Summary
Background
Accurate assessment of atopic dermatitis (AD) severity is critical when initiating and monitoring therapy. Use of existing research tools such as the Eczema Area and Severity Index (EASI) and Scoring Atopic Dermatitis (SCORAD) is complex and time‐consuming in clinical practice. A previous analysis found the product of validated Investigator’s Global Assessment (vIGA) and affected body surface area (BSA) to be an accurate and practical tool for routine assessment of paediatric AD.
Objective
To evaluate the IGAxBSA composite as an alternative to EASI or SCORAD for assessment of AD disease severity and disease responsiveness.
Methods
The relationship between IGAxBSA, EASI and SCORAD was assessed in a post hoc analysis of pooled data from the dupilumab clinical trial programme in adult and paediatric patients with moderate‐to‐severe AD who had received dupilumab or placebo, with or without topical corticosteroids (TCS). The trials are registered at ClinicalTrials.gov and EudraCT: LIBERTY AD SOLO 1 (NCT02277743, 2014‐001198‐15), LIBERTY AD SOLO 2 (NCT02277769, 2014‐002619‐40), LIBERTY AD SOLO‐CONTINUE (NCT02395133, 2014‐003384‐38), LIBERTY AD CHRONOS (NCT02260986, 2013‐003254‐24), LIBERTY AD CAFÉ (NCT02755649, 2015‐002653‐35), LIBERTY AD ADOL (NCT03054428, 2015‐004458‐16), LIBERTY AD PEDS (NCT03345914, 2016‐004997‐16), LIBERTY AD OLE (NCT01949311, 2013‐001449‐15) and LIBERTY AD PEDS OLE (NCT02612454, 2015‐001396‐40).
Results
Using datapoints from pooled dupilumab randomized controlled trials (n = 3473) and open‐label extension trials (n = 3045), we found that IGAxBSA correlated well with EASI and SCORAD, irrespective of treatment group and race (white, Asian, black). IGAxBSA correlated better with objective measures (EASI, SCORAD) than with patient‐ or caregiver‐reported subjective measures. IGAxBSA correlated strongly with EASI and SCORAD in assessing disease change over time (r = 0·90, r = 0·76, respectively; P < 0·0001), and concordance between IGAxBSA‐50/75/90 and EASI‐50/75/90 was excellent (88–94%).
Conclusions
IGAxBSA is a valid alternative for assessment of AD disease severity and response over time, compared with EASI or SCORAD in patients with AD, irrespective of race.
What is already known about this topic?
Accurate assessment of atopic dermatitis (AD) severity is critical for both initiating and monitoring therapy.
Existing metrics to measure the severity of AD, such as the Eczema Area and Severity Index (EASI) and Scoring Atopic Dermatitis (SCORAD), are complex and time‐consuming in clinical practice.
Previous analysis found the product of validated Investigator’s Global Assessment (vIGA) and involved body surface area (BSA) to be an accurate and practical tool for routine assessment of AD in small cohorts of patients.
What does this study add?
We confirmed, in a large sample of patient data, that IGAxBSA can be used as a surrogate metric for EASI and SCORAD in assessing disease severity and monitoring treatment response.
Factors such as race and treatment used did not affect the validity of IGAxBSA.
What are the clinical implications of this work?
IGAxBSA is an alternative measure for the rapid and routine assessment of disease severity and improvement over time in routine clinical practice
IGAxBSA is less complex and quicker to perform than EASI or SCORAD in patients with AD, regardless of race.
![]()
Linked Comment: M. Futamura. Br J Dermatol 2022; 186:397.
Plain language summary available online
Assessment of atopic dermatitis (AD) disease severity is important in selecting appropriate therapy, as well as in evaluating therapeutic responsiveness in disease change over time. 1 , 2 No practical, comprehensive and accurate assessment tool currently exists for physician‐reported evaluation of disease extent and severity in patients with AD in research and clinical settings. 3 Clinical trials often utilize Eczema Area and Severity Index (EASI) or Scoring Atopic Dermatitis (SCORAD) – both validated, widely used, multi‐item outcome instruments that measure the extent and severity of AD. 4 , 5 , 6 However, both EASI and SCORAD are complicated and time‐consuming to complete (taking up to 6–10 min each), making them impractical for routine clinical practice. 7 , 8 , 9 The Investigator’s Global Assessment (IGA) is a simple, validated physician assessment tool that measures overall disease severity and is an endpoint mandated by the US Food and Drug Administration in AD clinical studies. However, IGA fails to account for the extent of AD involvement, typically measured by the involved body surface area (BSA). 10
Previous studies have shown that the multiplied product IGAxBSA correlated well with EASI and other severity measures in patients with mild‐to‐severe AD. 3 , 11 However, one study was limited to a small (< 200 person), single‐site paediatric cohort and did not assess disease responsiveness over time. 3 Another study assessed a slightly larger cohort (n = 653), but also utilized the validated IGA measurement, vIGA, 11 and thus failed to test the tool with an alternative IGA instrument. Our aim in this analysis is to affirm the reliability of the IGAxBSA product as a simple and alternative measure for assessing disease severity, and also to test response to change using data from a large, multinational cohort of adult and paediatric patients who have participated in dupilumab clinical studies. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 As differences have been reported in the visual presentation of AD based on skin colour, particularly in black patients, 20 we also sought to address whether the IGAxBSA metric was comparable with EASI and SCORAD in racially diverse patients.
This study aimed to evaluate IGAxBSA composite as a simple, practical, alternative tool to EASI or SCORAD for the rapid assessment of disease severity and therapeutic responsiveness.
