Abstract
Background and Aim
Ulcerative colitis (UC) is usually detected by clinical symptoms, such as bleeding and diarrhea; however, it is rather difficult to assess during asymptomatic clinical remission (CR). Hence, there is a need for a biomarker that can reliably detect UC during remission. We previously reported on the utility of the prostaglandin E‐major urinary metabolite (PGE‐MUM) as a biomarker reflecting UC activity. In this study, we evaluated the effectiveness of the PGE‐MUM in the diagnosis of endoscopic, histological, and histo‐endoscopic mucosal remission of UC, comparing with fecal tests.
Methods
This prospective study was conducted at the Jikei University Hospital between August 2017 and January 2021. Patients with UC in CR scheduled to undergo colonoscopy were included. The association between the PGE‐MUM with endoscopic remission (ER), histological remission (HR), and complete mucosal healing (CMH, defined as histo‐endoscopic remission) was analyzed. We also compared the area under the curve (AUC) for the receiver operating characteristic curves between PGE‐MUM, fecal calprotectin (FC), and fecal immunochemical test (FIT).
Results
In total, 128 patients were analyzed. PGE‐MUM differed significantly in ER versus non‐ER (14.5 vs 16.7, P = 0.028), HR versus non‐HR (14.2 vs 17.4, P = 0.004), and CMH versus non‐CMH (14.3 vs 16.7, P = 0.021). There were no significant differences between the AUCs for PGE‐MUM, FC, and FIT for ER, HR, or CMH.
Conclusions
The PGE‐MUM can determine CMH in UC even during CR, regardless of the disease phenotype, indicating its clinical benefit for non‐invasive monitoring.
Keywords: fecal calprotectin, fecal immunochemical test, mucosal healing, prostaglandin E‐major urinary metabolite, ulcerative colitis
Introduction
Ulcerative colitis (UC) is the most common inflammatory bowel disease (IBD) with an incidence of 505 and 286 per 100 000 people in Europe and North America, respectively, 1 as well as in Asian countries in recent years. UC is an idiopathic disease characterized by erosions and ulcerations in the large intestine and an associated decline in the quality of life. Additionally, recurring inflammation in UC can lead to colon cancer. 2 , 3
The management of UC involves the initiation and maintenance of remission; remission is evaluated clinically, endoscopically, and histologically. Mucosal healing (MH), which is evaluated using endoscopy, is known as deep remission and is associated with less recurrence and a favorable prognosis. 4 In particular, patients with Mayo endoscopic subscore (MES) 1 had a higher risk of recurrence than those with MES 0. 5 Furthermore, it is imperative to achieve histological healing in addition to MH for the best UC prognosis. In a recent clinical trial, the achievement of histo‐endoscopic MH was defined as complete MH (CMH) as the secondary outcome. 6 In the sub‐analysis of that trial, the middle‐term prognosis of histo‐endoscopic MH was better than that of histological or endoscopic MH alone. 7
Therefore, patients with UC in clinical remission undergo regular colonoscopy, which is not well tolerated owing to the pain associated with the examination and the large amount of liquid preparation required. Clinical remission often does not reflect MH; hence, a biomarker that can precisely detect MH is warranted.
Fecal calprotectin (FC) has been widely proposed as a useful fecal marker for bowel inflammation, and fecal immunochemical test (FIT) has also been reported as a useful biomarker to determine MH. 8 However, FC measurement has certain disadvantages, including the absence of an absolute cut‐off value to diagnose remission, individual variation in remission line, and long turnaround time to obtain results (i.e. rapid test kits are not yet available in Japan). Furthermore, common to both fecal tests, patients must collect their feces and bring them to the hospital (i.e. self‐check kits are not yet available in Japan).
Prostaglandin E‐major urinary metabolite (PGE‐MUM) is a substance excreted in the urine as a metabolite of prostaglandin E2 (PGE2). It is increased in cases of inflammation at the deep site of the enteric mucosa. 9 , 10 , 11 , 12 Although the half‐life of PGE2 is too short to measure, 13 PGE‐MUM has high stability, and diurnal variation has not been observed. 14 Moreover, urine sample collection is noninvasive. PGE‐MUM levels are reportedly associated with the extent of colonic inflammation in UC, 15 demonstrating a strong correlation with the MES 16 and Matts' grading (Matts) 17 as the histological scores. PGE‐MUM could predict MH with MES ≤ 1. However, whether PGE‐MUM can predict MH in patients with UC in clinical remission has not been evaluated. If PGE‐MUM can detect MH or CMH in patients with UC in clinical remission, it can aid in reducing the use of colonoscopy for MH evaluation. Moreover, although PGE‐MUM may possess the same ability to diagnose MH in UC as other fecal biomarkers, to date, only one study exists on the comparison of PGE‐MUM with FIT, 18 and no studies have compared it with FC.
