5-year pickled radish. The resolved picture of the above compounds has been obtained through a combination of thin layer chromatography (TLC), HPLC method, ESI-MS and NMR technology. Through these methods, we identified six bioactive components of pickled radish, including 5-hydroxymethylfurfural (1); phytosterol: β-sitosterol (2) and its derivative β-sitosterol-3-O-glucose glycosides (3); Two fatty acids: α-linolenic acid (4) and 1-monopalmitin (5); chaenomic acid A (6). Among them, all other compounds except α-linolenic acid were discovered from radish for the first time.
5-hydroxymethylfurfural (5-HMF) is an intermediate product of Maillard reaction, and it is also a common indicator for measuring browning index in the field of food inspection. The radish contains sugar and organic acid. During the pickling process, with the loss of water, the pH decreases, and the sugars in it are easily dehydrated under the above conditions to produce 5-HMF. Numerous biologically active functions of 5-HMF have been confirmed. For example, 5-HMF could effectively ameliorate alcohol-induced liver injury (Li et al., 2015), against acute hypobaric hypoxia (Li et al., 2011) and alleviate LPS-induced RAW 267.4 cells inflammatory response (Kong et al., 2019).In a recent report, 5-HMF effectively isolated and identified from Symplocos chinensisf. Pilosa Ohwi was shown to be effective against nerve pain (Kim et al., 2021). However, it has been reported that the safety of 5-HMF is controversial, but as a common bioactive factor in fermented foods, 5-HMF deserves further study (Severin et al., 2010). Phytosterols are a class of bioactive molecules found in plants. More and more evidences show that plant sterols have a variety of nutritional benefits. Whereas, the body cannot produce sterols on its own, people need to get them from their diet. As the most common phytosterol in People's Daily diet, β-sitosterol is widely found in Rubiaceae, Cruciferae, Araceae and other plants. A recent study showed that β-sitosterol injections significantly improved chronic anxiety in naive Stressed mice (Kim et al., 2021).In addition, β-sitosterol has been reported to have antitumor (Cao et al., 2020), anti-inflammatory (Zhang et al., 2020), lipid-lowering (Navarro Del Hierro et al., 2021) and bacteriostatic activities (Evangelina et al., 2021). A glycosidic bond is formed between the –OH of β-sitosterol C-3 and glucose. The resulting β-sitosterol-3-O-glucose glycosides is also commonly found in many medicinal plants. β-sitosterol-3-O-glucose glycosides and β-sitosterol were often isolated and identified together from some fruits, vegetables, plants and Chinese herbal medicines with biological activity (Hussain et al., 2020, Kayo et al., 2020, Khan et al., 2020).
In addition, two fatty acid compounds were found in this study. α-Linolenic acid (4) has been shown to be present in radishes in previous studies. α-linolenic acid (ALA) is a kind of plant-derived polyunsaturated fatty acid (PUFA), which is essential fatty acid for human body and has been proved to have a positive effect on the prevention of cardiovascular disease (Stivala et al., 2013, Stivala et al., 2020). In recent years, new evidence has shown that dietary supplementation of ALA could inhibit thrombosis through intestinal flora (Saeedi Saravi et al., 2021). 1-monopalmitin (5) is a kind of 1-monopalmitin based on palmitoyl, and formed by the esterification of palmitic acid and glyceride. 1-monopalmitin has been identified in a variety of plant extracts and has been shown to have antiviral and antibacterial biological activity (Cheng et al., 2020, Nipun et al., 2021, Wang et al., 2020). In addition, 1-monopalmitin has been reported to be a metabolic marker for Parkinson's disease and lipid synthesis (Ke et al., 2020, Shah et al., 2019). Finally, we determined that the component (6) was chaenomic acid A. As a new oxylipins, chaenomic acid A has been reported to have strong anti-neuroinflammatory activity (Kim et al., 2014). In particular, methanol extract and ethyl acetate extract were compared by HPLC. We found that the chemical composition of pickled radishes in 5 years has changed compared with that of fresh radishes, but the types of compounds have not changed significantly. After pickling fresh radishes, the moisture decreases and the acidity increases. Among them, the content of β-sitosterol, α-linolenic acid and chaenomic acid A is reduced, and 5-HMF is a new product of radish after pickling. In addition, the three phenolic compounds 2, 6-dihydroxyacetophenone (DHAP), 4-hydroxyphenyl alcohol (4-HPEA) and 4-hydroxybenzaldehyde (HBA) that have been reported in our previous research were shown in the spectral image (Li et al., 2020). Among them, HBA already exists in fresh radishes, while DHAP and 4-HPEA were both found in pickled radishes.
