Abstract
The presence of invasive alien plants (IAPs) alters the composition of soil arbuscular mycorrhizal (AM) fungal communities. Although fundamental for plant development, plant responses to AM from invaded soils have not been widely explored, especially under environmental stress.
We compared plant growth, P accumulation, root colonization and the photosynthetic responses of the native AM‐dependent Plantago lanceolata growing in contact with AM fungi from communities invaded by Acacia dealbata Link (AMinv) or non‐invaded communities (AMnat) exposed to water and light restriction (shade).
Under optimal growing conditions, plants in contact with AMnat produced higher leaf biomass and accumulated more P. However, plant responses to different AM inocula varied as the level of stress increased. Inoculation with AMinv promoted plant growth and root length under light restriction. When plants grew in contact with AMnat under drought, leaf P increased under severe water restriction, and leaf and root P increased under intermediate water irrigation. Growing in contact with the AMnat inoculum promoted root P content in both full light and light restriction. Colonization rates of P. lanceolata roots were comparable between treatments, and plants maintained photosynthetic activity within similar ranges, regardless of the level of stress applied.
Our results suggest that origin of the inoculum (native soils versus invaded soils) did not affect the ability of AM species therein to establish effective mutualistic associations with P. lanceolata roots but did influence plant responses depending on the type and level of the abiotic stress.
Keywords: Abiotic stress, Acacia dealbata, arbuscular mycorrhiza, invasive alien plants, phosphorus acquisition, photosynthetic responses, plant growth
Under different light and water stress, the response of the native Plantago lanceolata was altered (specially P uptake) growing with mycorrhizal inoculum from the invasive Acacia dealbata in comparison with inoculum from native species.
INTRODUCTION
One of the main consequences and characteristics of the Anthropocene is the unprecedented increase in biological invasions in response to large‐scale phenomena, such as globalization, intensification of international trade and tourism and soil degradation. With consequences at the local, regional and global scale, the spread of invasive alien plants (IAPs) reduces and eliminates native species, limits plant biodiversity, modifies soil physicochemical parameters, nutrient cycling, water regimes and decreases the provision and quality of ecosystem services (Vilá et al. 2011; Pyšek et al. 2012; Simberloff et al. 2013; Banks et al. 2015). At the soil level, the presence of IAPs interferes with the structure and function of microbial communities, altering symbiotic relationships and the soil–plant exchange system (Van der Putten et al. 2007; Pringle et al. 2009; Vogelsang & Bever 2009; Tanner & Gange 2013; Inderjit & Cahill 2015).
When IAPs arrive in new territories they create novel or selective associations with soil microbial species (Richardson et al. 2000a; Moora et al. 2011; Nuñez & Dickie 2014; Kamutando et al. 2017; Le Roux et al. 2017) or bring their own mutualists (Correia et al. 2019; Kamutando et al. 2019). Soil microbiota and mycorrhiza in particular play a crucial role in determining the abundance and invasiveness of plant species (Levine et al. 2004). Arbuscular mycorrhizal (AM) fungi are located at the soil–root interface, expanding the range of plant influence and acting as intermediaries between plants and the soil matrix (Richardson et al. 2009). Through a symbiotic relationship established with more than 80% of terrestrial plants (Smith & Read 2010), AM fungi provide essential soil nutrients, mainly P and N, but also facilitate access and uptake of NH4+, NO3 ‐, Zn, Cu and K (Mathur et al. 2019) in exchange for C compounds derived from photosynthesis. Besides their contribution to plant nutrition, AM fungi also serve as a first defence, increasing plant resistance to biotic and abiotic stresses (Hajiboland et al. 2019; Diagne et al. 2020).
Such AM communities are fundamental to maintaining forest soil balance as they can represent ecological barriers to limit the entry and spread of IAPs (Janos et al. 2013). Nevertheless, their role in the invasion process can vary, acting as facilitators (positive effect), inhibitors (negative effect) or having a neutral effect (Klironomos 2003; Levine et al. 2004; Shah et al. 2009). Reciprocally, once established in the novel community, IAPs may, in turn, affect the structure of AM fungal communities (Cantor et al. 2011; Meinhardt & Gehring 2012; Guisande‐Collazo et al. 2016), generally decreasing the abundance of native mycorrhiza and altering the structural composition of the soil fungal community (Grove et al. 2017).
The AM fungi are ubiquitous and widely distributed among almost all terrestrial environments, but interactions with plants become especially relevant under stress conditions. Multiple studies indicate that AM fungi help plants to deal with drought stress (Augé 2001; Augé & Moore 2005; Boomsma & Vyn 2008; Zhu et al. 2012; Endresz et al. 2015; Begum et al. 2019; Mathur et al. 2019). Drought stress reduces plant growth, mainly by reducing photosynthetic capacity (Teskey et al. 2015; Mathur et al. 2019). In this sense, the association with AM fungi alleviates drought stress by regulating hormone balance, increasing water absorption through hyphae (Augé et al. 2007), contributing to osmotic adjustment (Porcel & Ruiz‐Lozano 2004), expanding antioxidant activity or increasing nutrient absorption (Fernández‐Lizarazo & Moreno‐Fonseca 2016). Plant association with AM fungi can also maintain photosynthetic capacity in shaded conditions (Shukla et al. 2009), improving responses to light deficiency (Liu et al. 2015; Koorem et al. 2017). On the other hand, light reduction limits C gain by the AM fungi due to a decrease in photosynthetic efficiency of host plants (Liu et al. 2015). Consequently, shady conditions can be further responsible for changes in AM community composition (Van Diepen et al. 2011; Shi et al. 2014; Liu et al. 2015).
Acacia dealbata Link is a N2‐fixing highly invasive species, native to Australia and Tasmania, that currently invades Mediterranean ecosystems in South Africa, South America and Europe (Fuentes‐Ramirez et al. 2011; Richardson & Rejmanek 2011; Souza‐Alonso et al. 2017). Here, A. dealbata severely impacts plant biodiversity, alters habitat conditions (including light intensity or water regime), soil physical‐chemical properties and nutrient cycling (Fuentes‐Ramírez et al. 2011; Lazzaro et al. 2014; Souza‐Alonso et al. 2014; Kamutando et al. 2017; Lorenzo et al. 2017). Besides reducing aboveground plant diversity (Fuentes‐Ramírez et al. 2011; Lorenzo et al. 2012; Lazzaro et al. 2014), the presence of A. dealbata also causes significant changes to the structure and function of the soil microbial community (Lorenzo et al. 2010; Souza‐Alonso et al. 2015; Kamutando et al. 2017, 2019). Across geographical regions and nutritional levels, A. dealbata establishes relationships with different soil bacterial and fungal communities (Kamutando et al. 2017, 2019). In general, the Acacia genus associates with AM and ectomycorrhizal (EM) fungi (Brundrett 2009), but although both types of mycorrhizae have been identified in its rhizosphere (Kamutando et al. 2017), A. dealbata does not obtain clear benefits from the association with AM fungi (Crisóstomo 2012). However, even when their mycorrhizal dependence is low, IAPs generally succeed in competition with obligate mycorrhizal plants by disturbing local AM communities (Mummey and Rillig 2006; Vogelsang et al. 2006; Pringle et al. 2009; Vogelsang & Bever 2009).
The lower abundance of AM species (Kamutando et al. 2017) or changes in the community structure after A. dealbata invasion (Guisande‐Collazo et al. 2016) represent direct consequences of the invasion process. Novel AM communities in soils invaded by A. dealbata reduced plant growth, P acquisition and physiological activity of AM‐dependent plants (Guisande‐Collazo et al. 2016). However, how these structural changes affect plant establishment under different environmental conditions has not been explored to date. In this work, we hypothesize that the origin of the AM community influences the growth and development of plants exposed to different environmental stresses. Therefore, we compared growth of the mycorrhizal‐dependent Plantago lanceolata growing in contact with AM fungi obtained from areas invaded by A. dealbata with plants growing with inoculum from a local, non‐invaded plant community, when plants were exposed to different environmental stresses.
MATERIAL AND METHODS
Experimental design: water and light stress experiments
The experiment was arranged in a fully randomized design. Seedlings of P. lanceolata were treated with two different inoculum sources (AM fungi from invaded and non‐invaded soils) and exposed to different stress conditions (water and light stress) as independent variables. These variables were applied separately, and therefore the study was structured in two parts that were carried out at the same time. The water regime reduction was considered as Experiment 1 and light reduction as Experiment 2. Water limitation and light deficit were selected as treatments since both stresses are naturally observed in areas invaded by A. dealbata (de Neergard et al. 2005; Lorenzo et al. 2017).
In Experiment 1, three irrigation levels were applied to induce water stress: irrigation at field capacity (control level, W100), half of field capacity (moderate stress, W50) and a quarter of field capacity (high stress, W25). The selected water regime was based on previous experiences in evaluating water limitation on the growth of AM‐inoculated plants (Mathur et al. 2019). In Experiment 2, two light intensities were applied: full light and light reduction (shade). To simulate effective light reduction, P. lanceolata seedlings were placed inside a metallic structure (2 × 1 × 1 m length/width/height) covered by a shading net (polyethylene mesh, 1 × 2 mm) resulting in a reduction of light intensity similar to that observed under the canopy of A. dealbata (80% PAR reduction; Lorenzo et al. 2012). Ten replicates per treatment were established for Experiment 1 (2 inoculum sources × 3 levels of water reduction × 10 replicates, n = 60) and Experiment 2 (2 inoculum sources × 2 levels of light intensity × 10 replicates, n = 40).