During clinical symptom manifestation, such as bloody stools and frequent diarrhea in patients with UC, biomarkers are not necessary because UC activity can be easily evaluated. A truly significant biomarker is one that can identify the presence of activity during clinical remission.
Therefore, this study focused solely on clinical remission and aimed to evaluate the effectiveness of PGE‐MUM in the determination of MH and CMH in patients with UC and compare PGE‐MUM with FC and FIT to determine MH and CMH.
Materials and methods
We conducted a prospective observational study at the Jikei University School of Medicine in Tokyo, Japan. Patients were included, regardless of their age or sex, if they were (i) diagnosed with UC more than 3 months ago, (ii) in clinical remission (Simple Clinical Colitis Activity Index ≤ 2), 19 and (iii) scheduled to undergo colonoscopy between August 2017 and January 2021. Patients with altered UC activity between the day of PGE‐MUM measurement and colonoscopy and those who received non‐steroidal anti‐inflammatory drugs (NSAIDs) on the day of the PGE‐MUM measurement were excluded. FC levels were measured and FIT was performed on the day of colonoscopy; PGE‐MUM was measured either the day before or the day following colonoscopy because the laxatives used in colonoscopy preparation could influence the PGE‐MUM levels. 20 Primary outcome measures included the association between the PGE‐MUM values and endoscopic remission (ER), histological remission (HR), and CMH as histo‐endoscopic remission in patients with UC in clinical remission, with the comparison of FC and FIT (primary analysis). The secondary outcome measure was the comparison of the diagnostic accuracy of PGE‐MUM with FC and FIT for determining ER, HR, and CMH (secondary analysis). Furthermore, the tertiary outcome measure was the comparison of the biomarkers' reliability (tertiary analysis).
Definition of the mucosal healing
In this study, ER was defined as an MES of 0. HR was defined as Matts grade ≤ 2, and CMH was defined as combined ER and HR.
Primary analysis
We analyzed the differences in the PGE‐MUM, FC, and FIT values between the two groups, which were classified as success or failure in achieving ER, HR, and CMH.
Secondary analysis
The accuracy of PGE‐MUM, FC, and FIT on achieving ER, HR, and CMH was compared using the area under the curve (AUC) of the receiver operating characteristic (ROC) curves. Additionally, the optimal cut‐off values for determining ER, HR, and CMH were evaluated.
Tertiary analysis
The differences in the median values based on the disease type (including pancolitis, left‐sided colitis, and proctitis) were compared for PGE‐MUM, FC, and FIT in all the patients and the ER, HR, and CMH groups.
Colonoscopy
All the colonoscopies were performed within 2 months of clinical remission diagnosis during medical examination. At least one biopsy specimen was obtained from the most inflamed site of the colon in all the patients except for those who had a reason for not having a biopsy sample taken (e.g. those who refused biopsy or used anticoagulants).
Three expert physicians (managed ≥ 500 UC cases) independently scored the endoscopic findings (MES) while blinded to the clinical information. The scores were determined following discussion among the three experts if the assigned scores differed for the individual patients. The highest score was used as the patient's MES. One expert pathologist who was blinded to the clinical information evaluated the Matts grade as the histological activity (Table S1) of UC. If more than two biopsy specimens were obtained, the highest score was used as the patient's Matts grade.
Measurement of fecal calprotectin and fecal immunochemical test
The first stool sample produced in the hospital following polyethylene glycol administration for colonoscopy preparation was collected. After defecating on a dedicated sheet on the stool seat, the patients collected a small piece of the stool sample in a container. Both calprotectin and FIT were measured in the stool samples. FC was measured with a fluorescence enzyme immunoassay (Elia Calprotectin [Thermo Fisher, Uppsala, Sweden]), and FIT was performed using OC‐Sensor PLEDIA (Eiken Chemical, Tokyo, Japan).
Prostaglandin E‐major urinary metabolite measurements
Prostaglandin E‐major urinary metabolite levels in urine samples were measured using a radioimmunoassay kit (Institute of Isotopes Co., Ltd, Budapest, Hungary). 9
Alkaline treatment was conducted by adding 100 μL of 1‐mol NaOH to 50 μL of the urine sample. Thereafter, the samples were stored at room temperature for 30 min. With this treatment, the PGE‐MUM in the sample was converted to bicyclic PGE‐MUM. After neutralization by adding 100 μL of 1‐mol hydrochloric acid, the treated urine sample was further diluted fivefold with 1000 μL of assay buffer (50‐mM phosphate buffer, pH 7.4, containing 0.1% gelatin and 0.1% sodium azide). A sample or standard (100 μL) was dispensed into a reaction tube, and 100 μL of 125 I‐bicyclic PGE‐MUM (approximately 680 Becquerel) and 100 μL of rabbit antiserum to bicyclic PGE‐MUM were added. After overnight incubation at 2–8°C, 250 μL of the separating agent containing paramagnetic particles coated with antirabbit immunoglobulin was added to each tube and was incubated for 15 min at room temperature. The bound fraction was separated by centrifugation, and the radioactivity of each tube was measured.
