Abstract
Aims
Management of kaposiform haemangioendotheliomas (KHE) with Kasabach–Merritt phenomenon is challenging in young infants who are subjected to developmental pharmacokinetic changes. Sirolimus, sometimes combined with corticosteroids, can be used as an effective treatment of KHE. Simultaneously, toxicities such as interstitial pneumonitis related to the use of sirolimus may be fatal. As infants have a very low CYP3‐enzyme expression at birth, which rises during ageing, we hypothesize that a reduced metabolization of sirolimus might lead to high sirolimus serum levels and low dose may be sufficient without the side effects.
Methods
A case series of 5 infants with kaposiform haemangioendothelioma with Kasabach–Merritt phenomenon was analysed retrospectively. All infants were treated with sirolimus 0.2 mg/m2 every 24 or 48 hours according to their age. Prednisone was added to the therapy for additional effect in 4 patients.
Results
In all patients, low dose of sirolimus led to therapeutic sirolimus levels (4–6 ng/mL). All infants (aged 4 days–7 months) had a complete haematological response, without serious adverse events. In all patients, the Kasabach–Merritt phenomenon resolved, the coagulation profile normalized and tumour size reduction was seen.
Conclusion
Low‐dose sirolimus treatment is safe for infants with kaposiform haemangioendothelioma and Kasabach–Merritt phenomenon. It is essential to realize that during the first months of life, metabolism is still developing and enzymes necessary to metabolise drugs like sirolimus still have to mature. To avoid toxic levels, the sirolimus dosage should be based on age and the associated pharmacological developments.
Keywords: kaposiform haemangioendothelioma, Kasabach–Merritt phenomenon, sirolimus, vascular tumour
What is already known about this subject
Kaposiform haemangioendothelioma (KHE) can lead to the life‐threatening Kasabach–Merritt phenomenon.
Sirolimus can be used to treat KHE, although serious toxicities have been described in (very) young infants.
The CYP3A4 enzyme is nearly absent in neonates and matures in the first months of life, reaching adult levels at 1 year.
What this study adds
Sirolimus treatment of infants with life‐threatening KHE using dosing and titration based on the hypothetical maturation of liver enzymes can be safe and effective.
Pharmacological insight in treatment of KHE in neonates using sirolimus leading to a safer administration without hampering efficacy of treatment.
1. INTRODUCTION
Kaposiform haemangioendothelioma (KHE) is a rare locally aggressive vascular tumour that acts as an infiltrative lesion, invading the skin, subcutaneous fat mass, and muscle planes. KHE occurs with Kasabach–Merritt phenomenon (KMP) in 42–71% of cases. 1 , 2 , 3 , 4 The KMP is characterized by a coagulopathy with profound thrombocytopenia, hypofibrinogaenemia and elevation of D‐dimers, which can be life‐threatening. A form of disseminated intravascular coagulation (DIC) can be seen in the patients having both the propensity of clotting and the high risk of bleeding. The morbidity and mortality rates are up to 37% in patients with KHE due to the KMP which may have an acute onset. 1 , 3 , 5 , 6 , 7 Knowledge about this clinical condition is often insufficient, due the fact that the prevalence of KHE is very rare—approximately 0.91/100 000 children. There is not much known about the aetiology of KHE. Regarding genetics, it has been described in 1 patient that there was a somatic balanced translocation between chromosomes 13 and 16. 8 Furthermore, in other patients, activating mutations have been found in GNAQ and its paralogues (such as GNA11 and GNA14). 9 , 10 , 11
Immediate recognition of KHE including the KMP is of utmost importance and can be lifesaving. 1 Treatment options include steroids, radiation therapy, vincristine, interferon, propranolol and other chemotherapeutic agents such as cyclophosphamide. In the past decade, more and more cases of KHE with KMP have been treated with the mechanistic target of rapamycin kinase inhibitor, sirolimus. Whereas steroids, vincristine and other chemotherapeutic agents were often toxic and not always successful, sirolimus seems to have a substantial better outcome. 12 , 13 , 14 , 15 , 16 , 17 , 18 Still, toxicity directly related to high plasma levels of sirolimus can lead to severe complications and even fatal outcomes as has been described previously. 12 , 19
A systematic review of Freixo et al. confirmed the effectiveness of sirolimus in the treatment of KHE with KMP. 20 In 95.5% of the 121 patients the KHE clinically improved and in 93% the coagulopathy normalized. Most frequently used target levels of sirolimus were 10–15 ng/mL (38.4%), 15–20 ng/mL (24.7%) and 5–15 ng/mL (15.1%). Side effects in patients with vascular anomalies included oral mucositis (21.9%), dyslipidaemia (16.5%), leukopenia (12.3%), gastrointestinal symptoms (10.2%) and rash/eczema (8.2%). In 5.5% of patients with oral sirolimus treatment, complications of infections were reported, such as fatal pneumoniae in 2 children aged 1 and 6 months with KHE. 19 It may be possible that 1 of these patients suffered from this sirolimus‐associated interstitial pneumonitis, as signs of infections were lacking and antibiotic therapy failed. It is well known that sirolimus‐associated interstitial pneumonitis is a rare serious and sometimes even fatal complication. 21 , 22 , 23 , 24 , 25
As in most drugs observed, there might be a relation between levels of sirolimus and the occurrence of (severe) adverse events (AEs). 26 For example, there is a correlation of the occurrence of thrombocytopenia, leukopenia and hypertriglyceridaemia and sirolimus levels >15 ng/mL. 27 No toxic values were seen when the steady state concentration was <10 ng/mL. This is also seen in our case series of patients with low flow vascular malformations showing that low‐dose sirolimus led to less (serious) AEs compared to intermediate‐high dose sirolimus. 28
In some case series of KHE patients described in the literature, a dose of 0.8 mg/m2 twice daily was prescribed, based on standard dosing for off label treatment of vascular anomalies (such as vascular malformation). This dose regimen led to supratherapeutic levels of >30 ng/mL in patients with KHE, which in turn led to serious AEs or even were fatal. 29 This may be a result of the slow clearance of sirolimus secondary to the maturation of the CYP3A pathway in neonates with an almost absent expression of this enzymes after birth and slowly increases during the first year of life. 30
For this reason, neonates seem to require a lower dose of sirolimus to maintain a serum level that is therapeutic effective without supratherapeutic or even toxic levels. 31
Our case series represents a retrospective evaluation of 5 patients with KHE and KMP who were treated with low‐dose sirolimus and prednisone for additional effect. Dosing of sirolimus was based on the hypothetical assumption of the enzymatic maturation of CYP3A4 in time that is needed for the metabolization of sirolimus. Developmental changes of enzymes in infants during first year of life have been described previously. 30 Uniform guidelines how to dose sirolimus in infants with KHE are lacking; however, based on the cases reported here, we postulate a treatment regimen based on the age of the infants that may be useful in clinical practice.
2. METHODS
A retrospective case review was performed with review of the medical report of patients with kaposiform haemangioendothelioma complicated with KMP, treated with low‐dose sirolimus and corticosteroids between January 2019 and June 2021. Informed consent was obtained from all patients' parents reviewing of their child's medical report. The treatment efficacy of sirolimus was rated by the coagulation profile, platelet count, and relevant reduction (clinical or magnetic resonance imaging [MRI]) of the size of the KHE after follow‐up >3 months. A complete response was defined as a platelet count >100 × 109/L, a normalization of the coagulation profile, and a size reduction more than 80% was seen. A partial response was defined as a platelet count of 40–100 × 109/L, an abnormal/normal coagulation profile, and size reduction between 30 and 80%. There was no response if the platelet count was <40 × 109/L, the coagulation profile was abnormal, and size reduction was reduced <30% or increased (adjusted criteria based on Wang et al. 12 ). Adverse events were assessed according to the Common Terminology Criteria for Adverse Events version 4.0.