The experimental inoculation was carried out using field‐collected plant roots as the natural source of AM inoculum (Klironomos & Hart 2002; Gu et al. 2011; Hassan et al. 2013; Ba et al. 2018) as these are considered viable infection units (Smith & Read 2010). Thus, AM inoculum was created from roots obtained from two different sources: native inoculum (roots from a mixture of native shrub species, hereinafter AMnat) and invasive inoculum (roots of A. dealbata, AMinv). To create the inoculum, roots of native and invasive species were collected during June 2017 in two nearby locations that previously shown structural differences in the AM fungi community (Guisande‐Collazo et al. 2016): (i) an area fully occupied by A. dealbata (Marcosende, Spain; 42°09'58.3" N, 8°41'23.1" W) and (ii) a native shrubland without the presence of A. dealbata (Morgadáns, Spain; 42°06'54.7" N, 8°43'15.9" W). Prior to A. dealbata invasion, both locations exhibited similar plant communities typical from Atlantic shrublands, composed of a mixture of annual grasses and perennial shrubs. At each location, small diameter (<0.5 cm) live roots from at least 25 different individuals were collected using a shovel and scissors and transported to the laboratory for preservation (4 °C). The AMinv inoculum was exclusively formed of fine roots obtained from A. dealbata, whereas the AMnat was formed of roots obtained from a selection of different native species (Table S1).
Experimental set‐up
The soil used as substrate for the greenhouse assay was collected in June 2017 from an agricultural field (Ribadelouro, Spain; 42°06'12.1" N, 8°39'13.0" W). Soil from the top layer (upper 20 cm) was randomly collected at 30 different points, stored in polyethylene bags and transported to the laboratory for further processing. Once in the laboratory, the soil was air‐dried for 1 week and sieved (0.5 cm mesh) to remove roots, small stones, coarse debris and other particles. Textural analysis classified the soil as sandy loam, and the following physical‐chemical properties were obtained: pH H2O (1:2.5, w:v) = 5.7; electrical conductivity (EC, 20 g soil saturated with distilled water) <42 mS cm‐1; bulk density 1.21 g cm‐3 (Carter & Gregorich 2007); organic matter 10.8%; total N 0.41% and available P 36 ppm (Olsen method; Page et al. 1982); assimilable K+ 362 ppm and exchangeable Mg+2, Ca+2, Na+, K+ and Al3+ of 0.66, 2.40, 0.10, 0.90, 1.10 cmol(+) kg‐1, respectively (Page et al. 1982).
To prepare an adequate substrate for plant growth, the soil was mixed with perlite (2–6 mm; Gramme Flower, Germany) in a ratio of 1:2 (soil:perlite, v/v) to reduce compaction produced by sterilization and to improve plant growth (Guisande‐Collazo et al. 2020). The mixture of soil and perlite was introduced into polyethylene bags (20 × 30 cm) and sterilized by autoclaving (121 °C, 20 min) for three consecutive days to inactivate the microbial community (Emam et al. 2014; Liao et al. 2015; Li et al. 2017). Roots of A. dealbata and of native species were then cut into small fragments (±1 cm) and homogeneously mixed with the autoclaved soil:perlite mixture in a 1:180 (root weight:soil weight) ratio. Then, plastic pots (5 × 5 × 15 cm), sterilized with 80% ethanol and UV light (30 min), were filled under sterile conditions (under laminar flow cabinet; FASTER, Italy).
Seeds of P. lanceolata were sterilized in a sodium hypochlorite solution (1%) and germinated in a growth chamber (72 h, 25 °C, dark conditions). After which two seedlings were planted in each pot and kept for 4 weeks under greenhouse conditions, without stress conditions, until the seedlings reached 5 cm (±1 cm) in height. After acclimatization, water restriction (Experiment 1) and light reduction (Experiment 2) were applied to P. lanceolata seedlings. Every 3 days, pots were watered with respective volumes of tap water to reach 100%, 50% and 25% of field capacity to maintain water regimes. Plants were maintained under semi‐controlled conditions in a greenhouse at the University of Vigo for 12 weeks (average 19 °C, relative humidity 72%).
Plant harvest and biometric measurements, nutrient content and mycorrhizal colonization
The day before harvest, different fluorescence parameters were measured: the fraction of light energy captured by PSII used to synthesize ATP and NADPH (ФII), the fraction of energy lost through unregulated processes (a proxy to evaluate photosynthetic efficiency) (ФNO), loss of regulated energy in the form of heat (ФNPQ), maximum performance of PSII in the light‐adapted state (Fv'/Fm') and electron flow of the antenna complexes (LEF). Fluorescence parameters have previously been used to evaluate plant physiological responses in AM inoculation experiments (Endresz et al. 2015; Guisande‐Collazo et al. 2016; Mathur et al. 2019). Fluorescence parameters were individually measured in the first fully developed leaf using the Multispec Q fluorimeter (version 1.0; PhotosynQ platform).
Before harvesting, the number of fully developed leaves was counted, and plants were carefully removed from the pots. Aerial and root length were measured for all individuals, then plants were randomly separated into two groups of equal size for destructive measurements. One group was used to measure biomass and nutrient content (leaves and roots), while the other group was used to estimate root colonization and osmolarity. Total biomass was measured by drying plant material (70 °C) until constant weight. Specific leaf area (SLA) was calculated as the leaf length/leaf DW ratio, where DW represents leaf dry weight. Biomass partitioning was calculated as leaf mass fraction (LMF = leaf DW/total plant DW) and root mass fraction (RMF = root DW/total plant DW).
To evaluate AM fungi colonization, the roots of five plants were separated, washed under tap water and cut into small fragments (±1 cm). Root fragments were stained following the Phillips & Hayman (1970) method, modified by Koske & Gemma (1989). Initially, fragments were rinsed in 2.5% KOH (1 h, 90 °C), washed and acidified in 1% HCl overnight. Fragments were then stained in acidified Coomassie blue solution (1 h, 60 °C), rinsed and transferred to a solution of glycerol:distilled water:lactic acid (85%) at a ratio of 50:48:2 (v/v/v). Root colonization was evaluated using a modified gridline intersection method (McGonigle et al. 1990). Each root fragment was examined to find where intersections cut any arbuscules, vesicles and hyphae at 100 equidistant points on each root fragment. Thus, intersections were counted in the following categories: negative (no fungal presence), arbuscules, vesicles and hyphae. Presence of arbuscles, vesicles and hyphae was calculated by dividing the presence of each structure by the total number of intersections examined. Total colonization was calculated as the proportion of positive intersections.
Plant phosphorus (P) content was measured using 0.5 g dry plant material (leaves and roots), which was digested in an acidic solution of HCl:HNO3 and subsequently calcined (3 h, 550 °C). After that, total P was quantified in the extract using ICP‐OES (Perkin Elmer Optima 4300 DV; PerkinElmer Inc., Waltham, MA, USA). Throughout the text, the term phosphorus content will be used to refer the amount of P (in g kg‐1) of plant dry weight. Leaf fragments of P. lanceolata plants were used to measure osmolarity, calculated based on total osmolyte content. Fragments were placed in a disposable plastic syringe (10 ml) and frozen at −20 °C until analysis. The syringe content was then pressed and 100 µl of the extract and collected into an Eppendorf tube (2 ml). The osmolarity was then measured in the solution using a Löser Type 6 cryoscopic osmometer.
Statistical analysis
Data normality and the homogeneity of variances were initially explored using the Kolmogorov‐Smirnov (K‐S) test and Levene's test, respectively. To identify the effect of the independent variables (stress level and inoculum origin) on the dependent variables (aerial and root length, biomass, leaves, SLA, LMF, RMF, total P, fluorescence parameters, osmolarity and root colonization), data were analysed with a two‐way ANOVA, using Tukey's HSD or Dunnet's T3 (where variances were not homogeneous) as the post‐hoc tests for treatment comparison. When interactions between independent variables were found, the effects were investigated through pairwise comparisons using Tukey's HSD test (water stress = 3 levels) or Student's t‐test (light stress = 2 levels). The statistical analyses were performed using IBM SPSS Statistics version 23.0 (Chicago, IL, USA) software for Macintosh.
RESULTS
Experiment 1. Water stress
Fluorescence parameters
The results of the two‐way ANOVA indicated that the irrigation level affected fluorescence parameters ФII (P = 0.005), ФNPQ (P = 0.001) and Fv'/Fm' (P = 0.002). Also, LEF was affected by the interaction between the water regime and the inoculum (P = 0.026) (Table 1). Plantago lanceolata showed similar Fv'/Fm' values under the different water regimes when grown in contact with AMnat. In contrast, plants that were associated with AMinv showed a decrease in the Fv'/Fm' value after exposure to severe water reduction (W25), compared to moderate reduction (W50) and well‐watered (W100) plants (Table 2). Nevertheless, Fv'/Fm' values were generally maintained in an adequate range (>0.75). On the other hand, ФII values were similar in plants treated with AMinv, and significant differences were evidenced when AMnat inoculum was used: decreasing with low irrigation levels compared to the W100 and W50 (20%; P = 0.03). The effect of AMinv on plants grown with water limitation was evident in ФNPQ values, which increased significantly under severe water restriction (W25). Finally, exposure to moderate and severe water restriction reduced LEF when plants grew in contact with AMnat. Under moderate irrigation, LEF values increased significantly in plants grown with AMinv compared to plants grown in contact with AMnat.
Table 1.
