Abstract
Aims
Greater aortic intima media thickness (aIMT), a marker of subclinical atherosclerosis, can identify individuals at risk of CVD. This systematic review with meta‐analysis compared aIMT in youth with type 1 diabetes and healthy controls.
Methods
A systematic search of published literature (to July 2021) was undertaken using electronic databases MEDLINE, EMBASE, Scopus, CINAHL and AMED. Eligible studies reported aIMT in participants aged <20 years with type 1 diabetes and healthy controls. Meta‐analysis was used to combine outcome data, presented as forest plots. Moderator analysis and metaregression were conducted to identify study and participant characteristics associated with aIMT. Publication bias was assessed by funnel plot inspection.
Results
Meta‐analysis of nine studies (n = 1030 with type 1 diabetes and n = 498 healthy control participants) indicated, with high heterogeneity (I2 98%), that youth with type 1 diabetes have higher aIMT compared with healthy controls (mean difference [95% CIs]: 0.11 [0.04, 0.18] mm, P = 0.003). Factors associated with greater aIMT in type 1 diabetes compared to controls included: use of a phased array probe versus linear array probe; longer diabetes duration; higher insulin dose; higher BMI z score and waist circumference; higher LDL cholesterol; higher triglycerides; and higher diastolic blood pressure.
Conclusions
Type 1 diabetes in youth is associated with higher aIMT compared with healthy control individuals. Longer duration of diabetes and major CVD risk factors were also associated with higher aIMT. Together, these findings provide a strong rationale for targeting modifiable risk factors in CVD prevention. Registered in PROSPERO on 8 August 2019 (CRD42019137559).
Keywords: aortic intima media thickness, subclinical atherosclerosis, systematic review, type 1 diabetes, youth
1. INTRODUCTION
Individuals with type 1 diabetes experience increased lifelong risk of CVD and cardiovascular related mortality. Identification of those at greatest risk of CVD is essential so that targeted preventative interventions can be initiated. The pathogenesis of CVD in type 1 diabetes is multifactorial, involving accelerated progression and increased severity of atherosclerosis, the underlying disease process that drives the majority of myocardial infarctions.
Markers of subclinical atherosclerosis are a recognized surrogate for CVD that can identify individuals at increased risk, 1 and include aortic intima media thickness (aIMT), carotid intima media thickness (cIMT), flow‐mediated dilation, and carotid‐femoral pulse wave velocity. With the exception of aIMT, these markers of subclinical atherosclerosis were comprehensively evaluated for their differences between people with type 1 diabetes and controls in a 2019 systematic review and meta‐analysis. 2 Consistent with early signs of CVD, this review found that people with type 1 diabetes had significantly higher cIMT (SMD: 0.89; 95% CI, 0.69–1.09; p < 0.001), significantly lower flow‐mediated dilation (SMD: −1.45%; 95% CI, −1.74 to −1.17; p < 0.001), and significantly higher carotid‐femoral pulse wave velocity (SMD: 0.57; 95% CI, 0.03–1.11; p < 0.001) compared with controls. These results highlight that type 1 diabetes is associated with accelerated atherosclerosis progression and early signs of CVD. We hypothesize that aIMT would also be higher in youth with type 1 diabetes compared with controls. However, the impact of type 1 diabetes on aIMT has not yet been reviewed.
aIMT appears to be a better noninvasive marker of preclinical atherosclerosis in children and adolescents, compared with other measures, partly due to arterial lesions (fatty streaks) manifesting earliest in the aorta. 3 Therefore, measurement of aIMT may enable detection of increased CVD risk earlier in disease progression. Furthermore, a younger age at diagnosis of type 1 diabetes is associated with increased risk of CVD and CVD related death, 4 highlighting the need to feasibly investigate subclinical risk of CVD in youth with type 1 diabetes.
The primary aim of this systematic review with meta‐analysis was to evaluate the difference between aIMT in youth with type 1 diabetes and healthy controls. Secondarily, this review aimed to utilize moderator analysis and meta‐regression to identify other factors which may influence aIMT in type 1 diabetes.
2. METHODS
The Preferred Reporting Items for Systematic Reviews and Meta‐analyses 5 was used to guide reporting of this systematic review and meta‐analysis, and the protocol 6 was registered with PROSPERO (CRD42019137559, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019137559).
2.1. Eligibility criteria
Eligible studies were published articles (abstracts were not included) written in English and reported a cross sectional assessment, irrespective of study design, setting and method of assessment, of aIMT in youth (mean age < 20 years) with type 1 diabetes and a healthy control group. Studies needed to report aIMT (mean/median and SD/SEM/95% CIs) of both participants with type 1 diabetes and healthy controls to be eligible for inclusion. No limitation was placed on the date of publication of the study.
2.2. Search strategy
A systematic search of published literature up to July 2021 was undertaken using electronic databases MEDLINE, EMBASE, Scopus, CINAHL and AMED by Benjamin J Varley, under guidance from a University of Sydney librarian. Keywords were searched as both Medical Subject Headings of the National Library of Medicine and as independent search terms, for example, aorta, intima and aIMT (supplementary file 1). Relevant truncations and adjacencies were used to enhance results. 7 The search was limited to studies in children and adolescents. Hand‐searching of reference lists was conducted to identify any missed studies. Screening of studies and removal of duplicates were conducted using Covidence online software (Vertitas Health Innovation Ltd, Australia). All records were independently assessed for inclusion by two reviewers (Benjamin J Varley, Reeja F Nasir and Megan L Gow) based on the defined criteria, first by title and abstract, then full text. Discrepancies were also resolved through discussion by the two reviewers (Benjamin J Varley and Reeja F Nasir).