2.1. Treatment regime
The used local protocol was based on the estimated expression of CYP3A4 in the liver depending on the age of the infant and closely therapeutic drug monitoring (TDM). 30 , 32 This protocol is described below. We used low target trough levels of sirolimus under 6 ng/mL, as even with a very low dose of sirolimus 0.2 mg/m2 every 48 hours sirolimus levels above 10 ng/mL were seen in the literature.
2.1.1. Sirolimus dosage
Preterm neonates (gestational age <37 weeks) and clinical need for treatment: Single starting dose of sirolimus 0.1 mg (independent of body surface area [BSA]), TDM after 24, 48 and 72 hours, depending on values. Following doses are depending on sirolimus trough levels (1–2 ng/mL); if subtherapeutic after 72 hours, increase dose to 0.1 mg/48 h. In case of supratherapeutic levels after 48 and 72 hours, decrease dose to 0.1 mg/96 h.
Age 1–4 weeks, a term born: Starting dose 0.1 mg/48 h (independent of BSA), dose adjustments based on target trough levels 2–4 ng/mL.
Age 1–3 months: Start dose 0.1 mg/48 h (independent of BSA), dose adjustments based on target trough levels 2–6 ng/mL.
Age 3–6 months: Start dose 0.2 mg/m2/24 h in 2 doses, dose adjustments based on target trough levels 2–6 ng/mL.
Age 6–12 months: Start dose 0.5 mg/m2/24 h in 2 doses, dose adjustments based on target trough levels 2–6 ng/mL.
After 12 months: Start dose 0.8 mg/m2/24 h in 2 doses, dose adjustments based on target trough levels 2–6 ng/mL.
2.1.2. Sirolimus TDM and titration
We performed, when the child was older than 1 week, a TDM at 24 hours after the second gift to prevent supratherapeutic levels at start of sirolimus. Thereafter, weekly TDM was performed to achieve the steady state concentration and maintain the target trough levels. In case sirolimus level was <2 ng/mL at age >4 weeks, dose frequency was increased to 1 dose per 24 hours. If sirolimus levels were above 6/ng/mL the dosing interval was extended to 1 dose per 72 or 96 hours.
We advise to use the propylene glycol‐free liquid soluble capsules in children younger than 6 months, since the oral solution of sirolimus contains propylene glycol, which can be associated with severe neurotoxicities in neonates. 33 , 34 , 35 , 36 , 37
Addition of prednisone 1 mg/kg/d in 2 doses to sirolimus for supplementary effect in patients younger than 3 months is recommended. For pneumocystis prophylaxis we started co‐trimoxazole according to guidelines.
2.2. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in https://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/2020. 68
3. RESULTS
In total, 5 patients were treated in the acute phase at the Radboud University Medical centre and 1 patient continued treatment in a later phase at the University Medical Centre Groningen. All patients were diagnosed with a kaposiform haemangioendothelioma with KMP at age between 4 days and 6 months. Patient characteristics are summarized in Table 1. Patients were treated for a median duration of 13.3 months (interquartile range [IQR] 4.1–27.4 months).
TABLE 1.
Clinical characteristics of the 5 patients with kaposiform haemangioendothelioma
| Patient | Age at initiation [sex] | Clinical presentation | Location | D‐dimers (ng/mL; highest value before start) | Platelet count (× 109/L; lowest value before start) | Medication | Sirolimus start dose | Target trough levels (ng/mL), mean ± SD | Treatment duration (wk) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 d [F] | KMP, deviation of trachea without compression and oedema. Rapidly progressive. | Thorax | 47 000 | 9 |
‐ prednisone ‐ Sirolimus ‐ co‐trimoxazole |
0.1 mg every 48 h | 3.8 ± 1.62 | 114 |
| 2 | 4 wk [F] | Respiratory distress, vascular malformation of cervical paraspinal muscles of thoracic wall, mediastinum, pleurae, KMP, anaemia, | Thorax | 77 370 | 12 |
‐ prednisone ‐ Sirolimus ‐ co‐trimoxazole |
0.1 mg every 48 h | 3.6 ± 2.10 | 119 |
| 3 | 7 mo [M] | Thrombopenia due to KMP, enlargement of clinical size of tumour. | Head/neck | 9930 | 103 |
‐ Sirolimus ‐ co‐trimoxazole |
0.1 mg every 12 h | 2.65 ±0.64 | 58 |
| 4 | 4 d [M] | KMP, hyperbilirubinemia, dysmature, tachypnoea, oxygen needed, progressive hypercapnia. Complication due to KHE: Sepsis, intraventricular haemorrhage grade I. | Lower extremity | 38 570 | 7 |
‐ prednisone ‐ Sirolimus ‐ co‐trimoxazole |
0.1 mg every 48 h | 3.6 ± 1.64 | 45 |
| 5 | 2 mo [M] | KMP, petechiae | Thorax | 10 930 | 27 |
‐ prednisone ‐ Sirolimus ‐ co‐trimoxazole |
0.1 mg 48 h | 3.5 ± 1.68 | 18 |
F = female; M = male; KMP = Kasabach–Merritt phenomenon; SD = standard deviation.
Treatment with low‐dose sirolimus and prednisone was started in all patients under the age of 8 months. Mean sirolimus levels during the whole treatment period was 3.5 ± 1.74 ng/mL (range 1.0–11.0 ng/mL), Figure 1. No supratherapeutic levels were found at start of the sirolimus treatment. T2‐weighted MR images are shown in Figure A1.
FIGURE 1.
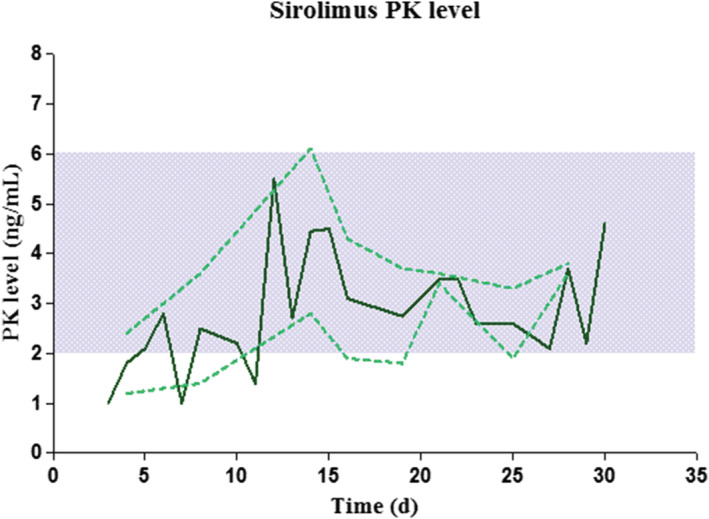
Mean sirolimus level of 5 patients after start sirolimus. Mean (dark green line) and standard error of mean (dotted green line) sirolimus level of all patients during sirolimus treatment (time after start sirolimus). The blue area represents the target levels of 2–6 ng/mL. PK = pharmacokinetic levels of sirolimus
In all patients, the initial response to treatment was observed in the first week by an increase in haemoglobin, fibrinogen, and platelet count (Figure 2). The median initial response time for platelet count was 6.0 days (IQR 1.50–19.00), for fibrinogen 2.0 days (IQR 1.25–4.25), and for haemoglobin median 2.0 days (range 1–3 d). The D‐dimer improvement occurred later after a median time of 15.0 days (IQR 2.00–32.00).
FIGURE 2.