Two‐way ANOVA results for Experiment 1, including independent variables (water reduction and inoculum origin) and their interaction for the studied variables: fluorescence, plant growth parameters, P content and mycorrhizal colonization.
| Water level (W) | Inoculum (I) | W × I | ||||
|---|---|---|---|---|---|---|
| F(x,y) | P | F(x,y) | P | F(x,y) | P | |
| Fluorescence parameters | ||||||
| фII | 5.905 | 0.005 | 0.323 | 0.573 | 2.596 | 0.086 |
| фNPQ | 11.138 | ≤0.001 | 0.944 | 0.337 | 1.143 | 0.329 |
| фNO | 0.061 | 0.941 | 1.768 | 0.191 | 2.735 | 0.076 |
| Fv'/Fm' | 7.118 | 0.002 | 1.782 | 0.189 | 1.270 | 0.291 |
| LEF | 7.697 | ≤0.001 | 1.648 | 0.206 | 4.002 | 0.026 |
| Plant growth parameters | ||||||
| Leaves (n) | 37.244 | ≤0.001 | 0.675 | 0.415 | 0.071 | 0.931 |
| Leaf length (cm) | 21.042 | ≤0.001 | 1.669 | 0.202 | 1.313 | 0.278 |
| Root length (cm) | 0.062 | 0.940 | 0.614 | 0.437 | 0.092 | 0.912 |
| Leaf biomass (g) | 11.382 | ≤0.001 | 0.334 | 0.566 | 1.090 | 0.358 |
| Root biomass (g) | 2.246 | 0.116 | 0.000 | 0.992 | 0.238 | 0.789 |
| LMF | 8.617 | ≤0.001 | 0.113 | 0.738 | 0.049 | 0.952 |
| RMF | 8.617 | ≤0.001 | 0.113 | 0.738 | 0.049 | 0.952 |
| SLA (m2 kg‐1) | 9.201 | ≤0.001 | 0.059 | 0.809 | 0.039 | 0.962 |
| Osmolarity (mosm kg‐1 H2O) | 31.364 | ≤0.001 | 1.689 | 0.216 | 1.014 | 0.390 |
| Phosphorus (P) content (g kg‐1) | ||||||
| Leaves | 45.238 | ≤0.001 | 3.417 | 0.087 | 0.194 | 0.826 |
| Roots | 249.614 | ≤0.001 | 47.585 | ≤0.001 | 29.223 | ≤0.001 |
| Mycorrhizal colonization (%) | ||||||
| Colonization | 0.103 | 0.958 | 1.171 | 0.682 | 1.979 | 0.140 |
| Arbuscules | 4.953 | 0.007 | 1.310 | 0.262 | 1.736 | 0.182 |
| Hyphae | 2.406 | 0.088 | 0.185 | 0.671 | 4.252 | 0.014 |
| Vesicles | 3.335 | 0.020 | 0.150 | 0.702 | 0.900 | 0.454 |
Values in bold indicate significant differences at P < 0.05. Quantum yield of PSII (ФII), quantum yield of non‐photochemical quenching (фNPQ), quantum yield of other unregulated (non‐photochemical) losses (фNO), maximum quantum yield of PSII primary photochemistry in the light‐adapted state (Fv'/Fm'), Linear Electron Flux (LEF), specific leaf area (SLA, and P) content in leaves and roots, leaf mass fraction (LMF) and root mass fraction (RMF).
Table 2.
Mean (±SE) of different fluorescence parameters: quantum yield of PSII (ФII), quantum yield of non‐photochemical quenching (фNPQ), quantum yield of other unregulated (non‐photochemical) losses (фNO), maximum quantum yield of PSII in light‐adapted state (Fv'/Fm') and linear electron flux (LEF) in P. lanceolata plants under different levels of water reduction (W100 = field capacity, W50 = 50% field capacity, W25 = 25% field capacity) and light reduction (direct light, shade).
| W100 | W50 | W25 | ||||
|---|---|---|---|---|---|---|
| AMnat | AMinv | AMnat | AMinv | AMnat | AMinv | |
| Water reduction | ||||||
| фII | 0.48 (±0.02) a | 0.49 (±0.019) | 0.48 (±0.02) a | 0.46 (±0.01) | 0.40 (±0.03) b | 0.45 (±0.02) |
| фNPQ | 0.18 (±0.09) | 0.14 (±0.01) B | 0.13 (±0.0.02) | 0.16 (±0.02) AB | 0.20 (±0.05) | 0.22 (±0.06) A |
| фNO | 0.35 (±0.07) | 0.36 (±0.06) | 0.35 (±0.08) | 0.38 (±0.04) | 0.41 (±0.09) | 0.33 (±0.04) |
| Fv'/Fm' | 0.77 (±0.02) | 0.78 (±0.01) A | 0.78 (±0.01) | 0.77 (±0.01) A | 0.76 (±0.03) | 0.75 (±0.03) B |
| LEF | 67.20 (±12.6) a | 61.10 (±12.4) | 46.9 (±6.7) b | 66.20 (±24.2)* | 41.80 (±8.4) b | 48.50 (±5.9) |
| Light | Shade | |||
|---|---|---|---|---|
| AMnat | AMinv | AMnat | AMinv | |
| Light reduction | ||||
| фII | 0.48 (±0.02) | 0.49 (±0.02) | 0.43 (±0.02)** | 0.50 (±0.015) |
| фNPQ | 0.18 (±0.03) | 0.14 (±0.005) | 0.18 (±0.01) | 0.15 (±0.01) |
| фNO | 0.35 (±0.02) | 0.36 (±0.02) | 0.39 (±0.01)* | 0.35 (±0.01) |
| Fv'/Fm' | 0.77 (±0.006) | 0.78 (±0.003) | 0.77 (±0.004) | 0.77 (±0.003) |
| LEF | 67.23 (±4.23) a | 61.07 (±4.14) A | 41.20 (±1.7) b,*** | 31.30 (±1.48) B |
Capital letters indicate significant differences between plants inoculated with AMinv and lowercase letters indicate significant differences in plants inoculated with AMnat after ANOVA using Tukey’s HSD or Student's t‐test at P ≤ 0.05. Asterisks indicate significant differences between AMnat and AMinv plants within the same stress level at *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001.
Biometric parameters, P content, osmolarity and root colonization
The two‐way ANOVA indicated that some variables related to plant growth were affected by water restriction, such as the number of leaves, leaf length, leaf biomass, LMF, RMF and SLA (Table 1). Moreover, osmolarity and leaf P content were also affected by the water regime. In the case of root P content, there was a significant interaction between factors. The reduction in water supply caused a significant decrease in leaf length and biomass of P. lanceolata, regardless of the inoculum origin (Fig. 1). However, the reduction in leaf length and biomass of plants growing in contact with AMinv was only evident when a severe water reduction was applied (W25). The different irrigation levels did not affect root length and root biomass, regardless of the inoculum used.
Fig. 1.
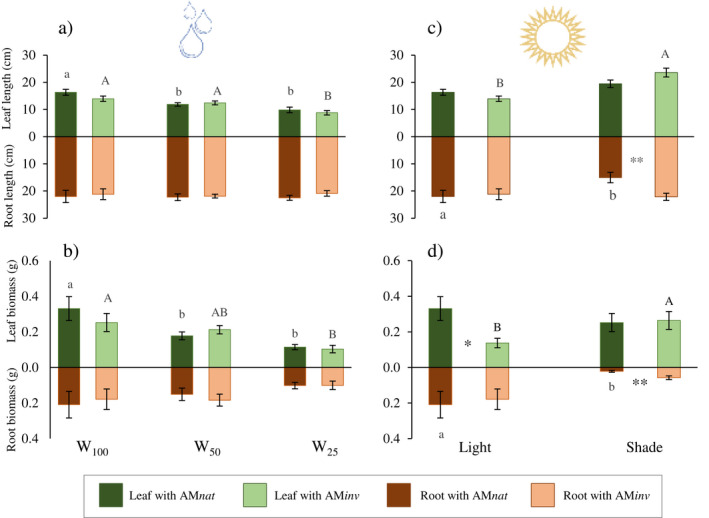
Mean (±SE) of (a) leaf and root length, (b) leaf and root biomass under water reduction (W100 = field capacity, W50 = 50% field capacity, W25 = 25% field capacity) and (c) leaf and root length, (d) leaf and root biomass under light reduction (direct light or shade). Capital letters indicate significant differences between plants inoculated with AMinv and lowercase letters indicate significant differences in plants inoculated with AMnat after ANOVA using Tukey's HSD test or Student's t‐test at P ≤ 0.05. Asterisks indicate significant differences between AMnat and AMinv plants within the same stress level at *P ≤ 0.05, **P ≤ 0.01 and *** P ≤ 0.001.
Leaf mass fraction (LMF) showed a progressive decrease in response to water supply reduction, being more pronounced in plants treated with AMinv. In contrast, RMF increased as the water content decreased (Table 3), which also led to a reduction in the number of leaves and the SLA of plants under water restriction. Both parameters showed a differential reduction trend according to the water regime applied W100 > W50 = W25 (for SLA) and W100 = W50 > W25 (for number of leaves).
Table 3.
Values (mean ± SE) of leaf mass fraction (LMF), root mass fraction (RMF), specific leaf area (SLA) and true leaves of P. lanceolata under different levels of water reduction (W100 = field capacity, W50 = 50% field capacity, W25 = 25% field capacity) and light reduction (direct light, shade).