2.3. Data extraction
Data were independently extracted from eligible studies by one reviewer into a purpose‐built database developed using REDCap electronic data capture tools hosted at The University of Sydney, 8 and cross‐checked for accuracy (Benjamin J Varley and Reeja F Nasir). Extracted data included study and participant characteristics, aIMT assessment methods, aIMT results, cIMT results and CVD risk markers including HbA1c, fasting glucose, LDL cholesterol, HDL cholesterol, total cholesterol, triglycerides, and systolic and diastolic BP. Where possible, authors of relevant papers were contacted to obtain missing data pertinent to this review or to confirm eligibility. Studies were excluded if the author could not be contacted after two attempts (n = 3).
2.4. Risk of bias
Studies were assessed for quality by two reviewers (Benjamin J Varley and Reeja F Nasir) using the Joanna Briggs Institute critical appraisal tools for cross sectional and cohort studies as appropriate 9 with discrepancies resolved through discussion between reviewers. Each study was given a score out of 8 for cross sectional and 11 for cohort studies, with one point being given for each item on the checklist that the study met. Publication bias was assessed by visual inspection of funnel plot symmetry, and using the classic fail‐safe N statistic 10 with interpretation based on the tolerance level suggested by Rosenthal. 11
2.5. Data synthesis
All included studies reported mean aIMT and standard deviation for both the participants with type 1 diabetes, and the control group. For our primary analysis, these data were combined in meta‐analysis using Revman, version 5.4 12 to calculate the mean difference in aIMT (mm) in youth with type 1 diabetes compared with controls, presented as a forest plot. When aIMT was assessed more than once in a study cohort (i.e. longitudinal data collection) the first aIMT measurement was used for meta‐analysis to optimize sample size of the study.
In our secondary analyses, for studies reporting cIMT, the mean difference in cIMT (mm) in youth with type 1 diabetes compared with controls was combined and calculated in meta‐analysis using Revman, version 5.4 and presented as a forest plot. Secondary analyses also included moderator analyses and metaregression which were conducted using the Comprehensive Meta‐Analysis package, version 3.0 (Biostat, Englewood, NJ). Categorical moderator analysis was conducted to identify study characteristics that may impact aIMT including aIMT assessment specifics such as the type of probe used (phased array vs. linear array), ultrasound frequency (1–10 MHz vs. >10 MHz), and manual (calipers) versus automatic software aIMT calculation. Meta‐regression of continuous variables was conducted to determine associations between type 1 diabetes participant characteristics and the difference in mean aIMT (mm) in controls compared to those with type 1 diabetes including: age (years), diabetes duration (years), insulin dose (IU/kg/day), BMI, BMI z score, waist circumference (cm), HbA1c, fasting glucose, LDL cholesterol, HDL cholesterol, total cholesterol, triglycerides, and systolic and diastolic BP.
Heterogeneity between studies was assessed using the I2 statistic. A random effects model was used due to assumed heterogeneity. p values of <0.05 were considered statistically significant.
3. RESULTS
3.1. Included studies
The literature search identified 8657 publications of which 1001 were retrieved for full text review (Figure 1). Overall, 11 articles met all inclusion criteria. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 While authors could not be contacted to confirm, three articles by Dayem et al. 13 , 14 , 15 appeared to represent the same cohort. Therefore, the 2018 study 15 was used as the primary study for data extraction (including aIMT and cIMT outcomes) due to it having the largest sample size. Additional data relevant to the review but not reported in the primary study were drawn from the two secondary studies 13 , 14 where possible. Therefore, nine unique studies 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 were included in this systematic review and meta‐analysis (Figure 1).
FIGURE 1.
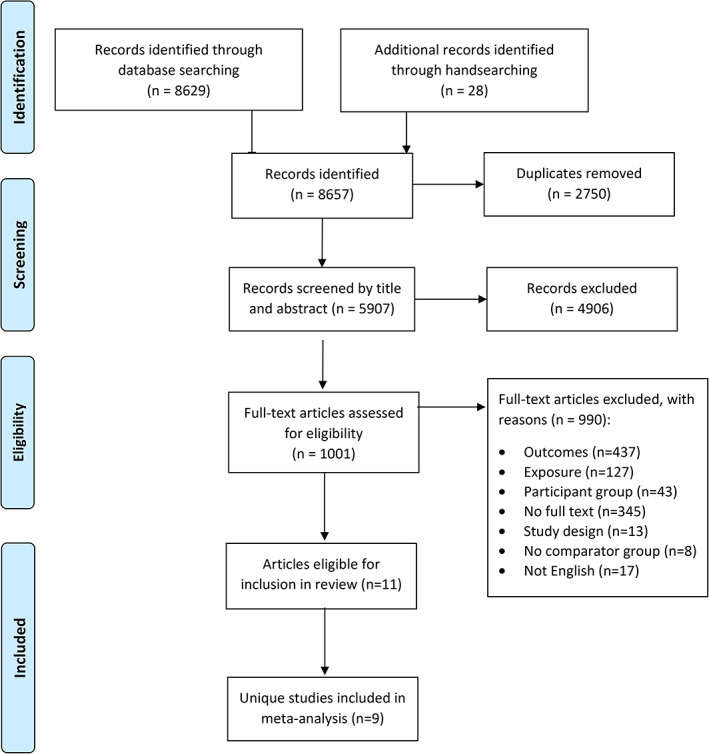
PRISMA flow diagram
3.2. Risk of bias
Included cross sectional studies achieved a quality score of between three and eight out of a possible eight, and the one cohort study had a score of eight out of a possible 11 (Table 1). No study was excluded due to study quality.