The PGE‐MUM concentrations were normalized to the creatinine concentration and expressed as μg/g·Cr.
Statistical analysis
For the primary analysis, Wilcoxon rank‐sum tests were used to compare the differences in PGE‐MUM, FC, and FIT values based on the success or failure of the achievement of ER, HR, and CMH. A common logarithm was used to analyze the FC values. For the secondary analysis, the AUCs of the ROC curves for PGE‐MUM, FC, and FIT were calculated to determine the achievement of ER, HR, and CMH. ROC analysis was used to compare the AUCs of the three markers. An optimum cut‐off was obtained by searching for the value with the maximum Youden index (sensitivity + specificity − 1). Kruskal–Wallis tests were used to compare the differences in the median values based on the PGE‐MUM disease type, and FC and FIT in the tertiary analysis. Statistical significance was set at P < 0.05, and the main results are expressed as means ± standard deviations or medians and interquartile ranges. All statistical analyses were performed using the STATA ver. 15.0 (Stata Corp, College Station, TX, USA).
Ethical considerations
This study was approved by the Jikei Hospital Ethics Committee (No. 8451) and was conducted in accordance with the tenets of the Declaration of Helsinki. All the participants provided written informed consent before participating in the study.
Results
A total of 143 patients were enrolled in this study. Although all the patients were examined using colonoscopy, 15 were excluded owing to unsuitable samples (n = 10 NSAIDs used on the day of PGE‐MUM measurement; n = 5 clearly altered UC activity). Of the remaining 128 patients (Fig. S1), urine samples were obtained from 114. The median period between the day of obtaining the urine sample and colonoscopy was 15 days. Although stool samples were obtained from 113 patients, three samples could not be measured. Biopsy specimens were obtained from 121 patients. The clinical characteristics of the patients are presented in Table S2.
Primary analysis
The median PGE‐MUM, FC, and FIT values were significantly different between the groups with: ER versus non‐ER were 14.5 (10.9–19.6) μg/g·Cr versus 16.7 (12.8–25.4) μg/g·Cr (P = 0.028) for PGE‐MUM, 21.7 (8.0–98.2) mg/kg versus 70.7 (13.6–336) mg/kg (P = 0.02) for FC, and 0 (0–2) ng/mL versus 2 (0–50) ng/mL (P = 0.002) for FIT (Fig. 1); HR versus non‐HR were 14.2 (9.9–19.6) μg/g·Cr versus 17.4 (13.5–28.8) μg/g·Cr (P = 0.004) for PGE‐MUM, 17.9 (5.6–57.9) mg/kg versus 121 (23.5–575) mg/kg (P < 0.001) for FC, and 0 (0–0) ng/mL versus 6 (0–94) ng/mL (P < 0.001) for FIT (Fig. 2); and CMH versus non‐CMH were 14.3 (9.9–17.6) μg/g·Cr versus 16.7 (12.8–25.4) μg/g·Cr (P = 0.021) for PGE‐MUM, 20.0 (5.6–57.9) mg/kg versus 68.3 (13.6–347) mg/kg (P = 0.003) for FC, and 0 (0–0) ng/mL versus 3 (0–0) ng/mL (P < 0.001) for FIT (Fig. 3).
Figure 1.