In addition, β-sitosterol and its derivative β -sitosterol-d-glucopyranoside has a high affinity for several key enzymes involved in oxidative stress through molecular docking. There were multiple hydrogen bonds between the hydroxyl group of β-sitosterol and the glycoside structure of β-sitosterol-3-O-glucose glycosides and the inner hydrophobic pocket of the protein, this was the key to their effective anchoring. β-sitosterol has previously been shown to slow CCl4-induced oxidative stress and liver damage (Devaraj et al., 2020). It was also reported that β-sitosterol relieves alcohol-induced oxidative stress by increasing the activity of antioxidant enzymes (Chen et al., 2020). It was clear that β-sitosterol and its derivatives β-sitosterol-3-O-glucose glycosides may be involved in preventing the deterioration caused by over-oxidation of pickled radish, which has potential antioxidant development value.
Conclusion
In this study, we identified 6 compounds of 5-year pickled radish, which were: 5-hydroxymethylfurfural (1); phytosterol: β-sitosterol (2) and its derivative β-sitosterol-3-O-glucose glycosides (3), as well as two fatty acids: α-linolenic acid (4) and 1-monopalmitin (5), and chaenomic acid A (6). These bioactive compounds were commonly found in many plants, but α-linolenic acid was found in pickled radish for the first time. Molecular docking analysis revealed that β-sitosterol and β-sitosterol-3-O-glucose glycosides have high affinity for multiple key enzymes involved in oxidation reactions. With this report, we hope to provide a scientific basis for the Chemical composition and nutritional benefits of pickled radish. Considering pickled radish is a kind of pickled food popular in the world, it is very important to reveal its nutritional composition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Natural Science Foundation of Fujian Province (2021N5003, 2021J01837), and the opening project of Fujian Provincial Engineering Technology Research Center of Marine Functional Food (No. 2900/Z820235), and the Key Project of Quanzhou City (No. 2021N018).
Contributor Information
Jian Li, Email: lijian2013@jmu.edu.cn.
Hui-Min David Wang, Email: davidw@dargon.nchu.edu.tw.
References
- Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Archives of Biochemistry and Biophysics. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Bergamini C., Cicoira M., Rossi A., Vassanelli C. Oxidative stress and hyperuricaemia: Pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. European Journal of Heart Failure. 2009;11:444–452. doi: 10.1093/eurjhf/hfp042. [DOI] [PubMed] [Google Scholar]
- Budak N.H., Aykin E., Seydim A.C., Greene A.K., Guzel-Seydim Z.B. Functional properties of vinegar. Journal of Food Science. 2014;79:R757–R764. doi: 10.1111/1750-3841.12434. [DOI] [PubMed] [Google Scholar]
- Cao Z.-Q., Wang X.-X., Lu L., Xu J.-W., Li X.-B., Zhang G.-R., et al. Corrigendum: β-sitosterol and gemcitabine exhibit synergistic anti-pancreatic cancer activity by modulating apoptosis and inhibiting epithelial-mesenchymal transition by deactivating Akt/GSK-3β signaling. Frontiers in Pharmacology. 2020;11:535–565. doi: 10.3389/fphar.2020.565535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R., Roy S. Exploration of the diversity and associated health benefits of traditional pickles from the Himalayan and adjacent hilly regions of Indian subcontinent. Journal of Food Science and Technology. 2018;55:1599–1613. doi: 10.1007/s13197-018-3080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheigh H.S., Park K.Y. Biochemical, microbiological, and nutritional aspects of kimchi (Korean fermented vegetable products) Critical Reviews in Food Science and Nutrition. 1994;34:175–203. doi: 10.1080/10408399409527656. [DOI] [PubMed] [Google Scholar]
