Abstract
Background and purpose
Anti‐acetylcholine receptor (AChR) antibodies (ab) in the serum are detected in most patients with generalized myasthenia gravis (MG) and used as a diagnostic tool. The aim of this study was to analyse a possible association between anti‐AChR‐ab serum levels and clinical improvement of MG.
Methods
The Maastricht University Medical Center is a centre of expertise for the treatment of MG. Between 1997 and 2020, more than 4000 anti‐AChR‐ab blood samples were measured for clinical care using a quantitative radioimmunoassay technique. These results, in combination with clinical status obtained from the patients’ electronic patient files, were retrospectively analysed by a single blinded clinician. Symptoms of MG were classified using the Myasthenia Gravis Foundation of America (MGFA) scale.
Results
In total, 90 anti‐AChR‐ab‐positive MG patients with 837 blood samples were included. The median follow‐up time was 72 months. The majority of the included patients were women (61.1%), were on immunosuppressive drug therapy (88.9%), and underwent a thymectomy (54.4%). Multilevel logistic regression analysis showed a significantly inverse association between change in anti‐AChR‐ab level and the odds of MGFA improvement (per 10% decrease of anti‐AChR‐ab level: odds ratio 1.21, 95% confidence interval 1.12–1.31; p < 0.001).
Conclusions
A change in anti‐AChR‐ab serum level is associated with clinical status in patients with MG. Analyses of anti‐AChR‐ab are not only useful for diagnostics but also in follow‐up of adult symptomatic patients with MG. The use of repetitive anti‐AChR‐ab serum levels might be valuable in long‐term monitoring for clinical improvement in patients with MG, however, further research is required for specific recommendations.
Keywords: anti‐acetylcholine receptor antibodies, clinical improvement, follow‐up, myasthenia gravis, radioimmunoassay
Multilevel logistic regression analysis showed a significant inverse association between change in anti‐acetylcholine receptor (AchR) antibody (ab) level and the odds of improvement, measured by the Myasthenia Gravis Foundation of America. A change in anti‐AChR‐ab serum level is associated with clinical status in patients with MG. Repetitive measurements of anti‐AChR‐ab serum levels can objectively assist in the follow‐up of a patient with MG.

INTRODUCTION
In myasthenia gravis (MG), the nicotinic acetylcholine receptor (AChR) is one of the targets for autoantibodies. MG is a neuromuscular autoimmune disease featuring fluctuating muscle weakness and exhaustion worsening upon exertion [1]. In MG, one of the pathological mechanisms in anti‐AChR‐positive patients is the loss of postsynaptic AChR due to anti‐AChR antibodies (ab). The decrease of functional AChR at the endplate leads to less binding of acetylcholine, which results in muscle weakness [2, 3]. Depending on the affected muscle groups, MG is more defined in ocular MG (diplopia and ptosis), bulbar MG (dysarthria and dysphagia), or generalized MG (muscle weakness to the limbs and neck). MG has high morbidity when treated inadequately. Serum anti‐AChR‐ab are present in 90% of patients with generalized MG and in 50% of patients with ocular MG [4]. One in 10 patients with MG develops a thymoma, and vice versa, one in four patients with a thymoma have MG. The presence of anti‐AChR‐ab is found in most patients with thymomatous MG [5, 6].
In clinical practice, the majority of anti‐AChR‐ab analyses are used to confirm the diagnosis of MG. Although MG is considered a life‐long autoimmune disease, there is no consensus on the use of anti‐AChR‐ab during the years of follow‐up. Previously, multiple studies have been performed on this topic. Some of these studies reported a significant association between change in anti‐AChR‐ab serum levels and clinical severity, especially after thymectomy or when patients used immunosuppressants [7, 8, 9, 10]. Other studies did not report a significant association, but these studies did not have consistent follow‐up durations, there was differential use of serial dilutions in the assay kit, or they included a relatively small number of patients [11, 12, 13].
Anti‐AChR‐ab are measured with a radioimmunoprecipitation assay (RIA). The sensitivity and specificity are high at 87% and 100%, respectively [14, 15]. For follow‐up of MG patients, quantitative values of the anti‐AChR‐ab level are essential and this often requires serial dilutions due to the restricted measuring range of both enzyme‐linked immunosorbent assay and RIA [16].
The primary aim of this retrospective study was to analyse the longitudinal association between reduction in serum anti‐AChR‐ab levels and clinical symptoms, using a clinically relevant follow‐up duration and the most accurate assay kit available, with inclusion of a substantial number of patients.
PATIENTS AND METHODS
In this retrospective study, pre‐existing data previously used for regular healthcare in the Maastricht University Medical Center (MUMC+) were obtained from the patients’ electronic patient files. The MUMC+ is a national centre of expertise for the treatment of MG and thymomas in the Netherlands. This study was approved by the medical ethical commission of the MUMC+ in 2020 (application number: 2018‐0865), no informed consent was necessary in this retrospective design. In clinical care, blood samples were analyzed for anti‐AChR‐ab concentration using an RIA (IBL International GmbH, Hamburg, Germany) in the central diagnostic laboratory of the MUMC+ between 1997 and 2020. Only patients who were at least 18 years old at the time of the first blood sample taken in the MUMC+ and who had two or more positive anti‐AChR‐ab blood samples (defined as >0.25 nmol/L) were included. Patients who had a negative test were excluded. Patients with subclinical MG were excluded because it is known that these patients have positive anti‐AChR‐ab at baseline, although the development of clinical MG may become manifest many months, or even years, later [6]. Patients who were in total clinical remission at the first anti‐AChR‐ab test, without relapse after the first test, were excluded as well because these patients were not able to change in terms of clinical improvement. In case of incomplete clinical data in the electronic patient files, the patient was excluded. A blinded clinician retrospectively determined the clinical status according to the Myasthenia Gravis Foundation of America (MGFA [17]) scale around the time of the blood sample, with a maximum variation of 1 month. The baseline for the results was defined as the time of the first measured anti‐AChR‐ab serum level in the MUMC+. Clinical improvement was determined, using a binary model (yes/no) and was based on the MGFA classification.