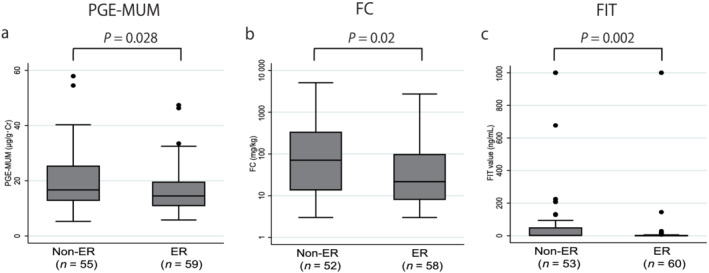
Comparison of the prostaglandin E‐major urinary metabolite (PGE‐MUM), fecal calprotectin (FC), and fecal immunochemical test (FIT) values between the endoscopic remission (ER) and non‐ER groups. The PGE‐MUM, FC, and FIT values were significantly lower in the ER group than those in the non‐ER group. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
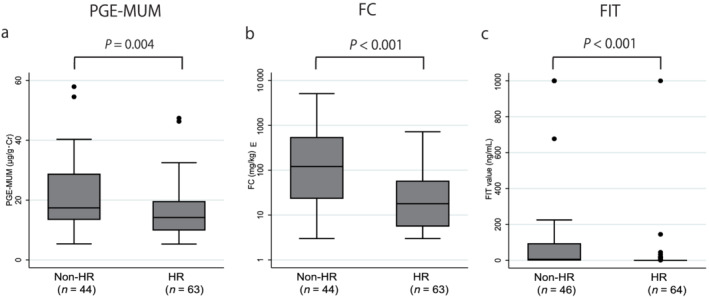
Comparison of the prostaglandin E‐major urinary metabolite (PGE‐MUM), fecal calprotectin (FC), and fecal immunochemical test (FIT) values between the histological remission (HR) and non‐HR groups. The PGE‐MUM, FC, and FIT values were significantly lower in the HR group than those in the non‐HR group. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.
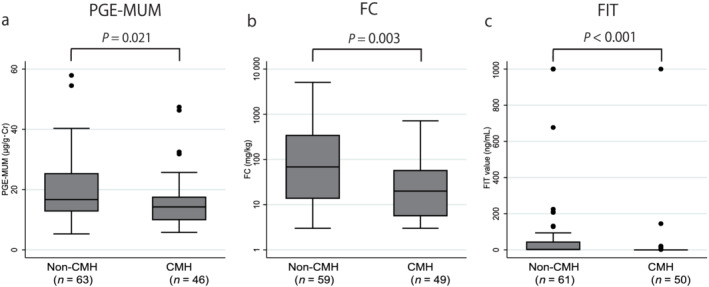
Comparison of the prostaglandin E‐major urinary metabolite (PGE‐MUM), fecal calprotectin (FC), and fecal immunochemical test (FIT) values between the complete mucosal healing (CMH) and non‐CMH groups. The PGE‐MUM, FC, and FIT values were significantly lower in the CMH group than those in the non‐CMH group. [Color figure can be viewed at wileyonlinelibrary.com]
Secondary analysis
The AUCs for the ROC curves of the PGE‐MUM, FC, and FIT for determining ER were 0.619 (95% confidence interval [CI] [0.515–0.723]), 0.629 (95% CI [0.524–0.738]), and 0.654 (95% CI [0.563–0.744]), respectively (P = 0.636) (Fig. 4). The AUCs of the ROC curves for PGE‐MUM, FC, and FIT to determine HR were 0.665 (95% CI [0.560–0.770]), 0.735 (95% CI [0.639–0.831]), and 0.726 (95% CI [0.636–0.815]), respectively (P = 0.818) (Fig. 5). The AUCs of the ROC curves for the PGE‐MUM, FC, and FIT to determine CMH were 0.630 (95% CI [0.523–0.737]), 0.668 (95% CI [0.566–0.769]), and 0.681 (95% CI [0.594–0.767]), respectively (P = 0.782) (Fig. 6). The optimal cut‐off value, sensitivity, specificity, positive predictive value, negative predictive value, and accuracy are shown in each figure.
Figure 4.
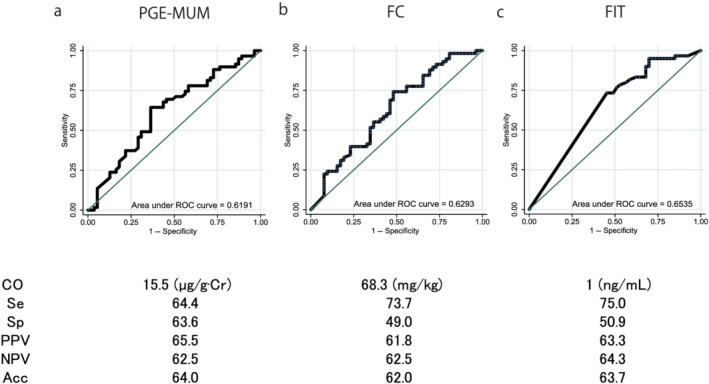
Comparison of the area under the curve of the receiver operating characteristic (ROC) curves, the optimal cut‐off (CO) values, the sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV), and the accuracy (Acc) of the three biomarkers for the determination of endoscopic remission: (a) prostaglandin E‐major urinary metabolite (PGE‐MUM), (b) fecal calprotectin (FC), and (c) fecal immunochemical test (FIT) values. The area under the curve of the ROC curves did not differ based on the type of exam conducted. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 5.