- Chen Z., Wu A., Jin H., Liu F. beta-Sitosterol attenuates liver injury in a rat model of chronic alcohol intake. Archives of Pharmacal Research. 2020;43:1197–1206. doi: 10.1007/s12272-020-01271-w. [DOI] [PubMed] [Google Scholar]
- Cheng J.C., Liaw C.C., Lin M.K., Chen C.J., Chao C.L., Chao C.H., et al. Anti-influenza virus activity and chemical components from the parasitic plant Cuscuta japonica Choisy on Dimocarpus longans Lour. Molecules. 2020;25:4427. doi: 10.3390/molecules25194427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D.H., Kim S.H., Myung N., Cho K.J., Chang M.J. The antihypertensive effect of ethyl acetate extract of radish leaves in spontaneously hypertensive rats. Nutrition Research and Practice. 2012;6:308–314. doi: 10.4162/nrp.2012.6.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj E., Roy A., Royapuram Veeraragavan G., Magesh A., Varikalam Sleeba A., Arivarasu L., et al. β-Sitosterol attenuates carbon tetrachloride–induced oxidative stress and chronic liver injury in rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 2020;393:1067–1075. doi: 10.1007/s00210-020-01810-8. [DOI] [PubMed] [Google Scholar]
- Dibenedetto A., Aresta M., di Bitonto L., Pastore C. Organic carbonates: Efficient extraction solvents for the synthesis of HMF in aqueous media with cerium phosphates as catalysts. ChemSusChem. 2016;9:118–125. doi: 10.1002/cssc.201501181. [DOI] [PubMed] [Google Scholar]
- Evangelina I.A., Herdiyati Y., Laviana A., Rikmasari R., Zubaedah C., Anisah, Kurnia D. Bio-mechanism inhibitory prediction of beta-sitosterol from Kemangi (Ocimum basilicum L.) as an Inhibitor of MurA enzyme of oral bacteria: In vitro and in silico study. Advances and Applications in Bioinformatics and Chemistry. 2021;14:103–115. doi: 10.2147/AABC.S301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Li J., Sivakumar D., Kim T.S., Patel S.K.S., Kalia V.C., et al. NADH oxidase from Lactobacillus reuteri: A versatile enzyme for oxidized cofactor regeneration. International Journal of Biological Macromolecules. 2019;123:629–636. doi: 10.1016/j.ijbiomac.2018.11.096. [DOI] [PubMed] [Google Scholar]
- He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cellular Physiology and Biochemistry. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- Hussain H., Ali I., Wang D., Mamadalieva N.Z., Hussain W., Csuk R., et al. 4-Benzyloxylonchocarpin and muracatanes A-C from Ranunculus muricatus L. and their biological effects. Biomolecules. 2020;10:1562. doi: 10.3390/biom10111562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayo M.T., Simo M.K., Tagatsing Fotsing M., Talla E., Laurent S., Elst L.V., et al. Antifungal potential of extracts, fractions and compounds from Uvaria comperei (Annonaceae) and Oxyanthus unilocularis (Rubiaceae) Natural Product Research. 2020;35:1–5. doi: 10.1080/14786419.2020.1828409. [DOI] [PubMed] [Google Scholar]
- Ke, S.A., Bo, Y.B., Bqy, A., Tian, X.A., Yfy, A., Zhz, C., & Cheng, C.A., (2020) Liraglutide attenuates renal tubular ectopic lipid deposition in rats with diabetic nephropathy by inhibiting lipid synthesis and promoting lipolysis. Pharmacological Research, 156, 104778 .doi: 10.1016/j.phrs.2020.104778. [DOI] [PubMed]
- Khan M.F., Nasr F.A., Noman O.M., Alyhya N.A., Ali I., Saoud M., et al. Cichorins D-F: Three new compounds from Cichorium intybus and their biological effects. Molecules. 2020;25:1461. doi: 10.3390/molecules25184160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.S., Kwon O.W., Kim S.Y., Choi S.U., Kim K.H., Lee K.R. Five new oxylipins from Chaenomeles sinensis. Lipids. 2014;49:1151–1159. doi: 10.1007/s11745-014-3953-0. [DOI] [PubMed] [Google Scholar]
