Abstract
It is well accepted that COVID-19-related mortality shows a strong age dependency. However, temporal changes in the age distribution of excess relative mortality between waves of the pandemic are less frequently investigated. We aimed to assess excess absolute mortality and the age-distribution of all-cause mortality during the second and third waves of the COVID-19 pandemic in Hungary compared to the same periods of non-pandemic years. Rate ratios for excess all-cause mortality with 95% confidence intervals and the number of excess deaths for the second (week 41 of 2020 through week 4 of 2021) and third waves (weeks 7–21 of 2021) of the COVID pandemic for the whole of Hungary compared to the same periods of the pre-pandemic years were estimated for 10-year age strata using Poisson regression. Altogether, 9771 (95% CI: 9554–9988) excess deaths were recorded during the second wave of the pandemic, while it was lower, 8143 (95% CI: 7953–8333) during the third wave. During the second wave, relative mortality peaked for ages 65–74 and 75–84 (RR 1.37, 95%CI 1.33–1.41, RR 1.38, 95%CI 1.34–1.42). Conversely, during the third wave, relative mortality peaked for ages 35–44 (RR 1.43, 95%CI 1.33–1.55), while those ≥65 had substantially lower relative risks compared to the second wave. The reduced relative mortality among the elderly during the third wave is likely a consequence of the rapidly increasing vaccination coverage of the elderly coinciding with the third wave. The hugely increased relative mortality of those 35–44 could point to non-biological causes, such as less stringent adherence to non-pharmaceutical measures in this population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00622-3.
Keywords: Coronavirus disease 2019, Coronavirus, Pandemics, Mortality, COVID-19
Introduction
Since the end of January 2019, when WHO Director-General declared the 2019 novel Coronavirus outbreak a public health emergency of international concern, more than 489 million people have been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and more than 6 million people have died of coronavirus disease 2019 (COVID-19) globally [26, 44].
It is well-accepted that the case fatality of COVID-19 gradually increases over age with a marked surge after age 65 [13, 27]. Consequently, national vaccine programs that have given high priority to those at highest risk due to their occupation or medical vulnerability prioritized elderly people (mostly those over 65 years of age). Given the fact that vaccines clearly decrease the risk of infection and COVID-19-related mortality [36] and that the elderly are often prioritized for vaccination, this could have led to a better protection and an increased survival in this population [42]. An indirect consequence of the prioritization of the elderly, however, is a potential shift of the age distribution of COVID-19 towards younger ages [33]. Indeed, recent studies indicate that adults aged 20–49 years and especially those 35–49 may play a central role in the resurgence of the COVID-19 pandemic [27]. Furthermore, a less often examined driving force of the pandemic could also be children, who could harbor the infection without severe symptoms and thus could lead to the disproportionate exposure of primary caregivers, who are often women [27, 34, 45].
The Hungarian vaccination program started at the end of 2020, coinciding with the beginning of the third wave of COVID-19. After health care workers, the elderly were prioritized, resulting in increasing vaccination coverage among them during the third wave [10]. Given the increasing vaccination coverage among the elderly in the third wave of the pandemic in Hungary and the effect of vaccination on both infection risk and disease severity, one can expect decreased relative risks of COVID-19-related mortality in the older ages in the third wave compared to the second wave of the pandemic. However, given the non-uniform testing for SARS-CoV-2 in the general population, reported COVID-19-related mortality rates could be influenced by indication bias, while all-cause mortality (although this also includes other changes coinciding with the pandemic) could better reflect the effect of vaccinations. Thus, the main goals of our study were (1) to estimate excess absolute mortality by age and (2) to assess the age-distribution of all-cause mortality during the second and third waves of the COVID-19 pandemic in Hungary compared to the same periods of non-pandemic years. As studies also indicate that children and primary caregivers (mostly women) may be driving up infection rates in resurging phases of the pandemic [27, 45], while men in general are affected more severely by the disease and have a higher mortality [2], our secondary goal was to examine sex differences of age-related all-cause mortality during the second and third waves of the COVID-19 pandemic in Hungary.
Materials and methods
Setting and participants
This ecological study uses weekly mortality data and yearly population structure for the whole of Hungary between 2015 and 2021 (total population 9.86 to 9.73 million people). Age and sex structure of the background population and weekly all-cause mortality by sex and age groups for Hungary for each year between 2015 and 2021 are publicly available through the website of the Hungarian Central Statistical Office [17, 19]. Thus, this study was exempt from institutional review approval.