| Inoculum | W100 | W50 | W25 | |
|---|---|---|---|---|
| Water Reduction | ||||
| LMF | Native | 0.697 (±0.055) a | 0.581 (±0.033) ab | 0.548 (±0.029) b |
| Invasive | 0.695 (±0.05) A | 0.557 (±0.029) B | 0.543 (±0.03) B | |
| RMF | Native | 0.30 (±0.055) b | 0.42 (±0.033) ab | 0.45 (±0.029) a |
| Invasive | 0.30 (±0.05) B | 0.44 (±0.029) AB | 0.46 (±0.03) A | |
| SLA (m2 kg‐1) | Native | 29.49 (±7.82) a | 19.25 (±2.04) b | 14.41 (±0.49) b |
| Invasive | 31.49 (±4.2) A | 19.33 (±1.59) B | 14.61 (±0.83) B | |
| Leaves (n) | Native | 8.5 (±0.79) a | 6.4 (±0.34) a | 3.8 (±0.33) b |
| Invasive | 8.7 (±0.6) A | 7.0 (±0.55) A | 4.1 (±0.53) B |
| Inoculum | Light | Shade | |
|---|---|---|---|
| Light reduction | |||
| LMF | Native | 0.71 (±0.05) b | 0.87 (±0.01) a,** |
| Invasive | 0.70 (±0.05) | 0.77 (±0.03) | |
| RMF | Native | 0.29 (±0.05) a | 0.13 (±0.01) b |
| Invasive | 0.3(±0.05) | 0.23 (±0.03)** | |
| SLA (m2 kg‐1) | Native | 22.04 (±2.64) b | 42.5 (±1.21) a |
| Invasive | 31.49 (±4.21) | 39.6 (±2.76) | |
| Leaves (n) | Native | 8.5 (±0.79) | 6.9 (±0.5) |
| Invasive | 8.7 (±0.6) | 8.0 (±0.22) |
Capital letters indicate significant differences between plants inoculated with AMinv and lowercase letters indicate significant differences in plants inoculated with AMnat after ANOVA using Tukey’s HSD or Student's t‐test at P ≤ 0.05. Asterisks indicate significant differences between AMnat and AMinv plants within the same stress level at * P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
Total P showed similar trends in both leaves and roots of P. lanceolata, regardless of the inoculum applied (Fig. 2). In general, there was a progressive reduction in total P as the water restriction increased. However, leaf P content remained stable under intermediate and severe water regimes (W50 = W25) when plants grew in contact with AMnat. In addition, differences in P content were evident between AMinv and AMnat: a significant P leaf reduction was observed when plants were exposed to W50 (20%; P = 0.039) and W25 (20%; P < 0.001), while root P was reduced even at optimal irrigation levels, W100 (22%; P < 0.001) and W50 (8%; P < 0.001). Osmolarity increased progressively as the water regime decreased (Fig. 3). However, plants associated with AMnat showed better responses in osmolyte accumulation (W100 = W50 < W25) compared to plants grown with AMinv (W100 < W50 < W25).
Fig. 2.
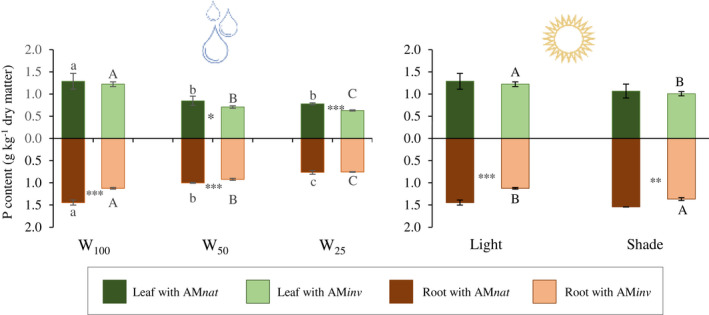
Mean (±SE) of leaf and root P content (g kg‐1) under (a) water reduction (W100 = field capacity, W50 = 50% field capacity, W25 = 25% field capacity) and (b) light reduction (direct light or shade). Capital letters indicate significant differences between plants inoculated with AMinv and lowercase letters indicate significant differences in plants inoculated with AMnat after ANOVA using Tukey's HSD test or Student's t‐test at P ≤ 0.05. Asterisks indicate significant differences between AMnat and AMinv plants within the same stress level at *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001.
Fig. 3.
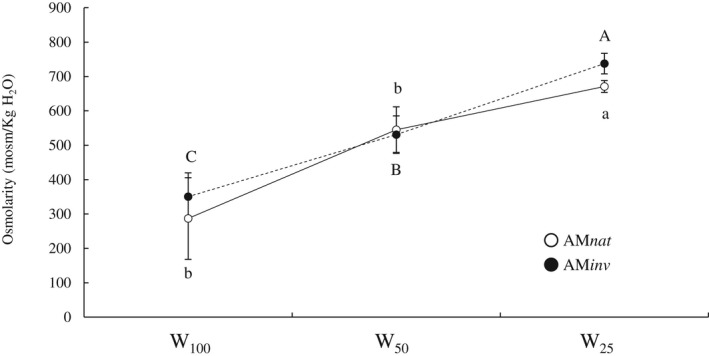
Mean (±SE) of osmolarity (mosm kg‐1 H2O) in different water regimes (W100 = field capacity, W50 = 50% field capacity, W25 = 25% field capacity). Capital letters indicate significant differences between plants inoculated with AMinv and lowercase letters indicate significant differences in plants inoculated with AMnat after ANOVA using Tukey's HSD test or Student's t‐test at P ≤ 0.05.
Although no significant differences in AM colonization were detected in roots of plants subjected to different water regimes, colonization percentages were, in all cases, >60% (Fig. 4). Under severe water restriction (W25), plants grown in contact with AMnat had a significant increase in the presence of arbuscules (%) compared to plants under moderate water restriction (W50) or no restriction (W100). Despite a decreasing trend in hyphae and vesicles as the water stress level increased, no significant differences were observed (Fig. 4).
Fig. 4.
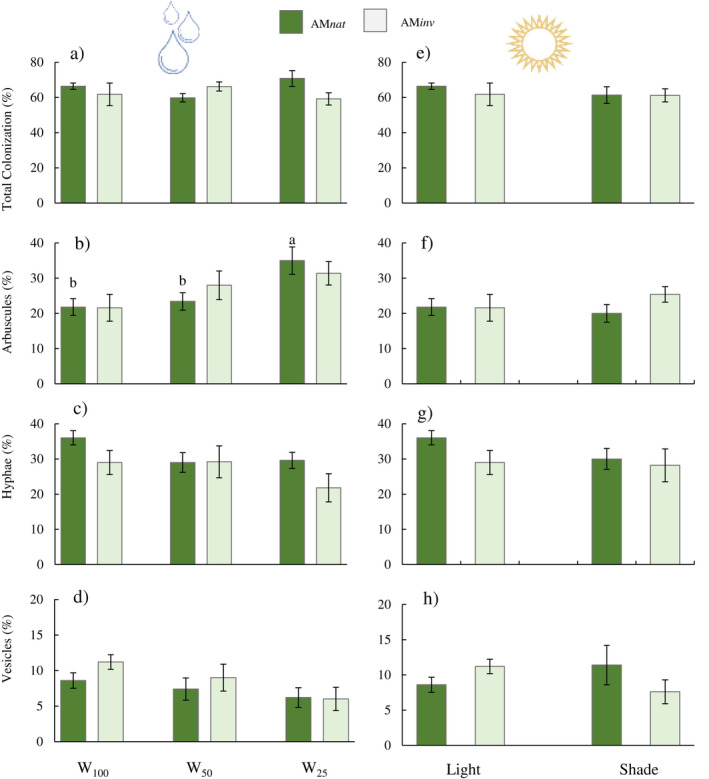
Mean (±SE) of (a) colonization, (b) arbuscules, (c) hyphae and (d) vesicles in P. lanceolata roots under drought stress (W100 = field capacity, W50 = 50% field capacity, W25 = 25% field capacity) or (e), (f), (g) and (h) under light stress (direct light or shade). Lowercase letters indicate significant differences in plants inoculated with AMnat after ANOVA using Tukey's HSD test or Student's t‐test at P ≤ 0.05.
Experiment 2. Light stress
Fluorescence measurements
As in Experiment 1, the two‐way ANOVA results indicated that sunlight reduction significantly affected the dependent variables (Table 4). Light reduction affected LEF (P < 0.001), whereas the origin of the inoculum influenced LEF (P = 0.004) and ФII (P = 0.036). The LEF decreased significantly in plants growing in the shade (Table 2) and, in turn, in those plants growing in contact with the AMinv. Additionally, under shade conditions, ФII values increased when P. lanceolata was grown with AMinv, while the opposite was observed for ФNO.
Table 4.
Two‐way ANOVA results for Experiment 2, including independent variables (light reduction and inoculum origin) and their interaction for the studied variables: fluorescence, plant growth parameters, P content and mycorrhizal colonization.
| Light (L) | Inoculum (I) | L × I | ||||
|---|---|---|---|---|---|---|
| F(x,y) | P | F(x,y) | P | F(x,y) | P | |
| Fluorescence parameters | ||||||
| фII | 1.690 | 0.203 | 4.779 | 0.036 | 2.756 | 0.107 |
| фNPQ | 2.790 | 0.105 | 3.765 | 0.061 | 0.712 | 0.405 |
| фNO | 0.091 | 0.765 | 1.570 | 0.219 | 2.183 | 0.149 |
| Fv'/Fm' | 1.477 | 0.233 | 1.188 | 0.284 | 0.014 | 0.905 |
| LEF | 82.790 | ≤0.001 | 9.620 | 0.004 | 0.018 | 0.894 |
| Plant growth parameters | ||||||
| Leaves (n) | 3.196 | 0.083 | 1.021 | 0.320 | 0.489 | 0.489 |
| Leaf length (cm) | 19.835 | <0.001 | 0.062 | 0.804 | 4.208 | 0.048 |
| Root length (cm) | 3.326 | 0.078 | 1.140 | 0.294 | 2.104 | 0.157 |
| Leaf biomass (g) | 0.005 | 0.945 | 4.892 | 0.034 | 1.956 | 0.172 |
| Root biomass (g) | 8.012 | 0.008 | 0.002 | 0.966 | 0.354 | 0.556 |
| LMF | 7.898 | 0.008 | 1.785 | 0.191 | 1.452 | 0.237 |
| RMF | 7.898 | 0.008 | 1.785 | 0.191 | 1.452 | 0.237 |
| SLA (m2 kg‐1) | 22.664 | ≤0.001 | 1.203 | 0.281 | 4.199 | 0.048 |
| Phosphorus (P) content (g kg‐1) | ||||||
| Leaves | 8.364 | 0.015 | 2.020 | 0.183 | 0.144 | 0.712 |
| Roots | 62.163 | ≤0.001 | 133.79 | ≤0.001 | 11.42 | 0.006 |
| Mycorrhizal colonization (%) | ||||||
| Colonization | 0.185 | 0.833 | 0.000 | 0.992 | 0.382 | 0.688 |
| Arbuscules | 0.073 | 0.930 | 0.901 | 0.354 | 3.851 | 0.038 |
| Hyphae | 1.270 | 0.303 | 1.052 | 0.317 | 4.754 | 0.020 |
| Vesicles | 3.428 | 0.052 | 0.610 | 0.444 | 1.959 | 0.167 |
Values in bold indicate significant differences at P < 0.05. The quantum yield of PSII (ФII), quantum yield of non‐photochemical quenching (фNPQ), quantum yield of other unregulated (non‐photochemical) losses (фNO), maximum quantum yield of PSII in the light‐adapted state (Fv'/Fm'), linear electron flux (LEF), specific leaf area (SLA) and P content in leaves and roots, leaf mass fraction (LMF) and root mass fraction (RMF).