Patients and methods
Study design
This post hoc analysis includes data from five randomized, double‐blind, placebo‐controlled, parallel‐group, phase III clinical trials (RCTs) and an open‐label, long‐term extension study (OLE) in adults: LIBERTY AD SOLO 1 (NCT02277743) and LIBERTY AD SOLO 2 (NCT02277769), 12 LIBERTY AD SOLO‐CONTINUE (NCT02395133), 18 LIBERTY AD CAFÉ (NCT02755649), 14 LIBERTY AD CHRONOS (NCT02260986) 19 and LIBERTY AD OLE (NCT01949311). 15 , 21 Also included were data from two RCTs and an OLE study in children (aged 6–11 years) and adolescents (aged 12–17 years): LIBERTY AD ADOL (NCT03054428), 22 LIBERTY AD PEDS (NCT03345914) 16 and LIBERTY AD PEDS OLE (NCT02612454). 17 Detailed methodology, primary efficacy and safety results have been reported previously for all studies. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19
Briefly, SOLO 1 and SOLO 2 were two identically designed RCTs that evaluated the efficacy and safety of dupilumab monotherapy treatment [300 mg weekly (qw) or every 2 weeks (q2w)] for 16 weeks in adult patients with moderate‐to‐severe AD. SOLO‐CONTINUE was an RCT extension study of SOLO 1 and SOLO2, in which high‐responding patients were rerandomized to continue their dupilumab regimen (300 mg qw or q2w), receive dupilumab 300 mg every 4 or 8 weeks, or receive placebo for an additional 36 weeks. The CAFÉ and CHRONOS RCTs evaluated dupilumab treatment (300 mg qw or q2w) with concomitant topical corticosteroids (TCS) in adults with moderate‐to‐severe AD for 16 and 52 weeks, respectively. CAFÉ included adult patients with AD with inadequate response or intolerance to ciclosporin A, or for whom this treatment was medically inadvisable. LIBERTY AD OLE is an OLE study in patients receiving dupilumab 300 mg qw.
The RCT LIBERTY AD ADOL included patients aged 12–17 years with moderate‐to‐severe AD, randomized to placebo or dupilumab monotherapy (200 mg q2w for patients with baseline weight < 60 kg, 300 mg q2w for baseline weight ≥ 60 kg, or 300 mg every 4 weeks regardless of baseline weight). The LIBERTY AD PEDS RCT included patients aged 6–11 years with severe AD, randomized to placebo or dupilumab plus TCS (100 mg q2w for patients with a baseline weight < 30 kg, 200 mg q2w baseline weight ≥ 30 kg, or 300 mg every 4 weeks regardless of baseline weight). In the PEDS OLE study, all patients received dupilumab. In the original protocol, the dose regimen was 2 mg kg−1 or 4 mg kg−1 qw; however, after protocol amendment the weight‐based dose was changed to a fixed dose of 300 mg every 4 weeks with an uptitration (200 mg q2w in patients with baseline weight < 60 kg, or 300 mg q2w in patients with baseline weight ≥ 60 kg) in case of inadequate response (defined as failure to achieve IGA 0 or 1) at week 16.
In this analysis, data were pooled as follows: from all RCTs for all dupilumab arms (with or without TCS) and all placebo arms (with or without TCS) for weeks 16 (SOLO 1, SOLO 2, CAFÉ, LIBERTY AD ADOL, LIBERTY AD PEDS), 36 (SOLO‐CONTINUE) and 52 (CHRONOS), and for all OLE patients with data up to 52 weeks (PEDS OLE) and up to 152 weeks (LIBERTY AD OLE).
All trials were approved by the respective institutional review boards and were conducted in accordance with the ethical principles outlined in the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, 23 and applicable regulatory requirements. All patients or carers provided written informed consent before participating in the trial.
Assessments
The assessments used in this post hoc analysis included physician‐assessed objective measures of AD severity [IGA, EASI, BSA, SCORAD and objective SCORAD (o‐SCORAD), which excluded two subjective measures, itch and sleep disturbance], patient‐reported measures of AD symptoms [Patient‐Oriented Eczema Measure (POEM) and Peak Pruritus numerical rating scale (NRS)] and quality of life [QoL; Dermatology Life Quality Index (DLQI) in adults and Children’s Dermatology Life Quality Index (CDLQI) in the paediatric population].
IGA consisted of a five‐point rating scale ranging from 0 (clear) to 4 (severe) based on an overall assessment of AD skin lesions (erythema and papulation/infiltration). 24 BSA was assessed using the rule of nines and/or the palmar rule and was calculated as the sum of all affected areas, ranging from 0% to 100%.
The IGAxBSA score was calculated by multiplying the IGA score by the BSA, giving a score range of 0 to 400 (maximum IGA = 4 and maximum BSA = 100). IGAxBSA scores were also assessed by improvement from baseline as follows: IGAxBSA score improvement of at least 50% (IGAxBSA 50), at least 75% (IGAxBSA 75) and at least 90% (IGAxBSA 90). IGAxBSA threshold severity values used were as previously defined by Suh et al. as follows: mild 0–39, moderate 30·1–130, severe 130·1–400. 3
EASI score calculation was based on the sum of AD signs (erythema, induration/papulation, excoriation, lichenification) for each body region (head and neck, upper extremities, trunk, lower extremities) multiplied by weighted area scores (range 0–72), with higher scores indicative of greater disease severity. EASI scores were also assessed by measuring improvement from baseline as follows: improvement from baseline to end of study treatment of at least 50% (EASI 50), at least 75% (EASI 75) or at least 90% (EASI 90). Previously reported EASI scores for severity strata were also used and were defined as mild ≤ 7, moderate > 7 to ≤ 21, severe > 21. 25 SCORAD scores were calculated based on the extent, intensity and symptoms of AD, with total SCORAD ranging from 0 to 103, and the objective component 0–83. Previously reported severity strata were used as follows: mild < 25, moderate 25–50, severe > 50. 26 Peak Pruritus NRS assessed average itch intensity during the previous 24 h using a 0–10‐point scale, and POEM, a seven‐item validated questionnaire, was used to assess AD symptoms (range 0–28). Lastly, DLQI or CDLQI, 10‐item questionnaires with range 0–30, were used to assess quality of life. The CDLQI questionnaire was provided to patients or caregivers of patients aged < 18 years.