TABLE 1.
Study and participant characteristics of included studies
| Author year of publication (Country; setting) | Quality score | n | Age, year | Percentager male | Mean aIMT (mm) | Max aIMT (mm) | Mean BMI; mean BMI z score | |
|---|---|---|---|---|---|---|---|---|
| El‐Asrar et al. 2016 (Egypt; hospital clinic) 17 | 5/8 | Type 1 diabetes | 60 | 11.5 ± 3.5 | 45 | 0.74 ± 0.11 | NR |
Group 1a: NR; 0.70 ± 1.33 Group 2b: NR; 0.74 ± 1.36 |
| Control | 30 | 10.7 ± 3.2 | 57 | 0.45 ± 0.01* | NR | NR | ||
| El Dayem et al. 2018 (Egypt; research clinic) 15 | 3/8 | Type 1 diabetes | 135 | 17.99 ± 2.59 | NR | 0.722 ± 0.108 | NR | 24.74 ± 4.07; NR |
| Control | 100 | 17.50 ± 2.67c | NR | 0.458 ± 0.046* | NR | 22.47 ± 6.45; NR | ||
| Ersoy et al. 2015 (Turkey; hospital clinic) 16 | 5/8 | Type 1 diabetes | 27 | 12.73 ± 2.98 | 52 | 0.63 ± 0.11 | NR | 19.15 ± 2.88; NR |
| Control | 30 | 11.46 ± 2.71 | 58 | 0.58 ± 0.12 | NR | 17.7 ± 3.71; NR | ||
| Harrington et al. 2010 (Australia; hospital clinic) 18 | 5/8 | Type 1 diabetes | 68 | 14.1 ± 2.5 | 54 | 0.57 ± 0.11 | 0.69 ± 0.14 | NR; 0.41 ± 0.74 |
| Control | 32 | 14.2 ± 3.0 | 47 | 0.50 ± 0.07* | 0.61 ± 0.09* | NR; 0.11 ± 0.92 | ||
| Jarvisalo et al. 2001 (Finland; hospital clinic) 23 | 6/8 | Type 1 diabetes | 44 | 11 ± 2 | 70 | 0.50 ± 0.09 | NR | NR; NR |
| Control | 28 | 11 ± 1 | 61 | 0.44 ± 0.05* | NR | NR; NR | ||
| Lilje et al. 2017 (USA; hospital clinic) 22 | 5/8 | Type 1 diabetes | 38 | 13.4 ± 3.4 | 63 | 0.60 ± 0.11 | NR | 21.4 ± 3.7; NR |
| Control | 38 | 12.7 ± 4.5 | 50 | 0.52 ± 0.10* | NR | 20.0 ± 3.6; NR | ||
| Maftei et al. 2014 (Australia; five research/ hospital centres) 21 | 8/8 | Type 1 diabetes | 406 | 14.2 ± 1.9 | NR | 0.56 ± 0.11 | 0.67 ± 0.13 | 22.0 ± 3.6; NR |
| Control | 57 | 14.0 ± 2.9 | NR | 0.51 ± 0.10* | 0.61 ± 0.12* | 20.9 ± 4.1; NR | ||
| Pena et al. 2016 (Australia; hospital clinic) 19 | 8/11 | Type 1 diabetes | 77 | 13.6 ± 2.6 | 52 | 0.56 ± 0.11 | 0.67 ± 0.14 | NR; 0.52 ± 0.83 |
| Control | 33 | 13.9 ± 2.9 | 45 | 0.50 ± 0.07* | 0.61 ± 0.09* | NR; 0.19 ± 0.89 | ||
| Zhang et al. 2019 (China; two hospital clinics) 20 | 5/8 | Type 1 diabetes | 175 | 12.5 ± 2.6 | 46 | 0.57 ± 0.06 | 0.69 ± 0.09 | 20.3 ± 2.0; 0.47 ± 0.33 |
| Control | 150 | 12.4 ± 2.7 | 47 | 0.51 ± 0.09* | 0.68 ± 0.08 | 19.8 ± 3.1; 0.26 ± 0.29 |
Note: Data presented as mean ± SD unless otherwise indicated. aT1D with microvascular complications; bT1D without microvascular complications; cdata extracted from secondary study (El Dayem et al. 2016); *denotes significantly different from type 1 diabetes group.
Abbreviations: aIMT, aortic intima media thickness; BMI, body mass index; n, number; NR, not reported; y, years.