- Kim, H.Y., Park, S.H., Zuo, G., Kim, K.H., Hwang, S.H., Suh, H.W., & Lim, S.S. (2021). Effect of extract and synthesized derivatives of isolated compound from Symplocos chinensis f. Pilosa Ohwi on neuropathic pain in mice. Molecules, 26,1639.doi:10.3390/molecules26061639. [DOI] [PMC free article] [PubMed]
- Kong F., Lee B.H., Wei K. 5-Hydroxymethylfurfural mitigates lipopolysaccharide-stimulated inflammation via suppression of MAPK, NF-kappaB and mTOR activation in RAW 264.7 cells. Molecules. 2019;24:275. doi: 10.3390/molecules24020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Liao Y., Zhou L., Zhang Y., Cheng J., Yuan Z., et al. Hematopoietic cell kinase (HCK) is essential for NLRP3 inflammasome activation and lipopolysaccharide-induced inflammatory response in vivo. Frontiers in Pharmacology. 2020;11 doi: 10.3389/fphar.2020.581011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumakura K., Kato R., Kobayashi T., Kimura N., Takahashi H., Takahashi A., et al. The salted radish takuan-zuke shows antihypertension effects in spontaneously hypertensive rats. Food Function. 2017;8:3491–3500. doi: 10.1039/c7fo00890b. [DOI] [PubMed] [Google Scholar]
- Lee W.B., Kwon H.C., Cho O.R., Lee K.C., Choi S.U., Baek N.I., et al. Phytochemical constituens of Cirsium setidens Nakai and their cytotoxicity against human cancer cell lines. Archives of Pharmacal Research. 2002;25:628–635. doi: 10.1007/Bf02976934. [DOI] [PubMed] [Google Scholar]
- Li H., Zhao M., Su G., Lin L., Wang Y. Effect of soy sauce on serum uric acid levels in hyperuricemic rats and identification of flazin as a potent xanthine oxidase inhibitor. Journal of Agricultural and Food Chemistry. 2016;64:4725–4734. doi: 10.1021/acs.jafc.6b01094. [DOI] [PubMed] [Google Scholar]
- Li J., Deng Q., Zhang Y., Wu D., Li G., Liu J., et al. Three novel dietary phenolic compounds from pickled Raphanus Sativus L. inhibit lipid accumulation in obese mice by modulating the gut microbiota composition. Molecular Nutrition & Food Research. 2021;65:e2000780. doi: 10.1002/mnfr.202000780. [DOI] [PubMed] [Google Scholar]
- Li J., Huang S.Y., Deng Q., Li G., Su G., Liu J., et al. Extraction and characterization of phenolic compounds with antioxidant and antimicrobial activities from pickled radish. Food and Chemical Toxicology. 2020;136 doi: 10.1016/j.fct.2019.111050. [DOI] [PubMed] [Google Scholar]
- Li J., Wakui R., Horie M., Nishimura Y., Nishiyama Y., Ikeno Y., et al. Feeding stimulant in Cinnamomum camphora for the common bluebottle, Graphium sarpedon nipponum (Lepidoptera: Papilionidae) Zeitschrift fur Naturforschung Section c-a Journal of Biosciences. 2010;65:571–576. doi: 10.1515/znc-2010-9-1007. [DOI] [PubMed] [Google Scholar]
- Li M.M., Wu L.Y., Zhao T., Wu K.W., Xiong L., Zhu L.L., et al. The protective role of 5-hydroxymethyl-2-furfural (5-HMF) against acute hypobaric hypoxia. Cell Stress Chaperones. 2011;16:529–537. doi: 10.1007/s12192-011-0264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Qu X.N., Han Y., Zheng S.W., Wang J., Wang Y.P. Ameliorative effects of 5-hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on alcoholic liver oxidative injury in mice. International Journal of Biological Macromolecules. 2015;16:2446–2457. doi: 10.3390/ijms16022446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.H., Chang S.T., Chang S.C., Chang H.T. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Natural Product Research. 2008;22:1085–1093. doi: 10.1080/14786410802267510. [DOI] [PubMed] [Google Scholar]
- Licandro H., Ho P.H., Nguyen T.K.C., Petchkongkaew A., Nguyen H.V., Chu-Ky S., et al. How fermentation by lactic acid bacteria can address safety issues in legumes food products? Food Control. 2020;110 doi: 10.1016/j.foodcont.2019.106957. [DOI] [Google Scholar]