Statistical analysis
Validation of the previously reported IGAxBSA severity strata (defined as mild: 0–30, moderate: 30·1–130, severe: 130·1–400) was performed using a simple kappa correlation, anchored to EASI and SCORAD severity strata. The IGAxBSA composite vs. EASI was compared for dupilumab (with or without TCS), placebo (with or without TCS) or combined treatment groups in adult, paediatric or combined populations using the Spearman correlation coefficient. In addition, IGAxBSA composite vs. EASI and SCORAD was compared in a subgroup analysis by racial stratification (white, Asian, black).
Evaluation of disease responsiveness to change was performed using Spearman correlations of change in IGAxBSA score vs. EASI, and IGAxBSA vs. SCORAD, from baseline to end of treatment (weeks 16, 36 and 52) in RCTs. Agreement between IGAxBSA and EASI was further assessed based on intraclass correlation coefficients and response concordance rates, using improvements from baseline of 50%, 75% and 90% as the response thresholds in both scales. Concordance was calculated as:
Patients were considered overrated if response was achieved based on IGAxBSA but not achieved based on EASI. Patients were considered underrated if response was achieved based on EASI, but not achieved based on IGAxBSA.
Spearman coefficient correlations were also used to compare IGAxBSA with other severity measures at baseline in the overall populations: IGA, BSA, EASI, SCORAD, o‐SCORAD, POEM, Pruritus NRS and DLQI/CDLQI. Analyses were performed using SAS v9.4 or higher (SAS Institute, Inc., Cary, NC, USA).
Results
Patients
In this analysis, 3473 patients (2861 adult and 612 paediatric) were included from the RCT studies, randomized to dupilumab (n = 2307) or placebo (n = 1166) with or without TCS. In addition, 3045 patients (2677 adult and 368 paediatric) from the OLE studies were included, all of whom received dupilumab with or without TCS at the discretion of the investigator. The baseline demographics were similar between patients in the dupilumab and placebo groups in the combined total population, as well as in the racial subgroups (Table 1; and Table S1; see Supporting Information). Disease characteristics were also relatively well balanced between the placebo and dupilumab groups, in both the overall population and the racial subgroups.
Table 1.
Baseline demographics and disease characteristics of the overall population
| Placebo‐controlled studies (randomized controlled trials) | OLE studies | |||
|---|---|---|---|---|
| Placebo or placebo + TCS | Dupilumab or dupilumab + TCS | Combined | Dupilumab | |
| Total number | 1166 | 2307 | 3473 | 3045 |
| Age (years), mean (SD) | 33·1 (16·1) | 33·3 (16·6) | 33·2 (16·4) | 35·5 (16·1) |
| Male, n (%) | 674 (57·8) | 1322 (57·3) | 1996 (57·5) | 1792 (58·9) |
| Duration of AD (years), mean (SD) | 25·0 (15·1) | 24·8 (15·6) | 24·9 (15·4) | 27·1 (15·7) |
| EASI score, mean (SD) | 32·0 (15·3) | 28·8 (16·2) | 29·9 (16·0) | 16·3 (14·8) |
| BSA score, mean (SD) | 53·2 (25·0) | 48·1 (26·1) | 49·8 (25·9) | 28·6 (25·5) |
| IGA score, n (%) | ||||
| IGA 3 | 495 (42·5) | 908 (39·4) | 1403 (40·4) | 1398 (45·9) |
| IGA 4 | 590 (50·6) | 1071 (46·4) | 1661 (47·8) | 531 (17·4) |
| Pruritus NRS, mean (SD) | 7·0 (2·2) | 6·6 (2·5) | 6·8 (2·4) | NAa |
| SCORAD, mean (SD) | 64·8 (18·9) | 60·7 (22·3) | 62·1 (21·3) | 42·0 (22·3) |
| o‐SCORAD, mean (SD) | 52·9 (15·7) | 49·5 (18·3) | 50·7 (17·5) | 35·0 (18·3) |
| POEM, mean (SD) | 19·4 (6·9) | 18·3 (7·7) | 18·7 (7·4) | 14·7 (8·0) |
| DLQI or CDLQI, mean (SD) | 13·8 (7·8) | 13·0 (8·0) | 13·3 (8·0) | 8·4 (7·1) |
AD, atopic dermatitis; BSA, body surface area; CDLQI, Children’s Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; NRS, numerical rating scale; OLE, open‐label extension; o‐SCORAD, objective Scoring Atopic Dermatitis; POEM, Patient‐Oriented Eczema Measure; SCORAD, Scoring Atopic Dermatitis; TCS, topical corticosteroids. aNot collected for OLE patients.
Correlation of IGAxBSA with Eczema Area and Severity Index
Data for all studies were pooled, as no differences were observed when data were stratified by treatment or age (data not shown). In the pooled analysis, a statistically significant (P < 0·0001) positive correlation coefficient was achieved between IGAxBSA and EASI in measuring disease severity across all timepoints (r = 0·95; Figure 1). Similarly, a strong correlation was demonstrated in patients stratified by race, with no observed differences between white, black and Asian patients (r = 0·96, r = 0·95, r = 0·94, respectively, P < 0·0001; Figure 2).
Figure 1.