Biometric measurements, P content and root colonization
Leaf biomass, root biomass, LMF, RMF and leaf and root P content were affected by the independent factors (Table 4). Leaf length, SLA and root P were also affected by the interaction between both factors.
Shade conditions influenced P. lanceolata plants differently, depending on the origin of the inoculum. In contact with AMinv, leaf length (40%; P < 0.001) and biomass (48%; P = 0.044) increased significantly, while root length (31%; P = 0.031) and root biomass (90%; P = 0.023) were reduced in plants treated with AMnat (Fig. 1). Under direct light exposure, plants grown in contact with AMnat had higher leaf biomass (Fig. 1). In contrast, under shade conditions, root length and root biomass of plants growing with AMinv were significantly enhanced compared to those with AMnat. Sunlight reduction increased LMF and SLA while decreasing RMF in plants grown in contact with AMnat (Table 3).
Regardless of the level of light exposure, root P content in plants treated with AMnat was significantly higher than in plants treated with AMinv. Plants that grew in shade had decreased aerial P in contact with AMinv but, in contrast, had increased root P (Fig. 2). These plants did not show significant differences in root colonization, percentage of arbuscules, hyphae or vesicles, regardless of the inoculum origin (Fig. 4). As in Experiment 1, colonization percentages were, in all cases, >60%.
DISCUSSION
From a simplistic perspective, mycorrhizae are often perceived as mere appendages to obtain valuable resources: a symbiotic association to exchange part of the photosynthetic production in return for essential nutrients. However, the role of mycorrhizae and mycorrhizal networks on ecosystem configuration is central by regulating competition, colonization, interplant resource transfers and cross‐scale ecosystem interactions (Simard et al. 2012). The mycorrhizal contribution is particularly relevant for plant growth under environmental stress conditions (Brooker et al. 2008). Plant responses depend largely on their symbiotic relationships and, consequently, it would be expected that responses to inoculation with a novel AM consortium would vary depending on plant stress level (Bever 2002). Therefore, we aimed to address the mycorrhizal influence on a dependent plant under different water and light regimes, simulating limiting conditions of habitats transformed by A. dealbata. In general, we observed that both light and water restriction influenced plant growth and responses, but P. lanceolata was also affected, to some extent, by the origin of the AM inoculum.
Photosynthesis and plant growth
The establishment of mutualist relationships with different AM sources modulates plant physiological responses, e.g. by alleviating structural and functional damage to the PSII reaction centre and electron transport under drought stress (Mathur et al. 2019). However, it is important to note that different AM species/communities lead to variable plant photosynthetic responses during stress, such as water limitation (Augé 2001). In general, P. lanceolata maintained similar photosynthetic activity despite reduced water supply. Improving plant hydraulic conductivity through root expansion by AM fungi—the so‐called mycorrhizosphere—alleviates drought stress (Augé et al. 2007; Mathur et al. 2019), also minimizing energy losses in the form of heat while protecting the electron flow of the antenna complexes (LEF) (Boomsma & Vyn 2008).
Commonly, plant photosynthetic activity decreases with drought stress; however, under mild or moderate water restriction it can remain temporarily stable or even increase (Morales et al. 2008), e.g. by increasing the rate of photorespiration (Massacci et al. 2008). Plants maintained similar photosynthetic activity across different water levels, regardless of the origin of the inoculum. In this sense, the different origin of the AM inoculum did not modify the photosynthetic response of P. lanceolata, since the photosynthetic efficiency (ФII) or Fv'/Fm'—indicators of damage to the photosynthetic apparatus—were maintained in ranges considered adequate. Nevertheless, the ФII value of plants inoculated with AMnat slightly decreased when plants were watered at 25% of field capacity. Nevertheless, plant growth and biomass production seemed to be more influenced by water availability (less availability, less growth) than by inoculum origin, since no differences were detected between plants treated with AMnat or AMinv.
Sunlight reduction generally decreases plant photosynthetic activity and photosynthesis rates (Ojanguren & Goulden 2013). Low light leads to insufficient ATP produced to allow C fixation and carbohydrate biosynthesis (Shao et al. 2014), reducing photoassimilate production and, consequently, plant growth and biomass (Mathur et al. 2018). As a consequence, limited C products constrain the mutualistic investment (Fellbaum et al. 2014) due to the significant energy requirements necessary to maintain the mutualist relationship, since AM receive up to 20% of primary plant production (Hobbie & Hobbie 2008; Smith & Read 2010). On the other hand, mycorrhization contributes, to some extent, to ameliorate photosynthetic responses (Zhu et al. 2012; Liu et al. 2015; Koorem et al. 2017; Mathur et al. 2019), and plants colonized by AM often show higher stress tolerance (Jung et al. 2012; Augé et al. 2015). Contrary to previous studies (Graham et al. 1982; Gehring 2003), the attenuation of natural irradiance did not reduce fungal colonization of P. lanceolata roots. Similar colonization rates, including essential organs for lipid storage in vesicles (Smith & Read 2010), suggests that P. lanceolata maintained the energy investment to preserve AM structures, even under restrictive treatments (W25, shade).
Nevertheless, although the mycorrhization levels were similar, our results indicated that growth responses of P. lanceolata were, to some extent, influenced by light conditions. The photosynthetic capacity (фII) and efficiency (фNO) of plants growing in contact with AMnat decreased compared to AMinv, which performed better under reduced irradiance. The adaptive response of plants to light restriction was reflected in the aerial length and biomass, suggesting a more beneficial role of symbiosis under limiting conditions (Zhang et al. 2015), in line with the stress‐gradient hypothesis (Brooker et al. 2008). Nevertheless, this effect also depends on the AM origin; plants in contact with AMinv could be more conservative in full light, prioritizing maintenance of the AM symbiosis over plant growth and the opposite under shade conditions. Growing in soils with AMinv allowed plants to maintain allocation patterns in shade, whereas plants in contact with AMnat relocated resources to the aerial parts, increasing LMF while reducing RMF. Although neither the quantum yield of PSII or the energy loss were affected, the allocation of plant resources to the aerial parts in AMnat plants, also the increase in SLA, would suggest an expansion of the available photosynthetic surface to compensate for the reduction in light.
Plant phosphorus content
From the plant point of view, the main advantage of investing in AM symbioses is the increased access to otherwise inaccessible soil nutrients, such as P or N, extending nutrient acquisition area beyond the limit of the root (Smith & Read 2010) and contributing up to 90% of the P obtained by the plant (Smith & Smith 2011). Phosphorus is taken up by extraradical hyphae, transported towards the root system and delivered to the plant via arbuscules (Smith & Read 2010), thus reducing plant dependence on soil environmental conditions for P uptake. In our study, water restriction caused a reduction in plant P content, which could be due to the decrease in P availability in water‐deficient soils (Gahoonia et al. 1994; García et al. 2008). However, plants in contact with AMnat tolerated water limitation better, maintaining root–stem transport, and therefore foliar P content. These plants had similar P levels under intermediate and severe water reduction ([P] at W50 = W25), unlike plants growing with AMinv ([P] at W50 > W25).
Although root colonization generally decreases under drought (García et al. 2008; Mathur et al. 2019), under light restriction (Koorem et al. 2017) or due to the contact with AM from invaded soils (Tanner & Gange 2013), in our case, the origin of the inoculum did not affect colonization, with similar infection levels and comparable AM structures regardless of the level of stress applied. As stated above, mycorrhizal relationships are maintained at a high metabolic cost, and the stress severity, e.g. drought level, influences the investment in mycorrhizal symbiosis (Augé 2001). Phosphorus solubility and availability decreases under water limitation and, therefore, increasing the number of arbuscules (75%) in W25 in plants growing with AMnat would suggest an additional effort to obtain P, probably at a higher metabolic cost (Roth & Paszkowski 2017). This increase contrasts with previous results that reported a reduction in the presence of arbuscules under drought (García et al. 2008).
Despite the structural change in the AM community in soils invaded by A. dealbata (Guisande‐Collazo et al. 2016), the level of mycorrhizal colonization observed suggests that P. lanceolata associates effectively with the AM community provided from the invader. Therefore, it could be argued that it was the origin of the inoculum (and the species within), rather than the root colonization level, that influenced the ability of P. lanceolata to obtain soil P under water limitation. Although AM species vary in the capacity to acquire and provide P to P. lanceolata (Pel et al. 2018), how a specific set of AM species would influence P acquisition under different stresses seems difficult to predict. Noteworthy, the increased P availability (x2) in shrublands invaded by A. dealbata across the region (Lorenzo et al. 2010; Souza‐Alonso et al. 2014) could reduce the need to invest in specific mechanisms for P acquisition, and the mutualistic relationship could be focused on complementing other requirements (plant defence, abiotic stress, water uptake, etc.) (Jung et al. 2012; Hajiboland et al. 2019; Li et al. 2019; Diagne et al. 2020). Nevertheless, the costs and benefits of symbiotic exchanges are complex and depend on the relative resource availability and their balance between both symbiotic partners (Grman 2012).
In Experiment 2, plants growing in contact with AMnat did not show different responses to different light regimes, but in all cases accumulated more P in roots than plants inoculated with AMinv. In this case, plant response also varied between different irradiance conditions, showing different allocation patterns in the light (leaf P > root P) or shade (root P > leaf P). This trend was also observed for C resource allocation, where higher root length and biomass were observed under shade but not under full light. The interaction between light and soil nutrients can affect preferential bidirectional allocation patterns of C and P (Zheng et al. 2015). In this sense, leaf expansion and thus, increased photosynthetic surface, could be interpreted as a response of P. lanceolata when associated with AMinv to acquire more photosynthates under low‐light conditions that can be further used to increase or maintain the bidirectional exchange with the AM.