Acetylcholine receptor antibody assay technique
All AChR‐ab assays were performed by trained laboratory technicians in the immunodiagnostic laboratory in the MUMC+. Quantitative assessment of anti‐AChR‐ab was performed on human serum according to the instructions of the manufacturer. The AChRs, labelled with I‐125‐alpha‐bungarotoxin, from human muscle were used as an antigen. Anti‐AChR‐ab present in the patients’ serum were bound to the labelled receptors. Radioactivity was determined using a gamma counter after excess labels were washed out. Based on a standard curve (0.2–8.0 nmol/L) the outcomes were translated into an anti‐AChR‐ab concentration (nmol/L). Results above 2.0 nmol/L were further stepwise diluted (1/10, 1/20, 1/100 and 1/500), as appropriate. The inter‐assay variation of the assay kit used was reported to be 7% (range 2.8%–13.1%) by IBL International GmbH [18]. Based on internal quality control in the MUMC+, the inter‐assay variation in clinical practice appeared to be 10%.
Statistical analysis
Descriptive statistics are reported as mean and standard deviation (SD), or median and range, as appropriate. Statistical analysis was performed with SPSS statistical software (IBM SPSS Statistics for Windows, version 25.0. Armonk, NY: IBM Corp) and R (version 3.6.1). Statistical significance was considered with α < 0.05. Percentage of change and baseline anti‐AChR‐ab serum level were used as continuous (per 10% decrease), and categorical (decrease ≥50%, decrease 0%–50%, and improvement) variables. In this study, the MGFA was used as a tool for measuring clinical improvement on the MGFA scale between each measurement (binary outcome: yes/no), not for associations with severity of disease. Multilevel logistic regression analyses were performed to assess the relationship between the percentage change in anti‐AChR‐ab concentration on clinical improvement. A random intercept was calculated for each participant to incorporate variability between subjects. These models were additionally adjusted for time since baseline measurement (months), age (years) and sex (male/female), immunosuppressive medication use (yes/no), thymectomy (yes/no) and time since thymectomy in days (continuous, centered). Results are reported as odds ratios (ORs) including 95% confidence intervals (CIs). Additional subgroup analyses were performed among those who had undergone thymectomy or were diagnosed with a thymoma (Subgroup analysis 1). Chi‐squared tests of independence and Student's t‐tests were performed to compare categorical and continuous variables. Additional binary logistic regression analyses were performed to estimate the association between the percentage of change in anti‐AChR‐ab concentration and clinical improvement (Subgroup analysis 2). A third subgroup analysis was performed to assess possible (baseline) differences between patients who had a fast‐ or slow response of anti‐AChR‐ab to immunosuppressive drugs over time (Subgroup analysis 3). Fast responders were defined as patients on immunotherapy, with an anti‐AChR‐ab serum level reduction of at least 50% in 12 months. Slow responders were defined as patients on immunotherapy, with less than 50% reduction in anti‐AChR‐ab serum level in 24 months. Figure S1 (Data S1) shows an example of the curve of two fast responders and two slow responders.
RESULTS
From January 1997 to December 2020, more than 4000 blood samples of 1912 patients were analyzed for anti‐AChR‐ab using an RIA at the MUMC+. A flowchart of this study is shown in Figure 1. The result of the test was positive (>0.25 nmol/L) in 481 patients. After exclusion, 90 patients with a total of 837 blood samples (mean 10.2, SD ±6.6) were included. The characteristics of these patients are shown in Table 1. Excluded patients had a negative anti‐AChR‐ab test (N = 1.431), had an anti‐AChR‐ab serum level measured with less than 12 months’ follow‐up (N = 348), had remission of MG without relapse after baseline (N = 7), had subclinical MG (N = 7), were children (N = 4), or had incomplete data (N = 25). Of the 90 included patients, the majority were women (61.1%), were on immunosuppressive drug therapy (88.9%), and underwent a thymectomy (54.4%). Intravenous immunoglobulins (IVIg) assessment or plasmapheresis was performed in seven patients during a myasthenic crisis or severe exacerbation. The median (range) follow‐up time was 72 (16–223) months. The median (range) time between diagnosis of MG and baseline measurement was 7 (0–300) months. The median (range) age at baseline was 53.5 (18–83) years. The baseline anti‐AChR‐ab concentration ranged from 0.36 to 487 nmol/L. In 7.8% of the patients, remission of MG (MGFA score 0) was reached at baseline, with a relapse of symptoms afterwards, meaning that these patients were already treated, before the anti‐AChR‐ab concentrations were analyzed in the MUMC+. Over half the study population (58.8%) had a baseline MGFA score in class I (ocular weakness) or II (mild weakness).
FIGURE 1.