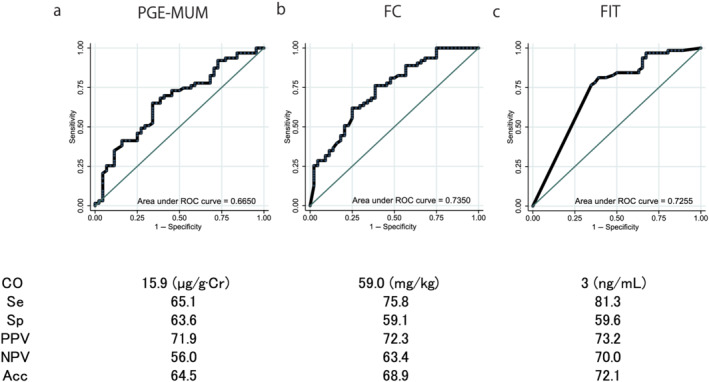
Comparison of the area under the curve of the receiver operating characteristic (ROC) curves, the optimal cut‐off (CO) values, the sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), and the accuracy (Acc) of the three biomarkers for the determination of histological remission: (a) prostaglandin E‐major urinary metabolite (PGE‐MUM), (b) fecal calprotectin (FC), and (c) fecal immunochemical test (FIT) values. The area under the curve of the ROC curves did not differ based on the type of exam conducted. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 6.
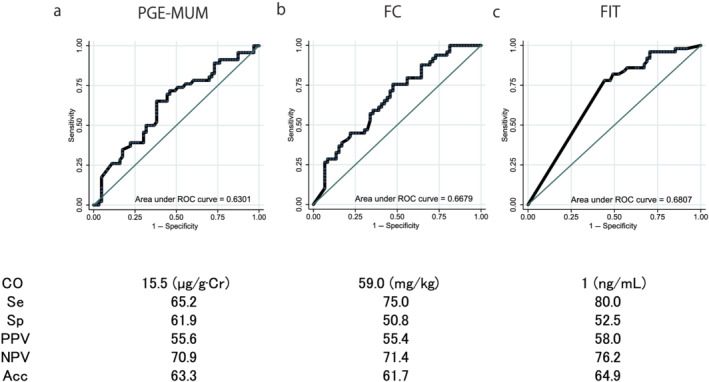
Comparison of the area under the curve of the receiver operating characteristic (ROC) curves, the optimal cut‐off (CO) values, the sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), and the accuracy (Acc) of the three biomarkers for the determination of complete mucosal healing: (a) prostaglandin E‐major urinary metabolite (PGE‐MUM), (b) fecal calprotectin (FC), and (c) fecal immunochemical test (FIT) values. The area under the curve of the ROC curves did not differ based on the type of exam conducted. [Color figure can be viewed at wileyonlinelibrary.com]
Tertiary analysis
The median value for PGE‐MUM did not significantly differ based on the disease type, whereas the FC value increased with the disease extent among all patients. FC significantly differed according to the disease type (Table 1). Similar results were obtained in the patients with ER, HR, and CMH.
Table 1.
Comparison of the median values from three exams based on the disease type: (a) all patients, (b) patients with endoscopic remission, (c) histological remission, and (d) complete mucosal healing
| Pancolitis | Left‐sided | Proctitis | P‐value † | |
|---|---|---|---|---|
| (a) All patients | n = 70 | n = 27 | n = 17 | |
| PGE‐MUM (μg/g·Cr) | 15.7 | 15.0 (26) | 15.1 | 0.911 |
| FC (mg/kg) | 54.3 (67) | 29.9 | 8.7 (15) | 0.002 |
| FIT (ng/mL) | 0 (68) | 0 | 0 (16) | 0.079 |
| (b) ER | n = 35 | n = 11 | n = 13 | |
| PGE‐MUM (μg/g·Cr) | 13.7 | 14.8 | 14.5 (12) | 0.738 |
| FC (mg/kg) | 37.5 (34) | 20.0 | 8.4 (12) | 0.014 |
| FIT (ng/mL) | 0 | 0 | 0 | 0.406 |
| (c) HR | n = 34 | n = 16 | n = 15 | |
| PGE‐MUM (μg/g·Cr) | 14.2 | 13.1 (14) | 15.1 | 0.728 |
| FC (mg/kg) | 34.1 | 16.9 | 8.0 (13) | 0.038 |
| FIT (ng/mL) | 0 | 0 | 0 (14) | 0.821 |
| (d) CMH | n = 28 | n = 9 | n = 13 | |
| PGE‐MUM (μg/g·Cr) | 13.9 (27) | 14.8 (7) | 14.5 (12) | 0.814 |
| FC (mg/kg) | 37.5 | 20.0 | 8.4 (12) | 0.021 |
| FIT (ng/mL) | 0 | 0 | 0 | 0.793 |
Kruskal–Wallis test.