The preservation of mycorrhizal structures in shade—and the associated energy cost—suggests that the stress level might not have been sufficiently intense (despite the 80% reduction in the natural irradiance) to affect plant growth or to produce noticeable changes. Considered globally, the benefit obtained in P acquisition was reduced when plants associated with the AMinv inoculum. Similar to observations in Experiment 1, it could be argued that fungal communities in AMnat and AMinv affected P. lanceolata differently under shade. Considering similar root infection levels, the association with AMinv favoured P. lanceolata growth, whereas AMnat was more effective in obtaining soil P.
Consequences of invasion and stress
In our study, P. lanceolata showed different responses to changes in water or light regime, probably due to differences in the type and intensity of the stress applied. In this sense, it is important to note that unidirectional negative consequences produced by the association with AM from invaded communities (Guisande‐Collazo et al. 2016; Zubek et al. 2016) were not observed, at least in growing plants under different stress conditions. The presence of IAPs, such as A. dealbata, with low dependence on native mutualisms is expected to induce changes, decrease mutualist efficiency over time and affect mutualist‐dependent species after disturbances (Vogelsang & Bever 2009). To some extent, plant responses were altered under stress, but instead of decreasing plant performance, we observed what can be considered an adapted response. Under optimal growth conditions, plants in contact with AMnat were slightly favoured (leaf biomass, P content). However, the influence of AMnat and AMinv seemed to be related to the level of stress applied (water stress > light stress), providing slight advantages to plants growing in contact with AMnat under drought stress and to plants associated with AMinv under light reduction. Thus, as stated above, responses of P. lanceolata to cope with different environmental stresses would be conditioned by the origin of the AM inoculum. It is well established that AM fungi show interspecific functional diversity (Munkvold et al. 2004; Mensah et al. 2015), with differences, e.g. in soil exploration efficiency. The specific composition of inocula, and the intrinsic characteristics of species therein, might harbour different physiological attributes that produce different responses and benefits in mycorrhized plants according to the level and the type of stress applied (Augé 2001; Manoharan et al. 2017; Pel et al. 2018; Li et al. 2019) or also related to biotic factors (Kiers et al. 2011; Fellbaum et al. 2014).
The presence of IAPs that do not depend on mycorrhiza disrupts the fungal community structure, negatively influencing native species that depend on AM symbioses (Tanner & Gange 2013). It is generally considered that IAPs take advantage of associations with soil microorganisms—in our case the low mycorrhizal dependence of A. dealbata—changing species composition and decreasing the effectiveness of the native mutualists over time (Vogelsang & Bever 2009). As a result, mutualistic‐dependent plants are adversely affected, especially under abiotic stress. In fact, the capacity of A. dealbata to modify its environment implies that changes occur on a much larger scale. It is not by chance that due to the ecosystem‐level changes produced, this and other Acacia species (Souza‐Alonso et al. 2017) are considered as transformer species (Richardson et al. 2000b). Nevertheless, the interpretation of the results and the ecological implications assumed should be considered with caution because of the limitations to the experimental design (limited number of target species, inoculum or sampling sites).
Our results indicated that the outcome of associating with AMnat or AMinv on plant performance is not unidirectional but is context dependent. Adapted responses of P. lanceolata could be related to its ability to associate with a wide range of AM species (Pel et al. 2018), mainly species of the genus Glomeromycota (Smith & Read 2010), and, at the same time, to the generalist character of AM species from different communities (invaded–native) to establish relationships with roots of different plants (Majewska et al. 2018). Here, the proportion, extent and number of AM structures, such as hyphae, vesicles or arbuscules, provide good insight into the plant–AM associative process. Considering that plant biomass and root mycorrhization are generally correlated (Zubek et al. 2016), our results separated plant performance from root colonization, suggesting that different sources of AM inocula and the level of stress applied do not limit the capacity of a generalist plant species such as P. lanceolata to associate with AM communities from areas invaded by A. dealbata.
CONCLUSIONS
Habitat transformation induced by A. dealbata lead to water and light limitation for native plants, but the association with AM fungi can alleviate these effects. Growing in contact with mycorrhiza from native and invaded soils, P. lanceolata responded differently to the reduction in water and light availability. Nevertheless, the source of the AM inoculum had different effects on the photosynthetic and growth responses of P. lanceolata under abiotic stresses, without noticeable effects on root colonization. Similar infection levels and fungal structures were evidenced across treatments, regardless of the type and level of stress. Hence, our results separated plant performance from root colonization, also suggesting that potential changes in the fungal community induced by A. dealbata and the level of stress applied did not affect the ability of the AM community of invaded areas to associate with AM‐dependent plants, such as P. lanceolata.
However, plant responses were affected to some extent by the origin of the AM inoculum used. Both AM inocula led to similar plant responses to drought stress, but the association with AMinv slightly improved plant growth under reduced light. Interestingly, inoculum origin influenced the capacity of the plants to maintain P supply, reducing plant P content when roots were associated with AM fungi from areas invaded by A. dealbata. With similar root colonization levels between treatments, the origin of the inoculum was the main factor influencing the ability of P. lanceolata to obtain soil P under water limitation.
Supporting information
Table S1. List of the native species present in the native plant community with no presence of A. dealbata. Species composition is typical from Atlantic shrublands, mainly dominated by a mixed composition of perennial shrubs, and with the presence of different annual grasses occupying the basal layer.
REFERENCES
- Augé R.M. (2001) Water relations, drought and vesicular arbuscular mycorrhizal symbiosis. Mycorrhiza, 11, 3–42. [Google Scholar]
- Augé R.M., Moore J.L. (2005) Arbuscular mycorrhizal symbiosis and plant drought resistance. In: Mehrotra V.S. (Ed), Mycorrhiza: role and applications. Allied Publishers, New Delhi, India, pp 136–157. [Google Scholar]
- Augé R.M., Toler H.D., Moore J.L., Cho K., Saxton A.M. (2007) Comparing contributions of soil versus root colonization to variations in stomatal behavior and soil drying in mycorrhizal Sorghum bicolor and Cucurbita pepo . Journal of Plant Physiology, 164, 1289–1299. [DOI] [PubMed] [Google Scholar]
- Augé R.M., Toler H.D., Saxton A.M. (2015) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta‐analysis. Mycorrhiza, 25, 13–24. [DOI] [PubMed] [Google Scholar]
- Ba L., Facelli E., Facelli J.M. (2018) Plant–mycorrhizal fungi feedbacks: potential accomplices of Avena barbata’s high invasiveness. Plant Ecology, 219, 1045–1052. [Google Scholar]
- Banks N.C., Paini D.R., Bayliss K.L., Hodda M. (2015) The role of global trade and transport network topology in the human‐mediated dispersal of alien species. Ecology Letters, 18, 188–199. [DOI] [PubMed] [Google Scholar]
- Begum N., Ahanger M.A., Su Y., Lei Y., Mustafa N.S.A., Ahmad P., et al. (2019) Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants, 8, 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever J.D. (2002) Negative feedback within a mutualism: host‐specific growth of mycorrhizal fungi reduces plant benefit. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269, 2595–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma C.R., Vyn T.J. (2008) Maize drought tolerance: potential improvements through arbuscular mycorrhizal symbiosis? Field Crops Research, 108, 14–31. [Google Scholar]
- Brooker R.W., Maestre F.T., Callaway R.M., Lortie C.L., Cavieres L.A., Kunstler G., et al. (2008) Facilitation in plant communities: the past, the present, and the future. Journal of Ecology, 96, 18–34. [Google Scholar]
- Brundrett M.C. (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant & Soil, 320, 37–77. [Google Scholar]
- Cantor A., Hale A., Aaron J., Traw M.B., Kalisz S. (2011) Low allelochemical concentrations detected in garlic mustard‐invaded forest soils inhibit fungal growth and AMF spore germination. Biological Invasions, 13, 3015–3025. [Google Scholar]
- Carter M.R., Gregorich E.G. (2007) Soil sampling and methods of analysis, 2nd edn. CRC Press, New York, USA. [Google Scholar]
- Correia M., Heleno R., da Silva L.P., Costa J.M., Rodríguez‐Echeverría S. (2019) First evidence for the joint dispersal of mycorrhizal fungi and plant diaspores by birds. New Phytologist, 222, 1054–1060. [DOI] [PubMed] [Google Scholar]