3.3. Study characteristics
The nine studies identified for inclusion in this systematic review and meta‐analysis are summarized in Table 1. Three of the nine studies were conducted in Australia, 18 , 19 , 21 two in Egypt, 15 , 17 and one each in Finland, 23 Turkey, 16 China 20 and the USA. 22 Eight of nine studies were conducted between 2010 and 2019 and one study was conducted in 2001. 23 One study presented longitudinal data from a cohort study, reporting both a baseline and follow up aIMT. 19 All other studies were cross sectional. One study was conducted in a research clinic, 15 seven were conducted in a hospital clinic 16 , 17 , 18 , 19 , 20 , 22 , 23 and the remaining five‐site multicentre study by Maftei et al. was conducted in both hospital and research centres. 21
3.4. Participant characteristics
Participant characteristics are described in Table 1. Control participants in included studies were described as being either ‘healthy’, 16 ‘healthy age‐matched’, 18 , 22 ‘healthy age‐, and gender‐matched’, 15 , 19 , 20 , 21 ‘healthy age‐, gender‐ and body size‐matched’, 23 or ‘healthy age‐, gender‐ and pubertal stage‐matched’. 17 The sample size of included studies ranged from 55 in the Turkish study 16 to 463 in the multicentre Australian study. 21 The age of participants at time of aIMT assessment ranged from 11.0 to 18.0 years in participants with type 1 diabetes and 10.7–17.5 years in healthy control participants. Mean BMI of participants with type 1 diabetes in included studies ranged from 19.2–24.7 kg/m2 and 17.7–22.5 kg/m2 in healthy controls; BMI z score ranged from 0.41–0.74 in participants with type 1 diabetes and 0.11–0.26 in healthy controls. Duration since diabetes onset at time of aIMT assessment was described by eight studies 15 , 17 , 18 , 19 , 20 , 21 , 22 , 23 and ranged from 4.4–10.9 years (Table 2). Ersoy et al. did not report duration of diabetes in relevant participants. 16 Six studies reported the prescribed mean insulin dose, with doses ranging from 0.95 to 1.7 IU/kg/day. 15 , 17 , 18 , 20 , 23
TABLE 2.
Clinical characteristics of participants with type 1 diabetes in included studies
| Study | Diabetes duration, mean ± SD y | Insulin therapy, mean ± SD dose U/kg/day | HbA1c, % (mmol/mol) |
|---|---|---|---|
| El‐Asrar et al. 2016 17 | 8.2 ± 1.7 | 1.7 ± 0.32 | 8.5 (69) |
| El Dayem et al. 2018 15 | 10.9 ± 3.5 a | 1.26 ± 0.44 a | 9.3 (78) |
| Ersoy et al. 2015 16 | NR | NR | NR |
| Harrington et al. 2010 18 | 5.4 ± 3.8 | 0.96 ± 0.3 | 8.6 (70) |
| Jarvisalo et al. 2001 23 | 4.4 ± 3.1 | 0.95 ± 0.24 | 8.8 (73) |
| Lilje et al. 2017 22 | 5.8 ± 4.3 | NR | 9.7 (83) |
| Maftei et al. 2014 21 | 6.7 ± 3.7 | NR | 8.5 (69) |
| Pena et al. 2016 19 | 5.9 ± 4.2 | NR (15 used continuous subcutaneous insulin infusion) | 8.6 (70) |
| Zhang et al. 2019 20 | 4.7 ± 2.4 | 1.03 ± 0.91 | 7.8 (62) |
data extracted from secondary study (El Dayem et al. 2016).
Abbreviations: NR, not reported; y, years.
In addition to aIMT assessment, all studies with the exception of the study by Ersoy et al., 16 also assessed and reported cIMT in participants with type 1 diabetes and controls. Other outcomes commonly reported in included studies were HbA1c (all studies), fasting glucose (five studies 16 , 17 , 18 , 19 , 20 ), lipid profile (eight studies 15 , 16 , 17 , 18 , 19 , 20 , 21 , 23 ), c‐reactive protein (four studies 17 , 18 , 19 , 20 ), albumin‐creatinine ratio (ACR, four studies 15 , 17 , 20 , 21 ) and systolic and diastolic BP (eight studies 15 , 17 , 18 , 19 , 20 , 21 , 22 , 23 ) (Table 3). All studies, except for the study by Jarvisalo et al. 23 reported either BMI or BMI z score. In addition to BMI and BMI z score, four studies 15 , 18 , 19 , 21 also reported waist circumference, two studies 15 , 20 reported waist‐to‐hip ratio and two studies 15 , 19 reported waist‐to‐height ratio. Participants with type 1 diabetes in included studies presented with poorer cardiometabolic health markers compared with healthy control participants including higher fasting glucose in 4/4 studies, higher HbA1c in 7/7 studies, at least one lipid outcome affected in 3/7 studies, higher systolic BP or diastolic BP in 3/7 studies, higher ACR in 1/2 studies, higher c‐reactive protein (CRP) in 3/3 studies and at least one weight related outcome increased in 3/7 studies (Table 3).
TABLE 3.
Secondary outcomes assessed and reported for participants with type 1 diabetes in included studies
| Study | Secondary outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | cIMT | ACR | Waist circumference | BMI/ BMI z | Waist to hip ratio | Waist to height ratio | Lipid profile | HbA1c | Blood pressure | CRP | |
| El‐Asrar et al. 2016 17 | • | y | • | • | • | • | • | • | |||
| El Dayem et al. 2018 15 | y | y | •a | y | x | y | y | y | x | ||
| Ersoy et al. 2015 16 | y | x | y | y | |||||||
| Harrington et al. 2010 18 | y | x | x | x | x | y | y | y | |||
| Jarvisalo et al. 2001 | y | x | y | x | |||||||
| Lilje et al. 2017 | x | x | • | x | |||||||
| Maftei et al. 2014 | x | • | y | y | y | y | y | ||||
| Pena et al. 2016 | y | x | x | x | x | x | y | y | y | ||
| Zhang et al. 2019 | y | y | x | y | x | x | y | x | y | ||
Note: White cell indicates reported by study, gray cell indicates not reported by study; y significantly ‘poorer’ outcome in participants with type 1 diabetes; x no significant between group difference; − mixed results; • between group difference not reported; adata extracted from 2016 secondary article by same authors.