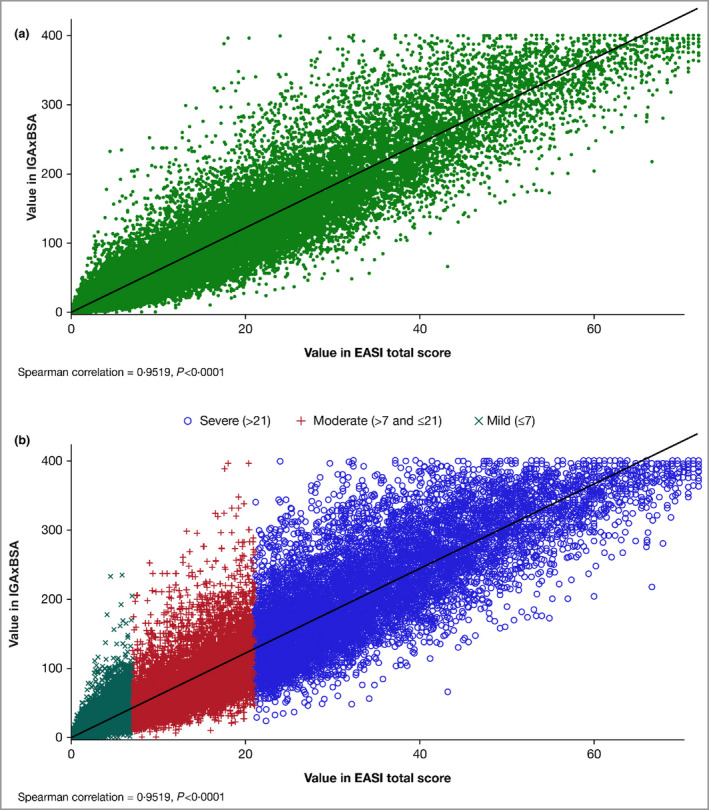
(a) Scatter plot of the correlation of disease severity metrics IGAxBSA vs. Eczema Area and Severity Index (EASI), all patients pooled (n = 36 657). Spearman correlation = 0·95, P < 0·0001. (b) Scatter plot of the correlation of disease severity metrics IGAxBSA vs. EASI by severity, all patients pooled (n = 36 657). Spearman correlation = 0·95, P < 0·0001. BSA, body surface area; IGA, Investigator’s Global Assessment.
Figure 2.
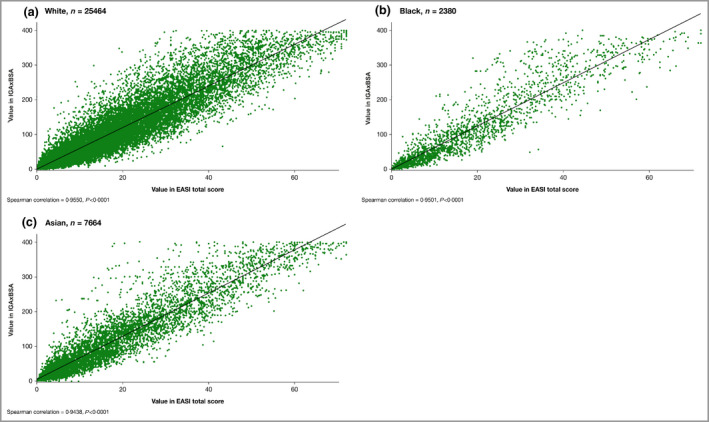
Scatter plot of the correlation of disease severity metrics IGAxBSA vs. Eczema Area and Severity Index (EASI), all patients pooled. (a) White, n = 25 464; Spearman correlation = 0·96, P < 0·0001; (b) black, n = 2380; Spearman correlation = 0·95, P < 0·0001; (c) Asian, n = 7664; Spearman correlation = 0·94, P < 0·0001. BSA, body surface area; IGA, Investigator’s Global Assessment.
Concordance between EASI 50/75/90 and IGAxBSA 50/75/90 up to week 52 is shown in Figure 3 for patients from the RCTs for pooled placebo treatment and pooled dupilumab treatment. There was an overall concordance (patients who achieved both EASI 50/75/90 and IGAxBSA 50/75/90 response or nonresponse) of between 88·0% and 93·9% at the end of treatment. Of nonconcordant patients, more were overrated with IGAxBSA (range 3·5–9·2%) vs. EASI than underrated (range 1·2–4·9%).
Figure 3.
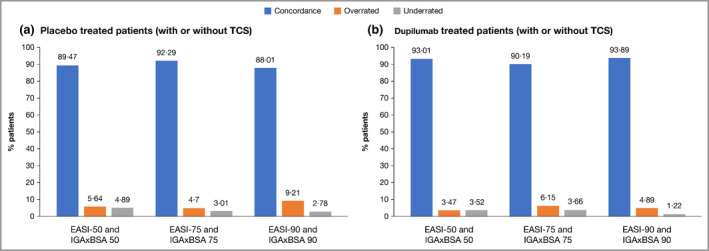
Concordance of IGAxBSA vs. EASI: patients in randomized controlled trials. (a) Placebo‐treated patients, with or without topical corticosteroids (TCS); (b) dupilumab‐treated patients, with or without TCS. BSA, body surface area; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment. Agreement between IGAxBSA and EASI scores was further assessed based on intraclass correlation coefficients and response concordance rates, using improvements from baseline of 50%, 75% and 90% as the response thresholds in both scales. Concordance was calculated as (number of patients without EASI response and without IGAxBSA response) + (number of patients with EASI response and with IGAxBSA response) / the total number of patients with sufficient data for evaluation. Patients were considered overrated if response was achieved based on IGAxBSA, but not achieved based on EASI. Patients were considered underrated if response was achieved based on EASI, but not achieved based on IGAxBSA. Note that the end of treatment visit for SOLO 1&2, CAFÉ, PEDs and ADOL is week 16, end of treatment visit for SOLO‐CONTINUE is week 36, and end of treatment visit for CHRONOS is week 52.
Correlation of IGAxBSA with Scoring Atopic Dermatitis
In the pooled analysis of all patients included in this analysis (RCT and OLE), a statistically significant (P < 0·0001) positive correlation coefficient was also achieved between IGAxBSA and SCORAD in measuring disease severity across all timepoints (r = 0·89; Figure 4). A strong correlation was demonstrated in patients stratified by race, with no observed differences among white, black and Asian patients (r = 0·90, r = 0·87, r = 0·88, respectively; P < 0·0001; Figure 5). Concordance between SCORAD 50/75/90 and IGAxBSA 50/75/90 up to week 52 is shown in Figure 6 for patients from the RCTs for pooled placebo‐treated and pooled dupilumab‐treated patients. Comparison with Figure 3 shows that there was lower overall concordance with SCORAD than with EASI at end of treatment. Of nonconcordant patients, again more were overrated with IGAxBSA (range 15·7–34·3%) than underrated (range 0–1·1%).
Figure 4.