- Crisóstomo J.A. (2012) Belowground mutualisms and plant genetic diversity: insights into the invasion process of Acacia dealbata and Acacia saligna . University of Coimbra, Portugal. PhD Thesis. [Google Scholar]
- de Neergaard A., Saarnak C., Hill T., Khanyile M., Berzosa A.M., Birch‐Thomsen T. (2005) Australian wattle species in the Drakensberg region of South Africa—an invasive alien or a natural resource? Agricultural Systems, 85, 216–233. [Google Scholar]
- Diagne N., Ngom M., Djighaly P.I., Fall D., Hocher V., Svistoonoff S. (2020) Roles of arbuscular mycorrhizal fungi on plant growth and performance: importance in biotic and abiotic stress regulation. Diversity, 12, 370. [Google Scholar]
- Emam T.M., Espeland E.K., Rinella M.J. (2014) Soil sterilization alters interactions between the native grass Bouteloua gracilis and invasive Bromus tectorum . Journal of Arid Environments, 111, 91–97. [Google Scholar]
- Endresz G., Mojzes A., Kalapos T. (2015) Deficit watering reduces plant growth to a smaller extent with arbuscular mycorrhizal association than without it for non‐invasive grass species but not for invasive grass species. Applied Ecology & Environmental Research, 13, 551–567. [Google Scholar]
- Fellbaum C.R., Mensah J.A., Cloos A.J., Strahan G.D., Pfeffer P.E., Kiers E.T., et al. (2014) Fungal nutrient allocation in common mycelia networks is regulated by the carbon source strength of individual host plants. New Phytologist, 203, 645–656. [DOI] [PubMed] [Google Scholar]
- Fernández‐Lizarazo J.C., Moreno‐Fonseca L.P. (2016) Mechanisms for tolerance to water‐deficit stress in plants inoculated with arbuscular mycorrhizal fungi. A review. Agronomía Colombiana, 34, 179–189. [Google Scholar]
- Fuentes‐Ramírez A., Pauchard A., Cavieres L.A., García R.A. (2011) Survival and growth of Acacia dealbata vs. native trees across an invasion front in south‐central Chile. Forest Ecology and Management, 261, 1003–1009. [Google Scholar]
- Gahoonia T.S., Raza S., Nielsen N.E. (1994) Phosphorus depletion in the rhizosphere as influenced by soil moisture. Plant & Soil, 159, 213–218. [Google Scholar]
- García I., Mendoza R., Pomar M.C. (2008) Deficit and excess of soil water impact on plant growth of Lotus tenuis by affecting nutrient uptake and arbuscular mycorrhizal symbiosis. Plant & Soil, 304, 117–131. [Google Scholar]
- Gehring C.A. (2003) Growth responses to arbuscular mycorrhizae by rain forest seedlings vary with light intensity and tree species. Plant Ecology, 167, 127–139. [Google Scholar]
- Graham J.H., Leonard R.T., Menge J.A. (1982) Interaction of light intensity and soil temperature with phosphorus inhibition of vesicular‐arbuscular mycorrhiza formation. New Phytologist, 91, 683–690. [Google Scholar]
- Grman E. (2012) Plant species differ in their ability to reduce allocation to non‐beneficial arbuscular mycorrhizal fungi. Ecology, 93, 711–718. [DOI] [PubMed] [Google Scholar]
- Grove S., Haubensak K.A., Gehring C., Parker I.M. (2017) Mycorrhizae, invasions, and the temporal dynamics of mutualism disruption. Journal of Ecology, 105, 1496–1508. [Google Scholar]
- Gu M., Chen A., Dai X., Liu W., Xu G. (2011) How does phosphate status influence the development of the arbuscular mycorrhizal symbiosis? Plant Signaling & Behavior, 6, 1300–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisande‐Collazo A., González L., Souza‐Alonso P. (2016) Impact of an invasive nitrogen‐fixing tree on arbuscular mycorrhizal fungi and the development of native species. AoB Plants, 8, plw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisande‐Collazo A., Gonzalez L., Souza‐Alonso P. (2020) Mineral amendments to improve plant growth after soil sterilization in allelopathy experiments. Allelopathy Journal, 49, 63–72. [Google Scholar]
- Hajiboland R., Joudmand A., Aliasgharzad N., Tolrá R., Poschenrieder C. (2019) Arbuscular mycorrhizal fungi alleviate low‐temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop and Pasture Science, 70, 218–233. [Google Scholar]
- Hassan S.E.D., Liu A., Bittman S., Forge T.A., Hunt D.E., Hijri M., et al. (2013) Impact of 12‐year field treatments with organic and inorganic fertilizers on crop productivity and mycorrhizal community structure. Biology & Fertility of Soils, 49, 1109–1121. [Google Scholar]
- Hobbie E.A., Hobbie J. (2008) Natural abundance of (15) N in nitrogen‐limited forest and tundra can estimate nitrogen cycling through mycorrhizal fungi: a review. Ecosystems, 11, 815–830. [Google Scholar]
- Inderjit, Cahill J.F (2015) Linkages of plant–soil feedbacks underlying invasion mechanisms. AoB Plants, 7, plv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janos D.P., Scott J., Aristizabal C., Bowman D.M. (2013) Arbuscular‐mycorrhizal networks inhibit Eucalyptus tetrodonta seedlings in rain forest soil microcosms. PLoS One, 8, e57716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.C., Martinez‐Medina A., Lopez‐Raez J.A., Pozo M.J. (2012) Mycorrhiza‐induced resistance and priming of plant defenses. Journal of Chemical Ecology, 38, 651–664. [DOI] [PubMed] [Google Scholar]
- Kamutando C.N., Vikram S., Kamgan‐Nkuekam G., Makhalanyane T.P., Greve M., Roux J.J.L., et al. (2017) Soil nutritional status and biogeography influence rhizosphere microbial communities associated with the invasive tree Acacia dealbata . Scientific Reports, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamutando C.N., Vikram S., Kamgan‐Nkuekam G., Makhalanyane T.P., Greve M., Le Roux J.J., et al. (2019) The functional potential of the rhizospheric microbiome of an invasive tree species, Acacia dealbata . Microbial Ecology, 77, 191–200. [DOI] [PubMed] [Google Scholar]
- Kiers E.T., Duhamel M., Beesetty Y., Mensah J.A., Franken O., Verbruggen E., et al. (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science, 333, 880–882. [DOI] [PubMed] [Google Scholar]
- Klironomos J.N. (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology, 8, 2292–2301. [Google Scholar]
- Klironomos J.N., Hart M.M. (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza, 12, 181–184. [DOI] [PubMed] [Google Scholar]
- Koorem K., Tulva I., Davison J., Jairus T., Öpik M., Vasar M., et al. (2017) Arbuscular mycorrhizal fungal communities in forest plant roots are simultaneously shaped by host characteristics and canopy‐mediated light availability. Plant & Soil, 410, 259–271. [Google Scholar]
- Koske R.E., Gemma J.N. (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycology Research, 92, 486–505. [Google Scholar]
- Lazzaro L., Giuliani C., Fabiani A., Agnelli A.E., Pastorelli R., Lagomarsino A., et al. (2014) Soil and plant changing after invasion: the case of Acacia dealbata in a Mediterranean ecosystem. Science of the Total Environment, 497, 491–498. [DOI] [PubMed] [Google Scholar]
- Le Roux J.J., Hui C., Keet J.H., Ellis A.G. (2017) Co‐introduction vs ecological fitting as pathways to the establishment of effective mutualisms during biological invasions. New Phytologist, 215, 1354–1360. [DOI] [PubMed] [Google Scholar]
- Levine J.M., Adler P.B., Yelenik S.G. (2004) A meta‐analysis of biotic resistance to exotic plant invasions. Ecological Letters, 7, 975–989. [Google Scholar]
- Li L., McCormack M.L., Chen F., Wang H., Ma Z., Guo D. (2019) Different responses of absorptive roots and arbuscular mycorrhizal fungi to fertilization provide diverse nutrient acquisition strategies in Chinese fir. Forest Ecology and Management, 433, 64–72. [Google Scholar]
- Li Y.‐P., Feng Y.‐L., Kang Z.‐L., Zheng Y.‐L., Zhang J.L., Chen Y.J. (2017) Changes in soil microbial communities due to biological invasions can reduce allelopathic effects. Journal of Applied Ecology, 54, 1281–1290. [Google Scholar]
- Liao H., Luo W., Peng S., Callaway R.M. (2015) Plant diversity, soil biota and resistance to exotic invasion. Diversity and Distributions, 21, 826–835. [Google Scholar]
- Liu Y., Mao L., Li J., Shi G., Jiang S., Ma X., et al. (2015) Resource availability differentially drives community assemblages of plants and their root‐associated arbuscular mycorrhizal fungi. Plant & Soil, 386, 341–355. [Google Scholar]
- Lorenzo P., Pazos‐Malvido E., Rubido‐Bará M., Reigosa M.J., González L. (2012) Invasion by the leguminous tree Acacia dealbata (Mimosaceae) reduces the native understory plant species in different communities. Australian Journal of Botany, 60, 669–675. [Google Scholar]
- Lorenzo P., Rodríguez J., González L., Rodríguez‐Echeverría S. (2017) Changes in microhabitat, but not allelopathy, affect plant establishment after Acacia dealbata invasion. Journal of Plant Ecology, 10, 610–617. [Google Scholar]
- Lorenzo P., Rodríguez‐Echeverría S., González L., Freitas H. (2010) Effect of invasive Acacia dealbata Link on soil microorganisms as determined by PCR‐DGGE. Applied Soil Ecology, 44, 245–251. [Google Scholar]
- Majewska M.L., Rola K., Stefanowicz A.M., Nobis M., Błaszkowski J., Zubek S. (2018) Do the impacts of alien invasive plants differ from expansive native ones? An experimental study on arbuscular mycorrhizal fungi communities. Biology and Fertility of Soils, 54, 631–643. [Google Scholar]
- Manoharan L., Rosenstock N.P., Williams A., Hedlund K. (2017) Agricultural management practices influence AMF diversity and community composition with cascading effects on plant productivity. Applied Soil Ecology, 115, 53–59. [Google Scholar]
- Massacci A., Nabiev S.M., Pietrosanti L., Nematov S.K., Chernikova T.N., Thor K., et al. (2008) Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas‐exchange analysis and chlorophyll fluorescence imaging. Plant Physiology and Biochemistry, 46, 189–195. [DOI] [PubMed] [Google Scholar]