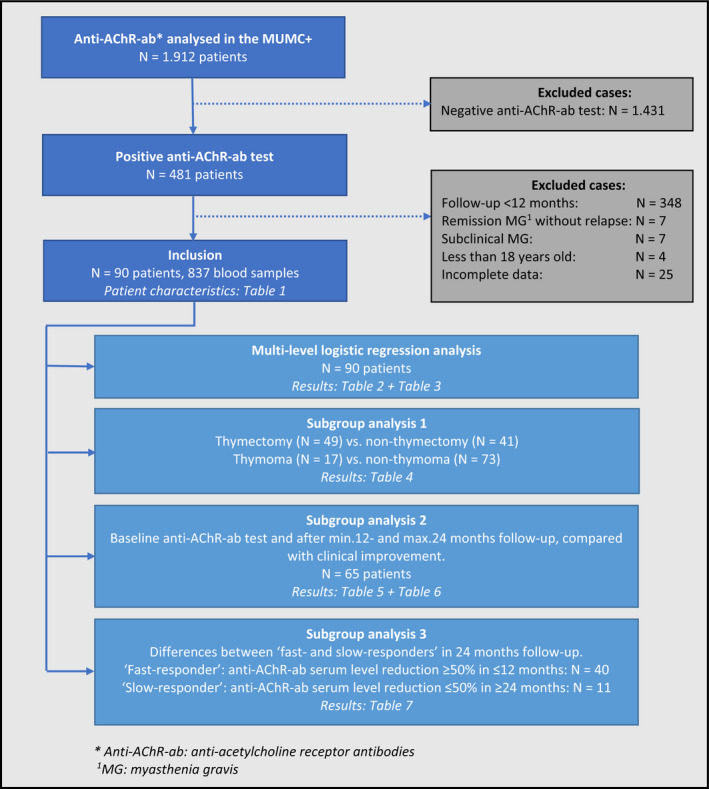
Flowchart of the study. AChR‐ab, anti‐acetylcholine receptor antibody; MG, myasthenia gravis [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Baseline patient characteristics of included myasthenia gravis patients
| Characteristics | Data |
|---|---|
| Patients, n | 90 |
| Females, n (%) | 55 (61.1) |
| Age at baseline a , median years (range) | 53.5 (18–83) |
| Duration of MG at baseline a , median months (range) | 7 (0–300) |
| Follow‐up, median months (range) | 72 (range: 16–223) |
| Therapy at baseline a , n (%) | |
| None | 0 (0.0) |
| Anticholinesterase monotherapy | 10 (11.1) |
| Immunosuppressive drug therapy | 80 (88.9) |
| MGFA classification at baseline a , n (%) | |
| 0 (remission) | 7 (7.8) |
| I | 21 (23.3) |
| IIA | 13 (14.4) |
| IIB | 19 (21.1) |
| IIIA | 10 (11.1) |
| IIIB | 11 (12.2) |
| IVA | 4 (4.4) |
| IVB | 3 (3.3) |
| V | 2 (2.2) |
| Remission of MG after baseline a , n (%), median months (range) | 76 (84.4), 19 (2–139) |
| Thymectomy, n (%) | 49 (54.4) |
| Thymectomy before baseline a , n (%) | 13 (26.5) |
| Thymectomy 0–12 months after baseline, n (%) | 34 (69.4) |
| Thymectomy >12 months after baseline, n (%) | 2 (4.1) |
| Thymoma, n (%) | 17 (18.9) |
| WHO histological type of thymoma, n (%) | |
| A | 0 (0.0) |
| AB | 4 (23.5) |
| B1 | 3 (17.7) |
| B2 | 5 (29.4) |
| B3 | 4 (23.5) |
| C | 0 (0.0) |
| Unknown | 1 (5.9) |
| Staging of thymoma, n (%) | |
| Early stage thymomas b | 12 (70.6) |
| Advanced stage thymomas c | 2 (11.8) |
| Unknown | 3 (17.6) |
Abbreviations: MG, myasthenia gravis; MGFA, Myasthenia Gravis Foundation of America; WHO, World Health Organization.
At baseline: first analyses of anti‐acetylcholine receptor antibody serum levels in the Maastricht University Medical Center.
Early‐stage thymomas: Masaoka‐Koga stages I and II/TNM < T3N0M0.
Advanced‐stage thymomas: Masaoka‐Koga stages III and IV/TNM ≥ T3N0M0.
During follow‐up, 76 patients (84.4%) went into remission (MGFA score 0) after a median (range) of 19 (2–139) months. Of the 14 patients who experienced no remission, the majority were women (64.3%), had immunosuppressive therapy (64.3%), and did not have a thymoma (85.7%). Furthermore, the group who experienced no remission had a shorter total follow‐up time, compared with the patients who did experience remission during follow‐up (46.4 vs. 85.8 months). Of the 14 patients without remission, 40% experienced an increment of the anti‐AChR‐ab during follow‐up, 46.7% had an unchanged anti‐AChR‐ab serum level and 13.3% experienced a decrease of anti‐AChR‐ab serum level. Around one‐third of the patients without remission had ocular MG (35.7%). In total, 49 patients (54.4%) underwent a thymectomy, of whom 17 patients were diagnosed with a thymoma. The thymomas were mostly diagnosed as type AB (23.5%), type B2 (29.4%) or type B3 (23.5%).
Multi‐level logistic regression analysis showed a significant inverse association between change in anti‐AChR‐ab and the odds of MGFA score improvement (per 10% decrease of anti‐AChR‐ab serum level: OR 1.21, CI 1.12–1.31; p < 0.001), as shown in Table 2. Similar results were observed when we additionally adjusted for confounders such as age (years) and sex (male/female; Model 2), and immunosuppressive medication use, thymectomy and time since thymectomy (Model 3). Patients who had undergone a thymectomy had a numerically lower OR than patients without a thymectomy (per 10% decrease of anti‐AChR‐ab serum level: OR 1.15, CI 1.05–1.25, p = 0.002, vs. OR 1.45, CI 1.17–1.80, p = 0.001), as shown in Table 3. However, no significant interaction was observed between the groups (p = 0.067). Patients without a thymoma had a numerically lower OR than patients with a thymoma (per 10% decrease of anti‐AChR‐ab serum level: OR 1.19, CI 1.10–1.30, p < 0.001 vs. OR 1.36, CI 1.10–1.69, p = 0.005).