The number of analyzed samples is shown in parentheses if it differs from the total number of each disease type in (a)–(d). Very few patients with right‐sided colitis were excluded from this analysis.
CMH, complete mucosal healing; ER, endoscopic remission; FC, fecal calprotectin; FIT, fecal immunochemical test; HR, histological remission; PGE‐MUM, prostaglandin E‐major urinary metabolite.
Discussion
Our study indicated that PGE‐MUM could diagnose ER and HR and could diagnose the simultaneous achievement of both histological and endoscopic MH in patients with UC in clinical remission, on par with fecal biomarkers. The diagnostic accuracy of PGE‐MUM, FC, and FIT was equivalent, indicating that PGE‐MUM was non‐inferior to conventional fecal biomarkers. Furthermore, all three examinations were useful in determining CMH, which indicated ER and HR.
Although we previously reported that PGE‐MUM was strongly correlated with UC activity beyond C‐reactive protein 15 and that PGE‐MUM had equivalent diagnostic ability to determine the mucosal condition of patients with UC, 18 these studies included many patients in the active phase. Therefore, the focus on patients with UC in clinical remission in this study is novel and meaningful. It is known that approximately 30% of the patients with ER have histological inflammation 21 ; thus, biopsy specimens are needed to evaluate the mucosal conditions in addition to regular colonoscopy. In fact, in this study, approximately 15% of the patients with ER did not achieve CMH. Hence, a reliable biomarker that can determine CMH is valuable for routine medical care and has the advantage of avoiding unnecessary colonoscopy and histological evaluation. The present study results do not eliminate the requirement for colorectal cancer surveillance with colonoscopy in patients with longstanding UC in clinical remission 22 ; however, they indicate that the mucosal conditions could be evaluated using PGE‐MUM instead of regular colonoscopy. Urine sample collection may be the least invasive method to evaluate MH; thus, our results could be beneficial for patients with UC.
This study is also valuable in comparing the three biomarkers for diagnosing ER, HR, and CMH. Although all three biomarkers were equivalent, fecal biomarkers had some disadvantages. FC is highly useful in determining MH; however, stool sample collection is a psychological burden for patients, especially those living in large and crowded cities. Further, if the FIT value is 0, it strongly indicates MH; however, a positive FIT value could be due to the presence of occult colonic bleeding, such as that attributed to hemorrhoids, diverticulosis, and colonic polyps. In fact, 6.7% of the patients with UC have hemorrhoids as a concomitant disease. 23 In addition, the optimal cut‐off value (1 or 3 ng/mL) diverged from the original cut‐off value (100 ng/mL). Therefore, FIT is not highly reliable despite the high AUC of the ROC, and its usefulness is limited.
In contrast, the major advantage of PGE‐MUM is that it is not affected by bleeding disorders and its convenient access for sample collection. Additionally, the PGE‐MUM assay reagent for fully automated chemiluminescent enzyme immunoassay (CLEIA) system 24 has recently become available. Although it took time to obtain the urine sample results, because PGE‐MUM was measured using radioimmunoassay (RIA), the CLEIA method enabled results to be obtained within a short period of time (less than 1 h but within a minimum of 40 min). Moreover, PGE‐MUM is highly stable 14 and has no diurnal variation; thus, urine samples obtained several days earlier would be acceptable for measurement. 24 Additionally, the PGE‐MUM value did not fluctuate according to the disease type, in contrast with FC, despite all the cases being in the remission phase; this also demonstrates the reliability of PGE‐MUM as a biomarker.
In this study, the optimal cut‐off PGE‐MUM value was 15.5 μg/g·Cr for determining ER, 15.9 μg/g·Cr for HR, and 15.5 μg/g·Cr for CMH. In a previous report, the cut‐off values for MES ≤ 1 and HR were 21.0 and 17.0 μg/g·Cr, respectively. 15 15.5 μg/g·Cr may be the optimal cut‐off value for diagnosing CMH; however, studies with larger sample sizes are warranted to validate this finding.
In this study, all the AUCs for the ROC curves of the PGE‐MUM, FC, and FIT for determining ER and HR were slightly lower compared with the results of previous studies, including our own, in which the AUCs for the ROC curves of PGE‐MUM, FC, and FIT for determining MH were reported as 0.90, 15 0.80, 25 0.94, 26 0.88, 25 and 0.96, 26 respectively. However, in the present study, all the three biomarkers showed 0.61–0.65 for ER, 0.67–0.74 for HR, and 0.63–0.68 for CMH. This could be because we focused solely on the patients with UC in clinical remission and did not include patients with clinical activity. Our study, nonetheless, demonstrated the usefulness of all the three biomarkers, especially PGE‐MUM, because the AUCs were high enough even though the study only included patients with clinical remission.