- Mathur S., Jain L., Jajoo A. (2018) Photosynthetic efficiency in sun and shade plants. Photosynthetica, 56, 354–365. [Google Scholar]
- Mathur S., Tomar R.S., Jajoo A. (2019) Arbuscular mycorrhizal fungi (AMF) protect photosynthetic apparatus of wheat under drought stress. Photosynthetic Research, 139, 227–238. [DOI] [PubMed] [Google Scholar]
- McGonigle T.P., Miller M.H., Evans D.G., Fairchild G.L., Swan J.A. (1990) A new method which gives an objective measure of colonization of roots by vesicular‐arbuscular mycorrhizal fungi. New Phytologist, 115, 495–501. [DOI] [PubMed] [Google Scholar]
- Meinhardt K.A., Gehring C.A. (2012) Disrupting mycorrhizal mutualisms: a potential mechanism by which exotic tamarisk outcompetes native cottonwoods. Ecological Applications, 22, 532–549. [DOI] [PubMed] [Google Scholar]
- Mensah J.A., Koch A.M., Antunes P.M., Kiers E.T., Hart M., Bücking H. (2015) High functional diversity within species of arbuscular mycorrhizal fungi is associated with differences in phosphate and nitrogen uptake and fungal phosphate metabolism. Mycorrhiza, 25, 533–546. [DOI] [PubMed] [Google Scholar]
- Moora M., Berger S., Davison J., Öpik M., Bommarco R., Bruelheide H., et al. (2011) Alien plants associate with widespread generalist arbuscular mycorrhizal fungal taxa: evidence from a continental‐scale study using massively parallel 454 sequencing. Journal of Biogeography, 38, 1305–1317. [Google Scholar]
- Morales F., Abadía A., Abadía J. (2008) Photoinhibition and photoprotection under nutrient deficiencies, drought and salinity. In: Demmig‐Adams B., Adams W., Mattoo A. (Eds). Photoprotection, photoinhibition, gene regulation, and environment. Springer, Dordrecht, the Netherlands, pp. 65–85. [Google Scholar]
- Mummey D.L., Rillig, M.C. (2006) The invasive plant species Centaurea maculosa alters arbuscular mycorrhizal fungal communities in the field. Plant and Soil, 288, 81–90. [Google Scholar]
- Munkvold L., Kjoller R., Vestberg M., Rosendahl S., Jakobsen I. (2004) High functional diversity within species of arbuscular mycorrhizal fungi. New Phytologist, 164, 357–364. [DOI] [PubMed] [Google Scholar]
- Nuñez M.A., Dickie I.A. (2014) Invasive belowground mutualist of woody plants. Biological Invasions, 16, 645–661. [Google Scholar]
- Ojanguren C.T., Goulden M.L. (2013) Photosynthetic acclimation within individual Typha latifolia leaf segments. Aquatic Botany, 111, 54–61. [Google Scholar]
- Page A.L., Miller R.H., Keeney D.R. (1982) Chemical and microbiological properties, 2nd edn. American Society of Agronomy and Soil Science Society of America, USA. [Google Scholar]
- Pel R., Dupin S., Schat H., Ellers J., Kiers E.T., van Straalen N.M. (2018) Growth benefits provided by different arbuscular mycorrhizal fungi to Plantago lanceolata depend on the form of available phosphorus. European Journal of Soil Biology, 88, 89–96. [Google Scholar]
- Phillips J.M., Hayman D.S. (1970) Improved procedures for clearing and staining parasitic and vesicular‐arbuscular mycorrhizal fungi for rapid assessment of infection. Mycological Research, 55, 158–161. [Google Scholar]
- Porcel R., Ruiz‐Lozano J.M. (2004) Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. Journal of Experimental Botany, 55, 1743–1750. [DOI] [PubMed] [Google Scholar]
- Pringle A., Bever J.D., Gardes M., Parrent J.L., Rillig M.C., Klironomos J. (2009) Mycorrhizal symbioses and plant invasions. Annual Review of Ecology, Evolution, and Systematics, 40, 699–715. [Google Scholar]
- Pyšek P., Jarošík V., Hulme P.E., Pergl J., Hejda M., Schaffner U., et al. (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species' traits and environment. Global Change Biology, 18, 1725–1737. [Google Scholar]
- Richardson A.E., Barea J.M., McNeill A.M., Prigent‐Combaret C. (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant & Soil, 321, 305–339. [Google Scholar]
- Richardson D.M., Allsopp N., D’Antonio C.M., Milton S.J., Rejmánek M. (2000a) Plant invasions—the role of mutualisms. Biological Reviews, 75, 65–93. [DOI] [PubMed] [Google Scholar]
- Richardson D.M., Pysek P., Rejmánek M., Barbour M.G., Panetta D., West C.J. (2000b) Naturalization and invasion of alien plants: concepts and definitions. Diversity and Distributions, 6, 93–107. [Google Scholar]
- Richardson D.M., Rejmánek M. (2011) Trees and shrubs as invasive alien species—a global review. Diversity and Distributions, 17, 788–809. [Google Scholar]
- Roth R., Paszkowski U. (2017) Plant carbon nourishment of arbuscular mycorrhizal fungi. Current Opinion in Plant Biology, 39, 50–56. [DOI] [PubMed] [Google Scholar]
- Shah M.A., Reshi Z.A., Khasa D.P. (2009) Arbuscular mycorrhizas: drivers or passengers of alien plant invasion. Botanical Reviews, 75, 397–417. [Google Scholar]
- Shao Q., Wang H., Guo H., Zhou A., Huang Y., Sun Y., et al. (2014) Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii . PLoS One, 9, e85996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G., Liu Y., Johnson N.C., Olsson P.A., Mao L., Cheng G., et al. (2014) Interactive influence of light intensity and soil fertility on root‐associated arbuscular mycorrhizal fungi. Plant & Soil, 378, 173–188. [Google Scholar]
- Shukla A., Kumar A., Jha A., Chaturvedi O.P., Prasad R., Gupta A. (2009) Effects of shade on arbuscular mycorrhizal colonization and growth of crops and tree seedlings in Central India. Agroforestry Systems, 76, 95–109. [Google Scholar]
- Simard S.W., Beiler K.J., Bingham M.A., Deslippe J.R., Philip L.J., Teste F.P. (2012) Mycorrhizal networks: mechanisms, ecology and modelling. Fungal Biology Reviews, 26, 39–60. [Google Scholar]
- Simberloff D., Martin J.‐L., Genovesi P., Maris V., Wardle D.A., Aronson J., et al. (2013) Impacts of biological invasions: what's what and the way forward. Trends in Ecology & Evolution, 28, 58–66. [DOI] [PubMed] [Google Scholar]
- Smith S.E., Read D.J. (2010) Mycorrhizal symbiosis. Academic Press, London, UK. [Google Scholar]
- Smith S.E., Smith F.A. (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annual Review of Plant Biology, 62, 227–250. [DOI] [PubMed] [Google Scholar]
- Souza‐Alonso P., Guisande‐Collazo A., González L. (2015) Gradualism in Acacia dealbata Link invasion: impact on soil chemistry and microbial community over a chronological sequence. Soil Biology & Biochemistry, 80, 315–323. [Google Scholar]
- Souza‐Alonso P., Novoa A., González L. (2014) Soil biochemical alterations and microbial community responses under Acacia dealbata Link invasion. Soil Biology & Biochemistry, 79, 100–108. [Google Scholar]
- Souza‐Alonso P., Rodríguez J., González L., Lorenzo P. (2017) Here to stay. Recent advances and perspectives about Acacia invasion in Mediterranean areas. Annals of Forest Science, 74, 55. [Google Scholar]
- Tanner R.A., Gange A.C. (2013) The impact of two non‐native plant species on native flora performance: potential implications for habitat restoration. Plant Ecology, 214, 423–432. [Google Scholar]
- Teskey R., Wertin T., Bauweraerts I., Ameye M., McGuire M.A., Steppe K. (2015) Responses of tree species to heat waves and extreme heat events. Plant, Cell & Environment, 38, 1699–1712. [DOI] [PubMed] [Google Scholar]
- Van der Putten W.H., Kowalchuk G.A., Brinkman E.P., Doodeman G.T.A., van der Kaaij R.M., Kamp A.F.D., et al. (2007) Soil feedback of exotic savanna grass relates to pathogen absence and mycorrhizal selectivity. Ecology, 88, 978–988. [DOI] [PubMed] [Google Scholar]
- Van Diepen L.T., Lilleskov E.A., Pregitzer K.S. (2011) Simulated nitrogen deposition affects community structure of arbuscular mycorrhizal fungi in northern hardwood forest. Molecular Ecology, 20, 799–811. [DOI] [PubMed] [Google Scholar]
- Vilà M., Espinar J.L., Hejda M., Hulme P.E., Jarošík V., Maron J.L., et al. (2011) Ecological impacts of invasive alien plants: a meta‐analysis of their effects on species, communities and ecosystems. Ecological Letters, 14, 702–708. [DOI] [PubMed] [Google Scholar]
- Vogelsang K.M., Bever J.D. (2009) Mycorrhizal densities decline in association with non‐native plants and contribute to plant invasion. Ecology, 90, 399–407. [DOI] [PubMed] [Google Scholar]
- Vogelsang K.M., Reynolds H.L., Bever J.D. (2006) Mycorrhizal fungal identity and richness determine the diversity and productivity of a tallgrass prairie system. New Phytologist, 172, 554–562. [DOI] [PubMed] [Google Scholar]
- Zhang H., Ziegler W., Han X., Trumbore S., Hartmann H. (2015) Plant carbon limitation does not reduce nitrogen transfer from arbuscular mycorrhizal fungi to Plantago lanceolata . Plant & Soil, 396, 369–380. [Google Scholar]
- Zheng C., Ji B., Zhang J., Zhang F., Bever J.D. (2015) Shading decreases plant carbon preferential allocation towards the most beneficial mycorrhizal mutualist. New Phytologist, 205, 361–368. [DOI] [PubMed] [Google Scholar]
- Zhu X.C., Song F.B., Liu S.Q., Liu T.D., Zhou X. (2012) Arbuscular mycorrhizae improve photosynthesis and water status of Zea mays L. under drought stress. Plant, Soil and Environment, 58, 186–191. [Google Scholar]
- Zubek S., Majewska M.L., Błaszkowski J., Stefanowicz A.M., Nobis M., Kapusta P. (2016) Invasive plants affect arbuscular mycorrhizal fungi abundance and species richness as well as the performance of native plants grown in invaded soils. Biology & Fertility of Soils, 52, 879–893. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of the native species present in the native plant community with no presence of A. dealbata. Species composition is typical from Atlantic shrublands, mainly dominated by a mixed composition of perennial shrubs, and with the presence of different annual grasses occupying the basal layer.