Abbreviations: ACR, albumin‐creatinine ratio; cIMT, carotid intima media thickness; CRP, c‐reactive protein.
3.5. Aortic intima media thickness
3.5.1. Methods of assessment; mean and max IMT
All nine studies included in this review reported mean aIMT. Four studies also reported maximum aIMT. 18 , 19 , 20 , 21 A linear array ultrasound probe was used in the assessment of aIMT in five studies, 15 , 18 , 20 , 22 , 23 a phased array probe in one study 17 and three studies 16 , 19 , 21 did not report the probe used. Reported ultrasound frequency used to assess aIMT ranged from 7.5 MHz in two studies 16 , 17 to 17 MHz in the study by Harrington et al.. 18 Eight studies reported assessing aIMT in the abdominal aorta 15 , 16 , 17 , 18 , 19 , 21 , 22 , 23 with three studies specifying assessment in the most distal 15 mm of the abdominal aorta, before bifurcation, 15 , 17 , 23 and four specifying assessment in the most distal 10 mm of the abdominal aorta. 18 , 19 , 21 , 22 One study did not report exact assessment location. 20 aIMT was calculated manually using calipers in four studies, 15 , 17 , 20 , 23 using edge detection software in four studies 18 , 19 , 21 , 22 and calculation method was not reported in the study by Ersoy et al.. 16
3.5.2. Mean aIMT in participants with type 1 diabetes versus healthy controls
Eight of nine studies included in this review reported mean aIMT to be significantly increased in participants with type 1 diabetes compared with healthy controls. 15 , 17 , 18 , 19 , 20 , 21 , 22 , 23 The study by Ersoy et al. was the only study to report no difference in aIMT between groups. 16
Meta‐analysis of nine studies comparing aIMT in 1030 participants with type 1 diabetes and 498 healthy controls demonstrated that the mean (95% CI) aIMT in participants with type 1 diabetes is 0.11 (0.04, 0.18) mm (p = 0.003, I2 98%) higher compared with healthy control participants (Figure 2). Funnel plots appeared symmetrical, and the N statistic estimated that 994 unpublished studies would be required for p > 0.05, and therefore, publication bias is unlikely.
FIGURE 2.
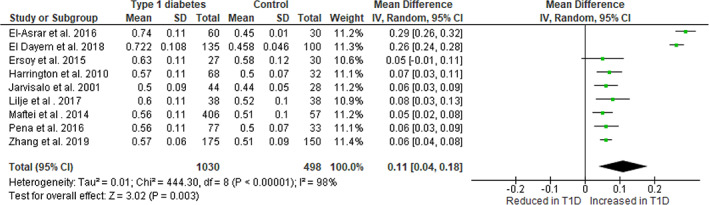
Meta‐analysis of aIMT in young people with type 1 diabetes versus healthy controls
3.6. Secondary analyses
3.6.1. cIMT
Eight of the nine studies included in this review also assessed cIMT. Of these eight studies, four 15 , 17 , 20 , 23 reported that participants with type 1 diabetes had significantly higher cIMT compared with healthy controls and four reported no difference between groups. 18 , 19 , 21 , 22 Meta‐analysis of the eight studies comparing cIMT in 764 participants with type 1 diabetes and 468 healthy controls demonstrated that the mean (95% CI) cIMT in youth with type 1 diabetes is 0.08 (0.02, 0.13) mm (p = 0.005, I2 98%) higher compared with healthy control participants (Figure 3). Funnel plots appeared symmetrical, and the N statistic estimated that 815 unpublished studies would be required for p > 0.05, and therefore, publication bias is unlikely. When calculating aIMT for these same eight studies the mean difference in aIMT was 0.12 (0.06, 0.18) mm in favor of higher aIMT in participants with type 1 diabetes.
FIGURE 3.
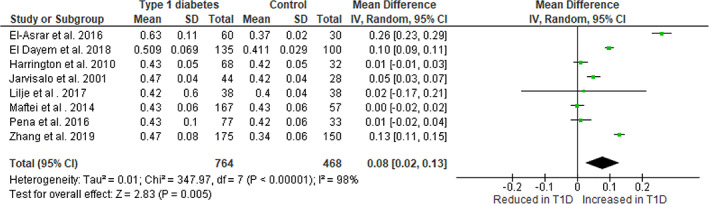
Meta‐analysis of cIMT in young people with type 1 diabetes versus healthy controls
3.6.2. Moderator analysis
Moderator analysis indicated that use of a phased array probe in one study, (El‐Asrar et al. 2016 17 ) was associated with a greater mean difference [95% CI] in aIMT in participants with type 1 diabetes versus healthy controls (0.29 [0.25, 0.33] mm higher in youth with type 1 diabetes), compared with five studies using a linear array probe (0.11 [0.04, 0.17] mm higher in youth with type 1 diabetes, p < 0.001) (Table 4). Use of calipers versus software to calculate aIMT did not influence findings, nor did frequency of ultrasound used (Table 4).