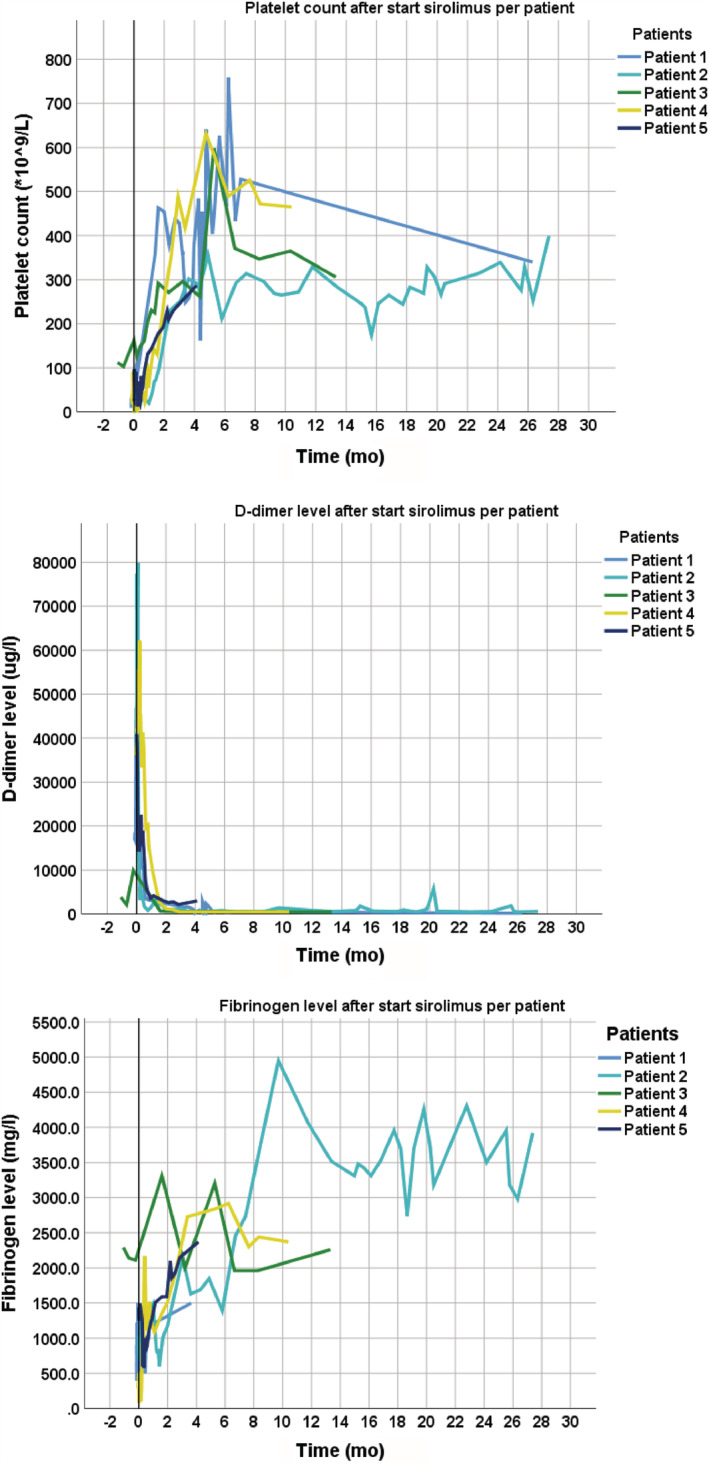
Laboratory results after start of low‐dose sirolimus per patient. Graphs of platelet count, D‐dimers and fibrinogen after start sirolimus
Table 2 shows the laboratory results before and after treatment. All patients had increased platelet count and fibrinogen, and decreased D‐dimer level after treatment. An increased haemoglobin after treatment was seen in 4 patients.
TABLE 2.
Haematological findings of each patient
| Patient | Platelet count (× 109/L) | Fibrinogen (mg/L) | Haemoglobin (mmol/L) | *D‐dimer (ng/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Before sirolimus therapy (lowest value) | After sirolimus therapy [mo] | Before sirolimus therapy (lowest value) | After sirolimus therapy [mo] | Before sirolimus therapy (lowest value) | After sirolimus therapy [mo] | Before sirolimus therapy (μg/L; highest value) | After sirolimus therapy [mo] | |
| 1 | 9 | 340 [26] | 400 | 1500 [7] | 6.2 | 6.7 [26] | 47 000 | 162 [26] |
| 2 | 12 | 399 [27] | 780 | 3920 [27] | 5.6 | 7.6 [27] | 77 370 | 560 [27] |
| 3 | 103 | 306 [13] | 2110 | 2260 [13] | 7.0 | 7.7 [13] | 9930 | <500 [13] |
| 4 | 7 | 465 [10] | 80 | 2370 [10] | 7.9 | 7.6 [10] | 38 570 | <500 [10] |
| 5 | 27 | 287 [4] | 1300 | 2370 [4] | 5.1 | 8.5 [4] | 40 930 | 2990 [4] |
| Total median (IQR) | 12 (9.0–27.0) | 340.0 (296.50–432.00) | 780 (400.0–1300.0) | 2370.0 (2287.50–3532.50) | 6.2 (5.6–7.0) | 7.6 (7.15–8.10) | 40 930 (38 570–47 000) | 500.0 (331.00–1775.00)* |
IQR = interquartile range.
D‐dimers measurements were sometimes limited with a lowest detection level of 500 μg/L, so 500 μg/lL was used.
In 4 patients a complete response—platelet count >100 × 109/L, median 28.0 days (IQR 16.50–44.00), normalisation of coagulation profile: D‐dimer <500 ng/mL: median of 109.5 days (IQR 91.25–184.00), fibrinogen >1000 mg/L median of 3.5 days (IQR 2.25–7.00), and clinical size reduction—was seen. The follow‐up of 1 patient (patient 5) was 4.13 months at the time of this report and D‐dimers highly decreased; however, they were not normalized yet, resulting in a partial response (Table 3).
TABLE 3.
Overview of efficacy and safety per patient treated with low‐dose sirolimus and corticosteroids
| Patient | Efficacy: response of treatment | Safety: Related adverse events (grade; relation to sirolimus) | Follow‐up (mo) | Response | |
|---|---|---|---|---|---|
| Coagulation profile and platelet count (× 109/L) | Size (clinical/MRI) | ||||
| 1 | Normal coagulation profile, platelet count >100 |
High clinical size reduction MRI: 80% reduction of vascular malformation |
No possible related to sirolimus occurred | 7.0 | CR |
| 2 |
Normal coagulation profile Platelet count >100 |
High clinical size reduction MRI: After 4 mo reduction of size. |
Hypertriglyceridaemia (II; 4), 2× viral infection (I; 3), elevation of liver enzymes (I; 4), hypophosphataemia (I; 4), lymphopenia (I; 3), upper airway infection (I; 3), 2× pneumonia (II; 3), upper airway infection (II; 3), viral infection (II; 3), diarrhoea (I; 3), gastroenteritis (I; 4), aphthous lesions (II; 4), anaemia (I; 3) | 24.2 | CR |
| 3 |
Normal coagulation profile Platelet count >100 |
Stabilization of size MRI: NA |
Viral infection (I; 3), conjunctivitis (II; 3) | 10.3 | CR |
| 4 |
Normal coagulation profile Platelet count >100 |
Moderate clinical size reduction of KHE. MRI: NA | Urinary tract infection (I; 3), viral gastroenteritis (II; 3) | 7.6 | CR |
| 5 | Up to normal coagulation profile platelet count >100 |
Clinical size reduction 50–80% MRI: NA |
No AE | 4.13 | PR—due to FU time improvement of symptoms |
MRI = magnetic resonance imaging; CR = complete response; PR = partial response; AE = adverse events—only possible/probable/definitely (3–5) related adverse events are mentioned. Severity grades 1–4 (1: mild; 2: moderate; 3: severe; 4: life‐threatening; 5: death related to AE—according to CTCAE 4.03. Relation to sirolimus use 1–6 (1: unrelated; 2: unlikely; 3: possibly; 4: probably; 5: definitely; 6: no info available). NA = not applicable—no MRI after start sirolimus was available; FU = follow‐up.