Based on visual inspection of the weekly mortality data of 2020 and 2021, we defined the second mortality wave of the pandemic as week 41 of 2020 through week 4 of 2021 (16 weeks), while the third wave was defined as weeks 7–21 of 2021 (15 weeks, data available on request). Given the time lag between the onset of clinical disease and mortality, waves defined by mortality data are different from those based on disease surveillance (Supplementary Fig. 1).
Outcomes
All-cause mortality (counts and rate ratios) during the second and third waves of the COVID-19 pandemic (2020 and 2021) were compared to all-cause mortality of the respective time periods of non-COVID-19 years (2015–2019) [17, 19].
Covariates
All-cause mortality and background population data were tabulated by sex and age using the following strata: 0–34, 35–44, 45–54, 55–64, 65–74, 75–84, and 85+ years of age.
Statistical analysis
Rate ratios for excess all-cause mortality with 95% confidence intervals and excess absolute weekly deaths were estimated for each age and sex stratum separately using generalized linear models (Poisson distribution with log link) with age- and sex-specific mortality counts as the dependent variable; age groups, sex, and time period (COVID-19 vs. control) as factors; and finally, the natural log transformed values of the age- and sex-specific background population as offset. The validity of the Poisson distribution of the outcome was tested by comparing the model-based and robust estimators. Since no major differences were observed between estimators, the results of the model-based confidence intervals were presented. Statistical significance was set at p<0.05. IBM SPSS Statistics 28.0.1.0 was used for statistical analysis.
Results
Altogether, 9771 (95% CI: 9554–9988) excess deaths were recorded during the second wave of the pandemic, while this number was substantially lower, 8143 (95% CI: 7953–8333) during the third wave. During the second and third waves of the COVID-19 pandemic, all age groups exhibited higher observed number of deaths compared to control years except for a non-significant excess for the 0–34 population and a significantly reduced number of deaths in the 85+ population in the third wave (Table 1).
Table 1.
Observed all-cause mortality during the 2nd and 3rd waves of the COVID-19 pandemic in Hungary and excess all-cause mortality compared to mortality of the same weeks of 2015–2019
| Second wave | Third wave | |||||
|---|---|---|---|---|---|---|
| Observed deaths | Excess deaths (95% CI) | Vaccination coverage (%)* | Observed deaths | Excess deaths (95% CI) | Vaccination coverage (%)* | |
| Age groups (years) | ||||||
| 85+ | 14685 | 3280 (2849–3711) | <10 | 11245 | −1300 (−164–−953) | 65 |
| 75-84 | 17189 | 4885 (4397–5364) | <10 | 14829 | 1189 (776–1602) | 65 |
| 65-74 | 13483 | 3585 (3199–3971) | <10 | 13409 | 2494 (2119–2851) | 50–65 |
| 55-64 | 6519 | 1048 (819–1258) | <10 | 6784 | 1172 (949–1376) | 35–50 |
| 45-54 | 2538 | 341 (227–454) | <10 | 2776 | 434 (320–548) | 20–30 |
| 35-44 | 766 | 160 (91–228) | <10 | 901 | 240 (175–306) | 20 |
| 0-34 | 524 | 58 (14–116) | <10 | 507 | 9 (−39–56) | 10–20 |
| Total | 55704 | 9771 (9554–9988) | <10 | 50451 | 8143 (7953–8333) | 30 |
Excess absolute weekly deaths were estimated for each age stratum separately using generalized linear models (Poisson distribution with log link) with age-specific mortality counts as the dependent variable; age groups and time period (COVID-19 vs. control) as factors; and the natural log transformed values of the age-specific background population as offset. The p-values are p < 0.001
95% CI: 95% confidence interval; COVID-19 coronavirus disease 2019
*Approximate cumulative uptake data of first vaccination are presented at the end of the respective wave [10]
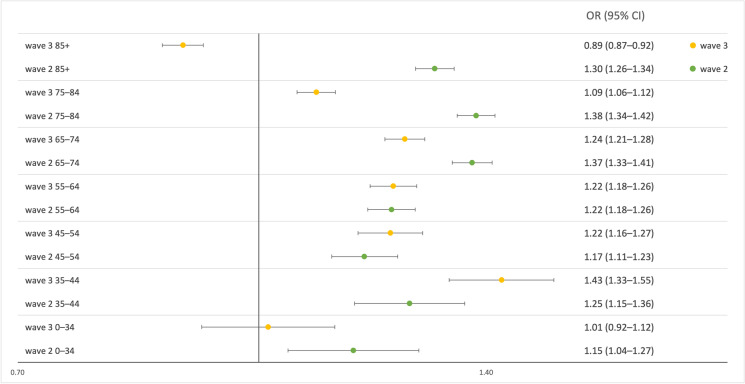
During the second wave, rate ratios were highest for ages 65–84. A lower risk was observed in those older than 85 years, while the risk was lowest in those below 65 years of age. A non-significant second peak for ages 35–44 can also be suspected. In contrast, during the third wave, rate ratios peaked between ages 35 and 44, followed by the age groups 45–74. Similarly, low results were observed for both the 75–84 and the 0–34 age groups, while the lowest risk was observed in the 85+ population (Fig. 1).