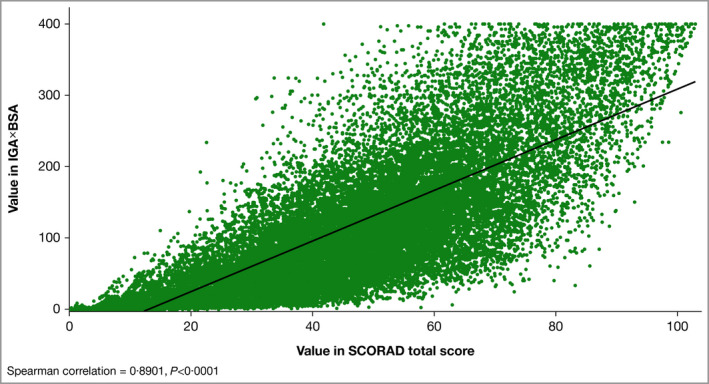
Scatter plot of the correlation of disease severity metrics IGAxBSA vs. Scoring Atopic Dermatitis (SCORAD), all patients pooled (n = 36 547). Spearman correlation = 0·89, P < 0·0001. BSA, body surface area; IGA, Investigator’s Global Assessment.
Figure 5.
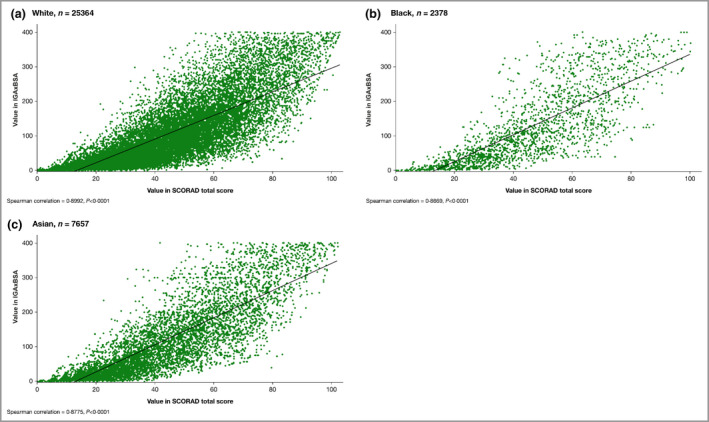
Scatter plot of the correlation of disease severity metrics IGAxBSA vs. Scoring Atopic Dermatitis (SCORAD), all patients pooled. (a) White, n = 25 364; Spearman correlation = 0·90, P < 0·0001; (b) black, n = 2378; Spearman correlation = 0·87, P < 0·0001; (c) Asian, n = 7657; Spearman correlation = 0·88, P < 0·0001. BSA, body surface area; IGA, Investigator’s Global Assessment.
Figure 6.
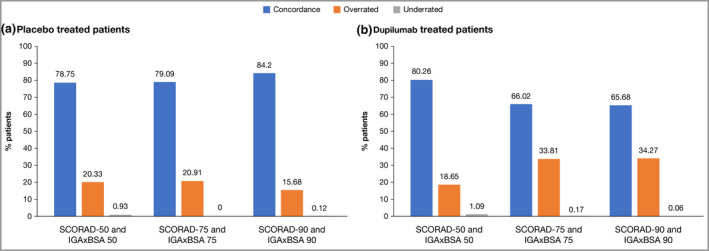
Concordance of IGAxBSA vs. Scoring Atopic Dermatitis (SCORAD): patients in randomized controlled trials. (a) Placebo‐treated patients, (b) dupilumab‐treated patients. BSA, body surface area; IGA, Investigator’s Global Assessment. Agreement between IGAxBSA and SCORAD scores was further assessed based on intraclass correlation coefficients and response concordance rates, using improvements from baseline of 50%, 75% and 90% as the response thresholds in both scales. Concordance was calculated as (number of patients without SCORAD response and without IGAxBSA response) + (number of patients with SCORAD response and with IGAxBSA response) / the total number of patients with sufficient data for evaluation. Patients were considered overrated if response was achieved based on IGAxBSA, but not achieved based on SCORAD. Patients were considered underrated if response was achieved based on SCORAD, but not achieved based on IGAxBSA. Note that the end of treatment visit for SOLO 1&2, CAFÉ, PEDs and ADOL is week 16, end of treatment visit for SOLO‐CONTINUE is week 36, and end of treatment visit for CHRONOS is week 52.
Responsiveness to change
There were strong and statistically significant correlations between IGAxBSA and EASI (r = 0·90; P < 0·0001) and between IGAxBSA and SCORAD (r = 0·76; P < 0·0001) in assessing disease change over time in patients from the RCT studies who received placebo or dupilumab, with or without TCS (Figure 7).
Figure 7.
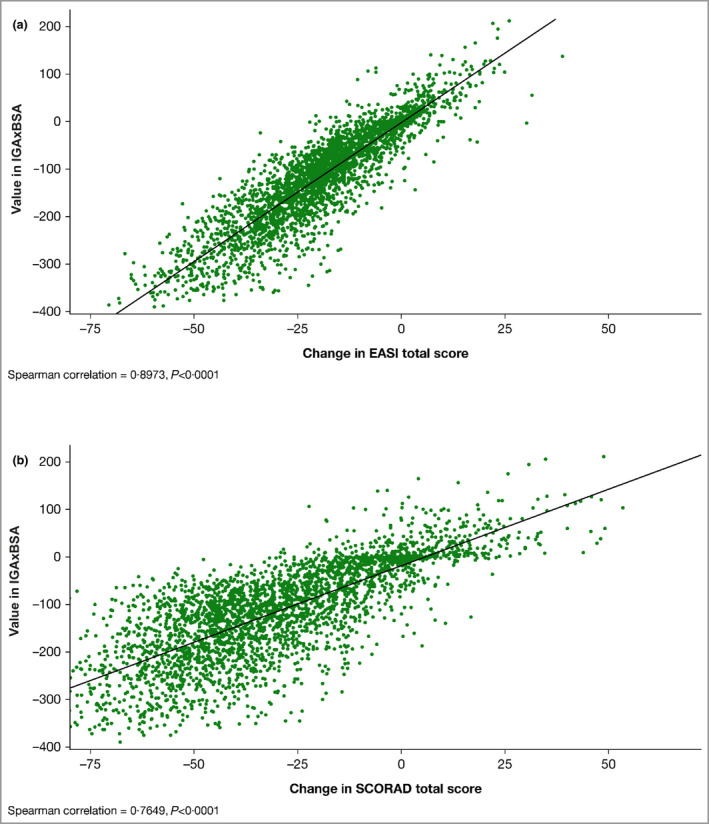
(a) Scatter plot of the correlation of disease severity metrics IGAxBSA vs. Eczema Area and Severity Index (EASI), change from baseline to end of treatment, randomized controlled trial (RCT) patients pooled, n = 3267. Spearman correlation = 0·90, P < 0·0001. (b) Scatter plot of the correlation of disease severity metrics IGAxBSA vs. Scoring Atopic Dermatitis, change from baseline to end of treatment, RCT patients pooled, n = 3250. Spearman correlation = 0·76, P < 0·0001. BSA, body surface area; IGA, Investigator’s Global Assessment. Note that the end of treatment visit for SOLO 1&2, CAFÉ, PEDs and ADOL is week 16, end of treatment visit for SOLO‐CONTINUE is week 36, and end of treatment visit for CHRONOS is week 52.