Overall clinical response, consisting of relevant decrease of the size or discoloration of the vascular tumour, was seen at median of 14 days (IQR 2.50–29.50).
Two patients did not experience any AEs, while 1 patient developed multiple mild–moderate AEs. In total, 19 possible or probable related to sirolimus AEs occurred (Table 3): infection (36.8%), metabolic/laboratory toxicity (21.1%), pulmonary/upper respiratory toxicity (21.1%), gastrointestinal toxicity (15.8%), and blood/bone marrow toxicity (5.3%). Of these only 8 grade II possible related to sirolimus AEs occurred and no serious AEs related to sirolimus occurred.
Two patients are described in detail to illustrate the effect of low‐dose sirolimus in changes in clinical symptoms and in MRI.
3.1. Patient 1
A term‐born female patient (birthweight 3250 g) with a tumour located at her left part of the thorax was diagnosed as KHE with KMP (Figure 3). After birth, haematological investigations showed a platelet count of 17 × 109/L and D‐dimers>16 000 μg/L. MRI revealed a large tumour mass in the left shoulder, upper left arm and neck region with tracheal deviation to the right (Figure 3). No biopsy with genetic analysis was performed. Prednisone 1 mg/kg/d administered by nasogastric tube was started on the day of birth. On day or life (DOL) 3, transfusions of platelets, fresh frozen plasma, fibrinogen and red blood cells were given to prevent intracranial haemorrhage during intubation and hospital transfer. Despite treatment, the tumour enlarged and the chest circumference (nipple line) increased from 38.5 cm (DOL4) to 39.7 cm (DOL5; Figure 3, Day 0—start of treatment). For this reason, sirolimus 0.1 mg/48 h with co‐trimoxazole prophylaxis was started at DOL5 and the dose of prednisone was increased to 2 mg/kg/d. An intravascular embolization of the KHE was planned in case of further deterioration, but, after only 2 days sirolimus, the platelet count were improved from 11 × 109/L to 42 × 109/L and a decrease of chest circumference was seen. After 3 days of treatment, the chest circumference was decreased to 38.1 cm (Figure 3 shows the decrease at Day 6 of treatment). After 4 days of treatment, the platelet count was further increased above 100 × 109/L, which made embolization unnecessary.
FIGURE 3.
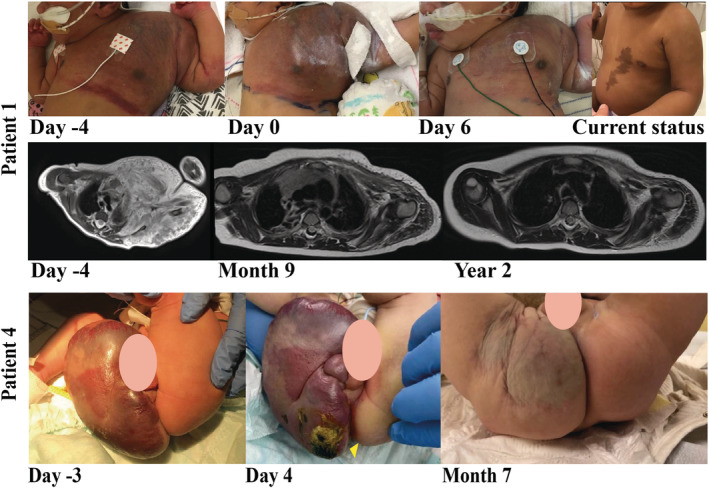
Photographs and T2‐weighted‐magnetic resonance images obtained from patients 1 and 4. Patient 1: photographs: before start sirolimus treatment, day at start sirolimus treatment, after 6 days of sirolimus treatment, and current status. T2‐weighted‐magnetic resonance images: before start sirolimus treatment, after 9 months of sirolimus treatment, and after 2 years of sirolimus treatment. Patient 4: photographs: before sirolimus treatment, after 4 days sirolimus treatment, and after 5 months sirolimus treatment
Two years later this patient received 0.4 mg sirolimus twice daily (1.4 mg/m2/24 h), achieving target trough levels of 4.5–5.5 ng/mL. There was an eminent size reduction of the KHE, resulting in a slight asymmetry of the left shoulder and arm. Lab stabilized with D‐dimers below 150 μg/L and platelets of 325 × 109/L. Figure 3 shows the significant effect of low‐dose sirolimus without any sirolimus related side effects.
3.2. Patient 4
A term male infant was born with a tumour of his scrotum, right buttock and lower extremity (Figure 3). The MRI (Figure A1) and biopsy confirmed the diagnosis of KHE. Genetic analysis showed no active mutations that have been described previously in KHE. At DOL2, the boy had a thrombopenia of 18 × 109/L. Platelets and plasma transfusions were given and treatment with prednisone 1 mg/kg/d was started at DOL3. On that day he developed fever and signs of infection/sepsis (pale colour). Treatment of flucloxacillin and gentamycin was started and he recovered. However, the haematological results deteriorated on DOL4: platelets even further decreased to 7 × 109/L; D‐dimers were 38.570 ng/mL; and fibrinogen under 500 mg/L. Therefore, 0.1 mg sirolimus per 48 hours was started (0.2 mg/m2/48 h) and platelet and plasma transfusion was given. At DOL6 this patient developed a right sided intraventricular haemorrhage grade I. Plasma, fibrinogen, platelet and erythrocyte transfusions were given. At DOL9 after cessation of his intravenously antibiotics co‐trimoxazole was restarted. This patient experienced elevated liver enzymes and alkalic phosphatase, which may be possibly caused by sirolimus or by total parenteral feeding, which may also lead to these elevations. At age 3 weeks, he developed a Staphylococcus aureus sepsis due to a lesion on his bottom (Figure 3, Day 4 of treatment). Treatment with flucloxacillin was started and the contaminated central venous line was removed.
After 3 months of treatment with low‐dose sirolimus (0.15 mg/48 h, 0.33 mg/m2/24 h) and prednisone (0.6 mg/kg/d decreased to 0.4 mg/kg/d after stabilizing D‐dimers), the coagulation profile normalized (platelets 487 × 109/L, D‐dimers <500 ng/mL).
A first clinical response of decrease in tumour size of his scrotum, right buttock and his right lower extremity was seen after 4 months of sirolimus treatment. Changes during sirolimus treatment are shown in Figure 3 (photographs).
4. DISCUSSION
This case series of infants with KHE with life‐threatening KMP shows that treatment with sirolimus using dosing and titration based on the hypothetical maturation of liver enzymes can be safe and effective. Sirolimus is mainly metabolized by the CYP3A4 enzyme. A significant smaller part is metabolized by the CYP3A5 and/or by the CYP2C8 enzyme. 38 Based on age, body weight and estimated expression of the CYP3A enzymes, treatment was initiated. Intensive therapeutic drug monitoring was used to adjust sirolimus dose either by increasing or decreasing the interval (see methods). All patients in our case series had a quick positive response using low‐dose sirolimus corresponding to findings in literature. 13 , 20 , 39 Table 4 shows that only 17 articles described a total of 30 patients younger than 3 months at start of sirolimus. Most frequently used target levels in this age is 10–15 ng/mL (n = 24/30; 80%).
TABLE 4.