Fig. 1.
Age-specific rate ratios and their 95% confidence intervals of all-cause mortality in the second (green markers) and the third waves (yellow markers) of the COVID-19 pandemic in Hungary compared to the same weeks in 2015–2019. Rate ratios for excess all-cause mortality with 95% confidence intervals for waves 2 and 3 were estimated for each age stratum separately using generalized linear models (Poisson distribution with log link) with age-specific mortality counts as the dependent variable; age groups and time period (COVID-19 vs. control) as factors; and the natural log transformed values of the age-specific background population as offset. Abbreviations: RR: rate ratio; 95% CI: 95% confidence interval
When comparing the second and third waves, we found a substantially lower relative mortality in the 65+ population during the third wave compared to the second wave. The rate ratios were similar for ages 45–64 and 0–34 years, whereas point estimates were higher for the 35–44 age groups during the third wave compared to the second wave (Fig. 1).
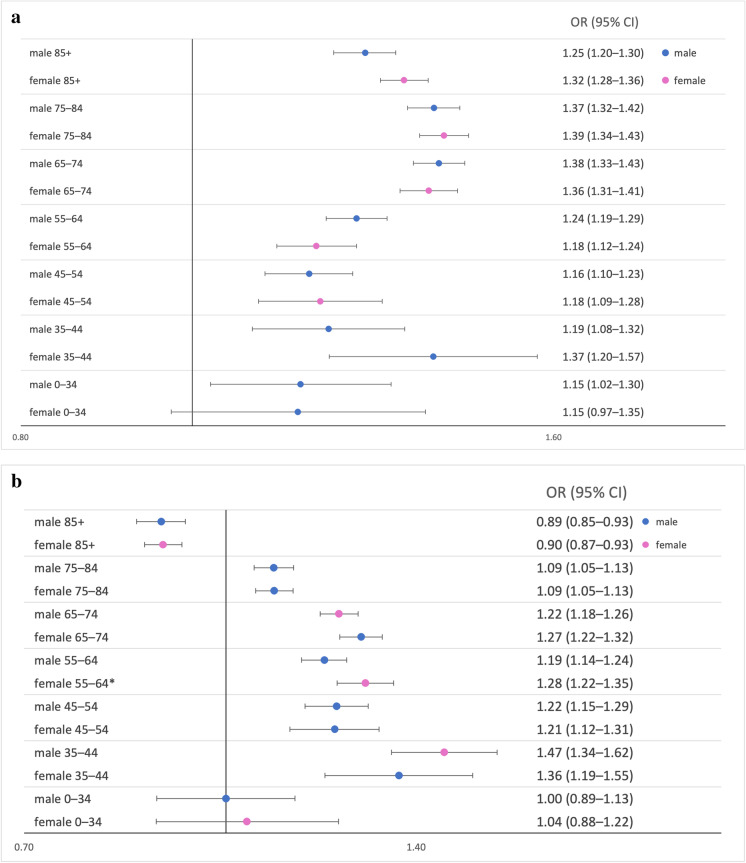
When examining the effect of sex, we found no significant differences except for a higher rate ratio in women compared to men aged 55–64 during the third wave (Fig. 2 Panel A and B).
Fig. 2.