TABLE 4.
Moderator analysis of the effect of type 1 diabetes on aortic intima media thickness
| Variable | Comparison (number of studies) | Mean difference in aIMT between type 1 diabetes and controls (95% CIs); I2 | P value for difference between comparison groups |
|---|---|---|---|
| Probe |
Linear array (5) Phased array (1) |
0.11 (0.04, 0.17) mm; 95% 0.29 (0.25, 0.33) mm; 0% |
<0.001 |
| Ultrasound frequency |
≤10 MHz (4) >10 MHz (3) |
0.17 (0.06, 0.29) mm; 96% 0.06 (0.05, 0.08) mm; 0% |
0.064 |
| Calculation method |
Calipers (4) Software (4) |
0.17 (0.05, 0.29) mm; 98% 0.06 (0.04, 0.08) mm; 0% |
0.088 |
3.6.3. Meta‐regression
Meta‐regression found that a longer diabetes duration, higher insulin dose/kg/day, higher BMI, BMI z score and waist circumference, higher LDL cholesterol, higher triglycerides, and higher diastolic BP in participants with type 1 diabetes were associated with a greater difference in aIMT between participants with type 1 diabetes and healthy controls (Table 5). Meta‐regression found no association between aIMT and participant age or HbA1c, fasting glucose, systolic BP, total cholesterol or HDL cholesterol levels.
TABLE 5.
Meta‐regression analysis of the effect of type 1 diabetes on aortic intima media thickness
| Variable, Unit | Number of studies | Change in aIMT (95% CI) | p value |
|---|---|---|---|
| Age, years | 9 | 0.014 (−0.016, 0.044) | 0.358 |
| Diabetes duration, years | 8 | 0.039 (0.020, 0.058) | <0.001 |
| Insulin dose, IU/kg/day | 5 | 0.335 (0.171, 0.499) | <0.001 |
| BMI, kg/m2 | 5 | 0.039 (0.011, 0.067) | 0.006 |
| BMI, z score | 4 | 0.789 (0.432, 1.147) | <0.001 |
| Waist circumference, cm | 4 | 0.018 (0.009, 0.027) | <0.001 |
| HbA1c, % | 7 | −0.005 (−0.131, 0.122) | 0.942 |
| Fasting glucose, mg/dl | 5 | 0.001 (−0.043, 0.045) | 0.967 |
| Systolic BP, mmHg | 7 | 0.016 (−0.001, 0.032) | 0.063 |
| Diastolic BP, mmHg | 7 | 0.010 (0.000, 0.019) | 0.049 |
| Total cholesterol, mg/dl | 8 | 0.004 (−0.001, 0.010) | 0.129 |
| LDL cholesterol, mg/dl | 7 | 0.006 (0.003, 0.009) | <0.001 |
| HDL cholesterol, mg/dl | 8 | −0.007 (−0.018, 0.004) | 0.226 |
| Triglycerides, mg/dl | 7 | 0.004 (0.001, 0.008) | 0.018 |
Abbreviations: aIMT, aortic intima media thickness; NR, not reported.
4. DISCUSSION
This is the first systematic review to report higher aIMT in children and adolescents with type 1 diabetes compared with healthy control individuals. Longer duration of diabetes and established CVD risk factors (including lipids, DBP and BMI z score) were associated with increased disparity in aIMT between groups. Together, these findings demonstrate evidence of early and progressive CVD risk in youth with type 1 diabetes and provide a strong rationale for targeting modifiable risk factors in CVD prevention.
The pathological process of atherosclerosis begins with the thickening of the vascular lining of susceptible blood vessels, with regions of the aorta impacted earliest in disease progression. 3 , 23 These changes have been observed as early as in utero with thickening occurring in the abdominal aorta in response to early life exposures such as growth restriction. 24 Additional studies have reported seeing changes in aIMT in younger children, suggesting that aIMT may be a better marker of early CVD risk in youth, compared with cIMT. 3 The findings of this review suggest that aIMT should be used in assessing vascular health of young people with type 1 diabetes.
This review extends findings from a 2019 systematic review, which showed that other markers of subclinical atherosclerosis, including cIMT and FMD, are also abnormal in youth with type 1 diabetes compared with controls. 2 Although a secondary outcome in our review, we similarly identified that cIMT was significantly thicker in participants with type 1 diabetes compared with healthy controls. The 2019 review reported a standardized mean difference in cIMT between groups in 51 studies of 0.89 (95% CI: 0.69–1.09). When we calculated standardized mean difference in cIMT using the data from the eight studies reporting cIMT in our review, we found a similar value of 0.99 (95% CI: 0.30–1.68). However, we found the magnitude of difference to be greater for aIMT as compared with cIMT (0.12 mm thicker for aIMT versus 0.08 mm thicker cIMT in the same eight studies). Indeed, four of eight studies included in our meta‐analysis did not report a significant difference in cIMT in participants with type 1 diabetes compared with healthy controls, compared with only one of nine for aIMT. This supports the earlier pathogenesis of atherosclerosis in the aorta compared to the carotid artery, and suggests that aIMT may have utility by identifying individuals with type 1 diabetes at greatest early risk for CVD and who will benefit from early prevention strategies.