Overview of literature of patients with KHE and KMP under 3 months treated with sirolimus categorized per target trough level
| Sirolimus level (ng/mL) | No of articles | Number of KHE cases; number of KMP | Age range | Overall response rate | First overall response (d) | Grade of toxicity | Number of AE/number of patients* |
|---|---|---|---|---|---|---|---|
| >15 | 1 66 | 1; 0 | 2.53 mo | 1.00 | 6 mo | Gr. II | N = 3/1 (mouth sores; 300%) |
| Gr. ≥III | None | ||||||
| 10–15 | 11 29 , 39 , 66 , 67 , 69 , 70 , 71 , 72 , 73 , 74 | 24; 21 | 4 d–3 mo | 0.96 | 2.2 d–7 wk | Gr. II | n = 5/17 (29.4%) |
| Gr. ≥III | n = 5/17 (29.4%) | ||||||
| 5–10 | 4 45 , 66 , 67 , 75 | 4; 3 | 3 d–3 mo | 1.00 | 12 d–6 mo | Gr. II | n = 4/4 (100%) |
| Gr. ≥III | n = 4/4 (100%) | ||||||
| 2–5 | 1 65 | 1; 1 | 2 d | 1.00 | 6 mo | Gr. II | n = UNK |
| Gr. ≥III | n = UNK | ||||||
| Total | 17 | 30; 25 | 2 d–3 mo | 0.998 | 2.2 d–6 mo | Gr. II | n = 21/22 (95.5%) |
| Gr. ≥III | n = 21/22 (95.5%) | ||||||
| Case series | 1 | 4; 4 | 4 d–2 mo | 1.00 | 1–3 d | Gr. II | n = 8/5 (160%) |
| Gr. ≥III | n = 0 (0%) |
KHE = kaposiform haemangioendothelioma; KMP = Kasabach–Merritt phenomenon; AE = adverse event; Gr. = Grade; UNK = Unknown; * articles which described AE, divided by the described patients under 3 months.
The clinical pharmacology in neonates is dynamic, contains many covariates and is influenced by multiple factors such as pharmacokinetic (PK) factors (adsorption, distribution, metabolism, elimination), ethnicity, (pharmaco‐)genetic profile, infections, comedication and chronic diseases. 32 Due to a large variability of the developmental maturation in PK, pharmacodynamics (PD), and other factors it is a challenge to develop an optimal dose regimen in neonates and paediatric patients. Standard fixed‐dose stratification or weight‐based dosing design frequently leads to exposures outside the narrow targeted range. 40
Emoto et al. also showed in the Emax model (nonlinear model frequently used in dose–response analyses) of infants aged <1 year that a high sirolimus blood level was observed in the first 8 hours after administration of sirolimus. 41 Unfortunately, in their study, no neonates aged <21 days were included. The CYP3A4 and CYP3A5 expression development, and other processes (e.g. developmental changes in distribution sites, gastrointestinal function and acquisition of renal function) influences both PK and PD aspects in infants and specifically in neonates. 30 , 42 , 43 , 44 For this reason, we recommend starting with a low dose of sirolimus and frequent TDM as maturation of enzymes and the development of all the other processes can differ by week. In addition, we advise lower target levels especially in neonates aged <4 weeks because they have a substantial lower metabolization of sirolimus leading to a prolonged exposure. In theory, the exponential growth after birth may be hypothetically caused by an high activity of mechanistic target of rapamycin kinase. For this reason the PD effect of sirolimus may be higher in the first month of age as well.
The changes in amount and activity of CYP3A enzymes during the neonatal life and variation in ethnicity/pharmacogenetic factors are of importance with respect to the initial dose.
To prevent toxic blood levels at initiation of sirolimus therapy it is important that the initial dose is as low as possible. A high initial sirolimus blood level is seen in several studies using high initial doses (0.14–0.8 mg/m2/d), leading to high supratherapeutic or even toxic sirolimus target levels >20 ng/mL. 12 , 45
Another retrospective study described a Chinese boy aged 6 months. He suffered of a multifocal KHE of his right shoulder, chest, lung, right axilla and was treated with an initial sirolimus dose of 1.6 mg/m2/24 h in 2 doses. 12 Despite his positive response to sirolimus, he died of complications probably due to sirolimus toxicities. Interestingly, this was the only patient described in this study who had initial sirolimus range above the normal target range (4–20 ng/mL), namely 30 ng/mL.
It is possible that there is an inter‐racial variability of the activity of the CYP3A4 and CYP3A5 enzyme, leading to higher sirolimus levels, which might result in more severe adverse events. It has been described that in Asian patients the genetic variants of CYP3A4/A5 (CYP3A4*1G, CYP3A5*1/*1 and *1/*3) are frequently found and associated with the need of higher dose of sirolimus to reach the therapeutic levels, 46 , 47 , 48 in contrast to patients with CYP3A5 *3/*3 genotypes frequently found in Caucasian patients, who may are more at risk of high sirolimus level. 49
In our case series, patient 5 needed a dose elevation in a short time. Since the patient has an Italic/Asian ethnicity it is possible that he had a functional variant from the CYP3A5 gene, which may lead to a higher quantity of this CYP3A5 enzymes. 50 , 51 However, there are contradictive reports, suggesting that there is no difference between Asian and Caucasian races. 49 , 52 , 53 Further research is necessary for investigation of the interracial differences and their pharmacogenetic role of the CYP3 enzymes in infants. In general, we have to realize that each patient is different with perhaps different genetic backgrounds. Therefore, we can only recommend a low starting dose and that sirolimus dosage has to be adapted based on TDM.
In addition to the mentioned PK aspects, frequent TDM is needed when prednisone is added to sirolimus treatment. Since corticosteroids are competitive inhibitors of CYP3A4, they may induce the CYP3A4 enzyme. 54 , 55 This might affect sirolimus PK leading to lower sirolimus levels. 56 By contrast, sirolimus may affect the PK of prednisone, leading to a decrease of maximum plasma concentration found in kidney transplant recipients. No significant effect was seen in doses sirolimus of ≤5 mg/m2/d. 57 More research is necessary to analyse the exact interaction of sirolimus and corticosteroids.
The pathophysiology of KMP and the effect of prednisone on this process remains unestablished and could be multifactorial. Platelet trapping by the abnormal proliferating endothelium within the KHE‐lesion leading to activation of coagulation cascades and eventually leading to clotting factors consumption may play a role in this process. 58 , 59 We speculate that the coagulation cascade leading to disseminated intravascular coagulopathy is mediated by the immune system. Due to inhibition of this system, prednisone may have an additional effect in the improvement of the coagulopathy and lesion size. It was the first‐choice treatment for KMP in the past. 60 , 61 , 62 , 63 Literature shows a variable response to prednisone ranging from no significant regression in lesion size to even complete remission. 61 , 62 , 64
The exact working mechanism of sirolimus in improvement of the coagulopathy is also unclear. It is possible that T cells play a role in this mediation; inhibition of the T cells by sirolimus may lead to improvement of the coagulation. It is possible that this happened in our patient 3, who was treated with sirolimus alone, showing a normalisation of his coagulopathy. Conversely, addition of prednisone to sirolimus may prevent the chance of the development of DIC as we have seen in our other patients in whom lowering of the prednisone dose increased the D‐dimers. For this possible additional effect to sirolimus on coagulopathy and tumour size, we advise to add prednisone in children younger than 1 year, at least in the acute phase of DIC.
No grade III AEs occurred, in contrast to other case series. 13 , 20 , 39 This can be explained by the lower initial dosage and the gradual sirolimus titration in the patients presented here. In an overview of the literature of patients with KHE and KMP aged <3 months treated with sirolimus (Table 4), only 1 study used trough levels of 2–5 ng/mL, so only limited information about AEs in this range is available. 65 Shan et al. treated 21 patients with KHE, without KMP (age 33 days–4.74 years) and analysed patients by target trough levels. No significant differences between the 3 concentration groups (>15, 10–15, 5–10 ng/mL) was seen. 66 However, all 3 patients younger than 3 months developed AEs grade I–III, some of which repeated multiple times during treatment. Czechowicz et al. showed in a case series that neonates often suffer severe effects of vascular anomalies and advised a sirolimus target range of 4–10 ng/mL. 67 Two patients had KHE with KMP: 1 patient aged 6 days with levels of 10–15 ng/mL died of complications of KMP without achieving a stable dose prior to death. The other patient had elevated triglycerides at a sirolimus target level of 8–10 ng/mL.