Age- and sex-specific rate ratios and their 95% confidence intervals of all-cause mortality in the second (panel A) and third (panel B) waves of the COVID-19 pandemic in Hungary compared to the same weeks in 2015–2019. Blue markers—male; Magenta markers—female. Rate ratios for excess all-cause mortality with 95% confidence intervals for waves 2 and 3 were estimated for each age and sex stratum separately using generalized linear models (Poisson distribution with log link) with age- and sex-specific mortality counts as the dependent variable; age groups, sex, and time period (COVID-19 vs. control) as factors; and finally, the natural log transformed values of the age and sex-specific background population as offset. *p<0.05 Abbreviations: RR: rate ratio; 95% CI: 95% confidence interval
Discussion
Compared to control years, a substantial number of excess deaths during both waves of the pandemic with larger absolute numbers during the second wave were observed compared to the third. An excess number of deaths and an increased risk of mortality were observed for all age-groups except fort the oldest age group in the third wave during both examined waves of the pandemic. Comparing the two waves, the highest risk was observed in the 65+ age groups during the second wave, while the 35–44 age group had the highest relative risk during the third wave. During the second wave, relative mortality increased up to ages 65–84, followed by a shallow decrease. In contrast, risk rates peaked at ages 35–44 and gradually decreased after that during the third wave. As for sex differences, significantly higher excess mortality among women aged 55–64 was found compared to males of the same age group in the third wave, although this difference was likely a chance finding, given the high number of tests performed in this analysis.
Our findings for the second wave, namely a continuous increase of all-cause mortality compared to the non-COVID years until ages 65–84, corroborate other observations on the age-dependent mortality of COVID-19 [32]. The age mortality association is partly explained by the more severe manifestations of COVID-19 among the elderly related to the higher occurrence of comorbidities, such as heart failure [35] and diabetes [41], or the age-related dysfunction of the heart-brain axis [22]. These observations are unlikely to be confounded by the vaccination program, as vaccination coverage was almost non-existent during the second wave [10]. Surprisingly, the relative mortality of the oldest people (85+) was lower than that of the 65–84 age groups. A likely explanation of this observation may be that the oldest population (and their families) followed non-pharmaceutical interventions more stringently, as certain studies indicate an increasing adherence to non-pharmaceutical measures with age [15, 20]. This is also corroborated by a study that found that those aged 80 and above were 2.3-fold more likely to isolate themselves than elderly between ages 60–70 [39].
When comparing the second to the third wave, a clear decline in relative mortality of the ≥65 population was observed. This is possibly related to the quickly increasing vaccination coverage in the elderly during the third wave. While some vaccinations were administered as early as the end of December 2020, most vaccinations among the elderly were administered only 2 months later, from February 2020 [43]. Given that elderly were prioritized in vaccination programs both worldwide and in Hungary, coverage rates showed a strong age-dependence during the third wave [40]. According to ECDC data, vaccination coverage reached 65% in the ≥70 age group, 50% in the 60–69, while it remained around 10–30% for younger age groups [10] (Table 1). Furthermore, vaccination acceptance also increases with age and the number of comorbidities (which is also age dependent), resulting in an even higher uptake in the multimorbid elderly age groups [21, 24]. Thus, the lower relative all-cause mortality observed among the elderly during the third wave compared to the second wave could reflect the effectiveness of the vaccination program in Hungary, similarly to other countries with similar policies [23, 33, 37, 42]. Given the ecological nature of our analysis, however, other explanations cannot be excluded, such as a selectively increasing adherence to non-pharmaceutical interventions in the elderly population over time [3], as some evidence suggests an increased compliance of wearing face masks among the elderly [38], or the absence of influenza activity during the pandemic years leading to an underestimation of the relative risk in the more severely affected elderly [25]. We suspect that the combination of the above factors could be an explanation of the lower-than-expected mortality in the 85+ population during the third wave.
The most interesting finding of our study is the increase in relative risk of mortality for the 35–44 age group in the third wave compared to the second wave. This finding is unlikely to be confounded by vaccinations, as vaccination coverage was around 20% in this age group even by the end of the third wave. While differential vaccination hesitancy by age could play a role in vaccination uptake in certain age groups, this is an unlikely explanation to our findings given that the 20% uptake in this age group is more likely to reflect a low supply of vaccines and not low demand in this age group [8]. Given the fact that children less frequently present severe symptoms of COVID-19 if infected and may carry relatively high viral loads in the upper respiratory tract in the early phase of the infection [45], it is not surprising that young children (0–8 years) may play an important part in the spread of infection among household contacts [34] and adults living with children up to 18 years of age [11]. However, this is not necessarily reflected in our findings, as the closure of daycare and primary educational facilities was more stringent during the third wave compared to the second wave of the pandemic in Hungary [9, 18]. The results are even more surprising when considering that the proportion of employees working from home also increased from 2 to 17% from April to May 2020 [18].