Validation of disease strata
Table 2 shows the correlation in IGAxBSA disease strata proposed by Suh et al. 3 (mild 0–30, moderate 30·1–130; severe 130·1–400) vs. previously reported EASI and SCORAD disease strata. A strong correlation was observed when IGAxBSA severity strata were anchored to EASI severity strata (mild ≤ 7, moderate > 7 to ≤ 21, severe > 21; Figure 1b), with an overall kappa correlation score of 0·74 (Table 2). A moderate correlation was found with IGAxBSA anchored to SCORAD (severity strata defined as mild < 25, moderate 25–50, severe > 50), with an overall kappa correlation score of 0·59 (Table 2).
Table 2.
Proposed severity threshold validation: simple kappa correlations of IGAxBSA vs. EASI and SCORAD for patients in randomized controlled trialsa
| Validation statistic | Placebo (with or without TCS), n = 1166 | Dupilumab (with or without TCS), n = 5352 | Total, N = 6518 |
|---|---|---|---|
| IGAxBSA vs. EASI | |||
| Kappa | 0·71 | 0·74 | 0·74 |
| Alpha’s standard error | 0·0053 | 0·0037 | 0·0030 |
| Kappa 95% upper limit | 0·73 | 0·75 | 0·74 |
| Kappa 95% lower limit | 0·70 | 0·73 | 0·73 |
| IGAxBSA vs. SCORAD | |||
| Kappa | 0·55 | 0·60 | 0·59 |
| Alpha’s standard error | 0·0063 | 0·0043 | 0·0035 |
| Kappa 95% upper limit | 0·56 | 0·61 | 0·60 |
| Kappa 95% lower limit | 0·54 | 0·59 | 0·59 |
BSA, body surface area; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; SCORAD, Scoring Atopic Dermatitis; TCS, topical corticosteroids. aEASI threshold values are mild ≤ 7, moderate 7–21, severe ≥ 21. Disease severity strata for IGAxBSA are mild 0–30, moderate 30·1–130, severe 130·1–400. SCORAD threshold values are mild < 25, moderate 25–50, severe > 50.
Correlation of IGAxBSA with disease severity measures at baseline
Correlations with disease severity measures at baseline (prior to treatment) are represented by heatmap analysis (Figure 8). IGAxBSA correlated better with the physician‐assessed objective measures BSA, EASI, IGA and SCORAD than with the patient‐ or caregiver‐reported subjective measures, such as Pruritus NRS, POEM or DLQI/CDLQI.
Figure 8.
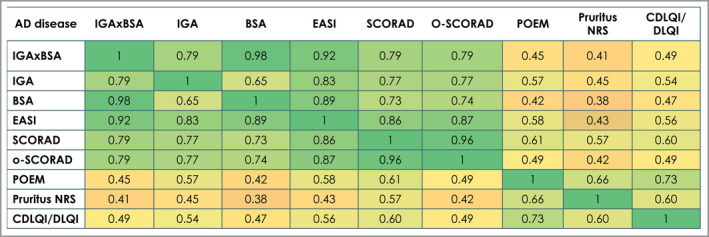
Heatmap correlation between disease measures in pooled studies (randomized controlled trials and open‐label extensions; Spearman correlations) from low to high (0–1), all correlations significant (P < 0·0001). Total N = 6518. BSA, body surface area; CDLQI, Children’s Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; NRS, numerical rating scale; o‐SCORAD, objective Scoring Atopic Dermatitis; POEM, Patient‐Oriented Eczema Measure; SCORAD, Scoring Atopic Dermatitis.
Discussion
The objective of this post hoc analysis was to evaluate the use of IGAxBSA as an alternative to EASI or SCORAD in assessing both AD severity and AD responsiveness in disease improvement in adult and paediatric patients who received dupilumab or placebo, with or without TCS. The results demonstrated a strong (using Spearman correlation coefficients) and statistically significant correlation between IGAxBSA and both EASI and SCORAD in capturing changes in AD disease severity. The correlations with EASI and/or SCORAD found in our study are consistent with those reported earlier in two smaller cohort studies of patients with mild‐to‐severe AD. 3 , 11
Using more than 36 000 datapoints from a multinational cohort of pooled adult and paediatric dupilumab placebo‐controlled RCT (n = 3473) and OLE studies (n = 3045), we confirmed that IGAxBSA correlated well with EASI and SCORAD score, irrespective of race (white, black, Asian), both at baseline and following treatment. Additionally, we found a strong agreement (87–90%) between disease improvement metrics of IGAxBSA 50/75/90 and EASI 50/75/90 in the RCT study population.