Moderator analysis and metaregression conducted as part of this systematic review identified several clinical variables associated with the observed difference in aIMT between participants with type 1 diabetes and controls. Longer duration of diabetes at time of aIMT assessment was associated with increased disparity between aIMT in healthy controls versus participants with type 1 diabetes. This supports the evidence demonstrating the progressive detrimental effect of type 1 diabetes on vascular health. Additionally, we identified that modifiable risk factors for the development of CVD were also associated with increased disparity between aIMT in type 1 diabetes versus controls. This included higher BMI z score, LDL cholesterol, triglycerides and diastolic BP. This confirms that targeting modifiable risk factors in children and adolescents with type 1 diabetes is important for preventing, or lowering the risk of, CVD.
There are several factors that should be considered in the use of aIMT as a measure of subclinical atherosclerosis in young people with type 1 diabetes. Firstly, increasing rates of obesity in the type 1 diabetes population, and in youth generally, may make measuring aIMT more difficult due to more interference associated with increased body tissue, particularly in older adolescents. Secondly, methods used to assess aIMT varied between included studies with some methods associated with increased disparities in aIMT between groups, emphasizing the need for adoption of standardized best‐practice methodologies for assessment of aIMT. 3 The use of biomarkers of vascular health may provide a meaningful addition to assessment of aIMT in studies of type 1 diabetes. In particular, studies included in this review and others suggest that angiopoietin‐2, osteopontin, copeptin and urinary albumin excretion may be viable markers of vascular health, and are associated with aIMT. 14 , 17 , 21 , 25 However, these were not evaluated in this review and more research is required to investigate the validity of these markers in youth with type 1 diabetes.
One study included in our review measured aIMT twice during puberty (at baseline and 2‐years later) finding no deterioration in aIMT over this time period. 19 This was not expected as puberty is a time where the presence of microvascular complications may accelerate, which could be anticipated to be mirrored by increased progression of subclinical atherosclerosis. Furthermore, this finding does not align with our findings from metaregression which indicated that longer diabetes duration was associated with higher aIMT in type 1 diabetes participants versus controls. Further research is needed to investigate how aIMT tracks in individuals with type 1 diabetes over various life stages. Longitudinal measures would also allow us to determine how specific treatments or interventions (including intensive insulin regimes and physical activity) may halt or reduce the thickening observed in the abdominal aorta of youth.
This analysis represents the most comprehensive study conducted to date comparing the aIMT of youth with type 1 diabetes versus healthy controls. Our metaregression analysis also enabled identification of several factors which were associated with increased aIMT in included studies. Despite these strengths, there are also some limitations to this study including that we only identified nine studies that represented data from six countries of youth with type 1 diabetes. Data from a further three studies could not be included in this review as authors did not provide the necessary data or information pertinent to their inclusion. The quality of included studies was mixed, suggesting that findings from this review should be interpreted with caution. Assessment of aIMT was variable across included studies and, while we examined this using moderator analysis, this factor does increase the heterogeneity of our meta‐analysis, which was high, and limits generalisability of our findings. We only included studies that assessed aIMT in young people with type 1 diabetes and healthy controls aged up to 20 years therefore findings cannot be extrapolated to the young adult population. Furthermore, due to availability of resources, only English language studies were included. Eight of nine studies included reported significantly higher aIMT in type 1 diabetes participants compared with controls, suggesting the possibility that null findings have not been published. However, our use of the N statistic indicated that the risk of publication bias in this review was low.
In conclusion, our study highlights that youth with type 1 diabetes have higher aIMT suggesting early vascular risk compared with healthy controls. This highlights the importance of optimizing treatment of youth with type 1 diabetes to prevent future CVD, and indicates the potential utility of aIMT to be used to identify those that may benefit from targeted CVD prevention strategies.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Megan L Gow, Benjamin J Varley and Maria E Craig contributed to conception and design of the work; Benjamin J Varley and Reeja F Nasir contributed to data acquisition; Megan L Gow performed data analysis; Megan L Gow, Maria E Craig and Michael R Skilton contributed to interpretation of results. Megan L Gow drafted the manuscript, all other authors revised it for intellectual content. All authors have read and approved the final version. Megan L Gow is the guarantor of this work.