There are some challenges to consider in future studies of PGE‐MUM. In theory, the factors that affect PGE‐MUM are the use of purgatives 20 and NSAIDs. However, it remains unclear whether other factors, such as TNF‐α antibodies and steroids, can impact PGE‐MUM. Additionally, the association of PGE‐MUM with Crohn's disease and other inflammatory diseases remains unclear; only associations with interstitial pneumonia 27 and chronic enteropathy associated with SLCO2AI gene (CEAS) 28 have been reported. Furthermore, our results do not indicate that surveillance colonoscopy is unnecessary, as colon cancer screening is still required. It is also necessary to clarify the association between the PGE‐MUM values and UC prognosis, and the risk of developing colon cancer. Despite these limitations, PGE‐MUM is a useful marker owing to its accessibility and high accuracy.
There are additional limitations pertaining to this study; first, the moderate sample size as the study was conducted at a single facility. Second, the evaluation of the endoscopic and histological findings are subject to human errors. Finally, the physical and environmental factors affecting PGE‐MUM have not been fully examined.
In conclusion, PGE‐MUM could be used as a potential biomarker to determine ER, HR, and CMH. PGE‐MUM is independent of the disease phenotype, such as inflammation severity and extent. The non‐invasive assessment of PGE‐MUM makes it comparable with FC and FIT in the diagnosis of ER and HR in UC even during the remission phase.
Supporting information
Figure S1. Flow diagram of the included patients.
Fifteen patients were excluded, and the remaining 128 patients were analyzed.
Abbreviations: UC, ulcerative colitis; NSAIDs, non‐steroidal anti‐inflammatory drugs; PGE‐MUM, prostaglandin E‐major urinary metabolite.
Table S1. Matts grading for histological evaluation.
Table S2. The characteristics of patients eligible for analysis.
Acknowledgments
The authors are grateful to Umeda K and Ouki T of Fujirebio Inc. for helping with the PGE‐MUM measurements. We would like to thank Editage (www.editage.com) for English language editing.
Sakurai, T. , Akita, Y. , Miyashita, H. , Miyazaki, R. , Maruyama, Y. , Saito, T. , Shimada, M. , Yamasaki, T. , Arhihiro, S. , Kato, T. , Matsuura, T. , Ikegami, M. , Okayasu, I. , and Saruta, M. (2022) Prostaglandin E‐major urinary metabolite diagnoses mucosal healing in patients with ulcerative colitis in remission phase. Journal of Gastroenterology and Hepatology, 37: 847–854. 10.1111/jgh.15782.
Declaration of conflict of interest: The authors declare that they have no competing interests in this study or its publication. TS, YA, HM, RM, YM, TS, MS, TY, SA, TK, TM, and MI: none. MS: scholarship/research grants from EA Pharma Co., Ltd., Zeria Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., and Mochida Pharmaceutical Co., Ltd.; honoraria (lecture fee) from AbbVie GK, Mitsubishi Tanabe Pharma, Janssen Pharma K.K., and Takeda Pharmaceutical Co., Ltd.
Financial support: No funding was received for this study. The present work was undertaken as part of the routine work of an organization.
References
- 1. Ng SC, Shi HY, Hamidi N et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population‐based studies. Lancet 2017; 390: 2769–2778. [DOI] [PubMed] [Google Scholar]
- 2. Danese S, Fiocchi C. Ulcerative colitis. N. Engl. J. Med. 2011; 365: 1713–1725. [DOI] [PubMed] [Google Scholar]
- 3. Farrell RJ, Peppercorn MA. Ulcerative colitis. Lancet 2002; 359: 331–340. [DOI] [PubMed] [Google Scholar]
- 4. Arai M, Naganuma M, Sugimoto S et al. The ulcerative colitis endoscopic index of severity is useful to predict medium‐to long‐term prognosis in ulcerative colitis patients with clinical remission. J. Crohns Colitis 2016; 10: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 5. Barreiro‐de Acosta M, Vallejo N, de la Igresia D et al. Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing (Mayo 0 vs 1): a longitudinal cohort study. J. Crohns Colitis 2016; 10 (1):13–19. [DOI] [PubMed] [Google Scholar]
- 6. Sands BE, Sandborn WJ, Panaccione R et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2019; 381: 1201–1214. [DOI] [PubMed] [Google Scholar]
- 7. Li K, Marano C, Zhang H et al. Relationship between combined histologic and endoscopic endpoints and efficacy of ustekinumab treatment in patients with ulcerative colitis. Gastroenterology 2020; 159: 2052–2064. [DOI] [PubMed] [Google Scholar]