In patients with moderate‐to‐severe AD, EASI and SCORAD are often used as outcome measurements. However, both are time‐consuming to assess routinely in the clinic. Traditionally, IGA reflects only lesion severity but not disease extent (area affected), and is best used in combination with other metrics. 10 , 27 More recently, the vIGA has been introduced, which incorporates a nonquantitative assessment of disease extent to categorize ‘severe’ disease. 28 However, the vIGA was not utilized in the dupilumab trials; BSA was assessed quantitatively. Thus, IGAxBSA, as measured in this analysis, reflects both the severity and the extent of disease. It can be performed quickly and efficiently in clinical practice, unlike more time‐consuming severity measures such as SCORAD and EASI.
In this study we confirmed the finding by Suh et al. 3 that IGAxBSA correlated better with objective measures such as EASI, o‐SCORAD, SCORAD, IGA and BSA than with patient‐ or caregiver‐reported subjective measures such as Peak Pruritus NRS, POEM and DLQI/CDLQI. This is likely a result of the extent of itch and sleep loss differing from disease intensity, as reported in other studies, and reflects the heterogeneity of AD presentations. 29 , 30 In addition, previous reports have shown that variations in patient and caregiver outcomes can be due to other factors, such as a patient coping strategy or coexisting conditions. 25 , 31 , 32 Our study also validated the previously proposed severity thresholds for IGAxBSA from a small paediatric cohort 3 (mild 0–30, moderate 30·1–130, severe 130·1–400), anchored to the EASI disease severity strata (mild, moderate and severe). 7 , 25 Previously proposed strata from the cohort study comparing vIGAxBSA against EASI severity strata in 653 patients used similar thresholds, including also a correlation to the EASI ‘clear’ 0 score (clear 0–0·1, almost clear 0·1–3·2, mild 3·2–39, moderate 39–142·7, severe 142·7–400), with a concordance of r = 0·81, P < 0·0001. 11 However, correlation of IGAxBSA with the commonly used SCORAD disease strata (mild < 25, moderate 25–50, severe > 50) 25 was only moderate; this is likely related to the inclusion of patient‐reported outcomes (itch and sleep) in the SCORAD scale and not in EASI. Additional studies are needed to define whether alternative strata can provide a stronger correlation with both SCORAD and EASI scores. 3
Lastly, we analysed the use of IGAxBSA as a tool to assess the overall response to treatment. In clinical studies this is often expressed as improvements in EASI of 50%, 75% or 90%. 33 We found excellent concordance between IGAxBSA and EASI improvement strata of 50%, 75% and 90%. However, concordance was higher in dupilumab‐treated patients. In addition, concordance appeared to weaken with higher extent of improvement. However, as this analysis used a pooled dataset combining multiple treatment arms, specific conclusions about treatments cannot be drawn. Lower concordance was observed in this analysis between SCORAD 50, 75 and 90 and IGAxBSA 50, 75 and 90. Recent analyses have linked ≥ 50% improvement in EASI and ≥ 35% improvement in SCORAD as equivalent, 34 and it would be interesting to analyse other possible SCORAD improvement strata vs. IGAxBSA. A limitation of this study is that it was a retrospective post hoc analysis.
In conclusion, these data support the use of IGAxBSA as an alternative measure for the rapid and routine assessment of disease severity and improvement over time in routine clinical practice compared with the more complex and time‐consuming measures EASI or SCORAD in patients with AD, regardless of race.
Author Contribution
Amy Paller: Conceptualization (equal); Investigation (equal); Methodology (equal); Writing‐review & editing (equal). Jerry Tan: Investigation (equal); Writing‐review & editing (equal). Jerry Bagel: Investigation (equal); Writing‐review & editing (equal). Ana B. Rossi: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Brad Shumel: Conceptualization (equal); Methodology (equal); Project administration (equal); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Haixin Zhang: Data curation (equal); Formal analysis (lead); Methodology (equal); Visualization (lead). Alvina Abramova: Conceptualization (equal); Funding acquisition (equal); Methodology (equal); Project administration (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
Supporting information
Table S1 Baseline demographics and disease characteristics by race, all patients pooled.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.
Acknowledgments
We thank the patients and investigators who participated in the studies, El‐Bdaoui Haddad, Linda Williams and Adriana Mello.
Funding sources This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Medical writing and editorial support was provided by Carolyn Ellenberger, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc., according to the Good Publication Practice guideline.
Conflicts of interest A.S.P. has served as an investigator and/or consultant for AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Forte, Incyte, Janssen, LEO, Lifemax, Novartis, Pfizer, Rapt, Regeneron and Sanofi. J.K.L.T. has served as an advisor, consultant, investigator and/or speaker for Lilly, Pfizer, Regeneron Pharmaceuticals, Inc. and Sanofi. J.B. is a consultant or investigator for AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and Regeneron; a speaker for AbbVie, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis and Regeneron; and an investigator for AbbVie, Celgene, DS Biopharma, Eli Lilly, Janssen, LEO Pharma, Novartis and Regeneron. A.B.R. is an employee of and may hold stock and/or stock options in Sanofi Genzyme. B.S. and H.Z. are employees and shareholders of Regeneron Pharmaceuticals, Inc. A.A. is a former employee and shareholder of Regeneron Pharmaceuticals, Inc.
Data availability Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication have been approved by major health authorities (e.g. FDA, EMA, PMDA), if there is legal authority to share the data and there is not a reasonable likelihood of participant re‐identification. Submit requests to https://www.clinicalstudydatarequest.com/Login.aspx?ReturnUrl=%2FSubmission.aspx%3Fgroupid%3DENQUIRY.
Regeneron Pharmaceuticals, Inc. is the former affiliation of A.A.