In summary, our case series showed that even with very low target trough levels of 2–6 ng/mL sirolimus is still effective in patients with KHE and KMP. Low‐dose sirolimus may decrease the need for platelet transfusions and can reduce the risk of morbidity and mortality, accompanied with a lower incidence of AEs. The overall response of our case series is comparable to other used target levels, while no grade III AEs were seen in contrast to the patients described previously.
5. CONCLUSIONS
Management of KHE with KMP is difficult and challenging in infants and especially in neonates, as developmental changes in the metabolization, pharmacogenetics and clearance of drugs play an important role. Treatment dosing and titration can be based on the developing metabolism in infants and other processes such as absorption, distribution and clearance. By frequent TDM, supratherapeutic levels of sirolimus can be avoided whereas the desired effect of treatment is reached. Our case series offers a proposal for treatment dose regimen covering the neonatal period and young infancy.
ETHICS APPROVAL
All caregivers of the patients included in this case series gave their informed consent for describing and images of their children.
COMPETING INTERESTS
All authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Dr Harbers reviewed the cases, drafted the initial manuscript, reviewed and revised the manuscript. Drs van der Salm, Drs Pegge, Dr Verhoeven, Dr L.A.G. Vrancken and Dr Fuijkschot were involved in diagnosis and/or treatment of the patients, and critically reviewed the manuscript.
Dr van der Vleuten, Prof. Dr Schultze Kool critically reviewed the manuscript for important intellectual content. Dr te Loo was the Principal Investigator of this case series and was involved in diagnoses and treatment of all patients, supervised data collection, and reviewed and revised the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
ACKNOWLEDGEMENT
No funding was secured for this study.
APPENDIX A.
FIGURE A1.
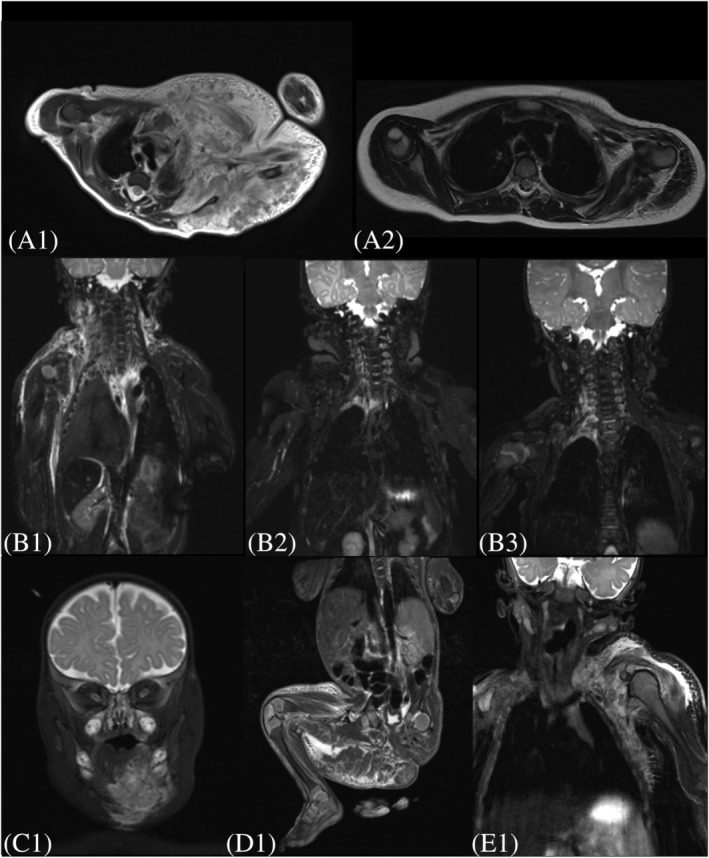
Representative T2‐weighted magnetic resonance imaging (MRI) of each patient with kaposiform haemangioendothelioma with Kasabach–Merritt phenomenon. (A1) MRI of patient 1 before start sirolimus treatment. (A2) MRI of Patient 1 after 2 years of sirolimus treatment. (B1) MRI of patient 2 before start sirolimus treatment. (B2) MRI of patient 2 after 4 months sirolimus treatment. (C) MRI of patient 3 before start sirolimus treatment. (D) MRI of patient 4 before start sirolimus treatment. (E) MRI of patient 5 before start sirolimus treatment
Harbers VEM, van der Salm N, Pegge SAH, et al. Effective low–dose sirolimus regimen for kaposiform haemangioendothelioma with Kasabach–Merritt phenomenon in young infants. Br J Clin Pharmacol. 2022;88(6):2769-2781. doi: 10.1111/bcp.15202
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach‐Merritt phenomenon in 107 referrals. J Pediatr. 2013;162(1):142‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ji Y, Yang K, Peng S, et al. Kaposiform haemangioendothelioma: clinical features, complications and risk factors for Kasabach‐Merritt phenomenon. Br J Dermatol. 2018;179(2):457‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lyons LL, North PE, Mac‐Moune Lai F, Stoler MH, Folpe AL, Weiss SW. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28(5):559‐568. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez V, Lee A, Witman PM, Anderson PA. Kasabach‐merritt phenomenon: case series and retrospective review of the mayo clinic experience. J Pediatr Hematol Oncol. 2009;31(7):522‐526. [DOI] [PubMed] [Google Scholar]
- 5. Kelly M. Kasabach‐Merritt phenomenon. Pediatr Clin North Am. 2010;57(5):1085‐1089. [DOI] [PubMed] [Google Scholar]
- 6. Ryan C, Price V, John P, et al. Kasabach‐Merritt phenomenon: a single centre experience. Eur J Haematol. 2010;84(2):97‐104. [DOI] [PubMed] [Google Scholar]
- 7. Sarkar M, Mulliken JB, Kozakewich HP, Robertson RL, Burrows PE. Thrombocytopenic coagulopathy (Kasabach‐Merritt phenomenon) is associated with Kaposiform hemangioendothelioma and not with common infantile hemangioma. Plast Reconstr Surg. 1997;100(6):1377‐1386. [DOI] [PubMed] [Google Scholar]
- 8. Zhou S, Wang L, Panossian A, Anselmo D, Wu S, Venkatramani R. Refractory Kaposiform Hemangioendothelioma Associated with the Chromosomal Translocation t(13;16)(q14;p13.3). Pediatr Dev Pathol. 2016;19(5):417‐420. [DOI] [PubMed] [Google Scholar]
- 9. Ayturk UM, Couto JA, Hann S, et al. Somatic Activating Mutations in GNAQ and GNA11 Are Associated with Congenital Hemangioma. Am J Hum Genet. 2016;98(6):1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lim YH, Bacchiocchi A, Qiu J, et al. GNA14 Somatic Mutation Causes Congenital and Sporadic Vascular Tumors by MAPK Activation. Am J Hum Genet. 2016;99(2):443‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ten Broek RW, Eijkelenboom A, van der Vleuten CJM, et al. Comprehensive molecular and clinicopathological analysis of vascular malformations: A study of 319 cases. Genes Chromosomes Cancer. 2019;58(8):541‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Z, Yao W, Sun H, et al. Sirolimus therapy for kaposiform hemangioendothelioma with long‐term follow‐up. J Dermatol. 2019;46(11):956‐961. [DOI] [PubMed] [Google Scholar]
- 13. Peng S, Yang K, Xu Z, Chen S, Ji Y. Vincristine and sirolimus in the treatment of kaposiform haemangioendothelioma. J Paediatr Child Health. 2019;55(9):1119‐1124. [DOI] [PubMed] [Google Scholar]
- 14. Schroeder U, Lauten M, Stichtenoth G, Gebhard MP, Buchholz M, Kaiser MM. Laryngomalacia and complicated, life‐threatening mTOR‐positive Kaposiform hemangioendothelioma cured by Supraglottoplasty and sirolimus. Klin Padiatr. 2014;226(6–7):362‐368. [DOI] [PubMed] [Google Scholar]
- 15. Lackner H, Karastaneva A, Schwinger W, et al. Sirolimus for the treatment of children with various complicated vascular anomalies. Eur J Pediatr. 2015;174(12):1579‐1584. [DOI] [PubMed] [Google Scholar]
- 16. Blatt J, Stavas J, Moats‐Staats B, Woosley J, Morrell DS. Treatment of childhood kaposiform hemangioendothelioma with sirolimus. Pediatr Blood Cancer. 2010;55(7):1396‐1398. [DOI] [PubMed] [Google Scholar]