While individuals between 25 and 54 years of age with children were more likely to work from home [12], and thus may in fact have a relatively lower risk of infection, people without children may have adhered less strictly to sanitary measures and may have been less worried about their own and relatives’ health during the COVID-19 pandemic [31]. Moreover, younger individuals, and especially those with lower income brackets, often express concerns regarding job security and income in relation to isolation and quarantine measures and are more likely to present for work even when symptomatic [38]. Moreover, younger individuals have lower compliance to personal hygiene and avoidance of contacts in general as well [14]. As a consequence, younger individuals may exhibit higher infection rates that should be taken into consideration in the planning of vaccine rollouts and non-pharmaceutical recommendations [6]. Another important difference between the second and third waves of the pandemic was that while the second wave is mostly unconfounded by influenza, the third wave coincides with the usual time period of the influenza epidemic [30]. However, the lack of influenza activity during the third wave of the pandemic would most likely decrease the relative risks in the third wave compared to the second wave (NNK). Another factor for consideration is the evolution of SARS-CoV-2 between the pandemic waves. While there is no reliable information of the distribution of the different SARS-CoV-2 variants before week 24 of 2021, according to data reported to the ECDC, until week 29, 2021 (weeks after the end of wave 3 in the present analysis), the predominant SARS_CoV-2 variant was alpha (B.1.1.7). Given this, it is unlikely that SARS_CoV-2 variants played an important role in our findings [7]. Even if due to misclassification some of the COVID-19 cases were caused by the delta variant, which is more contagious and causes more severe disease than other variants (CDC), this is unlikely to explain our findings of selectively increased mortality in the 35–44 age group, while the relative risk in the youngest age group (un-confounded by vaccination) was non-significantly lower in the third wave.
In contrast to our original hypothesis of infection rates and consequent mortality increase among women as primary caregivers, we found no strong support of sex differences in age-specific relative mortality. The only difference was a significantly higher rate ratio among women aged 55–64 compared to men in the same age group during the third wave. Since COVID-19-related mortality is in general higher as well as presentation is more severe in men in all age groups, this lack of difference between men and women could indirectly support lower infection rates among men [5, 28] [2]. While there is no strong support for a biological cause for this difference in infection rates, the fact that women more frequently work as informal or formal caregivers [1, 16] could lead to higher rates of infection among women. Furthermore, the significantly increased mortality of women aged 55–64 may be related to their role as informal caregivers of children during the closure of educational facilities. It must be noted, however, that given the high number of comparisons made in this analysis, the observed sex differences could signal Type I error.
A major strength of the study is the fact that our observations cover the whole Hungarian population, leading to an increase in statistical power and a decrease in selection bias. A subsequent strength is that the methodology used for the description of the background population and the outcome was the same for both the pandemic and the non-pandemic years. By selecting a hard endpoint (all-cause mortality), misclassification is also unlikely. Furthermore, our statistical model fits the data well, further increasing the reliability of our results.
Limitations of our study include its ecological nature as only population-level data was gathered. The lack of information on cause-specific mortality makes it impossible to assess the true effect and magnitude of COVID-19 on mortality. It is also impossible to disentangle deaths linked directly to COVID-19 from causes of deaths linked indirectly to COVID-19, such as increased suicide rates or deaths linked to excessive alcohol consumption or drug abuse. We also had no access to potentially important confounding factors influencing mortality, namely residence, ethnicity, or socioeconomic status. Furthermore, our results are especially affected by the composition of the background population, and background populations may have been estimated imprecisely. It must be also noted that our results can be biased by the change of mortality from year to year as well. For instance, in 2021, the influenza pandemic was less evident, and the influenza season coincides with the third wave. Finally, we tested different models to describe mortality in our population. While we found that our results were consistent with a Poisson distribution, it is possible that other assumptions would better fit the data.
Conclusion
We found that relative mortality increased with age in the second wave of the pandemic compared to the non-pandemic years. However, as vaccination coverage increased among the elderly in the third wave, the rate ratios in these age groups became lower compared to the second wave clearly signaling the effectiveness of vaccines. We also report the highest relative mortality in the 35–44 age group during the third wave that could point to the role of non-biological factors (such as less stringent adherence to non-pharmaceutical measures). Our results highlight the importance of vaccination coverage and non-pharmaceutical measures in the whole population with a special emphasis on younger people who are usually considered to be at low risk for COVID-19-related mortality.
Supplementary information
(PNG 182 kb)
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. AGT was supported by TKP2021-NKTA-47 provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the 2021 Thematic Excellence Programme funding scheme.