Plain language summary available online
References
- 1. Wollenberg A, Barbarot S, Bieber T et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32:657–82. [DOI] [PubMed] [Google Scholar]
- 2. Wollenberg A, Barbarot S, Bieber T et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol 2018; 32:850–78. [DOI] [PubMed] [Google Scholar]
- 3. Suh TP, Ramachandran D, Patel V et al. Product of Investigator Global Assessment and Body Surface Area (IGAxBSA): a practice‐friendly alternative to the Eczema Area and Severity Index to assess atopic dermatitis severity in children. J Am Acad Dermatol 2020; 82:1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanifin JM, Thurston M, Omoto M et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001; 10:11–18. [DOI] [PubMed] [Google Scholar]
- 5. Schmitt J, Spuls PI, Thomas KS et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014; 134:800–7. [DOI] [PubMed] [Google Scholar]
- 6. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993; 186:23–31. [DOI] [PubMed] [Google Scholar]
- 7. Leshem YA, Hajar T, Hanifin JM et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol 2015; 172:1353–7. [DOI] [PubMed] [Google Scholar]
- 8. Zhao CY, Tran AQ, Lazo‐Dizon JP et al. A pilot comparison study of four clinician‐rated atopic dermatitis severity scales. Br J Dermatol 2015; 173:488–97. [DOI] [PubMed] [Google Scholar]
- 9. Gånemo A, Svensson Å, Svedman C et al. Usefulness of Rajka & Langeland Eczema Severity Score in clinical practice. Acta Derm Venereol 2016; 96:521–4. [DOI] [PubMed] [Google Scholar]
- 10. Silverberg JI, Simpson EL, Ardeleanu M et al. Dupilumab provides important clinical benefits to patients with atopic dermatitis who do not achieve clear or almost clear skin according to the Investigator’s Global Assessment: a pooled analysis of data from two phase III trials. Br J Dermatol 2019; 181:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silverberg JI, Lei D, Yousaf M et al. Measurement properties of the product of investigator’s global assessment and body surface area in children and adults with atopic dermatitis. J Eur Acad Dermatol Venereol 2021; 35:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simpson EL, Bieber T, Guttman‐Yassky E et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375:2335–48. [DOI] [PubMed] [Google Scholar]
- 13. Blauvelt A, Gooderham M, Foley P et al. Long‐term management of moderate to severe atopic dermatitis with dupilumab and concomitant topical corticosteroids: a 1‐year randomized, placebo‐controlled phase 3 trial (CHRONOS). Intern Med J 2017; 47:25–67. [DOI] [PubMed] [Google Scholar]
- 14. de Bruin‐Weller M, Thaçi D, Smith CH et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trial. Br J Dermatol 2018; 178:1083–101. [DOI] [PubMed] [Google Scholar]
- 15. Deleuran M, Thaçi D, Beck LA et al. Dupilumab shows long‐term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open‐label extension study. J Am Acad Dermatol 2020; 82:377–88. [DOI] [PubMed] [Google Scholar]
- 16. Paller AS, Siegfried EC, Thaçi D et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double‐blinded, placebo‐controlled phase 3 trial. J Am Acad Dermatol 2020; 83:1282–93. [DOI] [PubMed] [Google Scholar]
- 17. Cork MJ, Thaçi D, Eichenfield LF et al. Dupilumab in adolescents with uncontrolled moderate‐to‐severe atopic dermatitis: results from a phase IIa open‐label trial and subsequent phase III open‐label extension. Br J Dermatol 2020; 182:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Worm M, Simpson EL, Thaçi D et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a randomized clinical trial. JAMA Dermatol 2020; 156:131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blauvelt A, de Bruin‐Weller M, Gooderham M et al. Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017; 389:2287–303. [DOI] [PubMed] [Google Scholar]
- 20. Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups – variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol 2018; 27:340–57. [DOI] [PubMed] [Google Scholar]
- 21. Beck LA, Thaçi D, Deleuran M et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an open‐label study of adults with moderate‐to‐severe atopic dermatitis. Am J Clin Dermatol 2020; 21:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simpson EL, Paller AS, Siegfried EC et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020; 156:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Battisti WP, Wager E, Baltzer L et al. Good publication practice for communicating company‐sponsored medical research: GPP3. Ann Intern Med 2015; 163:461–4. [DOI] [PubMed] [Google Scholar]
- 24. Futamura M, Leshem YA, Thomas KS et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol 2016; 74:288–94. [DOI] [PubMed] [Google Scholar]
- 25. Chopra R, Vakharia PP, Sacotte R et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol 2017; 177:1316–21. [DOI] [PubMed] [Google Scholar]
- 26. Wollenberg A, Oranje A, Deleuran M et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol 2016; 30:729–47. [DOI] [PubMed] [Google Scholar]
- 27. Paller AS, Bansal A, Simpson EL et al. Clinically meaningful responses to dupilumab in adolescents with uncontrolled moderate‐to‐severe atopic dermatitis: post‐hoc analyses from a randomized clinical trial. Am J Clin Dermatol 2020; 21:119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International Eczema Council . Validated Investigator Global Assessment scale for Atopic Dermatitis. Available at: https://www.eczemacouncil.org/assets/docs/Validated‐Investigator‐Global‐Assessment‐Scale_vIGA‐AD_2017.pdf (last accessed 22 November 2021).
- 29. Hon KL, Leung TF, Wong Y et al. Lesson from performing SCORADs in children with atopic dermatitis: subjective symptoms do not correlate well with disease extent or intensity. Int J Dermatol 2006; 45:728–30. [DOI] [PubMed] [Google Scholar]
- 30. Fishbein AB, Mueller K, Kruse L et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case–control study. J Am Acad Dermatol 2018; 78:336–41. [DOI] [PubMed] [Google Scholar]
- 31. Schmitt J, Langan S, Williams HC. What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol 2007; 120:1389–98. [DOI] [PubMed] [Google Scholar]
- 32. Vakharia PP, Chopra R, Sacotte R et al. Severity strata for five patient‐reported outcomes in adults with atopic dermatitis. Br J Dermatol 2018; 178:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Snast I, Reiter O, Hodak E et al. Are biologics efficacious in atopic dermatitis? A systematic review and meta‐analysis. Am J Clin Dermatol 2018; 19:145–65. [DOI] [PubMed] [Google Scholar]
- 34. Silverberg JI, Lei D, Yousaf M et al. What are the best endpoints for Eczema Area and Severity Index and Scoring Atopic Dermatitis in clinical practice? A prospective observational study. Br J Dermatol 2021; 184:888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline demographics and disease characteristics by race, all patients pooled.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.