TABLE 2.
Change in anti‐acetylcholine receptor antibody concentration (multivariable logistic regression)
| Obs | Event, n (%) | n | OR | 95% CI | p value | |
|---|---|---|---|---|---|---|
| Model 1 a | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 53 | 46 (87) | 4.39 | 1.79–10.74 | 0.001 | |
| Decrease 0%–50% | 158 | 96 (61) | Reference | Reference | Reference | |
| Increase | 84 | 29 (35) | 0.31 | 0.17–0.57 | <0.001 | |
| Continuous (per 10% decrease) | 295 | 171 (58) | 90 | 1.21 | 1.12–1.31 | <0.001 |
| Model 2 b | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 53 | 46 (87) | 4.11 | 1.67–10.15 | 0.002 | |
| Decrease 0%–50% | 158 | 96 (61) | Reference | Reference | Reference | |
| Increase | 84 | 29 (35) | 0.32 | 0.18–0.58 | <0.001 | |
| Continuous (per 10% decrease) | 295 | 171 (58) | 90 | 1.21 | 1.12–1.30 | <0.001 |
| Model 3 c | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 47 | 41 (87) | 3.65 | 1.40–9.54 | 0.008 | |
| Decrease 0%–50% | 143 | 88 (62) | Reference | Reference | Reference | |
| Increase | 77 | 27 (35) | 0.31 | 0.17–0.57 | <0.001 | |
| Continuous (per 10% decrease) | 267 | 156 (58) | 82 d | 1.19 | 1.10–1.29 | <0.001 |
Event was defined as improvement in MGFA score.
Abbreviations: AChR‐ab, anti‐acetylcholine receptor; MGFA, Myasthenia Gravis Foundation of America; n, number of individuals; Obs, number of observations.
Additionally adjusted for time since baseline measurement (months; continuous).
Model 1 additionally adjusted for age (years), and sex (male/female).
Model 2 additionally adjusted for use of immunosuppressive medication (yes/no), thymectomy (yes/no), and time since thymectomy (days (centered), continuous).
Lower number of patients due to missing values for immunosuppressive medication use, and/or (time since) thymectomy.
TABLE 3.
Change in anti‐acetylcholine receptor antibody concentration in patients with/without thymoma and/or thymectomy (multivariable logistic regression)
| Obs | Event n (%) | n | OR | 95% CI | p value | |
|---|---|---|---|---|---|---|
| No thymectomy | ||||||
| Model 1 a | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 27 | 22 (81) | 1.89 | 0.48–7.48 | 0.367 | |
| Decrease 0%–50% | 59 | 43 (73) | Reference | Reference | Reference | |
| Increase | 24 | 5 (21) | 0.05 | 0.01–0.27 | 0.001 | |
| Continuous (per 10% decrease) | 110 | 70 (64) | 41 | 1.45 | 1.17–1.80 | 0.001 |
| Model 2 b | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 27 | 22 (81) | 1.68 | 0.40–6.99 | 0.479 | |
| Decrease 0%–50% | 59 | 43 (73) | Reference | Reference | Reference | |
| Increase | 24 | 5 (21) | 0.05 | 0.09–0.26 | <0.001 | |
| Continuous (per 10% decrease) | 110 | 70 (64) | 41 | 1.45 | 1.17–1.80 | 0.001 |
| Thymectomy | ||||||
| Model 1 a | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 26 | 24 (92) | 10.78 | 2.38–48.73 | 0.002 | |
| Decrease 0%–50% | 99 | 53 (54) | Reference | Reference | Reference | |
| Increase | 60 | 24 (40) | 0.58 | 0.30–1.13 | 0.107 | |
| Continuous (per 10% decrease) | 185 | 101 (55) | 49 | 1.15 | 1.05–1.25 | 0.002 |
| Model 2 b | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 26 | 24 (92) | 10.49 | 2.32–47.38 | 0.002 | |
| Decrease 0%–50% | 99 | 53 (54) | Reference | Reference | Reference | |
| Increase | 60 | 24 (40) | 0.57 | 0.29–1.12 | 0.101 | |
| Continuous (per 10% decrease) | 185 | 101 (55) | 49 | 1.14 | 1.05–1.25 | 0.003 |
| p‐interaction c (thymectomy: yes vs. no) | 0.067 | |||||
| No thymoma | ||||||
| Model 1 a | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 39 | 33 (85) | 3.56 | 1.31–9.65 | 0.013 | |
| Decrease 0%–50% | 137 | 84 (61) | Reference | Reference | Reference | |
| Increase | 69 | 24 (35) | 0.30 | 0.16–0.58 | <0.001 | |
| Continuous (per 10% decrease) | 245 | 141 (58) | 73 | 1.19 | 1.10–1.30 | <0.001 |
| Model 2 b | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 39 | 33 (85) | 3.21 | 1.17–8.85 | 0.024 | |
| Decrease 0%–50% | 137 | 84 (61) | Reference | Reference | Reference | |
| Increase | 69 | 24 (35) | 0.31 | 0.16–0.60 | 0.001 | |
| Continuous (per 10% decrease) | 245 | 141 (58) | 73 | 1.18 | 1.09–1.28 | <0.001 |
| Thymoma | ||||||
| Model 1 a | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 14 | 13 (93) | 9.97 | 1.09–91.46 | 0.042 | |
| Decrease 0%–50% | 21 | 12 (57) | Reference | Reference | Reference | |
| Increase | 15 | 5 (33) | 0.38 | 0.10–1.51 | 0.170 | |
| Continuous (per 10% decrease) | 50 | 30 (60) | 17 | 1.36 | 1.10–1.69 | 0.005 |
| Model 2 b | ||||||
| Change in anti‐AChR‐ab concentration | ||||||
| Decrease ≥50% | 14 | 13 (93) | 10.33 | 1.11–96.18 | 0.040 | |
| Decrease 0%–50% | 21 | 12 (57) | Reference | Reference | Reference | |
| Increase | 15 | 5 (33) | 0.37 | 0.09–1.51 | 0.166 | |
| Continuous (per 10% decrease) | 50 | 30 (60) | 17 | 1.37 | 1.10–1.70 | 0.005 |
| p‐interaction c (thymoma: yes vs. no) | 0.147 | |||||
Event was defined as improvement in MGFA score.