ETHICS STATEMENT
This study is a systematic review, therefore ethics approval was not required. However, this systematic review has been prepared according to PRISMA guidelines.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
Megan L Gow is supported by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1158876), and Maria E Craig is supported by a NHMRC practitioner fellowship (APP1136735). Benjamin J Varley is funded by an Australian Government Research Training Program PhD scholarship. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Gow ML, Varley BJ, Nasir RF, Skilton MR, Craig ME. Aortic intima media thickness in children and adolescents with type 1 diabetes: A systematic review. Pediatr Diabetes. 2022;23(4):489‐498. doi: 10.1111/pedi.13322
Funding information NHMRC practitioner fellowship, Grant/Award Number: APP1136735; NHMRC Early Career Fellowship, Grant/Award Number: APP1158876
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Urbina EM, Williams RV, Alpert BS, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54(5):919‐950. doi: 10.1161/HYPERTENSIONAHA.109.192639 [DOI] [PubMed] [Google Scholar]
- 2. Wang P, Xu YY, Lv TT, et al. Subclinical atherosclerosis in patients with type 1 diabetes mellitus: a systematic review and meta‐analysis. Angiology. 2019;70(2):141‐159. doi: 10.1177/0003319718787366 [DOI] [PubMed] [Google Scholar]
- 3. Skilton MR, Celermajer DS, Cosmi E, et al. Natural history of atherosclerosis and abdominal aortic intima‐media thickness: rationale, evidence, and best practice for detection of atherosclerosis in the young. J Clin Med. 2019;8(8):1201. doi: 10.3390/jcm8081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rawshani A, Sattar N, Franzen S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register‐based cohort study. Lancet. 2018;392(10146):477‐486. doi: 10.1016/S0140-6736(18)31506-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Varley BJ, Craig ME, Gow ML. Identification of pre‐natal, pregnancy and childhood factors that influence measures of subclinical atherosclerosis, pulse wave velocity and aortic intima‐media thickness, in children and adolescents. Prospero. 2019;CRD42019137559. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=137559 [Google Scholar]
- 7. Bramer WM, de Jonge GB, Rethlefsen ML, Mast F, Kleijnen J. A systematic approach to searching: an efficient and complete method to develop literature searches. J Med Libr Assoc. 2018;106(4):531‐541. doi: 10.5195/jmla.2018.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moola S, Munn Z, Tufanaru C, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. https://synthesismanual.jbi.global; 2020. doi: 10.46658/JBIMES-20-01 [DOI] [Google Scholar]
- 10. Card NA. Applied Meta‐Analysis for Social Science Research. Guilford Press; 2012. [Google Scholar]
- 11. Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638‐641. doi: 10.1037/0033-2909.86.3.638 [DOI] [Google Scholar]
- 12. The Cochrane Collaboration . Review Manager (RevMan) [Computer program]. 2020; version 5.4.
- 13. Dayem SM, Battah AA, El Bohy AE. Assessment of increase in aortic and carotid intimal medial thickness in type 1 diabetic patients. Open Access Maced J Med Sci. 2016;4(4):630‐635. doi: 10.3889/oamjms.2016.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dayem SM, Battah AA, El Bohy AE, Yousef RN, Talaat A. Copeptin as a biomarker of atherosclerosis in type 1 diabetic patients. Open Access Macedonian J Med Sci. 2019;7(23):3975‐3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dayem SM, Battah AA, El Bohy AE, Habib SA. Determination of the best anthropometric measurements for early discovery of atherosclerosis in adolescent type 1 diabetic patients. Biosci Res. 2018;15(4):4087‐4096. [Google Scholar]
- 16. Ersoy M, Selcuk Duru HN, Elevli M, Ersoy O, Civilibal M. Aortic intima‐media thickness and mean platelet volume in children with type 1 diabetes mellitus. Iran J Pediatr. 2015;25(2):e368. doi: 10.5812/ijp.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El‐Asrar MA, Elbarbary NS, Ismail EA, Bakr AA. Circulating angiopoietin‐2 levels in children and adolescents with type 1 diabetes mellitus: relation to carotid and aortic intima‐media thickness. Angiogenesis. 2016;19(3):421‐431. doi: 10.1007/s10456-016-9517-6 [DOI] [PubMed] [Google Scholar]
- 18. Harrington J, Pena AS, Gent R, Hirte C, Couper J. Aortic intima media thickness is an early marker of atherosclerosis in children with type 1 diabetes mellitus. J Pediatr. 2010;156(2):237‐241. doi: 10.1016/j.jpeds.2009.08.036 [DOI] [PubMed] [Google Scholar]
- 19. Pena AS, Maftei O, Harrington J, et al. Lack of evidence for progression of atherosclerosis during puberty in type 1 diabetes. Pediatr Diabetes. 2016;17(3):199‐205. doi: 10.1111/pedi.12265 [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Zhang H, Li P. Cardiovascular risk factors in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2019;32(7):699‐705. doi: 10.1515/jpem-2018-0382 [DOI] [PubMed] [Google Scholar]
- 21. Maftei O, Pena AS, Sullivan T, et al. Early atherosclerosis relates to urinary albumin excretion and cardiovascular risk factors in adolescents with type 1 diabetes: adolescent type 1 diabetes cardio‐renal intervention trial (AdDIT). Diabetes Care. 2014;37(11):3069‐3075. doi: 10.2337/dc14-0700 [DOI] [PubMed] [Google Scholar]
- 22. Lilje C, Cronan JC, Schwartzenburg EJ, et al. Intima‐media thickness at different arterial segments in pediatric type 1 diabetes patients and its relationship with advanced glycation end products. Pediatr Diabetes. 2018;19(3):450‐456. doi: 10.1111/pedi.12557 [DOI] [PubMed] [Google Scholar]
- 23. Jarvisalo MJ, Jartti L, Nanto‐Salonen K, et al. Increased aortic intima‐media thickness: a marker of preclinical atherosclerosis in high‐risk children. Circulation. 2001;104(24):2943‐2947. doi: 10.1161/hc4901.100522 [DOI] [PubMed] [Google Scholar]
- 24. Lo Vasco VR, Salmaso R, Zanardo V, et al. Fetal aorta wall inflammation in ultrasound‐detected aortic intima/media thickness and growth retardation. J Reprod Immunol. 2011;91(1–2):103‐107. doi: 10.1016/j.jri.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 25. Abo El‐Asrar M, Ismail EAR, Thabet RA, Kamel AS, NehmedAllah S. Osteopontin as a marker of vasculopathy in pediatric patients with type 1 diabetes mellitus: relation to vascular structure. Pediatr Diabetes. 2018;19:1107‐1115. doi: 10.1111/pedi.12686 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