Declarations
Conflict of interest
Dr. Adam Tabak serves as Associate Editor for GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vince Fazekas-Pongor and Zsófia Szarvas contributed equally as co-first authors.
References
- 1.Baji P, Golicki D, Prevolnik-Rupel V, Brouwer WBF, Zrubka Z, Gulácsi L, Péntek M. The burden of informal caregiving in Hungary, Poland and Slovenia: results from national representative surveys. Eur J Health Econ. 2019;20:5–16. doi: 10.1007/s10198-019-01058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechmann N, Barthel A, Schedl A, Herzig S, Varga Z, Gebhard C, Mayr M, Hantel C, Beuschlein F, Wolfrum C, Perakakis N, Poston L, Andoniadou CL, Siow R, Gainetdinov RR, Dotan A, Shoenfeld Y, Mingrone G, Bornstein SR. Sexual dimorphism in COVID-19: potential clinical and public health implications. Lancet Diabetes Endocrinol. 2022;10:221–230. doi: 10.1016/S2213-8587(21)00346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger R, Christian C, English R, Maughan-Brown B, Rossouw L. Predictors of mask-wearing during the advent of the COVID-19 pandemic: evidence from South Africa. Transl. Behav Med. 2022;12(1):ibab132. [DOI] [PMC free article] [PubMed]
- 4.CDC. What You Need to Know About Variants. 2022 https://www.cdc.gov/coronavirus/2019-ncov/variants/about-variants.html. 2022.04.12.
- 5.Crimmins EM, Shim H, Zhang YS, Kim JK. Differences between men and women in mortality and the health dimensions of the morbidity process. Clin Chem. 2019;65:135–145. doi: 10.1373/clinchem.2018.288332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding X, Brazel DM, Mills MC. Factors affecting adherence to non-pharmaceutical interventions for COVID-19 infections in the first year of the pandemic in the UK. BMJ Open. 2021;11:e054200. doi: 10.1136/bmjopen-2021-054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ECDC. Data on SARS-CoV-2 variants in the EU/EEA. 2022a. https://www.ecdc.europa.eu/en/publications-data/data-virus-variants-covid-19-eueea. 2021.04.12.
- 8.ECDC. Data on COVID-19 vaccination in the EU/EEA. 2022b. https://www.ecdc.europa.eu/en/publications-data/data-covid-19-vaccination-eu-eea. 2022.06.17.
- 9.ECDC. Data on country response measures to COVID-19. 2022c. https://www.ecdc.europa.eu/en/publications-data/download-data-response-measures-covid-19. 2021.12.06.
- 10.ECDC. Country Overview Report. 2022d. https://covid19-country-overviews.ecdc.europa.eu/vaccination.html. 2022.01.19.
- 11.Forbes H, Morton CE, Bacon S, McDonald HI, Minassian C, Brown JP, Rentsch CT, Mathur R, Schultze A, DeVito NJ, MacKenna B, Hulme WJ, Croker R, Walker AJ, Williamson EJ, Bates C, Mehrkar A, Curtis HJ, Evans D, Wing K, Inglesby P, Drysdale H, Wong AYS, Cockburn J, McManus R, Parry J, Hester F, Harper S, Douglas IJ, Smeeth L, Evans SJW, Bhaskaran K, Eggo RM, Goldacre B, Tomlinson LA. Association between living with children and outcomes from covid-19: OpenSAFELY cohort study of 12 million adults in England. BMJ. 2021;372:n628. doi: 10.1136/bmj.n628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaffney AW, Himmelstein DU, Woolhandler S. Trends and disparities in teleworking during the COVID-19 pandemic in the USA: May 2020-February 2021. J Gen Intern Med. 2021;36:3647–3649. doi: 10.1007/s11606-021-07078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horváth VJ, Hajdú N, Vági O, Schnábel K, Szelke E, Körei AE, Békeffy M, Svébis MM, Domján BA, Berényi T, Takács I, Ungvári Z, Kun A, Tabák ÁG. Comparison of clinical characteristics of patients with pandemic SARS-CoV-2-related and community-acquired pneumonias in Hungary – a pilot historical case-control study. GeroScience. 2021;43:53–64. doi: 10.1007/s11357-020-00294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang YS, Jo HS. Associations between compliance with non-pharmaceutical interventions and social-distancing policies in Korea during the COVID-19 pandemic. J Prev Med Public Health. 2021;54:230–237. doi: 10.3961/jpmph.21.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney PM, Spillane M, Humphries R, Gannon A, Stamenic D, Bhuachalla CN, Hoevel P, Arensman E, O’Riordain M, Troya MI, Khashan AS, O’Reilly E, Buckley C, O’Connor L, Perry IJ. Compliance with local travel restrictions and face masks during first phase of COVID-19 pandemic in Ireland: a national survey. J Public Health. 2022;fdac017. [DOI] [PMC free article] [PubMed]
- 16.King T, Hewitt B, Crammond B, Sutherland G, Maheen H, Kavanagh A. Reordering gender systems: can COVID-19 lead to improved gender equality and health? Lancet (London, England) 2020;396:80–81. doi: 10.1016/S0140-6736(20)31418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.KSH. Halálozások száma nemek és korcsoportok szerint, hetente. 2021a. https://www.ksh.hu/stadat_files/nep/hu/nep0065.html. 2021.12.06.