Abbreviations: AChR‐ab, anti‐acetylcholine receptor antibody; n = number of individuals; Obs = number of observations.
Additionally adjusted for time since baseline measurement (months; continuous).
Model 1 additionally adjusted for age (years), and sex (male/female).
p‐interaction calculated based on continuous outcome (per 10% decrease), Model 2.
Subgroup analysis
Patients who had undergone a thymectomy were more often women (72%, p = 0.028) and were younger than the patients who had not undergone a thymectomy (median age 40.0 vs. 68.0 years; p < 0.001 [Table 4]). Furthermore, the patients who underwent a thymectomy experienced slower remission of MG, than the group who did not undergo a thymectomy (median 19 vs. 11 months; p = 0.027). In patients who underwent a thymectomy, 75% of the nonthymomatous and 100% of the thymomatous patients used immunosuppressive drugs.
TABLE 4.
Patient characteristics of subgroup with thymectomy and thymomas
| Characteristics | Thymomas | Thymectomy | ||||
|---|---|---|---|---|---|---|
| Yes | No | p value | Yes | No | p value | |
| Patients, n | 17 | 73 | 49 | 41 | ||
| Females, n (%) | 8 (47.1) | 47 (64.9) | 0.187 a | 35 (72.0) | 20 (48.8) | 0.028 |
| Age at baseline a , median years (range) | 49.0 (30–73) | 55.0 (18–83) | 0.200b | 40.0 (18–78) | 68.0 (35–83) | <0.001 |
| Immunosuppressive therapy, n (%) | 17 (100) | 63 (86.3) | 0.090 a | 41 (83.6) | 39 (95.1) | 0.085 |
| MGFA‐score at baseline a , n (%) | ||||||
| 0 (remission) | 1 (5.9) | 6 (8.2) | 3 (6.1) | 4 (9.8) | ||
| I | 4 (23.5) | 17 (23.3) | 9 (18.4) | 12 (29.2) | ||
| IIA | 2 (11.8) | 11 (15.1) | 7 (14.3) | 6 (14.6) | ||
| IIB | 3 (17.6) | 16 (21.9) | 15 (30.6) | 4 (9.8) | ||
| IIIA | 2 (11.8) | 8 (11.0) | 4 (8.2) | 6 (14.6) | ||
| IIIB | 4 (23.5) | 7 (9.6) | 7 (14.3) | 4 (9.8) | ||
| IVA | 0 (0.0) | 4 (5.5) | 2 (4.1) | 2 (4.9) | ||
| IVB | 1 (5.9) | 2 (2.7) | 1 (2.0) | 2 (4.9) | ||
| V | 0 (0.0) | 2 (2.7) | 1 (2.0) | 1 (2.4) | ||
| Severity of MG at baseline a , n (%) | 0.312 a | 0.354 | ||||
| MGFA 0—IIB | 10 (58.8) | 50 (68.5) | 34 (69.4) | 26 (63.4) | ||
| MGFA IIIA—IV | 7 (41.2) | 23 (31.5) | 15 (30.6) | 15 (36.6) | ||
| Remission of MG after baseline, n (%) | 15 (88.2) | 61 (83.6) | 0.632 a | 42 (85.7) | 34 (82.9) | 0.716 |
| Remission of MG after baseline, median months (range) | 13 (3–163) | 15 (2–66) | 0.459b | 19 (3–163) | 11 (2–35) | 0.027 |
Abbreviation: MG, myasthenia gravis; MGFA, Myasthenia Gravis Foundation of America.
At baseline: first analysis of anti‐acetylcholine receptor antibody serum levels in the Maastricht University Medical Center.
The second subgroup analysis was performed to compare the baseline anti‐AChR‐ab serum level with the first anti‐AChR‐ab serum level after at least 12 months’ follow‐up, with a maximum of 24 months’ follow‐up. In total, 65 out of 90 patients (72.2%) were included in this subgroup analysis. Patient characteristics are shown in Table 5 and the results in Table 6. A statistically significant inverse association was observed between change in anti‐AChR‐ab concentration and MGFA improvement (p = 0.004). Altogether, after 12 to 24 months of follow‐up, a 10% decrease in anti‐AChR‐ab concentration was associated with an increased odds for improvement on the MGFA clinical classification scale by a factor of 1.30 (OR 1.30, CI 1.09–1.56; p = 0.005).
TABLE 5.
Patient characteristics of subgroup with two anti‐acetylcholine receptor antibody test within 24 months after baseline a
| Characteristics | Data |
|---|---|
| Patients, n | 65 |
| Female, n (%) | 40 (61.5) |
| Age at baseline a , median years (range) | 57.0 (18–83) |
| Duration of MG at baseline a , median months (range) | 4 (0–204) |
| Follow‐up, median (range) | 16 (12–24) |
| Immunosuppressive drug therapy, n (%) | 53 (81.5) |
| MGFA classification at baseline a , n (%) | |
| I | 17 (26.1) |
| IIA | 10 (15.4) |
| IIB | 15 (23.1) |
| IIIA | 7 (10.8) |
| IIIB | 8 (12.3) |
| IVA | 3 (4.6) |
| IVB | 3 (4.6) |
| V | 2 (3.1) |
| Thymectomy, n (%) | 34 (52.3) |
| Thymoma, n (%) | 11 (16.9) |
Abbreviations: MG: myasthenia gravis; MGFA: Myasthenia Gravis Foundation of America.