- Manivannan A., Kim J.H., Kim D.S., Lee E.S., Lee H.E. Deciphering the nutraceutical potential of Raphanus sativus—A comprehensive overview. Nutrients. 2019;11:402. doi: 10.3390/nu11020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro Del Hierro J., Casado-Hidalgo G., Reglero G., Martin D. The hydrolysis of saponin-rich extracts from fenugreek and quinoa improves their pancreatic lipase inhibitory activity and hypocholesterolemic effect. Food Chemistry. 2021;338 doi: 10.1016/j.foodchem.2020.128113. [DOI] [PubMed] [Google Scholar]
- Nipun T.S., Khatib A., Ahmed Q.U., Nasir M.H.M., Supandi F., Taher M., et al. Preliminary phytochemical screening, in vitro antidiabetic, antioxidant activities, and toxicity of leaf extracts of Psychotria malayana jack. Plants (Basel) 2021;10:2688. doi: 10.3390/plants10122688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi Saravi S.S., Bonetti N.R., Pugin B., Constancias F., Pasterk L., Gobbato S., et al. Lifelong dietary omega-3 fatty acid suppresses thrombotic potential through gut microbiota alteration in aged mice. iScience. 2021;24 doi: 10.1016/j.isci.2021.102897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K., Koyano H., Yamamoto N., Yamada T. The effects of vegetable pickling conditions on the dynamics of microbiota and metabolites. PeerJ. 2021;9:e11123. doi: 10.7717/peerj.11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severin I., Dumont C., Jondeau-Cabaton A., Graillot V., Chagnon M.C. Genotoxic activities of the food contaminant 5-hydroxymethylfurfural using different in vitro bioassays. Toxicology Letters. 2010;192:189–194. doi: 10.1016/j.toxlet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Shah A., Han P., Wong M.Y., Chang R., Legido-Quigley C. Palmitate and stearate are increased in the plasma in a 6-OHDA model of Parkinson's disease. Metabolites. 2019;9:31. doi: 10.3390/metabo9020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stivala S., Reiner M.F., Lohmann C., Luscher T.F., Matter C.M., Beer J.H. Dietary alpha-linolenic acid increases the platelet count in ApoE-/- mice by reducing clearance. Blood. 2013;122:1026–1033. doi: 10.1182/blood-2013-02-484741. [DOI] [PubMed] [Google Scholar]
- Stivala S., Sorrentino S., Gobbato S., Bonetti N.R., Camici G.G., Luscher T.F., et al. Glycoprotein Ib clustering in platelets can be inhibited by alpha-linolenic acid as revealed by cryo-electron tomography. Haematologica. 2020;105:1660–1666. doi: 10.3324/haematol.2019.220988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terras F., Goderis I.J., Leuven F.V., Vanderleyden J., Broekaert C. In vitro antifungal activity of a radish (Raphanus sativus L.) seed protein homologous to nonspecific lipid transfer proteins. Plant Physiology. 1992;100:1055–1058. doi: 10.1104/pp.100.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang R., Zhang G., Chen F., Baocai X.U. In vitro antibacterial activities and mechanisms of action of fatty acid monoglycerides against four foodborne bacteria. Journal of Food Protection. 2020;83:331–337. doi: 10.4315/0362-028X.JFP-19-259. [DOI] [PubMed] [Google Scholar]
- Xiong L.X., Guo-Cheng S.U., Jiang F., Liu J.W., Zhou C.Y., Chen J.D., et al. Preliminary analysis, separation, and purification of the chemical components of minnan preserved radish produced in different years. Modern Food Science and Technology. 2016;32:309–314. doi: 10.13982/j.mfst.1673-9078.2016.5.046. [DOI] [Google Scholar]
- Zhang F., Liu Z., He X., Li Z., Shi B., Cai F. beta-Sitosterol-loaded solid lipid nanoparticles ameliorate complete Freund's adjuvant-induced arthritis in rats: Involvement of NF-small ka, CyrillicB and HO-1/Nrf-2 pathway. Drug Delivery. 2020;27:1329–1341. doi: 10.1080/10717544.2020.1818883. [DOI] [PMC free article] [PubMed] [Google Scholar]