- 18.KSH. Felértékelődött a távmunka a Covid19 árnyékában. 2021b https://www.ksh.hu/docs/hun/xftp/idoszaki/koronavirus-tavmunka/index.html. 2021.12.06.
- 19.KSH. Age-sex pyramid for the Hungarian population. 2021c. http://www.ksh.hu/interaktiv/korfak/orszag.html?lang=hu. 01.03.2021.
- 20.Lang R, Atabati O, Oxoby RJ, Mourali M, Shaffer B, Sheikh H, Fullerton MM, Tang T, Leigh JP, Manns BJ, Marshall DA, Ivers NM, Ratzan SC, Hu J, Benham JL. Characterization of non-adopters of COVID-19 non-pharmaceutical interventions through a national cross-sectional survey to assess attitudes and behaviours. Sci Rep. 2021;11:21751–21751. doi: 10.1038/s41598-021-01279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, Kimball S, El-Mohandes A. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lionetti V, Bollini S, Coppini R, Gerbino A, Ghigo A, Iaccarino G, Madonna R, Mangiacapra F, Miragoli M, Moccia F, Munaron L, Pagliaro P, Parenti A, Pasqua T, Penna C, Quaini F, Rocca C, Samaja M, Sartiani L, Soda T, Tocchetti CG, Angelone T. Understanding the heart-brain axis response in COVID-19 patients: A suggestive perspective for therapeutic development. Pharmacol Res. 2021;168:105581. doi: 10.1016/j.phrs.2021.105581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macchia A, Ferrante D, Angeleri P, Biscayart C, Mariani J, Esteban S, Tablado MR, de Quirós FGB. Evaluation of a COVID-19 vaccine campaign and SARS-CoV-2 infection and mortality among adults aged 60 years and older in a middle-income country. JAMA Netw Open. 2021;4:e2130800-e2130800. doi: 10.1001/jamanetworkopen.2021.30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malesza M, Wittmann E. Acceptance and intake of COVID-19 vaccines among Older Germans. J Clin Med. 2021;10(7):1388. [DOI] [PMC free article] [PubMed]
- 25.Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig RL, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016;16:481. doi: 10.1186/s12889-016-3128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modig K, Lambe M, Ahlbom A, Ebeling M. Excess mortality for men and women above age 70 according to level of care during the first wave of COVID-19 pandemic in Sweden: A population-based study. Lancet Reg Health – Europe. 2021;4:100072. [DOI] [PMC free article] [PubMed]
- 27.Monod M, Blenkinsop A, Xi X, Hebert D, Bershan S, Tietze S, Baguelin M, Bradley VC, Chen Y, Coupland H, Filippi S, Ish-Horowicz J, McManus M, Mellan T, Gandy A, Hutchinson M, Unwin HJT, van Elsland SL, Vollmer MAC, et al. Age groups that sustain resurging COVID-19 epidemics in the United States. Science. 2021;371(6536):eabe8372. [DOI] [PMC free article] [PubMed]
- 28.Nielsen J, Nørgaard SK, Lanzieri G, Vestergaard LS, Moelbak K. Sex-differences in COVID-19 associated excess mortality is not exceptional for the COVID-19 pandemic. Sci Rep. 2021;11:20815. doi: 10.1038/s41598-021-00213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NNK. Tájékoztató az influenza figyelőszolgálat adatairól - 2021.20. hét. 2022. https://www.antsz.hu/felso_menu/temaink/jarvany/influenza/influ_figyelosz_21/influ_2021_20.html. 2022.04.12.