At baseline: first analysis of anti‐acetylcholine receptor antibody serum levels in the Maastricht University Medical Center.
TABLE 6.
Change in anti‐acetylcholine receptor antibody concentration in patients with a follow‐up of 12–24 months (binary logistic regression)
| n | Event n (%) | OR | 95% CI | p‐value | |
|---|---|---|---|---|---|
| Model 1 a | |||||
| Change in anti‐AChR‐ab concentration | |||||
| Decrease ≥50% | 36 | 34 (94.4) | 7.29 | 1.31–40.57 | 0.023 |
| Decrease 0%–50% | 20 | 14 (70.0) | Reference | Reference | Reference |
| Increase | 9 | 5 (55.6) | 0.54 | 0.11–2.72 | 0.452 |
| Continuous (per 10% decrease) | 65 | 53 (81.5) | 1.30 | 1.09–1.56 | 0.005 |
| Model 2 b | |||||
| Change in anti‐AChR‐ab concentration | |||||
| Decrease ≥50% | 36 | 34 (94.4) | 6.28 | 1.09–36.19 | 0.040 |
| Decrease 0%–50% | 20 | 14 (70.0) | Reference | Reference | Reference |
| Increase | 9 | 5 (55.6) | 0.62 | 0.11–0.3.40 | 0.583 |
| Continuous (per 10% decrease) | 65 | 53 (81.5) | 1.27 | 1.06–1.53 | 0.011 |
Event was defined as improvement in Myasthenia Gravis Foundation of America score.
Abbreviations: AChR‐ab, anti‐acetylcholine receptor antibody; n = number of individuals; Obs, number of observations; OR, odds ratio.
Additionally adjusted for time since baseline measurement (months; continuous).
Model 1 additionally adjusted for age (years), and sex (male/female).
The third subgroup analysis focused on the possible different types of responders after the start of immunosuppressive therapy. In total, 53 out of 90 patients (58.9%) were included based on the use of immunosuppressive drugs with a minimum follow‐up of 24 months. Fast responders had an anti‐AChR‐ab serum level reduction of at least 50% in 12 months (N = 40). The slow responders had less than 50% reduction in anti‐AChR‐ab serum level in 24 months (N = 11). Only two out of 53 patients had an intermediate response and were excluded because they did not fit into one of the two defined groups. A specific overview of the two groups is shown in Table 7. The two groups significantly differed with respect to age (median age of fast responders 63.0 years vs. median age of slow responders 53.0 years; p = 0.042) and history of a thymectomy, with thymectomy more frequently performed in the group with slow responders compared to fast responders (73.3% vs. 41.5%; p = 0.035). There was no significant difference between the groups in severity of MG, or level of anti‐AChR‐ab at baseline.
TABLE 7.
Patient characteristics of subgroup ‘fast‐ and slow responders’ on immunosuppressive therapy
| Characteristics | Fast responders a | Slow responders b | p value |
|---|---|---|---|
| Patients, n | 40 | 11 | |
| Females, n (%) | 23 (57.5) | 7 (63.6) | 0.714 |
| Age, median years (range) | 63.0 (18–83) | 53.0 (22–69) | 0.042 |
| Level of anti‐AChR‐ab at baseline c , median (range) | 21 (1.4–202) | 14 (0.36–214) | 0.372 |
| Severity of MG at baseline c , n (%) | 0.08 | ||
| MGFA I – IIB | 21 (52.5) | 9 (81.8) | |
| MGFA IIIA – IV | 19 (47.5) | 2 (18.2) | |
| Thymectomy, n (%) | 17 (41.5) | 9 (81.8) | 0.021 |
| Thymectomy before baseline c | 3 (17.6) | 3 (33.3) | |
| Thymectomy 0–12 months after baseline | 13 (76.5) | 6 (66.7) | |
| Thymectomy >12 months after baseline | 1 (5.9) | 0 (0.0) | |
| Thymoma, n (%) | 7 (17.1) | 3 (27.3) | 0.47 |
| Remission of MG after baseline, n (%) | 38 (95.0) | 10 (90.1) | 0.61 |
| Remission of MG after baseline, median (range) | 11 (2–50) | 14 (4–45) | 0.418 |
Abbreviations: AChR‐ab, anti‐acetylcholine receptor antibody; MG: myasthenia gravis; MGFA: Myasthenia Gravis Foundation of America.
Fast responder: anti‐AChR‐ab serum level reduction ≥50% in ≤12 months.
Slow responder: anti‐AChR‐ab serum level reduction ≤50% in ≥24 months.
At baseline: first analyzation of anti‐AChR‐ab serum levels in the Maastricht University Medical Center.
DISCUSSION
In this retrospective cohort study, an inverse association between the percentage of change in anti‐AChR‐ab serum levels and clinical improvement was found in both bivariate and multilevel logistic regression analyses. This indicates that repetitive measurements of anti‐AChR‐ab serum levels can potentially be used to assist in the follow‐up of a patient with MG.