- 17. Ji Y, Chen S, Xiang B, et al. Sirolimus for the treatment of progressive kaposiform hemangioendothelioma: A multicenter retrospective study. Int J Cancer. 2017;141(4):848‐855. [DOI] [PubMed] [Google Scholar]
- 18. Adams DM, Trenor CC 3rd, Hammill AM, et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics. 2016;137(2):e20153257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ying H, Qiao C, Yang X, Lin X. A Case Report of 2 Sirolimus‐Related Deaths Among Infants With Kaposiform Hemangioendotheliomas. Pediatrics. 2018;141(Suppl 5):S425‐S429. [DOI] [PubMed] [Google Scholar]
- 20. Freixo C, Ferreira V, Martins J, et al. Efficacy and safety of sirolimus in the treatment of vascular anomalies: A systematic review. J Vasc Surg. 2020;71(1):318‐327. [DOI] [PubMed] [Google Scholar]
- 21. Morath C, Schwenger V, Ksoll‐Rudek D, et al. Four cases of sirolimus‐associated interstitial pneumonitis: identification of risk factors. Transplant Proc. 2007;39(1):99‐102. [DOI] [PubMed] [Google Scholar]
- 22. Garrean S, Massad MG, Tshibaka M, Hanhan Z, Caines AE, Benedetti E. Sirolimus‐associated interstitial pneumonitis in solid organ transplant recipients. Clin Transplant. 2005;19(5):698‐703. [DOI] [PubMed] [Google Scholar]
- 23. Wang WL, Yu LX. Acute respiratory distress attributed to sirolimus in solid organ transplant recipients. Am J Emerg Med. 2015;33(1):e121‐e124. [DOI] [PubMed] [Google Scholar]
- 24. Singer SJ, Tiernan R, Sullivan EJ. Interstitial pneumonitis associated with sirolimus therapy in renal‐transplant recipients. N Engl J Med. 2000;343(24):1815‐1816. [DOI] [PubMed] [Google Scholar]
- 25. Russell TB, Rinker EK, Dillingham CS, Givner LB, McLean TW. Pneumocystis Jirovecii Pneumonia During Sirolimus Therapy for Kaposiform Hemangioendothelioma. Pediatrics. 2018;141(Suppl 5):S421‐S424. [DOI] [PubMed] [Google Scholar]
- 26. Rossler J, Baselga E, Davila V, et al. Severe adverse events during sirolimus "off‐label" therapy for vascular anomalies. Pediatr Blood Cancer. 2021;68(8):e28936. [DOI] [PubMed] [Google Scholar]
- 27. Kahan BD, Napoli KL, Kelly PA, et al. Therapeutic drug monitoring of sirolimus: correlations with efficacy and toxicity. Clin Transplant. 2000;14(2):97‐109. [DOI] [PubMed] [Google Scholar]
- 28. Harbers VEM, Rongen G, van der Vleuten CJM, et al. Patients with Congenital Low‐Flow Vascular Malformation Treated with Low Dose Sirolimus. Adv Ther. 2021;38(6):3465‐3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cabrera TB, Speer AL, Greives MR, Goff DA, Menon NM, Reynolds EW. Sirolimus for Kaposiform Hemangioendothelioma and Kasabach‐Merritt Phenomenon in a Neonate. AJP Rep. 2020;10(4):e390‐e394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kearns GL, Abdel‐Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental Pharmacology — Drug Disposition, Action, and Therapy in Infants and Children. N Engl J Med. 2003;349(12):1157‐1167. [DOI] [PubMed] [Google Scholar]
- 31. Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82(4):381‐388. [DOI] [PubMed] [Google Scholar]
- 32. Allegaert K, van de Velde M, van den Anker J. Neonatal clinical pharmacology. Paediatr Anaesth. 2014;24(1):30‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zar T, Graeber C, Perazella MA. Recognition, treatment, and prevention of propylene glycol toxicity. Semin Dial. 2007;20(3):217‐219. [DOI] [PubMed] [Google Scholar]
- 34. Speth PA, Vree TB, Neilen NF, et al. Propylene glycol pharmacokinetics and effects after intravenous infusion in humans. Ther Drug Monit. 1987;9(3):255‐258. [DOI] [PubMed] [Google Scholar]
- 35. Shehab N, Lewis CL, Streetman DD, Donn SM. Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates. Pediatr Crit Care Med. 2009;10(2):256‐259. [DOI] [PubMed] [Google Scholar]
- 36. "Inactive" ingredients in pharmaceutical products: update (subject review). American Academy of Pediatrics Committee on Drugs. Pediatrics. 1997;99(2):268‐278. [DOI] [PubMed] [Google Scholar]
- 37. Pouwels PJW, van de Lagemaat M, van de Pol LA, Witjes BCM, Zonnenberg IA. Spectroscopic detection of brain propylene glycol in neonates: Effects of different pharmaceutical formulations of phenobarbital. J Magn Reson Imaging. 2019;49(4):1062‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Emoto C, Fukuda T, Venkatasubramanian R, Vinks AA. The impact of CYP3A5*3 polymorphism on sirolimus pharmacokinetics: insights from predictions with a physiologically‐based pharmacokinetic model. Br J Clin Pharmacol. 2015;80(6):1438‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang H, Guo X, Duan Y, Zheng B, Gao Y. Sirolimus as initial therapy for kaposiform hemangioendothelioma and tufted angioma. Pediatr Dermatol. 2018;35(5):635‐638. [DOI] [PubMed] [Google Scholar]
- 40. Momper JD, Wagner JA. Therapeutic drug monitoring as a component of personalized medicine: applications in pediatric drug development. Clin Pharmacol Ther. 2014;95(2):138‐140. [DOI] [PubMed] [Google Scholar]
- 41. Emoto C, Fukuda T, Johnson TN, Adams DM, Vinks AA. Development of a Pediatric Physiologically Based Pharmacokinetic Model for Sirolimus: Applying Principles of Growth and Maturation in Neonates and Infants. CPT Pharmacometrics Syst Pharmacol. 2015;4(2):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van den Anker JN. Developmental pharmacology. Dev Disabil Res Rev. 2010;16(3):233‐238. [DOI] [PubMed] [Google Scholar]
- 43. de Wildt SN. Profound changes in drug metabolism enzymes and possible effects on drug therapy in neonates and children. Expert Opin Drug Metab Toxicol. 2011;7(8):935‐948. [DOI] [PubMed] [Google Scholar]
- 44. Hines RN. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int J Pharm. 2013;452(1–2):3‐7. [DOI] [PubMed] [Google Scholar]
- 45. Koury J, Brown M, Sturtevant S, Wiley C, Felton L. Use of Sirolimus in a Premature Neonate With Kaposiform Hemangioedema. J Pediatr Pharmacol Ther. 2021;26(2):205‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang J, Dai Y, Liu Z, et al. Effect of CYP3A4 and CYP3A5 Genetic Polymorphisms on the Pharmacokinetics of Sirolimus in Healthy Chinese Volunteers. Ther Drug Monit. 2017;39(4):406‐411. [DOI] [PubMed] [Google Scholar]