- 30.NNK. Tájékoztató az influenza figyelőszolgálat adatairól. 2021. https://84.206.43.26/print/https://84.206.43.26/felso_menu/temaink/jarvany/influenza/influ_figyelosz_21/influ_2021_17.html. 2022.04.15.
- 31.Nowak BM, Miedziarek C, Pełczyński S, Rzymski P. Misinformation, Fears and Adherence to Preventive Measures during the Early Phase of COVID-19 Pandemic: a Cross-Sectional Study in Poland. Int J Environ Res Public Health. 2021;18:12266. doi: 10.3390/ijerph182212266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 33.Pastorino R, Pezzullo AM, Villani L, Causio FA, Axfors C, Contopoulos-Ioannidis DG, Boccia S, Ioannidis JPA. Change in age distribution of COVID-19 deaths with the introduction of COVID-19 vaccination. Environ Res. 2022;204:112342. doi: 10.1016/j.envres.2021.112342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paul LA, Daneman N, Schwartz KL, Science M, Brown KA, Whelan M, Chan E, Buchan SA. Association of age and pediatric household transmission of SARS-CoV-2 infection. JAMA Pediatr. 2021;175:1151–1158. doi: 10.1001/jamapediatrics.2021.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razeghian-Jahromi I, Zibaeenezhad MJ, Lu Z, Zahra E, Mahboobeh R, Lionetti V. Angiotensin-converting enzyme 2: a double-edged sword in COVID-19 patients with an increased risk of heart failure. Heart Fail Rev. 2021;26:371–380. doi: 10.1007/s10741-020-10016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rustagi V, Bajaj M, Tanvi, Singh P, Aggarwal R, AlAjmi MF, Hussain A, Hassan MI, Singh A, Singh IK. Analyzing the effect of vaccination over covid cases and deaths in asian countries using machine learning models. Front Cell Infect Microbiol. 2022:11:806265. [DOI] [PMC free article] [PubMed]
- 37.Sadarangani M, Abu Raya B, Conway JM, Iyaniwura SA, Falcao RC, Colijn C, Coombs D, Gantt S. Importance of COVID-19 vaccine efficacy in older age groups. Vaccine. 2021;39:2020–2023. doi: 10.1016/j.vaccine.2021.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seale H, Dyer CEF, Abdi I, Rahman KM, Sun Y, Qureshi MO, Dowell-Day A, Sward J, Islam MS. Improving the impact of non-pharmaceutical interventions during COVID-19: examining the factors that influence engagement and the impact on individuals. BMC Infect Dis. 2020;20:607. doi: 10.1186/s12879-020-05340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shati M, Alami A, Mortazavi SS, Eybpoosh S, Emamian MH, Moghadam M. Adherence to self-isolation measures by older adults during coronavirus disease 2019 (COVID-19) epidemic: a phone survey in Iran. Med J Islam Repub Iran. 2020;34:152–152. doi: 10.47176/mjiri.34.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava A, Rockman-Greenberg C, Sareen N, Lionetti V, Dhingra S. An insight into the mechanisms of COVID-19, SARS-CoV2 infection severity concerning β-cell survival and cardiovascular conditions in diabetic patients. Mol Cell Biochem. 2022;477:1681–1695. doi: 10.1007/s11010-022-04396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Victora CG, Castro MC, Gurzenda S, Medeiros AC, França GVA, Barros AJD. Estimating the early impact of vaccination against COVID-19 on deaths among elderly people in Brazil: analyses of routinely-collected data on vaccine coverage and mortality. eClinicalMedicine. 2021:38:101036. [DOI] [PMC free article] [PubMed]
- 43.Vokó Z, Kiss Z, Surján G, Surján O, Barcza Z, Pályi B, Formanek-Balku E, Molnár GA, Herczeg R, Gyenesei A, Miseta A, Kollár L, Wittmann I, Müller C, Kásler M. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary - the HUN-VE study. Clin Microbiol Infect. 2022;28(3):398–404. doi: 10.1016/j.cmi.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO. WHO Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int. 2021.09.22.
- 45.Yonker LM, Boucau J, Regan J, Choudhary MC, Burns MD, Young N, Farkas EJ, Davis JP, Moschovis PP, Bernard Kinane T, Fasano A, Neilan AM, Li JZ, Barczak AK. Virologic features of severe acute respiratory syndrome coronavirus 2 infection in children. J Infect Dis. 2021;224:1821–1829. doi: 10.1093/infdis/jiab509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 182 kb)