Although most patients did experience remission of MG during follow‐up, this could take many months to years, and change in immunosuppressive therapy is no exception. A patient without clinical improvement can present a challenge for clinicians to decide when a switch in immunosuppressive therapy is indicated, due to the time it generally takes before the effect is established in drugs such as azathioprine and mycophenolate mofetil. [19]. In this study, the MGFA scale was used as a tool for measuring clinical improvement between measurements (binary outcome: yes/no). Using classification such as the MGFA has limitations because scoring by a clinician is possibly subjective and the scale is also prone to influence by patients’ perception of the symptoms. Therefore, an improvement on the MGFA scale should not be interpreted as an indicator of severity of a disease for which more suitable measurement tools are available, such as Quantitative Myasthenia Gravis (QMG) combined with Myasthenia Gravis Activities of Daily Living (MG‐ADL) score [20]. Unfortunately, these tools were not available in the present study setting because of its retrospective nature. MG is a disease characterized by fluctuating muscle weakness over the course of a day. These daily fluctuations in symptoms can also influence the grading of disease severity. In addition, central fatigue is a frequently described symptom in MG and can be confused with peripheral muscle weakness [21]. Furthermore, the intake of acetylcholinesterase inhibitors can play an important factor in the imprecision of the rating scales due to their short interval of action [19]. The addition of a more objective measurement tool, such as anti‐AChR‐ab serum levels, that is not heavily subjective to daily fluctuations and barely invasive for a patient, is a tool that can support decisions regarding change in immunosuppressive therapy. An anti‐AChR‐ab serum level that shows a significant decline in concentration, indicates that the chosen therapy is working and should therefore be continued. Vice versa, no change or an increase in concentration indicate that a change in drug therapy could be beneficial in a particular patient, which can lead to faster remission, fewer exacerbations, a lower dose of immunosuppressive drugs, and fewer costs in clinical care. In this study, we did not focus on change in immunosuppressive therapies and further prospective research is necessary. It would be beneficial to examine more closely whether changes in anti‐AChR‐ab concentration can assist clinicians in deciding when exactly an immunosuppressive therapy should be changed. Moreover, specific types of therapy for the particular patient to reduce time to remission, exacerbations and costs should be analyzed in further research.
We found that patients who underwent a thymectomy experienced slower remission of MG than the group who did not undergo a thymectomy. Furthermore, the patients who had undergone a thymectomy were also more often classified as a ‘slow responder’; defined as a less than 50% reduction of anti‐AChR‐ab serum level in 24 months. A possible explanation could be that the thymectomy group was significantly younger and in a less stable phase of the disease. Moreover, the patients in the thymectomy group used fewer immunosuppressive drugs, which could be also a factor in the delayed achievement of MG remission. Severity of MG or the quantitative level of anti‐AChR‐ab were not significantly different between these patients and the fast responders. Another hypothesis could be that the thymectomy patients in our study were able to taper the prednisolone, but did not started azathioprine on time, to take over the effect of the prednisolone. Azathioprine as an adjunct to prednisolone is considered as a treatment that reduces the dose of prednisolone, has fewer treatment failures, longer remissions, and has fewer side effects [22]. Further research is necessary to observe the daily use of different types of immunosuppressive drugs combined with changes in anti‐AChR‐ab serum levels.
Anti‐AChR‐ab serum levels can also play a role in creating a correct indication for treatment‐resistant MG patients. For example, eculizumab, a humanized monoclonal antibody, is indicated mainly in patients with anti‐AChR‐ab positive MG who are treatment‐resistant to general immunosuppressive therapy [23, 24]. Although, previous research showed beneficial effects in this study population, cost‐effectiveness is a topic of discussion. Therefore, a more objective tool such as anti‐AChR‐ab serum level (combined with clinical examination tests) can possibly contribute to a reasoned indication for drug therapies in treatment‐resistant MG.
In this study, an RIA was used to accurately measure anti‐AChR‐ab concentration. If blood samples are diluted, the assay kit can determine concentrations from 0.25 to 500 nmol/L within a reasonable margin of error. By determining the precise concentration, it is possible to obtain an insight into the fluctuation of the concentration over time, instead of a binary outcome (positive/negative). Therefore, the use of anti‐AChR‐ab with serial dilutions is recommended in all patients with clinical MG.
The aim of this study was to investigate whether there was an association between anti‐AChR‐ab and clinical status in MG. Because this association is now found in several studies, it is important to achieve an international consensus about the use of anti‐AChR‐ab in follow‐up, in addition to the well‐known diagnostic value.
This study has some limitations which can be optimized in further research. A future prospective study is necessary to standardize follow‐up time and treatment, with precise information about doses and switch of immunosuppressive therapy. Furthermore, the inclusion of patients at the same point in the disease and the use of a combination of rating scales (e.g. QMG combined with MG‐ADL) are recommended in further research. Lastly, this study was not able to make specific recommendations on an individual level, but we support the continuation of research on this topic to achieve more personalized treatment of patients with MG.
In conclusion, this blinded retrospective cohort study found that a change in anti‐AChR‐ab serum level was associated with clinical status in MG. These results indicate that the use of anti‐AChR‐ab serum levels might be valuable as a long‐term monitor for clinical improvement in MG patients, and could possibly support clinicians in their decisions regarding continuing or changing immunosuppressive treatment. Repetitive measurements of anti‐AChR‐ab serum levels can objectively assist in the follow‐up of a patient with MG. A future prospective study is necessary to provide additional information about the influence of different immunosuppressive strategies on anti‐AChR‐ab serum levels to lead to more personalized treatment of patients with MG.
CONFLICT OF INTEREST
No conflict of interest to report.