- 47. Ozdemir V, Kalow W, Tang BK, et al. Evaluation of the genetic component of variability in CYP3A4 activity: a repeated drug administration method. Pharmacogenetics. 2000;10(5):373‐388. [DOI] [PubMed] [Google Scholar]
- 48. Le Meur Y, Djebli N, Szelag JC, et al. CYP3A5*3 influences sirolimus oral clearance in de novo and stable renal transplant recipients. Clin Pharmacol Ther. 2006;80(1):51‐60. [DOI] [PubMed] [Google Scholar]
- 49. van Schaik RH, van der Heiden IP, van den Anker JN, Lindemans J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin Chem. 2002;48(10):1668‐1671. [PubMed] [Google Scholar]
- 50. Dorji PW, Tshering G, Na‐Bangchang K. CYP2C9, CYP2C19, CYP2D6 and CYP3A5 polymorphisms in South‐East and East Asian populations: A systematic review. J Clin Pharm Ther. 2019;44(4):508‐524. [DOI] [PubMed] [Google Scholar]
- 51. Khan AR, Raza A, Firasat S, Abid A. CYP3A5 gene polymorphisms and their impact on dosage and trough concentration of tacrolimus among kidney transplant patients: a systematic review and meta‐analysis. Pharmacogenomics J. 2020;20(4):553‐562. [DOI] [PubMed] [Google Scholar]
- 52. Zochowska D, Wyzgal J, Paczek L. Impact of CYP3A4*1B and CYP3A5*3 polymorphisms on the pharmacokinetics of cyclosporine and sirolimus in renal transplant recipients. Ann Transplant. 2012;17(3):36‐44. [DOI] [PubMed] [Google Scholar]
- 53. Yang J, He MM, Niu W, et al. Metabolic capabilities of cytochrome P450 enzymes in Chinese liver microsomes compared with those in Caucasian liver microsomes. Br J Clin Pharmacol. 2012;73(2):268‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pichard L, Fabre I, Daujat M, Domergue J, Joyeux H, Maurel P. Effect of corticosteroids on the expression of cytochromes P450 and on cyclosporin A oxidase activity in primary cultures of human hepatocytes. Mol Pharmacol. 1992;41(6):1047‐1055. [PubMed] [Google Scholar]
- 55. Pichard L, Fabre I, Fabre G, et al. Cyclosporin A drug interactions. Screening for inducers and inhibitors of cytochrome P‐450 (cyclosporin A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab Dispos. 1990;18(5):595‐606. [PubMed] [Google Scholar]
- 56. Cattaneo D, Merlini S, Pellegrino M, et al. Therapeutic drug monitoring of sirolimus: effect of concomitant immunosuppressive therapy and optimization of drug dosing. Am J Transplant. 2004;4(8):1345‐1351. [DOI] [PubMed] [Google Scholar]
- 57. Jusko WJ, Ferron GM, Mis SM, Kahan BD, Zimmerman JJ. Pharmacokinetics of prednisolone during administration of sirolimus in patients with renal transplants. J Clin Pharmacol. 1996;36(12):1100‐1106. [DOI] [PubMed] [Google Scholar]
- 58. Hall GW. Kasabach‐Merritt syndrome: pathogenesis and management. Br J Haematol. 2001;112(4):851‐862. [DOI] [PubMed] [Google Scholar]
- 59. Kim T, Roh MR, Cho S, Chung KY. Kasabach‐merritt syndrome arising from tufted angioma successfully treated with systemic corticosteroid. Ann Dermatol. 2010;22(4):426‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhou SY, Li HB, Mao YM, Liu PY, Zhang J. Successful treatment of Kasabach‐Merritt syndrome with transarterial embolization and corticosteroids. J Pediatr Surg. 2013;48(3):673‐676. [DOI] [PubMed] [Google Scholar]
- 61. Maguiness S, Guenther L. Kasabach‐merritt syndrome. J Cutan Med Surg. 2002;6(4):335‐339. [DOI] [PubMed] [Google Scholar]
- 62. Wananukul S, Nuchprayoon I, Seksarn P. Treatment of Kasabach‐Merritt syndrome: a stepwise regimen of prednisolone, dipyridamole, and interferon. Int J Dermatol. 2003;42(9):741‐748. [DOI] [PubMed] [Google Scholar]
- 63. Esterly NB. Kasabach‐Merritt syndrome in infants. J Am Acad Dermatol. 1983;8(4):504‐513. [DOI] [PubMed] [Google Scholar]
- 64. Enjolras O, Riche MC, Merland JJ, Escande JP. Management of alarming hemangiomas in infancy: a review of 25 cases. Pediatrics. 1990;85(4):491‐498. [PubMed] [Google Scholar]
- 65. Ji Y, Chen S, Xia C, et al. Chronic lymphedema in patients with kaposiform hemangioendothelioma: incidence, clinical features, risk factors and management. Orphanet J Rare Dis. 2020;15(1):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shan Y, Tian R, Gao H, et al. Sirolimus for the treatment of kaposiform hemangioendothelioma: In a trough level‐dependent way. J Dermatol. 2021;48(8):1201‐1209. [DOI] [PubMed] [Google Scholar]
- 67. Czechowicz JA, Long‐Boyle JR, Rosbe KW, Mathes EF, Frieden IJ, Shimano KA. Sirolimus for management of complex vascular anomalies ‐ A proposed dosing regimen for very young infants. Int J Pediatr Otorhinolaryngol. 2018;105:48‐51. [DOI] [PubMed] [Google Scholar]
- 68. Alexander SPH, Fabbro D, Kelly E, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. Br J Pharmacol. 2019;176(Suppl 1):S364. [Google Scholar]
- 69. Ji Y, Chen S, Yang K, Xia C, Peng S. Development of Kasabach‐Merritt phenomenon following vaccination: More than a coincidence? J Dermatol. 2018;45(10):1203‐1206. [DOI] [PubMed] [Google Scholar]
- 70. Kai L, Wang Z, Yao W, Dong K, Xiao X. Sirolimus, a promising treatment for refractory Kaposiform hemangioendothelioma. J Cancer Res Clin Oncol. 2014;140(3):471‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tan X, Zhang J, Zhou S, Liu Z, Zhang T, Xia J. Successful management of steroid‐resistant vascular tumors associated with the Kasabach‐Merritt phenomenon using sirolimus. J Dermatol. 2018;45(5):580‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tribolet S, Hoyoux C, Boon LM, et al. A not so harmless mass: Kaposiform hemangioendothelioma complicated by a Kasabach‐Merritt phenomenon. Arch Pediatr. 2019;26(6):365‐369. [DOI] [PubMed] [Google Scholar]
- 73. Wang Y, Kong L, Sun B, Cui J, Shen W. Sirolimus for Kaposiform Hemangioendothelioma With Kasabach‐Merritt Phenomenon in Two Infants. J Craniofac Surg. 2020;31(4):1074‐1077. [DOI] [PubMed] [Google Scholar]
- 74. Wang H, Duan Y, Gao Y, Guo X. Sirolimus for Vincristine‐Resistant Kasabach‐Merritt Phenomenon: Report of Eight Patients. Pediatr Dermatol. 2017;34(3):261‐265. [DOI] [PubMed] [Google Scholar]
- 75. Alaqeel AM, Alfurayh NA, Alhedyani AA, Alajlan SM. Sirolimus for treatment of kaposiform hemangioendothelioma associated with Kasabach‐Merritt phenomenon. JAAD Case Rep. 2016;2(6):457‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