- 8. Nakarai A, Kato J, Hiraoka S et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am. J. Gastroenterol. 2013; 108: 83–89. [DOI] [PubMed] [Google Scholar]
- 9. Okayasu I, Ohnishi H, Sarandi I et al. Significant increase of prostaglandin E‐major urinary metabolite in male smokers: a screening study of age and gender differences using a simple radioimmunoassay. J. Clin. Lab. Anal. 2014; 28: 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raab Y, Sundberg C, Hällgren R, Knutson L, Gerdin B. Mucosal synthesis and release of prostaglandin E2 from activated eosinophils and macrophages in ulcerative colitis. Am. J. Gastroenterol. 1995; 90: 614–620. [PubMed] [Google Scholar]
- 11. Nataraj C, Thomas DW, Tilley SL et al. Receptors for prostaglandin E2 that regulate cellular immune responses in the mouse. J. Clin. Invest. 2001; 108: 1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chinen T, Komai K, Muto G et al. Prostaglandin E2 and SOCS1 have a role in intestinal immune tolerance. Nat. Commun. 2011; 2: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J. Clin. Immunol. 1983; 3: 295–315. [DOI] [PubMed] [Google Scholar]
- 14. Inagawa T, Imaki K, Masuda H, Morikawa Y, Hirata F, Tsuboshima M. Simplified immunoassays of prostaglandin E main metabolite in human urine. Adv. Prostaglandin Thromboxane Leukot. Res. 1983; 11: 191–196. [PubMed] [Google Scholar]
- 15. Arai Y, Arihiro S, Matsuura T et al. Prostaglandin E‐major urinary metabolite as a reliable surrogate marker for mucosal inflammation in ulcerative colitis. Inflamm. Bowel Dis. 2014; 20: 1208–1216. [DOI] [PubMed] [Google Scholar]
- 16. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987; 317: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 17. Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q. J. Med. 1961; 30: 393–407. [PubMed] [Google Scholar]
- 18. Ishida N, Miyazu T, Takano R et al. Prostaglandin E‐major urinary metabolite versus fecal immunochemical occult blood test as a biomarker for patient with ulcerative colitis. BMC Gastroenterol. 2020; 20: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut 1998; 43: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujiwara M, Okayasu I, Oritsu M et al. Significant increase in prostaglandin E‐main urinary metabolite by laxative administration: comparison with ulcerative colitis. Digestion 2000; 61: 201–206. [DOI] [PubMed] [Google Scholar]
- 21. Park S, Abdi T, Gentry M, Laine L. Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: systematic review and meta‐analysis. Am. J. Gastroenterol. 2016; 111: 1692–1701. [DOI] [PubMed] [Google Scholar]
- 22. Rutter MD, Saunders BP, Wilkinson KH et al. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut 2004; 53: 1813–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi YS, Kim DS, Lee DH et al. Clinical characteristics and incidence of perianal diseases in patients with ulcerative colitis. Ann Coloproctol 2018; 34: 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katagiri N, Wakabayashi M, Arihiro S et al. Development of fully automated chemiluminescent enzyme immunoassay system for measurement of prostaglandin E major urinary metabolites (PGE‐MUM). Jpn J Clin Lab Autom 2017; 42: 584–590. [Google Scholar]
- 25. Ma C, Lumb R, Walker EV et al. Noninvasive fecal immunochemical testing and fecal calprotectin predict mucosal healing in inflammatory bowel disease: a prospective cohort study. Inflamm. Bowel Dis. 2017; 23: 1643–1649. [DOI] [PubMed] [Google Scholar]
- 26. Kim DJ, Jeoun YM, Lee DW, Koo JS, Lee SW. Usefulness of fecal immunochemical test and fecal calprotectin for detection of active ulcerative colitis. Intest Res 2018; 16: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Horikiri T, Hara H, Saito N et al. Increased levels of prostaglandin E‐major urinary metabolite (PGE‐MUM) in chronic fibrosing interstitial pneumonia. Respir. Med. 2017; 122: 43–50. [DOI] [PubMed] [Google Scholar]
- 28. Matsuno Y, Umeno J, Esaki M et al. Measurement of prostaglandin metabolites is useful in diagnosis of small bowel ulcerations. World J. Gastroenterol. 2019; 25: 1753–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow diagram of the included patients.
Fifteen patients were excluded, and the remaining 128 patients were analyzed.
Abbreviations: UC, ulcerative colitis; NSAIDs, non‐steroidal anti‐inflammatory drugs; PGE‐MUM, prostaglandin E‐major urinary metabolite.
Table S1. Matts grading for histological evaluation.
Table S2. The characteristics of patients eligible for analysis.