AUTHOR CONTRIBUTION
Florit Marcuse: Data curation (lead); Formal analysis (lead); Investigation (lead); Project administration (lead); Writing – original draft (lead); Writing – review and editing (lead). Lloyd Brandts: Conceptualization (equal); Formal analysis (lead); Methodology (lead); Software (lead); Writing – original draft (supporting); Writing – review and editing (supporting). Daan Moens: Data curation (equal); Formal analysis (equal); Investigation (equal); Software (equal). Jan Damoiseaux: Resources (lead); Supervision (equal); Validation (equal); Visualization (equal). Monique Hochstenbag: Resources (equal); Supervision (equal); Validation (equal). Janneke G.J. Hoeijmakers: Conceptualization (equal); Resources (equal); Visualization (equal). Jos Maessen: Resources (equal); Supervision (equal); Visualization (equal). Marc De Baets: Conceptualization (lead); Investigation (equal); Project administration (equal); Resources (equal); Supervision (lead); Validation (equal); Visualization (equal).
INFORMED CONSENT
Approval of ethics committee (non‐WMO research).
Supporting information
Marcuse F, Brandts L, Moens D, et al. The association between anti‐acetylcholine receptor antibody level and clinical improvement in myasthenia gravis. Eur J Neurol. 2022;29:1187–1197. doi: 10.1111/ene.15238
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gilhus NE, Tzartos S, Evoli A, et al. Myasthenia gravis. Nat Rev Dis Primers. 2019;5:30. [DOI] [PubMed] [Google Scholar]
- 2. Sam C, Bordoni B. Physiology, acetylcholine. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK431128/ [PubMed] [Google Scholar]
- 3. Ruff RL, Lennon VA. How myasthenia gravis alters the safety factor for neuromuscular transmission. J Neuroimmunol. 2008;201–202:13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141‐149. [DOI] [PubMed] [Google Scholar]
- 5. Lucchi M, Ricciardi R, Melfi F, et al. Association of thymoma and myasthenia gravis: oncological and neurological results of the surgical treatment. Eur J Cardiothorac Surg. 2009;35(5):812‐816. [DOI] [PubMed] [Google Scholar]
- 6. Marcuse F, Hochstenbag M, Hoeijmakers JGJ, et al. Subclinical myasthenia gravis in thymomas. Lung Cancer. 2021;152:143‐148. [DOI] [PubMed] [Google Scholar]
- 7. Vincent A, Newsom‐Davis J, Newton P, Beck N. Acetylcholine receptor antibody and clinical response to thymectomy in myasthenia gravis. Neurology. 1983;33(10):1276‐1282. [DOI] [PubMed] [Google Scholar]
- 8. Oosterhuis HJ, Limburg PC, Hummel‐Tappel E, The TH. Anti‐acetylcholine receptor antibodies in myasthenia gravis. Part 2. Clinical and serological follow‐up of individual patients. J Neurol Sci. 1983;58(3):371‐385. [DOI] [PubMed] [Google Scholar]
- 9. Oosterhuis HJ, Limburg PC, Hummel‐Tappel E, Van den Burg W, The TH. Anti‐acetylcholine receptor antibodies in myasthenia gravis. Part 3. The effect of thymectomy. J Neurol Sci. 1985;69(3):335‐343. [DOI] [PubMed] [Google Scholar]
- 10. Heldal AT, Eide GE, Romi F, Owe JF, Gilhus NE. Repeated acetylcholine receptor antibody‐concentrations and association to clinical myasthenia gravis development. PLoS One. 2014;9(12):e114060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roses AD, Olanow CW, McAdams MW, Lane RJ. No direct correlation between serum antiacetylcholine receptor antibody levels and clinical state of individual patients with myasthenia gravis. Neurology. 1981;31(2):220‐224. [DOI] [PubMed] [Google Scholar]
- 12. Olanow CW, Wechsler AS, Roses AD. A prospective study of thymectomy and serum acetylcholine receptor antibodies in myasthenia gravis. Ann Surg. 1982;196(2):113‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanders DB, Burns TM, Cutter GR, Massey JM, Juel VC, Hobson‐Webb L. Does change in acetylcholine receptor antibody level correlate with clinical change in myasthenia gravis? Muscle Nerve. 2014;49(4):483‐486. [DOI] [PubMed] [Google Scholar]
- 14. Oger J, Frykman H. An update on laboratory diagnosis in myasthenia gravis. Clin Chim Acta. 2015;449:43‐48. [DOI] [PubMed] [Google Scholar]
- 15. Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26(11):1054‐1059. [DOI] [PubMed] [Google Scholar]
- 16. Damoiseaux J. Autoantibodies in the grocery shop: does quantity matter? Immunol Res. 2013;56(2):413‐419. [DOI] [PubMed] [Google Scholar]
- 17. Jaretzki A, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards1. Ann Thoracic Surg. 2000;70(1):327‐334. [DOI] [PubMed] [Google Scholar]
- 18. IBL International GmbH . Acetylcholine Receptor Autoantibodies (ARAb) RRA (RE21021/RE21023), version 2020‐02.
- 19. Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ. Treatment of myasthenia gravis. Neurol Clin. 2018;36(2):311‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomsen JLS, Andersen H. Outcome measures in clinical trials of patients with myasthenia gravis. Front Neurol. 2020;11:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruiter AM, Verschuuren JJGM, Tannemaat MR. Fatigue in patients with myasthenia gravis. A systematic review of the literature. Neuromuscul Disord. 2020;30(8):631‐639. [DOI] [PubMed] [Google Scholar]
- 22. Palace J, Newsom‐Davis J, Lecky B, MGS Group . A randomized double‐blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Neurology. 1998;50(6):1778‐1783. [DOI] [PubMed] [Google Scholar]
- 23. Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oyama M, Okada K, Masuda M, et al. Suitable indications of eculizumab for patients with refractory generalized myasthenia gravis. Ther Adv Neurol Disord. 2020;13:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


